Abstract
The term “click chemistry” describes a class of organic transformations that were developed to make chemical synthesis simpler and easier, in essence allowing chemists to combine molecular subunits as if they were puzzle pieces. Over the last 25 years, the click chemistry toolbox has swelled from the canonical copper-catalyzed azide–alkyne cycloaddition to encompass an array of ligations, including bioorthogonal variants, such as the strain-promoted azide–alkyne cycloaddition and the inverse electron-demand Diels–Alder reaction. Without question, the rise of click chemistry has impacted all areas of chemical and biological science. Yet the unique traits of radiopharmaceutical chemistry have made it particularly fertile ground for this technology. In this update, we seek to provide a comprehensive guide to recent developments at the intersection of click chemistry and radiopharmaceutical chemistry and to illuminate several exciting trends in the field, including the use of emergent click transformations in radiosynthesis, the clinical translation of novel probes synthesized using click chemistry, and the advent of click-based in vivo pretargeting.
Introduction
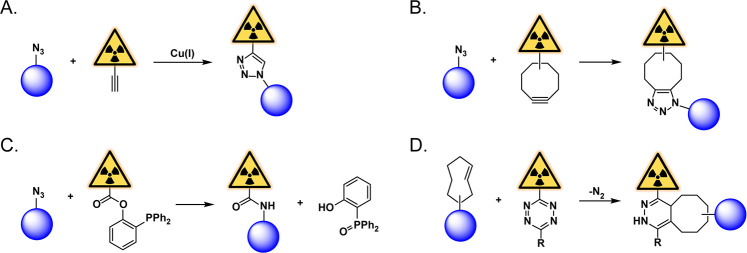
Click chemistry transformations—a suite of chemical reactions designed to be efficient, rapid, modular, clean, and water compatible—have revolutionized chemical and biological science over the past two decades.1,2 The technology is both powerful and democratizing, enabling the creation of complex molecules by scientists (like us!) outside of the synthetic elite. The reaction that started it all, the copper-catalyzed azide–alkyne cycloaddition (CuAAC), was developed independently by the groups of K. Barry Sharpless and Morten Meldal in the early aughts.3,4 The CuAAC reaction, a cousin of the thermally driven Huisgen cycloaddition, is the catalyzed ligation between an organic azide and a terminal alkyne to form a 1,4-disubstituted 1,2,3-triazole (Figure 1A). The immense popularity of the CuAAC reaction immediately fueled efforts to expand the utility of click chemistry. One intrinsic limitation of the CuAAC ligation is that its applicability in biological systems is hampered by both its ternary nature and the inherent toxicity of free copper cations. These obstacles were circumvented in 2004 by Carolyn Bertozzi’s development of the first bioorthogonal click reaction: the strain-promoted azide–alkyne cycloaddition (SPAAC).5 The SPAAC reaction is driven by the release of ring strain within a cyclic octyne, abrogating the need for a catalyst and rendering it compatible with biological systems (Figure 1B). While the CuAAC and SPAAC ligations are without a doubt the foremost representatives of click chemistry, an array of other potent click reactions has arrived on the scene in recent years, including the traceless Staudinger ligation (Figure 1C), the inverse electron-demand Diels–Alder cycloaddition (Figure 1D), and a handful of other equally elegant yet less often employed variants.6,7
Figure 1.
Click chemistry and radiochemistry. Schematics of (A) the copper-catalyzed azide–alkyne cycloaddition, (B) the strain-promoted azide–alkyne cycloaddition, (C) the traceless Staudinger ligation, and (D) the inverse electron-demand Diels–Alder reaction. The blue circle represents a target-binding vector.
The profound impact that click chemistry has had on the chemical and biological sciences was underscored just last year by the decision to award the 2022 Nobel Prize in Chemistry to the technology’s trio of founders: Sharpless, Meldal, and Bertozzi.8 And while it is difficult to find a discipline that has not been touched by click chemistry, some have inevitably been influenced more than others. Along these lines, radiopharmaceutical chemistry is a standout example, as the exigencies of radiochemistry align remarkably well with the advantages of click chemistry.9,10 Indeed, a list of the things that radiochemists need for the effective synthesis of radiopharmaceuticals—simplicity, selectivity, reliability, efficiency, rapidity, and biocompatibility—reads remarkably like a description of the fundamental benefits of click chemistry. In 2006, a team from ETH Zurich led by Mindt and Schibli became the first to harness click chemistry in service of radiochemistry, reporting on the use of the CuAAC ligation to create coordination scaffolds for 99mTc.11 Research at the intersection of these two fields has exploded in the years since, with click chemistry leveraged to create radiopharmaceuticals labeled with nuclides ranging from short-lived positron-emitters (i.e., 11C) to long-lived α-emitters (i.e., 225Ac) and based on vectors spanning from small molecules to nanoparticles. Furthermore, click chemistry has been harnessed for a surprising variety of applications in radiopharmaceutical chemistry, including the construction of vectors, bioconjugation, radiolabeling, the purification of probes, and in vivo pretargeting, just to name a few.
In 2016, we published an extensive review of the first decade of interplay between click chemistry and radiochemistry.12 This work is meant to be a companion for the original article, a critical and thorough update covering developments at the convergence of these two fields in the intervening years. In response to feedback on the original piece, we have altered course a bit in the organization of this review by pivoting to sections based on the type of click reaction rather than the application of the technology within radiopharmaceutical chemistry. Of course, we are not the only ones to write about the contribution of click chemistry to the radiopharmaceutical sciences, so we would be remiss if we did not direct the reader to a handful of reviews that can provide differing yet no less valuable vantage points.13−17 It is also important to note that a variety of other coupling reactions have been employed in radiopharmaceutical chemistry but will not be discussed here because they do not meet the definitional criteria for “click chemistry” as originally laid out by Kolb et al.18 For example, the Michael addition between maleimides and thiols is selective, but the thioether product is notoriously unstable in vivo; similarly, N-hydroxysuccinimidyl esters react efficiently with primary amines, but the former are prone to hydrolysis in water. In the end, it is our hope that this Review will provide a comprehensive portrait of the current state of this exciting discipline, illustrate how the radiopharmaceutical applications of click chemistry have grown more sophisticated in recent years, and inspire innovative new work at the junction of these two fields.
The Copper-Catalyzed Azide–Alkyne Cycloaddition
Before we begin detailing the role that the CuAAC reaction has played in radiochemistry, a brief refresher on the ligation is in order. As its name suggests, the CuAAC reaction is predicated on the Cu(I)-catalyzed union of an organic azide and a terminal alkyne to create a 1,4-disubstituted 1,2,3-triazole (Figure 1A). A wide variety of options exist for the catalyst, ranging from commercially available salts—e.g., the Sharpless–Folkin catalyst (CuSO4·5H2O reduced by sodium ascorbate)19,20—to Cu(I) complexes stabilized by ancillary ligands.21,22 Interestingly, silver-, ruthenium-, iridium-, and nickel-catalyzed azide–alkyne cycloadditions have also been reported,23 but the copper-mediated flavor remains the gold standard (no pun intended!). CuAAC reactions are simple, rapid, and tolerant of oxygen and water.24 The latter is particularly important in the context of radiopharmaceutical chemistry, as avoiding the presence of even trace organic solvents in clinical doses is vital.24 This water compatibility has also allowed for the radiolabeling of live cells,25 sensitive proteins,26,27 nanoparticles,28−30 metal–organic frameworks,31 and liposomes.32 While concerns about the biocompatibility of the Cu(I) cation have long hampered the use of the CuAAC reaction with biomolecules, the advent of catalysts bearing water-soluble Cu(I)-stabilizing ligands like tris(3-hydroxypropyltriazolylmethyl)amine (THPTA) can ameliorate these worries by protecting sensitive biomolecules from hydrolysis or oxidative damage due to free Cu cations.19,33−35
In recent years, the CuAAC reaction has been called upon to play a variety of roles in radiochemistry, ranging from creating coordination architectures for gamma-emitting radiometals to facilitating direct radiolabeling with alpha-emitting radiohalogens. In the following pages, we will cover these applications and discuss several particularly impressive and impactful examples in detail.
Assembling Precursors for Radiohalogenation
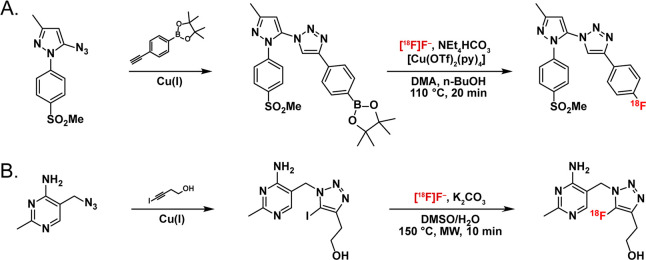
A common rule in the synthesis of radiopharmaceuticals is that the radiolabel should be installed last. As we will see later, this is not always the case but—given the expense of radionuclides and their inexorable decay—it is a pretty good rule of thumb. Along these lines, the reliability, selectivity, and modularity of the CuAAC reaction make it particularly useful for the synthesis of precursors for radiolabeling via the installation of prosthetic groups for radiohalogenation or, as we will discuss in the next section, chelators for radiometalation. Recent work by Litchfield et al. on the development of [18F]triacoxib, a COX-2-targeted PET tracer for the visualization of inflammation, provides an excellent example of the former (Figure 2A).36 Here, the authors first used CuAAC ligation to install an aryl group bearing a pinacol borane. The reaction was performed in methanol with 1 mol % of CuSO4·5 H2O and produced the desired 1,4-disubstituted triazole in a 74% isolated yield. Subsequently, this precursor was labeled with [18F]fluoride, affording [18F]triacoxib in 72 ± 14% isolated radiochemical yield and a molar activity exceeding 90 GBq/μmol. In a slightly different approach, the CuAAC ligation can be used to create 5-iodo-1,2,3-triazole-bearing precursors that can be radiofluorinated via heteroaromatic substitution to create tracers with 5-[18F]fluoro-1,2,3-triazole groups.37 The Swenson laboratory employed this method to synthesize a 5-[18F]fluoro-triazole-containing analogue of thiamin for the imaging of thiamin-dependent enzymes and achieved radiochemical yields (RCYs) of 10–16% in 40 min (Figure 2B).38 In a third example, a team at Weill Cornell Medical College used the CuAAC ligation to incorporate an N-propargyl-N,N-dimethylammoniomethyl trifluoroborate (propargyl-AMBF3) group into a Cy3-bearing, prostate-specific membrane antigen (PSMA)-targeting probe and subsequently labeled the product with [18F]fluoride via isotope exchange to create ACUPA-Cy3-[18F]F-BF3, a bimodal near-infrared fluorescence (NIRF)/PET imaging agent.26,27
Figure 2.
Synthesis of [18F]F-triacoxib (top) as well as a 5-[18F]fluoro-1,2,3-triazole-based tracer (bottom) for investigating the activity of cyclooxygenase-2 and thiamin-dependent enzymes, respectively, via PET.
Beyond its synthetic elegance, the ACUPA-Cy3-[18F]BF3 case study also illustrates a common phenomenon at the intersection of click chemistry and radiochemistry: the influence of the “footprint” of the click reaction—in this case, the 1,2,3-triazole—cannot be ignored. In this example, the authors noted that the rigid triazole unit conferred stability upon the product by preventing an unwanted intramolecular cyclization reaction. In other cases, click-derived 1,2,3-triazoles have been observed to increase the metabolic stability,39,40 binding affinity,40,41 and rigidity40,42 of radiopharmaceuticals as well as reduce defluorination43 and improve excretion profiles.44,45 However, the 1,2,3-triazole does not always help, as shown by the work of Kwon et al.46 In pursuit of tracers for the C-X-C chemokine receptor, the investigators created a pair of radiofluorination precursors: one in which a carboxy-ammoniomethyl-trifluoroborate (PepBF3) group was incorporated via traditional amide bond formation and another in which a propargyl-AMBF3 group was installed via CuAAC chemistry. While both routes proved amenable to 19F–18F isotope exchange, the triazole-bearing variant ultimately produced significantly higher renal uptake [7.6 ± 1.0 %ID/g (% injected dose per gram)] in a murine model of leukemia than its amide-containing cousin (2.2 ± 0.5 %ID/g). This observation has been reinforced by other work that has similarly found that the insertion of polar 1,2,3-triazoles units can accelerate the renal uptake of radiopharmaceuticals44,47−49 and occasionally increase their renal retention.50
Facilitating Two-Step Radiohalogenation
As we discussed above, radionuclides are typically incorporated into radiopharmaceuticals during the final step of synthesis. Yet a growing body of work has underscored many cases in which this approach is not the best choice. In these scenarios, the CuAAC ligation can be particularly useful, as azide- or alkyne-bearing prosthetic groups can be radiolabeled and then clicked to vectors in a one-pot two-step schema. This approach is especially popular in the synthesis of 18F-labeled agents, as evidenced by a large library of 18F-labeled clickable groups that includes [18F]fluorobenzyl azides,51,52 azide-32 and alkyne-modified18 [18F]fluoropyridines, [18F]fluoroalkyl azides,22,53−60 [18F]fluorocarbohydrate azides,35,45,61−65 [18F]fluoropolyoxyethylene azides,32,51,66−68 3-[18F]fluoropropyne,69 5-[18F]fluoropentyne,20,70 ethynyl-4-[18F]fluorobenzene,71 and [18F]AMBF3.72,73 In one recent example, Wang et al. reported the synthesis of a modular scaffold for combined near-infrared (NIR) fluorescence and PET imaging via the CuAAC-mediated modification of an azide-bearing NIR dye with an 18F-labeled alkyne.72 The work of Walker et al. provides a particularly fine example of the pros and cons of a two-step, CuAAC-driven approach to radiofluorination (Figure 3).74 Here, the investigators compared two strategies for the synthesis of 18F-labeled LLP2A, a tracer for delineating the expression of integrin α4β1. In the first approach, an arylboronate-ester-bearing precursor was directly labeled with [18F]F–. And in the second, a prosthetic group containing both an azide and an arylboronate ester was first 18F-labeled and then clicked. Ultimately, each approach offered its own advantages: while the latter boasted higher radiochemical conversion (∼30% vs ∼15%, as determined by HPLC), the former yielded a product with higher specific activity (∼1.3 vs ∼3.9 mCi/mol).
Figure 3.
One-step (A) and two-step (B) approaches to the synthesis of 18F-labeled LLP2A.
Perhaps the most obvious application of this approach is the radiolabeling of sensitive biomolecules (such as nucleic acids and proteins) that may decompose under the conditions needed for one-step radiolabeling.48,51,52 However, the most significant impact of this technology lies elsewhere: automation. Automated synthesis modules enable the reliable and reproducible production of radiopharmaceuticals while limiting radiation dose rates to personnel and (when needed) ensuring current good manufacturing practice (cGMP) standards.75,76 The robustness and modularity of the CuAAC reaction make it particularly attractive for this purpose. This is especially true because highly optimized radiosyntheses have been developed for several of the 18F-labeled synthons enumerated above, and these prosthetic groups can be used with any complementary click substrate. In some cases, automation is relied upon only for the synthesis of the 18F-labeled prosthetic group, and the click reaction itself is performed manually.32,35,77
Automated modules have also been leveraged to perform both steps of these CuAAC-based radiosyntheses. This strategy could be even more valuable in the context of clinical translation, as it goes even further to ensure reproducibility, reliability, and cGMP compliance. Iannone et al., for example, recently reported the fully automated synthesis of an 18F-labeled variant of PSMA-617 in which both the radiofluorination of an azide-based prosthetic group and its subsequent CuAAC reaction with an alkyne-modified analogue of PSMA-617 were performed on a Trasis AllinOne module (Figure 4A).68 The click reaction was performed using aqueous 0.5 M NH3 as the solvent and Cu(SO4)2·5 H2O as the catalyst, leading to 80% conversion in only 10 min (92% in 20 min). Likewise, Toms et al. developed a fully automated synthesis for [18F]F-FGlc-FAPI, a 18F-labeled alternative to the well-known fibroblast activation protein (FAP)-targeted imaging agent [68Ga]Ga-FAPI-04.35 Here, the radiofluorination of an azide-bearing sugar was followed by its CuAAC-mediated ligation with an alkyne-bearing small molecule, which ultimately produced the final product in 6–15% radiochemical yield and a molar activity of 30–200 GBq/μmol (Figure 4B). Finally, a pair of teams relied upon the CuAAC reaction to create different tracers for the visualization of reactive oxygen species (ROS). Zhang et al. synthesized [18F]DHMT in ∼7% radiochemical yield via the automated radiofluorination of 2-[18F]fluoroethylazide and its subsequent CuAAC ligation with an alkyne-bearing substrate (Figure 4C).59 In contrast, Daum and co-workers manually created ferrocene-based probes via the initial radiofluorination of an azide-modified sugar and its Cu-catalyzed reaction with an alkyne-containing partner (Figure 4D).61 Ultimately, the complete automation of the CuAAC ligation in a two-step one-pot approach represents the most promising path to clinical production for a diverse array of 18F-labeled, click-based radiopharmaceuticals.22,59,78
Figure 4.
Structures of (A) PSMA-, (B) FAP-, and (C,D) ROS-targeted radiotracers synthesized via the CuAAC reaction.
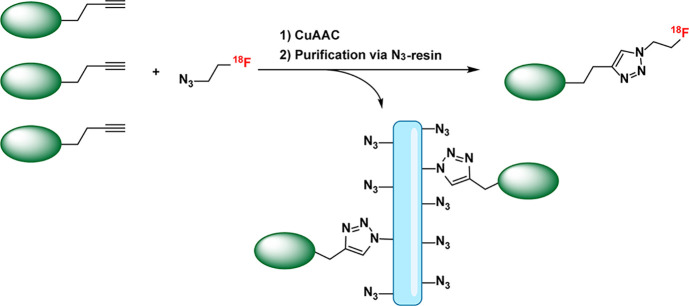
Innovative work by Pisaneschi et al. underscores yet another advantage afforded by this two-step approach to radiolabeling: an opportunity to improve specific activity (i.e., the amount of radioactivity per mole of compound).79 Specifically, the team used an inexpensive azide-coated resin as a scavenger to rapidly remove excess precursor during the automated CuAAC ligation between 2-[18F]fluoroethylazide and alkyne-bearing peptides (Figure 5). This approach removed >98% of the unreacted alkyne-modified precursor from the reaction solution in under 20 min at room temperature, ultimately yielding 18F-labeled radiotracers in radiochemical purities >99% and with specific activities more than 200-fold higher than could be achieved without the scavenger system.
Figure 5.
Maximizing specific activity during CuAAC-mediated radiolabeling by scavenging unreacted precursor. The green ovals represent target-binding molecules; the blue rectangle represents the resin.
Enabling One-Step Radiohalogenation
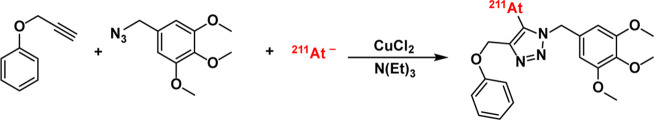
One particularly exciting way of harnessing the CuAAC ligation for radiolabeling stands apart from both methods discussed thus far. In 2019, Denk et al. reported the creation of compounds containing 211At-labeled 1,2,3-triazole rings by performing CuAAC conjugations in the presence of the α particle-emitting radiohalogen (Figure 6).80 The investigators hypothesize that 211At(I) is formed via oxidation by Cu(II) during the CuAAC reaction and that this astatonium cation undergoes electrophilic substitution with the triazole ring to provide the 5-[211At]astato-1,2,3-triazole product. The group optimized the synthesis to reach radiochemical yields >70% in 10 min using acetonitrile as the primary solvent and CuCl2 as the catalyst. Ultimately, the 211At-labeled radiopharmaceuticals synthesized in this manner seemed to be more stable to in vivo dehalogenation than analogous compounds radiohalogenated in other ways. Interestingly, similar approaches have been used to create radioiodinated 5-iodo-1,2,3-triazoles and 5-[18F]F-trifluoromethyl-1,2,3-triazoles.81−83
Figure 6.
The synthesis of 5-[211At]astato-1,2,3-triazoles via the CuAAC reaction.
Attaching Chelators for Radiometalation
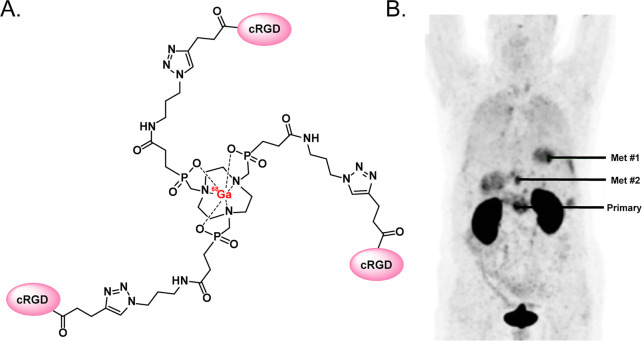
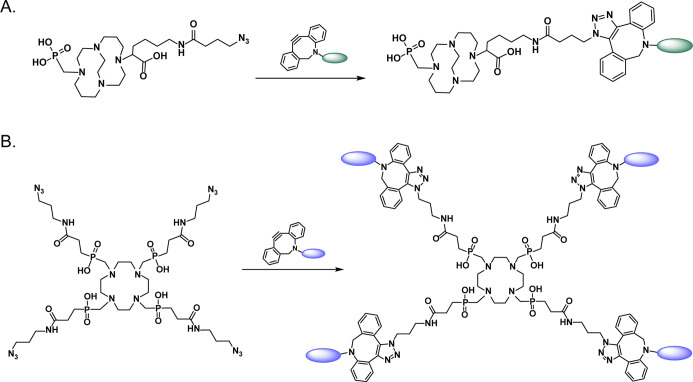
Just as the CuAAC reaction has been used to build precursors for subsequent radiohalogenation, it has also proven highly effective for the synthesis of chelator-bearing conjugates for radiometalation.84−97 In perhaps the best example of this application, Quigley et al. used the CuAAC ligation to create the precursor for Trivehexin, an αvβ6-targeting tracer in which an azide-bearing central triazacyclononane-triphosphinate (TRAP) chelator is linked to a trio of integrin-binding cyclic peptides via 1,2,3-triazoles (Figure 7A).98 This multimeric construct exhibits increased avidity for its target compared to monomeric analogues, and a 68Ga-labeled variant—[68Ga]Ga-Trivehexin—has been used for the PET imaging of both pancreatic adenocarcinoma and head and neck cancer. Indeed, first-in-human data suggest that [68Ga]Ga-Trivehexin is capable of effectively mapping the expression of αvβ6-integrin in patients (Figure 7B). Shifting to more preclinical work, Reissig et al. synthesized variants of macropa equipped with one or two propyne units and then used the CuAAC ligation to attach either one or two azide-bearing, prostate specific membrane antigen (PSMA)-targeting vectors to the chelator core.97 After labeling with 225Ac, the team found that the bivalent probe produced nearly 9-fold higher binding in vitro than the monovalent analogue as well as longer tumoral retention in a murine model of prostate cancer.
Figure 7.
(A) The structure of [68Ga]Ga-Trivehexin; (B) PET image acquired 120 min after the administration of 105 MBq of [68Ga]Ga-Trivehexin to a patient with αvβ6-expressing pancreatic ductal adenocarcinoma showing uptake in a primary tumor as well as several metastatic lesions. Panel (B) was reproduced with permission under Creative Commons Attribution 4.0 International License from ref (98). Published 2021 by Springer Nature.
It is important to note that the metallic catalyst of the CuAAC ligation can create a challenge in the construction of chelator-bearing precursors, as the inadvertent coordination of Cu(I/II) by the chelator could theoretically prevent subsequent radiolabeling reactions. In some cases—particularly those in which the chelator does not interact strongly with copper cations—this is not a problem. However, in others, the chelator may coordinate the copper catalyst more strongly than the radiometal in question.84 It is especially important to ensure that all traces of the catalyst have been removed prior to radiolabeling because the catalyst is present in much higher molar quantities (i.e., ∼0.05 to 10 equiv. relative to the chelator) than the radiometal (i.e., ∼10–2 to 10–7 equiv. relative to the chelator). Over the years, a variety of creative solutions to this issue have come to light. For example, Reissig et al. added sodium sulfide after the ligation to precipitate the copper catalyst as an insoluble copper sulfide salt that could be removed by filtration.97 Makarem et al. and others have used the CuAAC reaction to install protected chelators and have then purified away any residual catalyst prior to deprotection and radiolabeling.86 In a creative variation on this theme, Baranski et al. used metal ions rather than organic moieties to protect the chelator.87 In this case, the investigators used Fe(III) cations to occupy an alkyne-bearing variant of HBED-CC during its CuAAC-mediated attachment to a PSMA-targeting vector. Following the conjugation, the residual catalyst was purified away, and the Fe(III) cations were released from the chelator prior to radiolabeling via treatment with 1 M HCl. Liolios et al. used a similar approach to create a [68Ga]Ga-HBED-CC-based bivalent probe that targets both PSMA and gastrin-releasing peptide receptor (GRPr).99 Lee et al. took a different track by using temperature rather than protecting groups to prevent the inadvertent coordination of the catalyst.85 Here, the authors employed an alkyne-bearing propylene cross-bridged chelator that can only bind Cu cations at elevated temperatures (>80 °C) due to its rigid backbone. After the chelator was clicked to an azide-modified vector at room temperature and the product was purified, the conjugate was radiolabeled with 64Cu at high temperature without incident. Notni et al. have turned to transchelation to remove residual catalyst.93,89−92 After using the CuAAC ligation to attach targeting moieties to their azide-bearing variants of TRAP, residual copper cations are removed from the multimeric constructs via treatment with excess 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) followed by HPLC. This approach, however, is limited to chelators like TRAP that do not interact particularly strongly with Cu cations. Finally, still other groups have explored radiolabeling alkyne-modified chelators first and then clicking them to azide-modified biomolecules in an approach reminiscent of the one-pot two-step radiofluorination strategies described above.96
Creating Chelation Architectures
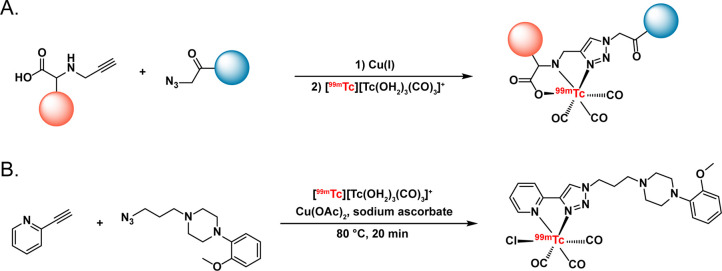
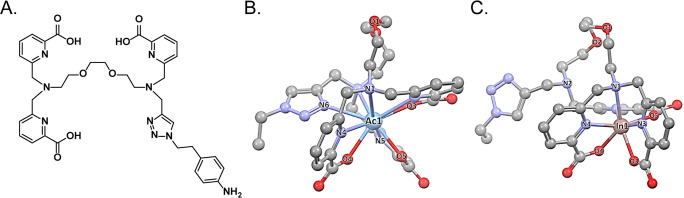
In an elegant twist on the idea of using click chemistry for the conjugation of chelators, the CuAAC reaction has also been used to help build coordination architectures. In this technique, a CuAAC ligation between azide- and alkyne-bearing substrates creates a bespoke chelator in which the 1,2,3-triazole participates in the coordination of the radiometal as a ligand with both σ-donor and π-acceptor character. This approach—dubbed “Click-to-Chelate” when it was first reported in 200611—has most famously been used to create tridentate chelators for radiolabeling with Tc(I) cores like [99mTc][Tc(CO)3(H2O)3]+ but has been employed with Re(I) synthons as well.100−102 Recently, Mindt and co-workers used this approach to synthesize 99mTc-labeled probes in which the radiometal is sequestered by a tripodal N2O-based architecture created via the reaction of Nα-propargyl lysine with an azide-bearing vector (Figure 8A).101,102 Others have taken a similar approach to creating probes in which 99mTc(I) is coordinated by N2O- and NSO-containing coordination structures, most notably a multimodal, PSMA-targeted theranostic designed to facilitate both SPECT imaging and photodynamic therapy.100,103−107 In yet another example, Erfani et al. created a 99mTc-labeled agent for the visualization of 5HT1A serotonin receptors in the brain by clicking together 2-ethynylpyridine and an azide derived from 1-(2-methoxyphenyl)piperazine (Figure 8B). The 99mTc-labeled probe displayed brain uptake of 1.4 ± 0.1 %ID/g 15 min post-administration with hotspots in the 5HT1A-active hippocampus.108 Finally, this approach has recently been extended beyond the realm of 99mTc and its congeners by Wharton et al. In this case, the investigators used the CuAAC reaction to create a bifunctional chelator, H3TPAN-triazole-Bn-NH2, in which the 1,2,3-triazole both coordinates the radiometal and attaches the chelator to the vector in question (Figure 9).109 This acyclic chelator offers several advantages over extant options, including its synthetic accessibility, lack of steric hindrance, and compatibility with both 225Ac and 111In.
Figure 8.
Schematics of (A) the use of the “Click-to-Chelate” method to create a tripodal N2O-based coordination environment for Tc(I) and (B) the use of the CuAAC reaction to create a 5HT1A serotonin receptor-targeted SPECT imaging agent. The red and blue circles represent the molecular subunits.
Figure 9.
Structure of H3TPAN-triazole-Bn-NH2 (A) as well as DFT-calculated structures of H3TPAN-triazole-Bn-NH2 bound to (B) Ac3+ and (C) In3+. Panels (B,C) were reproduced with permission from ref (109). Copyright 2022 American Chemical Society.
The Strain-Promoted Azide–Alkyne Cycloaddition
The strain-promoted azide–alkyne cycloaddition (SPAAC) was developed by Bertozzi and co-workers to circumvent the single largest limitation of the CuAAC ligation: the need for a copper catalyst that renders the cycloaddition incompatible with many biological systems. The key to the SPAAC reaction is the destabilization of one of the two reactants—the alkyne—via the introduction of ring strain, a change that lowers the activation barrier of the ligation and allows it to proceed without a catalyst (Figure 10). This simple yet critical shift makes the SPAAC ligation not only a two-component affair (unlike the CuAAC reaction) but also bioorthogonal, meaning it can be performed even within the “sea of functionality” of complex biological milieu. The SPAAC reaction, like its CuAAC progenitor, has found a variety of applications in radiopharmaceutical chemistry, including several that take advantage of its bioorthogonal nature.
Figure 10.
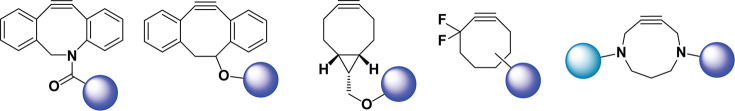
Structures of strained alkynes commonly used for the SPAAC ligation. From left to right: dibenzoazacyclooctyne [DBCO or DIBAC; also occasionally referred to as azadibenzocyclooctyne (ADIBO)], 4-dibenzocyclooctynol (DIBO), bicyclo[6.1.0]nonyne (BCN), difluorobenzocyclooctyne (DIFO), and 4,8-diazacyclononyne (DACN). The circles represent molecular cargoes.
Radiohalogenation
The straightforward, two-component nature of the SPAAC ligation has made it an attractive tool for radiohalogenation. Indeed, within the past few years, the reaction has been used to label small molecules,19,110,111 biomolecules,112 and polymers113 with radiohalogens including fluorine-18,114 iodine-125,115 and astatine-211.116 In 2018, for example, a group from Bristol-Meyers Squibb modified an anti-PD-L1 protein with a maleimide-bearing variant of azadibenzocyclooctyne (ADIBO) and subsequently reacted the conjugate with an azide-containing, radiofluorinated prosthetic group to create [18F]F-BMS-986192, an imaging agent capable of delineating PD-L1 expression in a murine model of nonsmall cell lung cancer (Figure 11A).117 In a similar vein, Zhou et al. created an 18F-labeled HER2-targeted single-domain antibody via the reaction of a radiofluorinated ADIBO with a VHH functionalized with an azide-bearing residualizing group.112 Switching the components around, Murrell et al. used a radiofluorinated ADIBO-based prosthetic group to radiolabel a pair of azide-bearing peptides that target growth hormone secretagogue receptor-1a (GHSR-1a) and gastrin-releasing peptide receptor (GRPR) in high yield and purity (64–66% and 81–88% RCY, respectively) (Figure 11B).114
Figure 11.
The SPAAC-mediated synthesis of 18F-labeled probes that target (A) PD-L1 and (B) GHSR-1a. The ribbon structure in part (A) represents an adnectin; the colored circles in part (B) represent a peptide.
Just last year, Li et al. reported on the use of a particularly elegant photoactivated variant of the SPAAC reaction for radiosynthesis.118 This strategy relies on a unique monocyclopropenone-caged dibenzocyclooctadiyne (MC-DIBOD) that contains one normal strained alkyne as well as a second triple bond masked as a cyclopropenone (Figure 12). The investigators demonstrated that this cross-linking reagent can undergo an initial SPAAC ligation with a radiofluorinated azide and then—after the second strained alkyne is unmasked by UV irradiation—a second strain-promoted cycloaddition with an azide-tagged biomolecule. The modularity of this approach is particularly impressive, as it could be used to combine virtually any azide-modified radioprecursor with a vast array of azide-functionalized targeting vectors.
Figure 12.
The use of a monocyclopropenone-caged dibenzocyclooctadiyne cross-linking reagent for a photoactivated SPAAC ligation. The blue circle represents a target-binding vector.
It is important to note, however, that the SPAAC reaction is not without its drawbacks as a radiosynthetic tool. To begin, the SPAAC ligation—like any [3 + 2] cycloaddition—can produce multiple regioisomers, a phenomenon that can lead to the inadvertent formation of mixtures of radiotracers with slightly different biochemical and biophysical behaviors. This is, of course, of particular concern in the context of small molecule radiopharmaceuticals in which regioisomeric differences can exert a greater influence on structure and function. Furthermore, the polycyclic and hydrophobic “footprint” of the SPAAC reaction can negatively influence the pharmacokinetic profiles of probes, particularly those based on small molecules. To wit, Kettenbach et al. observed dramatic differences in the in vivo behavior of two folate variants that were based on the same folate-azide building block but had been radiofluorinated with either a DBCO- or alkyne-based 18F-labeled precursor, ultimately attributing the significantly lower tumoral uptake of the former (0.5 ± 0.1 vs 1.7 ± 0.1 %ID/g) to its higher hydrophobicity.119 In their work on 18F-labeled PSMA inhibitors, Wang et al. noted that even the identity of a strained SPAAC prosthetic group can influence pharmacokinetics.120 Specifically, a PSMA-targeting probe synthesized using a bulkier, more hydrophobic 18F-labeled oxa-dibenzocyclooctyne ([18F]F-ODIBO) prosthetic group produced lower tumoral uptake (0.46 ± 0.02 %ID/g) in a murine model of metastatic prostate cancer than an analogue created using a smaller and less hydrophobic 18F-labeled bicyclo[6.1.0]nonyne (2.5 ± 0.3 %ID/g, p = 0.01) at 40 min. Clearly, this issue must be given credence when using the SPAAC reaction in the context of smaller vectors.
Chelator Installation and Radiometalation
Given the inherent challenges of using the CuAAC ligation for the conjugation of chelators (vide supra), it is not surprising that its copper-free cousin has proven popular as a tool for radiometalation. Most of the time, a “click-then-chelate” schema is used.121 For example, separate groups have leveraged the SPAAC ligation to create radiotracers by conjugating metal chelators for 64Cu (e.g., CB-TE1K1P)122 and 177Lu [e.g., DOTPI(azide)4 and DOTA-N3]123,124 to PMSA-targeting vectors for radiometalation (Figure 13).
Figure 13.
Leveraging the SPAAC ligation to create conjugates bearing chelators for radiometalation with 64Cu (A) and 177Lu (B). The green and blue ovals represent target-binding vectors.
In work by Choy et al., the group leveraged the SPAAC reaction to attach an azide-modified DOTA chelator to a PSMA inhibitor with or without an albumin-binding motif to explore the differences in circulating half-life and tumor uptake.124 Ultimately, the team found that the 177Lu-labeled variant with the albumin binding group boasted a longer serum half-life, increased tumor uptake, and greater median survival in a longitudinal therapy study. In 2019, Lodhi et al. flipped the usual script by pursuing a “chelate-then-click” approach.125 Here, the investigators first labeled an azide-bearing 2,2′-dipicolylamine chelator with [99mTc][Tc(CO)3(H2O)3]+ and then clicked this prosthetic group to an ADIBO-functionalized human serum albumin conjugate to produce a SPECT agent for blood pool imaging. The “chelate” and “click” steps were performed at 100 and 37 °C, respectively, providing an overall yield of >98%. Other groups have taken advantage of the modularity and ease of the SPAAC ligation to incorporate chelators into complex multimodality probes. Along these lines, Hensbergen et al. reacted diethylenetriaminepentaacetic acid (DTPA)-bearing derivatives of bicyclononyne (DTPA-BCN) and dibenzoazacyclooctyne (DTPA-DBCO) with an azide-containing scaffold containing both an NIR fluorophore and an arginine-glycine-aspartic acid (RGD) peptide, thereby creating conjugates that could be labeled with 111In for multimodal SPECT/NIRF imaging of integrin αvβ3 expression.126 Wang et al. likewise used the SPAAC ligation to append chelators for 99mTc and 64Cu to a construct functionalized with both biotin and a taxoid chemotherapeutic.127
Site-Specific Bioconjugation
The SPAAC ligation has proven particularly adept at facilitating site-specific antibody modifications. To provide some background, the overwhelming majority of radioimmunoconjugates are synthesized via the attachment of chelators to random lysines within the immunoglobulin. While this approach is facile, it inevitably leads to poorly defined and heterogeneous mixtures of radiotracers with various in vitro and in vivo behavior. Site-specific bioconjugation—i.e., the attachment of cargoes to a specific site or sites within a biomolecule—offers a solution to this problem and thus paves the way for the creation of well-defined, homogeneous radioimmunoconjugates with excellent immunoreactivity. The key to site-specific bioconjugation is selectivity, specifically the ability to modify individual sites within an immunoglobulin without perturbing the rest of the biomolecule. The exquisite selectivity of click chemistry seemingly fits this bill, but the CuAAC reaction’s metallic catalyst poses several intractable problems, including its potential reactivity with the immunoglobulin itself. Not surprisingly, the catalyst-free SPAAC ligation has stepped in to fill this gap and has been used with great success for the construction of site-specifically modified radioimmunoconjugates.
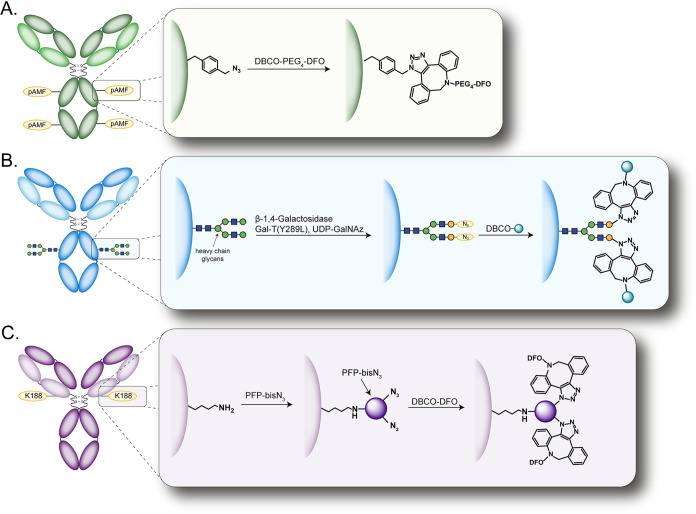
Almost all approaches to site-specific bioconjugation based on the SPAAC reaction rely upon the selective incorporation of azides—rather than strained alkynes—into the biomolecule, and a variety of creative methods have been developed to achieve this end (Figure 14).128 The use of genetic code expansion has been particularly popular.129,130 Ahn et al., for example, used a cell-free expression system to include a quartet of unnatural p-azidomethylphenylalanine (pAMF) residues and then leveraged the SPAAC reaction to modify these residues with DBCO-bearing variants of the chelators DO3A and desferrioxamine (DFO) for subsequent radiolabeling with 111In and 89Zr, respectively (Figure 14A).131 In an effort to eschew the expense and complexity of genetic engineering, other laboratories have turned to chemoenzymatic methods.132,133
Figure 14.
SPAAC-based approaches to site-specific bioconjugation based on (A) the incorporation of unnatural pAMF residues, (B) the chemoenzymatic manipulation of the heavy chain glycans, and (C) the installation of bifurcated azide-bearing prosthetic groups (PFP-bisN3).
Over the last several years, our teams have developed a chemoenzymatic approach for site-specific bioconjugation predicated on the use of a promiscuous galactosyltransferase to incorporate azide-bearing sugars into the heavy chain glycans of antibodies.134,135 The SPAAC reaction can be used to modify these azide-functionalized mAbs with a wide variety of cargoes, including toxins,136,137 fluorophores,138 and chelators (Figure 14B).136,139,140 This strategy has been shown to reliably and reproducibly yield well-defined radioimmunoconjugates with excellent in vivo behavior, and one such probe—a site-specifically modified variant of [89Zr]Zr-DFO-pertuzumab—was the subject of a recent first-in-human clinical trial in patients with HER2-positive metastatic breast, gastric, and bladder cancer at Memorial Sloan Kettering Cancer Center (NCT04692831).
Finally, two teams have recently published purely chemical approaches to SPAAC-mediated site-selective bioconjugation. Skovsgaard et al. employed an “affinity-guided protein conjugation” strategy to modify trastuzumab site-selectively with azide-bearing prosthetic groups that could then react with NOTA-ADIBO synthons.141,142 In 2022, Sarrett et al. described a site-specific bioconjugation strategy that relies upon the unique selectivity of perfluorophenyl (PFP) esters for one lysine residue (K188) within the light chain of IgG1κ.143 Here, the investigators site-specifically modified pertuzumab with a pair of bifurcated azide-bearing PFP esters, reacted this product with a DFO-functionalized variant of DBCO, and radiolabeled the chelator-modified radioimmunoconjugate with [89Zr]Zr4+. This approach ultimately produced a 89Zr-labeled radioimmunoconjugate with >99% RCY and excellent in vivo performance in a much simpler manner than either biologic-based solution described above (Figure 14C).
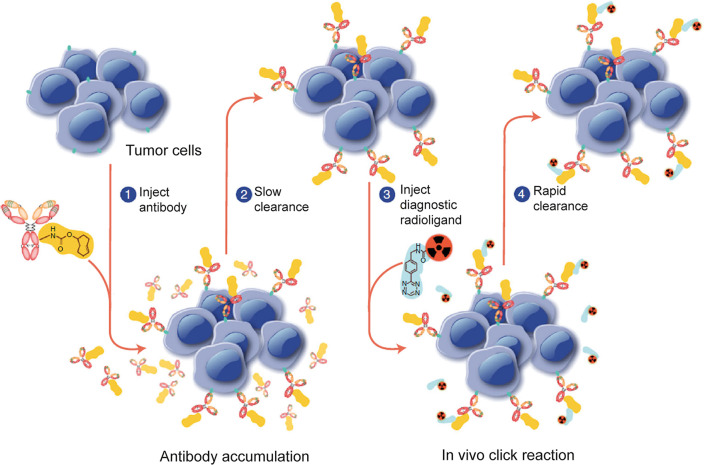
In Vivo Pretargeting
While radiolabeled antibodies have long held promise for nuclear medicine due to their exquisite specificity and affinity for cancer antigens, their long serum half-lives (i.e., 5+ days) can result in problematic radiation doses to healthy tissues, particularly in the context of radioimmunotherapy. In vivo pretargeting is a solution to this problem that seeks to address these dosimetric concerns while retaining the targeting advantages of full-length antibodies.15,144 The approach is predicated on decoupling the antibody and the radionuclide. The former is injected first and allowed to localize within the target tissue for several days prior to the administration of the latter, and a selective ligation is relied upon to bring the two components together within the body.144 This strategy not only limits the circulation of the radiolabeled antibody (thereby reducing dose rates to healthy tissues) but also enables the use of short-lived radionuclides that are normally incompatible with the biological half-life of immunoglobulins.144,145
The use of a molecular couple capable of rapid and selective in vivo ligations is essential to the success of any methodology for in vivo pretargeting. Not surprisingly, bioorthogonal click reactions have attracted attention in this sphere because of their modularity and selectivity. As we will see, the remarkable speed of the inverse electron-demand Diels–Alder reaction has made it particularly popular for this task (vide infra). However, others have worked to harness the SPAAC ligation for this purpose as well, even extending the technology beyond antibodies to vectors based on lipids,146 biological particles,147 nanoparticles,148−150 extracellular vesicles,151 and—somewhat counterintuitively—small molecules.152 Generally speaking, however, approaches to in vivo pretargeting that use the SPAAC ligation have produced moderate results at best, possibly because of the relatively sluggish kinetics of the ligation itself (i.e., k2 = ∼0.1 M1 s–1).153 Only a handful of more compelling reports have appeared in the literature. In 2022, for example, Qian et al. performed pretargeted PET imaging using the [68Ga]Ga-L-NETA-DBCO radioligand in conjunction with a multifunctional azide-bearing liposome loaded with doxorubicin for chemotherapy and 5-aminolevulinic acid for photodynamic therapy. In a murine model of colorectal cancer, in vivo pretargeting with this untargeted liposome produced only modest uptake in tumor tissue (0.87 ± 0.05 %ID/g at 2 h post-administration) that was nonetheless significantly higher than that of a control cohort (0.31 ± 0.03 %ID/g at the same time point).154
More impressively, Au et al. recently developed an approach to the pretargeted radioimmunotherapy (PRIT) of non-Hodgkin lymphoma (NHL) predicated on a DBCO-modified αCD20 mAb and a polyamidoamine-based dendrimer bearing 29 azides and 8 DOTA chelators.155 A fascinating longitudinal therapy study helped unravel this system’s complex mechanism of action. A control cohort that received the DBCO-modified mAb, alone, showed a median survival time (MST) of 42 days. However, when another cohort of mice were injected with the non-radioactive, natY-labeled polymer 24 h after the administration of the mAb, the MST nearly doubled to 81 days. Finally, in a third cohort that received PRIT with 0.28 GBq/kg of the 90Y-labeled polymer, all mice survived without paralysis until the termination of the study (150 days). Taken together, these data suggest that this treatment strategy exerts cytotoxicity on two separate levels: radiation-induced cell death due to the radiometal’s β-particles and apoptosis induced by the cross-linking of the membrane-bound αCD20.
Metabolic Cell Labeling
Without question, the Bertozzi lab’s elegant metabolic cell labeling experiments in zebrafish embryos are what truly put the SPAAC ligation “on the map.” It is not surprising, then, that molecular imaging scientists have tried to leverage glycoengineering and Cu-free click chemistry to facilitate cell tracking using fluorophores,156−158 radionuclides,159 or both.160 To highlight one example, the SPAAC reaction was recently harnessed to facilitate the real-time visualization of radiolabeled gut bacteria via PET.147 To this end, B. fragilis was cultured in broth supplemented with an azide-functionalized monosaccharide, leading to the presentation of the azide-modified sugars on the surface of the bacteria. The cells were then radiolabeled with [64Cu]Cu-NOTA-DBCO to create 64Cu-labeled bacteria that could be tracked in vivo and thus help elucidate whether gut bacteria increase the efficiency of immune checkpoint inhibitors. To provide some background, anti-PD-1/PD-L1 immunotherapy has improved outcomes for many cancer patients, yet only a fraction of patients respond, and predicting response remains challenging. Interestingly, recent studies have suggested an association between gut microbiota and the efficiency of immunotherapy, which led these authors to explore whether fecal bacterial transplantation can improve or rescue the effects of immune checkpoint inhibition. Wang et al. tested this hypothesis in a low-response 4T1 cancer mouse model receiving α-PD-1 treatment.147 They revealed that an antibiotic-induced disturbance to the mouse’s gut microbiota reduced the efficacy of anti-PD-1 treatment and that the reintroduction of B. fragilis via gavage enhanced the therapeutic effects of PD-1 blockade. The 64Cu-labeled bacteria allowed the team to quantitatively visualize the changes to each mouse’s microbiome, thereby helping to illustrate its impact on immune checkpoint inhibition therapy.
To give a second example, Lu et al. utilized metabolic glycoengineering and the SPAAC ligation to facilitate the monitoring of ovalbumin cytotoxic T lymphocytes (OVA-CTLs) in vivo and thus elucidate their antitumor efficacy within the context of combination therapy.159 The OVA-CTLs were metabolically primed with N-azidoacetylmannosamine and then radiolabeled with [64Cu]Cu-NOTA-DBCO. The group then treated mice bearing B16-OVA (murine melanoma) tumors with an encapsulated focal adhesion kinase inhibitor (FAKi)—PLGA-FAKi—followed by the adoptive transfer of the radiolabeled OVA-CTLs. PET imaging revealed an enhanced infiltration of the OVA-CTLs following treatment with PLGA-FAKi, suggesting that the latter could enhance the infiltration and efficacy of the former.
Vector Construction
As we bring this section to a close, it is important to note that there have been several cases in which SPAAC ligation has been used for the construction of radiopharmaceuticals without being directly involved in radiolabeling or the installation of chelators. Along these lines, the reaction has been leveraged to attach PEG chains to chelator-bearing conjugates,161,162 append antibodies and antibody fragments to radiolabeled liposomes,163 and modify tumor-targeting mAb with radiolabeled residualizing peptides.164 In one example, Ghosh et al. used the SPAAC reaction to append a DBCO-modified NIR fluorophore to an azide- and octreotide-modified chelator to create a platform for the fluorescence-guided surgery and 64Cu-based PET imaging of somatostatin receptor type 2-expressing cancers.165 In a particularly innovative study, Bart Cornelissen’s team at Oxford University equipped a DBCO-modified anti-green fluorescent protein-targeting mAb with an azide-bearing cell-penetrating peptide, as well as an azide-modified variant or the chelator DTPA.166 This immunoconjugate was then radiolabeled with 111In to create an immunoSPECT probe designed to explore the detection limits of the modality for intranuclear targets.
The Inverse Electron-Demand Diels–Alder Reaction
While the inverse electron-demand Diels–Alder (IEDDA) reaction has been known to chemists for decades,167,168 its advent as a tool for click chemistry had to wait until the seminal work of Joseph Fox and his team in the late 2000s.6 The reaction between an electron-poor diene (most famously a 1,2,4,5-tetrazine) and an electron-rich dienophile (most famously a trans-cyclooctene) is bioorthogonal and proceeds without a catalyst at room temperature and physiological pH.169−173 However, what sets this cycloaddition apart from its click contemporaries is its speed, as it offers rate constants exceeding 100 000 M–1 s–1 for the most reactive tetrazine (Tz) and trans-cyclooctene (TCO) pairs.171,173 Even set against the backdrop of an expanding field, the use of the IEDDA reaction in radiopharmaceutical chemistry has surged in recent years, with applications ranging from straightforward radiolabeling to in vivo pretargeting.
Radiolabeling with Halogens and 11C
The speed of IEDDA ligation has made it particularly popular as a tool for radiofluorination. Along these lines, a great deal of effort has been dedicated to creating 18F-labeled prosthetic groups based on both Tz and TCO.174−180 For example, Wang et al. synthesized a trio of 18F-labeled TCOs—[18F]F-TCO, [18F]F-sTCO, and [18F]F-dTCO—and combined them with a Tz-bearing variant of neurotensin, ultimately uncovering the influence of the TCO-prosthetic group on the in vivo behavior of these tracers in a murine model of prostate cancer (Figure 15).181,182 In 2019, Billaud et al. reported a particularly clever route to novel 18F-labeled TCOs that eschewed the radiofluorination of TCO-based precursors and instead radiolabeled cis-cyclooctenes that were then photoisomerized to create the desired trans products.183 Others have pursued creating 18F-labeled tetrazine moieties.184−186 Matthias Herth’s laboratory at the University of Copenhagen has been particularly successful in this regard. In 2021 alone, his team reported a versatile and high-yielding Cu-mediated approach to the direct radiofluorination of tetrazines starting from tin-bearing precursors as well as a novel strategy for the aliphatic 18F-labeled of these notoriously base-sensitive heterocycles (Figure 16).174 Following a separate strategy, Otaru et al. have issued a pair of reports on the use of Si–F chemistry and 19F–18F isotopic exchange for the radiofluorination of tetrazines, though the resultant 18F-labeled Tz exhibited suboptimal stability in vivo due to its rapid dehalogenation.187,188 A particularly meaningful development in the use of the IEDDA reaction for the synthesis of 18F-labeled tracers—especially in the context of clinical translation—was the automated synthesis of a radiofluorinated biologic on a GE FastLab platform.189 In this work, Allot et al. used the module to synthesize a radiofluorinated tetrazine ([18F]F-Box-Tz) and append it to a TCO-bearing variant of interleukin-2, yielding the final product in ∼20% radiochemical yield and >98% radiochemical purity in under 2 h.189 Finally, Hans Mikkula’s laboratory at TU Wien produced a creative and versatile “cross-isotopic” approach to the construction of radiotheranostics. Here, a DOTA- and alkyne-bearing tetrazine can be labeled with either 18F and a non-radioactive metal (e.g., 176Lu) or19F and a radiometal (e.g., 177Lu), thereby facilitating the creation of isotopologous radiopharmaceuticals for imaging and therapy upon reaction with TCO-functionalized scaffolds (Figure 17).190
Figure 15.
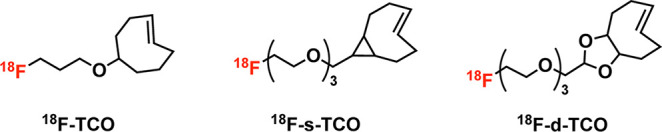
Structures of three 18F-labeled TCOs.
Figure 16.
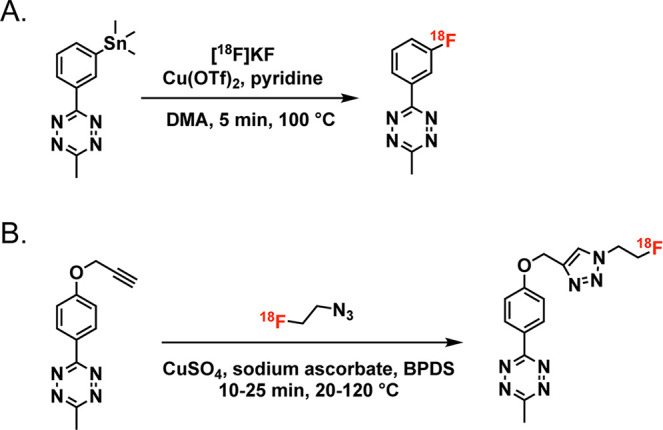
Two approaches to the radiofluorination of tetrazines: (A) the use of a stannylated precursor and (B) the CuAAC ligation.
Figure 17.
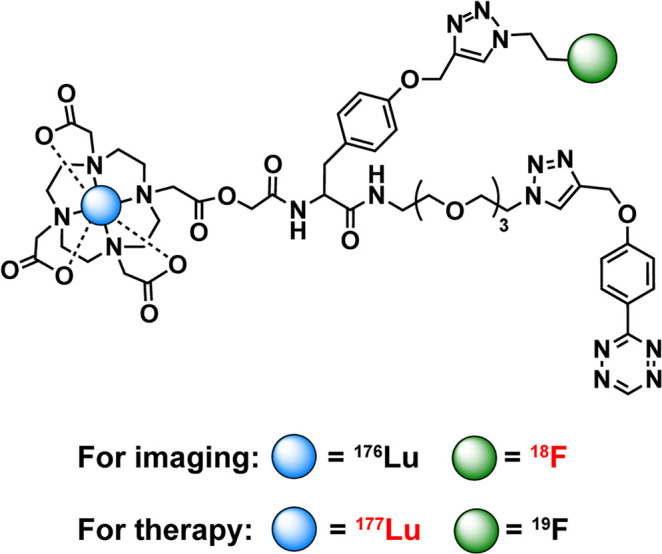
An IEDDA- and CuAAC-based “cross-isotopic” approach to the synthesis of radiotheranostics.
Moving to a much shorter-lived radionuclide, Garcia et al. created a Cu-mediated approach to using [11C]CO2 to synthesize 11C-labeled tetrazines that could then be clicked to TCO-bearing, dendrimeric PeptoBrush scaffolds.191 Another report from the same team reports the use of a different 11C-containing synthon—[11C]CH3I—to create a 11C-labeled Tz for pretargeted PET imaging (vide infra).192 Finally, as we discussed above, Denk et al. devised a creative CuAAC-based method to create (amongst other compounds) a library of tetrazines labeled with the alpha-emitting radiohalogen 211At.193
Chelator Installation and Radiometalation
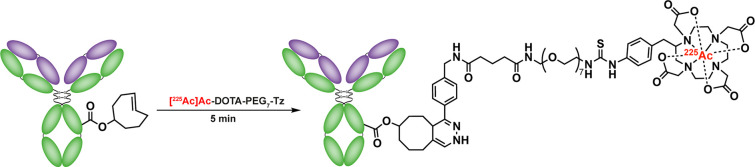
Researchers have also turned to the IEDDA ligation for the synthesis of radiometal-based radiopharmaceuticals, both through the use of prelabeled prosthetic groups and via the conjugation of chelators. A two-step protocol for the synthesis of 225Ac-labeled radioimmunoconjugates devised by Poty et al. clearly illustrates the advantages of the former approach (Figure 18).171 Here, a DOTA-PEG7-Tz synthon was first labeled with 225Ac, and the resultant 225Ac-labeled Tz was used to label a TCO-modified immunoconjugate of the CA19.9-targeting antibody hu5B1. Ultimately, this novel strategy produced radiochemical yields of 35–45%, two-to-three times higher than those offered by the previously published methodology based on an isothiocyanate-bearing [225Ac]Ac-DOTA prosthetic group. In a similar approach, another team used a [68Ga]Ga-DO3A-Tz to modify TCO-decorated microbubbles to create probes for multimodal ultrasound/PET imaging.172 With respect to the bioconjugation of chelators, Meimetis et al. synthesized a Tz-BODIPY-DFO probe whose fluorescence is activated upon reaction with a TCO-bearing mAb, thereby providing a quantitative readout of degree-of-labeling and enabling the synthesis of 89Zr-labeled radioimmunoconjugates for multimodal PET/NIRF imaging.169 In another innovative approach, a bifunctional 1,2,4,5-tetrazine core was created that could be orthogonally labeled at the 3- and 6-positions with different imaging reporters (e.g., fluorophores, chelators for radiometals, etc.) and then reacted with dienophile-functionalized targeting vectors, thus providing a versatile and modular route to multispectral or multimodal imaging agents.170
Figure 18.
An IEDDA-based approach to the synthesis of 225Ac-labeled radioimmunoconjugates.
Site-Specific Bioconjugation
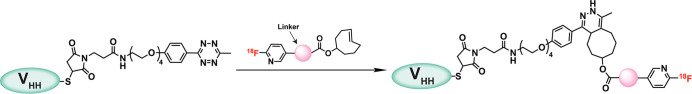
As we discussed above, the ability to attach radionuclides to specific sites within a biomolecule can be absolutely essential to ensuring the efficacy of a radiopharmaceutical. Not surprisingly, the IEDDA reaction has played an increasingly important role here as well. For example, Zhou et al. modified the C-terminal cysteine of a HER2-targeted VHH with a maleimide-bearing tetrazine and subsequently used the IEDDA reaction to install a TCO-functionalized prosthetic group containing both a 18F-labeled residualizing moiety and a renal brush border enzyme-cleavable linker (Figure 19).194 More specifically, the 18F-labeled protein was produced by adding a solution of Tz-modified 5F7GGC in PBS to a vial containing dried [18F]F-TCO and incubating the mixture for 10 min at 37 °C, which ultimately yielded the product in an overall radiochemical yield of 9% after gel filtration. The probe, [18F]F-5F7GGC, effectively visualized tumor tissue in a murine model of breast cancer and exhibited much more rapid renal clearance (and thus higher tumor-to-kidney activity concentration ratios) than an analogous probe without the cleavable linker. This technology has also been used to create well-defined dual modal tracers. Along these lines, Zettlitz et al. modified the terminal cysteines of a prostate stem cell antigen (PSCA)-targeting cys-diabody with a maleimide-bearing “dual modality linker” containing both a tetrazine and a near-infrared fluorophore. The investigators then reacted this Tz-bearing immunoconjugate with an 18F-labeled TCO, thereby producing a probe capable of multimodal PET/NIRF imaging in a murine model of prostate cancer.195 More recently, a European team used a tetrazine doubly functionalized with DTPA and an NIR fluorophore to modify an epidermal growth factor receptor (EGFR)-targeted VHH bearing a BCN moiety attached to its C-terminal cysteine. This multifunctional yet well-defined immunoconjugate was then labeled with [111In]In3+ to yield a probe capable of SPECT, NIRF imaging, and photodynamic therapy.196 Finally, several other groups have ably harnessed the IEDDA ligation for the site-specific radiolabeling of biomolecules, including probes based on peptides,186 proteins,197,198 affibody molecules,199 and even nanoparticles.200
Figure 19.
Harnessing the IEDDA reaction to site-specifically modify a HER2-targeted VHH with a 18F-labeling residualizing prosthetic group containing a renal brush border enzyme-cleavable linker.
In Vivo Pretargeting
On some level, the preceding paragraphs have felt like a preamble to our discussion of the area in which the IEDDA ligation has had its single largest impact on the radiopharmaceutical sciences: in vivo pretargeting. The reaction’s unique combination of speed and bioorthogonality makes it nearly ideal for in vivo chemistry, and pretargeting strategies based on the IEDDA ligation have been the subject of a great deal of work since Rossin and Robillard’s pioneering report in 2010.201 Because TCO exhibits greater in vivo stability than Tz, the overwhelming majority of IEDDA-based pretargeting strategies employ a TCO-modified immunoconjugate alongside a Tz-bearing radioligand (Figure 20).202 Since our last review, a remarkable surge in the field has yielded pretargeting systems featuring radioligands labeled with gamma-emitters (i.e., 99mTc and 111In),203−212 β+-emitters (i.e., 68Ga, 18F, 64Cu, 44Sc, and 89Zr),135,213−231 β–-emitters (i.e., 177Lu and 67Cu),232−237 and α-emitters (i.e., 212Pb, 211At, and 225Ac).238,239
Figure 20.
Schematic of IEDDA-based in vivo pretargeting. This image was reproduced with permission from ref (240). Copyright 2018 American Chemical Society.
While the earliest reports on IEDDA-based pretargeting focused primarily on proof-of-concept, a significant body of recent work has been dedicated to optimizing this approach to imaging and therapy. Teams in both the United States and Europe, for example, have worked to identify the parameters that make for the best Tz-based radioligands and—fortuitously—came to similar conclusions.224,241 In 2021, Steen et al. used an extensive library of 18F-labeled Tz radioligands to determine that high IEDDA reaction rates and low lipophilicity are both essential traits of an effective radioligand.224 Alternative approaches to maximizing tumoral activity concentrations have also been investigated. Summer et al. explored the use of radioligands bearing multiple Tz moieties and found that constructs with more reactive groups exhibited increased in vivo ligation yields but also heightened uptake in healthy, non-target tissues.242 Another strategy sought similar gains by increasing the number of TCOs attached to the mAb. Cook et al. synthesized immunoconjugates bearing TCO-functionalized dendrimers that brought the degree of labeling (DOL) up to 8 TCO/mAb. In a murine model of colorectal carcinoma, pretargeted PET with the dendrimer-bearing immunoconjugate produced significantly higher tumoral activity concentrations than an analogous immunoconjugate with a DOL of 2 TCO/mAb; unfortunately, however, the former also produced higher non-specific uptake in several healthy tissues. Other laboratories have worked to expand the utility of IEDDA-based pretargeting by combining it with other modalities. In a “double-click” approach, Adumeau et al. used the SPAAC reaction to append fluorophores to the heavy chain glycans of the huA33 antibody and then modified the immunoconjugate with TCOs, ultimately yielding a multimodal platform for pretargeted PET and NIRF imaging.225
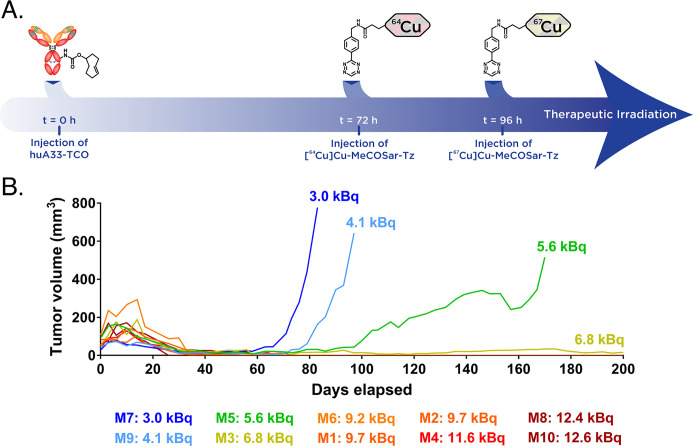
Even amongst these advances, arguably the most promising recent work in the field has been centered on pretargeted radioimmunotherapy (PRIT). Indeed, the pharmacokinetic advantages of pretargeting are critical in the context of radiopharmaceutical therapy, as the radiation dose rates to healthy tissues associated with traditional radioimmunoconjugates are even more problematic with therapeutic radionuclides than with their diagnostic cousins. The earliest report on the efficacy of IEDDA-based PRIT emerged in 2017 when Houghton et al. described the use of a system based on a TCO-modified immunoconjugate of the hu5B1 antibody (hu5B1-TCO) alongside a [177Lu]Lu-DOTA-PEG7-Tz radioligand to produce a dose-dependent therapeutic response in a murine model of pancreatic cancer.232 The most exciting extant work on IEDDA-based PRIT, however, has focused on α-emitters. Shah et al. were the first to report an IEDDA-based approach to αPRIT in late 2017 when they desribed a system predicated on a [212Pb]Pb-DOTA-Tz radioligand and a TAG72-targeting CC49-TCO immunoconjugate that significantly extended median overall survival in a murine model of colorectal carcinoma.238 More recently, an 225Ac-based approach to PRIT based on 5B1-TCO and 225Ac-DOTA-PEG7-Tz yielded very promising results in a murine model of pancreatic cancer, boasting a tumoral dose rate (1994 Gy/MBq) and a tumor-to-blood therapeutic index (24.1) higher than that of a traditional 225Ac-labeled radioimmunoconjugate (1377 Gy/MBq and 11.4, respectively).239 Finally, a theranostic approach to IEDDA-based PRIT based on the radioisotopic pair of 64Cu and 67Cu was recently developed in a murine model of colorectal cancer (Figure 21).237 Here, the initial administration of a TCO-bearing immunoconjugate (i.e., huA33-TCO) was followed by the injection of not one but two radioligands: first, a 64Cu-labeled probe for theranostic PET and then, 24 h later, a 67Cu-labeled Tz for therapy. Ultimately, the investigators found that the PET-derived tumoral uptake of the 64Cu-labeled Tz was predictive of response to therapy to the 67Cu-labeled radioligand.
Figure 21.
An IEDDA-based approach to theranostic pretargeting featuring 64Cu and 67Cu. (A) Temporal schematic of the sequential administration of two different tetrazine radioligands—[64Cu]Cu-MeCOSar-Tz and [67Cu]Cu-MeCOSar-Tz—after the injection of huA33-TCO; (B) plot illustrating the correlation between the activity concentration of the 64Cu-labeled Tz in the tumor as determined via PET and the subsequent response to therapy with the 67Cu-labeled Tz. This image was reproduced with permission under Creative Commons License CC BY-NC-ND 4.0 from ref (237). Published 2020 by National Academy of Sciences.
Despite the undeniable promise of IEDDA-based pretargeting, one critical (and elusive) step remains: clinical translation. Concerns over the feasibility of in vivo Tz/TCO ligations in humans were allayed somewhat by a recent study demonstrating the efficacy of pretargeted PET in large companion dogs with osteosarcoma, but this (of course) is no substitute for data from human patients.243 Thankfully, we will likely see some clinical results soon, as a trial focused on pretargeted PET with 5B1-TCO and [64Cu]Cu-SarAr-Tz in patients with pancreatic cancer (NCT05737615) has begun enrollment at Memorial Sloan Kettering Cancer Center.
Emergent Click Reactions
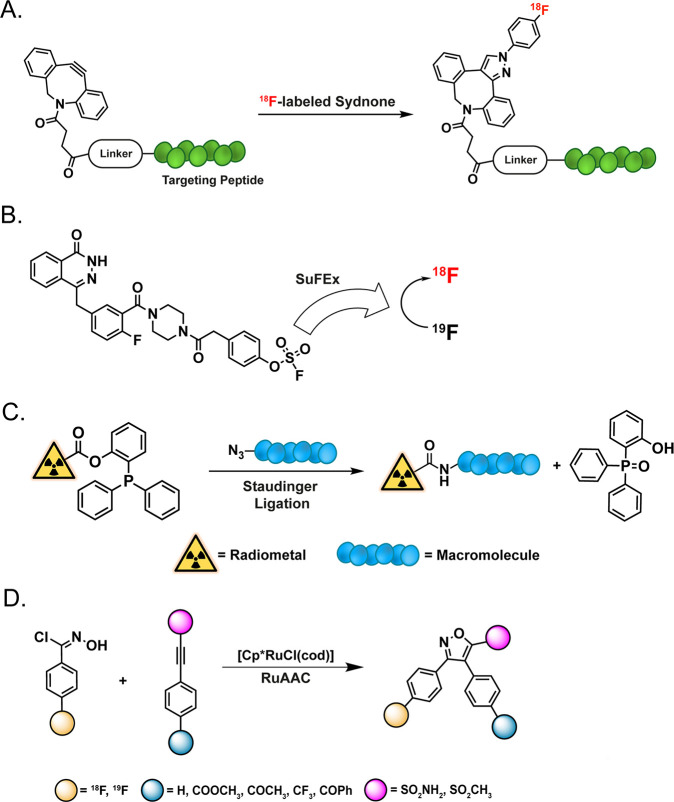
While the overwhelming majority of work at the intersection of radiochemistry and click chemistry has relied upon the CuAAC, SPAAC, and IEDDA ligations, a handful of other click reactions have also been leveraged for radiopharmaceutical chemistry.244 Just as we have seen above, radiofluorination has been a particularly common proving ground. Several groups have leveraged the strain-promoted sydnone–alkyne cycloaddition (SPSAC) for radiofluorination. For example, Narayanam et al. used this faster cousin of the SPAAC ligation to label a DIBAC-functionalized CD8-targeting peptide with 4-[18F]fluorophenyl sydnone (Figure 22A).245−247 While the synthetic strategy was no doubt effective, it should be noted that the SPSAC reaction’s sizable “footprint” has the potential to create hydrophobicity-related problems, especially in the context of smaller vectors. Interestingly, similar chemistry has also been applied to in vivo pretargeting, though with admittedly meager results.248 A fluoride-specific variant of click chemistry—dubbed sulfur–fluoride exchange (SuFEx) click chemistry—has, not surprisingly, also been used for radiofluorination. In a 2021 report in the Journal of the American Chemical Society, Zheng et al. describe the labeling of a diverse panel of aryl fluorosulfates with 18F via isotope exchange (Figure 22B).249 Impressively, this reaction is extremely rapid at room temperature and tolerant of a wide variety of reaction conditions and functional groups. Furthermore, it produces compounds in high radiochemical yields and molar activities with only cartridge-based purifications, underscoring its potential as a “next-generation” radiofluorination method. Still more groups have focused on 18F-labeling via the family of Staudinger reactions. While some have turned to labeling with the traceless Staudinger ligation between azides and phosphanes because it leaves no “footprint” in its wake, others have worked to optimize (and, critically, automate) the synthesis of 18F-labeled amines via the phosphine-mediated Staudinger reduction of azides (Figure 22C).250,251 Finally, Roscales et al. used the ruthenium-promoted 1,3-dipolar cycloaddition reaction between an 18F-labeled aromatic nitrile oxide—4-[18F]fluoro-N-hydroxybenzimidoyl chloride—and 4-ethynylbenzenesulfonamide to create a radiofluorinated variant of valdecoxib, a potent cyclooxygenase-2 inhibitor (Figure 22D).252 While this transformation likely does not have the broad applicability of radiofluorination via the CuAAC reaction, it could nonetheless be useful for the synthesis of 18F-labeled heterocycles.
Figure 22.
Approaches to radiofluorination based on (A) the strain-promoted sydnone–alkyne (SPSA) cycloaddition, (B) sulfur–fluoride exchange click chemistry (SuFEx), (C) the traceless Staudinger ligation, and (D) the ruthenium-promoted 1,3-dipolar cycloaddition.
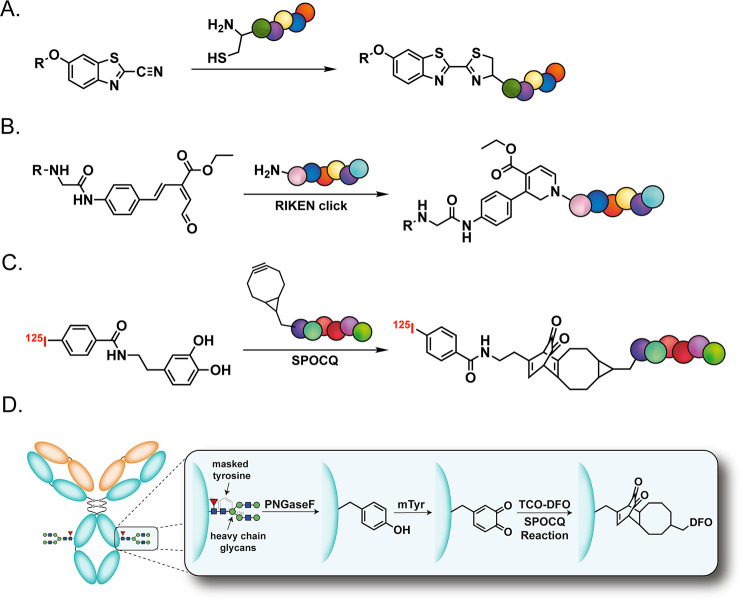
Of course, the use of these emergent click ligations has not been limited to fluorine-18. In 2020, for example, Chen et al. reported on the use of the click reaction between 2-cyanobenzothiazole and 1,2-aminothiol for the facile synthesis of chelator-bearing peptides that could be labeled with [68Ga]Ga3+ and [111In]In3+ (Figure 23A).253,254 Moving on, a Japanese team has continued to exploit the so-called RIKEN reaction—i.e., the mild and rapid 6π-azaelectrocyclization between α,β,γ,∂-unsaturated aldehydes and primary amines—for the construction of radiopharmaceuticals.255−257 Indeed, this transformation was used extensively alongside the SPAAC and IEDDA reactions in “double-click” strategies designed to append chelators (for 68Ga, 111In, and 67Cu) and boron clusters (for radiolabeling with 211At) to proteins and immunoglobulins (Figure 23B). It should be noted, however, that this reaction’s dependence on primary imines inevitably limits its site-specificity and -selectivity in many biological contexts. Finally, two different groups have turned to the strain-promoted oxidation-controlled quinone (SPOCQ) cycloaddition to aid in the synthesis of radiolabeled biomolecules. In one case, a team from South Korea derived an approach to radioiodination based on the reaction between a trio of BCN-functionalized biomolecules—c(RGDyK), human transferrin, and human serum albumin—with an 125I-labeled 1,2-catechol in the presence of an oxidant (Figure 23C).258 More recently, Rodriguez et al. have used the SPOCQ reaction in a chemoenzymatic approach to the site-selective radiolabeling of antibodies. Here, the heavy chain glycans of the mAb were first removed with PNGaseF, which facilitated the selective oxidation of a quartet of previously hidden tyrosine residues (Y296 and Y300) by mushroom tyrosinase.259 The resultant quinones were then reacted with a TCO-bearing variant of DFO, yielding a site-selectively modified immunoconjugate that was subsequently labeled with [89Zr]Zr4+ in a radiochemical yield of >95%. Ultimately, this approach offers a streamlined and less expensive alternative to extant methods for the chemoenzymatic modification of the Fc regions of full-length antibodies (Figure 23D).
Figure 23.
Strategies for the construction of radiopharmaceuticals based on (A) the click ligation between 2-cyanobenzothiazole and 1,2-aminothiol, (B) the RIKEN reaction, and (C,D) the strain-promoted oxidation-controlled quinone cycloaddition. The chains of colored circles in (A,B,C) represent biomolecules. Panel (D) was reproduced with permission under Creative Commons License CC BY 3.0 from ref (259). Published 2023 by Royal Society of Chemistry.
The Future
Perhaps above all else, the preceding pages serve to illustrate the sheer volume of work at the intersection of click chemistry and radiopharmaceutical chemistry since our last review in 2016. In many ways, the collaboration between these two disciplines has advanced considerably: the CuAAC and SPAAC ligations have become workhorses, the IEDDA reaction has progressed from fringe technique to reliable tool, and several emergent chemistries have been successfully harnessed for the construction of probes. Yet in other ways, we still have a long way to go. This is particularly true in the context of clinical translation. Several click-based radiopharmaceuticals have reached the clinic and produced promising results, most notably [18F]F-RGD-K5, [18F]F-fluoroethyltriazole-Tyr3-octreotate, and [68Ga]Ga-Trivehexin. Yet it is hard to escape the sense that a handful of clinical trials is a sparing representation of the impressive amount of preclinical work that has been done at the nexus of these two fields. It follows that an important priority in the near future—at least as we see it—must be expanding the impact of click chemistry on clinical nuclear medicine. The key will be pushing for translation in areas in which the use of click chemistry is not simply possible but rather essential. Put another way, we must identify the scenarios in which click chemistry truly adds value. Along these lines, we contend that automation,249,260,261 site-specific bioconjugation,143,262in vivo pretargeting,224,237 multimerization,98,263 and probe purification79,264 are the areas in which click chemistry has the best chance to improve clinical nuclear medicine. Novel click chemistries that are particularly well suited to radiosynthesis (e.g. SuFEx chemistry249,265 and the creation of [211At]astatotriazoles via the CuAAC ligation80) could also prove valuable in the clinic. In the end, it is our sincere hope that when we sit down to write the third installment of this review in several years, we will find that we must cover not only a wealth of basic science and preclinical innovations but also a host of new clinical agents that underscore how the combination of these two technologies has improved patient care.
Acknowledgments
We are grateful for generous support from the NIH (R01CA240963 and R01CA244327 to B.M.Z.; R01CA204167 and U01CA221046 to B.M.Z. and J.S.L.; and R35CA232130 to J.S.L.) and the Tow Foundation Fellowship Program (D.B.). The MSK Radiochemistry and Molecular Imaging Probes Core Facility and the MSK Small Animal Imaging Facility were supported in part by NIH P30 CA08748.
Author Contributions
¶ These authors contributed equally to this work.
The authors declare no competing financial interest.
This paper was publsished ASAP on September 22, 2023, with an error in Figure 1. This was corrected in the version published ASAP on October 13, 2023.
References
- Moses J. E.; Moorhouse A. D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36 (8), 1249–1262. 10.1039/B613014N. [DOI] [PubMed] [Google Scholar]
- Kaur J.; Saxena M.; Rishi N. An Overview of Recent Advances in Biomedical Applications of Click Chemistry. Bioconjug Chem. 2021, 32 (8), 1455–1471. 10.1021/acs.bioconjchem.1c00247. [DOI] [PubMed] [Google Scholar]
- Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective ″ligation″ of azides and terminal alkynes. Angew. Chem., Int. Ed. Engl. 2002, 41 (14), 2596–2599. . [DOI] [PubMed] [Google Scholar]
- Tornoe C. W.; Christensen C.; Meldal M. Peptidotriazoles on solid phase: [1, 2, 3]-triazoles by regiospecific copper(I)-catalyzed 1, 3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67 (9), 3057–3064. 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- Agard N. J.; Prescher J. A.; Bertozzi C. R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126 (46), 15046–15047. 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- Blackman M. L.; Royzen M.; Fox J. M. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc. 2008, 130 (41), 13518–9. 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon E.; Bertozzi C. R. Cell surface engineering by a modified Staudinger reaction. Science 2000, 287 (5460), 2007–2010. 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- The Nobel Prize in Chemistry 2022. NobelPrize.org; Nobel Prize Outreach AB 2023, 2023. https://www.nobelprize.org/prizes/chemistry/2022/summary (accessed 2023-05-17).
- Bauer D.; Sarrett S. M.; Lewis J. S.; Zeglis B. M. Click chemistry: a transformative technology in nuclear medicine. Nat. Protoc 2023, 18, 1659–1668. 10.1038/s41596-023-00825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeglis B. M.; Lewis J. S. Click Here for Better Chemistry. N Engl J. Med. 2022, 387 (24), 2291–2293. 10.1056/NEJMcibr2213596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindt T. L.; Struthers H.; Brans L.; Anguelov T.; Schweinsberg C.; Maes V.; Tourwe D.; Schibli R. ″Click to chelate″: synthesis and installation of metal chelates into biomolecules in a single step. J. Am. Chem. Soc. 2006, 128 (47), 15096–15097. 10.1021/ja066779f. [DOI] [PubMed] [Google Scholar]
- Meyer J. P.; Adumeau P.; Lewis J. S.; Zeglis B. M. Click Chemistry and Radiochemistry: The First 10 Years. Bioconjug Chem. 2016, 27 (12), 2791–2807. 10.1021/acs.bioconjchem.6b00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamat C.; Ramenda T.; Wuest F. Recent applications of click chemistry for the synthesis of radiotracers for molecular imaging. Mini-Reviews in Organic Chemistry 2009, 6 (1), 21–34. 10.2174/157019309787316148. [DOI] [Google Scholar]
- Nwe K.; Brechbiel M. W. Growing applications of ″click chemistry″ for bioconjugation in contemporary biomedical research. Cancer Biother Radiopharm 2009, 24 (3), 289–302. 10.1089/cbr.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven M.; Seimbille Y.; Dalm S. U. Therapeutic Applications of Pretargeting. Pharmaceutics 2019, 11 (9), 434. 10.3390/pharmaceutics11090434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq S.; Yun S. J.; Jeon J. Recent Advances in Bioorthogonal Click Chemistry for Efficient Synthesis of Radiotracers and Radiopharmaceuticals. Molecules 2019, 24 (19), 3567. 10.3390/molecules24193567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X.; Yan J.; Ding X.; Su C.; Xu Y.; Yang M. Recent Advances in Bioorthogonal Click Chemistry for Enhanced PET and SPECT Radiochemistry. Bioconjug Chem. 2023, 34 (3), 457–476. 10.1021/acs.bioconjchem.2c00583. [DOI] [PubMed] [Google Scholar]
- Kolb H. C.; Finn M. G.; Sharpless K. B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem., Int. Ed. Engl. 2001, 40 (11), 2004–2021. . [DOI] [PubMed] [Google Scholar]
- Abreu Diaz A. M.; Drumeva G. O.; Petrenyov D. R.; Carrier J. F.; DaSilva J. N. Synthesis of the Novel AT(1) Receptor Tracer [18F]Fluoropyridine-Candesartan via Click Chemistry. ACS Omega 2020, 5 (32), 20353–20362. 10.1021/acsomega.0c02310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P.; Han Y.; Zhu Y.; Hu K.; Huang S.; Tan J.; Wang M.; Wu H.; Tang G. Radiosynthesis and biological evaluation of fluorine-18 labeled N-acetylgalactosamine derivative [18F]FPGalNAc for PET imaging of asialoglycoprotein receptor-positive tumors. Nucl. Med. Biol. 2020, 88–89, 1–9. 10.1016/j.nucmedbio.2020.06.003. [DOI] [PubMed] [Google Scholar]
- Hein J. E.; Fokin V. V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39 (4), 1302–1315. 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann C. W.; Goh Y. W.; Parslow A. C.; Rigopoulos A.; Guo N.; Scott A. M.; Ackermann U.; White J. M. Synthesis and validation of [18F]mBPET-1, a fluorine-18 labelled mTOR inhibitor derivative based on a benzofuran backbone. EJNMMI Radiopharm Chem. 2020, 5 (1), 3. 10.1186/s41181-020-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra P.; Kaur R.; Singh G.; Singh H.; Singh G.; Pawan; Kaur G.; Singh J. Metals as “Click” catalysts for alkyne-azide cycloaddition reactions: An overview. J. Organomet. Chem. 2021, 944, 121846. 10.1016/j.jorganchem.2021.121846. [DOI] [Google Scholar]
- Nebra N.; Garcia-Alvarez J. Recent Progress of Cu-Catalyzed Azide-Alkyne Cycloaddition Reactions (CuAAC) in Sustainable Solvents: Glycerol, Deep Eutectic Solvents, and Aqueous Media. Molecules 2020, 25 (9), 2015. 10.3390/molecules25092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama Y.; Kusamori K.; Nishikawa M. Click Chemistry as a Tool for Cell Engineering and Drug Delivery. Molecules 2019, 24 (1), 172. 10.3390/molecules24010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommidi H.; Guo H.; Nurili F.; Vedvyas Y.; Jin M. M.; McClure T. D.; Ehdaie B.; Sayman H. B.; Akin O.; Aras O.; Ting R. 18 F-Positron Emitting/Trimethine Cyanine-Fluorescent Contrast for Image-Guided Prostate Cancer Management. J. Med. Chem. 2018, 61 (9), 4256–4262. 10.1021/acs.jmedchem.8b00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An F. F.; Kommidi H.; Chen N.; Ting R. A Conjugate of Pentamethine Cyanine and 18 F as a Positron Emission Tomography/Near-Infrared Fluorescence Probe for Multimodality Tumor Imaging. Int. J. Mol. Sci. 2017, 18 (6), 1214. 10.3390/ijms18061214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz M. B.; Renner A. M.; Ilyas S.; Lê K.; Guliyev M.; Krapf P.; Neumaier B.; Mathur S. 18F-Labeled magnetic nanovectors for bimodal cellular imaging. Biomater Sci. 2021, 9 (13), 4717–4727. 10.1039/D1BM00616A. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Liu H.; Yao D.; Li J.; Yang S.; Zhang C.; Chen W.; Wang D. 18F-labeled magnetic nanoparticles for monitoring anti-angiogenic therapeutic effects in breast cancer xenografts. J. Nanobiotechnology 2019, 17 (1), 105. 10.1186/s12951-019-0534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L.; Xiong C.; Zhou M.; Shi S.; Chow D. S.; Li C. Integrin αvβ3-Targeted [64Cu]CuS Nanoparticles for PET/CT Imaging and Photothermal Ablation Therapy. Bioconjug Chem. 2018, 29 (12), 4062–4071. 10.1021/acs.bioconjchem.8b00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y.; Xiao J.; Cong Y.; Liu J.; He Y.; Ross B. D.; Xu H.; Yin Y.; Hong H.; Xu W. PEGylated Nanoscale Metal-Organic Frameworks for Targeted Cancer Imaging and Drug Delivery. Bioconjug Chem. 2021, 32 (10), 2195–2204. 10.1021/acs.bioconjchem.1c00368. [DOI] [PubMed] [Google Scholar]
- Wagener K.; Worm M.; Pektor S.; Schinnerer M.; Thiermann R.; Miederer M.; Frey H.; Rösch F. Comparison of Linear and Hyperbranched Polyether Lipids for Liposome Shielding by 18 F-Radiolabeling and Positron Emission Tomography. Biomacromolecules 2018, 19 (7), 2506–2516. 10.1021/acs.biomac.8b00115. [DOI] [PubMed] [Google Scholar]
- Lin D.; Wallace M.; Allentoff A. J.; Donnelly D. J.; Gomes E.; Voronin K.; Gong S.; Huang R. Y.; Kim H.; Caceres-Cortes J.; Bonacorsi S. Jr Chemoselective Methionine Bioconjugation: Site-Selective Fluorine-18 Labeling of Proteins and Peptides. Bioconjug Chem. 2020, 31 (8), 1908–1916. 10.1021/acs.bioconjchem.0c00256. [DOI] [PubMed] [Google Scholar]
- Presolski S. I.; Hong V. P.; Finn M. G. Copper-Catalyzed Azide-Alkyne Click Chemistry for Bioconjugation. Curr. Protoc Chem. Biol. 2011, 3 (4), 153–162. 10.1002/9780470559277.ch110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms J.; Kogler J.; Maschauer S.; Daniel C.; Schmidkonz C.; Kuwert T.; Prante O. Targeting Fibroblast Activation Protein: Radiosynthesis and Preclinical Evaluation of an 18F-Labeled FAP Inhibitor. J. Nucl. Med. 2020, 61 (12), 1806–1813. 10.2967/jnumed.120.242958. [DOI] [PubMed] [Google Scholar]
- Litchfield M.; Wuest M.; Glubrecht D.; Wuest F. Radiosynthesis and Biological Evaluation of [18 F]Triacoxib: A New Radiotracer for PET Imaging of COX-2. Mol. Pharmaceutics 2020, 17 (1), 251–261. 10.1021/acs.molpharmaceut.9b00986. [DOI] [PubMed] [Google Scholar]
- Li L.; Xing X.; Zhang C.; Zhu A.; Fan X.; Chen C.; Zhang G. Novel synthesis of 5-iodo-1, 2, 3-triazoles using an aqueous iodination system under air. Tetrahedron Lett. 2018, 59 (39), 3563–3566. 10.1016/j.tetlet.2018.08.039. [DOI] [Google Scholar]
- Zhang X.; Basuli F.; Abdelwahed S.; Begley T.; Swenson R. Radiosynthesis of 5-[18F]Fluoro-1, 2, 3-triazoles through Aqueous Iodine-[18F]Fluorine Exchange Reaction. Molecules 2021, 26 (18), 5522. 10.3390/molecules26185522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenberg V.; Hermann S.; Galla F.; Schafers M.; Wunsch B.; Kolb H. C.; Szardenings K.; Lebedev A.; Walsh J. C.; Mocharla V. P.; Gangadharmath U. B.; Kopka K.; Wagner S. Radiolabeled hydroxamate-based matrix metalloproteinase inhibitors: How chemical modifications affect pharmacokinetics and metabolic stability. Nucl. Med. Biol. 2016, 43 (7), 424–437. 10.1016/j.nucmedbio.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Kumar Sharma A.; Satpati D.; Sharma R.; Das A.; Dev Sarma H.; Mukherjee A. Targeting HER2-receptors with 177Lu-labeled triazole stapled cyclic peptidomimetic. Bioorg Chem. 2023, 135, 106503. 10.1016/j.bioorg.2023.106503. [DOI] [PubMed] [Google Scholar]
- Grob N. M.; Schibli R.; Behe M.; Valverde I. E.; Mindt T. L. 1, 5-Disubstituted 1, 2, 3-Triazoles as Amide Bond Isosteres Yield Novel Tumor-Targeting Minigastrin Analogs. ACS Med. Chem. Lett. 2021, 12 (4), 585–592. 10.1021/acsmedchemlett.0c00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras M.; Testa A.; Fleming I. N.; Dall’Angelo S.; Andriu A.; Menta S.; Mori M.; Brown G. D.; Forster D.; Williams K. J.; Zanda M. High-Affinity ″Click″ RGD Peptidomimetics as Radiolabeled Probes for Imaging αv β3 Integrin. ChemMedChem. 2017, 12 (14), 1142–1151. 10.1002/cmdc.201700328. [DOI] [PubMed] [Google Scholar]
- Cai Z.; Mason N. S.; Anderson C. J.; Edwards W. B. Synthesis and preliminary evaluation of an 18F-labeled oleic acid analog for PET imaging of fatty acid uptake and metabolism. Nucl. Med. Biol. 2016, 43 (1), 108–115. 10.1016/j.nucmedbio.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Amor-Coarasa A.; Kelly J. M.; Singh P. K.; Ponnala S.; Nikolopoulou A.; Williams C. Jr; Vedvyas Y.; Jin M. M.; Warren J. D.; Babich J. W. [18 F]Fluoroethyltriazolyl Monocyclam Derivatives as Imaging Probes for the Chemokine Receptor CXCR4. Molecules 2019, 24 (8), 1612. 10.3390/molecules24081612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potemkin R.; Strauch B.; Kuwert T.; Prante O.; Maschauer S. Development of 18F-Fluoroglycosylated PSMA-Ligands with Improved Renal Clearance Behavior. Mol. Pharmaceutics 2020, 17 (3), 933–943. 10.1021/acs.molpharmaceut.9b01179. [DOI] [PubMed] [Google Scholar]
- Kwon D.; Lozada J.; Zhang Z.; Zeisler J.; Poon R.; Zhang C.; Roxin Á.; Lin K. S.; Perrin D.; Benard F. High-Contrast CXCR4-Targeted 18F-PET Imaging Using a Potent and Selective Antagonist. Mol. Pharmaceutics 2021, 18 (1), 187–197. 10.1021/acs.molpharmaceut.0c00785. [DOI] [PubMed] [Google Scholar]
- Mikkola K.; Yim C. B.; Lehtiniemi P.; Kauhanen S.; Tarkia M.; Tolvanen T.; Nuutila P.; Solin O. Low kidney uptake of GLP-1R-targeting, beta cell-specific PET tracer, 18F-labeled [Nle 14, Lys40]exendin-4 analog, shows promise for clinical imaging. EJNMMI Res. 2016, 6 (1), 91. 10.1186/s13550-016-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G.; Zhang H.; Jacobson O.; Wang Z.; Chen H.; Yang X.; Niu G.; Chen X. Combinatorial Screening of DNA Aptamers for Molecular Imaging of HER2 in Cancer. Bioconjug Chem. 2017, 28 (4), 1068–1075. 10.1021/acs.bioconjchem.6b00746. [DOI] [PubMed] [Google Scholar]
- Kelly J.; Amor-Coarasa A.; Nikolopoulou A.; Kim D.; Williams C. Jr; Ponnala S.; Babich J. W. Synthesis and pre-clinical evaluation of a new class of high-affinity 18F-labeled PSMA ligands for detection of prostate cancer by PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44 (4), 647–661. 10.1007/s00259-016-3556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H. T.; Pan J.; Lau J.; Zhang C.; Zeisler J.; Colpo N.; Bénard F.; Lin K. S. Radiolabeled R954 Derivatives for Imaging Bradykinin B1 Receptor Expression with Positron Emission Tomography. Mol. Pharmaceutics 2017, 14 (3), 821–829. 10.1021/acs.molpharmaceut.6b01055. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Devoogdt N.; Zalutsky M. R.; Vaidyanathan G. An Efficient Method for Labeling Single Domain Antibody Fragments with (18)F Using Tetrazine- Trans-Cyclooctene Ligation and a Renal Brush Border Enzyme-Cleavable Linker. Bioconjug Chem. 2018, 29 (12), 4090–4103. 10.1021/acs.bioconjchem.8b00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.; Jacobson O.; Zhu G.; Chen Z.; Liang S. H.; Tian R.; Yang Z.; Niu G.; Zhu X.; Chen X. PET imaging of EGFR expression using an 18F-labeled RNA aptamer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46 (4), 948–956. 10.1007/s00259-018-4105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Lisok A.; Chatterjee S.; Wharram B.; Pullambhatla M.; Wang Y.; Sgouros G.; Mease R. C.; Pomper M. G. [18F]Fluoroethyl Triazole Substituted PSMA Inhibitor Exhibiting Rapid Normal Organ Clearance. Bioconjug Chem. 2016, 27 (7), 1655–1662. 10.1021/acs.bioconjchem.6b00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss S. D.; Müller C.; Siwowska K.; Büchel J. I.; Schmid R. M.; Groehn V.; Schibli R.; Ametamey S. M. Reduced 18F-Folate Conjugates as a New Class of PET Tracers for Folate Receptor Imaging. Bioconjug Chem. 2018, 29 (4), 1119–1130. 10.1021/acs.bioconjchem.7b00775. [DOI] [PubMed] [Google Scholar]
- Denk C.; Wilkovitsch M.; Skrinjar P.; Svatunek D.; Mairinger S.; Kuntner C.; Filip T.; Fröhlich J.; Wanek T.; Mikula H. [18F]Fluoroalkyl azides for rapid radiolabeling and (Re)investigation of their potential towards in vivo click chemistry. Org. Biomol Chem. 2017, 15 (28), 5976–5982. 10.1039/C7OB00880E. [DOI] [PubMed] [Google Scholar]
- Jia L.; Li X.; Cheng D.; Zhang L. Fluorine-18 click radiosynthesis and microPET/CT evaluation of a small peptide-a potential PET probe for carbonic anhydrase IX. Bioorg. Med. Chem. 2019, 27 (5), 785–789. 10.1016/j.bmc.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Lu X.; Wang C.; Li X.; Gu P.; Jia L.; Zhang L. Synthesis and preliminary evaluation of 18F -icotinib for EGFR-targeted PET imaging of lung cancer. Bioorg. Med. Chem. 2019, 27 (3), 545–551. 10.1016/j.bmc.2018.12.034. [DOI] [PubMed] [Google Scholar]
- Schwegmann K.; Hohn M.; Hermann S.; Schäfers M.; Riemann B.; Haufe G.; Wagner S.; Breyholz H. J. Optimizing the Biodistribution of Radiofluorinated Barbiturate Tracers for Matrix Metalloproteinase Imaging by Introduction of Fluorescent Dyes as Pharmacokinetic Modulators. Bioconjug Chem. 2020, 31 (4), 1117–1132. 10.1021/acs.bioconjchem.9b00817. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Cai Z.; Li L.; Ropchan J.; Lim K.; Boutagy N. E.; Wu J.; Stendahl J. C.; Chu W.; Gropler R.; Sinusas A. J.; Liu C.; Huang Y. Optimized and Automated Radiosynthesis of [18F]DHMT for Translational Imaging of Reactive Oxygen Species with Positron Emission Tomography. Molecules 2016, 21 (12), 1696. 10.3390/molecules21121696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickute D.; Chen C.; Braga M.; Barnes C.; Wang N.; Allott L.; Aboagye E. O. Design, synthesis, and evaluation of a novel PET imaging agent targeting lipofuscin in senescent cells. RSC Adv. 2022, 12 (40), 26372–26381. 10.1039/D2RA04535D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum S.; Toms J.; Reshetnikov V.; Özkan H. G.; Hampel F.; Maschauer S.; Hakimioun A.; Beierlein F.; Sellner L.; Schmitt M.; Prante O.; Mokhir A. Identification of Boronic Acid Derivatives as an Active Form of N-Alkylaminoferrocene-Based Anticancer Prodrugs and Their Radiolabeling with 18F. Bioconjug Chem. 2019, 30 (4), 1077–1086. 10.1021/acs.bioconjchem.9b00019. [DOI] [PubMed] [Google Scholar]
- Toms J.; Reshetnikov V.; Maschauer S.; Mokhir A.; Prante O. Radiosynthesis of an 18F -fluoroglycosylated aminoferrocene for in-vivo imaging of reactive oxygen species activity by PET. J. Labelled Comp Radiopharm 2018, 61 (14), 1081–1088. 10.1002/jlcr.3687. [DOI] [PubMed] [Google Scholar]
- Collet C.; Maskali F.; Clément A.; Chrétien F.; Poussier S.; Karcher G.; Marie P. Y.; Chapleur Y.; Lamandé-Langle S. Development of 6-[18F]fluoro-carbohydrate-based prosthetic groups and their conjugation to peptides via click chemistry. J. Labelled Comp Radiopharm 2016, 59 (2), 54–62. 10.1002/jlcr.3362. [DOI] [PubMed] [Google Scholar]
- Maschauer S.; Einsiedel J.; Hübner H.; Gmeiner P.; Prante O. 18F- and 68Ga-Labeled Neurotensin Peptides for PET Imaging of Neurotensin Receptor 1. J. Med. Chem. 2016, 59 (13), 6480–6492. 10.1021/acs.jmedchem.6b00675. [DOI] [PubMed] [Google Scholar]
- Maschauer S.; Heilmann M.; Wängler C.; Schirrmacher R.; Prante O. Radiosynthesis and Preclinical Evaluation of 18F-Fluoroglycosylated Octreotate for Somatostatin Receptor Imaging. Bioconjug Chem. 2016, 27 (11), 2707–2714. 10.1021/acs.bioconjchem.6b00472. [DOI] [PubMed] [Google Scholar]
- Kang J.; Young Lee J.; Taş İ.; More K. N.; Kim H.; Park J. H.; Chang D. J. Radiosynthesis, biological evaluation and preliminary microPET study of 18F-labeled 5-resorcinolic triazolone derivative based on ganetespib targeting HSP90. Bioorg. Med. Chem. Lett. 2018, 28 (23–24), 3658–3664. 10.1016/j.bmcl.2018.10.035. [DOI] [PubMed] [Google Scholar]
- Lugato B.; Stucchi S.; Ciceri S.; Iannone M. N.; Turolla E. A.; Giuliano L.; Chinello C.; Todde S.; Ferraboschi P. A novel versatile precursor suitable for 18F-radiolabeling via ″click chemistry″. J. Labelled Comp Radiopharm 2017, 60 (10), 466–480. 10.1002/jlcr.3529. [DOI] [PubMed] [Google Scholar]
- Iannone M. N.; Stucchi S.; Turolla E. A.; Beretta C.; Ciceri S.; Chinello C.; Pagani L.; Todde S.; Ferraboschi P. Synthesis and automated fluorine-18 radiolabeling of new PSMA-617 derivatives with a CuAAC radiosynthetic approach. J. Labelled Comp Radiopharm 2022, 65 (3), 48–62. 10.1002/jlcr.3959. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Lee S. J.; Yook C. M.; Oh S. J.; Ryu J. S.; Lee J. J. Biological evaluation of new [18F]F-labeled synthetic amino acid derivatives as oncologic radiotracers. J. Labelled Comp Radiopharm 2016, 59 (10), 404–410. 10.1002/jlcr.3424. [DOI] [PubMed] [Google Scholar]
- Sun P.; Zhu Y.; Han Y.; Hu K.; Huang S.; Wang M.; Wu H.; Tang G. Radiosynthesis and biological evaluation of an fluorine-18 labeled galactose derivative [18F]FPGal for imaging the hepatic asialoglycoprotein receptor. Bioorg. Med. Chem. Lett. 2020, 30 (12), 127187. 10.1016/j.bmcl.2020.127187. [DOI] [PubMed] [Google Scholar]
- Sarasamkan J.; Fischer S.; Deuther-Conrad W.; Ludwig F. A.; Scheunemann M.; Arunrungvichian K.; Vajragupta O.; Brust P. Radiosynthesis of (S)-[18F]T1: The first PET radioligand for molecular imaging of α3β4 nicotinic acetylcholine receptors. Appl. Radiat. Isot. 2017, 124, 106–113. 10.1016/j.apradiso.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Weng J.; Lin J.; Ye D.; Zhang Y. NIR Scaffold Bearing Three Handles for Biocompatible Sequential Click Installation of Multiple Functional Arms. J. Am. Chem. Soc. 2020, 142 (6), 2787–2794. 10.1021/jacs.9b10467. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Pourghiasian M.; Radtke M. A.; Lau J.; Pan J.; Dias G. M.; Yapp D.; Lin K. S.; Benard F.; Perrin D. M. An organotrifluoroborate for broadly applicable one-step 18F-labeling. Angew. Chem., Int. Ed. Engl. 2014, 53 (44), 11876–11880. 10.1002/anie.201406258. [DOI] [PubMed] [Google Scholar]
- Walker D.; Li Y.; Roxin Á.; Schaffer P.; Adam M. J.; Perrin D. M. Facile synthesis and 18F -radiolabeling of α4β1-specific LLP2A-aryltrifluoroborate peptidomimetic conjugates. Bioorg. Med. Chem. Lett. 2016, 26 (20), 5126–5131. 10.1016/j.bmcl.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Bruton L.; Scott P. J. H. Automated Synthesis Modules for PET Radiochemistry. Handbook of Radiopharmaceuticals 2020, 437–456. 10.1002/9781119500575.ch13. [DOI] [Google Scholar]
- Krasikova R. Synthesis modules and automation in F-18 labeling. Ernst Schering Res. Found Workshop 2007, 64 (62), 289–316. 10.1007/978-3-540-49527-7_11. [DOI] [PubMed] [Google Scholar]
- Allott L.; Dubash S.; Aboagye E. O. [18F]FET-βAG-TOCA: The Design, Evaluation and Clinical Translation of a Fluorinated Octreotide. Cancers 2020, 12 (4), 865. 10.3390/cancers12040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannone M. N.; Stucchi S.; Turolla E. A.; Beretta C.; Ciceri S.; Chinello C.; Pagani L.; Todde S.; Ferraboschi P. Synthesis and automated fluorine-18 radiolabeling of new PSMA-617 derivatives with a CuAAC radiosynthetic approach. J. Labelled Comp Radiopharm 2022, 65 (3), 48–62. 10.1002/jlcr.3959. [DOI] [PubMed] [Google Scholar]
- Pisaneschi F.; Kelderhouse L. E.; Hardy A.; Engel B. J.; Mukhopadhyay U.; Gonzalez-Lepera C.; Gray J. P.; Ornelas A.; Takahashi T. T.; Roberts R. W.; Fiacco S. V.; Piwnica-Worms D.; Millward S. W. Automated, Resin-Based Method to Enhance the Specific Activity of Fluorine-18 Clicked PET Radiotracers. Bioconjug Chem. 2017, 28 (2), 583–589. 10.1021/acs.bioconjchem.6b00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk C.; Wilkovitsch M.; Aneheim E.; Herth M. M.; Jensen H.; Lindegren S.; Mikula H. Multifunctional Clickable Reagents for Rapid Bioorthogonal Astatination and Radio-Crosslinking. ChemPlusChem. 2019, 84 (7), 774. 10.1002/cplu.201900250. [DOI] [PubMed] [Google Scholar]
- Gao F.; Peng C.; Li J.; Zhuang R.; Guo Z.; Xu D.; Su X.; Zhang X. Radioiodinated progesterone derivative for progesterone receptor targeting with enhanced nucleus uptake via phenylboronic acid conjugation. J. Labelled Comp Radiopharm 2019, 62 (7), 301–309. 10.1002/jlcr.3741. [DOI] [PubMed] [Google Scholar]
- Yang R.; Wang D.; Chu T. Synthesis and bioevaluation of radioiodinated nitroimidazole hypoxia imaging agents by one-pot click reaction. Bioorg. Med. Chem. Lett. 2020, 30 (17), 127386. 10.1016/j.bmcl.2020.127386. [DOI] [PubMed] [Google Scholar]
- Yuan F.; Sun H.; Yang C.; Yang H.; Pan L.; Zhang X.; Tian R.; Li L.; Chen W.; Wu X.; Wu H. Difluorocarbene-derived rapid late-stage trifluoromethylation of 5-iodotriazoles for the synthesis of 18F-labeled radiotracers. Chin. Chem. Lett. 2023, 34 (6), 107960. 10.1016/j.cclet.2022.107960. [DOI] [Google Scholar]
- Vanasschen C.; Molnár E.; Tircsó G.; Kálmán F. K.; Tóth É.; Brandt M.; Coenen H. H.; Neumaier B. Novel CDTA-based, Bifunctional Chelators for Stable and Inert Mn(II) Complexation: Synthesis and Physicochemical Characterization. Inorg. Chem. 2017, 56 (14), 7746–7760. 10.1021/acs.inorgchem.7b00460. [DOI] [PubMed] [Google Scholar]
- Lee W.; Sarkar S.; Pal R.; Kim J. Y.; Park H.; Huynh P. T.; Bhise A.; Bobba K. N.; Kim K. I.; Ha Y. S.; Soni N.; Kim W.; Lee K.; Jung J. M.; Rajkumar S.; Lee K. C.; Yoo J. Successful Application of CuAAC Click Reaction in Constructing 64Cu-Labeled Antibody Conjugates for Immuno-PET Imaging. ACS Appl. Bio Mater. 2021, 4 (3), 2544–2557. 10.1021/acsabm.0c01555. [DOI] [PubMed] [Google Scholar]
- Makarem A.; Klika K. D.; Litau G.; Remde Y.; Kopka K. HBED-NN: A Bifunctional Chelator for Constructing Radiopharmaceuticals. J. Org. Chem. 2019, 84 (11), 7501–7508. 10.1021/acs.joc.9b00832. [DOI] [PubMed] [Google Scholar]
- Baranski A. C.; Schäfer M.; Bauder-Wüst U.; Wacker A.; Schmidt J.; Liolios C.; Mier W.; Haberkorn U.; Eisenhut M.; Kopka K.; Eder M. Improving the Imaging Contrast of 68Ga-PSMA-11 by Targeted Linker Design: Charged Spacer Moieties Enhance the Pharmacokinetic Properties. Bioconjug Chem. 2017, 28 (9), 2485–2492. 10.1021/acs.bioconjchem.7b00458. [DOI] [PubMed] [Google Scholar]
- Kaeopookum P.; Petrik M.; Summer D.; Klinger M.; Zhai C.; Rangger C.; Haubner R.; Haas H.; Hajduch M.; Decristoforo C. Comparison of 68Ga-labeled RGD mono- and multimers based on a clickable siderophore-based scaffold. Nucl. Med. Biol. 2019, 78–79, 1–10. 10.1016/j.nucmedbio.2019.09.002. [DOI] [PubMed] [Google Scholar]
- Notni J.; Reich D.; Maltsev O. V.; Kapp T. G.; Steiger K.; Hoffmann F.; Esposito I.; Weichert W.; Kessler H.; Wester H. J. In Vivo PET Imaging of the Cancer Integrin αvβ6 Using 68Ga-Labeled Cyclic RGD Nonapeptides. J. Nucl. Med. 2017, 58 (4), 671–677. 10.2967/jnumed.116.182824. [DOI] [PubMed] [Google Scholar]
- Notni J.; Steiger K.; Hoffmann F.; Reich D.; Kapp T. G.; Rechenmacher F.; Neubauer S.; Kessler H.; Wester H. J. Complementary, Selective PET Imaging of Integrin Subtypes α5β1 and αvβ3 Using 68Ga-Aquibeprin and 68Ga-Avebetrin. J. Nucl. Med. 2016, 57 (3), 460–466. 10.2967/jnumed.115.165720. [DOI] [PubMed] [Google Scholar]
- Quigley N. G.; Steiger K.; Richter F.; Weichert W.; Hoberück S.; Kotzerke J.; Notni J. Tracking a TGF-β activator in vivo: sensitive PET imaging of αvβ8-integrin with the Ga-68-labeled cyclic RGD octapeptide trimer Ga-68-Triveoctin. EJNMMI Res. 2020, 10 (1), 133. 10.1186/s13550-020-00706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley N. G.; Tomassi S.; Di Leva F. S.; Di Maro S.; Richter F.; Steiger K.; Kossatz S.; Marinelli L.; Notni J. Click-Chemistry (CuAAC) Trimerization of an αvβ6 Integrin Targeting Ga-68-Peptide: Enhanced Contrast for in-Vivo PET Imaging of Human Lung Adenocarcinoma Xenografts. Chembiochem 2020, 21 (19), 2836–2843. 10.1002/cbic.202000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzer A.; Pollmann J.; Schmidt A.; Reich D.; Wester H. J.; Notni J. Molar Activity of Ga-68 Labeled PSMA Inhibitor Conjugates Determines PET Imaging Results. Mol. Pharmaceutics 2018, 15 (9), 4296–4302. 10.1021/acs.molpharmaceut.8b00602. [DOI] [PubMed] [Google Scholar]
- Liu T.; Gan Q.; Zhang J. Macrocyclic triamine derived glucose analogues for 99mTc(CO)3 labeling: synthesis and biological evaluation as potential tumor-imaging agents. Chem. Biol. Drug Des 2017, 89 (2), 277–284. 10.1111/cbdd.12784. [DOI] [PubMed] [Google Scholar]
- Guo Z.; Gao M.; Song M.; Shi C.; Zhang P.; Xu D.; You L.; Zhuang R.; Su X.; Liu T.; Du J.; Zhang X. Synthesis and Evaluation of 99mTc-Labeled Dimeric Folic Acid for FR-Targeting. Molecules 2016, 21 (6), 817. 10.3390/molecules21060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hee C.; Ho D.; Karton A.; Nealon G.; Kretzmann J. A.; Norret M.; Iyer K. S. Macromolecular approach for targeted radioimmunotherapy in non-Hodgkin’s lymphoma. Chem. Commun. 2019, 55 (96), 14506–14509. 10.1039/C9CC06603A. [DOI] [PubMed] [Google Scholar]
- Reissig F.; Bauer D.; Zarschler K.; Novy Z.; Bendova K.; Ludik M. C.; Kopka K.; Pietzsch H. J.; Petrik M.; Mamat C. Towards Targeted Alpha Therapy with Actinium-225: Chelators for Mild Condition Radiolabeling and Targeting PSMA-A Proof of Concept Study. Cancers 2021, 13 (8), 1974. 10.3390/cancers13081974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley N. G.; Steiger K.; Hoberuck S.; Czech N.; Zierke M. A.; Kossatz S.; Pretze M.; Richter F.; Weichert W.; Pox C.; Kotzerke J.; Notni J. PET/CT imaging of head-and-neck and pancreatic cancer in humans by targeting the ″Cancer Integrin″ alphavbeta6 with Ga-68-Trivehexin. Eur. J. Nucl. Med. Mol. Imaging 2022, 49 (4), 1136–1147. 10.1007/s00259-021-05559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liolios C.; Schäfer M.; Haberkorn U.; Eder M.; Kopka K. Novel Bispecific PSMA/GRPr Targeting Radioligands with Optimized Pharmacokinetics for Improved PET Imaging of Prostate Cancer. Bioconjug Chem. 2016, 27 (3), 737–751. 10.1021/acs.bioconjchem.5b00687. [DOI] [PubMed] [Google Scholar]
- Radford L. L.; Papagiannopoulou D.; Gallazzi F.; Berendzen A.; Watkinson L.; Carmack T.; Lewis M. R.; Jurisson S. S.; Hennkens H. M. Synthesis and evaluation of Re/99mTc(I) complexes bearing a somatostatin receptor-targeting antagonist and labeled via a novel [N, S, O] clickable bifunctional chelating agent. Bioorg. Med. Chem. 2019, 27 (3), 492–501. 10.1016/j.bmc.2018.12.028. [DOI] [PubMed] [Google Scholar]
- Römhild K.; Fischer C. A.; Mindt T. L. Glycated 99mTc-Tricarbonyl-Labeled Peptide Conjugates for Tumor Targeting by ″Click-to-Chelate″. ChemMedChem. 2017, 12 (1), 66–74. 10.1002/cmdc.201600485. [DOI] [PubMed] [Google Scholar]
- Giammei C.; Balber T.; Benčurová K.; Cardinale J.; Berroterán-Infante N.; Brandt M.; Jouini N.; Hacker M.; Mitterhauser M.; Mindt T. L. Sorbitol as a Polar Pharmacological Modifier to Enhance the Hydrophilicity of 99mTc-Tricarbonyl-Based Radiopharmaceuticals. Molecules 2020, 25 (11), 2680. 10.3390/molecules25112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap S. Y.; Savoie H.; Renard I.; Burke B. P.; Sample H. C.; Michue-Seijas S.; Archibald S. J.; Boyle R. W.; Stasiuk G. J. Synthesis of a porphyrin with histidine-like chelate: an efficient path towards molecular PDT/SPECT theranostics. Chem. Commun. 2020, 56 (75), 11090–11093. 10.1039/D0CC03958F. [DOI] [PubMed] [Google Scholar]
- Das S.; Mathur A.; Sakhare N.; Mallia M. B.; Sarma H. D.; Sachdev S. S.; Dash A. Synthesis and biodistribution studies of 99mTc labeled fatty acid derivatives prepared via ″Click approach″ for potential use in cardiac imaging. J. Labelled Comp Radiopharm 2018, 61 (14), 1048–1057. 10.1002/jlcr.3681. [DOI] [PubMed] [Google Scholar]
- Tejería M. E.; Giglio J.; Dematteis S.; Rey A. Development and characterization of a 99mTc-tricarbonyl-labelled estradiol derivative obtained by ″Click Chemistry″ with potential application in estrogen receptors imaging. J. Labelled Comp Radiopharm 2017, 60 (11), 521–527. 10.1002/jlcr.3527. [DOI] [PubMed] [Google Scholar]
- Vats K.; Satpati D.; Sharma R.; Sarma H. D.; Banerjee S. Synthesis and comparative in vivo evaluation of 99mTc(CO)3-labeled PEGylated and non-PEGylated cRGDfK peptide monomers. Chem. Biol. Drug Des 2017, 89 (3), 371–378. 10.1111/cbdd.12844. [DOI] [PubMed] [Google Scholar]
- Vats K.; Sharma R.; Kameswaran M.; Sarma H. D.; Satpati D.; Dash A. Design, synthesis, and comparative evaluation of 99mTc(CO)3-labeled N-terminal and C-terminal modified asparagine-glycine-arginine peptide constructs. J. Pept Sci. 2019, 25 (7), e3192 10.1002/psc.3192. [DOI] [PubMed] [Google Scholar]
- Erfani M.; Malek H.; Sadat Ebrahimi S. E.; Hassanzadeh L. New 99mTc(CO)3-radiolabeled arylpiperazine pharmacophore as potent 5HT(1A) serotonin receptor radiotracer: Docking studies, chemical synthesis, radiolabeling, and biological evaluation. J. Labelled Comp Radiopharm 2019, 62 (4), 166–177. 10.1002/jlcr.3709. [DOI] [PubMed] [Google Scholar]
- Wharton L.; Zhang C.; Zeisler J.; Rodriguez-Rodriguez C.; Osooly M.; Radchenko V.; Yang H.; Lin K. S.; Benard F.; Schaffer P.; Orvig C. H3TPAN-Triazole-Bn-NH(2): Tripicolinate Clicked-Bifunctional Chelate for [225Ac]/[111In] Theranostics. Bioconjug Chem. 2022, 33 (12), 2381–2397. 10.1021/acs.bioconjchem.2c00465. [DOI] [PubMed] [Google Scholar]
- Murrell E.; Luyt L. G. Incorporation of Fluorine into an OBOC Peptide Library by Copper-Free Click Chemistry toward the Discovery of PET Imaging Agents. ACS Comb. Sci. 2020, 22 (3), 109–113. 10.1021/acscombsci.9b00146. [DOI] [PubMed] [Google Scholar]
- Kwon Y. D.; Lee J. Y.; La M. T.; Lee S. J.; Lee S. H.; Park J. H.; Kim H. K. Novel multifunctional 18F-labelled PET tracer with prostate-specific membrane antigen-targeting and hypoxia-sensitive moieties. Eur. J. Med. Chem. 2020, 189, 112099. 10.1016/j.ejmech.2020.112099. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Chitneni S. K.; Devoogdt N.; Zalutsky M. R.; Vaidyanathan G. Fluorine-18 labeling of an anti-HER2 VHH using a residualizing prosthetic group via a strain-promoted click reaction: Chemistry and preliminary evaluation. Bioorg. Med. Chem. 2018, 26 (8), 1939–1949. 10.1016/j.bmc.2018.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassner M.; Palmieri L.; Monnery B. D.; Verbrugghen T.; Deleye S.; Stroobants S.; Staelens S.; Wyffels L.; Hoogenboom R. The Label Matters: μPET Imaging of the Biodistribution of Low Molar Mass 89Zr and 18F-Labeled Poly(2-ethyl-2-oxazoline). Biomacromolecules 2017, 18 (1), 96–102. 10.1021/acs.biomac.6b01392. [DOI] [PubMed] [Google Scholar]
- Murrell E.; Kovacs M. S.; Luyt L. G. A Compact and Synthetically Accessible Fluorine-18 Labelled Cyclooctyne Prosthetic Group for Labelling of Biomolecules by Copper-Free Click Chemistry. ChemMedChem. 2018, 13 (16), 1625–1628. 10.1002/cmdc.201800334. [DOI] [PubMed] [Google Scholar]
- Choi M. H.; Shim H. E.; Nam Y. R.; Kim H. R.; Kang J. A.; Lee D. E.; Park S. H.; Choi D. S.; Jang B. S.; Jeon J. Synthesis and evaluation of an 125I-labeled azide prosthetic group for efficient and bioorthogonal radiolabeling of cyclooctyne-group containing molecules using copper-free click reaction. Bioorg. Med. Chem. Lett. 2016, 26 (3), 875–878. 10.1016/j.bmcl.2015.12.073. [DOI] [PubMed] [Google Scholar]
- Navarro L.; Berdal M.; Chérel M.; Pecorari F.; Gestin J. F.; Guérard F. Prosthetic groups for radioiodination and astatination of peptides and proteins: A comparative study of five potential bioorthogonal labeling strategies. Bioorg. Med. Chem. 2019, 27 (1), 167–174. 10.1016/j.bmc.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Donnelly D. J.; Smith R. A.; Morin P.; Lipovšek D.; Gokemeijer J.; Cohen D.; Lafont V.; Tran T.; Cole E. L.; Wright M.; Kim J.; Pena A.; Kukral D.; Dischino D. D.; Chow P.; Gan J.; Adelakun O.; Wang X. T.; Cao K.; Leung D.; Bonacorsi S. J. Jr; Hayes W. Synthesis and Biologic Evaluation of a Novel 18F-Labeled Adnectin as a PET Radioligand for Imaging PD-L1 Expression. J. Nucl. Med. 2018, 59 (3), 529–535. 10.2967/jnumed.117.199596. [DOI] [PubMed] [Google Scholar]
- Li M.; Ma X.; Molnar C. J.; Wang S.; Wu Z.; Popik V. V.; Li Z. Modular PET Agent Construction Strategy through Strain-Promoted Double-Click Reagent with Efficient Photoclick Step. Bioconjug Chem. 2022, 33 (11), 2088–2096. 10.1021/acs.bioconjchem.2c00427. [DOI] [PubMed] [Google Scholar]
- Kettenbach K.; Reffert L. M.; Schieferstein H.; Pektor S.; Eckert R.; Miederer M.; Rösch F.; Ross T. L. Comparison Study of Two Differently Clicked 18F-Folates-Lipophilicity Plays a Key Role. Pharmaceuticals 2018, 11 (1), 30. 10.3390/ph11010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; McNitt C. D.; Wang H.; Ma X.; Scarry S. M.; Wu Z.; Popik V. V.; Li Z. The efficiency of 18F labelling of a prostate specific membrane antigen ligand via strain-promoted azide-alkyne reaction: reaction speed versus hydrophilicity. Chem. Commun. 2018, 54 (56), 7810–7813. 10.1039/C8CC03999B. [DOI] [PubMed] [Google Scholar]
- Gai Y.; Jiang Y.; Long Y.; Sun L.; Liu Q.; Qin C.; Zhang Y.; Zeng D.; Lan X. Evaluation of an Integrin αvβ3 and Aminopeptidase N Dual-Receptor Targeting Tracer for Breast Cancer Imaging. Mol. Pharmaceutics 2020, 17 (1), 349–358. 10.1021/acs.molpharmaceut.9b01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedrow J. R.; Latoche J. D.; Day K. E.; Modi J.; Ganguly T.; Zeng D.; Kurland B. F.; Berkman C. E.; Anderson C. J. Targeting PSMA with a Cu-64 Labeled Phosphoramidate Inhibitor for PET/CT Imaging of Variant PSMA-Expressing Xenografts in Mouse Models of Prostate Cancer. Mol. Imaging Biol. 2016, 18 (3), 402–410. 10.1007/s11307-015-0908-7. [DOI] [PubMed] [Google Scholar]
- Wurzer A.; Vágner A.; Horváth D.; Fellegi F.; Wester H. J.; Kálmán F. K.; Notni J. Synthesis of Symmetrical Tetrameric Conjugates of the Radiolanthanide Chelator DOTPI for Application in Endoradiotherapy by Means of Click Chemistry. Front Chem. 2018, 6, 107. 10.3389/fchem.2018.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy C. J.; Ling X.; Geruntho J. J.; Beyer S. K.; Latoche J. D.; Langton-Webster B.; Anderson C. J.; Berkman C. E. 177Lu-Labeled Phosphoramidate-Based PSMA Inhibitors: The Effect of an Albumin Binder on Biodistribution and Therapeutic Efficacy in Prostate Tumor-Bearing Mice. Theranostics 2017, 7 (7), 1928–1939. 10.7150/thno.18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi N. A.; Park J. Y.; Kim K.; Kim Y. J.; Shin J. H.; Lee Y. S.; Im H. J.; Jeong J. M.; Khalid M.; Cheon G. J.; Lee D. S.; Kang K. W. Development of 99mTc-Labeled Human Serum Albumin with Prolonged Circulation by Chelate-then-Click Approach: A Potential Blood Pool Imaging Agent. Mol. Pharmaceutics 2019, 16 (4), 1586–1595. 10.1021/acs.molpharmaceut.8b01258. [DOI] [PubMed] [Google Scholar]
- Hensbergen A. W.; van Willigen D. M.; Welling M. M.; van der Wijk F. A.; de Korne C. M.; van Oosterom M. N.; Schottelius M.; Wester H. J.; Buckle T.; van Leeuwen F. W. B. Click Chemistry in the Design and Production of Hybrid Tracers. ACS Omega 2019, 4 (7), 12438–12448. 10.1021/acsomega.9b01484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; Vineberg J. G.; Honda T.; Ojima I. Design and synthesis of tumor-targeting theranostic drug conjugates for SPECT and PET imaging studies. Bioorg Chem. 2018, 76, 458–467. 10.1016/j.bioorg.2017.12.018. [DOI] [PubMed] [Google Scholar]
- Jeppesen T. E.; Kristensen L. K.; Nielsen C. H.; Petersen L. C.; Kristensen J. B.; Behrens C.; Madsen J.; Kjaer A. Site-Specific 64Cu Labeling of the Serine Protease, Active Site Inhibited Factor Seven Azide (FVIIai-N3), Using Copper Free Click Chemistry. Bioconjug Chem. 2018, 29 (1), 117–125. 10.1021/acs.bioconjchem.7b00649. [DOI] [PubMed] [Google Scholar]
- Wissler H. L.; Ehlerding E. B.; Lyu Z.; Zhao Y.; Zhang S.; Eshraghi A.; Buuh Z. Y.; McGuth J. C.; Guan Y.; Engle J. W.; Bartlett S. J.; Voelz V. A.; Cai W.; Wang R. E. Site-Specific Immuno-PET Tracer to Image PD-L1. Mol. Pharmaceutics 2019, 16 (5), 2028–2036. 10.1021/acs.molpharmaceut.9b00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Zhu H.; Zhang B.; Liu F.; Chen J.; Wang Y.; Wang Y.; Zhang Z.; Wu L.; Si L.; Xu H.; Yao T.; Xiao S.; Xia Q.; Zhang L.; Yang Z.; Zhou D. Synthesis of Site-Specific Radiolabeled Antibodies for Radioimmunotherapy via Genetic Code Expansion. Bioconjug Chem. 2016, 27 (10), 2460–2468. 10.1021/acs.bioconjchem.6b00412. [DOI] [PubMed] [Google Scholar]
- Ahn S. H.; Vaughn B. A.; Solis W. A.; Lupher M. L. Jr; Hallam T. J.; Boros E. Site-Specific 89Zr- and 111In-Radiolabeling and In Vivo Evaluation of Glycan-free Antibodies by Azide-Alkyne Cycloaddition with a Non-natural Amino Acid. Bioconjug Chem. 2020, 31 (4), 1177–1187. 10.1021/acs.bioconjchem.0c00100. [DOI] [PubMed] [Google Scholar]
- Adumeau P.; Sharma S. K.; Brent C.; Zeglis B. M. Site-Specifically Labeled Immunoconjugates for Molecular Imaging-Part 1: Cysteine Residues and Glycans. Mol. Imaging Biol. 2016, 18 (1), 1–17. 10.1007/s11307-015-0919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen L. K.; Christensen C.; Jensen M. M.; Agnew B. J.; Schjöth-Frydendahl C.; Kjaer A.; Nielsen C. H. Site-specifically labeled 89Zr-DFO-trastuzumab improves immuno-reactivity and tumor uptake for immuno-PET in a subcutaneous HER2-positive xenograft mouse model. Theranostics 2019, 9 (15), 4409–4420. 10.7150/thno.32883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S. J.; Bargh J. D.; Dannheim F. M.; Hanby A. R.; Seki H.; Counsell A. J.; Ou X.; Fowler E.; Ashman N.; Takada Y.; Isidro-Llobet A.; Parker J. S.; Carroll J. S.; Spring D. R. Site-selective modification strategies in antibody-drug conjugates. Chem. Soc. Rev. 2021, 50 (2), 1305–1353. 10.1039/D0CS00310G. [DOI] [PubMed] [Google Scholar]
- Cook B. E.; Membreno R.; Zeglis B. M. Dendrimer Scaffold for the Amplification of In Vivo Pretargeting Ligations. Bioconjug Chem. 2018, 29 (8), 2734–2740. 10.1021/acs.bioconjchem.8b00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adumeau P.; Vivier D.; Sharma S. K.; Wang J.; Zhang T.; Chen A.; Agnew B. J.; Zeglis B. M. Site-Specifically Labeled Antibody-Drug Conjugate for Simultaneous Therapy and ImmunoPET. Mol. Pharmaceutics 2018, 15 (3), 892–898. 10.1021/acs.molpharmaceut.7b00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toftevall H.; Nyhlen H.; Olsson F.; Sjogren J. Antibody Conjugations via Glycosyl Remodeling. Methods Mol. Biol. 2020, 2078, 131–145. 10.1007/978-1-4939-9929-3_9. [DOI] [PubMed] [Google Scholar]
- Sadiki A.; Kercher E. M.; Lu H.; Lang R. T.; Spring B. Q.; Zhou Z. S. Site-specific Bioconjugation and Convergent Click Chemistry Enhances Antibody-Chromophore Conjugate Binding Efficiency. Photochem. Photobiol. 2020, 96 (3), 596–603. 10.1111/php.13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adumeau P.; Raave R.; Boswinkel M.; Heskamp S.; Wessels H.; van Gool A. J.; Moreau M.; Bernhard C.; Da Costa L.; Goncalves V.; Denat F. Site-Specific, Platform-Based Conjugation Strategy for the Synthesis of Dual-Labeled Immunoconjugates for Bimodal PET/NIRF Imaging of HER2-Positive Tumors. Bioconjug Chem. 2022, 33 (3), 530–540. 10.1021/acs.bioconjchem.2c00049. [DOI] [PubMed] [Google Scholar]
- Vivier D.; Fung K.; Rodriguez C.; Adumeau P.; Ulaner G. A.; Lewis J. S.; Sharma S. K.; Zeglis B. M. The Influence of Glycans-Specific Bioconjugation on the FcgammaRI Binding and In vivo Performance of 89Zr-DFO-Pertuzumab. Theranostics 2020, 10 (4), 1746–1757. 10.7150/thno.39089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovsgaard M. B.; Jeppesen T. E.; Mortensen M. R.; Nielsen C. H.; Madsen J.; Kjaer A.; Gothelf K. V. Affinity-Guided Conjugation to Antibodies for Use in Positron Emission Tomography. Bioconjug Chem. 2019, 30 (3), 881–887. 10.1021/acs.bioconjchem.9b00013. [DOI] [PubMed] [Google Scholar]
- Mortensen M. R.; Skovsgaard M. B.; Okholm A. H.; Scavenius C.; Dupont D. M.; Rosen C. B.; Enghild J. J.; Kjems J.; Gothelf K. V. Small-Molecule Probes for Affinity-Guided Introduction of Biocompatible Handles on Metal-Binding Proteins. Bioconjug Chem. 2018, 29 (9), 3016–3025. 10.1021/acs.bioconjchem.8b00424. [DOI] [PubMed] [Google Scholar]
- Sarrett S. M.; Rodriguez C.; Rymarczyk G.; Hosny M. M.; Keinänen O.; Delaney S.; Thau S.; Krantz B. A.; Zeglis B. M. Lysine-Directed Site-Selective Bioconjugation for the Creation of Radioimmunoconjugates. Bioconjug Chem. 2022, 33 (9), 1750–1760. 10.1021/acs.bioconjchem.2c00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondon A.; Degoul F. Antibody Pretargeting Based on Bioorthogonal Click Chemistry for Cancer Imaging and Targeted Radionuclide Therapy. Bioconjug Chem. 2020, 31 (2), 159–173. 10.1021/acs.bioconjchem.9b00761. [DOI] [PubMed] [Google Scholar]
- Cheal S. M.; Chung S. K.; Vaughn B. A.; Cheung N.-K. V.; Larson S. M. Pretargeting: A Path Forward for Radioimmunotherapy. J. Nucl. Med. 2022, 63 (9), 1302–1315. 10.2967/jnumed.121.262186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Chu T. A two-step strategy to radiolabel choline phospholipids with 99mTc in S180 cell membranes via strain-promoted cyclooctyne-azide cycloaddition reaction. Bioorg. Med. Chem. Lett. 2016, 26 (22), 5472–5475. 10.1016/j.bmcl.2016.10.026. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhang C.; Lai J.; Zhao Y.; Lu D.; Bao R.; Feng X.; Zhang T.; Liu Z. Noninvasive PET tracking of post-transplant gut microbiota in living mice. Eur. J. Nucl. Med. Mol. Imaging 2020, 47 (4), 991–1002. 10.1007/s00259-019-04639-3. [DOI] [PubMed] [Google Scholar]
- Davis R. A.; Rippner D. A.; Hausner S. H.; Parikh S. J.; McElrone A. J.; Sutcliffe J. L. In Vivo Tracking of Copper-64 Radiolabeled Nanoparticles in Lactuca sativa. Environ. Sci. Technol. 2017, 51 (21), 12537–12546. 10.1021/acs.est.7b03333. [DOI] [PubMed] [Google Scholar]
- Wong P.; Li L.; Chea J.; Hu W.; Poku E.; Ebner T.; Bowles N.; Wong J. Y. C.; Yazaki P. J.; Sligar S.; Shively J. E. Antibody Targeted PET Imaging of 64Cu-DOTA-Anti-CEA PEGylated Lipid Nanodiscs in CEA Positive Tumors. Bioconjug Chem. 2020, 31 (3), 743–753. 10.1021/acs.bioconjchem.9b00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.; Sun L.; Li J.; Xu J.; Wan Z.; Ouyang Z.; Liang L.; Li S.; Zeng D. A multi-functional polymeric carrier for simultaneous positron emission tomography imaging and combination therapy. Acta Biomater 2018, 75, 312–322. 10.1016/j.actbio.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing B.; Qian R.; Jiang D.; Gai Y.; Liu Z.; Guo F.; Ren S.; Gao Y.; Lan X.; An R. Extracellular vesicles-based pre-targeting strategy enables multi-modal imaging of orthotopic colon cancer and image-guided surgery. J. Nanobiotechnology 2021, 19 (1), 151. 10.1186/s12951-021-00888-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J.; Su H.; Wang F.; Chu T. A pre-targeting strategy for imaging glucose metabolism using technetium-99m labelled dibenzocyclooctyne derivative. Bioorg. Med. Chem. Lett. 2019, 29 (14), 1791–1798. 10.1016/j.bmcl.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Dommerholt J.; Rutjes F. P. J. T.; van Delft F. L. Strain-Promoted 1, 3-Dipolar Cycloaddition of Cycloalkynes and Organic Azides. Topics in Current Chemistry 2016, 374 (2), 16. 10.1007/s41061-016-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian R.; Jing B.; Jiang D.; Gai Y.; Zhu Z.; Huang X.; Gao Y.; Lan X.; An R. Multi-antitumor therapy and synchronous imaging monitoring based on exosome. Eur. J. Nucl. Med. Mol. Imaging 2022, 49 (8), 2668–2681. 10.1007/s00259-022-05696-x. [DOI] [PubMed] [Google Scholar]
- Au K. M.; Tripathy A.; Lin C. P.; Wagner K.; Hong S.; Wang A. Z.; Park S. I. Bespoke Pretargeted Nanoradioimmunotherapy for the Treatment of Non-Hodgkin Lymphoma. ACS Nano 2018, 12 (2), 1544–1563. 10.1021/acsnano.7b08122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y.; Lee S.; Lee J.; Yhee J. Y.; Yoon H. I.; Park S.-J.; Koo H.; Moon S.-H.; Lee H.; Cho Y. W.; Kang S. W.; Lee S.-Y.; Kim K. Non-invasive stem cell tracking in hindlimb ischemia animal model using bio-orthogonal copper-free click chemistry. Biochem. Biophys. Res. Commun. 2016, 479 (4), 779–786. 10.1016/j.bbrc.2016.09.132. [DOI] [PubMed] [Google Scholar]
- Teng M.; Li Z.; Zhou Y.; Zhang Z.; Miao L.; Bai X.; Li Y.; Wang S. Real-Time Monitoring of CAR-T Cell Efficiency through a Biorthogonal Cycloaddition Labeling Strategy. Bioconjugate Chem. 2023, 34 (2), 443–452. 10.1021/acs.bioconjchem.3c00006. [DOI] [PubMed] [Google Scholar]
- Wang D.; Zhang Y.; Kleiner R. E. Cell- and Polymerase-Selective Metabolic Labeling of Cellular RNA with 2′-Azidocytidine. J. Am. Chem. Soc. 2020, 142 (34), 14417–14421. 10.1021/jacs.0c04566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D.; Wang Y.; Zhang T.; Wang F.; Li K.; Zhou S.; Zhu H.; Yang Z.; Liu Z. Metabolic radiolabeling and in vivo PET imaging of cytotoxic T lymphocytes to guide combination adoptive cell transfer cancer therapy. J. Nanobiotechnology 2021, 19 (1), 175. 10.1186/s12951-021-00924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Kang S.-W.; Ryu J. H.; Na J. H.; Lee D.-E.; Han S. J.; Kang C. M.; Choe Y. S.; Lee K. C.; Leary J. F.; Choi K.; Lee K.-H.; Kim K. Tumor-Homing Glycol Chitosan-Based Optical/PET Dual Imaging Nanoprobe for Cancer Diagnosis. Bioconjugate Chem. 2014, 25 (3), 601–610. 10.1021/bc500020g. [DOI] [PubMed] [Google Scholar]
- Hagemans I. M.; Wierstra P. J.; Steuten K.; Molkenboer-Kuenen J. D. M.; van Dalen D.; Ter Beest M.; van der Schoot J. M. S.; Ilina O.; Gotthardt M.; Figdor C. G.; Scheeren F. A.; Heskamp S.; Verdoes M. Multiscale imaging of therapeutic anti-PD-L1 antibody localization using molecularly defined imaging agents. J. Nanobiotechnology 2022, 20 (1), 64. 10.1186/s12951-022-01272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lühmann T.; Gutmann M.; Moscaroli A.; Raschig M.; Béhé M.; Meinel L. Biodistribution of Site-Specific PEGylated Fibroblast Growth Factor-2. ACS Biomater Sci. Eng. 2020, 6 (1), 425–432. 10.1021/acsbiomaterials.9b01248. [DOI] [PubMed] [Google Scholar]
- Hood E. D.; Greineder C. F.; Shuvaeva T.; Walsh L.; Villa C. H.; Muzykantov V. R. Vascular Targeting of Radiolabeled Liposomes with Bio-Orthogonally Conjugated Ligands: Single Chain Fragments Provide Higher Specificity than Antibodies. Bioconjug Chem. 2018, 29 (11), 3626–3637. 10.1021/acs.bioconjchem.8b00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. T.; Burvenich I. J.; Guo N.; Kocovski P.; Tochon-Danguy H.; Ackermann U.; O’Keefe G. J.; Gong S.; Rigopoulos A.; Liu Z.; Gan H. K.; Scott A. M. L-Tyrosine Confers Residualizing Properties to a d-Amino Acid-Rich Residualizing Peptide for Radioiodination of Internalizing Antibodies. Mol. Imaging 2016, 15, 153601211664753. 10.1177/1536012116647535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. C.; Rodriguez M.; Carmon K. S.; Voss J.; Wilganowski N. L.; Schonbrunn A.; Azhdarinia A. A Modular Dual-Labeling Scaffold That Retains Agonistic Properties for Somatostatin Receptor Targeting. J. Nucl. Med. 2017, 58 (11), 1858–1864. 10.2967/jnumed.116.187971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal M.; Dias G.; Kersemans V.; Sneddon D.; Faulkner S.; Cornelissen B. A Model System to Explore the Detection Limits of Antibody-Based Immuno-SPECT Imaging of Exclusively Intranuclear Epitopes. J. Nucl. Med. 2021, 62 (11), 1537–1544. 10.2967/jnumed.120.251173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer J.; Wiest H. Diels-Alder-Additionen mit “inversem” Elektronenbedarf. Angew. Chem. 1962, 74 (10), 353–353. 10.1002/ange.19620741006. [DOI] [Google Scholar]
- Boger D. L.; Robarge K. D. Divergent de novo synthesis of carbohydrates based on an accelerated inverse electron demand Diels-Alder reaction of 1-oxa-1, 3-butadienes. J. Org. Chem. 1988, 53 (24), 5793–5796. 10.1021/jo00259a040. [DOI] [Google Scholar]
- Meimetis L. G.; Boros E.; Carlson J. C.; Ran C.; Caravan P.; Weissleder R. Bioorthogonal Fluorophore Linked DFO-Technology Enabling Facile Chelator Quantification and Multimodal Imaging of Antibodies. Bioconjug Chem. 2016, 27 (1), 257–263. 10.1021/acs.bioconjchem.5b00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canovas C.; Moreau M.; Vrigneaud J. M.; Bellaye P. S.; Collin B.; Denat F.; Goncalves V. Modular Assembly of Multimodal Imaging Agents through an Inverse Electron Demand Diels-Alder Reaction. Bioconjug Chem. 2019, 30 (3), 888–897. 10.1021/acs.bioconjchem.9b00017. [DOI] [PubMed] [Google Scholar]
- Poty S.; Membreno R.; Glaser J. M.; Ragupathi A.; Scholz W. W.; Zeglis B. M.; Lewis J. S. The inverse electron-demand Diels-Alder reaction as a new methodology for the synthesis of 225Ac-labelled radioimmunoconjugates. Chem. Commun. (Camb) 2018, 54 (21), 2599–2602. 10.1039/C7CC09129J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Gil J.; Braga M.; Harriss B. I.; Carroll L. S.; Leow C. H.; Tang M. X.; Aboagye E. O.; Long N. J. Development of 68 Ga-labelled ultrasound microbubbles for whole-body PET imaging. Chem. Sci. 2019, 10 (21), 5603–5615. 10.1039/C9SC00684B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litau S.; Seibold U.; Wängler B.; Schirrmacher R.; Wängler C. iEDDA Conjugation Reaction in Radiometal Labeling of Peptides with 68Ga and 64Cu: Unexpected Findings. ACS Omega 2018, 3 (10), 14039–14053. 10.1021/acsomega.8b01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Vázquez R.; Battisti U. M.; Jørgensen J. T.; Shalgunov V.; Hvass L.; Stares D. L.; Petersen I. N.; Crestey F.; Löffler A.; Svatunek D.; Kristensen J. L.; Mikula H.; Kjaer A.; Herth M. M. Direct Cu-mediated aromatic 18F-labeling of highly reactive tetrazines for pretargeted bioorthogonal PET imaging. Chem. Sci. 2021, 12 (35), 11668–11675. 10.1039/D1SC02789A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Vázquez R.; Jørgensen J. T.; Bratteby K. E.; Shalgunov V.; Hvass L.; Herth M. M.; Kjær A.; Battisti U. M. Development of 18F-Labeled Bispyridyl Tetrazines for In Vivo Pretargeted PET Imaging. Pharmaceuticals 2022, 15 (2), 245. 10.3390/ph15020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen O.; Li X. G.; Chenna N. K.; Lumen D.; Ott J.; Molthoff C. F.; Sarparanta M.; Helariutta K.; Vuorinen T.; Windhorst A. D.; Airaksinen A. J. A New Highly Reactive and Low Lipophilicity Fluorine-18 Labeled Tetrazine Derivative for Pretargeted PET Imaging. ACS Med. Chem. Lett. 2016, 7 (1), 62–66. 10.1021/acsmedchemlett.5b00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratteby K.; Shalgunov V.; Battisti U. M.; Petersen I. N.; van den Broek S. L.; Ohlsson T.; Gillings N.; Erlandsson M.; Herth M. M. Insights into Elution of Anion Exchange Cartridges: Opening the Path toward Aliphatic 18F-Radiolabeling of Base-Sensitive Tracers. ACS Pharmacol Transl Sci. 2021, 4 (5), 1556–1566. 10.1021/acsptsci.1c00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruivo E.; Adhikari K.; Elvas F.; Fissers J.; Vangestel C.; Staelens S.; Stroobants S.; Van der Veken P.; Wyffels L.; Augustyns K. Improved stability of a novel fluorine-18 labeled TCO analogue for pretargeted PET imaging. Nucl. Med. Biol. 2019, 76–77, 36–42. 10.1016/j.nucmedbio.2019.11.001. [DOI] [PubMed] [Google Scholar]
- Ruivo E.; Elvas F.; Adhikari K.; Vangestel C.; Van Haesendonck G.; Lemière F.; Staelens S.; Stroobants S.; Van der Veken P.; Wyffels L.; Augustyns K. Preclinical Evaluation of a Novel (18)F-Labeled dTCO-Amide Derivative for Bioorthogonal Pretargeted Positron Emission Tomography Imaging. ACS Omega 2020, 5 (9), 4449–4456. 10.1021/acsomega.9b03584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti U. M.; Bratteby K.; Jørgensen J. T.; Hvass L.; Shalgunov V.; Mikula H.; Kjær A.; Herth M. M. Development of the First Aliphatic 18F-Labeled Tetrazine Suitable for Pretargeted PET Imaging-Expanding the Bioorthogonal Tool Box. J. Med. Chem. 2021, 64 (20), 15297–15312. 10.1021/acs.jmedchem.1c01326. [DOI] [PubMed] [Google Scholar]
- Wang M.; Svatunek D.; Rohlfing K.; Liu Y.; Wang H.; Giglio B.; Yuan H.; Wu Z.; Li Z.; Fox J. Conformationally Strained trans-Cyclooctene (sTCO) Enables the Rapid Construction of 18 F-PET Probes via Tetrazine Ligation. Theranostics 2016, 6 (6), 887–895. 10.7150/thno.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Vannam R.; Lambert W. D.; Xie Y.; Wang H.; Giglio B.; Ma X.; Wu Z.; Fox J.; Li Z. Hydrophilic 18F-labeled trans-5-oxocene (oxoTCO) for efficient construction of PET agents with improved tumor-to-background ratios in neurotensin receptor (NTR) imaging. Chem. Commun. 2019, 55 (17), 2485–2488. 10.1039/C8CC09747J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billaud E. M. F.; Shahbazali E.; Ahamed M.; Cleeren F.; Noël T.; Koole M.; Verbruggen A.; Hessel V.; Bormans G. Micro-flow photosynthesis of new dienophiles for inverse-electron-demand Diels-Alder reactions. Potential applications for pretargeted in vivo PET imaging. Chem. Sci. 2017, 8 (2), 1251–1258. 10.1039/C6SC02933G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvänen S.; Fang X. T.; Faresjö R.; Rokka J.; Lannfelt L.; Olberg D. E.; Eriksson J.; Sehlin D. Fluorine-18-Labeled Antibody Ligands for PET Imaging of Amyloid-β in Brain. ACS Chem. Neurosci. 2020, 11 (24), 4460–4468. 10.1021/acschemneuro.0c00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H.; Zhang H.; Wang M.; Vannam R.; Wang H.; Yan X.; Ouyang W.; Jia X.; Fox J. M.; Li Z. Improving Tumor-to-Background Contrast through Hydrophilic Tetrazines: The Construction of 18 F-Labeled PET Agents Targeting Nonsmall Cell Lung Carcinoma. Chemistry 2020, 26 (21), 4690–4694. 10.1002/chem.202000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Peng J.; Tang W.; Rawson J.; Karunananthan J.; Jung M.; Ma Y.; Shively J. E.; Kandeel F. Synthesis and evaluation of 18F-PTTCO-Cys40-Exendin-4 for PET imaging of ectopic insulinomas in rodents. Bioorg Chem. 2020, 98, 103718. 10.1016/j.bioorg.2020.103718. [DOI] [PubMed] [Google Scholar]
- Otaru S.; Imlimthan S.; Sarparanta M.; Helariutta K.; Wähälä K.; Airaksinen A. J. Evaluation of Organo [18F]Fluorosilicon Tetrazine as a Prosthetic Group for the Synthesis of PET Radiotracers. Molecules 2020, 25 (5), 1208. 10.3390/molecules25051208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaru S.; Niemikoski H.; Sarparanta M.; Airaksinen A. J. Metabolism of a Bioorthogonal PET Tracer Candidate [19F/18F]SiFA-Tetrazine in Mouse Liver Microsomes: Biotransformation Pathways and Defluorination Investigated by UHPLC-HRMS. Mol. Pharmaceutics 2020, 17 (8), 3106–3115. 10.1021/acs.molpharmaceut.0c00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allott L.; Amgheib A.; Barnes C.; Braga M.; Brickute D.; Wang N.; Fu R.; Ghaem-Maghami S.; Aboagye E. O. Radiolabelling an 18F biologic via facile IEDDA ″click″ chemistry on the GE FASTLab platform. React. Chem. Eng. 2021, 6 (6), 1070–1078. 10.1039/D1RE00117E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosecker V.; Denk C.; Maurer M.; Wilkovitsch M.; Mairinger S.; Wanek T.; Mikula H. Cross-Isotopic Bioorthogonal Tools as Molecular Twins for Radiotheranostic Applications. Chembiochem 2019, 20 (12), 1530–1535. 10.1002/cbic.201900042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Vazquez R.; Battisti U. M.; Shalgunov V.; Schafer G.; Barz M.; Herth M. M. [11 C]Carboxylated Tetrazines for Facile Labeling of Trans-Cyclooctene-Functionalized PeptoBrushes. Macromol. Rapid Commun. 2022, 43 (12), 2100655 10.1002/marc.202100655. [DOI] [PubMed] [Google Scholar]
- Stéen E. J. L.; Jørgensen J. T.; Petersen I. N.; Nørregaard K.; Lehel S.; Shalgunov V.; Birke A.; Edem P. E.; L’Estrade E. T.; Hansen H. D.; Villadsen J.; Erlandsson M.; Ohlsson T.; Yazdani A.; Valliant J. F.; Kristensen J. L.; Barz M.; Knudsen G. M.; Kjær A.; Herth M. M. Improved radiosynthesis and preliminary in vivo evaluation of the 11C-labeled tetrazine [11C]AE-1 for pretargeted PET imaging. Bioorg. Med. Chem. Lett. 2019, 29 (8), 986–990. 10.1016/j.bmcl.2019.02.014. [DOI] [PubMed] [Google Scholar]
- Denk C.; Svatunek D.; Mairinger S.; Stanek J.; Filip T.; Matscheko D.; Kuntner C.; Wanek T.; Mikula H. Design, Synthesis, and Evaluation of a Low-Molecular-Weight 11C-Labeled Tetrazine for Pretargeted PET Imaging Applying Bioorthogonal in Vivo Click Chemistry. Bioconjug Chem. 2016, 27 (7), 1707–1712. 10.1021/acs.bioconjchem.6b00234. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Meshaw R.; Zalutsky M. R.; Vaidyanathan G. Site-Specific and Residualizing Linker for 18 F Labeling with Enhanced Renal Clearance: Application to an Anti-HER2 Single-Domain Antibody Fragment. J. Nucl. Med. 2021, 62 (11), 1624–1630. 10.2967/jnumed.120.261446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettlitz K. A.; Waldmann C. M.; Tsai W. K.; Tavaré R.; Collins J.; Murphy J. M.; Wu A. M. A Dual-Modality Linker Enables Site-Specific Conjugation of Antibody Fragments for 18F-Immuno-PET and Fluorescence Imaging. J. Nucl. Med. 2019, 60 (10), 1467–1473. 10.2967/jnumed.118.223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard E.; Collado Camps E.; Canovas C.; Kip A.; Gotthardt M.; Rijpkema M.; Denat F.; Goncalves V.; van Lith S. A. M. Site-Specific Dual-Labeling of a VHH with a Chelator and a Photosensitizer for Nuclear Imaging and Targeted Photodynamic Therapy of EGFR-Positive Tumors. Cancers 2021, 13 (3), 428. 10.3390/cancers13030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Zhang X.; Wu H. Y.; Sun L.; Ma Y.; Xu J.; Lin Q.; Zeng D. 64Cu-Labeled Ubiquitin for PET Imaging of CXCR4 Expression in Mouse Breast Tumor. ACS Omega 2019, 4 (7), 12432–12437. 10.1021/acsomega.9b00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason C. A.; Carter L. M.; Mandleywala K.; de Souza Franca P. D.; Meyer J. P.; Mamun T.; Backer J. M.; Backer M. V.; Reiner T.; Lewis J. S. Imaging Early-Stage Metastases Using an 18F-Labeled VEGFR-1-Specific Single Chain VEGF Mutant. Mol. Imaging Biol. 2021, 23 (3), 340–349. 10.1007/s11307-020-01555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Pieve C.; Allott L.; Martins C. D.; Vardon A.; Ciobota D. M.; Kramer-Marek G.; Smith G. Efficient [18F]AlF Radiolabeling of ZHER3:8698 Affibody Molecule for Imaging of HER3 Positive Tumors. Bioconjug Chem. 2016, 27 (8), 1839–1849. 10.1021/acs.bioconjchem.6b00259. [DOI] [PubMed] [Google Scholar]
- Lumen D.; Näkki S.; Imlimthan S.; Lambidis E.; Sarparanta M.; Xu W.; Lehto V. P.; Airaksinen A. J. Site-Specific 111In-Radiolabeling of Dual-PEGylated Porous Silicon Nanoparticles and Their In Vivo Evaluation in Murine 4T1 Breast Cancer Model. Pharmaceutics 2019, 11 (12), 686. 10.3390/pharmaceutics11120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossin R.; Verkerk P. R.; van den Bosch S. M.; Vulders R. C.; Verel I.; Lub J.; Robillard M. S. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem., Int. Ed. 2010, 49 (19), 3375–3378. 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- Sarrett S. M.; Keinänen O.; Dayts E. J.; Dewaele-Le Roi G.; Rodriguez C.; Carnazza K. E.; Zeglis B. M. Inverse electron demand Diels-Alder click chemistry for pretargeted PET imaging and radioimmunotherapy. Nat. Protoc 2021, 16 (7), 3348–3381. 10.1038/s41596-021-00540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García M. F.; Zhang X.; Shah M.; Newton-Northup J.; Cabral P.; Cerecetto H.; Quinn T. 99mTc-bioorthogonal click chemistry reagent for in vivo pretargeted imaging. Bioorg. Med. Chem. 2016, 24 (6), 1209–1215. 10.1016/j.bmc.2016.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapuarachchige S.; Si G.; Huang C. T.; Lesniak W. G.; Mease R. C.; Guo X.; Gabrielson K.; Artemov D. Dual-Modality PET-SPECT Image-Guided Pretargeting Delivery in HER2(+) Breast Cancer Models. Biomacromolecules 2021, 22 (11), 4606–4617. 10.1021/acs.biomac.1c00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L.; Tan H.; Lin Q.; Si Z.; Mao W.; Wang T.; Fu Z.; Cheng D.; Shi H. A Pretargeted Imaging Strategy for Immune Checkpoint Ligand PD-L1 Expression in Tumor Based on Bioorthogonal Diels-Alder Click Chemistry. Mol. Imaging Biol. 2020, 22 (4), 842–853. 10.1007/s11307-019-01441-3. [DOI] [PubMed] [Google Scholar]
- Qiu L.; Lin Q.; Si Z.; Tan H.; Liu G.; Zhou J.; Wang T.; Chen Y.; Huang Y.; Yu T.; Jin M.; Cheng D.; Shi H. A Pretargeted Imaging Strategy for EGFR-Positive Colorectal Carcinoma via Modulation of Tz-Radioligand Pharmacokinetics. Mol. Imaging Biol. 2021, 23 (1), 38–51. 10.1007/s11307-020-01539-z. [DOI] [PubMed] [Google Scholar]
- Vito A.; Alarabi H.; Czorny S.; Beiraghi O.; Kent J.; Janzen N.; Genady A. R.; Al-Karmi S. A.; Rathmann S.; Naperstkow Z.; Blacker M.; Llano L.; Berti P. J.; Valliant J. F. A 99mTc-Labelled Tetrazine for Bioorthogonal Chemistry. Synthesis and Biodistribution Studies with Small Molecule trans-Cyclooctene Derivatives. PLoS One 2016, 11 (12), e0167425 10.1371/journal.pone.0167425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani A.; Bilton H.; Vito A.; Genady A. R.; Rathmann S. M.; Ahmad Z.; Janzen N.; Czorny S.; Zeglis B. M.; Francesconi L. C.; Valliant J. F. A Bone-Seeking trans-Cyclooctene for Pretargeting and Bioorthogonal Chemistry: A Proof of Concept Study Using 99mTc- and 177Lu-Labeled Tetrazines. J. Med. Chem. 2016, 59 (20), 9381–9389. 10.1021/acs.jmedchem.6b00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani A.; Janzen N.; Czorny S.; Ungard R. G.; Miladinovic T.; Singh G.; Valliant J. F. Preparation of tetrazine-containing [2 + 1] complexes of 99mTc and in vivo targeting using bioorthogonal inverse electron demand Diels-Alder chemistry. Dalton Trans 2017, 46 (42), 14691–14699. 10.1039/C7DT01497J. [DOI] [PubMed] [Google Scholar]
- Ferreira V. F. C.; Oliveira B. L.; D’Onofrio A.; Farinha C. M.; Gano L.; Paulo A.; Bernardes G. J. L.; Mendes F. In Vivo Pretargeting Based on Cysteine-Selective Antibody Modification with IEDDA Bioorthogonal Handles for Click Chemistry. Bioconjug Chem. 2021, 32 (1), 121–132. 10.1021/acs.bioconjchem.0c00551. [DOI] [PubMed] [Google Scholar]
- Nazarova L.; Rafidi H.; Mandikian D.; Ferl G. Z.; Koerber J. T.; Davies C. W.; Ulufatu S.; Ho J.; Lau J.; Yu S. F.; Ernst J.; Sadowsky J. D.; Boswell C. A. Effect of Modulating FcRn Binding on Direct and Pretargeted Tumor Uptake of Full-length Antibodies. Mol. Cancer Ther 2020, 19 (4), 1052–1058. 10.1158/1535-7163.MCT-19-1015. [DOI] [PubMed] [Google Scholar]
- Stéen E. J. L.; Jørgensen J. T.; Johann K.; Nørregaard K.; Sohr B.; Svatunek D.; Birke A.; Shalgunov V.; Edem P. E.; Rossin R.; Seidl C.; Schmid F.; Robillard M. S.; Kristensen J. L.; Mikula H.; Barz M.; Kjær A.; Herth M. M. Trans-Cyclooctene-Functionalized PeptoBrushes with Improved Reaction Kinetics of the Tetrazine Ligation for Pretargeted Nuclear Imaging. ACS Nano 2020, 14 (1), 568–584. 10.1021/acsnano.9b06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edem P. E.; Jørgensen J. T.; Nørregaard K.; Rossin R.; Yazdani A.; Valliant J. F.; Robillard M.; Herth M. M.; Kjaer A. Evaluation of a 68Ga-Labeled DOTA-Tetrazine as a PET Alternative to 111In-SPECT Pretargeted Imaging. Molecules 2020, 25 (3), 463. 10.3390/molecules25030463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Zhang J.; Miao Y.; Wen X.; Wang J.; Sun Y.; Chen Y.; Lin J.; Qiu L.; Guo K.; Chen H. Y.; Ye D. Enzyme-Mediated In Situ Self-Assembly Promotes In Vivo Bioorthogonal Reaction for Pretargeted Multimodality Imaging. Angew. Chem., Int. Ed. Engl. 2021, 60 (33), 18082–18093. 10.1002/anie.202103307. [DOI] [PubMed] [Google Scholar]
- Meyer J. P.; Tully K. M.; Jackson J.; Dilling T. R.; Reiner T.; Lewis J. S. Bioorthogonal Masking of Circulating Antibody-TCO Groups Using Tetrazine-Functionalized Dextran Polymers. Bioconjug Chem. 2018, 29 (2), 538–545. 10.1021/acs.bioconjchem.8b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summer D.; Petrik M.; Mayr S.; Hermann M.; Kaeopookum P.; Pfister J.; Klingler M.; Rangger C.; Haas H.; Decristoforo C. Hybrid Imaging Agents for Pretargeting Applications Based on Fusarinine C-Proof of Concept. Molecules 2020, 25 (9), 2123. 10.3390/molecules25092123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Ding B.; Qu C.; Li H.; Sun Y.; Gai Y.; Chen H.; Fang H.; Qian K.; Zhang Y.; Cheng Z.; Lan X. A thiopyrylium salt for PET/NIR-II tumor imaging and image-guided surgery. Mol. Oncol 2020, 14 (5), 1089–1100. 10.1002/1878-0261.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billaud E. M. F.; Belderbos S.; Cleeren F.; Maes W.; Van de Wouwer M.; Koole M.; Verbruggen A.; Himmelreich U.; Geukens N.; Bormans G. Pretargeted PET Imaging Using a Bioorthogonal 18F-Labeled trans-Cyclooctene in an Ovarian Carcinoma Model. Bioconjug Chem. 2017, 28 (12), 2915–2920. 10.1021/acs.bioconjchem.7b00635. [DOI] [PubMed] [Google Scholar]
- Goos J.; Davydova M.; Dilling T. R.; Cho A.; Cornejo M. A.; Gupta A.; Price W. S.; Puttick S.; Whittaker M. R.; Quinn J. F.; Davis T. P.; Lewis J. S. Design and preclinical evaluation of nanostars for the passive pretargeting of tumor tissue. Nucl. Med. Biol. 2020, 84–85, 63–72. 10.1016/j.nucmedbio.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen O.; Mäkilä E. M.; Lindgren R.; Virtanen H.; Liljenbäck H.; Oikonen V.; Sarparanta M.; Molthoff C.; Windhorst A. D.; Roivainen A.; Salonen J. J.; Airaksinen A. J. Pretargeted PET Imaging of trans-Cyclooctene-Modified Porous Silicon Nanoparticles. ACS Omega 2017, 2 (1), 62–69. 10.1021/acsomega.6b00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen O.; Fung K.; Pourat J.; Jallinoja V.; Vivier D.; Pillarsetty N. K.; Airaksinen A. J.; Lewis J. S.; Zeglis B. M.; Sarparanta M. Pretargeting of internalizing trastuzumab and cetuximab with a 18F-tetrazine tracer in xenograft models. EJNMMI Res. 2017, 7 (1), 95. 10.1186/s13550-017-0344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira P. M. R.; Mandleywala K.; Ragupathi A.; Carter L. M.; Goos J.; Janjigian Y. Y.; Lewis J. S. Temporal Modulation of HER2Membrane Availability Increases Pertuzumab Uptake and Pretargeted Molecular Imaging of Gastric Tumors. J. Nucl. Med. 2019, 60 (11), 1569–1578. 10.2967/jnumed.119.225813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X.; Gao K.; Huang H.; Gao R. Pretargeted Immuno-PET Based on Bioorthogonal Chemistry for Imaging EGFR Positive Colorectal Cancer. Bioconjug Chem. 2018, 29 (2), 250–254. 10.1021/acs.bioconjchem.8b00023. [DOI] [PubMed] [Google Scholar]
- Steen E. J. L.; Jorgensen J. T.; Denk C.; Battisti U. M.; Norregaard K.; Edem P. E.; Bratteby K.; Shalgunov V.; Wilkovitsch M.; Svatunek D.; Poulie C. B. M.; Hvass L.; Simon M.; Wanek T.; Rossin R.; Robillard M.; Kristensen J. L.; Mikula H.; Kjaer A.; Herth M. M. Lipophilicity and Click Reactivity Determine the Performance of Bioorthogonal Tetrazine Tools in Pretargeted In Vivo Chemistry. ACS Pharmacol Transl Sci. 2021, 4 (2), 824–833. 10.1021/acsptsci.1c00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adumeau P.; Carnazza K. E.; Brand C.; Carlin S. D.; Reiner T.; Agnew B. J.; Lewis J. S.; Zeglis B. M. A Pretargeted Approach for the Multimodal PET/NIRF Imaging of Colorectal Cancer. Theranostics 2016, 6 (12), 2267–2277. 10.7150/thno.16744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S.; Choi J. S.; Garcia M. A.; Xing Y.; Chen K. J.; Chen Y. M.; Jiang Z. K.; Ro T.; Wu L.; Stout D. B.; Tomlinson J. S.; Wang H.; Chen K.; Tseng H. R.; Lin W. Y. Pretargeted Positron Emission Tomography Imaging That Employs Supramolecular Nanoparticles with in Vivo Bioorthogonal Chemistry. ACS Nano 2016, 10 (1), 1417–1424. 10.1021/acsnano.5b06860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton J. L.; Zeglis B. M.; Abdel-Atti D.; Sawada R.; Scholz W. W.; Lewis J. S. Pretargeted Immuno-PET of Pancreatic Cancer: Overcoming Circulating Antigen and Internalized Antibody to Reduce Radiation Doses. J. Nucl. Med. 2016, 57 (3), 453–459. 10.2967/jnumed.115.163824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulie C. B. M.; Jørgensen J. T.; Shalgunov V.; Kougioumtzoglou G.; Jeppesen T. E.; Kjaer A.; Herth M. M. Evaluation of [64Cu]Cu-NOTA-PEG7-H-Tz for Pretargeted Imaging in LS174T Xenografts-Comparison to [111In]In-DOTA-PEG11-BisPy-Tz. Molecules 2021, 26 (3), 544. 10.3390/molecules26030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; Li Z.; Ma Y.; Ludwig J.; Kim H. S.; Zeng D. PET Imaging of CD8 via SMART for Monitoring the Immunotherapy Response. Biomed Res. Int. 2021, 2021, 6654262. 10.1155/2021/6654262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edem P. E.; Sinnes J. P.; Pektor S.; Bausbacher N.; Rossin R.; Yazdani A.; Miederer M.; Kjær A.; Valliant J. F.; Robillard M. S.; Rösch F.; Herth M. M. Evaluation of the inverse electron demand Diels-Alder reaction in rats using a scandium-44-labelled tetrazine for pretargeted PET imaging. EJNMMI Res. 2019, 9 (1), 49. 10.1186/s13550-019-0520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Lin Q.; Wang T.; Shi D.; Fu Z.; Si Z.; Xu Z.; Cheng Y.; Shi H.; Cheng D. Targeting Infiltrating Myeloid Cells in Gastric Cancer Using a Pretargeted Imaging Strategy Based on Bio-Orthogonal Diels-Alder Click Chemistry and Comparison with 89Zr-Labeled Anti-CD11b Positron Emission Tomography Imaging. Mol. Pharmaceutics 2022, 19 (1), 246–257. 10.1021/acs.molpharmaceut.1c00745. [DOI] [PubMed] [Google Scholar]
- Houghton J. L.; Membreno R.; Abdel-Atti D.; Cunanan K. M.; Carlin S.; Scholz W. W.; Zanzonico P. B.; Lewis J. S.; Zeglis B. M. Establishment of the In Vivo Efficacy of Pretargeted Radioimmunotherapy Utilizing Inverse Electron Demand Diels-Alder Click Chemistry. Mol. Cancer Ther 2017, 16 (1), 124–133. 10.1158/1535-7163.MCT-16-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinanen O.; Brennan J. M.; Membreno R.; Fung K.; Gangangari K.; Dayts E. J.; Williams C. J.; Zeglis B. M. Dual Radionuclide Theranostic Pretargeting. Mol. Pharmaceutics 2019, 16 (10), 4416–4421. 10.1021/acs.molpharmaceut.9b00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läppchen T.; Rossin R.; van Mourik T. R.; Gruntz G.; Hoeben F. J. M.; Versteegen R. M.; Janssen H. M.; Lub J.; Robillard M. S. DOTA-tetrazine probes with modified linkers for tumor pretargeting. Nucl. Med. Biol. 2017, 55, 19–26. 10.1016/j.nucmedbio.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Membreno R.; Cook B. E.; Fung K.; Lewis J. S.; Zeglis B. M. Click-Mediated Pretargeted Radioimmunotherapy of Colorectal Carcinoma. Mol. Pharmaceutics 2018, 15 (4), 1729–1734. 10.1021/acs.molpharmaceut.8b00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondon A.; Schmitt S.; Briat A.; Ty N.; Maigne L.; Quintana M.; Membreno R.; Zeglis B. M.; Navarro-Teulon I.; Pouget J. P.; Chezal J. M.; Miot-Noirault E.; Moreau E.; Degoul F. Pretargeted radioimmunotherapy and SPECT imaging of peritoneal carcinomatosis using bioorthogonal click chemistry: probe selection and first proof-of-concept. Theranostics 2019, 9 (22), 6706–6718. 10.7150/thno.35461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinanen O.; Fung K.; Brennan J. M.; Zia N.; Harris M.; van Dam E.; Biggin C.; Hedt A.; Stoner J.; Donnelly P. S.; Lewis J. S.; Zeglis B. M. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (45), 28316–28327. 10.1073/pnas.2009960117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M. A.; Zhang X.; Rossin R.; Robillard M. S.; Fisher D. R.; Bueltmann T.; Hoeben F. J. M.; Quinn T. P. Metal-Free Cycloaddition Chemistry Driven Pretargeted Radioimmunotherapy Using α-Particle Radiation. Bioconjug Chem. 2017, 28 (12), 3007–3015. 10.1021/acs.bioconjchem.7b00612. [DOI] [PubMed] [Google Scholar]
- Poty S.; Carter L. M.; Mandleywala K.; Membreno R.; Abdel-Atti D.; Ragupathi A.; Scholz W. W.; Zeglis B. M.; Lewis J. S. Leveraging Bioorthogonal Click Chemistry to Improve 225Ac-Radioimmunotherapy of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2019, 25 (2), 868–880. 10.1158/1078-0432.CCR-18-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Membreno R.; Cook B. E.; Fung K.; Lewis J. S.; Zeglis B. M. Click-Mediated Pretargeted Radioimmunotherapy of Colorectal Carcinoma. Mol. Pharmaceutics 2018, 15 (4), 1729–1734. 10.1021/acs.molpharmaceut.8b00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. P.; Kozlowski P.; Jackson J.; Cunanan K. M.; Adumeau P.; Dilling T. R.; Zeglis B. M.; Lewis J. S. Exploring Structural Parameters for Pretargeting Radioligand Optimization. J. Med. Chem. 2017, 60 (19), 8201–8217. 10.1021/acs.jmedchem.7b01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summer D.; Mayr S.; Petrik M.; Rangger C.; Schoeler K.; Vieider L.; Matuszczak B.; Decristoforo C. Pretargeted Imaging with Gallium-68-Improving the Binding Capability by Increasing the Number of Tetrazine Motifs. Pharmaceuticals 2018, 11 (4), 102. 10.3390/ph11040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitz C. A.; Delaney S.; Cook B. E.; Genady A. R.; Hoerres R.; Kuchuk M.; Makris G.; Valliant J. F.; Sadeghi S.; Lewis J. S.; Hennkens H. M.; Bryan J. N.; Zeglis B. M. Pretargeted PET of Osteodestructive Lesions in Dogs. Mol. Pharmaceutics 2022, 19 (9), 3153–3162. 10.1021/acs.molpharmaceut.2c00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio A.; Caille F.; Chevalier A.; Loreau O.; Horkka K.; Halldin C.; Schou M.; Camus N.; Kessler P.; Kuhnast B.; Taran F.; Audisio D. Late-Stage Isotopic Carbon Labeling of Pharmaceutically Relevant Cyclic Ureas Directly from CO2. Angew. Chem., Int. Ed. Engl. 2018, 57 (31), 9744–9748. 10.1002/anie.201804838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanam M. K.; Lai B. T.; Loredo J. M.; Wilson J. A.; Eliasen A. M.; LaBerge N. A.; Nason M.; Cantu A. L.; Luton B. K.; Xu S.; Agnew H. D.; Murphy J. M. Positron Emission Tomography Tracer Design of Targeted Synthetic Peptides via 18F-Sydnone Alkyne Cycloaddition. Bioconjug Chem. 2021, 32 (9), 2073–2082. 10.1021/acs.bioconjchem.1c00379. [DOI] [PubMed] [Google Scholar]
- Narayanam M. K.; Ma G.; Champagne P. A.; Houk K. N.; Murphy J. M. Synthesis of [18 F]Fluoroarenes by Nucleophilic Radiofluorination of N-Arylsydnones. Angew. Chem., Int. Ed. Engl. 2017, 56 (42), 13006–13010. 10.1002/anie.201707274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Audisio D.; Plougastel L.; Decuypere E.; Buisson D. A.; Koniev O.; Kolodych S.; Wagner A.; Elhabiri M.; Krzyczmonik A.; Forsback S.; Solin O.; Gouverneur V.; Taran F. Ultrafast Click Chemistry with Fluorosydnones. Angew. Chem., Int. Ed. Engl. 2016, 55 (39), 12073–12077. 10.1002/anie.201606495. [DOI] [PubMed] [Google Scholar]
- Richard M.; Truillet C.; Tran V. L.; Liu H.; Porte K.; Audisio D.; Roche M.; Jego B.; Cholet S.; Fenaille F.; Kuhnast B.; Taran F.; Specklin S. New fluorine-18 pretargeting PET imaging by bioorthogonal chlorosydnone-cycloalkyne click reaction. Chem. Commun. 2019, 55 (70), 10400–10403. 10.1039/C9CC05486C. [DOI] [PubMed] [Google Scholar]
- Zheng Q.; Xu H.; Wang H.; Du W. H.; Wang N.; Xiong H.; Gu Y.; Noodleman L.; Sharpless K. B.; Yang G.; Wu P. Sulfur [18F]Fluoride Exchange Click Chemistry Enabled Ultrafast Late-Stage Radiosynthesis. J. Am. Chem. Soc. 2021, 143 (10), 3753–3763. 10.1021/jacs.0c09306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamat C.; Gott M.; Steinbach J. Recent progress using the Staudinger ligation for radiolabeling applications. J. Labelled Comp Radiopharm 2018, 61 (3), 165–178. 10.1002/jlcr.3562. [DOI] [PubMed] [Google Scholar]
- Stéen E. J. L.; Shalgunov V.; Denk C.; Mikula H.; Kjær A.; Kristensen J. L.; Herth M. M. Convenient Entry to 18 F-Labeled Amines through the Staudinger Reduction. Eur. J. Org. Chem. 2019, 2019 (8), 1722–1725. 10.1002/ejoc.201801457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscales S.; Kniess T. Access to 18F-labelled isoxazoles by ruthenium-promoted 1, 3-dipolar cycloaddition of 4-[18F]fluoro-N-hydroxybenzimidoyl chloride with alkynes. J. Labelled Comp Radiopharm 2019, 62 (8), 393–403. 10.1002/jlcr.3708. [DOI] [PubMed] [Google Scholar]
- Chen K. T.; Nguyen K.; Ieritano C.; Gao F.; Seimbille Y. A Flexible Synthesis of 68Ga-Labeled Carbonic Anhydrase IX (CAIX)-Targeted Molecules via CBT/1, 2-Aminothiol Click Reaction. Molecules 2019, 24 (1), 23. 10.3390/molecules24010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. T.; Nieuwenhuizen J.; Handula M.; Seimbille Y. A novel clickable MSAP agent for dual fluorescence/nuclear labeling of biovectors. Org. Biomol Chem. 2020, 18 (31), 6134–6139. 10.1039/D0OB01222J. [DOI] [PubMed] [Google Scholar]
- Fujiki K.; Tanaka K. Biomolecular labeling based on lysine-clickable 6pi-azaelectrocyclization toward innovative cancer theranostics. Bioorg. Med. Chem. 2021, 42, 116238. 10.1016/j.bmc.2021.116238. [DOI] [PubMed] [Google Scholar]
- Fujiki K.; Yano S.; Ito T.; Kumagai Y.; Murakami Y.; Kamigaito O.; Haba H.; Tanaka K. A One-Pot Three-Component Double-Click Method for Synthesis of [67Cu]-Labeled Biomolecular Radiotherapeutics. Sci. Rep 2017, 7 (1), 1912. 10.1038/s41598-017-02123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto Y.; Pradipta A. R.; Mukai H.; Zouda M.; Watanabe Y.; Kurbangalieva A.; Ahmadi P.; Manabe Y.; Fukase K.; Tanaka K. Expanding the Applicability of the Metal Labeling of Biomolecules by the RIKEN Click Reaction: A Case Study with Gallium-68 Positron Emission Tomography. Chembiochem 2018, 19 (19), 2055–2060. 10.1002/cbic.201800335. [DOI] [PubMed] [Google Scholar]
- Mushtaq S.; Park S. H. Efficient 125I-radiolabeling of biomolecules using a strain-promoted oxidation-controlled cyclooctyne-1, 2-quinone cycloaddition reaction. Chem. Commun. 2020, 56 (3), 415–418. 10.1039/C9CC08982A. [DOI] [PubMed] [Google Scholar]
- Rodriguez C.; Delaney S.; Sebastiano J.; Sarrett S. M.; Cornejo M. A.; Thau S.; Hosny M. M.; Zeglis B. M. Site-selective radiolabeling using mushroom tyrosinase and the strain-promoted oxidation-controlled 1, 2-quinone cycloaddition. RSC Adv. 2023, 13 (26), 17705–17709. 10.1039/D3RA03486K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maujean T.; Marchand P.; Wagner P.; Riche S.; Boisson F.; Girard N.; Bonnet D.; Gulea M. Hetero-Diels-Alder click reaction of dithioesters for a catalyst-free indirect 18F-radiolabelling of peptides. Chem. Commun. 2022, 58 (79), 11151–11154. 10.1039/D2CC04148K. [DOI] [PubMed] [Google Scholar]
- Allott L.; Amgheib A.; Barnes C.; Braga M.; Brickute D.; Wang N.; Fu R.; Ghaem-Maghami S.; Aboagye E. O. Radiolabelling an 18F biologic via facile IEDDA ″click″ chemistry on the GE FASTLab platform. React. Chem. Eng. 2021, 6 (6), 1070–1078. 10.1039/D1RE00117E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Zalutsky M. R.; Vaidyanathan G. Labeling a TCO-functionalized single domain antibody fragment with 18F via inverse electron demand Diels Alder cycloaddition using a fluoronicotinyl moiety-bearing tetrazine derivative. Bioorg. Med. Chem. 2020, 28 (17), 115634. 10.1016/j.bmc.2020.115634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley N. G.; Steiger K.; Richter F.; Weichert W.; Hoberuck S.; Kotzerke J.; Notni J. Tracking a TGF-beta activator in vivo: sensitive PET imaging of alphavbeta8-integrin with the Ga-68-labeled cyclic RGD octapeptide trimer Ga-68-Triveoctin. EJNMMI Res. 2020, 10 (1), 133. 10.1186/s13550-020-00706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel B. J.; Gammon S. T.; Chaudhari R.; Lu Z.; Pisaneschi F.; Yang H.; Ornelas A.; Yan V.; Kelderhouse L.; Najjar A. M.; Tong W. P.; Zhang S.; Piwnica-Worms D.; Bast R. C. Jr; Millward S. W. Caspase-3 Substrates for Noninvasive Pharmacodynamic Imaging of Apoptosis by PET/CT. Bioconjug Chem. 2018, 29 (9), 3180–3195. 10.1021/acs.bioconjchem.8b00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter N.; Bertram J.; Drewes B.; Bahutski V.; Timmer M.; Schutz M. B.; Kramer F.; Neumaier F.; Endepols H.; Neumaier B.; Zlatopolskiy B. D. Convenient PET-tracer production via SuFEx 18F-fluorination of nanomolar precursor amounts. Eur. J. Med. Chem. 2022, 237, 114383. 10.1016/j.ejmech.2022.114383. [DOI] [PubMed] [Google Scholar]