Abstract
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder that affects millions of people worldwide. The characteristic pathological manifestation of AD includes the deposition of extracellular insoluble β amyloid plaques and intracellular neurofibrillary tangles formed from hyperphosphorylated tau protein. Cost effective and minimally invasive peripheral blood-based biomarkers are critical for early AD diagnosis. Currently, the plasma based two fraction of β amyloid peptide ratio (Aβ42/40) and phosphorylated tau (p-tau) are considered as blood-based biomarkers for AD diagnosis. Recent research indicates that oxidative stress (OS) occurs prior to amyloid plaque (Aβ) formation and abnormal tau phosphorylation in AD. The imbalance of the master antioxidant, glutathione (GSH), and prooxidants (iron, zinc, and copper)—plays a crucial role in AD neurodegeneration. We present peripheral blood-based OS related biomarkers that are mechanistically involved in the disease process and may serve as a novel screening tool for early detection of AD onset. This OS based approach may also provide a quick and cost efficient method to monitor the effects of disease-modifying therapies in AD clinical trials.
Keywords: Alzheimer’s disease; oxidative stress; peripheral blood; biomarker; antioxidants; glutathione; prooxidants, iron
Introduction
Alzheimer’s disease (AD) is a major progressive neurological disorder that leads to the gradual deterioration of cognitive reserve and impaired activities of daily living. The β amyloid and abnormal tau-phosphorylation hypotheses have dominated the pathogenesis of this disease. The cause, however, is not yet identified, and no drug is available for AD modification or cure. The hallmark of AD was considered the presence of senile plaques caused by aggregation of extracellular Aβ protein and intracellular formation of neurofibrillary tangles (NFTs) leading to hyperphosphorylation of τ protein.1 Biomarkers from cerebrospinal fluid (CSF) as well as from blood (plasma) involving amyloid peptide and phosphorylated tau are used in clinical settings. The CSF based biomarkers detection procedure is expensive and intrusive requiring a lumbar puncture, thus hindering wide clinical application. On the contrary, blood-based biomarkers detection procedure is less expensive and associated with minimal patient discomfort. Hence, blood-based biomarkers are preferred options in patient population.
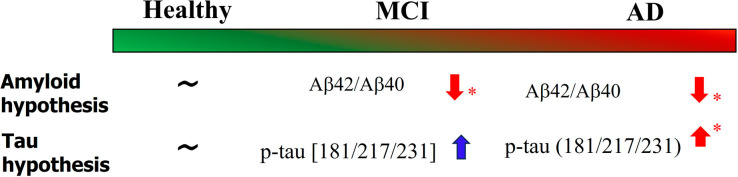
In AD, the serum level Aβ42/Aβ40 peptide ratio is assessed using immunoprecipitation and liquid chromatography–mass spectrometry assay. Studies have shown significant depletion of this Aβ42/Aβ40 ratio in mild cognitive impairment (MCI) and AD compared to healthy controls (HCs). Similarly, there is a significant elevation of p-tau 181/217/231 level in blood (plasma) of AD patients compared to HC. In the case of MCI, however, the increase of p-tau 181/217/231 level is not significant as depicted in Figure 1.
Figure 1.
Existing plasma-based biomarkers to screen MCI and AD patients. Tests with statistically significant outcomes are marked by an asterisk (∗). Significantly lower value of Aβ42/40 ratio was reported in amnestic-MCI patients compared to healthy subjects.2 P-tau 217 refers to phosphorylation of threonine residues at 217 amino acid position. In AD-affected brains, tau protein is present in the form of abnormal neurofibrillary tangles. Autopsy studies from AD-affected brains display a direct correlation between disease severity and tangle deposition.
In the past three decades, most of the clinical trials involving AD are based on amyloid and tau phosphorylation hypotheses, and to date, we do not have any reliable and safe disease modifying drugs. It is important to note that Aβ plaques have been reported in autopsy brains from individuals with no report of cognitive impairment prior to death. Hence, the presence of amyloid plaque in the brain does not necessarily correlate with abnormal clinical findings. The recently approved anti-amyloid drug (lecanemab) was reported to reduce amyloid load in early AD patients, slowing global decline in cognition. However, lecanemab and other similar drugs have adverse effects, as evidenced by cerebral edema and microintracerebral hemorrhages.3 Meta-analysis of brain imaging data involving anti-Aβ drugs has found drug-induced decrease in brain volume, accelerating hippocampal atrophy.3 This necessitates urgent attention for the development of potential drugs that have disease modifying properties.
Recent research highlighted that oxidative stress (OS), an indicator of imbalance in redox homeostasis, occurs prior to amyloid plaque (Aβ) formation and abnormal tau phosphorylation in AD.4 Hence it necessitates the validation of OS based blood biomarker for early detection and screening of AD patients. The redox imbalance and the generation of free radicals are the primary outcome of OS. We have thus suggested the mechanism of Aβ oligomerization and subsequent tau-mediated neurofibrillary tangles (NFTs) are due to OS induced neurotoxicity.4
OS in Amyloid Plaque Formation and Abnormal Tau Phosphorylation
Depletion of the brain antioxidant GSH and elevation of susceptibility (elevation of iron (Fe), zinc, and copper) in the hippocampus were demonstrated by state-of-the-art magnetic resonance spectroscopy and quantitative susceptibility measurement in AD patients. A transgenic AD mice model study validated depletion of GSH preceding amyloid plaque deposition. Similarly, GSH depletion initiates the dimerization of cysteine disulfide link in tau protein and subsequent NFT formation. The plausible mechanism is presented in our recent work.4
Pattern of Change of OS Based Biomarkers in Healthy and Patients Groups
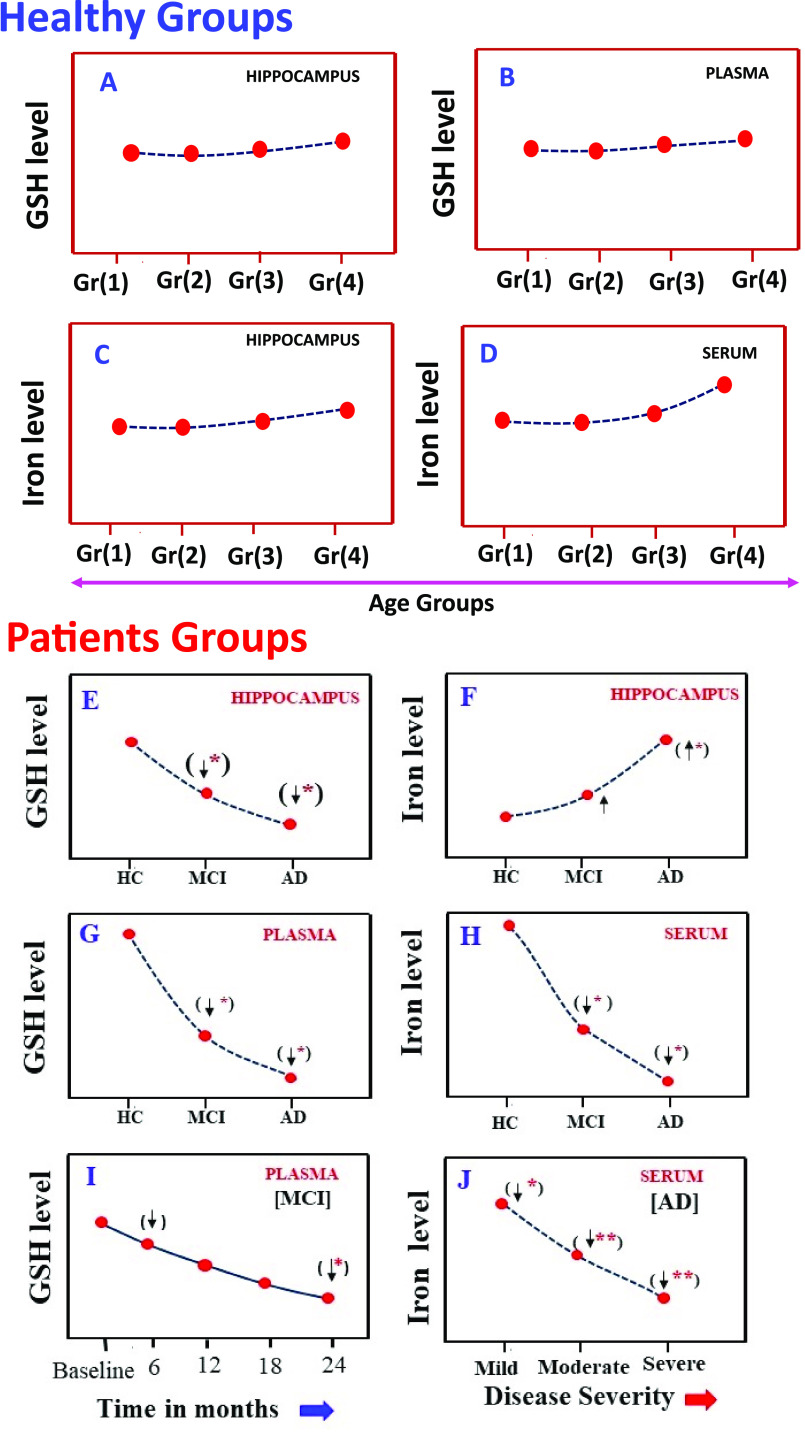
Although iron and GSH have been measured separately in clinical populations along with other metabolites, there is only one study that reported both GSH and iron levels in the blood as well as in the brain from the same study participants. It was found that GSH levels in the hippocampus and blood plasma respectively do not change in various healthy age groups (Figure 2A,B). Similarly, in the healthy age group, iron levels in the hippocampus do not change; however, blood serum iron levels increase nonsignificantly in the older age groups (Figure 2C,D).
Figure 2.
Distribution pattern of GSH and iron level of hippocampus and blood (plasma/serum) in healthy subjects and patients. Part A refers to left hippocampal GSH levels from four age groups of HC subjects, and part B refers to plasma GSH levels in the same study groups.6 Parts C and D refer to left hippocampal susceptibility and blood (serum) iron levels from the same study groups of healthy subjects (Gr(1), 20–30Y; Gr(2), 31–40Y; Gr(3), 41–50Y; Gr(4), 51–73Y). Parts E and F refer to GSH levels and iron levels in the hippocampus regions for the same study participants who were assessed, where again moving across HC to MCI to AD, a significant decrease in the GSH level and a significant increase in the iron level was observed.7 Parts G and H refer to modulation of plasma GSH and serum iron levels from study participants. Part I refer to longitudinal variations of GSH level in blood (plasma) of MCI patient within two years’ time frame.8 Part J refers to depletion of iron level in the blood (serum) of AD patients with severity (mild/moderate/severe). To date, there is no report where GSH (plasma) and iron (serum) levels are measured for the same clinical population.
In the patient group, there is a consistent significant GSH depletion pattern in the brain and blood plasma (Figure 2 E,G). It was consistently measured from a cross-sectional study involving a large MCI and AD population. There is a report of significant depletion of GSH levels in the hippocampus in MCI patients compared to HC; however, the iron level in hippocampus patients increases nonsignificantly in MCI group compared to HC. The increased level of iron, however, is significantly increased in the AD group (Figure 2E,F). Longitudinal study with MCI cohorts showed a significant depletion of GSH level (in plasma) within a two-year time frame from baseline (Figure 2 I). In that same longitudinal study, MMSE scores also decreased significantly. Autopsy studies have confirmed the presence of depleted GSH levels in the hippocampal area of MCI patients.5
The distribution patterns of iron in the brain and serum in AD are different. The state-of-the art quantitative susceptibility measurement of similar patients indicated that the iron level in the hippocampus is increased nonsignificantly in MCI; however, significant increment is observed in AD (Figure 2 F), which is supported by existing literature and autopsy studies. Various reports have indicated that serum iron level is depleted significantly from HC to MCI to AD. In the AD group, there is a significant depletion of iron based on severity (mild/moderate/severe) (Figure 2H,J). The mechanism of reciprocal relationship of iron in the brain and serum in AD patients is not known yet.
Based on significant depletion of serum iron in AD patients, researchers have established that anemia is a risk factor for cognitive loss. The Australian imaging biomarker and lifestyle cohort study reported that people with anemia were 2.40-fold more likely to have AD. This area of clinical research warrants closer investigation along with GSH levels.
Future Direction
In healthy subjects, both GSH (plasma) and iron (serum) on the same population are already investigated. In the next step, for clinical validation purposes, it is critical to measure both GSH (plasma) and iron (serum) levels concomitantly from different types of patients (MCI, AD, and Parkinson’s disease) compared to age-matched individuals without memory and movement-associated complaints. This requires urgent clinical validation of the laboratory findings in community settings with extensively worked up individuals for neuropsychological examinations and blood tests. This will immensely help in developing the cutoff range of GSH (plasma) and iron (serum) levels in the blood as biomarkers for detecting early AD in clinics. Furthermore, enriching the brain with GSH for maintaining cognitive reserve is also necessary as highlighted in earlier studies. Subsequently, a randomized clinical trial in this direction is urgently required.
Conclusion
Oxidative stress is the early event in AD, and biomarkers derived from this hypothesis are mechanistically associated with this disease. Validation will help to pave the way for clinical use in screening AD patients and may be helpful for monitoring efficacy of experimental drugs in clinical trials. We need greater cooperation among clinicians and researchers to initiate large scale clinical study with stringent inclusion and exclusion criteria. This viewpoint provides a critical analysis of our ongoing work and available literature. We could not cite all relevant works due to restriction on the total number of references.
Acknowledgments
Dr. Pravat K. Mandal thanks for partial financial support from the Ministry of Electronics and Information Technology (Grant 4(5)/2019-ITEA (Principal Investigator P.K.M.), and Department of Biotechnology, Government of India (Co-Coordinator, Dementia Science Program and Principal Investigator (NBRC component) to P.K.M.).
Author Contributions
P.K.M. (Principal Investigator) conceived the idea, contributed to the literature search and analysis, wrote the first draft of manuscript, and was involved in discussions, figure preparations, and overall quality check. J.M., A.G., and N.K.A. were involved in the analysis of the literature and discussions and involved in writing of the manuscript. R.B. was involved in the discussion and writing of the manuscript. A.K., A.S., G.K., and Y.A. were involved with literature review, analysis and figure preparation.
The authors declare no competing financial interest.
Published ASAP October 25, 2023; revised October 30, 2023 to correct production error in Figure 2.
References
- Liu P. P.; Xie Y.; Meng X. Y.; Kang J. S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct Target Ther 2019, 4, 29. 10.1038/s41392-019-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Grijalba V.; Romero J.; Pesini P.; Sarasa L.; Monleon I.; San-Jose I.; Arbizu J.; Martinez-Lage P.; Munuera J.; Ruiz A.; et al. Plasma Abeta42/40 Ratio Detects Early Stages of Alzheimer’s Disease and Correlates with CSF and Neuroimaging Biomarkers in the AB255 Study. J. Prev. Alzheimer's Dis. 2010, 6 (1), 34–41. 10.14283/jpad.2018.41. [DOI] [PubMed] [Google Scholar]
- Alves F.; Kalinowski P.; Ayton S. Accelerated Brain Volume Loss Caused by Anti-beta-Amyloid Drugs: A Systematic Review and Meta-analysis. Neurology 2023, 100 (20), e2114–e2124. 10.1212/WNL.0000000000207156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R. G.; Mandal P. K.; Maroon J. C. Oxidative Stress Occurs Prior to Amyloid Abeta Plaque Formation and Tau Phosphorylation in Alzheimer’s Disease: Role of Glutathione and Metal Ions. ACS Chem. Neurosci. 2023, 14 (17), 2944–2954. 10.1021/acschemneuro.3c00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R.; Piroddi M.; Galli F.; Butterfield D. A. Protein levels and activity of some antioxidant enzymes in hippocampus of subjects with amnestic mild cognitive impairment. Neurochem. Res. 2008, 33 (12), 2540–2546. 10.1007/s11064-008-9593-0. [DOI] [PubMed] [Google Scholar]
- Mandal P. K.; Dwivedi D.; Joon S.; Goel A.; Ahasan Z.; Maroon J. C.; Singh P.; Saxena R.; Roy R. G. Quantitation of Brain and Blood Glutathione and Iron in Healthy Age Groups Using Biophysical and In Vivo MR Spectroscopy: Potential Clinical Application. ACS Chem. Neurosci. 2023, 14, 2375. 10.1021/acschemneuro.3c00168. [DOI] [PubMed] [Google Scholar]
- Mandal P. K.; Goel A.; Bush A. I.; Punjabi K.; Joon S.; Mishra R.; Tripathi M.; Garg A.; Kumar N. K.; Sharma P.; et al. Hippocampal glutathione depletion with enhanced iron level in patients with mild cognitive impairment and Alzheimer’s disease compared with healthy elderly participants. Brain Commun. 2022, 4 (5), fcac215. 10.1093/braincomms/fcac215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. H.; Lane H. Y. Plasma Glutathione Levels Decreased with Cognitive Decline among People with Mild Cognitive Impairment (MCI): A Two-Year Prospective Study. Antioxidants (Basel) 2021, 10 (11), 1839. 10.3390/antiox10111839. [DOI] [PMC free article] [PubMed] [Google Scholar]