Abstract
Objective
Tuberculosis (TB) is a leading cause of death globally, with approximately 1.5 million deaths in 2020. TB often coexists with chronic communicable and non-communicable diseases, but data to determine the extent of comorbid diseases are limited. In this study, we aimed to assess the prevalence of TB multimorbidity and its risk factors in a tertiary hospital in Sierra Leone. This is a cross-sectional study of 240 adults with microbiologically-confirmed TB at Connaught Hospital in Freetown, between March and May 2022. Logistic regression analysis was used to identify factors associated with TB multimorbidity.
Results
The mean age of the patients was 37 years. More than two-thirds were males and about the same number had two or more chronic diseases. The most common were hypertension (47.9%) and diabetes (24.2%). Patients under 35 years of age were less likely to have TB multimorbidity (< 25 years: adjusted OR 0.07, 95%CI 0.01–0.6; 25–34 years: adjusted OR 0.2, 95%CI 0.01–0.9). We report a high prevalence of comorbid diseases among TB patients in the largest treatment center in Sierra Leone, with hypertension and diabetes being the most common. These findings support the current call for addressing comorbid non-communicable diseases in TB patients through integrated care.
Keywords: TB multimorbidity, Diabetes, Hypertension, Tuberculosis, Obesity, HIV
Introduction
Multimorbidity, defined as the co-existence of two or more chronic communicable and/or non-communicable diseases, is a growing global health problem [1]. In high-income countries, about 30% of adults have experienced multimorbidity at some point [2]. Although the exact burden of multimorbidity in low- and middle-income countries is unknown, prevalence in these countries is rising due to epidemiological transition caused by lifestyle changes, economic improvements, and changing environmental factors [3].
Tuberculosis (TB) is a leading infectious cause of death globally, with approximately 1.5 million TB deaths reported in 2020 [4]. TB often coexists with chronic communicable and non-communicable diseases, thus increasing management complexity and adversely affecting health and socioeconomic outcomes [5]. Despite these challenges with TB care, there are limited data on the prevalence of multimorbidity among TB patients in most countries of sub-Saharan Africa. A small number of observational studies have only considered individual chronic diseases in TB patients [6–8]. In a recent systematic review of TB multimorbidity, the prevalence of diabetes and HIV among TB patients in the African region was as high as 10.4% and 42.3%, respectively [9].
Sierra Leone, a low-income country in West Africa, has one of the highest TB burdens in the world [4, 10]. Superimposed on these challenges of chronic communicable disease is the high burden of hypertension (22%) and diabetes (10%) reported in the general population of Sierra Leone, which reinforces the need to know more about multimorbidity among TB patients [11, 12].
To date, however, there is no structure in the National TB Control Program to assess and manage multimorbidity in patients with TB, except HIV. In this study, we aimed to assess the prevalence of TB multimorbidity (hypertension, diabetes, obesity and HIV) and its risk factors among adult TB patients attending an urban tertiary hospital in Sierra Leone.
Materials and methods
Study design and setting
We used a cross-sectional design to collect primary data on TB multimorbidity in Connaught Hospital, which is Sierra Leone's national referral hospital with a capacity of 300 beds. The hospital is affiliated with the College of Medicine and Allied Health Sciences of the University of Sierra Leone [13]. Connaught Hospital's Chest Clinic provides outpatient and inpatient TB diagnosis and treatment services, including a Directly Observed Short-Term Treatment (DOTS) program. Owing to its location in the country's main referral hospital, the Chest Clinic provides services to the largest number of TB patients in Sierra Leone [14].
Study population, sampling and data collection
Between March 2022 and May 2022, we recruited non-randomly 240 participants at the Chest Clinic. Two research assistants, trained on the measurement of blood pressure and anthropometric parameters and phlebotomy, collected the data. We used a paper-based questionnaire to collect socio-demographic, clinical, anthropometric, and laboratory information.
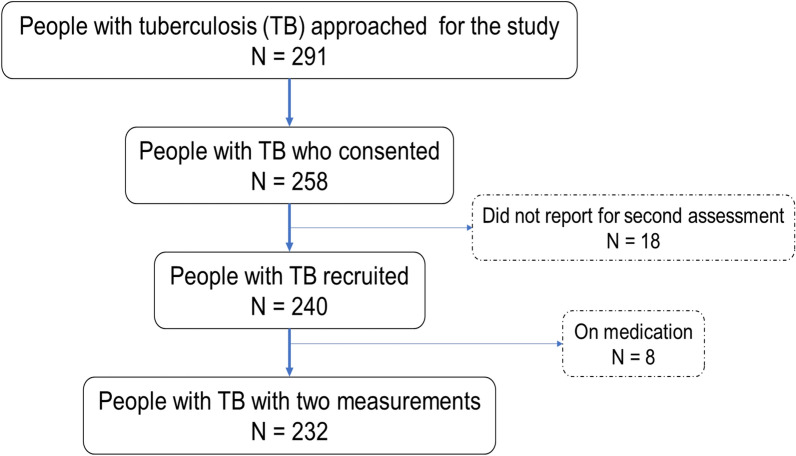
All microbiologically confirmed TB patients aged 18 years or older, including those newly diagnosed or receiving anti-TB treatment, were eligible, regardless of the treatment duration. Patients with extrapulmonary TB, and those who refused to consent to a second plasma glucose or blood pressure measurements were excluded from the study. Figure 1 shows the recruitment details of people with TB for the assessment of multimorbidity.
Fig. 1.
Recruitment details of people with tuberculosis for the study of multimorbidity
Assessment of blood pressure and fasting plasma glucose
We measured the blood pressure and fasting plasma glucose twice, at baseline and at least three days thereafter except where the result was unequivocally high or the patient was on medication.
We measured the blood pressure using a well-calibrated oscillometric manual device as recommended by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines [15]. The average of the two blood pressure measurements was used to categorize patients as follows: (1) normal: < 120/80 mmHg; (2) elevated: systolic blood pressure (SBP) > 120–129 mmHg and diastolic blood pressure (DBP) < 80 mmHg; (3) stage 1 hypertension: SBP > 130–139 mmHg or DBP ≥ 80–89; (2) stage 2 hypertension: SBP ≥ 140 mmHg or DBP ≥ 90 mmHg. We also considered patients to have hypertension if they were on antihypertensive medication, regardless of their average blood pressure records.
We screened for impaired glucose tolerance by measuring the fasting plasma glucose in accordance with the American Diabetes Association (ADA)’s criteria [16]. The average fasting plasma glucose after two measurements separated at least three days apart was used to classify fasting plasma glucose levels as follows: (1) prediabetes, defined as fasting plasma glucose level of 5.6–6.9 mmol/L (2) diabetes, defined as fasting plasma glucose level ≥ 7.0 mmol/L, or being on antidiabetic medications, regardless of the fasting plasma glucose.
Assessment of the body mass index and waist circumference
The body mass index (BMI) was defined as a person's weight in kilograms divided by their height in meters square and categorized accordingly into underweight (< 18 kg/m2), normal weight (18–25 kg/m2), overweight (25–30 kg/m2) and obesity (≥ 30 kg/m2) [17].
We used a tape measure to assess each patient's waist circumference. Abdominal obesity was defined as a waist circumference greater than 94 cm in men and 80 cm in women using a recent European consensus statement [18].
TB multimorbidity
We defined TB multimorbidity as the co-existence of TB with one or more of HIV, obesity, hypertension and diabetes mellitus.
Data analysis
Data analysis was performed using SPSS Version 28.0 (IBM Corp; Armonk, NY, USA). Categorical variables were reported as frequencies and percentages.
A logistic regression model was used to identify risk factors associated with TB multimorbidity. Variables that attained a p-value < 0.2 in the univariable analysis were included in the multivariable regression model. Associations were reported as crude (OR) and adjusted odds ratios (aOR) with 95% confidence intervals (CI), with statistical significance set at p < 0.05.
Results
Socio-demographic details
The mean age of the 240 patients enrolled in this study was 37 (SD 14) years, with a range of 18 to 83 years. Of these patients, 170 (70.8%) were males (sex ratio = 2.4), 116 (48.3%) were single, 174 (72.5%) worked in the informal sector, and 132 (55%) had secondary education. Cigarette smoking was reported by 51 (21.2%) patients (Table 1).
Table 1.
Socio-demographic characteristics of the participants (N = 240)
| Socio-demographic variables | Frequency | Percentage |
|---|---|---|
| Age (yr) | ||
| < 25 | 40 | 16.7 |
| 25–34 | 83 | 34.6 |
| 35–44 | 52 | 21.7 |
| 45–54 | 34 | 14.2 |
| ≥ 55 | 31 | 12.9 |
| Sex | ||
| Female | 70 | 29.2 |
| Male | 170 | 70.8 |
| Marital status | ||
| Single | 116 | 48.3 |
| Married | 105 | 43.8 |
| Separated/widowed/divorce | 19 | 7.9 |
| Occupation | ||
| Unemployed | 16 | 6.7 |
| Student | 30 | 12.5 |
| Informal sector | 174 | 72.5 |
| Formal sector | 10 | 4.2 |
| Retired | 10 | 4.2 |
| Level of education | ||
| None | 37 | 15.4 |
| Primary | 24 | 10.0 |
| Secondary | 132 | 55.0 |
| Tertiary | 47 | 19.6 |
| Smoking and substance use | ||
| Cigarette smoking | 51 | 21.4 |
| Alcohol use | 77 | 32.1 |
| Family history | ||
| Diabetes | 25 | 10.4 |
| Hypertension | 36 | 15.0 |
TB and comorbidity anthropometric, blood pressure and fasting glucose measurements
TB was diagnosed using Xpert MTB/Rif in 185 (77.1%) cases. Most (92.1%) cases were new TB diagnosis and many (69.6%) were in the intensive phase of TB therapy. Few (1.3%) patients were overweight, but a substantial proportion (11.7%) had truncal obesity (Waist circumference > 94 cm for men or > 80 cm for women) (Table 2).
Table 2.
TB details, anthropometry, plasma glucose and blood pressure (N = 240)
| Variable | Frequency | Percentage |
|---|---|---|
| Mode of TB diagnosis | ||
| Xpert MTB/Rif | 185 | 77.1 |
| AFB smear | 53 | 22.1 |
| Urinary TB LAM | 2 | 0.8 |
| Types of patients | ||
| New | 221 | 92.1 |
| Relapse | 14 | 5.8 |
| Treatment failure | 3 | 1.3 |
| Treatment interruption | 2 | 0.8 |
| Phase of TB therapy | ||
| Intensive phase | 167 | 69.6 |
| Continuation phase | 73 | 30.4 |
| Waist circumference (cm) | ||
| Men > 94 or women > 80 | 28 | 11.7 |
| Men ≤ 94 οr women ≤ 80 | 212 | 88.3 |
| BMI | ||
| Underweight | 202 | 84.2 |
| Normal weight | 34 | 14.2 |
| Overweight/obesity | 4 | 1.7 |
| Fasting plasma glucose (mmol/l) | ||
| Normal (≤ 5.5) | 58 | 24.2 |
| Prediabetes (5.6–6.9) | 124 | 51.7 |
| Diabetes (≥ 7 or using medication) | 58 | 24.2 |
| Blood pressure (mmHg) | ||
| Normal | 91 | 37.9 |
| Elevated | 31 | 12.9 |
| Stage 1 hypertension | 98 | 40.8 |
| Stage 2 hypertension | 20 | 8.3 |
| HIV status | ||
| Positive | 49 | 20.6 |
| Negative | 191 | 79.4 |
TB-LAM: Tuberculosis lipoarabinomannan BMI: Body mass index MTB: Mycobacterium tuberculosis HIV: Human immunodeficiency virus
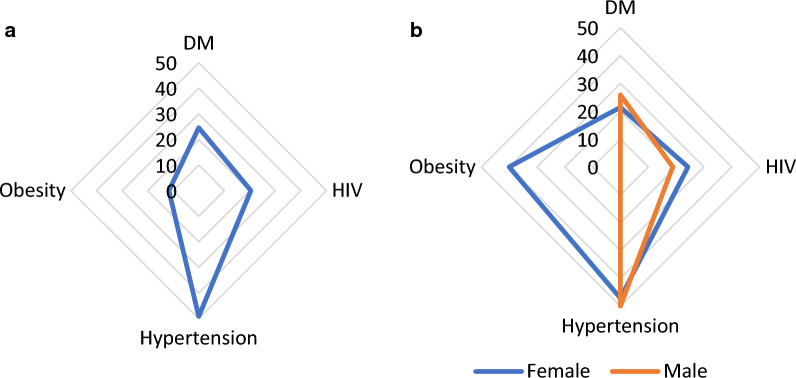
About 70.8% (95% CI: 63.8 to 75.7) of patients with TB had multimorbidity. Amongst TB patients with multimorbidity, the prevalence of comorbid illness was as follows: hypertension 49.1% (95% CI; 41.4 to 54.4), diabetes mellitus 24.2% (95% CI:18.9 to 30.1), HIV 20.4% (95% CI: 15.5 to 26.1) and truncal obesity 11.7% (95% CI: 7.9 to 16.4)). Three patients (1.3%) had all four assessed co-morbidities (Fig. 2).
Fig. 2.
Distribution of TB multimorbidity in percentage a in the total sample and b by sex
Factors associated with TB multimorbidity
In univariable analysis, young adults (< 25 years: OR 0.03, 95% CI 0.0–0.2; 24–34 years: OR 0.06, 95% CI 0.0–0.5; 35–44 years: OR 0.1, 95% CI 0.0–0.9), those with primary education (OR 0.27, 95% CI 0.1–0.9), or still studying (OR 0.04, 95% CI 0.002–0.78) were less likely to show TB multimorbidity. Patients who were not single (married: OR 2.3, 95% CI 1.3–4.1; divorced/separated/widowed: OR 5.4, 95% CI 1.2–24.4) or had family history of hypertension (OR 2.9, 95% CI 1.1–7.8) were more likely to have TB multimorbidity.
After adjusting for confounders, only age was significantly associated with TB multimorbidity, with patients under the age of 35 years (< 25 years: aOR 0.07, 95% CI 0.01–0.6; 25–34 years: aOR 0.2, 95% CI 0.01–0.9) being less likely to have two or more diseases (Table 3).
Table 3.
Bi- and multivariable analysis of TB multimorbidity and variables of interest
| Variables | TB Multimorbidity | Crude odds ratio (95% Confidence interval) |
P value | Adjusted odds ratio (95% Confidence interval) |
P value | |
|---|---|---|---|---|---|---|
| Yes N (%) 170(70.8) |
No N (%) 70 (29.2) |
|||||
| Sex | ||||||
| Female | 54(31.8) | 16(22.9) | 1.6 (0.8–2.9) | 0.170 | 1.9(0.9–4.0) | 0.116 |
| Male | 116(68.2) | 54(77.1) | 1 | 1 | ||
| Age | ||||||
| < 25 | 18(10.6) | 22(31.4) | 0.03(0.0–0.2) | 0.001 | 0.07 (0.01–0.6) | 0.019 |
| 25–34 | 53(31.2) | 30(42.9) | 0.06(0.0–0.5) | 0.007 | 0.2(0.01–0.9) | 0.047 |
| 35–44 | 40(23.5) | 12(17.1) | 0.1(0.0–0.9) | 0.04 | 0.2(0.02–1.7) | 0.1 |
| 45–54 | 29(17.1) | 5(7.1) | 0.2(0.0–1.8) | 0.1 | 0.3(0.03–3.3) | 0.4 |
| ≥ 55* | 30(17.6) | 1(1.4) | 1 | 1 | ||
| Marital status | ||||||
| Single | 71(41.8) | 45(64.3) | 1 | 1 | ||
| Married | 82(48.2) | 23(32.9) | 2.3(1.3–4.1) | 0.007 | 0.9(0.5–1.9) | 0.9 |
| Divorced/separated/widowed | 17(10) | 2(2.9) | 5.4(1.2–24.4) | 0.029 | 1.6(0.3–8.8) | 0.6 |
| Education | ||||||
| None | 31(18.2) | 6(8.6) | 1 | 1 | ||
| Primary | 14(8.2) | 10(14.3) | 0.3(0.1–1.1) | 0.03 | 0.4(0.1–1.3) | 0.1 |
| Secondary | 88(51.8) | 44(62.9) | 0.4(0.2–1.1) | 0.05 | 0.7(0.2–1.8) | 0.4 |
| Tertiary | 37(21.8) | 10(14.3) | 0.7(0.2–2.2) | 0.6 | 1.4(0.4–5.8) | 0.6 |
| Occupation | ||||||
| Unemployed | 12(7.1) | 4(5.7) | 0.1(0.01–2.8) | 0.2 | 1.1(0.1–10.3) | 0.9 |
| Student | 14(8.2) | 16(22.9) | 0.04(0.002–0.8) | 0.03 | 0.5(0.06–3.3) | 0.4 |
| Informal sector | 126(74.1) | 48(68.6) | 0.1(0.01–2.2) | 0.2 | 1.2(0.2–7.3) | 0.9 |
| Formal sector | 8(4.7) | 2(2.9) | 0.2(0.01–3.9) | 0.3 | 0.9(0.0–33.4) | 0.9 |
| Retired | 10(5.9) | 0 | 1 | 1 | ||
| Alcohol consumption | ||||||
| No | 113(66.5) | 50(71.4) | 1 | |||
| Yes | 57(33.5) | 20(28.6) | 1.3(0.7–2.3) | 0.5 | ||
| Cigarette smoking | ||||||
| No | 130(77.4) | 57(81.4) | 1 | |||
| Yes | 38(22.6) | 13(18.6) | 1.3(0.6–2.6) | 0.489 | ||
| Types of patients | ||||||
| New | 156(91.8) | 65(92.9) | 1 | |||
| Relapse/treatment failure/treatment interruption | 14(8.2) | 5(7.1) | 1.2(0.4–3.4) | 0.776 | ||
| Family history of Hypertension | ||||||
| No | 139(81.8) | 65(92.9) | 1 | 1 | ||
| Yes | 31(18.2) | 5(7.1) | 2.9 (1.1–7.8) | 0.035 | 2.37(0.81–6.92) | 0.114 |
| Family history of Diabetes | ||||||
| No | 152(89.4) | 63(90) | 1 | |||
| Yes | 18(10.6) | 7(10) | 1.06 (0.4–2.7) | 0.892 | ||
Discussion
This study is the first to examine multimorbidity in adult patients with TB cared for at a national referral hospital in Sierra Leone. Our study showed that 70.8% of TB patients in this hospital had one or more additional chronic diseases.
A number of studies have provided data on multimorbidity in Africa, but none has focused specifically on TB multimorbidity. In South Africa, a multimorbidity prevalence of 22% was reported in a peri-urban healthcare setting, although not exclusive for TB patients [18]. An earlier study reported a lower TB multimorbidity prevalence of 1.14% in Brazil, which may reflect a lower national prevalence of HIV or other comorbidities [19].
Among the comorbid diseases reported in the TB population, hypertension was the most common, with a reported prevalence of 49%, which was higher than the 22% prevalence in the general population of Sierra Leone. The ACC/AHA guidelines, which we used in this study, have a lower blood pressure threshold of > 80 mmHg for diastolic blood pressure, compared to the threshold of ≥ 90 mmHg defined in other guidelines [15, 20]. Thus, the difference in prevalence between the two studies could be explained by differences in diastolic blood pressure measurement thresholds, or may represent a true reflection of the hypertension burden in this population. Nonetheless, evidence from meta-analyses of observational studies suggests that elevated blood pressure and stages 1 or 2 hypertension, as defined in this study, are associated with increased cardiovascular-related mortality if left untreated [21]. Therefore, in settings of poor health-seeking behaviors, healthcare professionals should employ practical approach to detecting and managing blood pressures at lower thresholds to prevent cardiovascular disease risk, end-stage renal disease, and death.
Similar to hypertension, the prevalence of comorbid diabetes in TB patients in this study was higher than that reported in the general population of Sierra Leone [11]. The World Health Organization proposes a collaborative framework to integrate diabetes care into TB prevention and control services, as the two conditions can negatively impact each other. The recommendations were provisional as the evidence from which it was based was weak [22]. Thus, this study will add to the body of evidence on the need for the integration of diabetes care to TB services.
Despite the low national HIV seroprevalence of 1.7% [23], previous studies have reported a higher HIV burden among TB patients in the national referral hospital of Sierra Leone [14, 24]. Because of this and the fact that the hospital has concentrated HIV cases, it is not surprising that a high prevalence of HIV among tuberculosis patients is reported in this study [25].
Although abdominal obesity was the least comorbid condition among TB patients in this study, its high prevalence in this population is unexpected because TB patients are most often underweight. Nonetheless, this finding must be reported with caution due to the use of European thresholds to define obesity in this study [16].
Among patients with TB, young adults were less likely to have multimorbidity. The association between chronic comorbid disease and age is well established in the literature [25]. Previous studies reporting high prevalence of comorbidities in older populations support our finding that young adults under 35 are less likely to develop TB multimorbidity [19, 26]. In contrast to our study, a previous study reported a high incidence of TB among Brazilian women [19].
Our study has strengths and limitations. Data were collected sequentially from a diverse population in the largest TB treatment center in the country, and blood pressure and anthropometry were measured by trained personnel in accordance with international standards. However, as a single-center study conducted at a national referral hospital, the findings cannot be generalized to the general TB population. Nonetheless, these findings can be strengthened and used to advocate for the integration of chronic communicable and noncommunicable diseases in TB prevention and control.
Conclusion
In conclusion, we report a high prevalence of comorbid diseases among TB patients in the largest treatment center in Sierra Leone, with hypertension and diabetes being the most common. These findings support the current call for addressing comorbid non-communicable diseases in TB patients through integrated care in low-income countries, where the prevalence of non-infectious diseases is increasing.
Acknowledgements
We acknowledge the support provided by the staff and patients and patients’ relatives of the Chest Clinic of Connaught Hospital.
Abbreviations
- ACC
American College of Cardiology
- ADA
American Diabetes Association
- AHA
American Heart Association
- BMI
Body mass index
- BP
Blood pressure
- HIV
Human immunodeficiency virus
- MTB
Mycobacterium tuberculosis
- NCD
Non-communicable diseases
- TB
Tuberculosis
- WHO
World Health Organization
Author contributions
Conceptualization: SL, AFSA, PLV, GAY, GFD and JBWR. Methodology: SL, PLV and JEK. Formal analysis: AR O and EF. Data curation: PLV, JBK and AS. Supervision: SL and JEK. Resources: AS and SL. Writing-original draft preparation: SL, AFSA and EF. Writing-review and editing: GAY, SL, OA and AFSA.
Funding
Not applicable.
Data availability
The data is available at the University of Sierra Leone repository and will be available upon request.
Declarations
Ethics approval and consent to participate
The protocol was approved by the Institutional Review Board of the College of Medicine and Allied Health Sciences in accordance with the relevant guidelines and regulations and declaration of Helsinki. Written informed consent was obtained from each participant before participating in the study. If participants were illiterate, the study, and consent forms were explained verbally to them, and informed consent was given by legal guardian fingerprinting as approved by the Institutional Review Board of the College of Medicine and Allied Health Sciences, University of Sierra Leone. Participants who refused to consent were excluded from the study, but this did not affect their management.
Consent for publication
Not applicable.
Competing interests
EF receives his salary from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement (No 801076), through SSPH + Global PhD Fellowship Programme in Public Health Sciences (GlobalP3HS). GAY reports salary support from the National Institutes of Health/AIDS Clinical Trials Group under Award Numbers 5UM1AI068636-15, 5UM1AI069501-09 and AI068636(150GYD212), and consultancy fees from Pfizer. All other authors do not have any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siddiqi K, Stubbs B, Lin Y, Elsey H, Siddiqi N. TB multimorbidity: a global health challenge demanding urgent attention. Int J Tuberc Lung Dis. 2021;25(2):87–90. doi: 10.5588/ijtld.20.0751. [DOI] [PubMed] [Google Scholar]
- 2.Foguet-Boreu Q, Violan C, Roso-Llorach A, Rodriguez-Blanco T, Pons-Vigués M, Muñoz-Pérez MA, Pujol-Ribera E, Valderas JM. Impact of multimorbidity: acute morbidity, area of residency and use of health services across the life span in a region of south Europe. BMC Fam Pract. 2014;26(15):55. doi: 10.1186/1471-2296-15-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. Epub 2018 Nov 8. Erratum in: Lancet. 2019 Jun 22;393(10190): e44. Erratum in: Lancet. 2018 Nov 17;392(10160):2170. [DOI] [PMC free article] [PubMed]
- 4.World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020; online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf. Accessed 24 Oct 2022.
- 5.Creswell J, Raviglione M, Ottmani S, Migliori GB, Uplekar M, Blanc L, Sotgiu G, Lönnroth K. Tuberculosis and noncommunicable diseases: neglected links and missed opportunities. Eur Respir J. 2011;37(5):1269–1282. doi: 10.1183/09031936.00084310. [DOI] [PubMed] [Google Scholar]
- 6.Alebel A, Wondemagegn AT, Tesema C, Kibret GD, Wagnew F, Petrucka P, Arora A, Ayele AD, Alemayehu M, Eshetie S. Prevalence of diabetes mellitus among tuberculosis patients in Sub-Saharan Africa: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2019;19(1):254. doi: 10.1186/s12879-019-3892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seegert AB, Patsche CB, Sifna A, Gomes VF, Wejse C, Storgaard M, Rudolf F. Hypertension is associated with increased mortality in patients with tuberculosis in Guinea-Bissau. Int J Infect Dis. 2021;109:123–128. doi: 10.1016/j.ijid.2021.06.062. [DOI] [PubMed] [Google Scholar]
- 8.Nawaz H, Bibi S, Rabia M. Meta-analysis of diabetes mellitus prevalence among tuberculosis patients in Asia and Africa. J Pak Med Assoc. 2021;71(4):1200–1205. doi: 10.47391/JPMA.846. [DOI] [PubMed] [Google Scholar]
- 9.Jarde A, Romano E, Afaq S, Elsony A, Lin Y, Huque R, Elsey H, Siddiqi K, Stubbs B, Siddiqi N. Prevalence and risks of tuberculosis multimorbidity in low-income and middle-income countries: a meta-review. BMJ Open. 2022;12(9):e060906. doi: 10.1136/bmjopen-2022-060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sierra Leone Fourth Human Development Report 2019. https://www.undp.org/sites/g/files/zskgke326/files/migration/sl/undp_sle_NHDR-2019.pdf Accessed 30 Oct 2022.
- 11.Geraedts TJM, Boateng D, Lindenbergh KC, van Delft D, Mathéron HM, Mönnink GLE, Martens JPJ, et al. Evaluating the cascade of care for hypertension in Sierra Leone. Trop Med Int Health. 2021;26(11):1470–1480. doi: 10.1111/tmi.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundufu AJ, Bockarie CN, Jacobsen KH. The prevalence of type 2 diabetes in urban Bo, Sierra Leone, and in the 16 countries of the West Africa region. Diabetes Metab Res Rev. 2017;33:7. doi: 10.1002/dmrr.2904. [DOI] [PubMed] [Google Scholar]
- 13.Lakoh S, Jiba DF, Kanu JE, Poveda E, Salgado-Barreira A, Sahr F, Sesay M, Deen GF, Sesay T, Gashau W, Salata RA, Yendewa GA. Causes of hospitalization and predictors of HIV-associated mortality at the main referral hospital in Sierra Leone: a prospective study. BMC Public Health. 2019;19(1):1320. doi: 10.1186/s12889-019-7614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakoh S, Jiba DF, Adekanmbi O, Poveda E, Sahr F, Deen GF, Foray LM, Gashau W, Hoffmann CJ, Salata RA, Yendewa GA. Diagnosis and treatment outcomes of adult tuberculosis in an urban setting with high HIV prevalence in Sierra Leone: a retrospective study. Int J Infect Dis. 2020;96:112–118. doi: 10.1016/j.ijid.2020.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018 Jun;71(6): e13-e115. doi: 10.1161/HYP.0000000000000065. Epub 2017 Nov 13. Erratum in: Hypertension. 2018 Jun;71(6): e140-e144. PMID: 29133356. [DOI] [PubMed]
- 16.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 17.Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64. doi: 10.1016/j.clnu.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Oni T, Youngblood E, Boulle A, McGrath N, Wilkinson RJ, Levitt NS. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study. BMC Infect Dis. 2015;17(15):20. doi: 10.1186/s12879-015-0750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reis-Santos B, Gomes T, Macedo LR, Horta BL, Riley LW, Maciel EL. Prevalence and patterns of multimorbidity among tuberculosis patients in Brazil: a cross-sectional study. Int J Equity Health. 2013;20(12):61. doi: 10.1186/1475-9276-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry KM, Parker WA, Mchiza ZJ, Sewpaul R, Labadarios D, Rosen S, Stokes A. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011–2012. BMJ Glob Health. 2017;2(3):e000348. doi: 10.1136/bmjgh-2017-000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crim MT, Yoon SS, Ortiz E, Wall HK, Schober S, Gillespie C, Sorlie P, Keenan N, Labarthe D, Hong Y. National surveillance definitions for hypertension prevalence and control among adults. Circ Cardiovasc Qual Outcomes. 2012;5(3):343–51. doi: 10.1161/CIRCOUTCOMES.111.963439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO (2011) framework for the care and control of diabetes. Available at: https://apps.who.int/iris/handle/10665/44698 Accessed 29 Oct 2022.
- 23.Sierra Leone Demographic Health Survey (SLDHS) 2019. Available at: https://dhsprogram.com/pubs/pdf/PR122/PR122.pdf Accessed 30 Oct 2022.
- 24.Lakoh S, Jiba DF, Baldeh M, Adekanmbi O, Barrie U, Seisay AL, Deen GF, Salata RA, Yendewa GA. Impact of COVID-19 on Tuberculosis Case Detection and Treatment Outcomes in Sierra Leone. Trop Med Infect Dis. 2021;6(3):154. doi: 10.3390/tropicalmed6030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakoh S, Firima E, Jiba DF, Sesay M, Conteh MM, Deen GF. Low partner testing in high HIV prevalence setting in Freetown, Sierra Leone: a retrospective study. BMC Res Notes. 2019;12(1):629. doi: 10.1186/s13104-019-4662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10(2):142–151. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available at the University of Sierra Leone repository and will be available upon request.