Abstract
Background
Many community-acquired pleural infections are caused by facultative and anaerobic bacteria from the human oral microbiota. The epidemiology, clinical characteristics, pathogenesis, and etiology of such infections are little studied. The aim of the present prospective multicenter cohort study was to provide a thorough microbiological and clinical characterization of such oral-type pleural infections and to improve our understanding of the underlying etiology and associated risk factors.
Methods
Over a 2-year period, we included 77 patients with community-acquired pleural infection, whereof 63 (82%) represented oral-type pleural infections. Clinical and anamnestic data were systematically collected, and patients were offered a dental assessment by an oral surgeon. Microbial characterizations were done using next-generation sequencing. Obtained bacterial profiles were compared with microbiology data from previous investigations on odontogenic infections, bacteremia after extraction of infected teeth, and community-acquired brain abscesses.
Results
From the oral-type pleural infections, we made 267 bacterial identifications representing 89 different species. Streptococcus intermedius and/or Fusobacterium nucleatum were identified as a dominant component in all infections. We found a high prevalence of dental infections among patients with oral-type pleural infection and demonstrate substantial similarities between the microbiology of such pleural infections and that of odontogenic infections, odontogenic bacteremia, and community-acquired brain abscesses.
Conclusions
Oral-type pleural infection is the most common type of community-acquired pleural infection. Current evidence supports hematogenous seeding of bacteria from a dental focus as the most important underlying etiology. Streptococcus intermedius and Fusobacterium nucleatum most likely represent key pathogens necessary for establishing the infection.
Keywords: pleural infection, Fusobacterium nucleatum, Streptococcus intermedius, 16S rRNA, next-generation sequencing
Pleural empyema caused by bacteria from the oral microbiota is a common and distinct type of pleural infection. Hematogenous seeding of pathogens from an odontogenic infection to a locus minoris resistentiae in the lung is currently the most plausible pathogenesis.
Pleural empyema represents a diverse group of infections, where the underlying cause determines the spectrum of bacteria potentially involved. Until recently, empyema as a complication to bacterial pneumonia was considered most common [1]. However, it is now well established that many community-acquired pleural infections (CAPIs) are caused by facultative and anaerobic bacteria from the human oral microbiota not normally involved in pneumonia [2, 3]. Recent studies indicate that such oral-type pleural infections (OPIs) are in fact the most frequent type of pleural infections, but that their diagnosis is often dependent on molecular approaches [2, 4]. Bacterial culture remains negative in the majority of samples, presumably because of antibiotic treatment prior to sample collection or due to suboptimal conditions for fastidious bacteria [2, 4, 5].
The underlying causes of OPIs are not well investigated. Micro-aspiration of oral microbes is currently a leading explanation, but there are reasons to question this theory. Although anaerobic bacteria were previously considered important in aspiration pneumonia, this view has changed after the introduction of bronchoalveolar lavage and acknowledgment of a subepiglottic microbiota [6, 7]. Many patients with OPI have no radiologic signs of an underlying pneumonia [1, 2], and the bacteria involved are more strongly associated with dental infections than with the salivary microbiome [8–10].
In a recent retrospective study of pleural infections using 16S ribosomal RNA (rRNA) targeted next-generation sequencing (16S TNGS), we observed remarkable similarities between the microbiota of OPIs and that of oral-sinus–derived brain abscesses [2]. As already established for brain abscesses, we therefore suggested venous dissemination of bacteria from a dental focus as a plausible etiology also in such pleural infections [2]. We hypothesized that the same group of specialized facultative and anaerobic bacteria that, under certain conditions, spread via deoxygenated venous blood to cause a purulent infection in a highly oxygenated organ like the brain, are also capable of this in the pleural space. For both brain abscesses [11] and OPIs [2] we have suggested that Streptococcus intermedius, Fusobacterium nucleatum, and Aggregatibacter aphrophilus are key pathogens, meaning that the presence of at least 1 of them is necessary for establishing the infection.
The purpose of this study was to provide better data on the microbiology, clinical characteristics, and associated risk factors of OPI, and to investigate further the hypothesis of a venous route of infection from a dental focus.
METHODS
Study Design
This was a prospective multicenter cohort study with participation from 6 Norwegian hospitals (Akershus University Hospital, Førde Central Hospital, Haraldsplass Deaconess Hospital [HDH], Haukeland University Hospital [HUH], St Olav's University Hospital, and Stavanger University Hospital) with a total adult catchment population of 1 500 000. Based on our previous study [2], we estimated the annual incidence of CAPI to be 60–75 per year in this population. The study was approved by the regional ethical committee of South-East Norway (REK 31938).
Subjects
From January 2020 to December 2021, hospitalized patients aged 18 years and older with clinically suspected CAPI were assessed for inclusion. Patients qualified for inclusion if bacteria were confirmed in their pleural fluid by either culture and/or a broad range 16S rRNA gene polymerase chain reaction (PCR). Exclusion criteria were known cancer with potential lung involvement prior to admission, severe immunodeficiency, or pregnancy.
Definition of Oral-Type Pleural Infection
Oral-type pleural infection was defined as a pleural infection containing bacteria from the human oral microbiota not associated with pneumonia.
Data Collection
Predefined clinical data were registered systematically. These included age, sex, length of stay, readmissions, symptoms, duration of symptoms prior to admission, dental infections/treatments, thoracic trauma prior to onset of symptoms, relevant comorbidities, dental status, inflammatory parameters, diagnostic procedures, invasive treatment, antibiotic treatment, and mortality. Chest computed tomography (CT) scans were performed for all patients. For patients with microbiology indicating OPI, we encouraged CT of the sinuses and referral to the oral surgeon for dental assessment.
All pleural fluid samples were cultured at the site of inclusion. Residual sample material from the participating laboratories was shipped overnight with cooling elements to HUH. Sample material and/or extracted bacterial DNA was stored at −80°C until sequencing. 16S TNGS was performed at HUH using the MiSeq system (Illumina, Redwood City, CA, USA), as described previously [2]. Negative and positive sample processing controls were included with each run. For sequence data analysis, we used the RipSeq NGS software (Pathogenomix, Santa Cruz, CA, USA) [2]. Sequences representing contaminant bacterial DNA were filtered as described by Dyrhovden et al [12]. We did not include culture-independent methods for the detection of fungi.
Comparative Analyses of Microbiology Data
To further investigate the potential role of hematogenous seeding of bacteria from a dental focus in the etiology of OPI, we compared microbiological findings from this study with previous results from studies of the microbiology of dental infections, bacteremia following extraction of infected teeth, and the microbiology of brain abscesses. Data on the microbiota of 66 oral-sinus–derived brain abscesses were extracted from the 4 available studies [11, 13–15] that have used metagenomic approaches in the characterization of such infections (Supplementary Table 1). To define the most common bacteria in periapical tooth abscesses and severe periodontitis, we used data from 1 review [16] and 2 research papers [10, 17]. Data on bacteremia in relation to the treatment of dental infections were gathered from multiple publications [18–22].
Statistical Analyses
Statistical analyses were performed using the R programming language [23] version 2022.12.0+353. For qualitative values, baseline characteristics of patients with OPI and non-OPI were compared using a t test or Fisher's exact test, as appropriate. Quantitative values were tested for normality using a Quantile Quantile Plot and the Shapiro-Wilk test and compared using Mann-Whitney U test or unpaired t test, as appropriate. Phylogenetic and diversity analyses were performed using the R-package “phyloseq” [24] version 1.42.0. Permutational multivariate analysis of variance (PERMANOVA) was performed using the “adonis2” function in the R-package “vegan” [25] version 2.6-4. The figure comparing phylogenies of OPIs and brain abscesses was produced using the R-package “ggtree” [26] version 3.6.2. For neighbor-joining tree estimations of UniFrac analysis we used the R-package “ape” [27] version 5.7. Visualizations of phylogenetic trees were produced using the online tool “Interactive Tree of Life” [28].
RESULTS
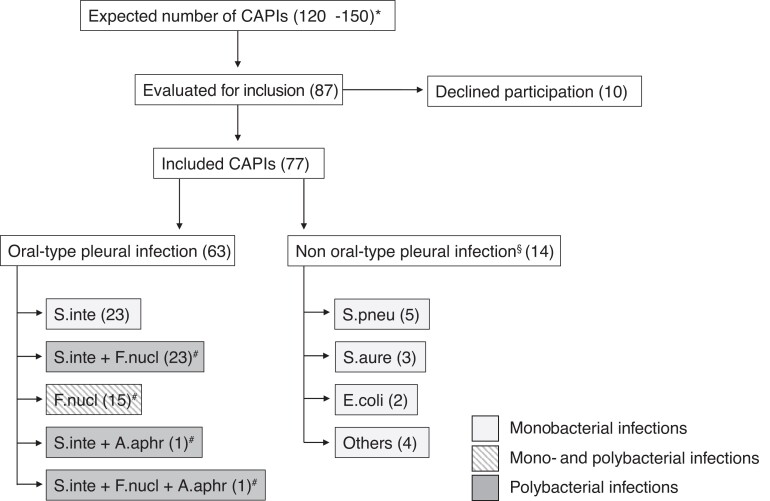
We included 77 unique patients with CAPI, of whom 63 (82%) had OPI as determined by 16S TNGS (Figure 1). Demographic and clinical characteristics for all patients, grouped as OPIs and non-OPIs, are provided in Table 1.
Figure 1.
Patient inclusion and distribution of key pathogens. *Precalculated number of patients based on reference [2]. §Related to pneumonia (10), pulmonary embolism (1), cholecystitis (1), systemic infection (1), or endocarditis (1). #Samples with F. nucleatum and/or A. aphrophilus often included many additional species not indicated in this figure (see Supplementary Table 2). Others: Bacillus cereus (1), Enterobacter cloacae (1), Listeria monocytogenes (1), and Streptococcus pyogenes (1). Abbreviations: A. aphr, A. aphrophilus; CAPIs, community-acquired pleural infections; E. coli, Escherichia coli; F. nucl, F. nucleatum; S. aure, Staphylococcus aureus; S. inte, S. intermedius; S. pneu, Streptococcus pneumoniae.
Table 1.
Characteristics and Clinical Data of All Included Patients
| OPI (n = 63) | Non-OPI (n = 14) | P | |
|---|---|---|---|
| Male | 45 (71%) | 12 (86%) | .334 |
| Age, y | 68 (±17, 73) | 68 (±15, 71) | .787 |
| Length of stay, d | 19 (±10, 17) | 19 (±10, 16) | .969 |
| Readmission within 1 montha | 4 (7%) | 1 (8%) | 1 |
| Duration of symptoms prior to admission (OPI n = 55), d | 18 (±24, 9) | 15 (±12, 11) | .731 |
| Initial symptoms | |||
| Pleuritic pain (OPI, n = 60) | 35 (57%) | 4 (28%) | .099 |
| Dyspnea (OPI, n = 60) | 25 (41%) | 5 (36%) | .952 |
| Dry cough (OPI, n = 60) | 25 (41%) | 3 (21%) | .290 |
| Fever | 12 (20%) | 3 (21%) | 1 |
| Productive cough (OPI, n = 60) | 7 (12%) | 2 (14%) | 1 |
| Symptoms during course of disease (OPI, n = 62) | |||
| Pleuritic pain | 53 (86%) | 7 (50%) | .007 |
| Dyspnea | 45 (73%) | 13 (93%) | .166 |
| Dry cough | 45 (73%) | 10 (71%) | 1 |
| Fever | 38 (61%) | 11 (79%) | .354 |
| Productive cough | 19 (31%) | 4 (29%) | 1 |
| Comorbidities | |||
| Intravenous drug injection | 4 (7%) | 1 (7%) | 1 |
| Alcohol abuse | 1 (2%) | 1 (7%) | .332 |
| Abuse of other drugs | 1 (2%) | 0 | 1 |
| Chronic obstructive pulmonary disease | 15 (24%) | 1 (7%) | .277 |
| Heart failure | 10 (16%) | 2 (14%) | 1 |
| Diabetes mellitus | 8 (13%) | 3 (21%) | .410 |
| Dementia | 3 (5%) | 1 (7%) | .560 |
| Kidney failure | 7 (11%) | 0 | .338 |
| Immune deficiencies | 0 | 0 | |
| Malignancy | 2 (3%) | 2 (14%) | .149 |
| Malignancy involving lungsb | 1 (2%) | 1 (7%) | .333 |
| Immunosuppressive treatment | 2 (3%) | 0 | 1 |
| Other pulmonary diseases | 2 (3%) | 3 (21%) | .039 |
| None of the above comorbidities | 26 (41%) | 4 (29%) | .563 |
| Chest trauma | |||
| Reported blunt chest trauma | 12 (22%) | 0 | .059 |
| Inflammatory markers at admission | |||
| C-reactive protein, mg/L | 221 (±115, 200) | 219 (±112, 200) | .999 |
| Leukocytes, 109/L | 19 (±11, 17) | 22 (±14, 17) | .853 |
| Neutrophil count (OPI, n = 51; non-OPI, n = 12), 109/L | 17 (±11, 15) | 21 (±12, 17) | .381 |
| Treatment | |||
| Days from admission to pleural drainage | 2.7 (±3·2, 2·0) | 3 (±3, 1) | .999 |
| Intercostal tube | 61 (97%) | 14 (100%) | 1 |
| Duration of intercostal tube drainage (OPI, n = 61), d | 10 (±7, 9) | 10 (±6, 9) | .688 |
| Fibrinolysis | 45 (71%) | 4 (29%) | .005 |
| Thoracotomy | 3 (5%) | 0 | 1 |
| Duration of intravenous antibiotics,a d | 19 (±10, 18) | 17 (±9, 14) | .265 |
| Total duration of antibiotic treatment,a d | 36 (±15, 34) | 34 (±12, 31) | .986 |
| Outcome | |||
| In-hospital death | 7 (11%) | 1 (7%) | 1 |
| Death within 1 year from admission | 11 (17%) | 4 (29%) | .455 |
| Days from admission to death | 43 (±53, 18) | 93 (±108, 62) | .513 |
Data are presented as no. (%) of patients for categorical variables and mean (±SD, median) for continuous variables. P values were obtained using chi-square or Fisher’s exact test to compare categorical variables and t test or Mann-Whitney U test (if required) to compare continuous variables.
Abbreviations: OPI, oral-type pleural infection; non-OPI, non–oral-type pleural infection.
aIn-hospital deaths not included in analysis.
bMalignancy not known at hospital admission and patients were therefore not excluded.
The coronavirus disease 2019 (COVID-19) pandemic affected inclusion and access to nonessential investigations such as CT of the sinuses and oral surgeon assessment. In 2 hospitals (HDH and HUH), inclusion is believed to have been complete. These 2 hospitals registered, in total, 42 included and 4 nonincluded empyemas, whereof 39 (85%) represented OPIs. With a combined adult catchment population of approximately 440 000, this equals an annual incidence for CAPI of 5.2 per 100 000 and for OPI 4.4 per 100 000. In the remaining 4 hospitals, inclusions were more sporadic (total of 35 patients; combined catchment area: ∼1 million).
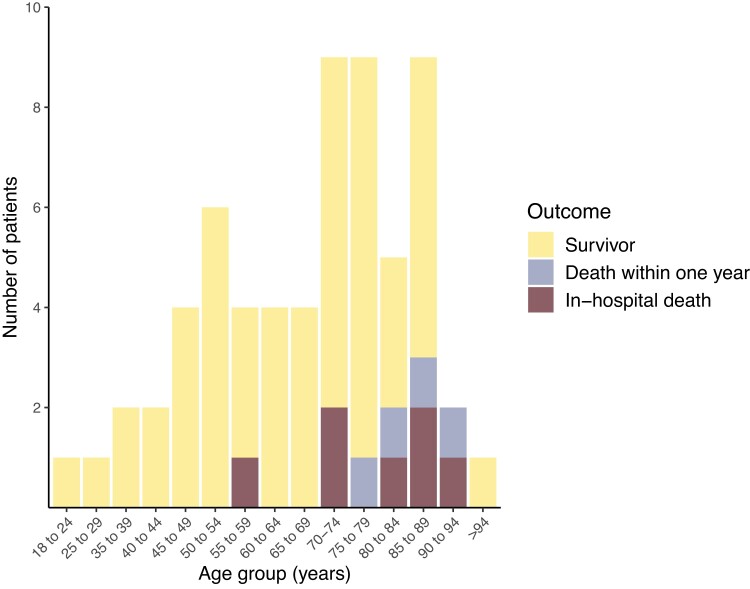
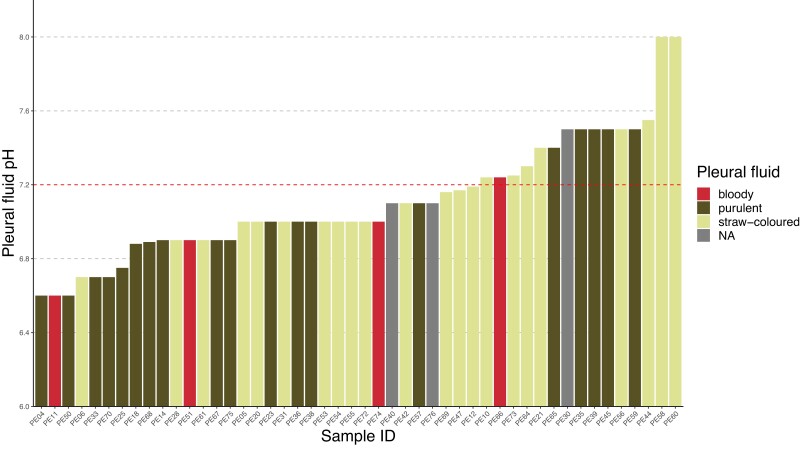
Most patients with OPI presented with classic symptoms and elevated infection parameters (Table 1). The most common initial presentations were pleuritic pain, dyspnea, and dry cough. Fifty-three out of 61 (87%) patients with OPI reported 1 or more of these as their first symptoms. These were also the most common symptoms during the course of the disease. For patients with OPI, we found an in-hospital mortality of 11% and a 1-year mortality of 17%. Mortality was confined to patients with advanced age (Figure 2) and/or significant comorbidities. Pleural fluid macroscopic appearance and pH are presented in Figure 3.
Figure 2.
Patient outcome in relation to age for all oral-type pleural infections.
Figure 3.
Macroscopic appearance and pH value of oral-type pleural infections. pH value was reported for 48 out of 63 (76%) patients. For 3 of these patients, the macroscopic appearance was not reported. Thirteen (27%) of the 48 oral-type pleural infections had a pH ≥7.2. Abbreviation: NA, not available.
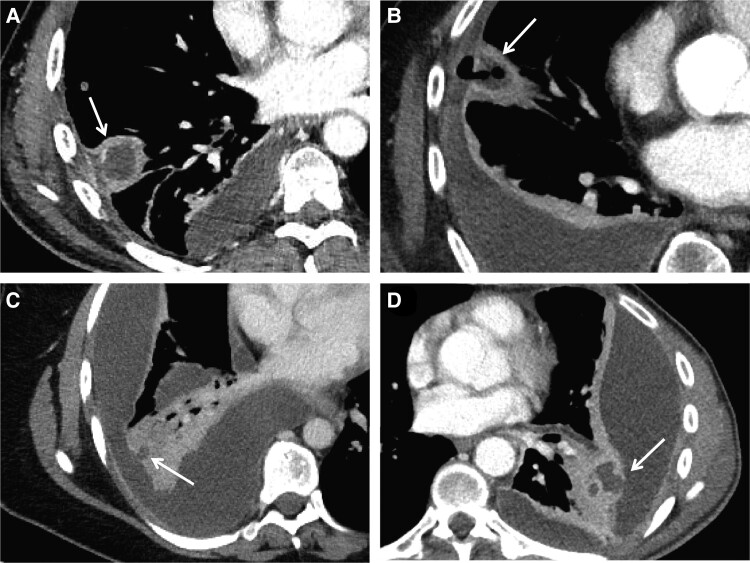
A systematic presentation of relevant findings by chest CT scan for all patients is provided in Table 2. Among the 63 patients with OPI, 24 (38%) also had radiological evidence of a lung abscess as described in the radiologist report. The CT images indicated communication between the abscess and the pleural empyema for several of these patients (Figure 4). Fourteen (22%) patients with OPI had no reported lung parenchymal involvement except for atelectasis. The corresponding number for the 14 non-OPIs was 3 (21%).
Table 2.
Chest Computed Tomography Scan Findings and Diagnostic Suggestions by the Radiologist as Described in the Radiology Reports
| Oral-Type Pleural Infection (n = 63) | Non–Oral-Type Pleural Infection (n = 14) | P | |
|---|---|---|---|
| Location | |||
| Right side only | 31 (49%) | 9 (64%) | .468 |
| Left side only | 25 (40%) | 1 (7%) | .027 |
| Both sides | 7 (11%) | 4 (29%) | .107 |
| Findings as reported by radiologist | |||
| Pleural fluid | 63 (100%) | 14 (100%) | 1 |
| Atelectasis | 49 (80%) | 10 (71%) | .728 |
| Unspecified opacifications | 27 (43%) | 8 (57%) | .500 |
| Nodule/pulmonary mass | 29 (46%) | 3 (21%) | .165 |
| Consolidations | 23 (37%) | 5 (36%) | 1 |
| Ground-glass opacities | 4 (6%) | 1 (7%) | 1 |
| Reticular opacifications | 1 (2%) | 0 | 1 |
| Mediastinal lymphadenopathy | 14 (22%) | 4 (29%) | .728 |
| Hilar lymphadenopathy | 11 (18%) | 3 (21%) | .711 |
| Emphysema | 4 (6%) | 1 (7%) | 1 |
| Venous thrombosis masses | 0 | 1 (7%) | 1 |
| Diagnostic suggestion by radiologist | |||
| Pleural empyema | 38 (60%) | 9 (64%) | 1 |
| Lung abscess | 24 (38%) | 3 (21%) | .355 |
| Pneumonia | 15 (24%) | 3 (21%) | 1 |
| Malignancy | 8 (13%)a | 1 (7%) | 1 |
| Necrotizing pneumonia | 7 (11%) | 0 | .338 |
| Bronchiolitis | 1 (2%) | 0 | 1 |
| Pulmonary embolism | 0 | 1 (7%) | 1 |
Data are presented as no. (%) of patients. P values were obtained using chi-square or Fisher’s exact test. The first chest CT scan results after admission are presented.
Abbreviation: CT, computed tomography.
aNone of the 8 patients where the radiologist suggested that the initial chest CT scan findings could represent malignancy were later confirmed to actually have a malignant disease. These initial radiologic findings probably represented a consolidation of infectious origin.
Figure 4.
Axial contrast-enhanced CT images from 4 patients with oral-type pleural infection showing communications between a lung abscess and the pleural empyema. A, Patient PE66. CT image with a mediastinal setting showing a consolidated opacity (33 × 29 mm) with central hypodensity and peripheral contrast enhancement (arrow) in the right lower lobe that communicates with the pleural space. In the adjacent pleura, there is a pocket with central hypodense content and peripheral contrast enhancement that extends along the pleura approximately 30 mm in craniocaudal direction. B, Patient PE60. CT image with a mediastinal setting showing a consolidated opacity (arrow) with central hypodensity (∼20 × 20 mm) and air bubbles that continue into the lateral pleural cavity. The pleural fluid here communicates with the larger posteriorly located fluid accumulation. C, Patient PE42. CT image with a mediastinal setting showing lobular organized pleural fluid in the right hemithorax. In the lower lobe is a consolidated opacity with a small hypodense area (arrow) of 8-mm diameter that communicates with the adjacent pleural fluid. D, Patient PE56. CT image with a mediastinal setting showing several apparently encapsulated fluid loculations in the left part of the thorax that compress adjacent lung tissue. They are lenticular in shape and provide compression of adjacent lung tissue. Medial to the largest loculation is a consolidated opacity containing several pockets with central hypodensity and peripheral contrast enhancement. The largest of these pockets (arrow) communicates with the adjacent pleural fluid. Abbreviation: CT, computed tomography.
Two hospitals (HUH and HDH) had regular access to an oral surgeon throughout the study. Of 35 included patients with OPI, they referred 25 to orodental assessment. Among these, 20 (80%) had odontogenic infections such as apical abscesses or severe periodontitis, of whom 18 had 1 or more teeth extracted (range: 1–8; median: 2; average: 2.5). Among the 5 patients without an oral focus, 1 had a focus in the small intestine, whereas 4 presented no obvious alternative foci. Among those not assessed by a dental surgeon, 2 died during the hospital stay. The remaining 8 patients either had a general health condition that did not allow for assessment by a dental surgeon, they declined a dental assessment, or the doctor in charge did not refer them. Four of these patients had a mention of poor dental health in their medical record. Among the 28 patients with OPI from the other hospitals, 11 had a dental status assessment. Of these, 7 had a possible dental focus and 3 had 1 or more teeth removed. Eighteen patients had a CT of the sinuses, all of which were negative.
From the OPIs, we made 267 bacterial identifications representing 89 species from 47 genera (Supplementary Table 2). Culture identified only 44 (16%) bacteria, and 31 (50%) of the 16S rRNA gene PCR-positive empyemas were culture negative. We identified S. intermedius and/or F. nucleatum in all samples, including monobacterial infections (Figure 1). Only 20 species were identified in 3 or more samples (ie, had a prevalence ≥5%). From these, 17 are also among the most common bacteria in dental apical abscesses (n = 15) and/or associated with severe periodontitis (n = 9) (Table 3). Among the remaining 3, both Slackia exigua and Campylobacter gracilis have been recovered from dental abscesses and found to be associated with periodontitis in other studies [29, 30]. Fifteen of the 20 species have been reported as causes of bacteremia, 13 specifically in relation to extraction of infected teeth (Table 3). Among the 47 most common species in apical abscesses according to Siqueira and Rôças [16] and George et al [10], 30 were recovered from 1 or more OPI in the present investigation (Supplementary Table 3).
Table 3.
The Most Common Bacteria in Oral-Type Pleural Infections and Their Presence in Dental Infections and Bacteremia
| Most Common Species in OPIa | No.b | Common in AA |
Associated With PD |
Growth in BC |
Growth in BC-DE |
|---|---|---|---|---|---|
| Streptococcus intermedius | 43 | x | … | x | x |
| Fusobacterium nucleatum | 39 | x | … | x | x |
| Parvimonas micra | 18 | x | x | x | x |
| Eubacterium brachy | 11 | x | x | … | … |
| Porphyromonas endodontalis | 7 | x | x | … | … |
| Campylobacter gracilis | 6 | (x) | (x) | x | x |
| Prevotella oris | 6 | x | … | x | x |
| Dialister pneumosintes | 5 | x | … | x | x |
| Streptococcus constellatus | 5 | x | x | x | x |
| Campylobacter rectus | 4 | x | … | x | x |
| Filifactor alocis | 4 | x | x | x | x |
| Lancefieldella rimae | 4 | x | … | x | … |
| Prevotella conceptionensis | 4 | … | … | … | … |
| Slackia exigua | 4 | (x) | (x) | x | x |
| Tannerella forsythia | 4 | x | x | x | x |
| Treponema lecithinolyticum | 4 | … | x | … | … |
| Eikenella corrodens/exigua | 4 | x | … | x | x |
| Peptostreptococcaceae(XI)(G-4) HMT369 c | 3 | … | x | … | … |
| Peptostreptococcus stomatis | 3 | x | x | x | … |
| Prevotella intermedia | 3 | x | … | x | x |
x = most common according to references 10, 16, and 17; (x) = common according to references 29 and 30.
Abbreviations: AA, apical abscess; BC, blood culture; BC-DE, blood culture drawn in relation to dental extraction; OPI, oral-type pleural infection; PD, periodontitis.
aPrevalence ≥5%.
bNumber of detections in this study.
cFor undescribed oral species, we applied the provisional Human Microbial Taxon-taxonomy (www.homd.org).
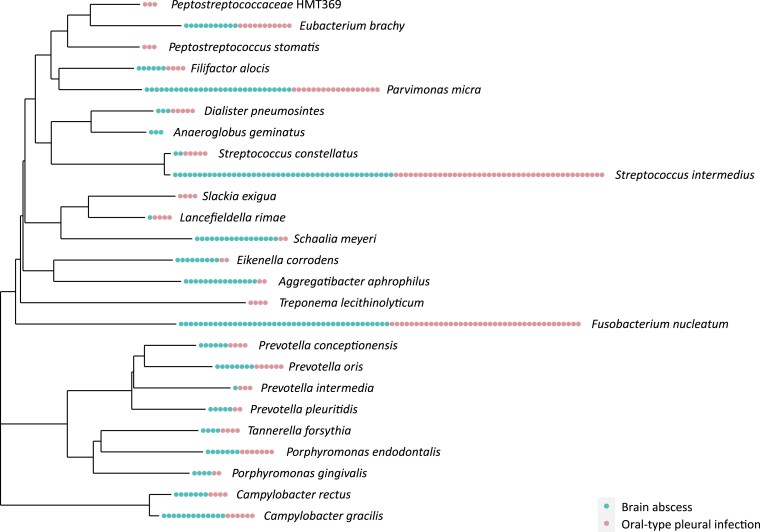
A comparison of the 25 species present in 3 or more (≥5%) samples of either OPI (20 species) and/or sinus-oral–derived brain abscesses (18 species) shows a species overlap of 80% (Figure 5). We also found a high concordance in the number of detections, except for the facultative bacteria A. aphrophilus, Eikenella corrodens, and Schaalia meyeri that seem to be more prevalent in brain abscesses. Results from PERMANOVA showed a significant difference (P = .004) between the 2 groups of infections in the unweighted UniFrac analysis (Supplementary Table 4A). In the weighted UniFrac analysis that is more influenced by the dominant species in each sample (typically, the key pathogens eventually together with various combinations of the other species represented in Figure 5), PERMANOVA showed no significant difference (P = .100). Samples from the 2 types of infections appeared indistinguishable in phylogenetic trees based on both weighted and unweighted UniFrac analysis (Supplementary Figure 1). We conducted the same comparative analyses for OPIs with and without lung abscess. We found no significant differences in the weighted (P = .208) or unweighted (P = .074) UniFrac analyses (Supplementary Table 4B), and samples appeared indistinguishable in the phylogenetic trees (Supplementary Figure 2).
Figure 5.
Phylogenetic tree of all species present in 3 or more (≥5%) samples of either oral-type pleural infection (20 species) and/or sinus-oral–derived brain abscesses (18 species). Each dot represents 1 sample containing the associated bacterium and is colored according to the type of infection.
DISCUSSION
This is the first study designed to investigate the epidemiology, microbiology, clinical characteristics, and risk factors associated with OPIs. We found that OPI was the most common type of CAPI, with an annual incidence of 4.4 per 100 000 inhabitants. The observed dominance of S. intermedius and F. nucleatum in OPIs is in line with our previous findings and strengthens our hypothesis that these are key pathogens necessary for establishing the infections. We place OPI into the broader context of purulent infections of odontogenic origin by finding a high prevalence of dental infections among these patients, and by demonstrating substantial similarities between the microbiota of such empyemas and the microbiota of dental infections.
Route of Infection in Oral-Type Pleural Infections
The strong association with deep-sited odontogenic infections is an argument for a hematogenous route of infection in OPIs. The fact that the most common bacteria in OPIs have been cultured from blood in relation to extraction of infected teeth and that the same bacteria are able to spread hematogenously to establish purulent infections in the brain with microbial compositions indistinguishable from that of the pleural infections strongly supports this hypothesis.
Most OPIs (70%) contained members from the 20 most frequent species only. Streptococcus intermedius and/or F. nucleatum were the sole pathogens in 31 (50%) samples and the dominant bacteria in 50 (81%). Considering the more than 1000 species in the human oral microbiota, such sample homogeneity supports a specialized route of infection restricting the number of pathogens, rather than an unselective micro-aspiration.
Key Pathogens and Microbial Patterns in Oral-Type Pleural Infections
Among the 63 OPIs, 23 contained S. intermedius as the sole key pathogen—all monomicrobial. Fusobacterium nucleatum was the sole key pathogen in 15 samples, of which 4 were monomicrobial. Twenty-four samples contained both S. intermedius and F. nucleatum, whereof 6 harbored these 2 species only and 18 were more complex (Figure 1). Based on these findings, we suggest that F. nucleatum is essential for the formation of polymicrobial empyemas. Such polymicrobial empyemas contain predominantly anaerobic bacteria, except for S. intermedius and the sporadic facultative genera Actinomyces, Eikenella, and Schaalia. This mirrors periodontitis research where F. nucleatum is considered crucial in the formation of complex plaques due to co-aggregating capabilities that protect other anaerobes from oxidative stress [31]. It also represents an adjustment of our previous understanding of F. nucleatum as essential only for the formation of an anaerobe component [2]. Based on a few samples from this and our previous studies [2, 11], we believe A. aphrophilus to represent a third, more unusual key pathogen that can also support the formation of polymicrobial infections.
Risk Factors for Oral-Type Pleural Infections
Pleural empyema is associated with advanced age, male gender, alcoholism, and intravenous drug abuse [32–34]. A common argument is that this is due to increased risk of micro-aspiration in these groups. However, these are also risk factors for the development of brain abscess. We suggest that the actual underlying risk factor for both conditions could be odontogenic infections, which are associated with all of the factors mentioned above. In our population, 80% of patients referred to the oral surgeon were diagnosed with significant dental infections.
For brain abscesses, a main pathogenic factor is the presence of an ischemic or devitalized locus minoris resistentiae [35]. We have previously hypothesized that this could also be important in the pathogenesis of OPIs, and that a blunt chest trauma with concomitant contusion of the peripheral lung parenchyma could represent a predisposing factor [2]. Older age, male sex, intravenous drug abuse, and alcoholism are all associated with an increased risk of thoracic trauma due to falls, manual work, or violence. Among our patients with OPI, 12 (22%) reported such trauma in the days prior to the onset of symptoms. Since many of the old and debilitated patients struggled to recall details from the weeks prior to hospitalization, this might represent an underreporting. According to their medical records, 9 more patients had a fall tendency due to hemiparesis, severe ataxia, multiple sclerosis, or epilepsy. Vascular events, unrecognized tumors, and recent lower respiratory tract infections might also form deoxygenated loci serving as potential predilection sites for hematogenous seeding of bacteria. A recent epidemiological study found an increased incidence of empyema in the winter season and the authors suggested a possible link between influenza and empyema [36]. However, the potential role of a locus minoris resistentiae needs to be further investigated.
One of our patients without a dental focus was diagnosed with a necrotic ileum and lower jejunum. The small intestinal microbiota contains many of the bacteria common in OPI, including S. intermedius and F. nucleatum [37]. This elderly patient reported falling from a chair some days prior to the onset of chest pain. For this patient we assume that bacteria have spread hematogenously from the small intestine to a locus minoris resistentiae in the lung.
Oral-Type Pleural Infections and Lung Abscess
Lung abscess and pleural empyema are generally considered separate conditions. However, most lung abscesses contain the same oral pathogens found in OPIs and poor dental health is recognized as a major risk factor [38]. Concurrent lung abscess and pleural empyema has been described in a small proportion of patients in studies of lung abscesses [38, 39] and pleural empyemas [2, 40].
In the present study, 24 (38%) patients with OPI had radiologic signs of a lung abscess. A likely pathogenesis for the pleural infection in these patients is leakage of bacteria from a ruptured abscess into the pleural cavity. Notably, there were no significant differences between the microbiota of abscess-related empyemas and that of empyemas without radiological evidence of an abscess (Supplementary Figure 2, Supplementary Table 4B).
We suggest that OPI and lung abscess caused by oral bacteria might represent different, sometimes overlapping, presentations of the same disease. Following hematogenous seeding to a predilection site in the lung, these bacteria establish a purulent infection leading to a consolidation/abscess that can, depending on factors like the proximity to the pleura and the immune status of the patient, erode into the pleural cavity. If bacteria spread directly to a predilection site in the pleura, it might result in an infection without involvement of the lung. This could explain why a significant group of pleural infections have no evidence of concurrent parenchymal infection [41].
Assessment of Strengths and Weaknesses
The strengths of our study include the prospective design aimed specifically at describing the subgroup of OPIs and evaluating hypotheses generated from a previous retrospective material. Other important qualities include complete microbiological characterizations by 16S TNGS and a dental status assessment by an oral surgeon for 58% of patients. We discuss our findings in the context of purulent infections of odontogenic origin and provide new evidence to support the role of hematogenous bacterial seeding from a dental focus in the formation of OPI. This theory may explain acknowledged risk factors and is built on observations that can be re-evaluated in future research.
Our study also has limitations. Inclusion was incomplete in 4 centers, resulting in fewer than expected patients. Although microbial patterns and clinical presentations were highly consistent and probably not affected by this, it clearly reduces the strength of our incidence data. The incidence of CAPI in this study was somewhat higher than the incidence of “no surgery–no cancer” empyema in a recent retrospective French study (5.2 vs 4.7/100 000) [42]. It is a potential bias that 42% of the patients did not have a professional assessment of their dental status. It might be that patients with obvious dental problems were more often referred to the dental surgeon. Lack of data on the dental status for the 14 patients with non-OPI is also a weakness.
We conclude that OPI is the most common type of CAPI and that S. intermedius and F. nucleatum most likely represent key pathogens necessary for establishing the infection. Current evidence supports hematogenous seeding of bacteria from a dental focus as the most important underlying etiology.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Ruben Dyrhovden, Department of Microbiology, Haukeland University Hospital, Bergen, Norway.
Tomas Mikal Eagan, Department of Clinical Science, University of Bergen, Bergen, Norway; Department of Thoracic Medicine, Haukeland University Hospital, Bergen, Norway.
Øystein Fløtten, Department of Clinical Science, University of Bergen, Bergen, Norway; Department of Thoracic Medicine, Haukeland University Hospital, Bergen, Norway.
William Siljan, Department of Pulmonary Medicine, Akershus University Hospital, Lørenskog, Norway.
Truls Michael Leegaard, Division of Medicine and Laboratory Sciences, Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Microbiology and Infection Control, Akershus University Hospital, Akershus, Norway.
Bjørnar Bø, Department of Pulmonary Medicine, Stavanger University Hospital, Stavanger, Norway.
Hilde Fardal, Department of Microbiology, Stavanger University Hospital, Stavanger, Norway.
Fredrik Grøvan, Department of Medicine, Haraldsplass Deaconess Hospital, Bergen, Norway.
Arne Kildahl-Andersen, Department of Thoracic Medicine, St Olavs Hospital, Trondheim University Hospital, Trondheim, Norway.
Kjersti Wik Larssen, Department of Medical Microbiology, St Olavs Hospital, Trondheim University Hospital, Trondheim, Norway.
Rune Tilseth, Department of Medicine, Førde Central Hospital, Førde, Norway.
Reidar Hjetland, Department of Microbiology, Førde Central Hospital, Førde, Norway.
Sigbjørn Løes, Department of Maxillofacial Surgery, Haukeland University Hospital, Bergen, Norway; Faculty of Health Sciences, UiT The Arctic University of Norway, Tromsø, Norway.
Frode Lindemark, Department of Thoracic Medicine, Haukeland University Hospital, Bergen, Norway.
Marit Tellevik, Department of Microbiology, Haukeland University Hospital, Bergen, Norway.
Rebecca Breistein, Department of Microbiology, Haukeland University Hospital, Bergen, Norway.
Øyvind Kommedal, Department of Microbiology, Haukeland University Hospital, Bergen, Norway.
Notes
Author Contributions. R. D. and Ø. K. conceived and designed the study and prepared the ethics protocol. T. M. E., Ø. F., W. S., B. B., F. G., A. K.-A., R. T., S. L., and F. L. enrolled patients and collected samples and clinical data. T. M. L., H. F., K. W. L., F. G., and R. H. were responsible for the study at the local hospitals and responsible for the local microbiological diagnostic procedures and sending of samples to HUH for 16S rRNA next-generation sequencing (TNGS). M. T. and R. B. performed the 16S rRNA TNGS preparation and analysis. R. D. and Ø. K. analyzed the 16S rRNA TNGS data and performed the statistical analysis. R. D. and Ø. K. wrote the first draft of the manuscript. Ø. K. supervised the study. All authors revised and approved the final version of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. R. D. and Ø. K. verified the underlying data of the study.
Financial support. This work was supported by the Department of Microbiology, Haukeland University Hospital, which funded 16S rRNA TNGS analysis of samples.
Data sharing. The 16S rRNA TNGS data have been deposited in the European Nucleotide Archive (ENA) at European Molecular Biology Laboratory-European Bioinformatics Institute (EMBL-EBI) under accession number PRJEB62005 (https://www.ebi.ac.uk/ena/browser/view/PRJEB62005) and will be made available upon publication. Other source data for this study are available from the corresponding author upon request. Not all patient data are publicly available due to restrictions from the regional ethical committee.
References
- 1. Corcoran JP, Wrightson JM, Belcher E, DeCamp MM, Feller-Kopman D, Rahman NM. Pleural infection: past, present, and future directions. Lancet Respir Med 2015; 3:563–77. [DOI] [PubMed] [Google Scholar]
- 2. Dyrhovden R, Nygaard RM, Patel R, Ulvestad E, Kommedal O. The bacterial aetiology of pleural empyema. A descriptive and comparative metagenomic study. Clin Microbiol Infect 2019; 25:981–6. [DOI] [PubMed] [Google Scholar]
- 3. Hassan M, Cargill T, Harriss E, et al. The microbiology of pleural infection in adults: a systematic review. Eur Respir J 2019; 54:1900542. [DOI] [PubMed] [Google Scholar]
- 4. Kanellakis NI, Wrightson JM, Gerry S, et al. The bacteriology of pleural infection (TORPIDS): an exploratory metagenomics analysis through next generation sequencing. Lancet Microbe 2022; 3:e294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maskell NA, Batt S, Hedley EL, Davies CW, Gillespie SH, Davies RJ. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Resp Crit Care 2006; 174:817–23. [DOI] [PubMed] [Google Scholar]
- 6. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest 1999; 115:178–83. [DOI] [PubMed] [Google Scholar]
- 7. Knudsen KS, Lehmann S, Nielsen R, et al. The lower airways microbiota and antimicrobial peptides indicate dysbiosis in sarcoidosis. Microbiome 2022; 10:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Böttger S, Zechel-Gran S, Schmermund D, et al. Microbiome of odontogenic abscesses. Microorganisms 2021; 9:1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siqueira JF, Rôças IN. The microbiota of acute apical abscesses. J Dent Res 2009; 88:61–5. [DOI] [PubMed] [Google Scholar]
- 10. George N, Flamiatos E, Kawasaki K, et al. Oral microbiota species in acute apical endodontic abscesses. J Oral Microbiol 2016; 8:30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kommedal Ø, Wilhelmsen MT, Skrede S, et al. Massive parallel sequencing provides new perspectives on bacterial brain abscesses. J Clin Microbiol 2014; 52:1990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dyrhovden R, Rippin M, Øvrebø KK, Nygaard RM, Ulvestad E, Kommedal Ø. Managing contamination and diverse bacterial loads in 16S rRNA deep sequencing of clinical samples: implications of the law of small numbers. Mbio 2021; 12:e00598-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stebner A, Ensser A, Geißdörfer W, Bozhkov Y, Lang R. Molecular diagnosis of polymicrobial brain abscesses with 16S-rDNA-based next-generation sequencing. Clin Microbiol Infect 2021; 27:76–82. [DOI] [PubMed] [Google Scholar]
- 14. Masalma MA, Lonjon M, Richet H, et al. Metagenomic analysis of brain abscesses identifies specific bacterial associations. Clin Infect Dis 2012; 54:202–10. [DOI] [PubMed] [Google Scholar]
- 15. Hansen KH, Justesen US, Kelsen J, Møller K, Helweg-Larsen J, Fuursted K. Diagnostics with clinical microbiome-based identification of microorganisms in patients with brain abscesses—a prospective cohort study. APMIS 2021; 129:641–52. [DOI] [PubMed] [Google Scholar]
- 16. Siqueira JF, Rôças IN. Microbiology and treatment of acute apical abscesses. Clin Microbiol Rev 2013; 26:255–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abusleme L, Dupuy AK, Dutzan N, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J 2013; 7:1016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bahrani-Mougeot FK, Paster BJ, Coleman S, Ashar J, Barbuto S, Lockhart PB. Diverse and novel oral bacterial species in blood following dental procedures. J Clin Microbiol 2008; 46:2129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008; 117:3118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bein T, Brem J, Schüsselbauer T. Bacteremia and sepsis due to Prevotella oris from dentoalveolar abscesses. Intensive Care Med 2003; 29:856–856. [DOI] [PubMed] [Google Scholar]
- 21. Rajasuo A, Perkki K, Nyfors S, Jousimies-Somer H, Meurman JH. Bacteremia following surgical dental extraction with an emphasis on anaerobic strains. J Dent Res 2004; 83:170–4. [DOI] [PubMed] [Google Scholar]
- 22. Könönen E, Bryk A, Niemi P, Kanervo-Nordström A. Antimicrobial susceptibilities of Peptostreptococcus anaerobius and the newly described Peptostreptococcus stomatis isolated from various human sources. Antimicrob Agents Chemother 2007; 51:2205–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. Available at: https://www.R-project.org/.
- 24. McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013; 8:e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oksanen J, Simpson GL, Blanchet FG, et al. Vegan: community ecology package; 2022. Available at: https://github.com/vegandevs/vegan.
- 26. Yu G, Smith DK, Zhu H, Guan Y, Lam TT. Ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol 2017; 8:28–36. [Google Scholar]
- 27. Paradis E, Schliep K. Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019; 35:526–8. [DOI] [PubMed] [Google Scholar]
- 28. Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 2021; 49:W293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hashimura T, Sato M, Hoshino E. Detection of Slackia exigua, Mogibacterium timidum and Eubacterium saphenum from pulpal and periradicular samples using the polymerase chain reaction (PCR) method. Int Endod J 2001; 34:463–70. [DOI] [PubMed] [Google Scholar]
- 30. Siqueira JF, Rôças IN. Campylobacter gracilis and Campylobacter rectus in primary endodontic infections. Int Endod J 2003; 36:174–80. [DOI] [PubMed] [Google Scholar]
- 31. Steeves CH, Potrykus J, Barnett DA, Bearne SL. Oxidative stress response in the opportunistic oral pathogen Fusobacterium nucleatum. Proteomics 2011; 11:2027–37. [DOI] [PubMed] [Google Scholar]
- 32. Bedawi EO, Ricciardi S, Hassan M, et al. ERS/ESTS statement on the management of pleural infection in adults. Eur Respir J 2023; 61:2201062. [DOI] [PubMed] [Google Scholar]
- 33. Cargill TN, Hassan M, Corcoran JP, et al. A systematic review of comorbidities and outcomes of adult patients with pleural infection. Eur Respir J 2019; 54:1900541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chalmers JD, Singanayagam A, Murray MP, Scally C, Fawzi A, Hill AT. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax 2009; 64:592–7. [DOI] [PubMed] [Google Scholar]
- 35. Brook I. Brain abscess and other focal pyogenic infections of the central nervous system. In: Cohen JP, William G, Opal SM, editors. Infectious Diseases. 4th ed. Amsterdam: Elsevier Ltd, 2017:198–207.e1. [Google Scholar]
- 36. Arnold DT, Hamilton FW, Morris TT, et al. Epidemiology of pleural empyema in English hospitals and the impact of influenza. Eur Respir J 2021; 57:2003546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villmones HC, Svanevik M, Ulvestad E, et al. Investigating the human jejunal microbiota. Sci Rep 2022; 12:1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takayanagi N, Kagiyama N, Ishiguro T, Tokunaga D, Sugita Y. Etiology and outcome of community-acquired lung abscess. Respiration 2010; 80:98–105. [DOI] [PubMed] [Google Scholar]
- 39. Monteiro R, Alfaro TM, Correia L, Simão A, Carvalho A, Costa JN. Lung abscess and thoracic empyema: retrospective analysis in an internal medicine department. Acta Medica Port 2011; 24(Suppl 2):229–40. [PubMed] [Google Scholar]
- 40. Huang H-C, Chen H-C, Fang H-Y, Lin Y-C, Wu C-Y, Cheng C-Y. Lung abscess predicts the surgical outcome in patients with pleural empyema. J Cardiothorac Surg 2010; 5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Franklin J, Talwar A, Addala D, et al. CT Appearances of pleural infection: analysis of the second Multi-Centre Intra-pleural Sepsis Trial (MIST 2) cohort. Clin Radiol 2021; 76:436–42. [DOI] [PubMed] [Google Scholar]
- 42. Bobbio A, Bouam S, Frenkiel J, et al. Epidemiology and prognostic factors of pleural empyema. Thorax 2021; 76:1117–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.