Abstract
Biofilm infection is a major contributor to wound chronicity. The establishment of clinically relevant experimental wound biofilm infection requires the involvement of the host immune system. Iterative changes in the host and pathogen during the formation of such clinically relevant biofilm can only occur in vivo. The swine wound model is recognized for its advantages as a powerful pre-clinical model. There are several reported approaches for studying wound biofilms. In vitro and ex vivo systems are deficient in terms of the host immune response. Short-term in vivo studies involve acute responses and, thus, do not allow for biofilm maturation, as is known to occur clinically. The first long-term swine wound biofilm study was reported in 2014. The study recognized that biofilm-infected wounds may close as determined by planimetry, but the skin barrier function of the affected site may fail to be restored. Later, this observation was validated clinically. The concept of functional wound closure was thus born. Wounds closed but deficient in skin barrier function may be viewed as invisible wounds. In this work, we seek to report the methodological details necessary to reproduce the long-term swine model of biofilm-infected severe burn injury, which is clinically relevant and has translational value. This protocol provides detailed guidance on establishing an 8 week wound biofilm infection using P. aeruginosa (PA01). Eight full-thickness burn wounds were created symmetrically on the dorsum of domestic white pigs, which were inoculated with (PA01) at day 3 post-burn; subsequently, noninvasive assessments of the wound healing were conducted at different time points using laser speckle imaging (LSI), high-resolution ultrasound (HUSD), and transepidermal water loss (TEWL). The inoculated burn wounds were covered with a four-layer dressing. Biofilms, as established and confirmed structurally by SEM at day 7 post-inoculation, compromised the functional wound closure. Such an adverse outcome is subject to reversal in response to appropriate interventions.
Introduction
Biofilm infection complicates burn and chronic wounds and causes chronicity1,2,3,4,5. In microbiology, biofilm mechanisms are primarily studied, with a focus on the microbes1,6. The lessons learned from these studies are of paramount importance from a biological science standpoint but may not necessarily be applicable to clinically relevant pathogenic biofilms6,7,8. Clinically relevant biofilm structural aggregates should include microbial as well as host factors8,9,10. Such a microenvironment allows for the inclusion of host-microbe iterative interactions, which are critical to developing a clinically relevant biofilm7,8. In such a process, the participation of immune cells and blood-borne factors is critical11,12. The host-microbe interactions underlying clinical pathogenic biofilms, as seen in chronic wounds, occur over a long period of time. Thus, any experimental approach aimed at developing a translationally relevant model of biofilm infection must account for these factors. So, we sought to develop a clinically reproducible swine chronic biofilm infection model.
While human studies clearly represent the best approach to studying healing outcomes, often they are not best suited to addressing the underlying mechanisms and new mechanistic paradigms. Ethical concerns limit the use of study designs requiring the collection of multiple biopsies from a chronic wound at different time points. It is, therefore, critical to have a well-established and reproducible animal model to enable invasive studies for the thorough examination of biofilm fate7,13. The selection of an animal model depends on several factors, including scientific/translational relevance and logistics. The porcine system is widely acknowledged to be the most translationally valuable experimental model to study human skin wounds7. Thus, this work reports an established swine model of biofilm-infected full-thickness burn injury. This work is based on several original publications reported in the literature2,7,13,14,15,16,17. In this study, a clinical isolate of multidrug-resistant Pseudomonas aeruginosa (PA01) was chosen to infect the wound. P. aeruginosa is a common cause of wound infections2,18,19,20. It is a Gram-negative bacterium that can be difficult to treat due to its resistance to some antibiotics11,19,21. None of the swine biofilm models reported so far involved 8 week long-term studies22,23,24,25,26. Chronic wounds are those that remain open for 4 weeks or more14,27,28. There are no other chronic wound biofilm models reported in the literature. This work addresses the notion of functional wound closure2,7,13,15,17,29.
Protocol
All the animal studies were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) #21147. The study was conducted at the Laboratory Animal Resource Center (LARC), Indiana University. We used a female domestic white pig (70–80 lb) in this protocol.
1. Animal acclimatization
Upon the arrival of the pigs to the facility, house the animals individually within the same room for at least 3 days for acclimatization and social interaction.
Feed the pigs a well-balanced diet. Decide the amount fed based on weight, and follow the recommendations from the manufacturer.
Ensure that the animal is fasted for 6–12 h prior to the procedure to prevent nausea, vomiting, and the aspiration of stomach fluids while under anesthesia.
2. Surgery room setup
Prepare the anesthesia machine, and make sure it is ready with the rebreathing circuit.
- Arrange the room for surgery, as described below (Figure 1A).
- Cover the procedure table with a sterile drape, and place a circulating water blanket underneath to aid in thermoregulation.
- Set up a table with induction supplies and surgery preparation materials. Set up a table with the burner devices and control boxes. Set up the imaging equipment, and make sure that it is turned on.
Figure 1: Setup for the procedure.

(A) Surgical table preparation. (B) Ear vein cannulation for IV fluids and drug administration. (C) Thermal blanket covering to protect the pig from hypothermia during the procedure. (D) Burner and timer setup.
3. Sedation of the pig
Sedate the pig with an intramuscular injection of TKX (Telazol 4.4 mg/kg; ketamine 2.2 mg/kg; xylazine 2.2 mg/kg) at a dose of 1 mL/50 lb. Maintain the pig in the procedure room on 1%−3% isoflurane delivered via a mask.
-
Administer the (pre-operative) analgesics to the pigs according to the IACUC protocol; some examples are as follows: buprenorphine 0.3 mg/mL, 0.01–0.05 mg/kg IM; carprofen 50 mg/mL, 4 mg/kg IM or SQ; fentanyl transdermal 100 mcg/h placed on the pinna of the ear; gabapentin 300 mg capsules, 3–10 mg/kg PO.
NOTE: For all burn and biopsy procedures, 1 dose of gabapentin will be given the day prior to surgery and 1 dose of carprofen will be given on the day of the procedure. For the Main burning procedure, a fentanyl patch will be placed, and 1 full dose of buprenorphine will be given during surgical prep.
4. Induction of anesthesia
Sterilize the ear with alternating 2% chlorhexidine scrub and alcohol at least three times. Insert A 22–18 G 1 in intravenous catheter into the marginal ear vein, and confirm the blood flow. Flush the catheter with saline, and fix the catheter with surgical tape (Figure 1B).
- Intubate the pig with an appropriately sized endotracheal tube (7–9 mm) once muscle relaxation has been achieved by the inhalation of anesthesia via the mask. Check the muscle relaxation by a loss of jaw tone and a palpebral reflex being observed.
- Open the tube, and test the cuff leak using a syringe of air. Insert the tube with the aid of a laryngoscope30.
-
Inflate the cuff, and secure the tube once proper placement is confirmed. Connect the pig to the rebreathing circuit.NOTE: The tube is tied into place over the snout, and roll gauze is used to secure it. Auscultation of the chest is performed with a stethoscope to confirm the proper placement of the tube.NOTE: During anesthesia, air is supplied every 5–10 min by closing the pop-off valve and depressing the rebreathing bag until the pressure manometer reaches 20 mm/Hg to prevent positional atelectasis.
- Monitor the animal and the depth of anesthesia.
- Connect the pig to a multi-parameter monitor. The monitor will continuously read the oxygen saturation (SpO2), pulse rate, end tidal carbon dioxide (EtCO2), respiratory rate, and temperature. Record the vitals every 10 min throughout the procedure.
-
Assess the depth of anesthesia by testing the pain reflexes with a hind leg toe pinch prior to beginning the wounding.NOTE: When necessary, adjust the anesthetic vaporizer to administer additional anesthesia, or wait for a few minutes. Check the pain reflexes and palpebral reflexes regularly throughout the surgery.
5. Animal preparation for burn wounding
Disconnect the pig from the anesthesia machine, and move it to the procedure table. Place the pig in the sternal recumbency position, and make sure to secure all the connected lines and tubes (Figure 1C).
Reconnect the pig to the anesthesia machine, and maintain the O2 at 0.8–1.5 L/min and the isoflurane at 1%−3% until the end of the procedure.
Administer IV fluids (LRS) to the pig at a drip rate of 8–10 mL/kg/h. Monitor the anesthesia as in step 4.3.
6. Antiseptic preparation and marking of the skin burn site
- Prepare the wound area by shaving and applying the hair removal cream, as described below (Figure 2).
- Shave the pig dorsum in an area of approximately 25 cm width from the vertebral column all the way to the axilla on both sides using electrical clippers.
- Apply the hair removal cream to the clipped area, and allow to sit for 3–7 min. Remove the cream along with the hair using clean absorbent towels.
- Preparation of the burn site
- Scrub the area to be wounded with alternating 2% chlorhexidine scrub and 70% isopropyl alcohol at least three times for approximately 5 min. Ensure the scrub is applied in a bullseye pattern (starting at the center and moving outward in a spiral) by personnel wearing sterile gloves.
- Mark the wound sites using a sterile burn template and a surgical skin marker (Figure 2B). Mark six to eight wounds (2 in × 2 in) symmetrically on the dorsum.
- Cover the areas around the marked sites with a sterile drape to reduce contamination (Figure 2C).
Figure 2: Surgical site sterilization and marking.
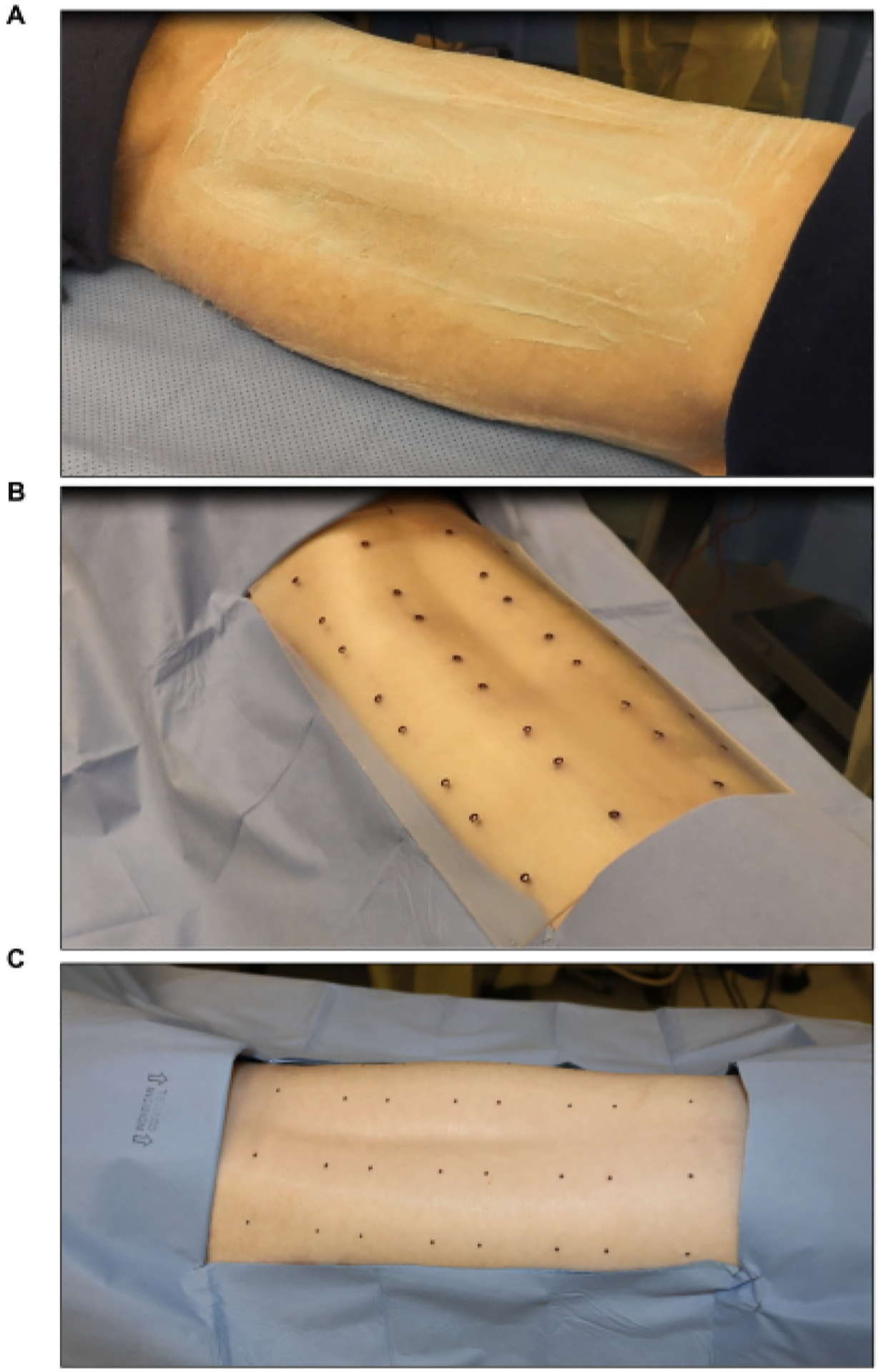
(A) Hair clipping and sterilization. (B) Marking of the burn site using a sterile eight-wound standard template (each wound is 2 in × 2 in). (C) Final marking using a sterile skin marker.
7. Burn wounding procedure
- Use a burn device, such as an in-house-fabricated custom burner consisting of a 2 in × 2 in stainless steel block (weight: 352 g) connected to a metal stylus, an electronic microstat, and an electronic scale (total weight: 1,714 g; Figure 3).
Create a full-thickness burn wound of 2 in × 2 in by using heated stainless-steel blocks connected to the burn device and placing them on the skin for 60 s (Figure 3B, C). During the application of the burn, use the electronic scale to ensure uniform pressure is being applied by the burner.
Figure 3: Burn wound induction.
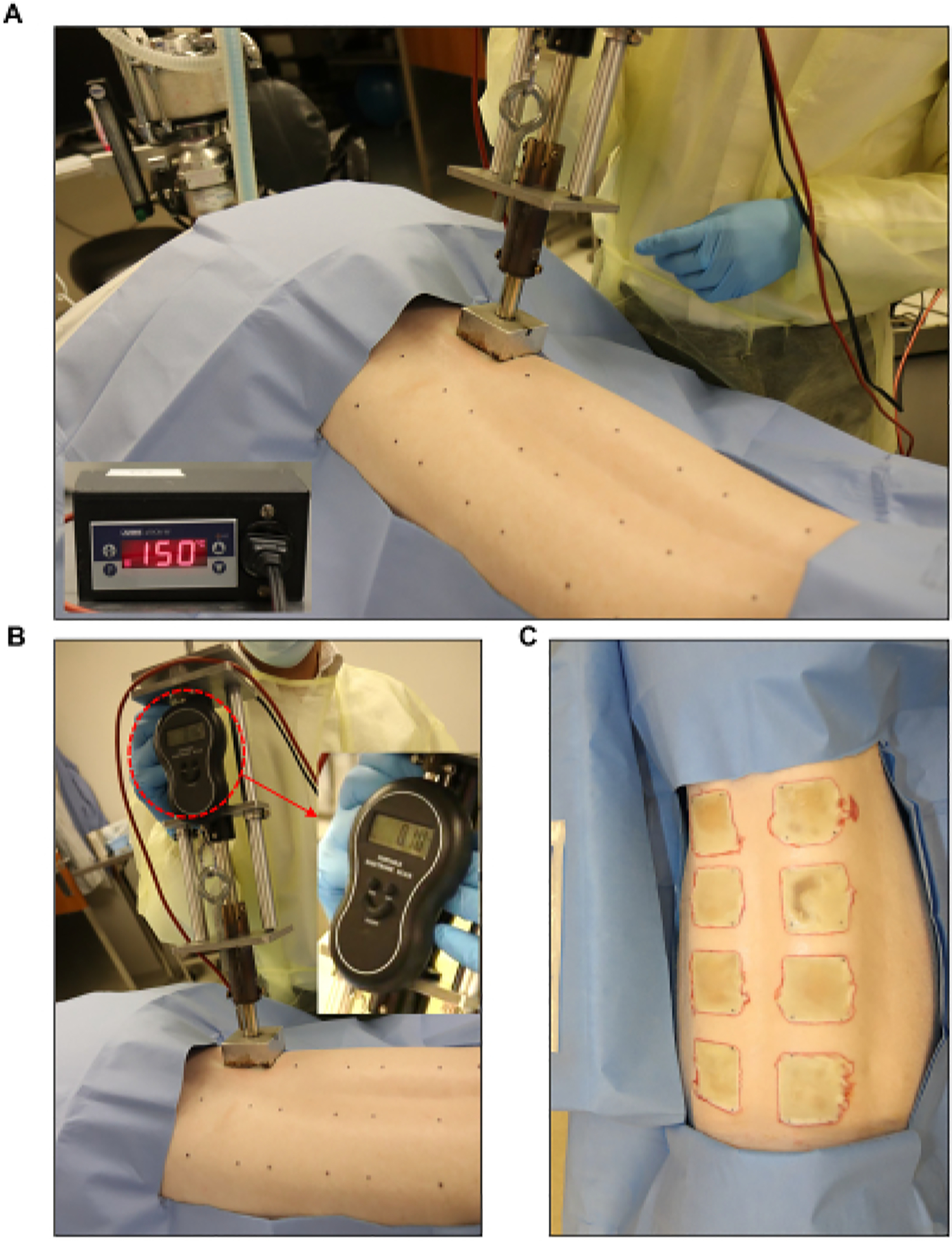
(A,B) Standardized burner with a pressure gauge and automated controller unit (2 in × 2 in) applied to the pre-marked wound site. (C) The whole back showing the eight full-thickness burn wounds.
8. Burn wound assessment and imaging
- Digital photography
- Image the wounds using a DSLR camera and an electro-focus short back focus (EFS) 17–55 mm ultrasonic wide-angle lens and a flashlight.
- Take a digital photo of the whole pig back, including a placard with the pig identification, timepoint, and date. Then, take images for each wound separately showing a placard with the pig ID, wound ID and timepoint, and a ruler.
-
Calculate the wound area as the percentage of the original wound size at each timepoint of collection until day 56.NOTE: In this work, the wound area was calculated at each timepoint (d0, d7, d14, d28, and d56) as a percentage of the original wound area on d0.
- Laser speckle imaging (LSI)
- For laser speckle imaging, use a blood perfusion imager based on the laser speckle contrast analysis (LASCA) technology to assess the wound microvascular perfusion in real-time.
- Take the images of all the wounds in a single recording. Adjust the measured value of the working distance from the laser camera to the wound so that it is consistent for the imaging of each wound (Figure 4A).
- Record the perfusion by a series of images taken over a span of 10–15 s. After a wound is imaged, the recording automatically pauses, and the recording is resumed once the camera is adjusted for the subsequent wound. Each time the recording pauses, a marker is added to identify the wound.
- Transepidermal water loss (TEWL)
- Measure the TEWL for each wound using a standard unit, a TEWL probe, and software (Figure 4B). For each wound, place a clean probe cover over the probe tip, which will be in contact with the wound tissue.
- Place the probe gently and evenly onto the skin, and start the reading by pressing the Start button on the unit.
- Measure each wound five times, first in the center and then on each corner. Then, export all the readings to a spreadsheet (Figure 4B).
- Harmonic ultrasound (HUSD)
- Perform HUSD mapping by scanning the wound with an ultrasound (US) probe from the midline (vertebral column) starting from normal skin toward the lateral side of the pig where there is normal skin again. Follow this scanning pattern for each wound in both B-mode and tissue elastography mode using the ultrasound machine (Figure 4C).
-
For B-mode scanning, apply sterile ultrasound gel to the wound area, and apply some on the ML-615 high-resolution probe. Annotate each recording with the wound identification label. Start the recording, and move the probe slowly from the midline down the wound until the normal skin on the other side is reached.NOTE: After finishing the scanning, the recording is saved and exported from the machine for analysis.
-
For elastography, switch the ultrasound machine to elasto mode by pressing the Elasto button. Scan the wound again in the same manner as in the B-mode scanning, ensuring that uniform pressure of the probe is maintained to allow for the elastography color indicator (green bars) to remain visible throughout the recording.NOTE: Appropriate pressure can be determined by the scale bar on the recording, which appears green when the correct contact is being made (Figure 4D).
- Change the annotation after each wound is imaged in both B-mode and elasto mode (two recordings per wound). Change the comment in the software to include the information for the next wound, and repeat the process for the subsequent wounds.
-
Figure 4: Noninvasive burn wound imaging and assessment.
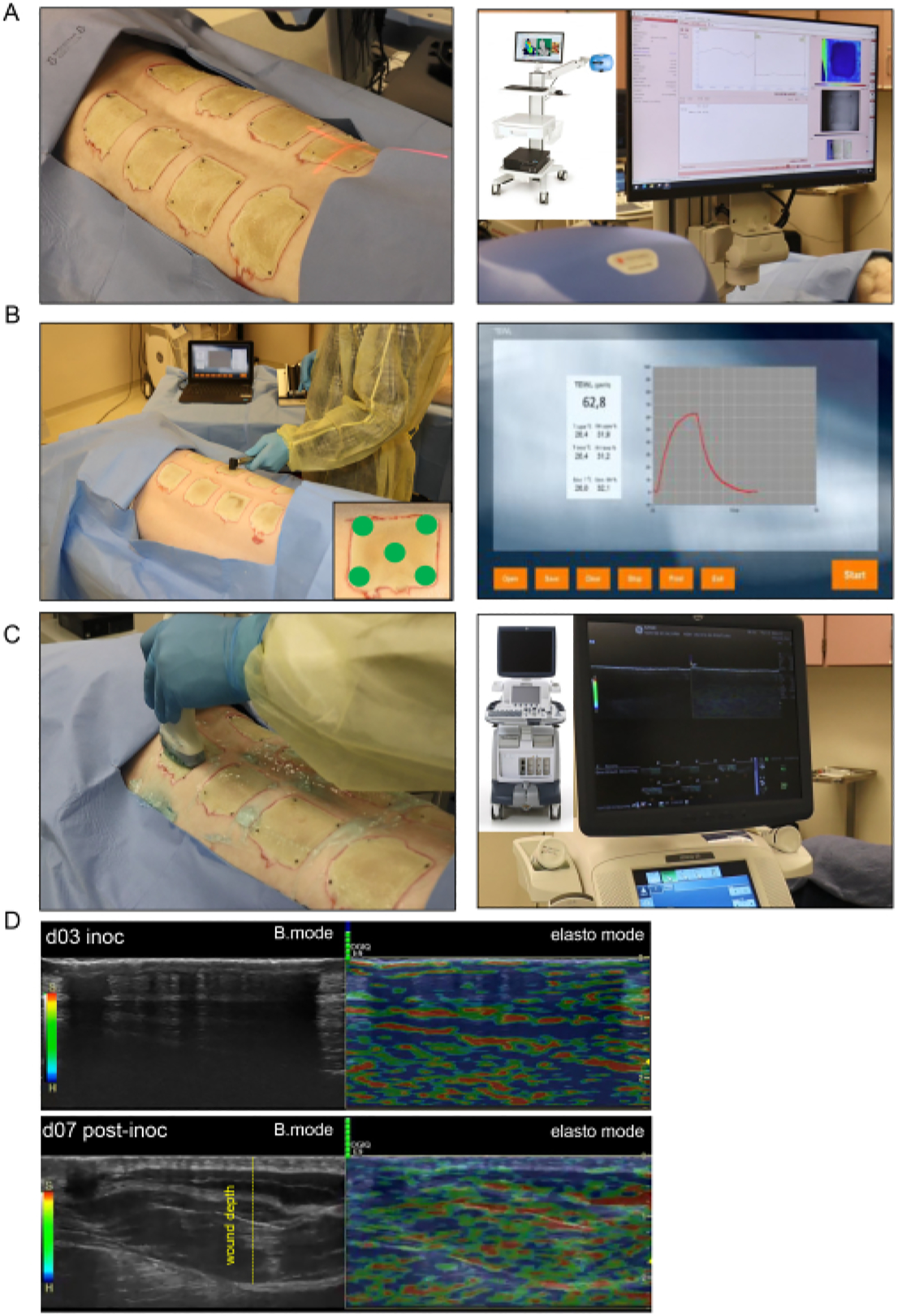
(A) Laser speckle imaging (LSI) with proper orientation of the laser beam indicator to the center of the wound is shown in the left-side image; the right-side image shows the LSI device and the real-time skin vascular perfusion map. (B) Transepidermal water loss (TEWL) probe application to the wound site at five different spots (four wound corners and the center demonstrated in lower-right corner image) is shown in the left-side image; the right-side image is a representative real-time captured screen of the TEWL measurement. (C) Harmonic ultrasound scanning of the burn wound using a high-resolution 16 MHz ultrasound probe is shown on the left side; the right-side image shows the ultrasound device and the real-time screen recording. (D) Structural (B-mode images, grayscale ultrasound) and biomechanical (elastography, color ultrasound) images of the burn wound site at the inoculation day and day 7 post-inoculation. The wound depth is indicated by the yellow dashed line.
9. Bandaging and dressing
Cover the burn wounds individually with transparent film dressings or the test dressing (Figure 5A, B). Place a larger transparent film dressing over the entire wound area (Figure 5C).
Apply a second layer of roll gauze loosely around the entire trunk of the pig to absorb any fluid exudate that comes from the wounds. Roll the pig back and forth from its side to slightly on its back to wrap the bandaging material around the pig.
-
Cover the gauze loosely with a layer of flexible elastic bandage (Figure 5D). Ensure that the bandage is not too tight, as applying it too tightly can restrict the breathing and put pressure on the abdomen, which can result in rectal prolapse or different complications.
NOTE: The elastic bandage is stretchy and can easily be overtightened during application. Pulling it off the roll and allowing it to lay over the edge of the previous wrap can help to prevent overtightening.
Cover the elastic bandage with a final layer of 4 in elastic tape (Figure 4E). Again, ensure the application is not too tight, but make sure that the dressing is secured at the top and bottom edge to prevent it from slipping down as the pig moves around post-procedure.
Figure 5:
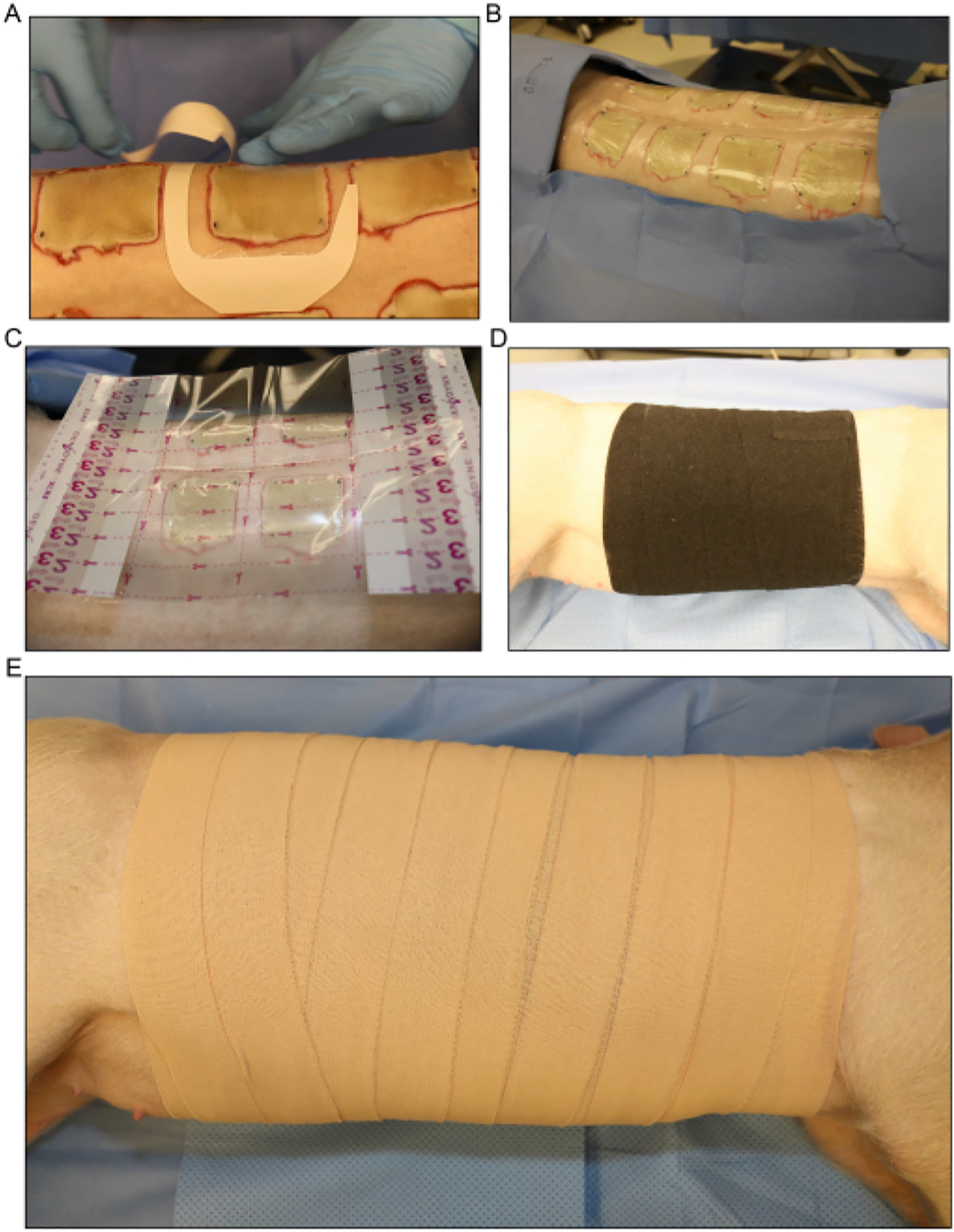
Wound dressing and bandaging. (A) Application of the transparent film dressing for each wound separately. (B) All the dorsal inoculated burn wounds are covered with the first layer of dressing. (C) A larger transparent film dressing is placed over the entire wound area.(D) Application of the second layer of gauze and a loose layer of stretchy elastic bandage around the entire trunk of the pig to absorb any fluid exudate that comes from the wounds. (E) Covering of the entire wound area with a final layer of 4 in adhesive dressing.
10. Animal recovery and postoperative care
- Recovery
- Discontinue the anesthetic gas upon completion of the wounding, imaging procedure, and bandaging. Allow the pig to remain on oxygen for at least 5 min.
- Move the pig, after returning to the primary enclosure, from the transport/lift table to a foam recovery mat in the cage. Raise the automatic waterer, and remove the j-feeder to prevent injury to the pig during recovery.
- Cover the pig with blankets (including a warm air blanket) if hypothermia is present. Monitor and record the vitals including the temperature, pulse, respiration rate, and SpO2 every 10–15 min.
- Continuously monitor the pig until it is able to maintain sternal recumbency independently. Once the pig is fully recovered, lower the nipple waterer, and then the pig can also be fed.
- Pain assessment
- Perform a post-operative pain assessment using a modified Glasgow pain scoring form. Ensure the pain assessments are completed by either lab or LARC staff at least every 12 h for the first 3–4 days postoperatively. The frequency of pain scoring is determined by the attending veterinarian. If the animal scores above 5, administer rescue analgesia (buprenorphine or hydromorphone).
- Provide analgesia by administering a dose of buprenorphine 0.01–0.05 mg/kg IM pre-procedure, with a second dose given 8–12 h later.
- Place a fentanyl patch (100 mcg/h) on the pinna of the ear prior to the burn wounding.
- Inject carprofen 4 mg/kg IM or SQ pre-procedure, and then once daily IM, SQ, or PO for 2 days or as directed by the LARC veterinarian.
- Give gabapentin 3–10 mg/kg orally, with a dose being given the day prior to the procedure, the morning of the procedure, the evening following the procedure, and then every 12 h for 3–5 days.
- Diet
- Ensure the pigs are recovered, and then allow free access to water and food according to their weight-based ration twice daily.
- Provide food enrichment (fresh fruit and vegetables, frozen fruit, marshmallows, yogurt, pudding, etc.), and use these to entice eating if a decreased appetite is observed.
- Dressing change
- Change the bandages at least once weekly or more often if the bandages become soiled or to accommodate treatment strategies.
- Change the bandages after imaging while still under anesthesia, or sedate the pig with only TKX for a dressing change.
- To replace the bandage, start by carefully removing the soiled bandage using Lister bandage scissors or trauma shears, being mindful to not allow the outside of the dressing to come into contact with the wounds.
-
Clean the area around the wounds if needed using 0.9% NaCl on clean gauze, and dry the area gently. Follow the procedure steps for bandaging outlined in section 9.NOTE: If experimental dressings are being applied, these can be applied prior to covering the wounds with the transparent film dressing.
- Imaging frequency
- Obtain imaging (digital photos, LSI, TEWL, and HUSD) at various timepoints throughout the study. Collect imaging data on day −3 (burn wounding), day 0 (inoculation), and day 7, day 14, day 28, day 35, and day 56 post-inoculation.
11. Biofilm preparation and inoculation
- Inoculum preparation
- Prepare a starter plate from a glycerol freezer stock of Pseudomonas aeruginosa (PA01) for a pure culture of the bacterium. Grow a P. aeruginosa culture in low-salt Luria−Bertani agar (LBA), and incubate at 37 °C overnight.
- Inoculate 5 mL of low-salt Luria−Bertani broth (LBB) with a single P. aeruginosa colony on the next day, and incubate overnight at 37 °C with shaking at 200 rpm.
- To obtain log phase cells, inoculate 200 μL of the overnight culture into 5 mL of LBB, and incubate in the shaker at 200 rpm at 37 °C for 2.5 h.
-
Measure the optical density at 600 nm (OD600) using a spectrophotometer. Prepare serial dilutions up to 1 × 10−9 using 100 μL from the culture in 900 μL of sterile LBB.NOTE: We started with undiluted samples and ended with 1 × 107 CFU/mL.We obtained countable colonies in the 1 × 107 dilution, so we considered this dilution as the ending dilution.
- Spread 100 μL of each dilution on LBA, and incubate overnight at 37 °C. As per standard microbiological protocols, use dilutions showing countable colonies (30–300) for the colony count, and obtain the colony forming units (CFU).
- Inoculation of the wound
- Inoculate 200 μL from the overnight culture into 5mL of LB broth, and incubate in the shaker at 37 °C for 2.5 h.
- Measure the optical density of the day culture at 600 nm (OD600). For PA01 inoculation, use 3 × 108 CFU/mL (250 μL of 1 × 108 CFU/mL PA01 is inoculated per wound). Transport the inoculum to the animal facility in a biohazard container.
-
Disperse the inoculum across the surface of the exposed wounds on day 3 post-burn using a pipette, and spread evenly using a disposable spreader (Figure 6). Keep the wounds open for approximately 15 min before bandaging.NOTE: All surgical procedures, inoculation, tissue biopsies, imaging, and bandaging are done under general anesthesia as in sections 3 and 4.
-
Confirming the establishment of infection
NOTE: To confirm that the wounds have become successfully infected following the inoculation, several approaches are utilized, and wound samples are compared to samples collected from normal skin; below are some examples.- For the pathology-based analysis of samples collected at different timepoints, use the count of colony forming units to estimate an infection (CFU; Figure 7E, F).
- Collect 6 mm of wound tissues by punch biopsy. Label and weigh empty 5 mL round-bottom tubes. Transfer the samples to the tubes, and weigh the tubes with the samples.
- Dice the tissue with a scalpel on a sterile surface. Perform all the steps in a BSL2 hood. NOTE: To make sure that the tissues are easily homogenized, the size should be very small (but not less than 0.5 mm)
- Put the sample in the tube, and add 1mL of PBS. Mix and grind the tissue using a hard tissue grinding probe.
- Serially dilute (undiluted to 1 × 10−5) the homogenate, and plate 50 μL of each dilution in selective (Pseudomonas Isolation Agar, PIA) and nonselective (LBA) media.
- Incubate all the dilutions under aerobic conditions at 37 °C for 18–24 h. Image the plates with proper lighting conditions.
- Select plates with 30–300 colonies, if none of the plates have reached that concentration, use the undiluted plate. Use ImageJ to count the colony numbers, and calculate the CFU per plate by multiplying the average value by the final dilution factor.
-
Acquire the images from samples collected from day 7 post-inoculation and other timepoints using scanning electron microscopy (SEM) to confirm the presence of the bacterial biofilms (Figure 7G).NOTE: Day 7 post-inoculation was selected because it is the day of establishment of biofilm infection and the beginning of burn eschar softening, which allows the penetration of the US waves and, thus, the visualization of the deeper tissues. In Figure 4, check the day 3 US burn wound image, which shows the thick leathery eschar that prevents the US waves from passing through to the deeper tissues.
- Stain the sections of the wound biopsies with specific antibodies against P. aeruginosa to confirm the presence of the specific bacteria, as shown in a previous publication13 (Figure 7H).
- Perform next-generation sequencing (NGS), as published in Sinha et al.31. Quantitate the bacteria 16srRNA from the infected wounds and the normal uninfected skin samples collected at different timepoints starting at day 7 post-inoculation until the end of the study.
Figure 6: Bacterial inoculation.
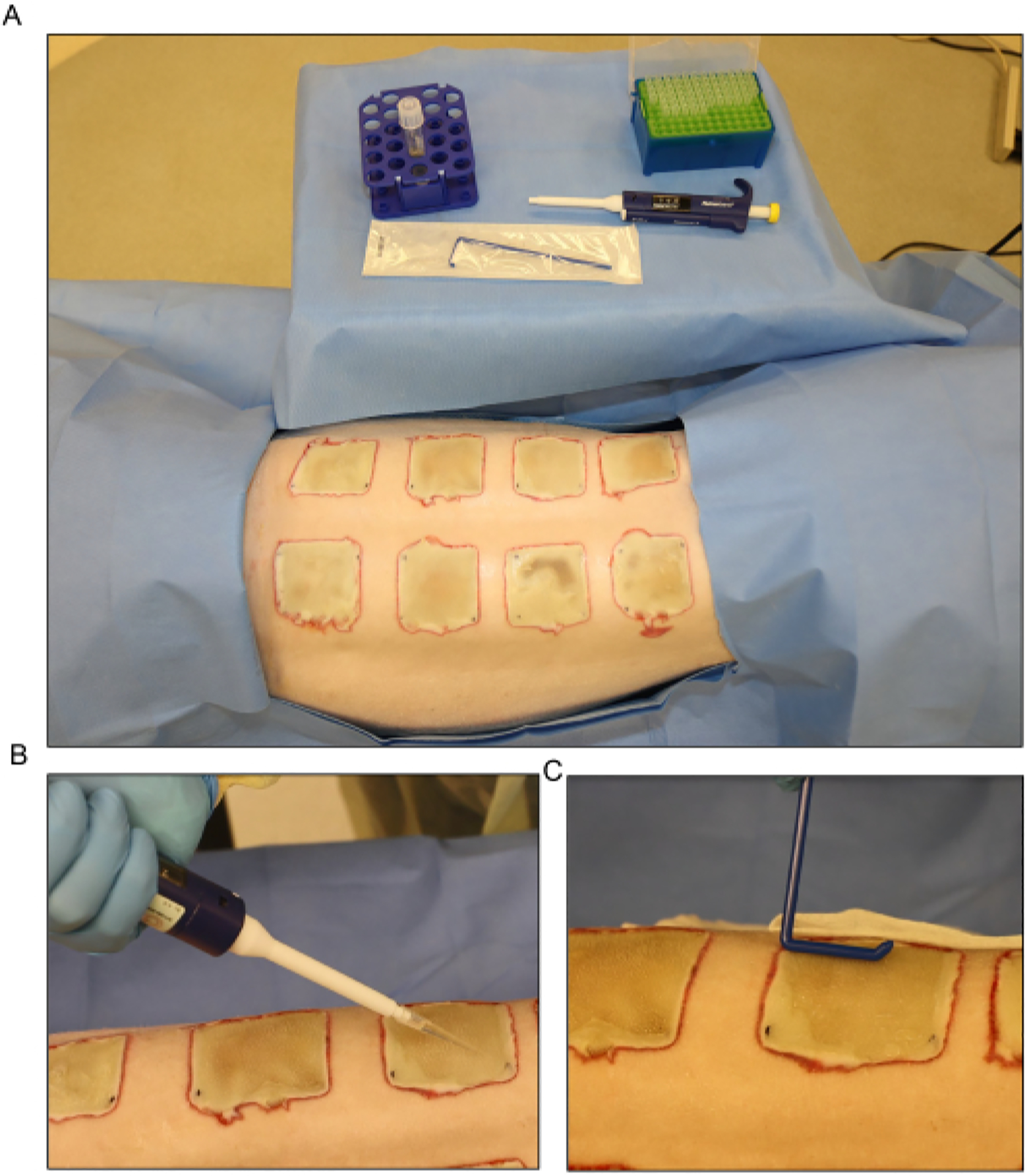
(A) Setup for the Pseudomonas aeruginosa (PA01) inoculation at day 3 post-burn. (B) Topical application of the inoculum with a pipette using a 500 μL volume for each wound. (C) The inoculum is dispersed across the wound surface evenly using a sterile disposable spreader.
Figure 7: Wound healing progress and biofilm confirmation.
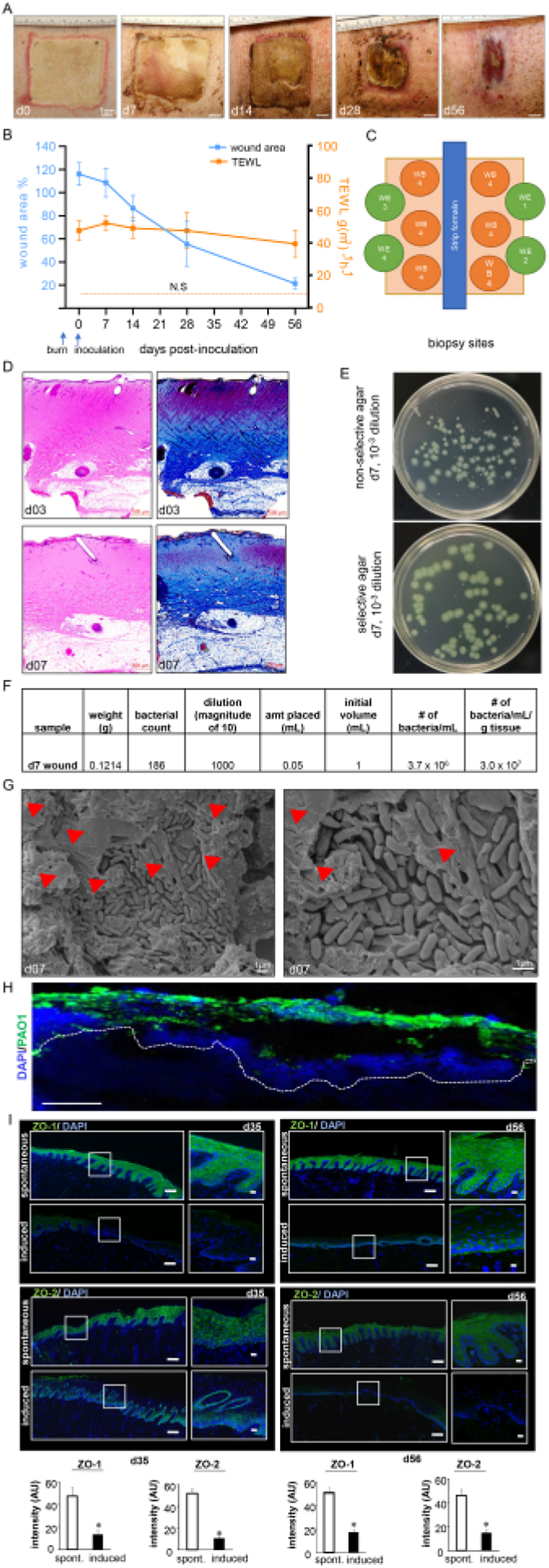
(A) Representative images of the wound closure over the timeline of the study. Scale bar = 1 cm. (B) Quantitation of the wound area and TEWL measurements over the timeline of the study (n = 6). The data are represented as mean ± SD. N.S. refers to the TEWL value of normal skin. (C) Schematic diagram showing different wound biopsy sites. D. H&E staining with its corresponding Masson’s trichrome staining showing distortion and necrosis of all the skin layers at day 3 post-burn and day 7 post-inoculation. Scale bar = 500 μm. (E) Representative digital images of non-selective agar (Luria-Bertani agar) and selective agar (Pseudomonas Isolation Agar) with bacterial colonies grown from porcine wound bed tissue. The selective medium enables the accurate counting of the PA01 colonies only. (F) A sample colony forming unit (CFU) calculation from the colony counts taken from processed day 7 post-inoculation wound biopsies is shown. (G) Representative scanning electron microscopy (SEM) images of the inoculated burn wounds at day 7 post-inoculation showing the established PA01 biofilm, with a zoomed-in image on the right side. Scale bar = 1 μm. The red arrowheads point to extracellular polymeric substances (EPS). (H) P. aeruginosa on the burn wounds were visualized using anti-Pseudomonas (green) antibody; the immunofluorescence images of the day 7 post-inoculation wound biopsies show heavy colonization of the wound tissues by P. aeruginosa. Scale bar = 100 μm. (I) Representative mosaic (scale bar = 200 μm) and corresponding zoomed-in (scale bar = 50 μm) images of ZO-1- and ZO-2-stained sections on day 35 and day 56 post-inoculation, demonstrating reduced expression of the proteins following the induced infection. The OCT-embedded frozen sections (10 μm) were stained using anti-ZO-1 (green) or anti-ZO-2 (green). The sections were counterstained using DAPI. The bar graphs present the quantitation of the ZO-1 and ZO-2 signal intensity. The data are presented as mean ± SD (n = 3); * p < 0.05 compared to spontaneous ones. Mann-Whitney or Kruskal-Wallis one-way analysis of variance tests were performed to test the significance. Figure 7H,I has been modified from Roy et al.13.
12. Biopsy collection
-
Collect the tissue biopsies for analysis following imaging on day 7, day 14, day 28, and day 56 post-inoculation. Collect biopsies from each wound only one time to minimize the interference with the healing process.
NOTE: All surgical procedures, inoculation, tissue biopsies, imaging, and bandaging are done under general anesthesia as in sections 3 and 4.-
Infiltrate the area around the wound with 0.5% bupivacaine. Cut a 3–4 mm wide strip from one edge of the wound to the other, keeping small margins of normal skin in both sides, using a disposable scalpel with a size 10 blade. Place the strip into a labeled conical tube filled with 4% buffered formalin for fixation.NOTE: For early timepoint imaging and biopsy procedures, a full dose of buprenorphine will be given during surgical prep. For late timepoint biopsy procedures, a half dose of buprenorphine will be given during surgical prep. After all burn and biopsy procedures, gabapentin will be given BID for up to 7 days as advised by the attending veterinarian. Carprofen will be given for days post-op or as advised by the attending veterinarian.
- Cut a 6 mm punch biopsy from the wound (either from the wound bed or wound edge). Collect from the wound edge, including part of the normal skin and the wound bed, for different types of analysis.
- Remove the sample using sterilized forceps and dissecting scissors. Place the biopsy sample into the appropriate tube or cassette for processing and analysis.
- For CFU, SEM, RNA, and FPPE, preserve the samples in tubes with an appropriate buffer. For example, samples can be placed into OCT in cassettes for laser capture microscopy (LCM) and immunohistochemistry (IHC).
- Achieve hemostasis after the samples are collected by gently pressing the wound with a sterile gauze. Cover the wound with non-adherent dressing, and bandage as in section 9.
-
13. Euthanasia and tissue collection
Sedate the pig on the day of euthanasia with TKX, and anesthetize with isoflurane. Place an intravenous catheter in the marginal ear vein following the steps outlined in section 3. Intubate the pig following the steps in section 4.
Remove the bandage once the pig is anesthetized, and clean the area around the wounds.
Complete digital photography, LSI, TEWL, and HUSD imaging. Collect the samples from the wounds and normal skin following the steps outlined in section 12.
Once all the required samples are collected, humanely euthanize the pig while still under anesthesia via an intravenous injection of commercially available euthanasia solution (sodium pentobarbital). Use a stethoscope to auscultate to confirm the cessation of the heartbeat and spontaneous respiration.
Perform a secondary method of euthanasia, as required by SOM IACUC, by using a scalpel to induce pneumothorax. Transfer the pig carcass into a barrel, and transport to the freezer to be picked up for incineration.
Representative Results
A standardized burn device was used to create full-thickness burn wounds at 150 °C for 1 min, which resulted in a homogenous deep burn with a uniform margin of erythema and inflammation (Figure 3 and Figure 7). Each pig received eight full-thickness burn wounds on their back, as depicted in Figure 3C.
The non-invasive real-time assessment of the burn wounds by B-mode high-resolution ultrasound to confirm the wound depth and progression of wound healing over time showed the destruction of all skin layers up to the subcutaneous fat (Figure 4). Laser speckle imaging (LSI) was used for further characterization of the wound perfusion (Figure 4A).
The burn wounds showed a thick pyogenic membrane on the wound surface by day 7 post-inoculation, thus confirming the infection and the establishment of the burn wound biofilm (Figure 7A). Digital planimetry showed an increased wound area at day 3 post-inoculation with PAO1 due to the inflammatory response at the wound site and margins (Figure 7A,B). Although the wound area started to shrink by day 14 post-inoculation, incomplete healing to approximately 25% of the original wound size was observed at day 56, indicating the chronicity of the wounds (Figure 7B). Wound chronicity and impaired wound healing were further confirmed by the TEWL, which showed high transepidermal water loss. The TEWL results reflected the loss of skin barrier function compared to normal skin at all measured the timepoints, thus indicating functional impairment of the burn wound healing (Figure 7B). This was also confirmed by the suppression of the tight junctional proteins ZO-1 and 213 and the impairment of the restoration of skin barrier function, as reflected in the high TEWL values seen at day 35 (mid) and day 56 (late) despite the visual wound closure (Figure 7I).
The burn depth was further validated by H&E staining, which showed distortion and necrosis of all the histological skin layers, as shown in Figure 7C. The established biofilm of PA01 was further validated at day 7 post-inoculation by CFU (Figure 7E,F), SEM imaging (Figure 7G), and immunofluorescence staining (Figure 7H).
Discussion
This report provides a detailed protocol for setting up a swine model of chronic wound biofilm infection for experimental studies. Several swine biofilm models have been reported previously22,23,24,25,26, but none of them are swine models involving 8 week long-term studies. Chronic wounds are those that remain open for 4 weeks or more14,27,28. There are no other chronic wound biofilm models reported in the literature. This work addresses the notion of functional wound closure2,7,13,15,17,29. A study conducted in 2014 was the first to report that biofilm-infected wounds may close without the restoration of barrier function7. The measurement of the skin barrier function in the healing wound using transepidermal water loss (TEWL) is reported in this work.
Anatomically and physiologically, the porcine skin, compared to the skin of other small animals, is a closer match to the human skin32,33,34. Both pig and human skin has a thick epidermis33, and the dermal-epidermal thickness ratio ranges from 10:1 to 13:1 in pig, which is comparable to humans34,35. Histologically and biomechanically, the skin of humans and pigs shows similarities in the rete-ridges, subdermal fat, dermal collagen, hair distribution, adnexal structures, and blood vessel size and distribution36,37,38. Functionally, both pigs and humans share similarities in the composition of the lipid, protein, and keratin components of the epidermal layer, as well as comparable immunohistological patterns37,38. The porcine immune system, compared to that of other small animals, shares higher similarities with the human immune system, meaning pigs are an appropriate model for studies on the host interactions that are integral to the complexities of the pathological biofilm in wound infections39. The critical assessment of the pros and cons offered by various animal models has led to the consensus that pigs represent an efficient model for studying wound healing34,38. Additionally, domestic pigs spontaneously develop chronic bacterial infections, as observed in humans10. The burn device used to create the wounds is an advanced and automated burn device that delivers heat energy based on a temperature read out from the targeted skin site22,40. Such an approach improves the rigor and reproducibility of the burn injury. The use of human clinical isolates of bacteria to infect the pig wounds adds value as a pre-clinical model.
Burn injuries are complex and cause several systemic perturbations20,41. Thus, it is important to resuscitate the pig with adequate fluids and prevent hypothermia during anesthesia and recovery. Several factors can interfere with the wound healing, including the post-burn nutrition, fluids, and pain42. Close monitoring of the nutrition and pain assessments is, therefore, of importance. Post-burn pain can be severe and influence the animal’s behavior and diet. Interventions to address behavioral concerns must be actively considered. Regular and continuous pain scoring and management is imperative. A thorough pain assessment sheet with a very detailed pain management plan is included in this protocol. To avoid cross-contamination between the wounds, special attention should be made to apply the first layer of the dressing on each wound separately. Critical care should be taken in handling all the biohazardous materials and when performing the thorough disinfection of the equipment, tools, and entire surgical room. The application of multiple layers of the dressing prevents the pig from exposing the wounds during their effort to rub or scratch the itching back.
The pig in the current model was not compromised by underlying metabolic disorders (e.g., diabetes), and, therefore, the effect being studied was purely the impact of the bacterial biofilm infection on wound healing. However, the model lends itself to the induction of diabetes (using streptozotocin for example) and could be used to study biofilm infection in relation to an underlying metabolic disorder. The other limitation of the model is the controlled infection setting using P. aeruginosa, a bacterium. It is expected that the normal skin micro-flora of the pig may also be growing in the wound and could impact healing. Further analysis using NGS or other advanced techniques to delineate the microbial content of the wound is necessary. The current model could also be applied to mixed infections with differing microbial species (e.g., fungal, viral, etc.). This is an important element, as clinically relevant wounds are likely to be populated by mixed microbes, which may impact wound healing differentially.
There are many potential advantages in this model, including the similarity to the complexity and long-term sequelae of human chronic wounds, the automated and reproducible burn process, and the use of clinically isolated bacterial species. The use of multiple non-invasive imaging modalities represents a powerful approach for collecting useful physiological data characterizing the wound. Finally, the assessment of the functional wound healing via the restoration of skin barrier function based on TEWL is critical. In conclusion, a robust, simple, detailed, and easy-to-use protocol to develop a biofilm-infected severe burn injury using a porcine model system is shown in this work.
Supplementary Material
Acknowledgments
We would like to thank the Laboratory Animal Resource Center (LARC), Indiana University, for their support and the veterinarian care of the animals during the study. This work was partly supported by the National Institutes of Health grants NR015676, NR013898, and DK125835 and the Department of Defense grant W81XWH-11-2-0142. In addition, this work benefited from the following National Institutes of Health awards: GM077185, GM069589, DK076566, AI097511, and NS42617.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/65301.
Disclosures
The authors declare no competing interests.
References
- 1.Goodwine J et al. Pyruvate-depleting conditions induce biofilm dispersion and enhance the efficacy of antibiotics in killing biofilms in vitro and in vivo. Scientific Reports. 9 (1), 3763 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sen CK, Roy S, Mathew-Steiner SS, Gordillo GM Biofilm management in wound care. Plastic and Reconstructive Surgery. 148 (2), 275e–288e (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Church D, Elsayed S, Reid O, Winston B, Lindsay R Burn wound infections. Clinical Microbiology Reviews. 19 (2), 403–434 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen TT, Gilpin DA, Meyer NA, Herndon DN Current treatment of severely burned patients. Annals of Surgery. 223 (1), 14–25 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksson E et al. Chronic wounds: Treatment consensus. Wound Repair and Regeneration. 30 (2), 156–171 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebeaux D, Chauhan A, Rendueles O, Beloin C From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens. 2 (2), 288–356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganesh K et al. Chronic wound biofilm model. Advances in Wound Care. 4 (7), 382–388 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjarnsholt T et al. The in vivo biofilm. Trends in Microbiology. 21 (9), 466–474 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Stewart PS Biophysics of biofilm infection. Pathogens and Disease. 70 (3), 212–218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen LK, Johansen ASB, Jensen HE Porcine models of biofilm infections with focus on pathomorphology. Frontiers in Microbiology. 8, 1961 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mah TF, O’Toole GA Mechanisms of biofilm resistance to antimicrobial agents. Trends in Microbiology. 9 (1), 34–39 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez JF, Hahn MM, Gunn JS Chronic biofilm-based infections: Skewing of the immune response. Pathogens and Disease. 76 (3), fty023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy S et al. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. Journal of Pathology. 233 (4), 331–343 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sen CK Human wound and its burden: Updated 2020 Compendium of Estimates. Advances in Wound Care. 10 (5), 281–292 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barki KG et al. Electric field based dressing disrupts mixed-species bacterial biofilm infection and restores functional wound healing. Annals of Surgery. 269 (4), 756–766 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dusane DH et al. Electroceutical treatment of Pseudomonas aeruginosa biofilms. Scientific Reports. 9 (1), 2008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy S et al. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Annals of Surgery. 271 (6), 1174–1185 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghanbari A et al. Inoculation density and nutrient level determine the formation of mushroom-shaped structures in Pseudomonas aeruginosa biofilms. Scientific Reports. 6, 32097 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin R, Cheng J, Wang J, Li P, Lin J Treatment of Pseudomonas aeruginosa infectious biofilms: Challenges and strategies. Frontiers in Microbiology. 13, 955286 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norbury W, Herndon DN, Tanksley J, Jeschke MG, Finnerty CC Infection in burns. Surgical Infections. 17 (2), 250–255 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitz F et al. Molecular detection of drug-resistance genes of bla(OXA-23)-bla(OXA-51) and mcr-1 in clinical isolates of Pseudomonas aeruginosa. Microorganisms. 9 (4), 786 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis SC et al. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair and Regeneration. 16 (1), 23–29 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Breuing K, Kaplan S, Liu P, Onderdonk AB, Eriksson E Wound fluid bacterial levels exceed tissue bacterial counts in controlled porcine partial-thickness burn infections. Plastic and Reconstructive Surgery. 111 (2), 781–788 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Nusbaum AG et al. Effective method to remove wound bacteria: Comparison of various debridement modalities in an in vivo porcine model. Journal of Surgical Research. 176 (2), 701–707 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Hirsch T et al. Enhanced susceptibility to infections in a diabetic wound healing model. BMC Surgery. 8, 5 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roche ED et al. Increasing the presence of biofilm and healing delay in a porcine model of MRSA-infected wounds. Wound Repair and Regeneration. 20 (4), 537–543 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Hartoch RS, McManus JG, Knapp S, Buettner MF Emergency management of chronic wounds. Emergency Medical Clinics of North America. 25 (1), 203–221 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Mustoe T Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. American Journal of Surgery. 187 (5A), 65S–70S (2004). [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharya M et al. Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proceedings of the National Academy of Sciences of the United States of America. 115 (28), 7416–7421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chum H, Pacharinsak C Endotracheal intubation in swine. Lab Animal. 41 (11), 309–311 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Sinha M et al. Pseudomonas aeruginosa theft biofilm require host lipids of cutaneous wound. Annals of Surgery. 277 (3), e634–e647 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan GY et al. Severe burn injury in a swine model for clinical dressing assessment. Journal of Visualized Experiments. (141), e57942 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Sullivan TP, Eaglstein WH, Davis SC, Mertz P The pig as a model for human wound healing. Wound Repair and Regeneration. 9 (2), 66–76 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Meyer W, Schwarz R, Neurand K The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Current Problems in Dermatology. 7, 39–52 (1978). [DOI] [PubMed] [Google Scholar]
- 35.Vardaxis NJ, Brans TA, Boon ME, Kreis RW, Marres LM Confocal laser scanning microscopy of porcine skin: implications for human wound healing studies. Journal of Anatomy. 190 (Pt 4), 601–611 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinrich W, Lange PM, Stirtz T, Iancu C, Heidemann E Isolation and characterization of the large cyanogen bromide peptides from the alpha1- and alpha2-chains of pig skin collagen. FEBS Letters. 16 (1), 63–67 (1971). [DOI] [PubMed] [Google Scholar]
- 37.Marcarian HQ, Calhoun ML Microscopic anatomy of the integument of adult swine. American Journal of Veterinary Research. 27 (118), 765–772 (1966). [PubMed] [Google Scholar]
- 38.Sullivan TP, Eaglstein WH, Davis SC, Mertz P The pig as a model for human wound healing. Wound Repair and Regeneration. 9 (2), 66–76 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Dawson HD et al. Structural and functional annotation of the porcine immunome. BMC Genomics. 14, 332 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JY, Dunham DM, Supp DM, Sen CK, Powell HM Novel burn device for rapid, reproducible burn wound generation. Burns. 42 (2), 384–391 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielson CB, Duethman NC, Howard JM, Moncure M, Wood JG Burns: Pathophysiology of systemic complications and current management. Journal of Burn Care and Research. 38 (1), e469–e481 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowan MP et al. Burn wound healing and treatment: Review and advancements. Critical Care. 19 (1), 243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


