Objective.
Hypertensive disorders of pregnancy (HDP), including pre-eclampsia, eclampsia and gestational hypertension, are associated with excessive inflammation and are a leading cause of perinatal morbidity and mortality. Soluble urokinase plasminogen activator receptor (suPAR) belongs to the three-finger toxin family of proteins and acts a biomarker of chronic inflammation associated with autoimmune, renal, and cardiovascular disease. Compared with non-pregnant individuals, suPAR levels are elevated in the first trimester and may be involved in the pathogenesis of pregnancy complications that are in part immune-mediated, including HDP1. Notably, two small European studies support that elevated suPAR levels during late pregnancy may be associated with preeclampsia2–4, although this finding did not hold when suPAR was measured before 20 weeks1, 4, 5, suggesting that elevated suPAR levels may reflect a heightened inflammatory response in preeclamptic pregnancies rather than serving as a pre-clinical indicator. No data currently exist on the trajectory of suPAR across pregnancy. In the present study, we investigated if and how plasma suPAR levels change across gestation and examined whether this change and the levels in each trimester varied between women with and without HDP.
Study Design.
Participants included pregnant individuals enrolled in the New York University Children’s Health and Environment Study (NYU CHES), a prospective birth cohort designed to study an array of exposures and conditions relevant to maternal and child health. Maternal blood was collected at up to three time points during pregnancy and plasma suPAR levels were analyzed by enzyme-linked immunosorbent assay. Information on maternal HDP was abstracted from electronic medical records. Study participants with suPAR data in any trimester and information about HDP were eligible for inclusion (n=393); 64 non-HDP participants who had chronic hypertension (n=5), gestational diabetes mellitus (n=55), lupus (n=1), type 1 diabetes (n=1) or type 2 diabetes (n=2) were excluded, resulting in a final analytic sample of 329. The study was approved by the institutional review board of NYU Langone Health and all participants provided written informed consent.
We first regressed suPAR levels on gestational age at the time of sample collection to assess change over the course of pregnancy. We did this for the sample overall and stratified by HDP status. Among the subset of participants with repeated measures, we used paired Wilcoxon signed-rank tests to assess the within-person change in suPAR across trimesters in both groups. Finally, we used Wilcoxon signed-rank tests to assess whether suPAR levels in each trimester and averaged over pregnancy were different among participants with and without HDP.
Results.
The sample is multi-ethnic with 38.6% self-identifying as non-Hispanic white. Participants had a mean±standard deviation (SD) age of 31.3±5.7 years at delivery with a range from 18-49 years; 22% of the sample was older than age 35 years. The median (interquartile range, IQR) pre-pregnancy body mass index (BMI) was 24.4 (6.2) kg/m2 and ranged from 16.8-50.1; 44% of the sample was overweight or obese defined by a BMI ≥ 25. The majority had at least a high school degree (90.1%) and reported never smoking cigarettes (92.9%). Participants with HDP (n=44) were older and had higher BMI; other participant characteristics did not significantly vary by HDP status. suPAR levels did not significantly differ between those with and without HDP at any gestational timepoint (Table 1), although the association was marginal when considering the third trimester such that those with HDP had higher suPAR levels (2.43 ng/mL vs. 2.12 ng/mL, p=0.11). In the sample overall, suPAR levels decreased by 1.1% per week of advancing gestation (p-value< 0.001); however, when stratified by HDP status, suPAR levels only significantly decreased among those without HDP (1.2% per week, p<0.001), while remaining more stable among the cases (0.8% per week, p=0.17) (Figure 1). This finding was also apparent when examining the subset of participants with repeated measures. Among those with paired samples that did not have HDP, the median suPAR level in early gestation (2.79 ng/mL) was significantly higher than late gestation (2.30 ng/mL) with a p-value <0.001 and large effect size r=0.634. In contrast, among those with paired samples and HDP, the median suPAR level in early gestation (2.37 ng/mL) was not significantly different than late gestation (2.45 ng/mL) with a p-value=0.578 and small effect size r=0.256. It is notable however that the sample size of participants with repeated measures and HDP was small (n=7) and the timing of HDP onset was variable across participants.
Table 1.
suPAR levels (ng/mL) among participants enrolled in NYU CHES.
| Sampling period | No HDP | Yes HDP | p-value |
|---|---|---|---|
| <18 weeks gestation (n=268) | 2.71 (0.97) | 2.89 (0.83) | 0.25 |
| 18-24 weeks gestation (n=85) | 2.34 (0.63) | 2.32 (0.32) | 0.76 |
| >24 weeks gestation (n=115) | 2.12 (0.79) | 2.43 (0.91) | 0.11 |
| Pregnancy average (n=329) | 2.54 (0.86) | 2.61 (0.88) | 0.10 |
P-values for the difference in suPAR levels by HDP status were computed using Wilcoxon-rank sum tests.
Values are median (interquartile range).
Figure 1.
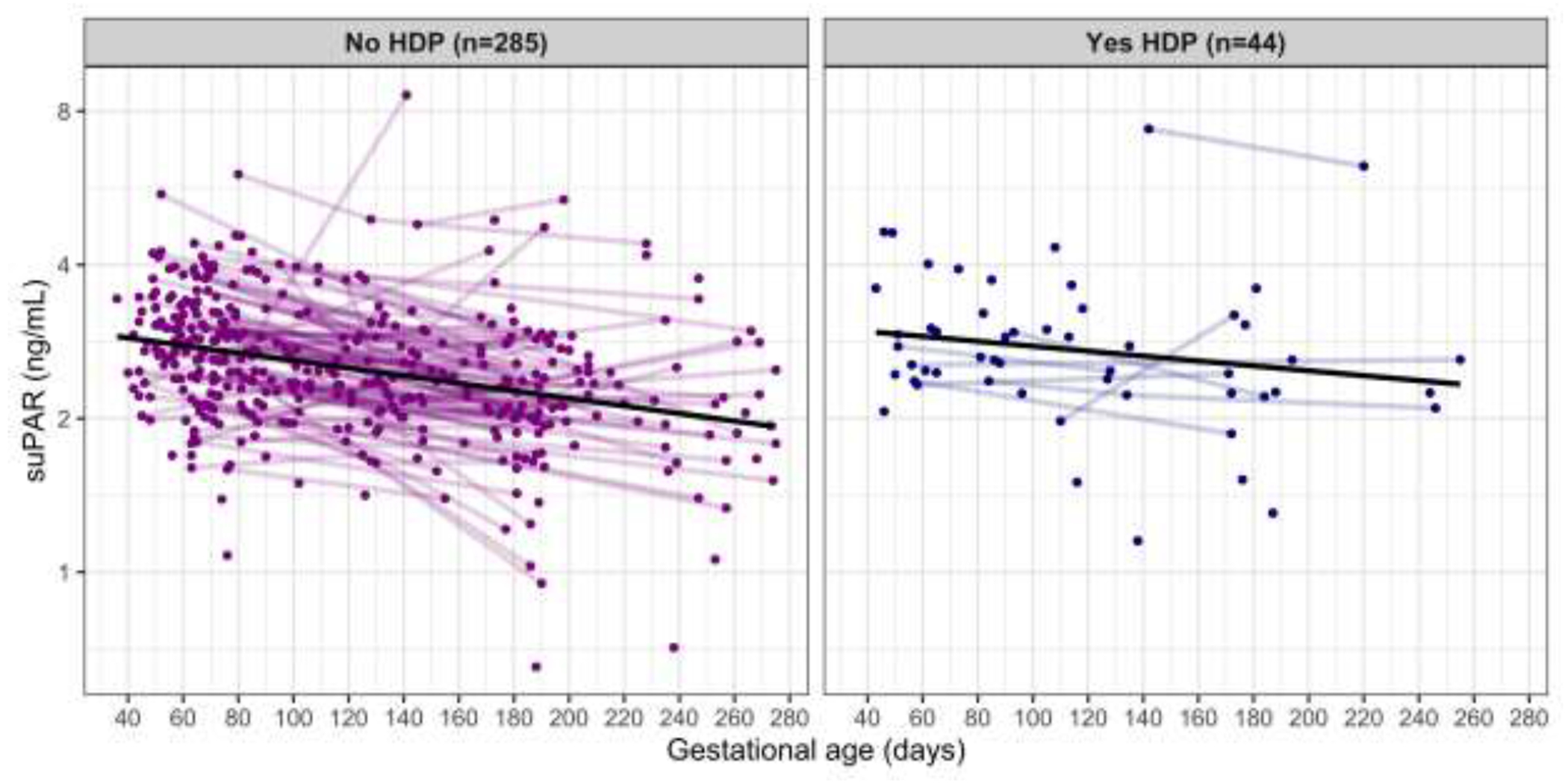
suPAR by gestational age at sample collection stratified by hypertensive disorder of pregnancy (HDP) status.
Each point represents a suPAR measurement. Points that are connected are repeated measurements within the same participant. The black line illustrates the overall trend.
Conclusions.
Although HDP is a primary cause of morbidity and mortality in pregnancy, predictive biomarkers are lacking. suPAR levels decrease with advancing gestation among healthy women, but remain stable in women with HDP, which may reflect a heightened inflammatory state. Additional research is needed to understand how suPAR correlates with other biomarkers of HDP and whether stable suPAR levels can predict HDP accurately in clinical practice.
Funding:
NYU CHES is supported by institutional funds of NYU Grossman School of Medicine as well as the NIH (UG3/UH3OD023305, R01ES032214). During preparation of this manuscript, WC was supported by R00ES032029 and LGK was supported by R00ES030403.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors report no conflict of interest.
Presentation: The 42nd Annual Pregnancy Meeting, Society for Maternal-Fetal Medicine, San Francisco, CA, February 6-11, 2023
Blinded conflict of interest statement: The authors report no conflict of interest.
Contributor Information
Whitney COWELL, Department of Pediatrics, NYU Grossman School of Medicine, New York, NY.
Meghana LIMAYE, Department of Obstetrics and Gynecology, NYU Grossman School of Medicine, New York, NY.
Sara G. BRUBAKER, Department of Obstetrics and Gynecology, NYU Grossman School of Medicine, New York, NY.
Linda G. KAHN, Department of Pediatrics, NYU Grossman School of Medicine, New York, NY.
Jochen REISER, Department of Internal Medicine, Rush Medical College, Chicago, IL.
Jenna SILVERSTEIN, Department of Obstetrics and Gynecology, NYU Grossman School of Medicine, New York, NY.
Laura MALAGA-DIEGUEZ, Department of Pediatrics, NYU Grossman School of Medicine, New York, NY.
Shilpi S. MEHTA-LEE, Department of Obstetrics and Gynecology, NYU Grossman School of Medicine, New York, NY.
Leonardo TRASANDE, Division of Environmental Pediatrics, Department of Pediatrics, NYU Grossman School of Medicine, New York, NY.
References
- 1.Odden N, Henriksen T, Morkrid L. Serum soluble urokinase plasminogen activator receptor (suPAR) in early pregnancy prior to clinical onset of preeclampsia. Acta Obstet Gynecol Scand 2012;91:1226–32. [DOI] [PubMed] [Google Scholar]
- 2.Toldi G, Biro E, Szalay B, et al. Soluble urokinase plasminogen activator receptor (suPAR) levels in healthy pregnancy and preeclampsia. Clin Chem Lab Med 2011;49:1873–6. [DOI] [PubMed] [Google Scholar]
- 3.Jalkanen V, Tihtonen K, Jalkanen K, Jalkanen AJ, Uotila J, Karlsson S. Serum levels of soluble urokinase plasminogen activator receptor are elevated in severe pre-eclampsia. J Clin Gynecol Obstet 2016;5:101–05. [Google Scholar]
- 4.Odden N, Roland M, Lorentzen B, Morkrid L, Henriksen T. suPAR levels in normal- and preeclamptic prengnacies. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health 2013:67–99. [DOI] [PubMed] [Google Scholar]
- 5.Haedersdal S, Salvig JD, Aabye M, et al. Inflammatory markers in the second trimester prior to clinical onset of preeclampsia, intrauterine growth restriction, and spontaneous preterm birth. Inflammation 2013;36:907–13. [DOI] [PubMed] [Google Scholar]


