Abstract
Background
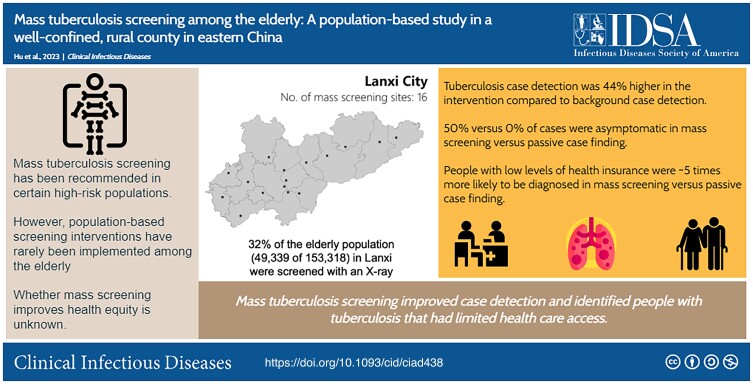
Mass tuberculosis (TB) screening has been recommended in certain high-risk populations. However, population-based screening interventions have rarely been implemented. Whether mass screening improves health equity is unknown.
Methods
We implemented a mass TB screening intervention among elderly persons (>60 years old) in Lanxi County, China. Standardized questionnaires, physical examinations, and chest radiographs (CXRs) were administered to all participants. Systematic testing with computed tomography, smear, culture, or Xpert was performed among persons with an abnormal CXR. We assessed TB prevalence per 100 000 persons and constructed multivariable regression models among subgroups that were and were not screened. Medical insurance was categorized as participation in either a basic program with limited coverage or a more comprehensive coverage program.
Results
In total, 49 339 individuals (32% of the elderly population in Lanxi) participated in the screening. One hundred fifteen screened persons were diagnosed with TB (233 cases per 100 000 persons), significantly higher than persons not screened (168 cases among 103 979 person-years; prevalence-to-case notification ratio, 1.44 [95% confidence interval {CI}, 1.14–1.83]). This increase was largely driven by diagnosis of asymptomatic disease during mass screening (n = 57 [50% of participants with TB]). Participants with basic medical insurance were much more likely to be diagnosed through mass screening than by passive detection (adjusted odds ratio, 4.52 [95% CI, 1.35–21.28]).
Conclusions
In a population-based, mass TB screening intervention encompassing >30% of the elderly population in a county in rural China, case finding was 44% higher than background detection, driven by diagnosis of TB without recognized symptoms. Importantly, mass screening identified TB in people with limited healthcare options who were less likely to be found through background case detection.
Keywords: tuberculosis, mass screening, active case finding, elderly
In a mass tuberculosis screening intervention encompassing >30% of the elderly population in rural China, case detection was 44% higher than passive case finding, driven by diagnosis of asymptomatic tuberculosis. Mass screening identified patients with tuberculosis with limited healthcare options.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/mass-tuberculosis-screening-among-the-elderly-a-population-based-study-in-a-well-confined-rural-community-in-eastern-china-cda1ea08-0ea1-4224-965e-b4f8efa847eb
(See the Editorial Commentary by Wingfield on pages 1476–9.)
Tuberculosis (TB) is the leading bacterial cause of death globally [1]. Case detection of TB prior to 2020 was poor, estimated at approximately 66%; however, after the coronavirus disease 2019 (COVID-19) pandemic, a substantial reduction in people with TB was observed and notified in 2020 and 2021 [2, 3]. These health impacts have threatened global goals of a 90% reduction in TB incidence by 2035 [4]. To meet this ambitious goal and recover from recent health infrastructure impacts, enhancing TB case detection through more comprehensive, active interventions may be necessary.
Active TB case finding through mass screening has been proposed to improve case detection in high-burden settings [5, 6]. In China, most cases of TB are identified when people with TB-related symptoms (eg, cough, fever) seek healthcare services. Recent studies show the importance of targeting high-risk populations such as people with human immunodeficiency virus and exposed persons [7–9]. A recent study found that multiple rounds of untargeted mass TB screening of the general population in Vietnam reduced overall TB prevalence in the community [10]. Despite these encouraging results, several questions remain regarding the utility of mass TB screening, including the prevalence threshold at which it remains high-yield and cost-effective. Few large-scale, population-level mass screening interventions have been implemented and reported. The effectiveness of mass screening among high-risk populations, such as the elderly, is unexplored. Mass screening may theoretically improve health equity, but no empirical evidence exists to confirm this assumption.
In 2021, we implemented a population-wide, mass TB screening program among an elderly population, including physical examinations and chest radiographs (CXRs) on all individuals. We aimed to evaluate this active, mass TB screening and assess whether mass screening was more likely than background screening to find TB among persons with limited health coverage.
METHODS
Study Setting
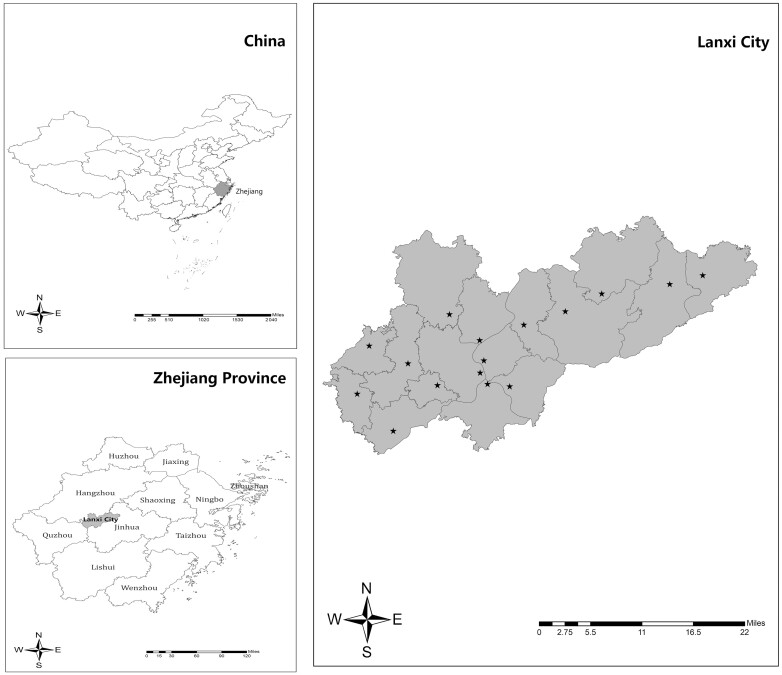
In 2021, we performed mass TB screening in Lanxi County in Zhejiang Province, China (Figure 1). Lanxi County is located in the central-west region of Zhejiang. In 2020, Lanxi had a population of 574 801 (population density, 438 persons/km2), with an estimated 153 318 elderly persons, accounting for 27% of the broader general population [11]. Outmigration is low in Lanxi County, especially among the elderly population.
Figure 1.
Location and the sites of the mass screening intervention in Lanxi County, China. Stars (★) indicate the 16 mass screening sites in Lanxi City.
Study Design, Procedures, and Participants
Mass TB screening was carried out among the registered local elderly population in Lanxi County. This intervention was initiated because, although there has been a decline in overall burden and incidence of TB in Zhejiang Province and China, the absolute number of people with TB among the elderly population has remained at a high level. A health information campaign related to the TB screening was implemented prior to the intervention to increase participation.
In China, individuals >65 years of age or persons of any age with diabetes are provided a series of healthcare examinations at no individual cost. This service is called the Basic Public Health Service Project, a national program provided free of charge to residents. This program is available for all residents but has a focus on children, pregnant women, the elderly, and patients with chronic diseases, in response to the main present health problems of urban and rural residents. In addition to this health package, CXRs were provided to all eligible persons in Lanxi County. After the health check-up at a local community health clinic, participants were then asked to provide informed consent for participation in the study and subsequently given a CXR. Each CXR was uploaded to 2 independent imaging departments in local general hospitals to derive and analyze for imaging diagnoses. Initial imaging results were interpreted by a specialized radiologist at the local community hospital; the image was uploaded to use a uniform, electronic recording system from a medical alliance of several hospitals.
The physician in attendance from the medical alliance then rechecked the imaging result. If the initial and subsequent imaging interpretations were distinct, the results were then discussed by a clinical group of specialists consisting of county, city, and provincial pulmonary physicians. CXR abnormalities suggestive of TB mainly included (1) cavitation and cavitary nodule; (2) foci of exudation proliferation; (3) tuberculoma and tuberculoma; (4) miliary; (5) pleural effusions or pleural thickening; and (6) combinations of these abnormalities. Participants with CXRs suggestive of TB were referred to a local designated hospital for further examination. Participants with CXR findings suggestive of other types of diseases were also referred for care and a further health examination. Persons with suggestive CXR findings were further examined first through computed tomography and sputum smear testing. If either was positive or further suggestive, testing including culture and Xpert were performed for case identification in a local designated hospital.
Separately, we collected information on elderly persons diagnosed with TB found through passive detection in the corresponding time period through the county-level TB registry. Tuberculosis is a reportable disease in China, and all persons with suspected TB (including those diagnosed in either the public or private sectors) are mandated to be reported in a timely fashion to the local Center for Disease Control and Prevention (CDC). All persons with diagnosed TB are managed through the Chinese Tuberculosis Information Management System [12]; therefore, patient diagnoses can be tracked over time. We collected all data from these registries from January 2021 to the end of December 2021.
Study Definitions
The diagnosis of pulmonary TB was based on a combination of bacteriological results, epidemiological history, clinical manifestations, and chest imaging [13]. Bacteriological examinations included sputum smear, sputum culture, and molecular biological tests. Standard-course TB treatment was provided regardless of symptom status of persons with TB.
The survey population was divided into 2 locally relevant categories: health insurance coverage for urban workers and a basic health insurance for urban residents. The former is considered at a significant economic advantage based on substantially higher reimbursement rates, a higher limit line of health coverage, and a lower payout line. Health insurance coverage was collected through county-wide health records. Body mass index (BMI) was categorized into subgroups relevant for Asian populations including <18.5 kg/m² (underweight), 18.5–23.9 kg/m² (normal weight), and ≥24 kg/m² (overweight) [14]. Prior TB was defined as having a confirmed or reported diagnosis/treatment of TB [15]. Alcohol consumption in the past year was categorized into 3 groups: nondrinkers, occasional drinkers (less than twice a week), and frequent drinkers (at least twice a week). Diabetes was defined as a prior diagnosis (collected through health records) or through a positive fasting glucose test [16]. We defined smoking based on separate categories including never smoker, prior smoker, and current smoker.
Statistical Analysis
Social and demographic data were summarized by frequency or proportion for categorical or ordinal variables, respectively. Age was categorized as 60–69, 70–79 and ≥80 years. TB prevalence per 100 000 persons was calculated overall and for each stratified subgroup. We calculated the number of elderly persons needed to screen (NNS) to detect 1 TB case. The NNS was calculated by dividing the number of screened individuals by the number of persons with detected TB [6].
We compared the presence and duration of symptoms prior to TB diagnosis between persons detected through mass screening and passive detection. We compared sputum smear grade among persons diagnosed with TB through either the mass screening or passive case registry. Last, we assessed whether participants diagnosed with TB during mass screening and passive detection had differential healthcare access (as measured by health insurance status).
We calculated the prevalence-to-case notification ratio as the prevalence of new cases of TB per 100 000 people divided by the number of newly reported cases of TB per 100 000 people per year in 2021. This ratio provides an approximate indicator of case detection or poor access to healthcare. We calculated this ratio overall and by specific high-risk subgroups of participants [17].
Univariable and multivariable analyses were conducted using binary logistic regression models of variables for both mass screening and passive detection. Odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were calculated.
Ethics Approval
This study was approved by the Ethics Committee of the Zhejiang Provincial CDC. All participants signed the informed consent provided by Lanxi County CDC. All protocols employed in this research followed the Law of the Prevention and Treatment of Infectious Diseases in the People's Republic of China. All participants diagnosed with TB were provided with standardized treatment and fully anonymized before further analysis.
RESULTS
In total, 49 339 persons participated in the mass screening intervention in 2021 (Table 1). The screening coverage rate was 32.2% of the elderly population in the county (49 339 of 153 318). Among all elderly in Lanxi County, females were more likely to participate in the mass screening compared to males (35% vs 30% of all females and males in the county participated; P < .001). The mass screening intervention was also more likely to be attended by people with diabetes mellitus (53% vs 30% of all persons with and without diabetes in the county participated; P < .0001) and less likely to be attended by persons with a prior TB diagnosis (23% and 33% of all persons with and without a prior TB diagnosis in the county participated; P < .0001) (Supplementary Table 1).
Table 1.
Demographic Characteristics and Tuberculosis Identification From Mass Tuberculosis Screening of the Elderly in Lanxi County
| Variables | Tuberculosis Cases, No. | Screened, No. | Cases per 100 000 Persons, No. | P Valuea |
|---|---|---|---|---|
| No. of participants | 115 | 49 339 | 233.1 | |
| Age, y | .34 | |||
| 60–69 | 45 | 21 395 | 210.3 | |
| 70–79 | 60 | 22 476 | 267.0 | |
| ≥80 | 10 | 5468 | 182.9 | |
| Sex | <.01 | |||
| Male | 90 | 22 854 | 393.8 | |
| Female | 25 | 26 485 | 94.4 | |
| Educational level | .13 | |||
| No literacy | 26 | 15 541 | 167.3 | |
| Elementary and middle school | 86 | 32 125 | 267.7 | |
| High school | 3 | 1657 | 181.1 | |
| College and above | 0 | 16 | 0 | |
| Nationality | 1.00 | |||
| Han | 115 | 49 168 | 233.9 | |
| Other | 0 | 171 | 0 | |
| Body mass index, kg/m2 | <.01 | |||
| <18.5 | 12 | 2090 | 574.2 | |
| 18.5–23.9 | 83 | 31 230 | 262.6 | |
| ≥24 | 20 | 15 966 | 131.5 | |
| Missing | 0 | 53 | 0 |
aWe used Pearson χ2 or Fisher's Exact test to derive P values for all categorical variables. For continuous variables, we used Wilcoxon rank-sum tests for comparison of 2-sample medians.
Among screened participants, 43% (n = 21 395) were 60–69 years of age and 46% (n = 22 476) were 70–79 years of age. Fifty-four percent of participants were female (n = 26 485), and 78% were farmers (n = 38 260). In total, 6659 (13%) had diabetes and 1406 (3%) had previously diagnosed TB.
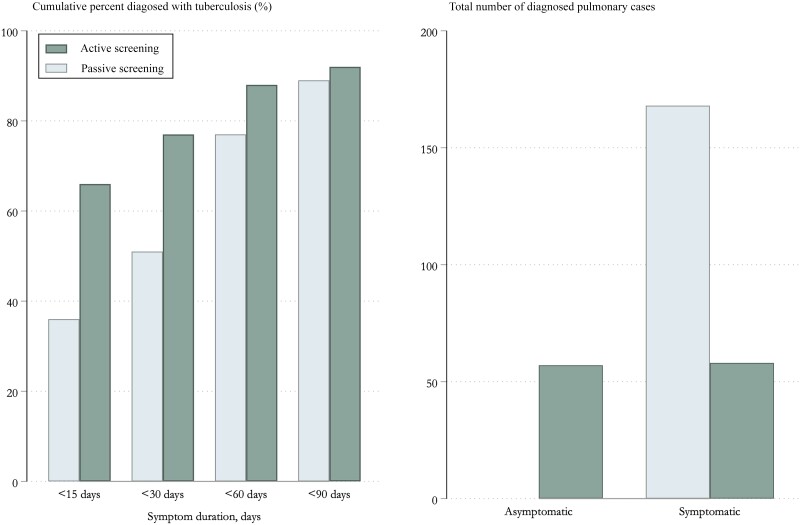
A total of 599 persons with abnormal CXRs suggestive of TB were found (Supplementary Table 2), and 588 of them (98%) were ultimately referred to the designated hospital. In total, 115 persons with TB were identified from screening (a prevalence of 233 [95% CI, 194–279] cases per 100 000 persons). Of these, 78 persons were smear-positive (68%) while 7 (6%) were smear negative, culture positive; 30 persons (26%) were bacteriologically negative. Among persons diagnosed with bacteriologically negative TB, 14 with pulmonary TB had no TB-related symptoms. Half of persons with TB had no symptoms (n = 57). Among those with symptoms (n = 58), 30 (52%) had chronic cough (>14 days’ duration), 28 had expectoration (48%), 3 (5%) had hemoptysis, 3 (5%) had fever, and 2 (3%) had fatigue. Other TB-related symptoms such as chest pain, anorexia, weight loss, and night sweats were unobserved.
The total NNS to detect 1 case of TB was 430 (95% CI, 357–526). TB prevalence per 100 000 persons was much higher among males (394 in males vs 94 in females) and participants with a BMI <18.5 kg/m2 (574 vs 263 and 132 in participants with BMI 18.5–23.9 kg/m2 and ≥24 kg/m2, respectively). Persons with diabetes were diagnosed almost 2 times more often than people without diabetes (391 vs 209 cases per 100 000 persons; risk difference, 182 cases per 100 000 persons).
Among 103 979 elderly persons not screened in Lanxi County in 2021, a total of 168 were passively diagnosed—a notification rate of 162 (95% CI, 139–188) cases per 100 000 persons. Of these, 129 were smear positive (77%) while 2 (1%) were smear negative, culture positive. Thirty-seven persons (22%) were bacteriologically negative on all tests. All passively diagnosed persons with TB had at least 1 TB-related symptom. Persons with diabetes were passively diagnosed almost 2 times more often than people without diabetes (374 [95% CI, 248–560] vs 149 [95% CI, 127–175] cases per 100 000 persons; risk difference, 225 cases per 100 000 persons [95% CI, 179–278]).
The prevalence-to-case notification ratio was 1.44 (95% CI, 1.14–1.83) overall. Among persons with diabetes, the TB notification rate was similar to TB prevalence (prevalence-to-case notification ratio, 1.04 [95% CI, .59–1.84]; Table 2). Among persons with prior TB, there were few overall cases (n = 1). The prevalence-to-notification ratio was higher for males (1.71 [95% CI, 1.20–2.44]) than for females (1.07 [95% CI, .56–2.05]), suggesting that case detection was poor among males in this setting, Last, younger age groups, specifically participants aged 60–69 years, had a high prevalence-to-case notification ratio (1.77 [95% CI, 1.09–2.89]). The prevalence-to-notification ratio among other age groups was close to 1.
Table 2.
Tuberculosis Prevalence-to-Case-Notification Ratio Among Distinct High-Risk Elderly Groups Undergoing Active and Passive Case Finding in Lanxi County
| Target Population | Population, No. | Tuberculosis Cases, No. | Cases per 100 000 Persons |
Prevalence-to-Case Notification Ratio (95% CI) |
|---|---|---|---|---|
| All participants | ||||
| Passive detection | 103 979 | 168 | 161.6 | 1 (referent) |
| Active screening | 49 339 | 115 | 233.1 | 1.44 (1.14–1.83) |
| Male | ||||
| Passive detection | 53 822 | 124 | 230.4 | 1 (referent) |
| Active screening | 22 854 | 90 | 393.8 | 1.71 (1.20–2.44) |
| Female | ||||
| Passive detection | 50 157 | 44 | 87.7 | 1 (referent) |
| Active screening | 26 485 | 25 | 94.4 | 1.07 (.56–2.05) |
| 60–69 y old | ||||
| Passive detection | 60 743 | 72 | 118.5 | 1 (referent) |
| Active screening | 21 395 | 45 | 210.3 | 1.77 (1.09–2.89) |
| 70–79 y old | ||||
| Passive detection | 26 433 | 70 | 264.8 | 1 (referent) |
| Active screening | 22 476 | 60 | 267 | 1.01 (.64–1.59) |
| ≥80 y old | ||||
| Passive detection | 16 803 | 26 | 154.7 | 1 (referent) |
| Active screening | 5468 | 10 | 182.9 | 1.18 (.45–3.08) |
| Diagnosed diabetes | ||||
| Passive detection | 5877 | 22 | 374.3 | 1 (referent) |
| Active screening | 6659 | 26 | 390.5 | 1.04 (.59–1.84) |
| Prior TB diagnosisa | ||||
| Passive detection | 4729 | 0 | 0 | 1 (referent) |
| Active screening | 1406 | 1 | 71.1 | … |
Abbreviations: CI, confidence interval; TB, tuberculosis.
aPrior TB was defined as having a confirmed or reported diagnosis/treatment of TB.
Overall, persons diagnosed through mass screening had a significantly shorter symptom duration before diagnosis than did persons diagnosed through passive case detection (Figure 2). Of 115 persons diagnosed through mass screening, 76 (66%) had symptoms for <15 days, compared with 60 of 168 (36%) persons diagnosed through programmatic passive case finding (risk difference, 30% [95% CI, 19%–41%]; P < .0001). Eighty-nine (77%) persons diagnosed through active case finding had symptoms for <30 days compared with 86 (51%) persons diagnosed through programmatic passive case finding (risk difference, 26% [95% CI, 15%–36%]; P < .0001). A comparable percentage of persons diagnosed with TB through mass screening and passive case finding had symptoms for <90 days (92% vs 89%; P = .45).
Figure 2.
The presence and duration of symptoms before diagnosis among participants diagnosed with tuberculosis during a mass screening intervention compared to participants diagnosed from background detection.
TB diagnosis was more common through mass screening compared to passive detection for people who smoked (adjusted OR [AOR], 2.43 [95% CI, 1.15–5.17]) or had diabetes (AOR, 4.72 [95% CI, 2.17–10.91]), but not for age (Supplementary Table 3). Persons with limited health insurance were also much more likely detected through mass screening compared to passive detection (AOR, 4.52 [95% CI, 1.35–21.28]) (Table 3).
Table 3.
Characteristics of Persons With Tuberculosis Diagnosed Within the Mass Tuberculosis Screening Intervention or as Part of Background Passive Case Detection
| Variable | Passive Case Findinga | Mass Screeninga | P Value | Odds Ratio (95% CI) |
AOR (95% CI) |
|---|---|---|---|---|---|
| No. of participants | 168 | 115 | … | … | |
| Age, y | .099 | ||||
| 60–69 | 73 (43.5) | 45 (39.1) | 1 (Referent) | … | |
| 70–79 | 69 (41.1) | 60 (52.2) | 1.41 (.85–2.35) | … | |
| ≥80 | 26 (15.5) | 10 (8.7) | 0.62 (.26–1.38) | … | |
| Sex | .409 | ||||
| Male | 123 (73.2) | 90 (78.3) | 1 (Referent) | … | |
| Female | 45 (26.8) | 25 (21.7) | 0.76 (.43–1.32) | … | |
| Smoking status | .003 | ||||
| Never | 133 (79.2) | 70 (60.9) | 1 (Referent) | 1 (Referent) | |
| Prior | 8 (4.8) | 7 (6.1) | 1.66 (.56–4.82) | 1.57 (.48–4.91) | |
| Current | 27 (16.1) | 38 (33.0) | 2.67 (1.52–4.78) | 2.43 (1.15–5.17) | |
| Alcohol consumption | .01 | ||||
| Never | 130 (77.4) | 72 (62.6) | 1 (Referent) | 1 (Referent) | |
| Occasional | 14 (8.3) | 10 (8.7) | 1.29 (.53–3.03) | 1.30 (.49–3.35) | |
| Frequently | 24 (14.3) | 33 (28.7) | 2.48 (1.37–4.56) | 1.34 (.60–2.95) | |
| Diabetes mellitus | <.001 | ||||
| No | 156 (92.9) | 85 (73.9) | 1 (Referent) | 1 (Referent) | |
| Yes | 12 (7.1) | 30 (26.1) | 3.64 (2.07–6.44) | 4.72 (2.17–10.91) | |
| Comorbidityb | .035 | ||||
| No | 100 (59.5) | 53 (46.1) | 1 (Referent) | 1 (Referent) | |
| Yes | 68 (40.5) | 62 (53.9) | 1.72 (1.07–2.79) | 1.34 (.78–2.32) | |
| Medical insurance | .01 | ||||
| Urban workers | 20 (11.9) | 3 (2.6) | 1 (Referent) | 1 (Referent) | |
| Urban residents | 148 (88.1) | 112 (97.4) | 5.05 (1.68–21.79) | 4.52 (1.35–21.28) |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval.
aPercentages refer to within-characteristic column totals. Percentages may not total 100% because within-column percentages were rounded to the nearest integer.
bThis category refers to prior medical history of epilepsy (other mental history) and disease of the heart, lung, liver, spleen, kidney, and/or other major organs. Information about these diseases was collected through a combination of self-reporting through surveys and through physician workups.
DISCUSSION
Although elderly persons represent a critical population to the TB epidemic in China and other southeastern Asian countries, there are few studies evaluating the effectiveness of TB interventions among this population [12, 18]. In a large, mass TB screening intervention covering >30% of elderly persons in Lanxi County, we found a TB prevalence exceeding 200 cases per 100 000 persons, 44% greater compared with passive detection. In our mass screening intervention, 50% of persons with TB were asymptomatic, encompassing most of the detection gap between background passive case detection. Importantly, mass screening was multifold more likely to detect persons with low health coverage and limited healthcare options, suggesting that this intervention is identifying difficult-to-reach, in-need populations.
After implementation of our mass TB screening intervention, TB was high at 233 cases per 100 000 persons. This prevalence is significantly higher than background notification rates among the elderly in China (88 cases per 100 000 person-years; China TB surveillance report 2021) and Zhejiang Province (75 cases per 100 000 person-years) but reflects the high burden of Lanxi County. We specifically targeted this mass screening intervention to this county, one of the highest-burden counties of the province. When comparing mass screening to passive detection, the prevalence-to-case notification ratio was 1.44, suggesting a low case detection rate or poor access to healthcare among this elderly age group. This detection gap was not explained by high-risk groups such as persons with diabetes or prior TB. Although persons with diabetes had a higher prevalence of TB within the mass screening intervention, their prevalence-to-case notification ratio was close to 1, indicating strong case detection among this group in the general population.
We found a substantial proportion of asymptomatic and subclinical TB, similar to prior prevalence surveys [19]. In our study, the detection gap between mass screening and passive detection was primarily due to asymptomatic and/or subclinical diagnoses. Additionally, the symptom duration of persons with TB found in mass screening was consistently lower than that found in programmatic passive case finding. This indicates that this intervention is highly effective at detecting persons with TB early in their disease progression and limiting healthcare system delays and health referrals [20, 21]. In China, all persons with TB, regardless of symptoms, are given a full TB disease treatment regimen. The high proportion of asymptomatic TB diagnosed from this mass intervention suggests that, at a minimum, CXR or bacteriological testing is necessary. People with asymptomatic TB may end up being ineffectively treated with standardized clinical therapy if only symptom screening is used to exclude disease.
Importantly, we found that persons with TB diagnosed through mass screening were more likely to lack adequate healthcare and have limited healthcare access. This result is important, demonstrating the potential that mass screening interventions may reach difficult-to-find, high-risk populations that are often underserved. Our results are distinct from a large household contact tracing study in Peru, which found that socioeconomic status did not differ in households of persons with TB diagnosed from active compared to passive case finding [22]. Whether active case finding improves health equity is likely to be largely contextual and dependent on the coverage of the intervention, background health services, and who participates in the intervention.
Despite widescale preliminary health campaigns prior to the TB screening, only 32% of the elderly population joined the mass TB screening. Although ambitious, our initial program goal was to cover the entire elderly population in Lanxi City. Our study represents tens of thousands of people actively screened and an increased uptake of case detection within the city; however, a higher screening coverage rate would have been preferable to increase effectiveness of the program as a population-based tool. Additional research is needed to understand why people do not participate in such screenings and the TB yield among this population. In our cohort, females were more likely to be screened compared to males despite the higher TB burden among males. The TB prevalence for males was roughly 4 times higher than females among those screened, consistent with previous prevalence studies [23, 24]. The reasoning for this increased burden is unclear, but selection biases may be present if certain populations present to the screening and not others. People with diabetes were also much more likely to participate in the screening, potentially due to substantial prior experience with the healthcare system [25–27]. Although preferentially targeting persons who were previously treated with TB has been proposed [28], our study found few secondary cases among this group. This contrasts with a recent prevalence survey from South Africa, which found that 27% of bacteriologically positive TB was from persons with previously treated TB [29]. Our low yield among this high-risk population is likely due to the surprising result that people with prior TB were less likely to participate in the screening. Less than 25% of all elderly persons with prior TB in the county were screened. Reasons for this result are unclear but may be due to community stigma or the view that recurrent TB is unlikely. In future rounds of mass screening, it is vital to screen these special high-risk groups missed in this first round.
There are limitations to this study. First, some characteristics were only available for persons with TB (eg, smoking and diabetes status), limiting our ability to further ascertain differences between participants who did or did not develop disease. Second, although CXRs were performed at a mass scale, artificial intelligence–based technology was not implemented. Although CXRs have high sensitivity, misclassification of CXR findings may have been possible, due to poor specificity [30]. Our focus on the elderly as our study population may accentuate misclassification further, potentially leading to overdiagnosis among individuals with bacteriologically negative test results. There was a high proportion of persons diagnosed with TB who were bacteriologically negative, likely due to the difficulty of sputum collection among the elderly. Third, participants eligible for the mass screening had to travel to a local community clinic, resulting in lack of attendance and missed cases. Persons may be more likely to have disease compared to a community screening not contingent on travel to a healthcare setting. Alternatively, persons included in the screening may be healthier if some of the elderly population could not attend a community clinic due to incapacitation. Last, notification rates may be subject to biases around migration. However, this population is generally stable without much migration and movement. Therefore, this bias is likely minimal if present.
In conclusion, a large, mass TB screening among the elderly in a rural eastern Chinese county contributed to overall case detection in this important, high-risk population. Compared with passive case finding, mass screening detected persons with TB who had limited healthcare options. Half of persons with TB during mass screening were asymptomatic, encompassing most of the detection gap compared to background detection. Further rounds of mass screening are needed to evaluate whether overall TB prevalence among this population can be reduced in subsequent years.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Zhengfang Hu, Department of Communicable Disease Control and Prevention, Lanxi Municipal Center for Disease Control and Prevention, Jinhua, Zhejiang Province, People's Republic of China.
Kui Liu, Department of Tuberculosis Control and Prevention, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang Province, People's Republic of China.
Meng Zhou, Department of Public Health, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, People's Republic of China.
Xineng Jiang, Department of Communicable Disease Control and Prevention, Lanxi Municipal Center for Disease Control and Prevention, Jinhua, Zhejiang Province, People's Republic of China.
Yaling Feng, Department of Communicable Disease Control and Prevention, Lanxi Municipal Center for Disease Control and Prevention, Jinhua, Zhejiang Province, People's Republic of China.
Zhicheng Yu, Department of Communicable Disease Control and Prevention, Lanxi Municipal Center for Disease Control and Prevention, Jinhua, Zhejiang Province, People's Republic of China.
Yuhao Li, Department of Public Health, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, People's Republic of China.
Songhua Chen, Department of Tuberculosis Control and Prevention, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang Province, People's Republic of China.
Qian Wu, Department of Tuberculosis Control and Prevention, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang Province, People's Republic of China.
Wei Wang, Department of Tuberculosis Control and Prevention, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang Province, People's Republic of China.
C Robert Horsburgh, Department of Epidemiology, School of Public Health, Boston University, Boston, MA, USA.
Yu Zhang, Department of Tuberculosis Control and Prevention, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang Province, People's Republic of China.
Lin Zhou, Department of Tuberculosis Control and Prevention, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang Province, People's Republic of China.
Bin Chen, Department of Tuberculosis Control and Prevention, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang Province, People's Republic of China; Key Laboratory of Vaccine, Prevention and Control of Infectious Disease of Zhejiang Province, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang Province, People's Republic of China.
Chonggao Hu, Department of Tuberculosis Control and Prevention, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang Province, People's Republic of China.
Leonardo Martinez, Department of Epidemiology, School of Public Health, Boston University, Boston, MA, USA.
Notes
Author contributions. Conception and/or design: X. J., C. H., K. L., and B. C. Study management: Z. H., Y. F., C. R. H., Q. W., K. L., W. W., and B. C. Analysis of the data: Y. L., X. J., Z. Y., S. C., W. W., K. L., L. M., and Q. W. Interpretation of study results: all authors. Drafting of the manuscript for important intellectual content: M. Z., Y. Z., L. Z., K. L., and L. M. Review and editing of manuscript and approval of the final version to be published: all authors.
Acknowledgments. The authors acknowledge and thank the Lanxi Municipal Center for Disease Control and Prevention (CDC), local community healthcare centers, and tuberculosis-designated hospitals for the implementation of this screening work.
Data availability. All data and materials are included in this article. The corresponding author can provide data upon reasonable request after all studies and substudies have been completed.
Financial support. This work was supported by the National-Zhejiang Health Commission Major S&T Project (grant number WKJ-ZJ-2118) and the Zhejiang Provincial Medical and Health Project (grant numbers 2021KY618 and 2020KY520).
References
- 1. Churchyard G, Kim P, Shah NS, et al. What we know about tuberculosis transmission: an overview. J Infect Dis 2017; 216(Suppl_6):S629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Global tuberculosis report 2022. 2022. Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022. Accessed 4 August 2023.
- 3. Pai M, Dewan P. Testing and treating the missing millions with tuberculosis. PLoS Med 2015; 12:e1001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ho J, Fox GJ, Marais BJ. Passive case finding for tuberculosis is not enough. Int J Mycobacteriol 2016; 5:374–8. [DOI] [PubMed] [Google Scholar]
- 5. Kagujje M, Chilukutu L, Somwe P, et al. Active TB case finding in a high burden setting; comparison of community and facility-based strategies in Lusaka, Zambia. PLoS One 2020; 15:e0237931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burke RM, Nliwasa M, Feasey HR, et al. Community-based active case-finding interventions for tuberculosis: a systematic review. Lancet Public Health 2021; 6:e283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinez L, Woldu H, Chen C, et al. Transmission dynamics in tuberculosis patients with human immunodeficiency virus: a systematic review and meta-analysis of 32 observational studies. Clin Infect Dis 2021; 73:e3446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martinez L, Sekandi JN, Castellanos ME, Zalwango S, Whalen CC. Infectiousness of HIV-seropositive patients with tuberculosis in a high-burden African setting. Am J Respir Crit Care Med 2016; 194:1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bohlbro AS, Hvingelby VS, Rudolf F, Wejse C, Patsche CB. Active case-finding of tuberculosis in general populations and at-risk groups: a systematic review and meta-analysis. Eur Respir J 2021; 58:2100090. [DOI] [PubMed] [Google Scholar]
- 10. Marks GB, Nguyen NV, Nguyen PT, et al. Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med 2019; 381:1347–57. [DOI] [PubMed] [Google Scholar]
- 11. Lanxi Municipal People's Government . Lanxi City 2020 seventh national population census main data bulletin. 2021. Available at: http://www.lanxi.gov.cn/art/2021/5/18/art_1229288054_3844520.html#. Accessed 4 August 2023.
- 12. Liu K, Xie Z, Xie B, et al. Bridging the gap in end tuberculosis targets in the elderly population in eastern China: observational study from 2015 to 2020. JMIR Public Health Surveill 2022; 8:e39142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Health Commission of the People's Republic of China . Diagnosis of tuberculosis (WS 288-2017). 2017. Available at: http://www.nhc.gov.cn/wjw/s9491/201712/a452586fd21d4018b0ebc00b89c06254.shtml. Accessed 4 August 2023.
- 14. National Health Commission of the People's Republic of China . Adult weight determination. 2013. Available at: http://www.nhc.gov.cn/wjw/yingyang/201308/a233d450fdbc47c5ad4f08b7e394d1e8.shtml. Accessed 4 August 2023.
- 15. Kamara RF, Saunders MJ, Sahr F, et al. Social and health factors associated with adverse treatment outcomes among people with multidrug-resistant tuberculosis in Sierra Leone: a national, retrospective cohort study. Lancet Global Health 2022; 10:e543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diabetes Society of Chinese Medical Association . Chinese guidelines for the prevention and treatment of type 2 diabetes mellitus (2020 edition). Chin J Diabetes 2021; 41:482–548. [Google Scholar]
- 17. Borgdorff MW. New measurable indicator for tuberculosis case detection. Emerg Infect Dis 2004; 10:1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu K, Peng Y, Zhou Q, et al. Assessment of active tuberculosis findings in the eastern area of China: a 3-year sequential screening study. Int J Infect Dis 2019; 88:34–40. [DOI] [PubMed] [Google Scholar]
- 19. Stuck L, van Haaster AC, Kapata-Chanda P, Klinkenberg E, Kapata N, Cobelens F. How “subclinical” is subclinical tuberculosis? An analysis of national prevalence survey data from Zambia. Clin Infect Dis 2022; 75:842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez L, Xu L, Chen C, et al. Delays and pathways to final tuberculosis diagnosis in patients from a referral hospital in urban China. Am J Trop Med Hyg 2017; 96:1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sekandi JN, Zalwango S, Martinez L, et al. Four degrees of separation: social contacts and health providers influence the steps to final diagnosis of active tuberculosis patients in urban Uganda. BMC Infect Dis 2015; 15:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saunders MJ, Tovar MA, Collier D, et al. Active and passive case-finding in tuberculosis-affected households in Peru: a 10-year prospective cohort study. Lancet Infect Dis 2019; 19:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med 2009; 6:e1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lienhardt C, Fielding K, Sillah JS, et al. Investigation of the risk factors for tuberculosis: a case-control study in three countries in West Africa. Int J Epidemiol 2005; 34:914–23. [DOI] [PubMed] [Google Scholar]
- 25. Liu Q, You N, Wen J, et al. Yield and efficiency of a population-based mass tuberculosis screening intervention among persons with diabetes in Jiangsu Province, China. Clin Infect Dis 2023; 77:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. You N, Pan H, Zeng Y, et al. A risk score for prediction of poor treatment outcomes among tuberculosis patients with diagnosed diabetes mellitus from eastern China. Sci Rep 2021; 11:11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Q, You N, Pan H, et al. Glycemic trajectories and treatment outcomes of patients with newly diagnosed tuberculosis: a prospective study in eastern China. Am J Respir Crit Care Med 2021; 204:347–56. [DOI] [PubMed] [Google Scholar]
- 28. Marx FM, Hesseling AC, Martinson N, Theron G, Cohen T. National survey in South Africa reveals high tuberculosis prevalence among previously treated people. Lancet Infect Dis 2022; 22:1273. [DOI] [PubMed] [Google Scholar]
- 29. Moyo S, Ismail F, Van der Walt M, et al. Prevalence of bacteriologically confirmed pulmonary tuberculosis in South Africa, 2017–19: a multistage, cluster-based, cross-sectional survey. Lancet Infect Dis 2022; 22:1172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mungai BN, Joekes E, Masini E, et al. "If not TB, what could it be?" Chest X-ray findings from the 2016 Kenya Tuberculosis Prevalence Survey. Thorax 2021; 76:607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.