Abstract
Objective:
We described the evolution of SARS-CoV-2 source of infection in a cohort of healthcare workers (HCWs) of Quebec, Canada, during the first three pandemic waves. We also estimated their household secondary attack rate (SAR) and its risk factors.
Design:
Cross-sectional surveys.
Participants:
HCWs with a SARS-CoV-2 infection confirmed by polymerasa chain reaction and diagnosed between March 2020 and May 2021.
Methods:
We collected demographic, clinical, vaccination, and employment information, self-reported perceived source of infection, and transmission to household members during the first three pandemic waves. SAR was calculated for households with ≥2 members where the HCW was the index case. A Poisson regression model estimated the association between risk factors and SAR.
Results:
Among the 11,670 HCWs completing the survey, 91%, perceived their workplace as the source of infection during the first wave (March–July 2020), 71% during the second wave (July 2020–March 2021), and 40% during the third wave (March–May 2021). Conversely, HCWs reported an increasing proportion of household-acquired infections with each wave from 4% to 14% and 33%, respectively. The overall household SAR of 7,990 HCWs living with ≥1 person was 30% (95%CI: 29–30). SAR increased with the presence of symptoms, older age, and during Alpha-variant predominant period.
Conclusions:
HCWs and their household members were largely affected during the first pandemic waves of COVID-19, but the relative importance of occupational exposure changed overtime. Pandemic preparedness in healthcare settings is essential to protect HCWs from emerging biological hazard exposures.
Introduction
Healthcare workers (HCWs) have been on the frontline of the COVID-19 pandemic facing a higher risk of infection than the general population. 1 Enhanced and evolving infection prevention and control (IPC) measures have been deployed to protect HCWs and their patients from virus exposure at their workplace, including prioritized vaccination, personal protective equipment (PPE), and repeat testing. 2 The sources of SARS-CoV-2 infection among HCWs and the relative importance of occupational versus household and community acquisition have changed through different pandemic waves along with IPC measures, public health measures, and the resulting SARS-CoV-2 circulation in the population. Most publications on the source of acquisition of infection in HCWs to date are based on cross-sectional seroprevalence studies, are focused on hospital settings, and/or have evaluated the source of COVID-19 acquisition only before the widespread availability of vaccination. 3–8
The aims of this study were (1) to describe the evolution and characteristics associated with workplace versus household or community-acquired SARS-CoV-2 infection among a population-based cohort of HCWs of the province of Quebec, Canada, during the first three COVID-19 pandemic waves, from March 2020 to May 2021, (2) to estimate the secondary transmission from infected HCWs to their household members, and (3) to explore the risk factors for household transmission.
Methods
Study design and population
The study population has been described elsewhere. 9,10 In summary, a survey was conducted among HCWs with a PCR-confirmed SARS-CoV-2 infection diagnosed between March 1, 2020, and May 31, 2021, excluding June 14 to July 11, 2020, due to the low incidence during that period. Vaccination of HCWs started on December 14, 2020. 11 Included HCWs needed to live in the province of Quebec, speak French or English, and have worked in a healthcare setting during the 14 d prior to their PCR test.
Data collection
Data about infected HCWs were obtained from the provincial COVID-19 database, which includes all PCR-confirmed cases in the province since the beginning of the pandemic. HCWs fulfilling inclusion criteria were contacted by phone from May 6, 2020, to July 31, 2021, and invited to complete a self-administered online (or by phone if preferred) questionnaire. It collected demographic and employment characteristics, the vaccination status (for cases occurring after December 14, 2020), and the severity of the disease. Questions about workplace exposures to COVID-19 during the 14 d before illness onset included: working in a ward with suspected or confirmed COVID-19 patients, providing care to a COVID-19 patient at <2 m or having a coworker diagnosed with COVID-19. Collected self-perceived most likely sources of infection included: patients, coworkers, unknown (patients or coworkers) but assumed to be the workplace, household, community, or other/unknown/several sources. Collected information about household transmission included the number and age of household members, if they had COVID-19 symptoms and were PCR-confirmed, and who was the first COVID-19 case in the household.
We additionally analyzed anonymized administrative data on COVID-19-related sick leaves from October 1, 2020, to May 31, 2021, obtained from the Ministry of Health and including all publicly paid HCWs except physicians. The source of acquisition as work related or unrelated was attributed to each facility by the employer and the ICP team through an epidemiological investigation.
Data analysis
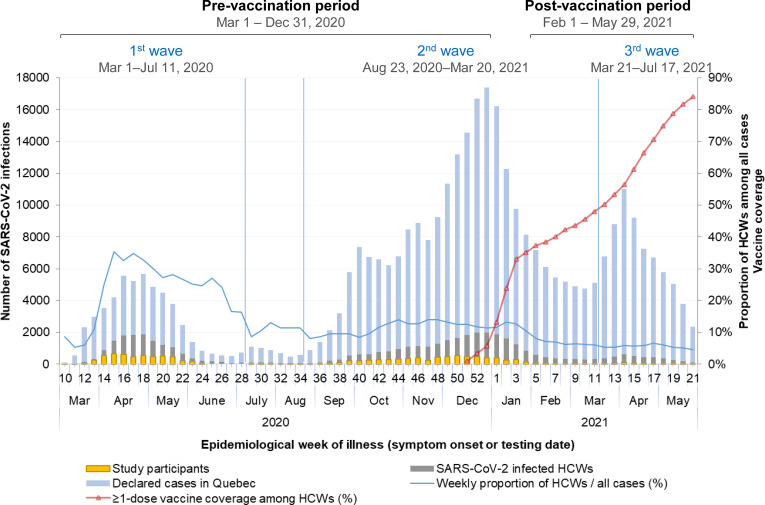
Proportions of HCWs reporting each source of infection by period and by HCW’s characteristics were compared using a chi-square test. Cochran-Armitage tests assessed the temporal trends regarding workplace-acquired infection by month, by pandemic wave (first wave from March 1 to July 11, 2020, interwave period/ second wave from July 12, 2020, to March 20, 2021, and third wave from March 21 to May 29, 2021), and by vaccination period (prevaccination from March 1 to December 31, 2020, and post-vaccination from February 1 to May 29, 2021). Vaccination periods were defined considering vaccine coverage and the 14 d necessary to confer protection (Figure 1).
Figure 1.
Weekly number of overall, healthcare workers and survey participants with a PCR-confirmed SARS-CoV-2 infection, and vaccine coverage among healthcare workers in Quebec from March 1, 2020, to May 29, 2021. Note: HCW, healthcare worker.
Secondary attack rate (SAR) was estimated for households of ≥2 persons where the HCW was the first (or the only) infected member. Household members were considered confirmed cases if they had a positive PCR test result, except during the first wave when diagnosis was based on compatible symptoms and an epidemiological contact with a PCR-confirmed case due to restricted testing access for non-HCWs and public health measures limiting other potential diagnoses in this period. SAR was calculated by dividing the number of secondary cases by the total number of household members. Potential determinants of household transmission (age, sex, presence of symptoms, vaccination status, and variant period) were evaluated by modeling the rate of secondary transmission using a single Poisson regression model with one observation per household, where the outcome is the number of infected members and the logarithm of the number of members is entered as an offset term. The exponential of the coefficients was interpreted as the SAR ratio.
We also calculated the monthly proportion of HCWs’ households in which the first reported case was the HCW, another adult, a child, or unknown, to explore the evolution of community transmission as indicated by a non-HCW being the source of infection in the household.
Ethical aspects
This survey, conducted under the legal mandate of the National Director of Public Health of Quebec under the Public Health Act, was also approved by the research ethics committee of the CHU de Québec – Laval University, and all participants gave consent at the recruitment stage.
Results
COVID-19 epidemic among HCWs in Quebec
In the province of Quebec, where around 600,000 persons (7% of the total population) work in the healthcare and social assistance sector, 12 45,214 (13%) of the 361,556 PCR-confirmed SARS-CoV-2 infections diagnosed from March 2020 to the end of May 2021 were HCWs. The percentage of HCWs among cases decreased from 27% during the first wave to 11% and 6% in the second and third waves, while the average weekly number of infected HCWs decreased from 812 to 777 and 381, respectively (Figure 1).
Study participants
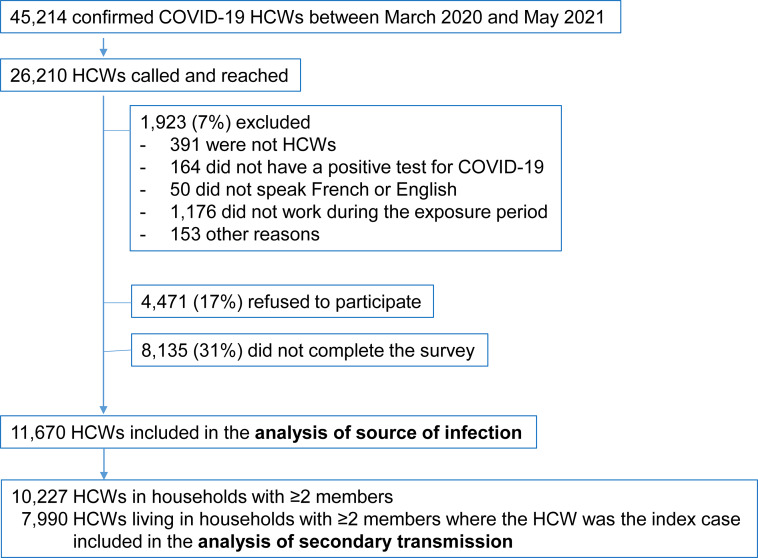
From the 45,214 HCWs with confirmed COVID-19, 26,210 (57.6%) were reached by phone. Among those, 1,923 (7.3%) were excluded for the following reasons: not having worked during the 14 d prior to illness, not being HCW, not having a positive PCR test, and not speaking French/English or other languages (e.g., not living in Quebec or teleworking). Additionally, 4,471 (17.1%) refused to participate and 8,135 (31.0%) who agreed to participate did not complete the electronic survey, leaving 11,670 HCWs who completed the survey during the first (4,542), second (6,701) or third (427) waves, according to their date of first positive PCR result (Figure 2). Overall, 79.0% were women and 21.0% were men; 45.2% were 18–39 yr old, 48.2% were 40–59 yr old, and 6.6% were ≥60 yr old. Most HCW cases worked as nurses/nurse assistants (29.3%), or as patient support assistants (responsible for providing basic care to patients, including hygiene, feeding, or mobilization) (30.2%) (Table 1).
Figure 2.
Population flowchart of healthcare workers participating to the epidemiologic survey. Note: HCW, healthcare worker.
Table 1.
Self-reported source of infection by period and characteristics of healthcare workers participating to the survey (March 2020 to May 2021)
| Self-reported perceived source of infection | ||||||||
|---|---|---|---|---|---|---|---|---|
| At work a | At home | Other or unknown | ||||||
| Total | Total at work | By patient | By coworker | Unknown (by patient or coworker) | ||||
| N (col %) | N (line %) | line % | line % | line % | N (line %) | N (line %) | P b | |
| Overall | 11,670 | 9,067 (77.7) | 29.9 | 9.5 | 38.3 | 1,223 (10.5) | 1,380 (11.8) | |
| Pandemic wave | <.01 | |||||||
| 1 (March–July 2020) | 4,542 (38.9) | 4,144 (91.2) | 33.7 | 9.8 | 47.8 | 175 (3.9) | 223 (4.9) | |
| 2 (July 2020–March 2021) | 6,701 (57.4) | 4,754 (70.9) | 28.2 | 9.4 | 33.3 | 909 (13.6) | 1,038 (15.5) | |
| 3 (March–May 2021) | 427 (3.7) | 169 (39.6) | 14.8 | 8.9 | 15.9 | 139 (32.6) | 119 (27.9) | |
| Period c | <.01 | |||||||
| Prevaccination | 9,848 (84.4) | 7,959 (80.8) | 30.5 | 9.7 | 40.7 | 855 (8.7) | 1,034 (10.5) | |
| Since-vaccination started | 1,208 (10.4) | 669 (55.4) | 33.4 | 8.8 | 29.3 | 69 (11.2) | 106 (17.3) | |
| Age of healthcare worker | 0.10 | |||||||
| 18–39 | 5,269 (45.2) | 4,047 (76.8) | 29.7 | 9.2 | 37.9 | 559 (10.6) | 663 (12.6) | |
| 40–59 | 5,630 (48.2) | 4,410 (78.3) | 30.3 | 9.5 | 38.5 | 612 (10.9) | 608 (10.8) | |
| ≥60 | 771 (6.6) | 610 (79.1) | 27.8 | 12.1 | 39.3 | 52 (6.7) | 109 (14.1) | |
| Type of employment | <.01 | |||||||
| Admin/management | 968 (8.3) | 564 (58.3) | 6.0 | 21.8 | 30.5 | 204 (21.1) | 200 (20.7) | |
| Nurse/nurse assistants | 3,424 (29.3) | 2,891 (84.4) | 35.6 | 7.9 | 41.0 | 263 (7.7) | 270 (7.9) | |
| Patient support assistant | 3,524 (30.2) | 3,055 (86.7) | 42.1 | 5.8 | 38.8 | 168 (4.8) | 301 (8.5) | |
| Housekeeping | 416 (3.6) | 339 (81.5) | 19.5 | 8.7 | 53.4 | 29 (7.0) | 48 (11.5) | |
| Physician | 418 (3.6) | 319 (73.6) | 29.2 | 8.6 | 38.5 | 54 (12.9) | 45 (10.8) | |
| Psychosocial worker | 355 (3.0) | 204 (57.5) | 17.2 | 12.4 | 27.9 | 80 (22.5) | 71 (20.0) | |
| Other | 2,565 (22.0) | 1,695 (66.1) | 18.0 | 12.1 | 36.0 | 425 (16.6) | 445 (17.4) | |
| Working experience | <.01 | |||||||
| 2,054 (17.6) | 1,655 (80.6) | 31.4 | 7.6 | 41.6 | 163 (7.9) | 236 (11.5) | ||
| ≥1 year | 9,607 (82.3) | 7,403 (77.1) | 29.5 | 10.0 | 37.6 | 1,060 (11.0) | 1,144 (11.9) | |
| Facility | <.01 | |||||||
| Acute-care hospital | 3,838 (32.9) | 2,998 (78.1) | 26.3 | 10.7 | 41.1 | 424 (11.1) | 416 (10.8) | |
| Long-term care facility | 3,715 (31.8) | 3,342 (90.0) | 38.0 | 6.8 | 45.1 | 140 (3.8) | 233 (6.3) | |
| Private seniors’ residences | 1,242 (10.6) | 1,014 (81.6) | 37.0 | 10.0 | 34.7 | 77 (6.2) | 151 (12.2) | |
| Health centers/clinics | 1,254 (10.7) | 689 (54.9) | 18.5 | 11.0 | 25.4 | 278 (22.2) | 287 (22.9) | |
| Other assisted residences | 380 (3.3) | 287 (75.5) | 34.5 | 10.5 | 30.5 | 43 (11.3) | 50 (13.2) | |
| Rehabilitation centers | 348 (3.0) | 227 (65.2) | 23.3 | 12.4 | 29.6 | 63 (18.1) | 58 (16.7) | |
| Other facilities | 893 (7.7) | 510 (57.1) | 17.9 | 11.4 | 27.8 | 198 (22.2) | 185 (20.7) | |
Total at work is the sum of by patient, by coworker, and unknown (by patient of coworker).
Chi-square test comparing the five mutually exclusive self-reported sources of infection.
Prevaccination period: from March to December 2020; post-vaccination period: from February to May 2021.
Infection source among SARS-CoV-2-infected HCWs
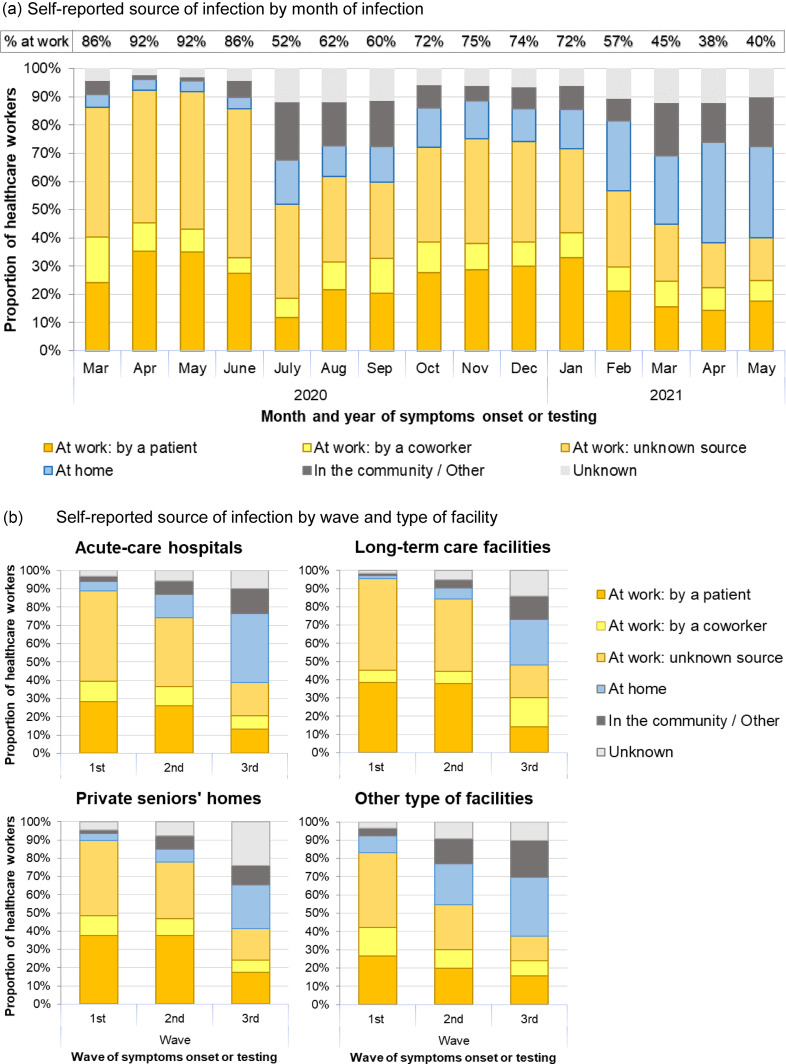
The proportion of infected HCWs who perceived the workplace as the source of their infection decreased from 91% for the first wave to 71% for the second and 40% for the third wave (trend test p < 0.001) (Figure 3a, Table 1). Patient support assistants reported most frequently the workplace as the perceived infection source (87%), followed by nurses (84%) and housekeeping staff (82%) (Table 1). Except for administrative staff, patients were considered the most frequent source of infection by all types of workers: by ~40% of patient support assistants, ~35% of nurses, ~30% of physicians, and ~20% of housekeeping staff and psychosocial workers. The proportion considering patients as their infection source decreased from 34% in the first wave to 15% in the third wave. In contrast, the proportion perceiving coworkers as their infection source did not change over the study period (9–10%) (Figure 3a, Table 1). During the first wave, similar high proportions (∼90%) of staff from acute-care hospitals (ACH), long-term care facilities (LTCF), and private seniors’ homes perceived SARS-CoV-2 infection as workplace acquired. These proportions decreased in all types of facilities over the waves, but HCWs from LTCF reported more often the workplace as source of infection than workers from ACH during the second and third waves (Figure 3b, Table 1).
Figure 3.
Self-reported perceived source of infection by month, wave, and type of facility among healthcare workers participating to the survey (March 2020 to May 2021).
Conversely, the proportion reporting household-acquired infection increased from 4% during the first wave to 14% and 33% in the second and third waves, respectively (Table 1). HCWs from ACH reported more often a household-acquired infection than those in LTCFs in the first (5% vs 1%), second (13% vs 6%), and third waves (38% vs 25%) (Figure 3b).
The employer-based data from October 2020 to May 2021 showed similar trends, with the highest proportion of workplace-related infections occurring during the peak of the second wave and a sharp decrease from February 2021 onward, during the post-vaccination period (Supplementary Figure 1). For the same period, employer-based data consistently reported lower proportions of workplace-related infections compared with self-reported perceived source. Patient-facing occupations (nurses, auxiliary nurses, and patient support assistants) had a higher proportion of workplace-related infections (61%–73%) compared with 25% among administrative staff.
Household transmission
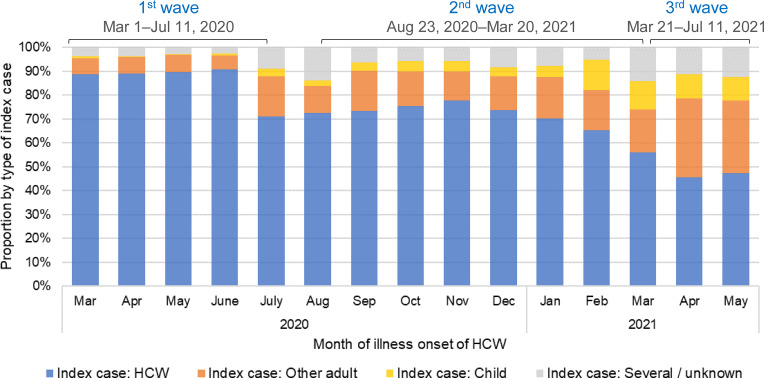
Among the 10,227 HCW participants not living alone, 78.1% (7,990) reported being the first or the only case in the household (Figure 2), a proportion decreasing from 87.9% in the first wave to 73.6% and 47.7% in the second and third waves (p < 0.001), respectively (Figure 4). An adult other than the HCW was the first case in 12.7% of households (7.9%, 14.8%, and 29.8% in each wave, respectively), whereas children accounted for only 3.1% of first cases (0.4%, 4.5%, and 10.5% in each wave, respectively). In 3.0% of the households, the first case was unknown or several members started symptoms simultaneously.
Figure 4.
Type of index case (HCW, other adult, or child) in households of infected HCWs where at least two people were living. Note: HCW, healthcare worker.
When the HCW was the first case, 57.4% of households had no onward transmission and 42.6% had at least one infected household member. The overall SAR was 29.7% (95%CI: 29.1–30.4). The SAR was 29.3% (95%CI: 28.6–30.0) during the first pandemic year (March 2020 to January 2021), increasing thereafter to 42.0% (95%CI: 36.7–47.5) between April and May 2021, during the Alpha-variant predominance. In univariate analyses, older age (≥60 yr), the presence of symptoms of the index case, and households of two people (including the index case) were associated with higher SAR (Table 2). In the model adjusted for age, sex, and period, household transmission was two times higher for symptomatic versus asymptomatic index cases (SAR ratio = 2.0, 95%CI: 1.8–2.3), while vaccination with ≥1 dose was associated with a 25% reduction in transmission compared to unvaccinated cases (SAR ratio = 0.8, 95%CI: 0.6–0.9) (Table 3).
Table 2.
Secondary attack rate in HCWs’ households and proportion of households without secondary transmission according to the characteristics of the index case
| HCWsN (%) | Household membersN | Infected membersN | Secondary attack rate% (95% CI) | Households without secondary transmission% | |
|---|---|---|---|---|---|
| All households with at least two members and the HCW being the first or the only case | 7,990 | 18,445 | 5,481 | 29.7% (29.1, 30.4) | 57.4 |
| 1. Characteristics of the HCW index case | |||||
| Sex | |||||
| Women | 6,422 (80.4) | 14,794 | 4,317 | 29,2% (28.4, 29.9) | 58.3 |
| Men | 1,568 (19.6) | 3,651 | 1,164 | 31.9% (30.4, 33.4) | 53.6 |
| Age | |||||
| 18–44 years | 4,809 (60.2) | 11,749 | 3,257 | 27.7% (26.9, 28.5) | 59.5 |
| 45–59 years | 2,756 (34.5) | 6,079 | 1,979 | 32.6% (31.4, 33.7) | 54.2 |
| ≥60 years | 425 (5.3) | 617 | 245 | 39.7% (35.8, 43.7) | 54.1 |
| Vaccination status | |||||
| Unvaccinated | 7,652 (95.8) | 17,690 | 5,271 | 29.8% (29.1, 30.5) | 57.1 |
| 1-dose days 0–13 before illness | 188 (2.4) | 436 | 114 | 26.1% (22.1, 30.5) | 64.9 |
| 1-dose ≥14 days before illness | 144 (1.8) | 300 | 88 | 29.3% (24.2, 34.8) | 63.2 |
| 2-doses | 6 (0.1) | 19 | 8 | 42.1% (20.3, 66.5) | 33.3 |
| Presence of symptoms | |||||
| Asymptomatic | 786 (9.8) | 1,797 | 283 | 15.7% (14.1, 17.5) | 76.3 |
| Symptomatic | 7,204 (90.2) | 16,648 | 5,198 | 31.2% (30.5, 31.9) | 55.3 |
| 2. Number and characteristics of household members | |||||
| Number of household members | |||||
| 1 | 2,936 (36.7) | 2,936 | 1,131 | 38.5% (36.8, 40.3) | 61.5 |
| 2 | 1,694 (21.2) | 3,388 | 992 | 29.3% (27.8, 30.8) | 60.1 |
| 3 | 1,994 (25.0) | 5,982 | 1,692 | 28.3% (27.1, 29.4) | 53.9 |
| ≥4 (maximum 12) | 1,366 (17.1) | 6,139 | 1,666 | 27.1% (26.0, 28.3) | 50.3 |
| Age of household members | |||||
| Children (0–17 years) | 3,958 | 7,450 | 1,768 | 23.7% (22.8, 24.7) | 54.7 |
| Adults (≥18 years) | 7,513 | 10,995 | 3,713 | 33.8% (32.9, 34.7) | 56.4 |
| 3. Period (SARS-CoV-2 strain circulation) | |||||
| March 2020–January 2021 (original Wuhan strain) | 7,629 | 17,650 | 5,170 | 29.3% (28.6, 30.0) | 57.6 |
| February–March 2021 (beginning of Alpha-variant transmission) | 215 | 462 | 171 | 37.0% (32.6, 41.6) | 56.7 |
| April–May 2021 (predominance of Alpha variant) | 146 | 333 | 140 | 42.0% (36.7, 47.5) | 44.5 |
Note. CI, confidence interval; HCW, healthcare worker.
aVaccination with at least 1 dose 14 or more days before illness onset.
Table 3.
Risk factors for secondary household transmission from SARS-CoV-2-infected HCWs
| SAR ratio (95% CI) | ||
|---|---|---|
| Unadjusted | Adjusted | |
| Male sex | 1.09 (1.02, 1.17) | 1.08 (1.01, 1.15) |
| Age | ||
| 18–44 years | Reference | Reference |
| 45–59 years | 1.17 (1.11, 1.24) | 1.18 (1.12, 1.25) |
| ≥60 years | 1.43 (1.26, 1.63) | 1.46 (1.28, 1.66) |
| Vaccination status | ||
| Unvaccinated | Reference | Reference |
| 1-dose days 0–13 before illness onset | 0.88 (0.73, 1.06) | 0.83 (0.69, 1.01) |
| ≥1-dose ≥14 days before illness onset | 1.01 (0.83, 1.24) | 0.75 (0.60, 0.94) |
| Presence of symptoms | 1.98 (0.76, 2.23) | 2.03 (1.80, 2.29) |
| Period | ||
| March–January 2020 (original Wuhan strain) | Reference | Reference |
| February 2020–March 2021 (onset of Alpha variant) | 1.26 (1.09, 1.47) | 1.36 (1.16, 1.59) |
| April–May 2021 (predominance of Alpha variant) | 1.44 (1.21, 1.70) | 1.71 (1.42, 2.06) |
Note. CI, confidence interval; SAR, secondary attack rate.
Discussion
In the province of Quebec, during the first three COVID-19 pandemic waves, occupational exposure was perceived by SARS-CoV-2-infected HCWs as the most frequent source of infection. Its relative importance decreased over time dropping by half from 91% to 40%, while the contribution of household-acquired infections increased eightfold from 4% to 33%.
Since the beginning of the pandemic, reports worldwide have described that HCWs were at greater risk of infection than the general population, attributing this to their occupational exposure when caring for patients. 1 Similar to our results, the proportion of HCWs among all diagnosed cases in Canada and in the USA was highest in the first wave (19% and 16%, respectively). It was lower but stable between July 2020 and January 2021 and decreased to 7% in Canada and 5% in the USA from January to May 2021, indirectly suggesting that their risk was approaching that of the general population. 13,14 During the first pandemic wave, mitigation measures limited the contacts and exposures to infection of the general population. However, PCR testing was limited and more readily available to HCWs, likely overestimating the relative proportion of HCWs among all cases. After the first wave, PCR testing became widely available to the population reducing the importance of this bias. The weekly number of reported HCW infections was, however, still high during the peak of the second wave, but decreased sharply from February to May 2021 despite the peak in community cases during the third wave.
Parallel to the decrease in SARS-CoV-2 infections, our study shows that the most common perceived source of infection shifted from the workplace to the community/household from February 2021 onward. This coincided temporally with the expected effect of vaccination against COVID-19 in HCWs, 15–17 but also with the expected effect of the vaccination among LTCF residents and other vulnerable persons at risk of hospitalization, who were also prioritized at the beginning of Quebec immunization campaign. 11,18 Other factors, such as the improvement of IPC measures, may have also contributed to this relative reduction of SARS-CoV-2 occupational transmission. 4,19 The end of the lockdown with the loosening of strict public health measures and reopening of schools was associated with a higher community and household transmission of SARS-CoV-2 over the waves. 20
Identification of the source of infection is challenging. Although household exposures are more obvious, occupational exposures leading to an infection may be more difficult to demonstrate or confirm. Multiple encounters may happen in the workplace during the incubation period with known or unknown contagious patients and/or coworkers during which different levels of IPC measures may have been applied. Similarly, exposures in the community are often difficult to identify. A study in two US hospitals using structured interviews to determine the infection source reported that only 6% of infections among HCWs were hospital-acquired, but classified as “unknown source” for those whose only known exposure was a contact with a COVID-19 patient while wearing PPE. 21 Reports about the importance of the workplace exposure in hospitals are contradictory 3,5,6,22 and no studies compared the evolution before/after the COVID-19 vaccine deployment. Although employer-based data may underestimate occupational exposure (e.g., undetected PPE lapses), self-reporting of workplace-acquired infections may overestimate this exposure if attributed by HCWs who did not identify any alternative source of infection or if associated with financial advantages. This latter bias should have had a minimal impact in our study, since the survey was confidential and participants did not get any benefit by reporting occupational exposure.
In our study, proportions of self-reported source of infection varied with time but also by occupation and facility. HCWs with closer and prolonged contacts with patients (auxiliary nurses and patient support assistants) perceived more often the workplace as an infection source, consistent with employer-generated data and other studies reporting a higher frequency of SARS-CoV-2 infections among these categories. 3,23–27 During the first pandemic wave, similar high proportions (∼90%) of staff from hospitals and LTCF reported workplace-acquired COVID-19. The relative decrease in the subsequent waves was more pronounced in hospitals than in LTCF and more pronounced for patients than for coworker exposure. These observations are consistent with the reduction in the number of COVID-19 hospitalizations and outbreaks in healthcare facilities following the vaccination campaign. 18,20 Few studies have compared the risk of transmission in ACH versus LTCF. Akinbami et al reported higher seroprevalence at the end of the first wave among staff in nursing homes (13%) than in hospitals (5%), 7 while our previous study in Quebec showed that working in LTCF or private seniors’ homes increased the risk of SARS-CoV-2 infection compared to ACH. 10 Conversely, the relatively higher proportion of HCWs with household-acquired infections among ACH workers might be a reflection of this lower workplace risk.
HCWs participating in the study and not living alone infected 30% of their household members, increasing from 29% during the first months of the pandemic to 42% during the period of Alpha-variant predominance. This is higher than the overall household SAR of 19% (95% CI, 16%–22%) reported by a meta-analysis summarizing 87 studies published through June 2021. 28 Similar to our results, they described an increasing pattern overtime; the household SAR was 20% in studies from March to June 2020, compared with 31% in studies from July 2020 to March 2021. This may be attributable to better ascertainment of secondary cases over time as PCR testing became widely available, 29,30 as well as changes in variant circulation. We identified secondary cases through both epidemiological link (during first wave) and laboratory confirmation (during the second and third waves). Consistent with our data, an updated meta-analysis stratified by variant circulation reported higher household SAR for Alpha variant (38%) than for the original wild-type strain (19%), 31 probably partially attributable to the higher transmissibility of this variant. 32,33
The self-reported the presence of symptoms in the index HCW case was associated with a twofold increase in the household SAR (31% for symptomatic versus 16% for asymptomatic index cases), in line with studies showing that symptomatic COVID-19 cases are more contagious than asymptomatic cases. 28,34–38
We found a 25% risk reduction of SAR when the index case was vaccinated with at least one dose versus unvaccinated after adjustment for the period of variant circulation. As vaccination coverage increased in parallel with the Alpha-variant circulation, punctual SAR estimates reflected both factors and are difficult to disentangle. An analysis restricted to the Alpha-dominated period would have been useful but was not possible due to the limited sample size. One meta-analysis that included 12 studies, from January 2021 to January 2022, reported a nonsignificantly lower risk of household transmission when the index case was vaccinated (23% for fully vaccinated versus 36% for unvaccinated), while the summary SAR including 4 studies during Alpha-variant circulation was significantly lower with fully vaccinated (11%) versus unvaccinated (36%) index cases. 31
Our study has several limitations. Like most studies about occupational COVID-19 acquisition, ours is a series of three cross-sectional surveys based on self-reported perceived source of infection. During the third pandemic wave, fewer HCWs were infected by COVID-19 and participated in our study, limiting our power to estimate the impact of vaccination. Finally, our results are not representative of the situation after May 2021, with the circulation of new emerging and more transmissible variants, a very high proportion of adults vaccinated with two or more doses, and a large proportion of adults already infected at least once. 39
In conclusion, HCWs and their household members were largely affected during the first pandemic waves of COVID-19, but the relative and the absolute occupational risk lowered contemporaneously with the start of vaccination and the improvement of ICP measures. These findings underscore the importance of pandemic preparedness in healthcare settings since ICP measures may be the only feasible intervention to protect HCWs from emerging biological hazard exposures until targeted vaccination becomes available.
Supporting information
Carazo et al. supplementary material
Acknowledgments
We thank Eric Bouchard (Ministère de la santé et des services sociaux du Québec) for his collaboration in preparing employer data on occupational source of COVID-19, which helped in the interpretation of our results. We would also like to thank all the healthcare workers who participated in this study.
Supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/ash.2023.442.
click here to view supplementary material
Financial support
This work was supported by the Ministère de la santé et des services sociaux du Québec.
Competing interests
SC and GDS report funding from the Ministère de la santé et des services sociaux du Québec to conduct this work, paid to their institution. GD reports participation as a medical advisor on the provincial public health scientific committee on COVID-19 for Quebec Ministry of Health. DT is supported by a research career award from the Fonds de recherche du Québec-Santé, paid to his institution. All other authors report no potential conflicts.
References
- 1. Gómez-Ochoa SA, Franco OH, Rojas LZ, et al. COVID-19 in healthcare workers: A living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol 2021;190:161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Islam MS, Rahman KM, Sun Y, et al. Current knowledge of COVID-19 and infection prevention and control strategies in healthcare settings: A global analysis. Infect Control Hosp Epidemiol 2020;15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilkins JT, Gray EL, Wallia A, et al. Seroprevalence and correlates of SARS-CoV-2 antibodies in health care workers in Chicago. Open Forum Infect Dis 2020;8:ofaa582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vitrat V, Maillard A, Raybaud A, et al. Effect of professional and extra-professional exposure on seroprevalence of SARS-CoV-2 infection among healthcare workers of the french alps: A multicentric cross-sectional study. Vaccines 2021;9:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacob JT, Baker JM, Fridkin SK, et al. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open 2021;4:e211283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker JM, Nelson KN, Overton E, et al. Quantification of occupational and community risk factors for SARS-CoV-2 seropositivity among health care workers in a large U.S. Health Care System. Ann Intern Med 2021;174:649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akinbami LJ, Chan PA, Vuong N, et al. Severe acute respiratory syndrome Coronavirus 2 seropositivity among healthcare personnel in hospitals and nursing homes, Rhode Island, USA, July–August 2020. Emerg Infect Dis 2021;27:823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dimcheff DE, Schildhouse RJ, Hausman MS, et al. Seroprevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection among Veterans Affairs healthcare system employees suggests higher risk of infection when exposed to SARS-CoV-2 outside the work environment. Infect Control Hosp Epidemiol 2021;42:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carazo S, Laliberté D, Villeneuve J, et al. Characterization and evolution of infection control practices among severe acute respiratory coronavirus virus 2 (SARS-CoV-2)–infected healthcare workers in acute-care hospitals and long-term care facilities in Québec, Canada, Spring 2020. Infect Control Hosp Epidemiol 2022;43:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carazo S, Villeneuve J, Laliberté D, et al. Risk and protective factors for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection among healthcare workers: A test-negative case-control study in Québec, Canada. Infect Control Hosp Epidemiol 2023;44:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Comité sur l’immunization du Québec, 2020. Stratégie de vaccination contre la COVID-19: report de la 2e dose en contexte de pénurie. https://www.inspq.qc.ca/publications/3098-strategie-vaccination-2e-dose-covid. Accessed June 10, 2021.
- 12. Government of Canada. Health and Social Services, 2024. Sectoral profile and 2022-2024 outlook in Quebec. https://www.jobbank.gc.ca/trend-analysis/job-market-reports/quebec/sectoral-profile-health. Accessed July 20, 2023.
- 13. Canadian Institute for Health Information, 2022. COVID-19 cases and deaths in health care workers in Canada. https://www.cihi.ca/en/covid-19-cases-and-deaths-in-health-care-workers-in-canada. Accessed April 15, 2022.
- 14. Centers for Disease Control and Prevention, 2022. COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#health-care-personnel. Accessed April 15, 2022.
- 15. Daniel W, Nivet M, Warner J, Podolsky DK. Early evidence of the effect of SARS-CoV-2 vaccine at one medical center. N Engl J Med 2021;384:1962–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021;397:1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhee C, Wang R, Ye S, et al. Decline in SARS-CoV-2 infections among health care workers at 2 hospitals following rollout and administration of mRNA vaccines. Open Forum Infect Dis 2021;8:ofab204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fortin É, De Wals P, Talbot D, et al. Impact of the first vaccine dose on COVID-19 and its complications in long-term care facilities and private residences for seniors in Québec, Canada. Can Commun Dis Rep 2022;48:164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Serres G, Carazo S, Villeneuve J, et al. 2022. Enquête épidémiologique sur les travailleurs de la santé atteints par la COVID-19. Québec: Institut national de santé publique du Québec. https://www.inspq.qc.ca/sites/default/files/publications/3192-enquete-epidemiologique-travailleurs-sante-covid-19.pdf. Accessed April 15, 2022.
- 20. Institut national de santé publique du Québec, 2022. Données Covid-19 au Québec. 2022. https://www.inspq.qc.ca/covid-19/donnees. Accessed April 15, 2022.
- 21. Rhee C, Baker MA, Tucker R, et al. Sources of exposure identified through structured interviews of healthcare workers who test positive for severe acute respiratory coronavirus virus 2 (SARS-CoV-2): A prospective analysis at two teaching hospitals. Antimicrob Steward Healthc Epidemiol 2021;1:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belan M, Charmet T, Schaeffer L, et al. SARS-CoV-2 Exposures of healthcare workers from primary care, long-term care facilities and hospitals: A nationwide matched case-control study. Clin Microbiol Infect 2022;28:1471–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jespersen S, Mikkelsen S, Greve T, et al. Severe acute respiratory syndrome Coronavirus 2 seroprevalence survey among 17 971 healthcare and administrative personnel at hospitals, prehospital services, and specialist practitioners in the Central Denmark Region. Clin Infect Dis 2021;73:e2853–e2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wratil PR, Schmacke NA, Osterman A, et al. In-depth profiling of COVID-19 risk factors and preventive measures in healthcare workers. Infection 2022;50:381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Plaat DA, Madan I, Coggon D, et al. Risks of COVID-19 by occupation in NHS workers in England. Occup Environ Med 2022;79:176–183. [DOI] [PubMed] [Google Scholar]
- 26. Poletti P, Tirani M, Cereda D, et al. Seroprevalence of and risk factors associated with SARS-CoV-2 infection in health care workers during the early COVID-19 pandemic in Italy. JAMA Netw Open 2021;4:e2115699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Modenese A, Casolari L, Rossi G, et al. Factors associated with SARS-CoV-2 infection risk among healthcare workers of an Italian University Hospital. Healthcare 2021;9:1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Factors associated with household transmission of SARS-CoV-2: An updated systematic review and meta-analysis. JAMA Netw Open 2021;4:e2122240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwartz KL, Achonu C, Buchan SA, et al. Epidemiology, clinical characteristics, household transmission, and lethality of severe acute respiratory syndrome coronavirus-2 infection among healthcare workers in Ontario, Canada. PLoS One 2020;15:e0244477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paul LA, Daneman N, Brown KA, et al. Characteristics associated with household transmission of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) in Ontario, Canada: A cohort study. Clin Infect Dis 2021;73:1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Madewell ZJ, Yang Y, Longini IM, Halloran ME, Dean NE. Household secondary attack rates of SARS-CoV-2 by variant and vaccination status. JAMA Netw Open 2022;5:e229317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021;372:eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lindstrøm JC, Engebretsen S, Kristoffersen AB, et al. Increased transmissibility of the alpha SARS-CoV-2 variant: Evidence from contact tracing data in Oslo, January to February 2021. Infect Dis 2022;54:72–77. [DOI] [PubMed] [Google Scholar]
- 34. Byambasuren O, Cardona M, Bell K, et al. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: Systematic review and meta-analysis. J Assoc Med Microbiol Infect Dis Can 2020;5:223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu P, Liu F, Chang Z, Lin Y, et al. Assessing asymptomatic, presymptomatic, and symptomatic transmission risk of severe acute respiratory syndrome Coronavirus 2. Clin Infect Dis 2021;73:e1314–e1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyahara R, Tsuchiya N, Yasuda I, et al. Familial clusters of Coronavirus disease in 10 prefectures, Japan, February−May 2020. Emerg Infect Dis 2021;27:915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li F, Li YY, Liu MJ, et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: A retrospective observational study. Lancet Infect Dis 2021;21:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tibebu S, Brown KA, Daneman N, Paul LA, Buchan SA. Household secondary attack rate of COVID-19 by household size and index case characteristics. medRxiv 2021;doi: 10.1101/2021.02.23.21252287v1. [DOI] [Google Scholar]
- 39. Skowronski DM, Kaweski SE, Irvine MA, et al. Serial cross-sectional estimation of vaccine- and infection-induced SARS-CoV-2 seroprevalence in British Columbia, Canada. CMAJ 2022;194:E1599–E1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Carazo et al. supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/ash.2023.442.
click here to view supplementary material