Abstract
At present, hepatic arterial infusion chemotherapy (HAIC) for the treatment of hepatocellular carcinoma (HCC) is often applied to patients who are not suitable or are unwilling to undergo surgical treatment. However, to the best of our knowledge, the efficacy and safety of HAIC combined with immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs) in HCC have not been fully demonstrated. Published studies involving the treatment of patients with HCC with HAIC, ICIs and TKIs were searched from public databases, including PubMed, Embase, the Cochrane Library and Sinomed. Efficacy and safety data for each study, including progression-free survival (PFS), overall survival (OS) and adverse events (AEs) were collected. The present study included 17 treatment groups from 15 studies, including 1,987 patients with HCC in the systematic review. The target population was dominated by those unsuitable for surgical treatment, with Barcelona Clinic Liver Cancer stage B or C, Eastern Cooperative Oncology Group performance status ≤2 and Child-Pugh score A or B. The results showed that the longest estimated median PFS (95% CI) in the HAIC + ICI/TKI therapy group (group C) was 9.37 months (95% CI, 6.81–11.93); in the HAIC therapy group (group B) was 7.45 months (95% CI, 6.45–8.46); and in the ICIs + other systemic therapies group (group A) was 5.92 months (95% CI, 5.31–6.54). There was no significant difference in the expected OS among the three groups, which may be because OS events were not reached in numerous studies during the follow-up time. The incidence of treatment-related adverse effects, such as increased AST [14/221 (6.33%)], increased ALT [13/221 (5.88%)], and decreased platelet count [13/221 (5.88%)], was not significantly increased in group C when compared with groups A or B (P>0.05). In conclusion, the effectiveness of HAIC + ICI/TKI for the treatment of advanced HCC was better than that of ICIs + other systemic therapies or HAIC alone. In addition, the incidence of AEs above grade 3 was not significantly higher compared with that in the other treatment groups, and the safety profile was good.
Keywords: hepatic arterial infusion chemotherapy, immune checkpoint inhibitors, tyrosine kinase inhibitors, hepatocellular carcinoma, adverse events
Introduction
Hepatocellular carcinoma (HCC) ranks sixth among the most common tumors worldwide and is responsible for ~700,000 deaths each year (1). In China, the high incidence and mortality rate of HCC are associated with the rapid progression of malignant tumors caused by hepatitis B viral infection (2). Liver resection, liver transplantation and local ablation are common curative approaches for early-stage patients. However, for most patients, HCC is already of an intermediate or advanced stage at the time of initial diagnosis; therefore, surgery is not an option for treatment (3). The effectiveness of systemic drug therapies has been limited; therefore, the emergence of targeted and immunotherapy agents has held promise for the non-surgical treatment of HCC. Among them, immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs) have been widely used in the treatment of advanced HCC (4).
Hepatic arterial infusion chemotherapy (HAIC) has been used as a palliative chemotherapy in the treatment of patients with intermediate or advanced HCC (5). HAIC enables the direct delivery of chemotherapeutic drugs through the transplantable port system to the feeding arteries of the liver tumor at higher local drug concentrations. Notably, the incidence of adverse drug reactions in response to systemic chemotherapy is relatively high, including during the use of the FOLFOX regimen (fluorouracil, leucovorin, and oxaliplatin) in patients with advanced HCC (6). HAIC has a stronger first-pass effect than systemic chemotherapy, thus ensuring antitumor efficacy and minimizing the toxicity associated with systemic chemotherapy (7). It is necessary to explore and evaluate novel therapies in the clinical treatment of HCC. At present, because of the lack of data available from clinical drug trials, HAIC for advanced liver cancer is not recommended by Grade I specialists in the American Association for the Study of Liver Diseases, European Association for Liver Diseases or Asian Association for the Study of Liver Diseases (8–10). However, HAIC has been used in some Asian countries, particularly in China, Japan and South Korea, as a method to improve the prognosis of patients with advanced HCC, and it has been included in the relevant treatment guidelines (11). A number of studies have reported that HAIC therapy serves an increasingly important role in the treatment of liver cancer by improving its antitumor targeting ability, reducing the impact on the surrounding normal tissues and reducing the incidence of serious adverse events (AEs) (7,12).
Previous studies have evaluated the therapeutic effect of HAIC in palliative and adjuvant chemotherapy in patients with HCC (7,12). However, the results of analyses on the efficacy and safety of HAIC vary significantly among different studies (12,13). Moreover, clinical trials of ICI/TKI treatment for advanced HCC have yielded a series of significant results with moderate treatment-related AEs (14–19). Therefore, the primary objective of the present systematic review and meta-analysis was to evaluate the prognostic outcome and safety analysis of combining HAIC with ICIs/TKIs in patients with HCC.
Materials and methods
Search strategy and literature selection
Using PubMed (https://pubmed.ncbi.nlm.nih.gov/), the Cochrane Library (https://www.cochranelibrary.com/), Embase (https://www.embase.com/), and Sinomed (http://www.sinomed.ac.cn/), clinical literature on the treatment of HCC published before March 2023 was searched. The key words searched included ‘hepatocellular carcinoma’, ‘HCC’, ‘HAIC’, ‘hepatic arterial infusion chemotherapy’, ‘ICI’, ‘PD-1’, ‘PD-L1’, ‘efficacy’ and ‘safety’, and the corresponding Chinese key words were also searched. Furthermore, the search was supplemented by manually reviewing the reference lists of the retrieved articles. Two authors independently conducted the screening of the research literature; in the case of a disagreement, this was discussed with a third author and resolved.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Study subjects were patients with unresectable HCC; ii) officially released clinical data, including registered clinical trial data and investigator-initiated clinical study data; iii) first-line treatment strategies for advanced liver cancer; iv) treatment options included ICI combination therapy (PD-1/PD-L1 inhibitors), HAIC therapy and HAIC combined with ICI/TKI therapy; v) the study literature had complete efficacy or safety data. The exclusion criteria were as follows: i) Study literature of non-HCC, including cholangiocarcinoma and mixed liver cancer; ii) non-clinical study literature, such as basic research and case reports; iii) studies of second-line or multiline therapy; iv) the treatment strategy did not meet the requirements of this study; v) efficacy or safety data were not available. The literature screening process is shown in Fig. 1. All treatments were divided into three groups: ICIs + other systemic therapies (group A), HAIC therapy alone (group B), and HAIC + ICI/TKI therapy (group C).
Figure 1.
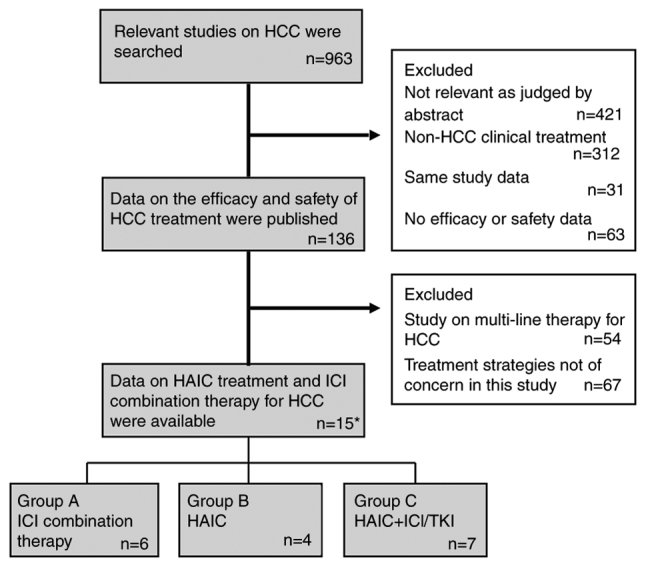
Flow chart of literature screening. *Two articles with different treatment groups were included in the analysis. HAIC, hepatic arterial infusion chemotherapy; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; TKI, tyrosine kinase inhibitor.
Literature quality evaluation and data extraction
The risk of bias was assessed using the Cochrane Collaboration (version 5.1.0; The Cochrane Institute) (20). Two authors assessed each study for bias and scored it as follows: Low risk of bias, high risk of bias, or ambiguity. Ambiguity was defined as the lack of information on ascertainment bias or uncertainty about bias. Through detailed screening of the included literature, author information, year of publication, study population, study design information, treatment groups, sample size, progression-free survival (PFS), overall survival (OS) and adverse event (AE) information were extracted. This information was collected, checked by the second author and then analyzed. Ultimately, the literature included in the present study met the criteria for meta-analysis.
Statistical analysis
SPSS 20.0 statistical software (IBM Corp.) was used to organize and analyze the data. Count data are presented as frequency and percentage, and comparisons for occurrence of AEs between groups were performed using the χ2 test. A routine meta-analysis was performed using STATA 14.0 software (StataCorp LLC). The expected median PFS (mPFS) and OS (mOS) with 95% CIs were calculated using a random effects model, and were used as the primary study endpoints to assess the treatment effect of the trial group (group C) vs. the control group (group A and B). P<0.05 was considered to indicate a statistically significant difference.
Results
General information
In the present systematic review, a total of 17 treatment groups from 15 studies were assessed, including 1,987 patients with HCC; specific information is shown in Table I (14–19,21–29). The study was divided into three treatment groups: The ICIs + other systemic therapies group, the HAIC therapy alone group and the HAIC + ICI/TKI therapy group. The year of publication for all studies was between 2020 and 2022. With the exception of one study that included global population data (atezolizumab + bevacizumab), the remaining study populations were Chinese. Of the 17 treatment groups within 15 studies, five groups underwent randomized controlled clinical trials, five groups underwent cohort studies, and the remaining seven groups underwent single-arm studies. Of these, there were 1,068 subjects in group A, 472 subjects in group B and 447 subjects in group C. The target population in all groups in the present study was largely dominated by patients unsuitable for surgical treatment, with Barcelona Clinic Liver Cancer stage B or C, Eastern Cooperative Oncology Group performance status ≤2 or Child-Pugh score A or B, as shown in Table I. The inclusion criteria remained consistent for the target population in the three groups; therefore, the efficacy and safety of the treatments could be compared between the three groups in the present study.
Table I.
Basic information of each study on the first-line treatment of advanced HCC.
| A, Group A | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| First author, year | Study population | Study design | Treatment group | Sample size | Target patients | (Refs.) |
| Finn, 2020 | Global | RCT | Atezolizumab/bevacizumab | 336 | 1. Inoperable-resected advanced HCC; 2. Child-Pugh score A, B or C; 3. ECOG score 0–1 | (14) |
| Ren, 2021 | Chinese | RCT | Sintilimab/bevacizumab | 380 | 1. Inoperable-resected advanced HCC; 2. Child-Pugh score ≤7; 3. ECOG score 0–1 | (15) |
| Liu, 2021 | Chinese | Cohort study | Camrelizumab/sorafenib | 35 | 1. Child-Pugh score A or B; 2. ECOG score 0–1 | (16) |
| Mei, 2021 | Chinese | Cohort study | Lenvatinib/PD-1 inhibitors | 25 | 1. BCLC stage B or C; 2. Child-Pugh score A or B | (17) |
| Qin, 2022 | Chinese | RCT | Camrelizumab/apatinib | 272 | 1. BCLC stage B or C; 2. Child-Pugh score A; 3. ECOG score 0–1 | (18) |
| Chen, 2022 | Chinese | Single-arm | Sintilimab/anlotinib | 20 | 1. BCLC stage B or C; 2. Child-Pugh score ≤7; 3. ECOG score 0–1 | (19) |
|
| ||||||
| B, Group B | ||||||
|
| ||||||
| First author, year | Study population | Study design | Treatment group | Sample size | Target patients | (Refs.) |
|
| ||||||
| Mei, 2021 | Chinese | Cohort study | HAIC: Oxaliplatin/leucovorin/fluorouracil | 148 | 1. BCLC stage B or C; 2. Child-Pugh score A | (21) |
| Wu, 2022 | Chinese | Single-arm | HAIC: Oxaliplatin/raltitrexed | 35 | 1. Patients with HCC for trans arterial chemoembolization failed; 2. Child-Pugh score ≤6; 3. ECOG PS ≤1 | (22) |
| Lyu, 2021 | Chinese | RCT | HAIC: Oxaliplatin/leucovorin/fluorouracil | 130 | 1. Patients with advanced HCC; 2. Child-Pugh score ≤7; 3. ECOG PS ≤2 | (23) |
| Li, 2021 | Chinese | RCT | HAIC: Oxaliplatin/leucovorin/fluorouracil | 159 | 1. Unresectable late stage HCC; 2. BCLC stage A-B; 3. Maximum lesion ≥7 cm (Response Evaluation Criteria in Solid Tumours 1.1); 4. Child-Pugh score A; 5. ECOG score 0–1 | (24) |
|
| ||||||
| C, Group C | ||||||
|
| ||||||
| First author, year | Study population | Study design | Treatment group | Sample size | Target patients | (Refs.) |
|
| ||||||
| Mei, 2021 | Chinese | Cohort study | HAIC + PD-1 inhibitors | 81 | 1. BCLC stage B or C; 2. Child-Pugh score A | (21) |
| Xin, 2022 | Chinese | Single-arm | HAIC + atezolizumab/bevacizumab | 52 | 1. First-treated and untreated advanced HCC; 2. BCLC stage C; 3. Child-Pugh score A | (25) |
| Xu, 2022 | Chinese | Single-arm | HAIC + PD-1/lenvatinib | 61 | 1. Not suitable for surgical treatment; 2. BCLC stage B or C; 3. ECOG PS ≤2; 4. Child-Pugh score A or B | (26) |
| Luo, 2022 | Chinese | Single-arm | HAIC + PD-1/TKIs | 145 | 1. Patients with unresectable advanced HCC | (27) |
| Mei, 2021 | Chinese | Cohort study | HAIC + PD-1/lenvatinib | 45 | 1. Not suitable for surgical treatment; 2. BCLC stage B or C; 3. Child-Pugh score A or B | (17) |
| Lai, 2022 | Chinese | Single-arm | HAIC + toripalimab/lenvatinib | 36 | 1. Patients with unresectable advanced HCC | (28) |
| Liu, 2021 | Chinese | Single-arm | HAIC + PD-1/TKIs | 27 | 1. BCLC stage C (presence of vascular invasion); 2. Child-Pugh score A or B | (29) |
BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; HAIC, hepatic artery infusion chemotherapy; HCC, hepatocellular carcinoma; PS, performance status; RCT, randomized controlled trial; TKI, tyrosine kinase inhibitor.
Effectiveness endpoint: PFS
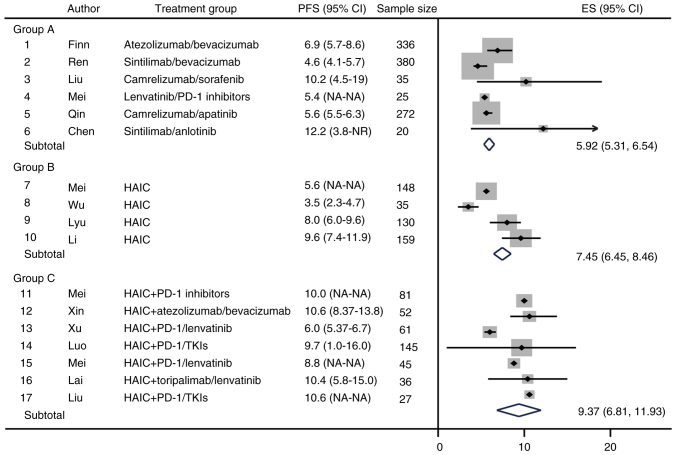
The present study performed a meta-analysis on one of the efficacy endpoints of the included studies, median PFS (mPFS). The results showed that the estimated mPFS (95% CI) in group C was 9.37 months (6.81–11.93); in group B was 7.45 months (6.45–8.46); and in group A was 5.92 months (5.31–6.54) (Fig. 2). Specifically, the efficacy of sintilimab combined with anlotinib was best in group A with a mPFS of 12.2 months. However, sintilimab combined with anlotinib for HCC was designed as a single-arm study with no data from the control group (19). In group B, HAIC therapy mainly consisted of oxaliplatin/leucovorin/fluorouracil, with the longest mPFS of 9.6 months. In group C, HAIC + ICI/TKI treatment the longest median PFS was 10.6 months (Fig. 2). These results indicated that for the PFS of patients with advanced HCC, HAIC + ICI/TKI had the best efficacy, followed by HAIC alone, while ICIs + other systemic therapies was poor.
Figure 2.
Forest plot of PFS as the primary study endpoint. HAIC, hepatic arterial infusion chemotherapy; PFS, progression-free survival; TKI, tyrosine kinase inhibitor; ES, effect size; NA, not available; NR, not reached.
Effectiveness endpoint: OS
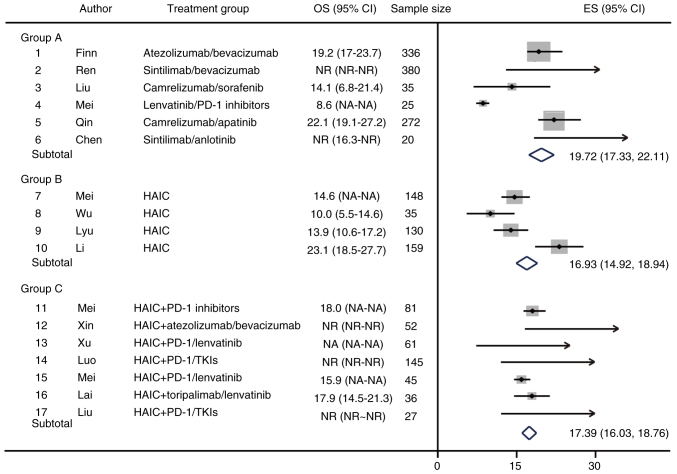
A meta-analysis of OS as a primary endpoint for the included studies was also performed. In group A, the expected median OS (mOS) (95% CI) was 19.72 months (17.33–22.11); camrelizumab and apatinib had the best efficacy, with a mOS of 23.1 months (Fig. 3). However, in group A, the mOS was not achieved in response to sintilimab/bevacizumab and sintilimab/anlotinib therapies, and the median follow-up time were 14.0 and 16.3 months, respectively. In group B, the expected mOS (95% CI) was 16.93 months (14.92–18.94) and the longest median OS was 23.1 months. Furthermore, in group C, mOS was not reached in three studies, with median follow-up times of 15.6, 12.5 and 12.9 months, respectively. One study of HAIC + PD-1/lenvatinib did not provide OS outcome data. The results of the remaining study showed an expected median OS (95% CI) in group C of 17.39 months (16.03–18.76) (Fig. 3). In group C, the expected mOS was longer than that in group B, but shorter than that in group A, and the possible reason is that the OS events in a number of studies were not reached during the follow-up time.
Figure 3.
Forest plot of OS as the primary study endpoint. HAIC, hepatic arterial infusion chemotherapy; OS, overall survival; TKI, tyrosine kinase inhibitor; ES, effect size; NA, not available; NR, not reached.
Safety data: Occurrence of AEs above level 3
The occurrence of treatment-related AEs was collected from the included studies, and AEs were summarized according to the groups A, B and C, as shown in Table II. Statistical analysis of AEs above grade 3 with an incidence of >5% in group C were statistically analyzed. Among them, the occurrence of AEs was different between group C and group A/B (control group). In group C, the incidence of hypertension [11/176 (6.25%)] was significantly different from that in group A [207/1,036 (19.98%)] (P<0.001) or in group B [0/128 (0%)] (P=0.004). Most of the remaining AEs above grade 3 in group C were not significantly different compared with that in the other two groups. For example, increased AST [14/221 (6.33%)], increased alanine transaminase (ALT) [13/221 (5.88%)], and decreased platelet count [13/221 (5.88%)] in this group were not significantly higher than those in groups A (P>0.05). Moreover, group C was not statistically different compared with group B (P>0.05)f regarding increased ALT and decreased platelet count, whereas group C had a significantly lower incidence of increased AST than group B (6.33% vs. 13.13%; P=0.003). Therefore, the types of AEs in the HAIC + ICI/TKI group were not significantly different from those in the other groups, indicating that this treatment has a good safety profile.
Table II.
Summary of grade 3 or above AEs.
| AE | Group A | Group B | Group Ca | P value, group C vs. A | P value, group C vs. B |
|---|---|---|---|---|---|
| Hypertension | 207/1,036 (19.98%) | 0/128 (0%) | 11/176 (6.25%) | <0.001 | 0.004 |
| Proteinuria | 47/1,001 (4.70%) | 0/128 (0%) | 5/115 (4.35%) | - | - |
| Decreased WBC count | 11/732 (1.50%) | 15/285 (5.26%) | 2/97 (2.06%) | - | - |
| Fatigue | 12/769 (1.56%) | 0/320 (0%) | 3/221 (1.36%) | - | - |
| Hand-foot syndrome | 36/1,061 (3.39%) | 0/128 (0%) | 3/169 (1.78%) | - | - |
| Increased blood bilirubin | 57/1,061 (5.37%) | 8/320 (2.50%) | 4/221 (1.81%) | - | - |
| Pyrexia | 6/769 (0.78%) | 0/285 (0%) | 1/185 (0.54%) | - | - |
| Increased AST | 77/1,026 (7.50%) | 42/320 (13.13%) | 14/221 (6.33%) | 0.544 | 0.003 |
| Increased ALT | 53/1,026 (5.17%) | 19/320 (5.94%) | 13/221 (5.88%) | 0.666 | 0.979 |
| Decreased neutrophil count | 24/697 (3.44%) | 14/285 (4.91%) | 8/221 (3.62%) | - | - |
| Decreased platelet count | 77/1,061 (7.26%) | 20/320 (6.25%) | 13/221 (5.88%) | 0.467 | 0.861 |
| Nausea/vomiting | 4/769 (5.20%) | 14/320 (4.38%) | 6/221 (2.71%) | - | - |
| Decreased appetite | 6/734 (0.82%) | 1/163 (0.61%) | 1/97 (1.03%) | - | - |
| Abdominal pain | 8/744 (1.08%) | 2/285 (0.70%) | 8/221 (3.62%) | - | - |
| Diarrhea | 16/661 (2.42%) | 3/285 (1.05%) | 2/221 (0.90%) | - | - |
| Rash | 2/769 (0.26%) | 0/128 (0%) | 2/133 (1.50%) | - | - |
| Cutaneous vascular hyperplasia | 7/272 (2.57%) | - | 1/88 (1.14%) | - | - |
AE, adverse events; ALT, alanine transaminase; AST, aspartate aminotransferase; WBC, white blood cell.
Differences AEs with an incidence of >5% between group C and group A/B (control group) were assessed using the χ2 test.
Ranking of treatment-related AEs among the groups
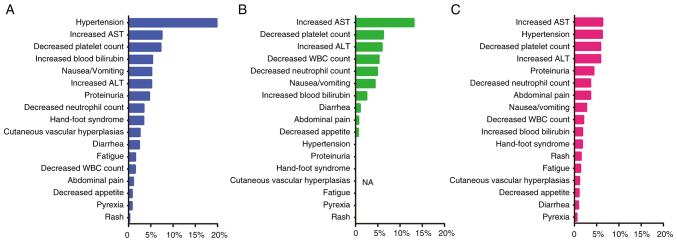
The present study ranked the occurrence of AEs above level 3 by groups, as shown in Fig. 4. The results showed that in group A, the top treatment-related AEs were: Hypertension, increased AST, decreased platelet count, increased blood bilirubin and nausea/vomiting. In group B, the occurrence of AEs above grade 3 were different from those in group A; the top AEs in this group were: Increased AST, decreased platelet count, increased ALT, decreased white blood cell count and decreased neutrophil count. In group C, the overall incidence of AEs above grade 3 was low, and the ranking was as follows: Increased AST, hypertension, decreased platelet count, increased ALT and proteinuria.
Figure 4.
Occurrence of grade 3 or above adverse events among the different groups. (A) Group A, ICI combination therapy group; (B) group B, HAIC therapy group; (C) group C, HAIC + ICI/tyrosine kinase inhibitor therapy group. ALT, alanine transaminase; AST, aspartate aminotransferase; HAIC, hepatic arterial infusion chemotherapy; ICI, immune checkpoint inhibitor; WBC, white blood cell.
Discussion
Due to the lack of clinical randomized controlled trials directly comparing different treatments of HCC, the present meta-analysis used data from three treatment groups, generated from 17 published treatment groups, to indirectly evaluate the efficacy and safety of ICI + other systemic therapy, HAIC alone and HAIC + ICI/TKI therapy.
HAIC was first proposed in Japan, and has been used in Japan, South Korea and other Asian countries for >30 years. Notably, it is recommended by the Japanese Society for Liver Disease consensus clinical practice guidelines as the standard treatment for liver cancer with portal venous tumor thrombosis (30). Currently, there is no universal standard for HAIC therapy, either with a single drug or with a combination of different drugs. The most widely used regimens include cisplatin monotherapy, the FAIT regimen (interferon and fluorouracil) and the FOLFOX regimen (31–33).
The present results showed that group C had the longest estimated mPFS (95% CI) at 9.37 months (6.81–11.93); much higher than that in groups A and B. However, there was no significant difference in the expected mOS among the three groups, which may be because the OS events in a number of studies were not reached during the follow-up time. For example, in group C, the median follow-up time of three studies was 15.6, 12.5 and 12.9 months, respectively, but the mOS was not reached. It is clear that the final mOS of these studies may be markedly higher than the follow-up duration, thus directly affecting the overall OS outcome in this group. OS is usually recommended as the primary endpoint of phase 2/3 clinical trials for the treatment of advanced liver cancer. However, OS also has limitations, such as the need for long-term follow-up to obtain the number of events. Surrogate endpoints are often used by the Food and Drug Administration and National Medical Products Administration for the approval of drug marketing applications under an accelerated plan. In the clinical study design of advanced liver cancer, surrogate endpoints are widely used, such as PFS (34). In a number of studies regarding the clinical treatment of tumors, there is a significant association between PFS and OS (35,36).
In order to analyze the occurrence of AEs above grade 3 in group C, the occurrence of AEs in groups A and B were compared in the present study. The results showed that the incidence of AEs above grade 3 in group C was not significantly different compared with those in the other two groups, showing a good safety profile. In other studies, the effectiveness and high safety profile of HAIC combined with ICI/TKI therapy has been verified in the treatment of advanced HCC (21,27).
The present study has several limitations. First, only 17 treatment groups from 15 studies met the inclusion criteria for the meta-analysis, and potential bias in these studies may have influenced the study findings. As the pathogenesis and prevalence of HCC vary widely across regions and populations, the population in the present study was mostly Chinese. In addition, the target population of the present study included patients with intermediate/advanced HCC with no surgical options and systemic medication strategies preferred for first-line therapy. Notably, these facts may limit the generalizability of the present findings. Second, some of the included study designs were observational, which led to some selection bias. In addition, the present meta-analysis lacks a direct ‘head-to-head’ design of the three treatment options, thus the relative differences between the groups could only be obtained by indirect comparisons. Moreover, among the OS results in the present study, several treatment groups had short follow-up times and did not reach mOS within these times. These factors all had a direct effect on the final result.
In conclusion, the present study demonstrated that, in patients with advanced HCC, treatment with HAIC + ICI/TKI therapy significantly improved the PFS compared with the other treatment options. Although mOS was not reached because of the follow-up times of the available studies, the available data indicated that the OS of group C was not significantly different from that in the other treatment groups. In addition, the safety data showed that the treatment-related AEs in group C were not significantly higher compared with those in the other treatment groups. Therefore, the results of the present study suggested that HAIC combined with ICI/TKI therapy has a notable efficacy and a high safety profile in the treatment of patients with advanced HCC.
Acknowledgements
Not applicable.
Funding Statement
This study was supported by the Natural Science Foundation of Jiangsu Province (grant no. BK20200275) and the Foundation of Jinling Hospital (grant no. 22LCZLXJS57).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XL conceived of and designed the study. ZL, YX and WQ performed data acquisition, data analysis and manuscript preparation. PL, YZ, HL and YG assisted with data acquisition, data analysis and statistical analysis. YX and WQ confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Tian T, Song C, Jiang L, Dai J, Lin Y, Xu X, Yu C, Ge Z, Ding Y, Wen Y, et al. Hepatitis B virus infection and the risk of cancer among the Chinese population. Int J Cancer. 2020;147:3075–3084. doi: 10.1002/ijc.33130. [DOI] [PubMed] [Google Scholar]
- 3.Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345–1362. doi: 10.1016/S0140-6736(22)01200-4. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Pan J, Gao F, Xu W, Li H, Qi X. Efficacy and Safety of PD-1/PD-L1 inhibitors in advanced hepatocellular carcinoma: A systematic review and meta-analysis. Adv Ther. 2023;40:521–549. doi: 10.1007/s12325-022-02371-3. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Zhang J, Wang Y, Shu G, Lou C, Du Z. HAIC vs. TACE for patients with unresectable hepatocellular carcinoma: A systematic review and meta-analysis. Medicine (Baltimore) 2022;101:e32390. doi: 10.1097/MD.0000000000032390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CC, Yang TS, Yen CJ, Cheng R, Liu J, Hsu C. Safety and preliminary efficacy of ramucirumab in combination with FOLFOX4 in patients with advanced hepatocellular carcinoma: A nonrandomized, open-label, phase Ib study. Oncologist. 2020;25:e1921–e1929. doi: 10.1002/onco.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Z, Tong Y, Yang L, He X, Bao G, Du X. Identifying optimal therapies in patients with advanced hepatocellular carcinoma: A systematic review and network meta-analysis. Transl Gastroenterol Hepatol. 2022;7:38. doi: 10.21037/tgh-20-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver. Electronic address, corp-author. easloffice@easloffice.eu: Corrigendum to ‘EASL clinical practice guidelines: Management of hepatocellular carcinoma’ [J Hepatol 69 (2018) 182–236] J Hepatol. 2019;70:817. doi: 10.1016/j.jhep.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Benson AB, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, Anders R, Are C, Brown D, Chang DT, et al. Guidelines Insights: Hepatobiliary cancers, version 2.2019. J Natl Compr Canc Netw. 2019;17:302–310. doi: 10.6004/jnccn.2019.0019. [DOI] [PubMed] [Google Scholar]
- 10.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Qin X, Jiang H, Sun C, Wu M, Xu Z, Lu T, Ma X, Han Z. Comparison of hepatic arterial infusion chemotherapy and transarterial chemoembolization for advanced hepatocellular carcinoma: A systematic review and meta-analysis. J Gastrointestin Liver Dis. 2022;31:336–343. doi: 10.15403/jgld-4455. [DOI] [PubMed] [Google Scholar]
- 13.Kong S, Yu H, Wang H, Song J, Yan J. Hepatic arterial infusion chemotherapy combined with sorafenib vs. sorafenib alone for advanced hepatocellular carcinoma: A systematic review and meta-analysis. Clin J Gastroenterol. 2023 Sep 23; doi: 10.1007/s12328-023-01860-4. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 15.Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) vs. sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22:977–990. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, You N, Li J, Wu K, Peng X, Wang Z, Wang L, Zhu Y, Zheng L. Camrelizumab plus sorafenib vs. sorafenib monotherapy for advanced hepatocellular carcinoma: A retrospective analysis. Front Oncol. 2021;11:694409. doi: 10.3389/fonc.2021.694409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mei J, Tang YH, Wei W, Shi M, Zheng L, Li SH, Guo RP. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib vs. PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. 2021;11:618206. doi: 10.3389/fonc.2021.618206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin S. Camrelizumab combined with apatinib for first-line treatment of unresectable or metastatic hepatocellular carcinoma: Total population and Chinese subgroup results. http://meeting.csco.org.cn/Resource/ CSCO Annual Meeting 2 oral report. 2022 Available: [Google Scholar]
- 19.Chen X, Li W, Wu X, Zhao F, Wang D, Wu H, Gu Y, Li X, Qian X, Hu J, et al. Safety and efficacy of sintilimab and anlotinib as first line treatment for advanced hepatocellular carcinoma (KEEP-G04): A single-arm phase 2 study. Front Oncol. 2022;12:909035. doi: 10.3389/fonc.2022.909035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board of the Cochrane Collaboration Back Review Group Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976) 2003;28:1290–1299. doi: 10.1097/00007632-200306150-00014. [DOI] [PubMed] [Google Scholar]
- 21.Mei J, Li SH, Li QJ, Sun XQ, Lu LH, Lin WP, Zheng L, Chen MS, Shi M, Wei W, Guo RP. Anti-PD-1 immunotherapy improves the efficacy of hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:167–176. doi: 10.2147/JHC.S298538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Zheng S, Zhang Z, Chen G, Chen X, Zheng T, Guo X, Chen H, Wang M, Xie X, Zhang B. Hepatic arterial infusion chemotherapy with oxaliplatin plus raltitrexed as an alternative option in advanced hepatocellular carcinoma patients with failure of, or unsuitability for, transarterial chemoembolization. Medicina (Kaunas) 2022;58:1343. doi: 10.3390/medicina58101343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, Deng HJ, He M, Mu LW, Zhao M. Arterial chemotherapy of oxaliplatin plus fluorouracil vs. sorafenib in advanced hepatocellular carcinoma: A biomolecular exploratory, Randomized, phase III trial (FOHAIC-1) J Clin Oncol. 2022;40:468–480. doi: 10.1200/JCO.21.01963. [DOI] [PubMed] [Google Scholar]
- 24.Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs. transarterial chemoembolization for large hepatocellular carcinoma: A Randomized phase III trial. J Clin Oncol. 2022;40:150–160. doi: 10.1200/JCO.21.00608. [DOI] [PubMed] [Google Scholar]
- 25.Xin Y, Cao F, Yang H, Zhang X, Chen Y, Cao X, Zhou X, Li X, Zhou J. Efficacy and safety of atezolizumab plus bevacizumab combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Front Immunol. 2022;13:929141. doi: 10.3389/fimmu.2022.929141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y, Fu S, Mao Y, Huang S, Li D, Wu J. Efficacy and safety of hepatic arterial infusion chemotherapy combined with programmed cell death protein-1 antibody and lenvatinib for advanced hepatocellular carcinoma. Front Med (Lausanne) 2022;9:919069. doi: 10.3389/fmed.2022.919069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo L, Xiao Y, Zhu G, Huang A, Song S, Wang T, Ge X, Xie J, Deng W, Hu Z, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors and tyrosine kinase inhibitors for unresectable hepatocellular carcinoma: A tertiary medical center experience. Front Oncol. 2022;12:1004652. doi: 10.3389/fonc.2022.1004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai Z, He M, Bu X, Xu Y, Huang Y, Wen D, Li Q, Xu L, Zhang Y, Wei W, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68–77. doi: 10.1016/j.ejca.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Liu BJ, Gao S, Zhu X, Guo JH, Kou FX, Liu SX, Zhang X, Wang XD, Cao G, Chen H, et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy. 2021;13:1395–1405. doi: 10.2217/imt-2021-0192. [DOI] [PubMed] [Google Scholar]
- 30.Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines) Hepatol Res. 2015;45 doi: 10.1111/hepr.12464. [DOI] [PubMed] [Google Scholar]
- 31.Long F, Chen S, Li R, Lin Y, Han J, Guo J, Chen Y, Li C, Song P. Efficacy and safety of HAIC alone vs. HAIC combined with lenvatinib for treatment of advanced hepatocellular carcinoma. Med Oncol. 2023;40:147. doi: 10.1007/s12032-023-02012-x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Ouyang D, Huang Z, Che X. Hepatic arterial infusion chemotherapy vs. sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombus: An updated meta-analysis and systematic review. Front Oncol. 2023;13:1085166. doi: 10.3389/fonc.2023.1085166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng K, Zhu X, Fu S, Cao G, Li WQ, Xu L, Chen H, Wu D, Yang R, Wang K, et al. Sorafenib plus hepatic arterial infusion chemotherapy vs. sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: A Randomized trial. Radiology. 2022;303:455–464. doi: 10.1148/radiol.211545. [DOI] [PubMed] [Google Scholar]
- 34.Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, Lencioni R, Greten TF, Kudo M, Mandrekar SJ, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology. 2021;73((Suppl 1)):S158–S191. doi: 10.1002/hep.31327. [DOI] [PubMed] [Google Scholar]
- 35.Wang ZX, Wu HX, Xie L, Lin WH, Liang F, Li J, Yang ZM, Xu RH. Exploration of modified progression-free survival as a novel surrogate endpoint for overall survival in immuno-oncology trials. J Immunother Cancer. 2021;9:e002114. doi: 10.1136/jitc-2020-002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llovet JM, Montal R, Villanueva A. Randomized trials and endpoints in advanced HCC: Role of PFS as a surrogate of survival. J Hepatol. 2019;70:1262–1277. doi: 10.1016/j.jhep.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.