Abstract
Background
Postural abnormalities involving the trunk are referred to as axial postural abnormalities and can be observed in over 20% of patients with Parkinson's disease (PD) and in atypical parkinsonism. These symptoms are highly disabling and frequently associated with back pain and a worse quality of life in PD. Despite their frequency, little is known about the pathophysiology of these symptoms and scant data are reported about their clinical predictors, making it difficult to prompt prevention strategies.
Objectives
We conducted a scoping literature review of clinical predictors and pathophysiology of axial postural abnormalities in patients with parkinsonism to identify key concepts, theories and evidence on this topic.
Methods
We applied a systematic approach to identify studies, appraise quality of evidence, summarize main findings, and highlight knowledge gaps.
Results
Ninety‐two articles were reviewed: 25% reported on clinical predictors and 75% on pathophysiology. Most studies identified advanced disease stage and greater motor symptoms severity as independent clinical predictors in both PD and multiple system atrophy. Discrepant pathophysiology data suggested different potential central and peripheral pathogenic mechanisms.
Conclusions
The recognition of clinical predictors and pathophysiology of axial postural abnormalities in parkinsonism is far from being elucidated due to literature bias, encompassing different inclusion criteria and measurement tools and heterogeneity of patient samples. Most studies identified advanced disease stage and higher burden of motor symptoms as possible clinical predictors. Pathophysiology data point toward many different (possibly non‐mutually exclusive) mechanisms, including dystonia, rigidity, proprioceptive and vestibular impairment, and higher cognitive deficits.
Keywords: axial postural abnormalities, Pisa syndrome, Camptocormia, Antecollis, Parkinsonisms, clinical predictors, pathophysiology
Postural abnormalities involving the trunk are referred to as axial postural abnormalities and can be observed in over 20% of patients with Parkinson's disease (PD) during the disease course. 1 Similarly, patients with other forms of parkinsonism, including multiple system atrophy (MSA), Lewy body dementia (DLB), supranuclear gaze palsy (PSP), can present axial postural abnormalities, with even higher prevalence, especially in MSA (up to 67%). 2 , 3 Axial postural abnormalities are part of the group of axial symptoms affecting parkinsonian patients and consist of abnormal trunk or neck flexion in the upright position, often worsened by prolonged standing or walking. 1 , 2 , 4 , 5 These symptoms proved to be highly disabling for patients, and associated with more back pain, increased risk of falling and a worse quality of life. 2 , 5 , 6 Noteworthy, axial postural abnormalities can be also observed as an idiopathic entity in people without parkinsonism or other known neurological diseases, with some forms like mild–moderate anterior trunk flexion being more common in the elderly population. 7
Recently, an expert‐based consensus was reached for the harmonization of nosology and cut‐off values to define and categorize axial postural abnormalities in PD patients 1 , 4 ; accordingly, camptocormia was defined as an involuntary anterior trunk flexion with thoracic fulcrum >45° or lumbar fulcrum >30°, Pisa syndrome as an involuntary lateral trunk flexion >10°, and antecollis as an involuntary anterior neck flexion >45°. The terms “anterior trunk flexion,” with thoracic (≥25° to ≤45°) or lumbar fulcrum (>15° to ≤30°), “lateral trunk flexion” (≥5° to ≤10°), and “anterior neck flexion” (>35° to ≤45°) were chosen for milder postural abnormalities. 1 , 4
Despite its frequency and the relevant impact on patients’ quality of life, there is only little evidence about prevention and management of axial postural abnormalities. The difficulty of developing prevention and management strategies can be due to the scant knowledge about pathophysiology of these symptoms and scarce data about their clinical predictors. As most axial motor symptoms, axial postural abnormalities are typically no or poorly responsive to dopaminergic therapies. 2 No other specific treatment has been found to manage these symptoms, with the exception of physiotherapy, which proved to be helpful for the improvement of camptocormia 8 , 9 and Pisa syndrome 10 , 11 although its efficacy in the long term is currently not sustained by strong literature evidence. 2 Thus, recommendations for management and prevention of these disabling symptoms remain an unmet need. 4 The possibility to identify adequate strategies for treatment and prevention would be increased by a better knowledge on the underlying pathophysiological mechanisms and on the clinical predictors (or risk factors) for their development. To pursue the aim of identifying key concepts, examining emerging evidence, and highlighting knowledge gaps on this topic, we conducted a scoping literature review of clinical predictors and pathophysiology of axial postural abnormalities in patients with parkinsonism.
Methods
Protocol
We performed a scoping review according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses extension. 12 , 13 , 14 Given the limited scientific evidence on this topic, the interest on both clinical predictors and the broad scope of pathophysiological mechanisms, and the existence of different axial postural abnormalities, along with the high heterogeneity of studies, we opted for a scoping review instead of a traditional systematic review.
Step 1: Identification of Research Questions
The aim of this scoping review was to analyze the number, quality, and bias of studies investigating clinical predictors and pathophysiology of axial postural abnormalities in patients with PD, to identify current knowledge gaps, and to present a roadmap for future studies.
Step 2: Identification of Studies
Information Sources
We searched the electronic databases PubMed, OVID Medline, Scopus, and Google Scholar from their inception to June 2022. The search terms were MeSH terms for movement disorders and the main forms of parkinsonism: Parkinson's disease, multiple system atrophy, progressive supranuclear palsy, dementia, Lewy body, parkinsonian disorders, corticobasal syndrome. The keywords for axial postural abnormalities were: Pisa syndrome, camptocormia, cervical dystonia, retrocollis, and anterocollis/antecollis. Other terms were: postural syndrome, spinal curvatures, stooped posture, anterior trunk flexion, forward trunk flexion, lateral trunk flexion, and axial manifestation. The full electronic search strategy is presented in the Supplementary Material in Data S1.
Eligibility Criteria
Studies were included if they reported data on clinical predictors and/or pathophysiology of axial postural abnormalities in patients with PD or atypical Parkinsonism, encompassing MSA, dementia with Lewy Bodies (DLB), PSP, and corticobasal syndrome. Studies reporting drug‐induced axial postural abnormalities (ie, stooped posture, camptocormia, anterocollis, Pisa syndrome) were also included. Only studies referring to human subjects and published in English were considered. No restrictions were applied to patient sex, age, ethnicity, disease duration or severity. We excluded systematic and narrative reviews, case report/single case studies, conference abstracts, commentaries, and letters to the editor. Also excluded were studies involving patients with non‐degenerative causes of Parkinsonism like normal pressure hydrocephalus, vascular parkinsonism, and drug‐induced parkinsonism. 15 The reference list of each record was searched to screen for additional studies not captured by the original search strategy.
Step 3: Study Selection
The records were processed with systematic review management software (Covidence, https://www.covidence.org); duplicate records were eliminated. Six reviewers (D.G., M.D., D.S., C.G., Y.L., C.A.) independently and in pairs screened titles and abstracts by inclusion and exclusion criteria. A third reviewer (V.L., R.W., M.S., M.A‐W., J.N., C.A.A.) was called to solve uncertainties and disagreements until consensus was reached. The full text of titles and abstracts was obtained and reviewed.
Step 4: Charting the Data
All authors reported in Step 3 worked in pairs to extract the data. The second person in the pair verified the extracted information. Data were collected and tabulated on an extraction form.
Step 5: Collating, Summarizing, and Reporting Results
We summarized the extracted data and applied qualitative and quantitative descriptive methods to obtain information on: first author, year of publication, study design, number of patients, age, diagnosis, disease stage, type of axial postural abnormality, prevalence, main pathophysiological assessment, clinical predictors, main finding, main study limitations, and quality appraisal. Two investigators independently performed the quality appraisal of each study, which was rated “good”, “fair”, or “poor” as per the National Heart, Lung, and Blood Institute tools (Research Triangle Institute International. National Heart, Lung, and Blood Institute Quality Appraisal Tools, in accordance with Cochrane handbook recommendations).
Moreover, the full text of all included articles was evaluated for the level of evidence of the results. Evidence levels were attributed as follows, according to the Oxford Centre for Evidence‐Based Medicine (CEBM) criteria: (1) RCTs, prospective, homogeneity of results, interventional; (2) Cohort studies with acceptable quality, number of probands and controls adequate to hypothesis, and reasonable homogeneity of results; (3) Case–control or low‐quality cohort study; (4) Case report, cross‐sectional study; (5) Expert opinion (https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009).
Results
Included and Excluded Studies
Of the total of 4735 records screened, 1964 were excluded because of duplicates and 2405 because they were irrelevant to the topic of this study. A total of 92 records were selected for qualitative data synthesis. Figure 1 illustrates the literature search and the study selection. Of the 92 records, 23 (25%) studies dealt with clinical predictors and 69 (75%) with pathophysiology of axial postural abnormalities in parkinsonism (Supplementary Tables S1 and S2).
Figure 1.
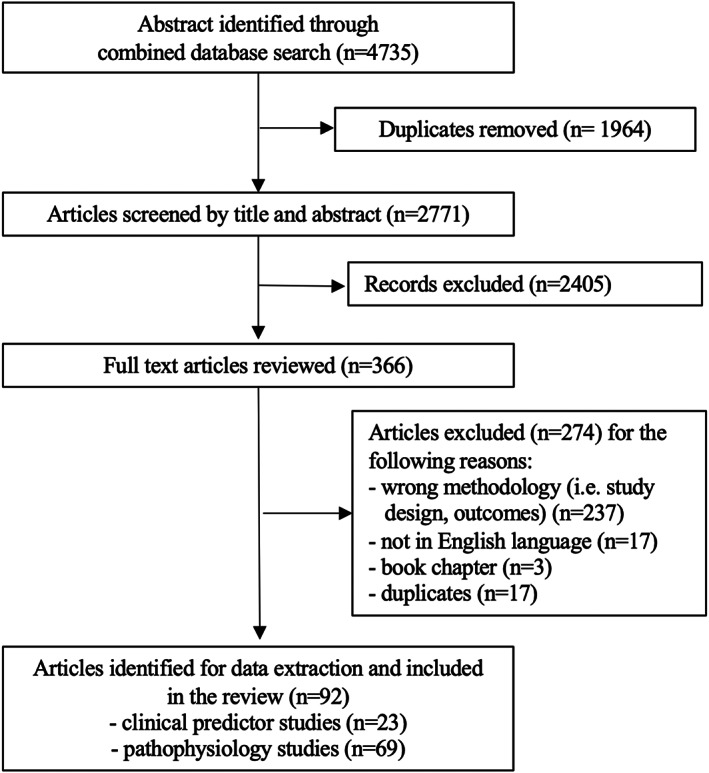
Study flow chart: phases of the scoping review.
Clinical Predictors: Main Findings
The majority of studies on clinical predictors of axial postural abnormalities in parkinsonism involved cohorts of PD patients (21 studies) 1 , 5 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 or MSA patients (two studies). 35 , 36 The total number of PD patients was 11,609 and the total number of MSA patients was 1448. Overall, camptocormia (or mild forms) was present in 973 patients, Pisa syndrome (or mild forms) in 462, antecollis (or mild forms) in 149, and retrocollis in 21. The study designs were various: 14 were observational cross‐sectional studies, 1 , 5 , 16 , 19 , 23 , 24 , 25 , 26 , 29 , 30 , 33 , 34 , 35 , 36 one study was retrospective, 17 three studies were prospective, 18 , 21 , 31 four were case–control, 20 , 22 , 27 , 28 and one was case series. 32
The clinical predictors for axial postural abnormalities varied and in the analyses were either unadjusted or adjusted for confounding variables (Supplementary Table S1). Many studies shared the criteria of advanced stage of disease and greater symptom severity (ie, high UPDRS part III score) as independent clinical predictors for the development of axial postural abnormalities in PD and MSA (Supplementary Table S1). Other clinical, less frequently reported predictors (adjusted and unadjusted for potential confounding variables) were male gender, older age, older age at PD onset, longer disease duration, higher doses of levodopa equivalent daily dose and dopamine agonists, history of falls, back pain, and comorbid orthopedic spinal lesions, as well as a higher score of axial symptoms and a higher probability of having an akinetic‐rigid phenotype. Also, the presence of motor fluctuations was reported as a potential predictor of axial postural abnormalities in two studies, which not survived to analyses including confounders. The major limitations were pitfalls in study design, small sample size, diverse diagnostic criteria to define axial postural abnormalities, and variety of measurement methods. A goniometer, wall goniometer, smartphone, or photographs were used in 14 studies, 1 , 5 , 18 , 19 , 20 , 21 , 25 , 26 , 27 , 28 , 30 , 33 , 34 , 36 software‐based measurement using Kinovea® in one study, 16 and the Cobb angle in one study. 23 Seven studies reported no use of instruments, tools, or bony reference points in the evaluation of axial postural abnormalities. 17 , 22 , 24 , 29 , 31 , 32 , 35 The score of quality appraisal was good in 43% (n = 10), fair in 35% (n = 8), and poor in 22% (n = 5) of studies (Supplementary Table S1).
The majority of studies regarding clinical predictors have a low level of evidence (class 4: 19 studies), two studies were categorized as class 3b, and two studies as class 2b.
Pathophysiology: Main Findings
The majority of studies on the pathophysiology of axial postural abnormalities in parkinsonism involved cohorts of PD patients (n = 63 studies) 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 or a cohort of patients with MSA (n = 1 study) 100 and mixed populations including PD, MSA, PSP, DLB (n = 5 studies). 101 , 102 , 103 , 104 , 105 The total number of patients was 2181 for PD, 29 for MSA, and 34 for other mixed sample populations. Camptocormia (or mild forms of anterior trunk flexion) was present in 801 patients, Pisa syndrome (or mild forms of lateral trunk flexion) in 666, and antecollis (or mild forms of anterior neck flexion) in 114. There was a wide range of study designs (in some cases more than one study design): 14 were observational cross‐sectional, 12 were retrospective, four were prospective, 22 were case–control, one study was a controlled‐intervention, 10 were case series, and six had a before‐after design.
The pathophysiological mechanisms were explained by a central hypothesis (i.e. imbalance in basal ganglia functioning leading to dystonia/rigidity, altered sensory‐motor integration, higher cognitive function deficits) and a peripheral hypothesis (ie, alteration of the musculoskeletal system).
The main study limitations were pitfalls in study design, small sample size, different diagnostic criteria to define axial postural abnormalities, and use of one or more different measurement methods: standard goniometric measurement with a wall goniometer, smartphone, protractor, inclinometer or photographs was used in 22 studies, 40 , 47 , 48 , 50 , 51 , 57 , 58 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 72 , 73 , 77 , 78 , 81 , 82 , 98 , 101 software‐based measurement with NeuroPostureApp, Kinovea, MB Ruler® software, or Image J was used in 12, 37 , 38 , 39 , 41 , 44 , 45 , 46 , 49 , 51 , 52 , 56 , 97 , 99 Cobb angle, X‐ray in nine, 42 , 43 , 55 , 71 , 79 , 81 , 83 , 84 , 85 a spinal mouse electronic measuring device in one study 91 ; 26 studies reported no instrument, tool or bony reference points for evaluating axial postural abnormalities. 53 , 54 , 59 , 67 , 68 , 69 , 70 , 74 , 75 , 76 , 80 , 86 , 87 , 88 , 89 , 90 , 92 , 93 , 94 , 95 , 96 , 100 , 102 , 103 , 104 , 105 The quality appraisal was good in 29% (n = 20), fair in 43% (n = 30), and poor in 28% (n = 19) studies. Various pathophysiological mechanisms and study methodologies were described (Supplementary Table S2).
The majority of studies regarding pathophysiology have a low level of evidence (class 4: 42 studies), 21 studies were categorized as class 3b, five studies as class 2b, and 1 study as class 1b.
Clinical Predictors
Our interpretation of the literature on clinical predictors was complicated by the heterogeneity of the studies and, in general, by the low quality of the evidence. The studies analyzed diverse relationships, associations, risk factors or correlations between clinical variables; however, the study aim (eg, prediction of an outcome or identification of causal mechanisms), was often not clearly stated. The study design sometimes did not match the stated aim or the data analysis failed to predict the outcome or to demonstrate the causal effect. While the majority of studies involved persons with PD, the patient sample was heterogeneous for disease severity and stage, ranging from 2 to 5 on the Hoehn & Yahr scale. Surprisingly, some studies did not even report the disease stage.
One possible issue identified in the literature is that many studies focused on a general presence of axial postural abnormality involving the trunk or neck. Since it is not clear whether abnormal anterior trunk flexion, lateral trunk flexion or anterior neck flexion share the same pathophysiological mechanisms, the search for clinical predictors (other than of their pathophysiological underpinnings) should be better analyzed separately for any form of axial postural abnormality. Moreover, the diagnostic criteria and especially the threshold of trunk flexion degrees for defining an axial postural abnormality (and thus the inclusion in the study as a patient with a postural abnormality) differed across studies and were not stated in many cases. Also, the variability in the tools and the reference bone points for measuring axial postural abnormalities adds further variability. All the above‐reported aspects render it difficult to draw robust conclusions from the current literature and represent a possible relevant bias, which could also explain the high variability in frequency of axial postural abnormalities across studies.
Despite the literature limitations, some findings appear to be robustly represented in most studies. Specifically, the findings converge on an advanced stage of disease and greater motor symptom severity as clinical predictors for axial postural abnormalities. The relationship between these associations is unclear: while the mechanisms correlated with the development of axial symptoms are typical of advanced disease stages and thus are associated with more severe motor symptoms, severe axial postural abnormalities (eg, Pisa syndrome and camptocormia) highly affect the motor burden of patients, leading to higher scores of UPDRS. More prospective longitudinal studies are needed not only to confirm these associations but also to elucidate the causal factors for the development of axial postural abnormalities.
Pathophysiology
Several different pathophysiological mechanisms have been hypothesized in patients with axial postural abnormalities. Central mechanisms (ie, imbalance in basal ganglia functioning, higher cognitive function deficits, altered sensory‐motor integration, proprioception, vestibular information) and peripheral processes (ie, alteration of the musculoskeletal system) have been investigated. Additional clues to the pathophysiology of axial postural abnormalities are given by small sample size studies suggesting their reversibility after deep‐brain stimulation, repetitive trans‐spinal magnetic stimulation, unilateral pallidotomy, and after withdrawal of pharmacological therapies.
The key concepts related to the pathophysiology of axial postural abnormalities can be resumed as the possibly concomitant presence of dystonia, impaired proprioception, deficits of higher cognitive functions and of the vestibular system. While most studies and most experts in the field currently point toward central mechanisms as the prime of the development of axial postural abnormalities in parkinsonism, various studies based on electromyographic findings, 41 , 45 , 78 , 81 , 86 , 88 , 91 , 94 , 99 , 101 , 102 , 103 , 104 , 105 muscle biopsy, 80 , 86 , 88 , 91 , 94 , 102 , 105 and muscle imaging 43 , 45 , 51 , 60 , 68 , 69 , 76 , 78 , 81 , 86 , 88 , 89 , 91 , 94 , 96 , 100 , 105 indicate the presence of myopathy in trunk muscles of patients with axial postural abnormalities.
Below we discuss the main theories regarding the pathogenesis of axial postural abnormalities, starting from the findings of the various studies included in this scoping review, always keeping in mind the relevant limitations of the current body of literature disclosed in the Results of this review.
Central Mechanisms
Dystonia
An abnormal trunk muscle hyperactivity proved by electromyographic findings has been consistently observed in patients with axial postural abnormalities. 45 , 51 , 57 , 61 , 63 , 73 , 74 , 75 , 78 , 81 , 84 , 85 , 92 , 99 , 102 , 103 The hypothesis for dystonic muscle activation as a possible cause of axial postural abnormalities is supported by anecdotal studies suggesting that the abnormalities can be alleviated by sensory tricks in some cases 106 and can be improved by botulinum toxin type‐A or lidocaine administration—as described for both camptocormia 73 , 82 and Pisa syndrome. 51 However, these hypotheses have attracted criticism related to contradictory electromyographic findings between studies and absence of clinical characteristics of dystonia (ie, overflow, twisting movements, sensory trick for Pisa syndrome). 25 In patients with Pisa syndrome, electromyography findings have been inconsistent concerning the side of hyperactivity of paraspinal and non‐paraspinal muscles. Some studies demonstrated hyperactivity of paraspinal muscles ipsilateral to the leaning side, whereas others found continuous activity contralateral to the leaning side. 57 , 61 , 63 , 74 , 81 , 84 , 85 However, definitive evidence for a possible dystonic origin of the Pisa syndrome is lacking. Botulinum toxin administered to the paraspinal muscles contralateral to the flexion side was reported efficacious in improving flexion and pain in some patients (though this would not be expected from a mechanical perspective), 51 suggesting that the action of botulinum may extend beyond simple de‐contraction of pulling muscles.
In patients with camptocormia with thoracic fulcrum, the bilateral abdominal internal and the external oblique muscles, together with the rectus abdominis muscles may be involved, 45 whereas rectus abdominis and iliopsoas hyperactivity is more frequently observed in patients with camptocormia with lumbar fulcrum. 45 , 73 The paraspinal muscles (ie, iliocostalis lumborum, longissimus and multifidus) and the rectus femoris to a lesser extent were also found to be hyperactive. 99 Evidence for dystonic activity of non‐paraspinal muscles is partially supported by improvement of camptocormia with thoracic fulcrum after injection of lidocaine in the oblique external muscle. 73 However, scientific evidence for clinical benefit is scant and this approach is also very limited in clinical practice.
Antecollis has been suggested to be a clue for MSA, but it also occurs in other parkinsonian conditions, including PD. 102 Neck voluntary movement is limited, but muscle strength appears normal when the residual range of motion is tested during active neck extension. 92 , 107 Muscle hyperactivity in anterior (sternocleidomastoid muscles and plathysma) and posterior neck muscles (eg, semispinalis, splenius capitis, trapezius, levator scapulae) have been reported. 2 , 102 , 103 In most cases, the activation of posterior neck muscles, which are considered “erector spinae” (ie, spine extensors), can be considered compensatory.
Overall, the low number of studies, the lack of objective measures to identify and quantify dystonia, and the difficulties in standardization of needle EMG studies renders dystonia a possible, yet unconfirmed pathogenic mechanisms for axial postural abnormalities in parkinsonism. It is generally recognized that a dystonic phenomenon could be an early and short‐lived factor contributing to the development of axial postural abnormalities, while secondary factors such as changes in soft tissue, muscle or spinal cord may develop later in more advanced stages. Further studies are needed to systematically explore the electromyographic patterns of paraspinal and non‐paraspinal muscles in very early and in advanced stages of axial postural abnormalities to better understand dystonic pathophysiology and develop tailored pharmacological 108 and non‐pharmacological interventions 109 for treatment and prevention.
Rigidity
Few studies to date have suggested in what way rigidity (persistent increase in muscle tone that is not velocity dependent) affects axial muscle tone in patients with PD, and no studies have systematically evaluated rigidity in patients with axial postural abnormalities. Patients with axial postural abnormalities demonstrate in most cases to have a complete or almost complete resolution of trunk or neck flexion when lying on the bed, while when sitting or standing the paravertebral muscles may be highly active. 96 , 99 Differently from dystonia, parkinsonian rigidity should be consistently present at rest. However, none of the literature studies found in our systematic search used direct measures of axial tone owing to technical difficulties. 110 , 111
Impaired Proprioception, Vestibular System and Visuospatial Cognition
Persons with PD have shown an impaired proprioception, and a deficit in proprioception could ensue in poor signal integration while controlling posture. 112 , 113 Some studies found that patients with camptocormia or Pisa syndrome have a greater velocity of body sway than patients without axial postural abnormalities. 49 , 72 Clinical observation indicates that patients with camptocormia with a lower fulcrum may have more severe gait and postural control deficits than patients with a thoracic fulcrum or patients without camptocormia. 49 These findings suggest that misalignment of posture can contribute to increased postural instability.
Another pivotal aspect for postural control is the vestibular system. PD patients with Pisa syndrome may have central, peripheral, or mixed vestibular dysfunction, while less data is available for camptocormia. 42 , 83 Vitale and collaborators described peripheral unilateral vestibular hypofunction ipsilateral to the leaning side and contralateral to the side that was most affected by PD in a small sample (n = 11) of patients with PD. 83 In PD with Pisa syndrome there may also be a bilateral alteration of cervical vestibular evoked myogenic potentials, pointing toward impairment of the vestibulospinal pathway. 58 A vestibular dysfunction in the roll plane may be associated with deviation of the subjective visual vertical, 37 , 44 , 46 , 48 , 57 estimated in about 67% of PD patients with Pisa syndrome. 65 A recent case–control study found worse performance on the subjective visual vertical perception in PD patients with Pisa syndrome compared to PD controls without axial postural abnormalities, but not in those with camptocormia when compared to their controls. 114 In the same study, reduced awareness of axial postural abnormality was observed in >60% of patients with PS or CC, suggesting a possible role for altered proprioception. There is a lack of available information for possible vestibular dysfunctions in patients with antecollis.
The control of posture is a semi‐automated processing requesting a complex interaction between motor and cognitive networks, especially involving attentional and executive functions domains. 113 However, the association between cognitive deficits and axial postural abnormalities remains controversial. In a small sample of PD, no difference in executive functions evaluated with the Montreal Cognitive Assessment and the Frontal Assessment Battery were recorded between patients with and without Pisa syndrome. 79 Two studies observed lower scores on tasks exploring executive functions, perceptual visuospatial functions, and language for patients with Pisa syndrome compared to those without axial postural abnormalities. 52 , 66 Only a few studies assessed the cognitive functions in patients with camptocormia compared with PD patients without postural abnormalities, 27 , 33 , 114 but none have specifically evaluated cognition in patients with antecollis. Whether these dysfunctions result from axial postural abnormalities or are a contributing factor remains to be investigated; nonetheless, there is growing evidence for the involvement of visuospatial abilities in patients with Pisa syndrome and not in camptocormia. 114
Peripheral Mechanisms
Empirical findings show that myopathy has a homogenous but not specific appearance, and the etiology of myopathy remains unclear, but ongoing research marks it as a secondary myopathic process. 41 The data on the beneficial effects of deep brain stimulation on camptocormia 60 suggest a central process and secondary muscle damage caused by altered central control of body posture. 80 These phenomena probably reflect a continuum from early myopathic changes (ie, edema and swelling, contrast enhancement without degenerative muscle changes) in the early stages of camptocormia to progression to muscle atrophy and fatty degeneration in the end stage (ie, atrophy, fatty changes, marked connective tissue increase). In fact, the exact etiology of muscular changes is unclear. It is hypothesized that they may be the consequence of muscle disuse or denervation secondary to prolonged trunk misalignment driven by central mechanisms. Moreover, there is evidence that patients can present axial postural abnormalities without myopathic changes in the paraspinal and non‐paraspinal muscles. 87 , 96
A primary myopathy has been suggested to explain Pisa syndrome, 81 but no pathological studies are available to endorse this hypothesis. Tassorelli and colleagues found more pronounced muscular atrophy on the leaning side, with a cranio‐caudal gradient involving the multifidus and the latissimus dorsi muscles. 81 An MRI study confirmed atrophy of the lumbar paraspinal muscles with greater fatty degeneration more prevalent on the leaning side. 78 The observed muscular changes are probably caused by secondary mechanisms: the atrophy ipsilateral to the bending side may be due to muscle disuse, while contralateral muscle atrophy might be secondary to the stretching stress. 78
Peak trunk extension force was much lower in PD patients with camptocormia than in healthy controls but did not differ from PD patients without camptocormia. However, the mechanical efficiency of muscular activation (especially at the thoracic level) of trunk extension was markedly reduced in PD patients with camptocormia compared to PD without postural alterations and to healthy controls, which suggests impairment of neuromuscular recruitment. 41 Clinically, patients with camptocormia can benefit from the support of a high‐frame walker to support and re‐align the trunk during stance and walking. 115 Further studies are needed to understand the role of muscular atrophy and weakness in PD patients with and without axial postural abnormalities.
Several methodological and technical problems limit the assessment of myopathy and have inevitably produced discrepancies between studies. Electromyography of the paraspinal muscles is technically difficult and findings from muscle biopsy and MRI studies may reflect age‐related changes because there are no reliable age‐matched control data in the literature. 116 For further research in empirically proven myopathic changes in trunk muscles, other more commonly available methods such as ultrasound are needed to monitor the time course of myopathy and related trunk misalignment in combination with age‐matched control data. This is particularly important for informing treatment options.
Finally, axial postural abnormalities could be more frequent in patients with concomitant history of back surgery, degenerative spinal conditions or severe spine trauma. 5 , 33 , 43 , 79 , 82 , 87 , 88 , 89 , 90 , 95 , 96 , 106 These complications may have mechanical effects on bones or soft tissue, predisposing to the development of axial postural abnormalities. 117 Many patients complain of other symptoms, such as fatigue, pain (producing compensatory postures), and strenuous exercise. 88 , 91 The role of chronic pain, in particular, should be explored as a potential trigger of axial postural abnormalities in parkinsonism. 6 , 25 , 84
Pathophysiology Clues from Therapeutic Approaches
There are anecdotal studies reporting on drug‐induced axial postural abnormalities in people with parkinsonism. Many studies involving small patient samples have explored the relationship between the use of dopamine agonists and the onset of axial postural abnormalities. The subacute onset of axial postural abnormalities usually improved after withdrawal of the agonist. Antecollis has been observed to appear 2 weeks later after initiation of pramipexole and pergolide therapy in patients with PD. 118 Pisa syndrome has been observed after the initiation or a dose increase of dopaminergic medication such as levodopa, levodopa/carbidopa, levodopa/benserazide, and levodopa/carbidopa/entacapone 32 or the addition of rasagiline to levodopa treatment. 85 A reduction, switching to another dopaminergic medication or their withdrawal led to improvement of posture. 32 In non‐PD patients the use of dopamine receptor blockers or cholinesterase inhibitors has been frequently associated with Pisa syndrome. Occasionally, Pisa syndrome appears after initiation of an antipsychotic or arises insidiously in antipsychotic‐treated patients for unknown reasons. 119 These findings would indicate a central dysregulation of dopaminergic networks in the pathogenesis of axial postural abnormalities. However, in most cases of axial postural abnormalities, there is no evidence of influence of the therapies on the onset or persistence of axial postural abnormalities in parkinsonian patients and whether the rare drug‐induced cases of antecollis, camptocormia or Pisa syndrome should be considered a group apart is still a matter of debate.
Other clues from therapeutic studies endorsing the role of basal ganglia in sustaining axial postural abnormalities derive from the improvement in axial postural abnormalities after high‐frequency deep brain stimulation of the bilateral subthalamic nuclei. 39 , 59 , 64 Also, repetitive trans‐spinal magnetic stimulation of spinal pathways may have an immediate beneficial effect on camptocormia, according to one study. 77 Moreover, a possible neuronal dysfunction in the midbrain, as evidenced by tests of saccadic eye movement 90 and smaller midbrain axial surface, 89 has been observed in patients with camptocormia. Finally, case series observed the onset of Pisa syndrome to the right side following 4, 8, and 9 years after surgery (left pallidotomies) in patients with PD. 120
Conclusions
The recognition of clinical predictors and pathophysiology of axial postural abnormalities in parkinsonism is far from being elucidated due to literature bias, encompassing different inclusion criteria and measurement tools, heterogeneity of patient samples and the common habit of considering axial postural abnormalities as a unique entity, without differentiating forward trunk flexion, lateral trunk flexion or forward neck flexion. While we were able to map out the current literature on axial postural abnormalities many research gaps still exist. To date, the most promising aspects to analyze are related to healthy (age‐related) vs. PD‐associated changes in posture, the role of rigidity of axial muscles on posture, and the impact of proprioception, the vestibular system, and higher cognitive functions. Also, the role of therapies acting on basal ganglia and dopaminergic networks needs to be clarified. Consensus among movement disorder experts on the nosology and the cut‐off criteria for axial postural abnormalities has recently been reached, 4 and this can be a starting point for the harmonization of inclusion criteria in future studies.
Regarding risk factors for the development of axial postural abnormalities, the best literature evidence suggests that more severe motor symptoms, especially with higher involvement of axial symptoms, are associated with the development of both camptocormia and Pisa syndrome. At the same time, it is interesting as some PD nonmotor features like REM sleep behavior disorder or lower smell function have been associated with a higher probability of developing axial postural abnormalities. Altogether, these findings seem to suggest that a more severe PD phenotype can be at higher risk of developing these axial symptoms. The fact that the disease severity is linked to the development of axial postural abnormalities is also endorsed by many class IV evidence studies highlighting a correlation with higher Hoehn and Yahr stages and longer disease duration. Moreover, older age is another risk factor frequently reported in the literature.
Regarding pathophysiology, the highest evidence points toward an alteration of the visual vertical perception and the elaboration of multimodal information provided by basal‐ganglia networks as pathogenic mechanisms associated with both Pisa syndrome and camptocormia. This evidence is also supported by the numerous studies showing an improvement of posture after deep brain stimulation. The role of vestibular system imbalance is also possibly implicated as well as the presence of peripheral abnormalities in axial muscles (both myopathic changes and strength alterations). The latter can be a consequence (eg, a peripheral process related to early‐onset and short‐lived dystonic phenomenon) or a trigger for the development of axial postural abnormalities when altered central integration of proprioceptive signals coming from the periphery are present.
However, current body of research holds more open questions than answers. Further studies on predictors, pathophysiology, management, and prevention of axial postural abnormalities are needed. In conclusion, systematic and standardized screening and assessment of axial postural abnormalities is recommended to provide patients with prompt treatment. 4 Prospective, long‐term studies with large samples are urgently needed to explore the potential risk (casual) factors involved in their development.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
C.A.A.: 1A, 1B, 1C, 3A, 3B
C.G.: 1A, 1B, 1C, 3A, 3B
J.N.: 1A, 1B, 1C, 3B
C.A.: 1A, 1B, 1C, 3B
D.G.: 1B, 1C, 3B
M.L.D.: 1B, 1C, 3B
D.S.: 1B, 1C, 3B
Y.L.: 1B, 1C, 3B
M.A.W.: 1B, 1C, 3B
M.S.: 1B, 1C, 3B
R.W.: 1B, 1C, 3B
V.T.L.: 1B, 1C, 3B
G.I.: 1B, 1C, 3B
S.C.: 1B, 1C, 3B
M.M.: 1B, 1C, 3B
B.R.B.: 1B, 1C, 3B
T.C.: 1B, 1C, 3B
R.D.: 1B, 1C, 3B
K.D.: 1B, 1C, 3B
A.F.: 1B, 1C, 3B
H.T.: 1B, 1C, 3B
L.L.: 1B, 1C, 3B
N.G.M.: 1B, 1C, 3B
C.M.: 1B, 1C, 3B
Y.U.: 1B, 1C, 3B
R.B.: 1A, 1C, 3A, 3B
M.T.: 1A, 1B, 1C, 3A, 3B
Disclosures
Ethical Compliance Statement: The authors confirm that [the approval of an institutional review board]/[patient consent] was not required for this work. For this work, no informed consent was obtained. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: The authors declare that there are no funding sources or conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: R.B. receives a salary from Chulalongkorn University and stipend from the Royal Society of Thailand, has received consultancy and/or honoraria/lecture fees from Abbott, Boehringer‐Ingelheim, Britannia, Ipsen, Novartis, Teva‐Lundbeck, Takeda, and Otsuka pharmaceuticals; he has received research funding from the Newton Fund, the UK Government, Thailand Science and Research Innovation Bureau, Thailand Research Fund, Crown Property Bureau, Chulalongkorn University, and the National Science and Technology Development Agency; he holds patents for a laser‐guided walking stick, portable tremor device, nocturnal monitoring, and electronic Parkinson's disease symptom diary as well as copyright on a Parkinson's mascot, dopamine lyrics and teaching video clips for common nocturnal and gastrointestinal symptoms for Parkinson's disease. A.F. reports the following: Consultancies from Abbvie, Medtronic, Boston Scientific, Sunovion, Ipsen, Merz; Advisory Boards of Abbvie, Boston Scientific, Ceregate, Inbrain, Ipsen, Medtronic, Jazz, Biogen/Sage; Honoraria from Abbvie, Medtronic, Boston Scientific, Sunovion, UCB, Ipsen; grants from University of Toronto, Weston foundation, Abbvie, Medtronic, Boston Scientific, Michael J. Fox Foundation, European Union, MSA coalition, Dystonia Medical Research Foundation. L.L. reports the following: Consultancies and Honoraria from Bial, Zambon, Abbvie and Grants from University of Turin, Zambon, Abbvie. N.M. reports the following: advisory Boards for Angelini, GW Pharmaceuticals and Grants from UCB Pharma, Desitin, Eisai, LivaNova, Angelini, GW Pharmaceuticals. M.M. reports the following: Consultant: St Jude/Abbott, Honoraria: Glaxo Research grants: Glaxo, Allergan, TEVA. Royalties: Springer, Random House, Cambridge University press, Humana Press. Editor honorarium Wiley & Son. Movement Disorders Society.
Supporting information
Table S1. Main characteristics of studies on clinical predictors for axial postural abnormalities in patients with Parkinsonism.
Table S2. Main characteristics of studies on the pathophysiology of axial postural abnormalities in patients with Parkinsonism.
Data S1. Electronic search strategy.
Acknowledgment
The authors wish to thank Rossella Targa (librarian) for her assistance in developing the literature search strategy.
Carlo Alberto Artusi and Christian Geroin are equally contributed to this work.
Contributor Information
Christian Geroin, Email: christian.geroin@univr.it.
Michele Tinazzi, Email: michele.tinazzi@univr.it.
References
- 1. Tinazzi M, Gandolfi M, Ceravolo R, et al. Postural abnormalities in Parkinson's disease: an epidemiological and clinical multicenter study. Mov Disord Clin Pract 2019;6(7):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doherty KM, van de Warrenburg BP, Peralta MC, Silveira‐Moriyama L, Azulay JP, Gershanik OS, Bloem BR. Postural deformities in Parkinson's disease. Lancet Neurol 2011;10(6):538–549. [DOI] [PubMed] [Google Scholar]
- 3. Ashour R, Jankovic J. Joint and skeletal deformities in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Mov Disord 2006;21(11):1856–1863. [DOI] [PubMed] [Google Scholar]
- 4. Tinazzi M, Geroin C, Bhidayasiri R, et al. Task force consensus on nosology and cut‐off values for axial postural abnormalities in parkinsonism. Mov Disord Clin Pract 2022;9:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Margraf NG, Granert O, Hampel J, Wrede A, Schulz‐Schaeffer WJ, Deuschl G. Clinical definition of Camptocormia in Parkinson's disease. Mov Disord Clin Pract 2017;4(3):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geroin C, Artusi CA, Gandolfi M, et al. Does the degree of trunk bending predict patient disability, motor impairment, falls, and Back pain in Parkinson's disease? Front Neurol 2020;11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koele MC, Lems WF, Willems HC. The clinical relevance of Hyperkyphosis: a narrative review. Front Endocrinol (Lausanne) 2020;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Volpe D, Giantin MG, Manuela P, Filippetto C, Pelosin E, Abbruzzese G, Antonini A. Water‐based vs. non‐water‐based physiotherapy for rehabilitation of postural deformities in Parkinson's disease: a randomized controlled pilot study. Clin Rehabil 2017;31(8):1107–1115. [DOI] [PubMed] [Google Scholar]
- 9. Gandolfi M, Tinazzi M, Magrinelli F, et al. Four‐week trunk‐specific exercise program decreases forward trunk flexion in Parkinson's disease: a single‐blinded, randomized controlled trial. Parkinsonism Relat Disord 2019;64:268–274. [DOI] [PubMed] [Google Scholar]
- 10. Bartolo M, Serrao M, Tassorelli C, et al. Four‐week trunk‐specific rehabilitation treatment improves lateral trunk flexion in Parkinson's disease. Mov Disord 2010;25(3):325–331. [DOI] [PubMed] [Google Scholar]
- 11. Tassorelli C, De Icco R, Alfonsi E, et al. Botulinum toxin type a potentiates the effect of neuromotor rehabilitation of Pisa syndrome in Parkinson disease: a placebo controlled study. Parkinsonism Relat Disord 2014;20(11):1140–1144. [DOI] [PubMed] [Google Scholar]
- 12. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015;13(3):141–146. [DOI] [PubMed] [Google Scholar]
- 14. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med 2018;169(7):467–473. [DOI] [PubMed] [Google Scholar]
- 15. Keener AM, Bordelon YM. Parkinsonism. Semin Neurol 2016;36(4):330–334. [DOI] [PubMed] [Google Scholar]
- 16. Pongmala C, Artusi CA, Zibetti M, et al. Postural abnormalities in Asian and Caucasian Parkinson's disease patients: a multicenter study. Parkinsonism Relat Disord 2022;97:91–98. [DOI] [PubMed] [Google Scholar]
- 17. Yoritaka A, Shimo Y, Hatano T, Hattori N. Motor/nonmotor symptoms and progression in patients with Parkinson's disease: prevalence and risks in a longitudinal study. Parkinsons Dis 2020;2020:2735361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu K, Ou R, Hou Y, et al. Predictors of Pisa syndrome in Chinese patients with Parkinson's disease: a prospective study. Parkinsonism Relat Disord 2019;69:1–6. [DOI] [PubMed] [Google Scholar]
- 19. Liu K, Ou R, Wei Q, et al. Pisa syndrome in Chinese patients with Parkinson's disease. Front Neurol 2019;10:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ando Y, Fujimoto KI, Ikeda K, et al. Postural abnormality in Parkinson's disease: a large comparative study with general population. Mov Disord Clin Pract 2019;6(3):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ou R, Liu H, Hou Y, et al. Predictors of camptocormia in patients with Parkinson's disease: a prospective study from Southwest China. Parkinsonism Relat Disord 2018;52:69–75. [DOI] [PubMed] [Google Scholar]
- 22. Ameghino L, Bruno V, Merello M. Postural disorders and antiparkinsonian treatments in Parkinson disease: an exploratory case‐control study. Clin Neuropharmacol 2018;41(4):123–128. [DOI] [PubMed] [Google Scholar]
- 23. Ye X, Lou D, Ding X, et al. A clinical study of the coronal plane deformity in Parkinson disease. Eur Spine J 2017;26(7):1862–1870. [DOI] [PubMed] [Google Scholar]
- 24. Cervantes‐Arriaga A, Rodriguez‐Violante M, Morales‐Briceno H, Neri‐Nani G, Millan‐Cepeda R, Velazquez‐Osuna S. Frequency and clinical correlates of postural and striatal deformities in Parkinson's disease. Clin Neurol Neurosurg 2016;142:140–144. [DOI] [PubMed] [Google Scholar]
- 25. Tinazzi M, Fasano A, Geroin C, et al. Pisa syndrome in Parkinson disease: an observational multicenter Italian study. Neurology 2015;85(20):1769–1779. [DOI] [PubMed] [Google Scholar]
- 26. Song W, Guo X, Chen K, et al. Camptocormia in Chinese patients with Parkinson's disease. J Neurol Sci 2014;337(1–2):173–175. [DOI] [PubMed] [Google Scholar]
- 27. Ou R, Guo X, Song W, et al. Characteristics of non‐motor symptoms in patients with Parkinson's disease exhibiting camptocormia. Gait Posture 2014;40(3):447–450. [DOI] [PubMed] [Google Scholar]
- 28. Oeda T, Umemura A, Tomita S, Hayashi R, Kohsaka M, Sawada H. Clinical factors associated with abnormal postures in Parkinson's disease. PloS One 2013;8(9):e73547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kashihara K, Imamura T. Frequency and clinical correlates of retrocollis in Parkinson's disease. J Neurol Sci 2013;324(1–2):106–108. [DOI] [PubMed] [Google Scholar]
- 30. Seki M, Takahashi K, Koto A, et al. Camptocormia in Japanese patients with Parkinson's disease: a multicenter study. Mov Disord 2011;26(14):2567–2571. [DOI] [PubMed] [Google Scholar]
- 31. Sato Y, Iwamoto J, Honda Y. Vitamin D deficiency‐induced vertebral fractures may cause stooped posture in Parkinson disease. Am J Phys Med Rehab 2011;90(4):281–286. [DOI] [PubMed] [Google Scholar]
- 32. Cannas A, Solla P, Floris G, et al. Reversible Pisa syndrome in patients with Parkinson's disease on dopaminergic therapy. J Neurol 2009;256(3):390–395. [DOI] [PubMed] [Google Scholar]
- 33. Tiple D, Fabbrini G, Colosimo C, Ottaviani D, Camerota F, Defazio G, Berardelli A. Camptocormia in Parkinson disease: an epidemiological and clinical study. J Neurol Neurosurg Psychiatry 2009;80(2):145–148. [DOI] [PubMed] [Google Scholar]
- 34. Yoshii F, Moriya Y, Ohnuki T, Ryo M, Takahashi W. Postural deformities in Parkinson's disease – Mutual relationships among neck flexion, fore‐bent, knee‐bent and lateral‐bent angles and correlations with clinical predictors. J Clin Mov Disord 2016;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang L, Cao B, Zou Y, et al. Prevalence of and factors associated with postural deformities in Chinese patients with multiple system atrophy. Parkinsonism Relat Disord 2019;64:324–327. [DOI] [PubMed] [Google Scholar]
- 36. Zhang LY, Cao B, Wei QQ, et al. Camptocormia in patients with multiple system atrophy at different disease durations: frequency and related factors. BMC Neurol 2021;21(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mikami K, Shiraishi M, Kamo T. Effect of subjective vertical perception on lateral flexion posture of patients with Parkinson's disease. Sci Rep 2022;12(1):1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lai Y, Song Y, Huang P, et al. Subthalamic stimulation for Camptocormia in Parkinson's disease: Association of Volume of tissue activated and structural connectivity with clinical effectiveness. J Parkinsons Dis 2021;11(1):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liang S, Yu Y, Li H, Wang Y, Cheng Y, Yang H. The study of subthalamic deep brain stimulation for Parkinson disease‐associated Camptocormia. Med Sci Monit 2020;26:e919682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yao MS, Zhou LC, Tan YY, et al. Gait characteristics and brain activity in Parkinson's disease with concomitant postural abnormalities. Aging Dis 2020;11(4):791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolke R, Kuhtz‐Buschbeck JP, Deuschl G, Margraf NG. Insufficiency of trunk extension and impaired control of muscle force in Parkinson's disease with camptocormia. Clin Neurophysiol 2020;131(11):2621–2629. [DOI] [PubMed] [Google Scholar]
- 42. Tang H, Chen Y, Cen Z, Ouyang Z, Lou D, Tan Y, Luo W. The link between lateral trunk flexion in Parkinson's disease and vestibular dysfunction: a clinical study. Int J Neurosci 2021;131(6):521–526. [DOI] [PubMed] [Google Scholar]
- 43. Park HY, Ha KY, Kim YH, et al. Spinal surgery for Parkinson disease with Camptocormia: propensity score‐matched cohort study with degenerative sagittal imbalance (DSI). Clin Spine Surg 2020;33(10):E563–E571. [DOI] [PubMed] [Google Scholar]
- 44. Mikami K, Shiraishi M, Kamo T. Subjective postural vertical in Parkinson's disease with lateral trunk flexion. Acta Neurol Scand 2020;142(5):434–442. [DOI] [PubMed] [Google Scholar]
- 45. Magrinelli F, Geroin C, Squintani G, et al. Upper camptocormia in Parkinson's disease: neurophysiological and imaging findings of both central and peripheral pathophysiological mechanisms. Parkinsonism Relat Disord 2020;71:28–34. [DOI] [PubMed] [Google Scholar]
- 46. Brugger F, Walch J, Hagele‐Link S, Abela E, Galovic M, Kagi G. Decreased grey matter in the postural control network is associated with lateral flexion of the trunk in Parkinson's disease. Neuroimage Clin 2020;28:102469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bissolotti L, Ruggeri J, Rota M, Calza S, Cosimo C. Muscle echo intensity of abdominal wall in Parkinson's disease and healthy controls: a cross sectional study. Neurol Sci 2020;41(11):3201–3207. [DOI] [PubMed] [Google Scholar]
- 48. Mikami K, Shiraishi M, Kamo T. Subjective vertical position allows prediction of postural deterioration in patients with Parkinson's disease. Parkinsons Dis 2019;2019:1875435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Geroin C, Gandolfi M, Maddalena I, Smania N, Tinazzi M. Do upper and lower Camptocormias affect gait and postural control in patients with Parkinson's disease? An Observational Cross‐Sectional Study. Parkinsons Dis 2019;2019:9026890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Formaggio E, Masiero S, Volpe D, Demertzis E, Gallo L, Del Felice A. Lack of inter‐muscular coherence of axial muscles in Pisa syndrome. Neurol Sci 2019;40(7):1465–1468. [DOI] [PubMed] [Google Scholar]
- 51. Artusi CA, Bortolani S, Merola A, et al. Botulinum toxin for Pisa syndrome: an MRI‐, ultrasound‐ and electromyography‐guided pilot study. Parkinsonism Relat Disord 2019;62:231–235. [DOI] [PubMed] [Google Scholar]
- 52. Artusi CA, Montanaro E, Tuttobene S, Romagnolo A, Zibetti M, Lopiano L. Pisa syndrome in Parkinson's disease is associated with specific cognitive alterations. Front Neurol 2019;10:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fujioka S, Yoshida R, Nose K, et al. A new therapeutic strategy with istradefylline for postural deformities in Parkinson's disease. Neurol Neurochir Pol 2019;53(4):291–295. [DOI] [PubMed] [Google Scholar]
- 54. Sugiyama T, Mochizuki H, Hara Y, et al. Disinhibited blink reflex recovery is related to lateral trunk flexion in Parkinson disease. J Clin Neurophysiol 2018;35(4):346–350. [DOI] [PubMed] [Google Scholar]
- 55. Okazaki M, Sasaki T, Yasuhara T, et al. Characteristics and prognostic factors of Parkinson's disease patients with abnormal postures subjected to subthalamic nucleus deep brain stimulation. Parkinsonism Relat Disord 2018;57:44–49. [DOI] [PubMed] [Google Scholar]
- 56. Margraf NG, Wolke R, Granert O, et al. Consensus for the measurement of the camptocormia angle in the standing patient. Parkinsonism Relat Disord 2018;52:1–5. [DOI] [PubMed] [Google Scholar]
- 57. Huh YE, Kim K, Chung WH, Youn J, Kim S, Cho JW. Pisa syndrome in Parkinson's disease: pathogenic roles of verticality perception deficits. Sci Rep 2018;8(1):1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Di Lazzaro G, Schirinzi T, Giambrone MP, et al. Pisa syndrome in Parkinson's disease: evidence for bilateral Vestibulospinal dysfunction. Parkinsons Dis 2018;2018:8673486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Artusi CA, Zibetti M, Romagnolo A, Rizzone MG, Merola A, Lopiano L. Subthalamic deep brain stimulation and trunk posture in Parkinson's disease. Acta Neurol Scand 2018;137(5):481–487. [DOI] [PubMed] [Google Scholar]
- 60. Sakai W, Nakane S, Urasaki E, et al. The cross‐sectional area of Paraspinal muscles predicts the efficacy of deep drain stimulation for Camptocormia. J Parkinsons Dis 2017;7(2):247–253. [DOI] [PubMed] [Google Scholar]
- 61. Di Martino S, Unti E, Tramonti C, et al. Efficacy of a combined therapeutic approach in the management of Pisa syndrome. NeuroRehabilitation 2017;41(1):249–253. [DOI] [PubMed] [Google Scholar]
- 62. Lee KH, Kim JM, Kim HS. Back extensor strengthening exercise and backpack wearing treatment for Camptocormia in Parkinson's disease: a retrospective pilot study. Ann Rehabil Med 2017;41(4):677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Geroin C, Squintani G, Morini A, et al. Pisa syndrome in Parkinson's disease: electromyographic quantification of paraspinal and non‐paraspinal muscle activity. Funct Neurol 2017;32(3):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yamada K, Shinojima N, Hamasaki T, Kuratsu J. Subthalamic nucleus stimulation improves Parkinson's disease‐associated camptocormia in parallel to its preoperative levodopa responsiveness. J Neurol Neurosurg Psychiatry 2016;87(7):703–709. [DOI] [PubMed] [Google Scholar]
- 65. Gandor F, Basta D, Gruber D, Poewe W, Ebersbach G. Subjective visual vertical in PD patients with lateral trunk flexion. Parkinsons Dis 2016;2016:7489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vitale C, Falco F, Trojano L, et al. Neuropsychological correlates of Pisa syndrome in patients with Parkinson's disease. Acta Neurol Scand 2016;134(2):101–107. [DOI] [PubMed] [Google Scholar]
- 67. Mensikova K, Kaiserova M, Vastik M, Kurcova S, Kanovsky P. Treatment of camptocormia with continuous subcutaneous infusions of apomorphine: 1‐year prospective pilot study. J Neural Transm (Vienna) 2015;122(6):835–839. [DOI] [PubMed] [Google Scholar]
- 68. Nakane S, Yoshioka M, Oda N, et al. The characteristics of camptocormia in patients with Parkinson's disease: a large cross‐sectional multicenter study in Japan. J Neurol Sci 2015;358(1–2):299–303. [DOI] [PubMed] [Google Scholar]
- 69. Margraf NG, Rohr A, Granert O, Hampel J, Drews A, Deuschl G. MRI of lumbar trunk muscles in patients with Parkinson's disease and camptocormia. J Neurol 2015;262(7):1655–1664. [DOI] [PubMed] [Google Scholar]
- 70. Khallaf ME, Fayed EE. Early postural changes in individuals with idiopathic Parkinson's disease. Parkinsons Dis 2015;2015:369454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kataoka H, Sawa N, Ueno S. Identification of a new target muscle for treatment in patients with Parkinson's disease who have lateral trunk flexion? J Neurol Sci 2015;358(1–2):435–439. [DOI] [PubMed] [Google Scholar]
- 72. Geroin C, Smania N, Schena F, et al. Does the Pisa syndrome affect postural control, balance, and gait in patients with Parkinson's disease? An observational cross‐sectional study. Parkinsonism Relat Disord 2015;21(7):736–741. [DOI] [PubMed] [Google Scholar]
- 73. Furusawa Y, Hanakawa T, Mukai Y, et al. Mechanism of camptocormia in Parkinson's disease analyzed by tilt table‐EMG recording. Parkinsonism Relat Disord 2015;21(7):765–770. [DOI] [PubMed] [Google Scholar]
- 74. Frazzitta G, Balbi P, Gotti F, et al. Pisa syndrome in Parkinson's disease: Electromyographic aspects and implications for rehabilitation. Parkinsons Dis 2015;2015:437190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Iijima M, Osawa M, Uchiyama S, Kitagawa K. Pramipexole‐induced antecollis in patients with Parkinson's disease: two cases and literature review. eNeurologicalSci 2015;1:21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ondo WG, Haykal HA. Paraspinal muscle asymmetry in Parkinson's disease. Int J Neurosci 2014;124(2):93–96. [DOI] [PubMed] [Google Scholar]
- 77. Arii Y, Sawada Y, Kawamura K, et al. Immediate effect of spinal magnetic stimulation on camptocormia in Parkinson's disease. J Neurol Neurosurg Psychiatry 2014;85(11):1221–1226. [DOI] [PubMed] [Google Scholar]
- 78. Tinazzi M, Juergenson I, Squintani G, et al. Pisa syndrome in Parkinson's disease: an electrophysiological and imaging study. J Neurol 2013;260(8):2138–2148. [DOI] [PubMed] [Google Scholar]
- 79. Doherty KM, Davagnanam I, Molloy S, Silveira‐Moriyama L, Lees AJ. Pisa syndrome in Parkinson's disease: a mobile or fixed deformity? J Neurol Neurosurg Psychiatry 2013;84(12):1400–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wrede A, Margraf NG, Goebel HH, Deuschl G, Schulz‐Schaeffer WJ. Myofibrillar disorganization characterizes myopathy of camptocormia in Parkinson's disease. Acta Neuropathol 2012;123(3):419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tassorelli C, Furnari A, Buscone S, et al. Pisa syndrome in Parkinson's disease: clinical, electromyographic, and radiological characterization. Mov Disord 2012;27(2):227–235. [DOI] [PubMed] [Google Scholar]
- 82. Furusawa Y, Mukai Y, Kobayashi Y, Sakamoto T, Murata M. Role of the external oblique muscle in upper camptocormia for patients with Parkinson's disease. Mov Disord 2012;27(6):802–803. [DOI] [PubMed] [Google Scholar]
- 83. Vitale C, Marcelli V, Furia T, et al. Vestibular impairment and adaptive postural imbalance in parkinsonian patients with lateral trunk flexion. Mov Disord 2011;26(8):1458–1463. [DOI] [PubMed] [Google Scholar]
- 84. Di Matteo A, Fasano A, Squintani G, et al. Lateral trunk flexion in Parkinson's disease: EMG features disclose two different underlying pathophysiological mechanisms. J Neurol 2011;258(5):740–745. [DOI] [PubMed] [Google Scholar]
- 85. Fasano A, Di Matteo A, Vitale C, et al. Reversible Pisa syndrome in patients with Parkinson's disease on rasagiline therapy. Mov Disord 2011;26(14):2578–2580. [DOI] [PubMed] [Google Scholar]
- 86. Spuler S, Krug H, Klein C, et al. Myopathy causing camptocormia in idiopathic Parkinson's disease: a multidisciplinary approach. Mov Disord 2010;25(5):552–559. [DOI] [PubMed] [Google Scholar]
- 87. Abe K, Uchida Y, Notani M. Camptocormia in Parkinson's disease. Parkinsons Dis 2010;2010:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Margraf NG, Wrede A, Rohr A, et al. Camptocormia in idiopathic Parkinson's disease: a focal myopathy of the paravertebral muscles. Mov Disord 2010;25(5):542–551. [DOI] [PubMed] [Google Scholar]
- 89. Bonneville F, Bloch F, Kurys E, et al. Camptocormia and Parkinson's disease: MR imaging. Eur Radiol 2008;18(8):1710–1719. [DOI] [PubMed] [Google Scholar]
- 90. Bloch F, Houeto JL, Tezenas du Montcel S, et al. Parkinson's disease with camptocormia. J Neurol Neurosurg Psychiatry 2006;77(11):1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lepoutre AC, Devos D, Blanchard‐Dauphin A, et al. A specific clinical pattern of camptocormia in Parkinson's disease. J Neurol Neurosurg Psychiatry 2006;77(11):1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kashihara K, Ohno M, Tomita S. Dropped head syndrome in Parkinson's disease. Mov Disord 2006;21(8):1213–1216. [DOI] [PubMed] [Google Scholar]
- 93. Jacobs JV, Dimitrova DM, Nutt JG, Horak FB. Can stooped posture explain multidirectional postural instability in patients with Parkinson's disease? Exp Brain Res 2005;166(1):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Schabitz WR, Glatz K, Schuhan C, et al. Severe forward flexion of the trunk in Parkinson's disease: focal myopathy of the paraspinal muscles mimicking camptocormia. Mov Disord 2003;18(4):408–414. [DOI] [PubMed] [Google Scholar]
- 95. Holler I, Dirnberger G, Pirker W, Auff E, Gerschlager W. Camptocormia in idiopathic Parkinson's disease: [(123)I]beta‐CIT SPECT and clinical characteristics. Eur Neurol 2003;50(2):118–120. [DOI] [PubMed] [Google Scholar]
- 96. Djaldetti R, Mosberg‐Galili R, Sroka H, Merims D, Melamed E. Camptocormia (bent spine) in patients with Parkinson's disease – characterization and possible pathogenesis of an unusual phenomenon. Mov Disord 1999;14(3):443–447. [DOI] [PubMed] [Google Scholar]
- 97. Schlenstedt C, Bosse K, Gavriliuc O, et al. Quantitative assessment of posture in healthy controls and patients with Parkinson's disease. Parkinsonism Relat Disord 2020;76:85–90. [DOI] [PubMed] [Google Scholar]
- 98. Sako W, Nishio M, Maruo T, et al. Subthalamic nucleus deep brain stimulation for camptocormia associated with Parkinson's disease. Mov Disord 2009;24(7):1076–1079. [DOI] [PubMed] [Google Scholar]
- 99. Margraf NG, Rogalski M, Deuschl G, Kuhtz‐Buschbeck JP. Trunk muscle activation pattern in parkinsonian camptocormia as revealed with surface electromyography. Parkinsonism Relat Disord 2017;44:44–50. [DOI] [PubMed] [Google Scholar]
- 100. Diederich NJ, Goebel HH, Dooms G, Bumb A, Huber F, Kompoliti K, Meinck HMM. Camptocormia associated with focal myositis in multiple‐system atrophy. Mov Disord 2006;21(3):390–394. [DOI] [PubMed] [Google Scholar]
- 101. Revuelta GJ, Montilla J, Benatar M, et al. An (1) (8)F‐FDG PET study of cervical muscle in parkinsonian anterocollis. J Neurol Sci 2014;340(1–2):174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. van de Warrenburg BP, Cordivari C, Ryan AM, et al. The phenomenon of disproportionate antecollis in Parkinson's disease and multiple system atrophy. Mov Disord 2007;22(16):2325–2331. [DOI] [PubMed] [Google Scholar]
- 103. Savica R, Kumar N, Ahlskog JE, Josephs KA, Matsumoto JY, McKeon A. Parkinsonism and dropped head: dystonia, myopathy or both? Parkinsonism Relat Disord 2012;18(1):30–34. [DOI] [PubMed] [Google Scholar]
- 104. Revuelta GJ, Benatar M, Freeman A, Wichmann T, Jinnah HA, DeLong MR, Factor SA. Clinical subtypes of anterocollis in parkinsonian syndromes. J Neurol Sci 2012;315(1–2):100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wanschitz JV, Sawires M, Seppi K, Boesch S, Loescher WN, Schocke M, Poewe W. Axial myopathy in parkinsonism. Mov Disord 2011;26(8):1569–1571. [DOI] [PubMed] [Google Scholar]
- 106. Azher SN, Jankovic J. Camptocormia: pathogenesis, classification, and response to therapy. Neurology 2005;65(3):355–359. [DOI] [PubMed] [Google Scholar]
- 107. Yoshiyama Y, Takama J, Hattori T. The dropped head sign in parkinsonism. J Neurol Sci 1999;167(1):22–25. [DOI] [PubMed] [Google Scholar]
- 108. Dressler D, Altavista MC, Altenmueller E, et al. Consensus guidelines for botulinum toxin therapy: general algorithms and dosing tables for dystonia and spasticity. J Neural Transm (Vienna) 2021;128(3):321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gandolfi M, Tinazzi M, Magrinelli F, et al. Four‐week trunk‐specific exercise program decreases forward trunk flexion in Parkinson's disease: a single‐blinded, randomized controlled trial. Mov Disord 2019;34:S263–S274. [DOI] [PubMed] [Google Scholar]
- 110. Ferreira‐Sanchez MDR, Moreno‐Verdu M, Cano‐de‐la‐Cuerda R. Quantitative measurement of rigidity in Parkinson s disease: a systematic review. Sensors (Basel) 2020;20(3):880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wright WG, Gurfinkel V, Nutt J, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson's disease: direct measurements of trunk and hip torque. Exp Neurol 2007;208(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vaugoyeau M, Azulay JP. Role of sensory information in the control of postural orientation in Parkinson's disease. J Neurol Sci 2010;289(1–2):66–68. [DOI] [PubMed] [Google Scholar]
- 113. Schoneburg B, Mancini M, Horak F, Nutt JG. Framework for understanding balance dysfunction in Parkinson's disease. Mov Disord 2013;28(11):1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Artusi CA, Montanaro E, Erro R, et al. Visuospatial deficits are associated with Pisa syndrome and not Camptocormia in Parkinson's disease. Mov Disord Clin Pract 2022;10:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schroeteler FE, Fietzek UM, Ziegler K, Ceballos‐Baumann AO. Upright posture in parkinsonian camptocormia using a high‐frame walker with forearm support. Mov Disord 2011;26(8):1560–1561. [DOI] [PubMed] [Google Scholar]
- 116. Gdynia HJ, Sperfeld AD, Unrath A, Ludolph AC, Sabolek M, Storch A, Kassubek J. Histopathological analysis of skeletal muscle in patients with Parkinson's disease and ‘dropped head’/‘bent spine’ syndrome. Parkinsonism Relat Disord 2009;15(9):633–639. [DOI] [PubMed] [Google Scholar]
- 117. Bonanni L, Thomas A, Varanese S, Scorrano V, Onofrj M. Botulinum toxin treatment of lateral axial dystonia in parkinsonism. Mov Disord 2007;22(14):2097–2103. [DOI] [PubMed] [Google Scholar]
- 118. Uzawa A, Mori M, Kojima S, Mitsuma S, Sekiguchi Y, Kanesaka T, Kuwabara S. Dopamine agonist‐induced antecollis in Parkinson's disease. Mov Disord 2009;24(16):2408–2411. [DOI] [PubMed] [Google Scholar]
- 119. Suzuki T, Matsuzaka H. Drug‐induced Pisa syndrome (pleurothotonus): epidemiology and management. CNS Drugs 2002;16(3):165–174. [DOI] [PubMed] [Google Scholar]
- 120. van de Warrenburg BP, Bhatia KP, Quinn NP. Pisa syndrome after unilateral pallidotomy in Parkinson's disease: an unrecognised, delayed adverse event? J Neurol Neurosurg Psychiatry 2007;78(3):329–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Main characteristics of studies on clinical predictors for axial postural abnormalities in patients with Parkinsonism.
Table S2. Main characteristics of studies on the pathophysiology of axial postural abnormalities in patients with Parkinsonism.
Data S1. Electronic search strategy.


