Abstract
Mammals and higher vertebrates including humans have only three members of the carotenoid cleavage dioxygenase family of enzymes. This review focuses on the two that function as carotenoid oxygenases. β-Carotene 15,15’-dioxygenase (BCO1) catalyzes the oxidative cleavage of the central 15,15’ carbon-carbon double of β-carotene bond by addition of molecular oxygen. The product of the reaction is retinaldehyde (retinal or β-apo-15-carotenal). Thus, BCO1 is the enzyme responsible for the conversion of provitamin A carotenoids to vitamin A. It also cleaves the 15,15’ bond of β-apocarotenals to yield retinal and of lycopene to yield apo-15-lycopenal. β-Carotene 9’,10’-dioxygenase (BCO2) catalyzes the cleavage of the 9,10 and 9’,10’ double bonds of a wider variety of carotenoids, including both provitamin A and non-provitamin A carotenoids, as well as the xanthophylls, lutein and zeaxanthin. Indeed, the enzyme shows a marked preference for utilization of these xanthophylls and other substrates with hydroxylated terminal rings. Studies of the phenotypes of BCO1 null, BCO2 null, and BCO1/2 double knockout mice and of humans with polymorphisms in the enzymes, has clarified the role of these enzymes in whole body carotenoid and vitamin A homeostasis. These studies also demonstrate the relationship between enzyme expression and whole body lipid and energy metabolism and oxidative stress.
In addition, relationships between BCO1 and BCO2 and the development or risk of metabolic diseases, eye diseases and cancer have been observed. While the precise roles of the enzymes in the pathophysiology of most of these diseases is not presently clear, these gaps in knowledge provide fertile ground for rigorous future investigations.
1. INTRODUCTION
1.1. Historical Background
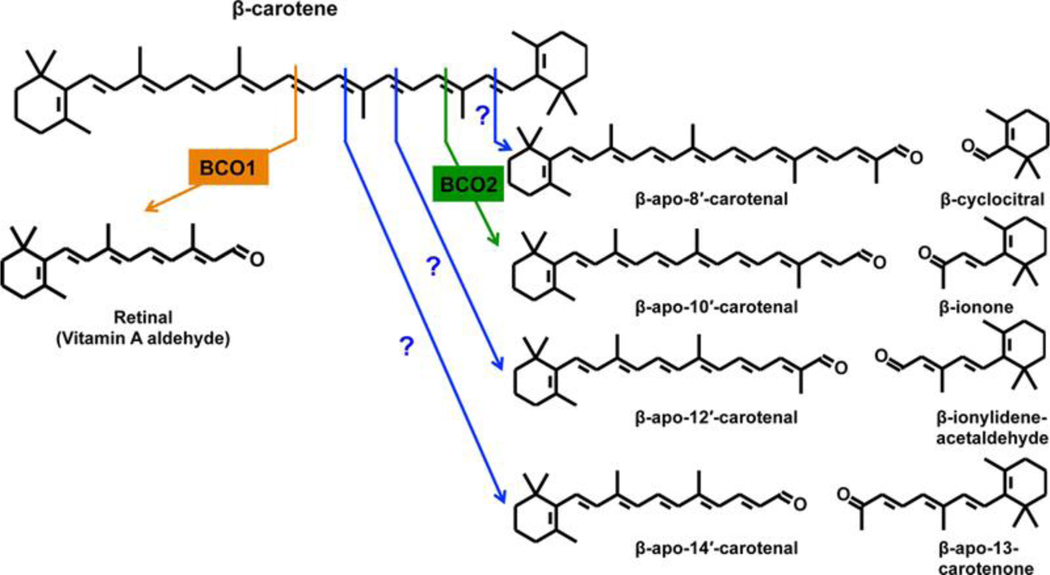
Early studies on carotenoid metabolism in animals focused on provitamin A carotenoids and their enzymatic conversion to vitamin A. The first in vitro studies published in 1965 used extracts from rat liver and intestinal mucosa to demonstrate enzymatic oxidative cleavage of β-carotene at the central double bond to yield retinal [1,2]. In 2000, the enzyme responsible for the conversion of β-carotene to retinal was identified in Drosophila [3] and chicken [4], and was initially named β-carotene-15,15′-oxygenase (BCO1). BCO1 has also been identified in humans [5–7], mice [8,9], rat [10], cows [11], zebrafish [12] and C. elegans [13]. In 2001, another carotenoid cleavage enzyme that catalyzes eccentric cleavage, β-carotene-9′,10′-oxygenase (BCO2), was identified in human, mouse and zebrafish [14]. The action of BCO1 and BCO2 on β-carotene are shown in Figure 1.
Figure 1.
Central and eccentric cleavages of β-carotene. Oxidative cleavage of β-carotene at the 15, 15’ double bond is catalyzed by the enzyme β-carotene 15, 15’-oxygenase 1 (BCO1) and leads to the generation of two molecules of retinal (β-apo-15-carotenal). The cleavage at the 9’, 10’ double bond is catalyzed by β-carotene 9’,10’-oxygenase 2 (BCO2) and leads to the formation of β-apo-10’-carotenal and β-ionone. Cleavage at other double bonds can occur non-enzymatically but may also be enzymatic, as noted by the question marks in blue.
1.2. Scope
As discussed in the review by Polikov et al. in this issue, carotenoid cleavage oxygenase family members are present in all kingdoms of life. Mammals and higher vertebrates have only 3 family members. These are BCO1 and BCO2, which catalyze oxidative cleavage of carbon-carbon double bonds. The third is the retinoid isomerohydrolase that catalyzes the concerted hydrolysis of all-trans retinyl ester to yield 11-cis-retinol in the visual cycle [15]. The details of the role of this enzyme in vision is presented in the reviews on carotenoids in vision in this issue and is not covered here. The focus of this review is on the enzymatic properties and functions of BCO1 and BCO2 in higher vertebrates and mammals, including humans. Details of the protein structures and enzyme reaction mechanisms are presented in the review by Kiser in this issue. A number of previous reviews have summarized earlier work on the role of mammalian carotenoid oxygenases in carotenoid and vitamin A homeostasis [16,17], the molecular regulation of BCO1 [18,19], and aspects of the substrate specificity and properties of BCO1 and BCO2 [20]. This review will focus mainly on research published in the last decade.
2. PROPERTIES AND SUBSTRATE SPECIFICITY OF ISOLATED ENZYMES
2.1. β-Carotene 15, 15’ oxygenase (BCO1)
In 2013, Kowatz et al. reported on the properties and cellular localization of human BCO1 expressed in Sf9 insect cells [21]. They reported that the enzyme functioned as a monomer with a MW of 60 kDa. The enzyme had a Km of 14 μM, and a Vmax of 390 pmoles of retinal x min−1 x mg−1 using β-carotene as the substrate. That same year we reported that human BCO1 expressed in E. coli and purified to homogeneity had a Km of 17 μM and Vmax of 3290 pmoles of retinal x min−1 x mg−1 [22]. The enzyme was also able to catalyze the 15,15’ bond cleavage of α-carotene, β-cryptoxanthin, β-apo-8’-, −10’-, −12’-, −14’-carotenals, and all-trans lycopene but not zeaxanthin nor lutein, which contain hydroxylated β- and ε-rings [22]. Amengual et al. (2013) further showed that human BCO1 expressed in Sf9 cells also cleaved the 15,15’ bond of β-apo-10’-carotenol and β-apo-12’-carotenoic acid [23]. This substrate specificity suggests that BCO1 has an active site that can only accommodate carotenoids with an unsubstituted β-ring (as in α- and β-carotene, β-cryptoxanthin or the β-apo-carotenals) or an uncyclized terminus that can adopt such a conformation (as in lycopene). This suggestion was indeed demonstrated in the recent elegant work by von Lintig and colleagues [24] in which they studied the products formed from β-cryptoxanthin by human BCO1 and mouse BCO2 (as discussed below).
2.2. β-Carotene 9’, 10’ oxygenase (BCO2)
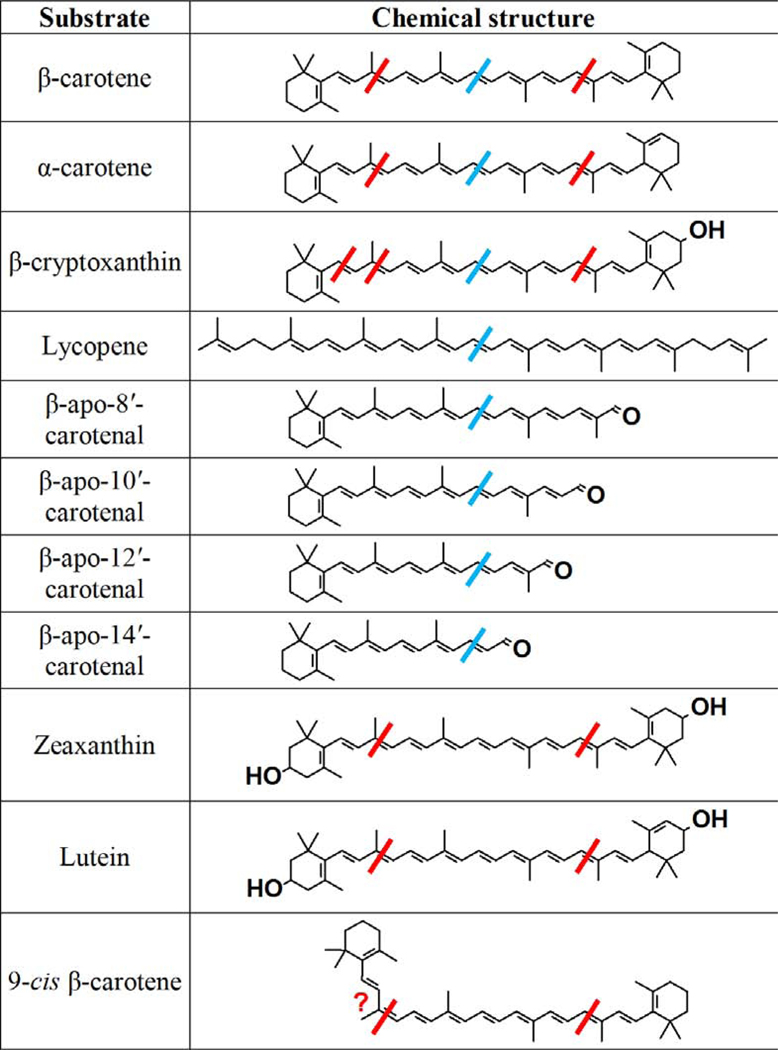
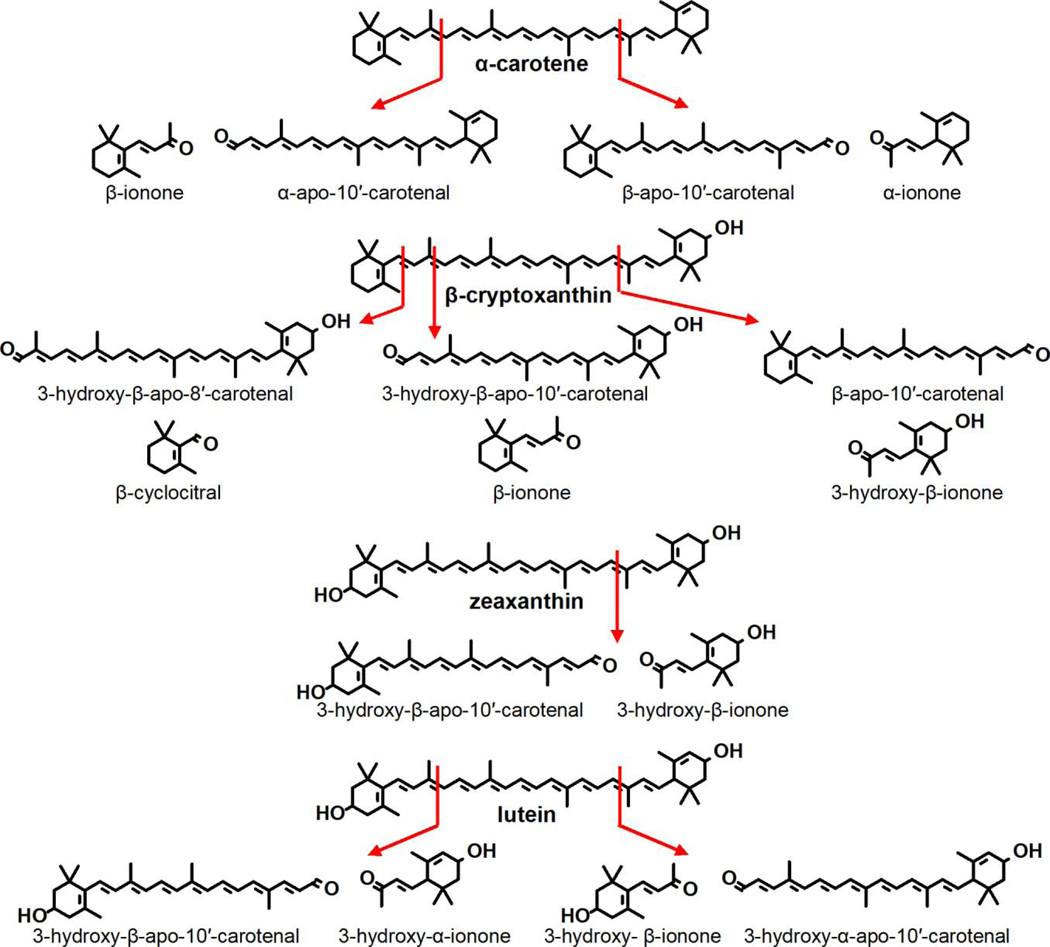
In contrast to BCO1, BCO2 is a mitochondrial protein that can utilize a wide variety of substrates and cleave them at the 9’,10’ bond. Kelly et al. [24] showed that while BCO2 can catalyze the cleavage of both the 9,10 bond and the 9’10’ bond of β-cryptoxanthin there is an order of magnitude higher catalytic efficiency for cleavage at the latter. Thus, BCO2 shows strong substrate specificity for substrates with 3-hydroxy-β-ionone rings. This explains why both mouse, ferret and chicken BCO2 catalyze cleavage of the xanthophylls, lutein and zeaxanthin [25–27]. Our work on purified chicken BCO2 agrees with that of Kelly et al. on the substrate specificity of mouse BCO2. Namely, we observed the cleavage of the 9,10 and 9’,10’ bond of both provitamin A carotenoids (viz., β-carotene, α-carotene, and β-cryptoxanthin) and of the xanthophylls, zeaxanthin and lutein [27]. Likewise, we found that the 3-hydroxy substrates are much preferred to β-carotene with its non-hydroxylated rings. Figure 2 shows the cleavages catalyzed by vertebrate BCO1 and BCO2 as determined by the various studies cited here. Figure 3 shows the products of BCO2-catalyzed cleavage of substrates other than β-carotene.
Figure 2.
Cleavage sites of various substrates by BCO2 and BCO1. The red bars represent the cleavages observed with BCO2, and the blue bars, BCO1.
Figure 3.
Structures of the 9,10 and 9’,10’ cleavage products of α-carotene, β-cryptoxanthin, zeaxanthin, lutein by avian BCO2.
While it is clear that purified murine and avian BCO2 catalyze efficiently the oxidative cleavage of xanthophylls, whether the human enzyme does so is still a matter of some debate. Before turning our attention to expressing the avian enzyme, we attempted to do the same with the five isoforms of human BCO2 but were unable to isolate active enzyme [27]. All five isoforms have a GKAA insertion that differentiates them from other BCO2 vertebrate orthologs including mouse and chicken. However, another primate, the macaque also has the GKAA insertion, and yet that protein displays robust activity [28]. Nonetheless, Li et al. (2014) have shown that deletion of the GKAA cannot convert their human BCO2 to an active enzyme, and conversely demonstrated that insertion of GKAA into mouse BCO2 was able to inactivate it [29]. Their structural homology modeling did suggest that the insertion led to a structural change that might be relevant to activity. Consistent with this hypothesis, they found that the binding affinities of xanthophylls to the human enzyme was 10–40-fold weaker than those for the mouse enzyme, causing them to conclude that the lack of activity of the human enzyme leads to the accumulation macular carotenoids in the human retina. In contrast, structural homology modeling data from Babino et al. [28] indicated that the GKAA in the human enzyme did not significantly alter the overall structure as compared to the macaque protein. Similarly, no change in overall structure was observed when the GKAA insertion was deleted from the two proteins. Thus, they suggest that the presence of the insertion into primate BCO2s does not cause inactivation of the enzyme. The bulk of available evidence is consistent with that suggestion. Given that an important general function of BCO2 is protection of mitochondrial oxidative stress [26], it is unclear how loss of function could be tolerated in humans. Thus, it will be important to clarify the exact nature of the activity of BCO2 in humans and primates, and its regulation in various tissues.
3. FUNTIONAL STUDIES OF VETEBRATE BCO1 AND BCO2 USING TRANSGENIC AND KNOCKOUT MODELS
As with many enzymes, understanding of the physiological roles of BCO1 and BCO2 benefits from the careful study of the phenotype of knockout and transgenic animal models. Johannes von Lintig and his colleagues, who have generated BCO1 null, BCO2 null, and double knockout mice and generously provided them to investigators in the field, initiated this work. They themselves have conducted seminal investigations of these models to assess the role of the carotenoid dioxygenases in mammalian nutrition, physiology, and pathophysiology. In this section, we review the recent literature using these models beginning with the insights provided on the enzymes’ roles in carotenoid and vitamin A homeostasis. We then discuss phenotypes of BCO1/2 null animals that are not obvious and/or directly related to carotenoid and retinoid metabolism and action or to the known substrates or products of these enzymes. Finally, we consider briefly recent work on BCO1 and/or BCO2 in animals other than mice.
3.1. Phenotypic Changes in BCO1 Knockout Mice
One of the first important insights provided by the study of BCO1 knockout mice was unambiguous demonstration of the primary pathway of vitamin A biosynthesis from dietary provitamin A carotenoids. Thus, BCO1 deficiency completely abolished vitamin A production from dietary β-carotene, indicating that BCO1 is both necessary and sufficient for the process, and further indicating that other possible pathways are of little physiological relevance [30]. BCO1 deficiency also had more modest but important effects on whole body lipid metabolism. Specifically, the knockout mice developed fatty livers, altered levels of serum lipids, and elevation of PPAR-activated genes related to adipogenesis. This suggested that accumulation of tissue carotenoids (normally converted to vitamin A), or their metabolites, may play a role as regulators of lipid metabolism in both liver [30] and adipocytes [31]. Another report demonstrated elevation of hepatic triglyceride accumulation and elevation in PPARγ in BCO1 deficient mice and demonstrated that BCO1 (both mRNA and protein) was highly enriched in hepatic stellate cells as compared to hepatocytes [32]. BCO1 deficient mice also have significantly altered lipid and retinoid metabolism in the heart associated with compromised heart function, characterized by reduced contractility [33]. Thus, BCO1 deficiency leads to multiple tissues with aberrant carotenoid, retinoid, and lipid metabolism and signaling.
3.2. Phenotypic Changes in BCO2 Knockout Mice
Generation of the BCO2 null [26] and the BCO1/BCO2 double knockout mouse [23] has provided novel and important insight into the possible physiological role of this carotenoid oxygenase and the consequences of either β-carotene or xanthophyll accumulation in tissues. In addition to demonstrating the mitochondrial localization of the enzyme and its broader substrate specificity as discussed above, Amengual et al. [26] suggested an important role for BCO2 in protecting mitochondria from oxidative stress. Thus, carotenoid (xanthophyll) accumulated in several tissues, including liver. In liver, this induced mitochondrial dysfunction and reduced rates of respiration. The normal presence of BCO2 thus initiates the degradation of the carotenoids and protects the mitochondria from oxidative damage.
Ford et al. [34] found that BCO2 knockout mice accumulate lycopene after consuming a tomato-supplemented diet containing primarily all-trans lycopene, suggesting that it is a substrate for BCO2. However, this hypothesis is not consistent with the observed substrate specificity of purified chicken BCO2, which did not utilize all-trans lycopene as a substrate [27], as also reported by Hu et al. [35] for the ferret enzyme. However, ferret BCO2 did cleave 5-cis- and 13-cis-lycopene [35], so the accumulated lycopene seen by Ford et al. could be due to accumulation of cis-isomers.
3.3. Effects of BCO1 and BCO2 on Carotenoid and Lipid Metabolism
BCO1 knockout mice have elevated expression of BCO2 and vice versa [23,36,37]. Upon β-carotene supplementation, BCO1 KO mice accumulate β-apo-10’-carotenol, the alcohol form of the BCO2 cleavage product of β-carotene, namely β-apo-10’-carotenal [23,26]. BCO2 knockout mice accumulate 3,3’-didehydrozeaxanthin and 3-dehydrolutein upon supplementation with zeaxanthin and lutein, respectively [26]. This is consistent with the biochemical findings that BCO1 cannot utilize xanthophylls as substrates.
Using the BCO1/BCO2 double knockout the von Lintig laboratory was able to use whole genome microarray analyses to assess the effects of either β-carotene or zeaxanthin feeding on the liver transcriptome [38]. β-Carotene accumulation resulted increases in hepatic triglycerides and cholesterol, whereas zeaxanthin accumulation resulted in increased serum cholesterol levels. Accumulation of either carotenoid slightly reduced both whole body respiration and energy expenditure. Both the changes in lipid and energy metabolism could be interpreted in terms of the transcriptomic and pathway analyses, and the approach offers to reveal more insight into roles of carotenoids in whole body metabolism.
3.4. Effects of BCO1 and BCO2 on Hepatic Steatosis and Liver Cancer
Given the rather consistent observations that BCO1/2 ablation in mice leads to fatty liver and changes in lipid metabolism, it is not surprising that investigators have used these mouse models to study the underlying mechanisms involved in the development of hepatic steatosis and its sequellae including formation of liver tumors. The carotenoids most studied in this regard are lycopene and more recently β-cryptoxanthin. Indeed, the review by Wang in this issue discusses this extensive literature in detail. Here we only briefly summarize a few recent studies to demonstrate the complexity of the mechanisms by which BCO1/2 impact the development of metabolic disorders and cancer. In one recent study, Wang and colleagues [39] studied the induction of hepatic steatosis in male BCO1/2 double knockout mice. The knockout animals showed increased liver steatosis and increases in hepatic triglycerides and cholesterol levels as previously demonstrated by the work of Palczewski et al. [38] just discussed. Wang and colleagues also observed elevations in cholesterol metabolism, fatty acid synthesis, and oxidative stress [39]. Importantly they observed decreases in the nuclear farnesoid X receptor (FXR) and decreases in microRNAs (miR-33 and miR-122) that are downstream targets of FXR and activators of sirtuin 1 (SIRT1). They propose that the BCO1/2 knockout inactivates the FXR/miR34a/SIRT1 signaling pathway and that the role of BCO1/2 may be independent of their carotenoid cleavage activity. Subsequent work by the same group [40] showed that supplementation of β-cryptoxanthin inhibits dietary-induced fatty liver disease via different mechanisms in wild type and BCO1/2 knockout mice. They again give evidence that FXR and SIRT1 are involved in this differential response. The very complex emerging picture of the roles of BCO1 and/or BCO2 in disease calls for continued research.
3.5. Summary of Murine Knockout Studies
In summary, studies of the metabolism of carotenoid and retinoids in in BCO1 null, BCO2 null and double knockout mice has provided important insight into the roles that these mammalian carotenoid oxygenases play in whole body physiology due to their tissue distribution, subcellular localization, and utilization of specific carotenoid substrates. The phenotypes of the various knockout mice in terms of more global effects on the transcriptome, metabolome, signaling pathways, and ultimately on the development of disease are more difficult to understand in terms of the current knowledge of the enzymology of the carotenoid oxygenases. It may be that there are yet unrecognized substrates for these enzymes that act either directly, or act when converted to metabolic products. These relatively small molecules could affect physiology in any of the ways that other hormones and bioactive molecules do. This review argues that a search for these other substrates would be worthwhile. Alternatively, it may be that the proteins themselves have direct activity unrelated to their function as enzymes. For example, there is evidence that BCO2 can act directly as a tumor suppressor in prostate cancer (see reference [41]). These two alternatives are not mutually exclusive and both deserve continued investigation.
4. ROLE OF BCO1 IN HUMAN CAROTENOID METABOLISM
4.1. Correlation with Circulating Carotenoid Concentrations
Evidence of a carotenoid cleavage enzyme influencing circulating β-carotene and vitamin A concentrations in humans has been observed for many years. Case studies dating back to 1958 identified individuals presenting with carotenemia (orange skin resulting from excess deposition of β-carotene in subcutaneous adipose stores) but typical β-carotene intakes, in combination with either deficient blood retinol concentrations (i.e. < 0.70 μM) [42–45], or marginal but clinically “sufficient” retinol concentrations [42,46,47]. A number of these studies also tested first degree relatives of the patients in question, and observed the same disparity in high β-carotene and lower-than-average blood retinol concentrations, highlighting the heritability of this phenomena [42,43,47]. It is now believed that this disparity can be attributed to single nucleotide polymorphisms (SNPs) in the BCO1 gene, which influence the ability of the BCO1 enzyme to produce vitamin A from β-carotene [48].
Focused efforts to identify SNPs in or near BCO1 influencing circulating carotenoid and/or and retinol concentrations in humans have been published since 2009. This review will focus on “functional variants”, i.e. BCO1 SNPs which correlate with or influence carotenoid concentrations in humans. However, it should be noted that the references cited herein also investigated multiple BCO1 SNPs for which no relationship was observed. Ferrucci et al. initially used a genome-wide association approach to identify common genetic variants associated with circulating carotenoid concentrations in 1,190 participants of the InCHIANTI study [49]. Polymorphisms in 5 BCO1 SNPs were significantly associated with plasma β-carotene and/or lutein concentrations (Table 1). Follow-up analyses in a subset of participants from the Women’s Health and Aging Studies I and the placebo arm of the α-Tocopherol, β-Carotene Cancer Prevention Study found the most consistent association with G-allele carriers of rs6564851, increasing plasma β-carotene and decreasing lutein concentrations in all groups studied [49]. The same SNP was also significantly associated with increased circulating α-carotene, and decreased lycopene and zeaxanthin concentrations, with no association for β-cryptoxanthin nor retinol concentrations [49]. This BCO1 SNP (rs6564851) is the best studied to date in all populations. GG homozygotes in a group of 92 healthy Japanese adults also had significantly higher circulating β-carotene concentrations as compared to T allele carriers [50]. Likewise, a study of the TwinsUK cohort (n = 310) confirmed previous observations of circulating plasma lutein concentrations and BCO1 SNP rs6564851[51]. A study of postmenopausal women (n = 1643) of the Carotenoids in Age-Related Eye Disease Study also found serum concentrations of both lutein and zeaxanthin to be correlated with rs6564851, as well as rs11645428 and rs7500996 [52].
Table 1.
Previously conducted studies of BCO1 SNPs with significant relationships to carotenoid and retinol concentrations and/or disease
| SNP Accession Number | Location relative to BCO11 | Alleles | Second Allele Frequency | Second Allele Effect2 | Measure(s) | Population Studied |
|---|---|---|---|---|---|---|
| rs48892864 | upstream | C/T | 0.50 | + | circulating BC, AC | NHS [53] |
| rs4889293 | within (intron variant) | C/G | 0.42 | + | circulating AC | NHS [53] |
| rs6420424 | upstream | G/A | 0.34, 0.49 | + | circulating BC | inCHIANTI, WHAS [49] |
| G/A | 0.45 | - | post-prandial ROL/BC | Healthy UK women (n = 28) [58] | ||
| rs65648513,4 | upstream | T/G | 0.36, 0.48, 0.39 | + | circulating BC, AC, LYC | inCHIANTI, WHAS, ATBC (placebo arm only)[49] |
| T/G | 0.36, 0.48, 0.39 | - | circulating LUT, ZEA | inCHIANTI, WHAS, ATBC (placebo arm only)[49] | ||
| T/G | 0.50 | + | circulating BC, AC, BCrypt | NHS [53] | ||
| T/G | 0.50 | - | circulating LUT/ZEA | NHS [53] | ||
| T/G | 0.48 | - | post-prandial ROL/BC | Healthy UK women (n = 28) [58] | ||
| T/G | not available | + | BC uptake after 54 d supplementation | Healthy UK women (n=85)[59] | ||
| T/G | 0.82 | + | circulating BC, daily BC intake | Healthy Japanese men & women (n=92)[50] | ||
| C/A | 0.49 | + | circulating LUT+ZEA | CAREDS postmenopausal women (n=1663) [52] | ||
| T/G | 0.57 | + | circulating LYC after ~25 days tomato juice supplementation; prostate BC and LYC | American men with prostate cancer (n=47)[60] | ||
| rs6564863 | within (intron variant) | G/A | 0.34 | + | MPOD | CAREDS postmenopausal women (n=1585) [65] |
| rs7196470 | downstream | C/A | 0.24 | - | post-prandial BC | Healthy French men (n = 33)[54] |
| rs7500996 | within (intron variant) | A/G | 0.18 | + | circulating LUT+ZEA | CAREDS postmenopausal women (n=1663) [52] |
| rs7501331 | within (missense variant) | C/T | 0.24 | - | circulating BC, AC | NHS [53] |
| C/T | 0.76 | - | circulating LUT | Healthy French men & women (n=28) [66] | ||
| C/T | 0.76 | + | MPOD after 6 mo. LUT supplementation | Healthy French men & women (n=28) [66] | ||
| C/T | 0.24 | + | circulating BC | Healthy UK women (n = 28) [57] | ||
| C/T | 0.24 | - | postprandial ROL/BC | Healthy UK women (n = 28) [57] | ||
| C/T | 0.29 | - | prostate BC after ~25 days tomato juice supplementation | American men with prostate cancer (n=47)[60] | ||
| rs8044334 | upstream | T/G | 0.24, 0.36, 0.35 | + | circulating BC | inCHIANTI, WHAS, ATBC (placebo arm only)[49] |
| rs10048138 | within | G/A | 0.14 | + | circulating LUT+ZEA | NHS [53] |
| G/A | 0.31 | + | post-prandial ROL/BC | Healthy UK women (n = 28) [58] | ||
| rs11645428 | upstream | G/A | 0.47, 0.33, 0.28 | - | circulating BC | inCHIANTI, WHAS, ATBC (placebo arm only)[49] |
| G/A | 0.33 | + | MPOD | CAREDS postmenopausal women (n=1585) [65] | ||
| G/A | 0.33 | + | circulating LUT+ZEA | CAREDS postmenopausal women (n=1663) [52] | ||
| G/A | 0.33 | - | AMD | CAREDS postmenopausal women (n=1663) [52] | ||
| G/A | 0.29 | + | post-prandial ROL/BC | Healthy UK women (n = 28) [58] | ||
| rs129349223 | within (missense variant) | A/T | 0.44 | + | circulating BC, AC, LUT+ZEA | NHS [53] |
| A/T | 0.44 | - | circulating ROL | NHS [53] | ||
| A/T | 0.44 | - | circulating BC and LYC after ~25 days of tomato juice supplementation; prostate BC and LYC | American men with prostate cancer (n=47)[60] | ||
| rs16955008 | downstream | C/A | 0.12 | + | AMD | CAREDS postmenopausal women (n=1663) [52] |
| rs56389940 | downstream | C/A | 0.36 | + | circulating LUT+ZEA | NHS [53] |
Location (i.e. upstream, within, or downstream) relative to the coding region of BCO1, and impact on the BCO1 gene (if known), verified via the NIH SNP database (https://www.ncbi.nlm.nih.gov/snp/) utilizing the Genome Reference Consortium Human Build 38 (https://www.ncbi.nlm.nih.gov/assembly/GCF_000001405.39).
Effect direction refers to the second allele noted in the previous column.
Significant multi-SNP interaction on circulating BC and ROL and breast cancer risk in the population after adjustment for alcohol and pack-years of smoking in NCI BPC population [67]
Trend for multi-SNP interaction with provitamin A and vitamin A intakes and CRC risk, after adjustment for age, smoking, alcohol, and NSAIDS in DDCH population [68]
Additional BCO1 SNPs of interest were also identified by Hendrickson et al., who used both GWAS and TaqMan genotyped data of 1563 subjects of the Nurses’ Health Study (NHS) [53]. Of the initial 224 BCO1 SNPs studied, 2–3 SNPs of greatest influence on circulating carotenoid concentrations were sub-selected for each compound, and given a weighted gene score. This predictive model was then validated in a new (validation) set of 781 NHS subjects, and 15% – 48% of the difference in plasma carotenoid concentrations between the highest and lowest plasma concentration quintiles were explained by these weighted scores [53].
Notably, BCO1 SNPs explained a greater percentage of the variation in circulating carotenoid concentrations than carotenoid dietary intakes as assessed by food frequency questionnaire in the Japanese [50] and NHS [53] cohorts (dietary intake x genotype was not assessed by Ferrucci et al. [49]). Thus, genetic polymorphisms may be a better predictor of plasma carotenoid status than traditional dietary assessment, and these results should be replicated in additional populations to confirm the utility of BCO1 SNPs in determining carotenoid status.
4.2. Postprandial and Short-Term Dietary Interventions
Functional variants of BCO1 have also been shown to influence inter-individual differences of post-prandial carotenoid bioavailability following controlled dosing. In a study of 33 healthy French men, 69% of the inter-individual difference in newly-absorbed β-carotene (0.4 mg dose) could be attributed to SNPs in 12 genes, one of which one was BCO1 rs7196470 [54]. However, no relationship between BCO1 SNPs and postprandial lycopene nor lutein absorption was observed in the same population [55,56]. Another study by Leung et al. tested the effect of a single 120 mg dose of BC on the triglyceride-rich lipoprotein (TRL) fraction of β-carotene and newly formed retinol (measured as retinyl esters) 3 hours following consumption in 28 healthy women [57]. SNP in BCO1 rs7501331 alone and in combination with rs12934922 led to a stepwise reduction in newly formed vitamin A relative to β-carotene in the TRL fraction [57]. A secondary analysis of the same study observed 1.5–2.5 fold more newly converted vitamin A for carriers of the BCO1 SNPs rs11645428-AA, rs6420424-GG, and rs6564851-TT, as compared to individuals carrying the alternative homozygous allele (i.e. rs11645428-GG, rs6420424-AA, and rs6564851-GG)[58].
SNPs in BCO1 have also been shown to influence changes in plasma carotenoid concentrations over a short-term period of prospective dietary intervention. Healthy women in the United Kingdom consumed one of three formulations of β-carotene (delivering 7 mg/day, n ~ 28 per formulation) for 46 days [59]. Significant interaction between plasma β-carotene and SNPs in BCO1 rs6564851 were observed for all three β-carotene formulations [59]. Likewise, a study in prostate cancer subjects also observed the correlation between BCO1 rs6564851 as well as rs12934922 and change in plasma lycopene and β-carotene following 0, 1, or 2 cans of tomato juice (20.6 mg lycopene + 1.2 mg β-carotene/can) daily for ~25 days [60]. Genotype alone was significantly correlated with change in plasma β-carotene, while genotype x diet interaction was correlated with change in plasma lycopene concentrations. Following prostatectomy, β-carotene and lycopene concentrations were also measured, with significant correlations observed with genotype and prostate lycopene and β-carotene concentrations, and a genotype x diet interaction also observed with prostate β-carotene concentrations [60].
Although only a few studies have been conducted to-date, it is clear that BCO1 SNPs influence postprandial carotene and retinol concentrations, as well as circulating and prostate carotene concentrations following a short-term dietary intervention. However, the effect of BCO1 SNPs on postprandial and short-term xanthophyll concentrations remains underexplored. Likewise, the ability of carotenoid supplementation to “overcome” a low provitamin A absorption and conversion phenotype arising from one or more BCO1 SNPs remains largely unknown.
4.3. Correlation with Macular Pigment Optical Density
Particular interest exists in regards to BCO1 SNPs and macular pigment optical density (MPOD) due to the role lutein and zeaxanthin are believed to play in protection from high-intensity blue light. The relationship between xanthophylls, and a carotenoid cleavage enzyme for which they do not serve as a substrate, may initially seem perplexing [22,23]. However, the downstream product retinoic acid has been shown to impact expression of uptake transporters (i.e. scavenger receptor class B type I, SR-B1) of the small intestine [19]. SR-B1 is the main transporter which effects both dietary carotene and xanthophyll absorption in human intestinal cells [61,62].
Feigl et al. studied BCO1 SNPs in 24 individuals with AMD vs. 20 healthy controls. The healthy controls with rs6564851 GG, rs11645428 GG, or rs6420424 AA genotypes had significantly lower MPOD compared to those with the other genotypes, while no relationship was observed between the same SNPs and MPOD in those with AMD [63]. In contrast, a second study by the same group in a different healthy cohort (n = 46 healthy men and women) found contrary results, i.e. no relationship between the same BCO1 SNPs and MPOD [64]. The disparity between these two studies may be due to the differences in the average age of the healthy controls (56 vs. 23 years of age, respectively).
A couple studies have also been conducted in post-menopausal women from the Carotenoids in Age-Related Eye Disease Study. In one study of CAREDS subjects who had both MPOD tested and genotyping performed (n = 1585) [65], BCO1 SNPs rs11645428 and rs6564863 were significantly associated with MPOD and together explained 1% of the variability in MPOD observed [65]. Follow-up work from the same group investigated the relationship between these SNPs and age-related macular degeneration (AMD), a disease for which evidence suggests increased MPOD (reflective of increased macular concentrations of lutein and zeaxanthin) may be protective. In this second CAREDS study (n = 1663), women with one or two A alleles of rs11645428 had 20% lower odds for a diagnosis of AMD [52].
Studies of lutein and zeaxanthin supplementation x BCO1 SNP interaction have reported mixed results on MPOD. Although baseline MPOD was significantly correlated with rs6564851 in the a subset of subjects in the TwinsUK cohort (n = 310), no correlation was observed with this SNP and MPOD following lutein and zeaxanthin supplementation for 6 mo. (i.e. a supplement consumed 3 x daily containing 18 mg lutein and 2.4 mg zeaxanthin) [51]. In contrast, a placebo-controlled, randomized study of 28 subjects from the SU.VI.MAX consuming lutein (10 mg/day for 6 mo.) found a higher MPOD response in subjects heterozygous for rs7501331, as compared to those who were homozygous for the dominant allele [66].
Taken together, these studies suggest that certain BCO1 SNPs may influence lutein and zeaxanthin concentrations, MPOD and/or AMD, and supplementation may alter status. However, the disparity of results suggests that subgroups of “benefit” (defined by age, disease status, or SNP) should be clearly defined in future work.
4.4. Relationship to Cancer Risk
Three studies have investigated how BCO1 SNPs are associated with cancer risk [67–69]. Hendrickson et al. examined the association between 4 BCO1 SNPs (see Table 1) and both plasma BC and ROL levels in a subset of subjects from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium (NCI BPC3) [67]. No interaction was observed between plasma carotenoids concentrations and individual BCO1 SNPs. However, a significant interaction between plasma provitamin A carotenoids (i.e. β-carotene, α-carotene, β-cryptoxanthin) and plasma retinol-weighted multi-SNP scores (rs6564851 + rs12934922) was found on estrogen receptor positive breast cancer risk adjusted for alcohol intake [67]. A significant interaction between provitamin A- and retinol-weighted multi-SNP scores were also observed for overall breast cancer risk following adjustment for pack-years of smoking (no interaction was observed for plasma xanthophylls) [67]. The Danish Diet, Cancer and Health (DDCH) cohort examined prospective dietary data collected on provitamin A and vitamin A consumption followed by 15 years of follow-up on colorectal cancer risk [68]. No interaction was observed between any one of the 3 BCO1 SNPs studied and dietary vitamin A intake on colorectal cancer risk. However, a statistical trend emerged for an interaction between dietary vitamin A and multiple SNPs (i.e. rs4889286 x rs6564851) on colorectal cancer risk [68]. Finally, a study conducted in an ethnic Han Chinese population investigated the influence of dietary patterns and 3 BCO1 SNPs (i.e. rs6564851, rs12934922, and rs7501331) on lung cancer risk [69]. Immediately following lung cancer diagnosis, in-person interviews were used to collect dietary information in 1,166 cases paired with 1,179 age- and gender-matched healthy controls [69]. A dose-dependent inverse association between a “fruit and vegetable” dietary pattern and odds ratio of lung cancer diagnosis was observed, irrespective of the SNP genotypes for any one of the 3 SNPs investigated (no multi-SNP interactions nor carotenoid intakes were studied in relation to the SNPs in this study) [69]. Due to the historical evidence suggesting a relationship between carotenoid intake and reduced cancer risk, additional studies in this area are certainly warranted.
4.5. Relationship to Other Physiological Processes
SNPs in BCO1 have also been investigated in relation to other processes of human health and disease, with mixed influence reported. Plasma concentrations of high density lipoproteins (HDL) adjusted for sex and body weight were positively correlated with BCO1 rs6564851 in two independent populations of male and female Caucasians in Sacramento, California (n = 249) and Beltsville, Maryland (n = 532) [70]. The mechanism(s) behind such a relationship are not clear. Likewise, the interaction of BCO1 polymorphisms and serum carotenoid status were observed in relation to HDL, metabolic disease, and symptoms of depression in African-American adults, finding no statistically significant correlation [71]. The potential inverse relationship between BCO1 rs6564851 and Type 2 diabetes (T2D) has also been explored. T2D is often accompanied with reduced circulating β-carotene concentrations, a phenomena hypothesized by Perry et al. to be causal [72]. Data collected from the InCHIANTI (9.4% T2D) and the Uppsala Longitudinal Study of Adult Men (ULSAM) populations was used to study the effect of rs6564851 on T2D incidence using a Mendelian randomization approach, with the model validated in Diabetes Genetics Replication And Metaanalysis (DIAGRAM) Consortium (n = 4,549 T2D cases and 5,579 healthy controls)[72]. No association was observed, thus rs6564851 is likely not a causal factor in T2D development[72]. Finally, BCO1 rs7501331 was also significantly associated with decreased odds of polycystic ovary syndrome diagnosis in a cohort of Polish women (n = 294 cases, 78 controls) [73].
5. ROLE OF BCO2 IN HUMAN AND ANIMAL CAROTENOID METABOLISM
The cell type-specific expression of BCO2 has been previously reported in humans [74], but much remains to be learned about the relative importance of functional variants in the gene itself [28], as well as post-transcriptional regulation [29], as discussed earlier. Here we focus solely on BCO2 SNPs, and due to the limited number of studies on BCO2 SNPs in humans, we summarize results of all variants (i.e. both functional and non-functional). Three studies published by Borel and colleagues have investigated a potential relationship between SNPs in BCO2 and postprandial carotenoid concentrations following a single meal [54–56]. Bioavailability of lutein, lycopene, and β-carotene in the newly absorbed TRL fraction of blood were found to be unrelated to BCO2 SNPs in 33 healthy male subjects [54–56]. Likewise, two SNPs in BCO2 were not correlated with circulating lutein or MPOD, but weakly associated with AMD, in the CAREDS cohort described previously [52,65].
In other mammals, BCO2 SNPs play a particularly important role in adipose color. Sheep which presented with yellow fat were also found to have a nonsense mutation in the BCO2 gene, believed to be the primary driver of lutein accumulation these tissues [75]. Likewise, a deletion mutation in another portion of the gene is responsible for the “yellow-fat” trait observed in rabbits, which results in ~3x more β-carotene and 14x more xanthophylls accumulating in perirenal adipose stores [76]. Similarly, a null mutation in the bovine BCO2 gene has also been linked to increased concentrations of β-carotene in both the milk and the circulating serum of lactating animals [77]. Collectively, these results demonstrate that mammals which carry these BCO2 mutations are apparently healthy (i.e. no obvious signs of tissue or organ damage, are able to reproduce), and both xanthophylls and β-carotene can be cleaved by BCO2 within a mammalian system.
A different phenomena has been observed in domestic chickens, where the species typically used in Occidental regions for commercial production of eggs and meat has yellow legs, while its junglefowl ancestors have gray/white legs [78]. Surprisingly, Eriksson and colleagues revealed that in the yellow-legged phenotype carrying both the “white” and “yellow” alleles, tissue-specific expression of BCO2 transcripts occurs, presumably inactivating BCO2 in the skin (i.e. permitting carotenoid accumulation and thus the yellow color) while BCO2 in the liver remains active (i.e. resulting in carotenoid catabolism) [78]. It is also possible that differential transcript expression could occur in different humans.
6. SUMMARY AND PERSPECTIVES
Multiple experiments have demonstrated the importance of BCO1 in catalyzing the oxidative cleavage of the central 15,15’ carbon-carbon double of carotenoids containing an un-substituted β-ionone ring, as well as the 15,15’ bond of β-apo-carotenals, to produce retinal. This reaction is essential for vitamin A production from provitamin A carotenoids, and the loss of BCO1 in rodent models has been shown to impact both vitamin A metabolism and whole body lipid and energy metabolism. BCO1 has also been shown to cleave lycopene to produce apo-15-lycopenal, although the relevance of this metabolite is less understood. In contrast, BCO2 catalyzes asymmetric carotenoid cleavage, i.e. cleavage of the 9,10 and 9’,10’ double bonds, of a variety of carotenes and xanthophylls (and potentially other substrates). BCO1 null mice accumulate metabolites believed to arise from BCO2 cleavage, a hypothesis supported by the increase in BCO2 expression in these models. BCO1/2 double knockout mice have more dramatic changes in liver lipid accumulation and dysregulated energy metabolism, highlighting their importance in maintaining lipid homeostasis.
The majority of human studies conducted to-date have focused on the relationship between BCO1 SNPs (especially rs6564851) with circulating or postprandial carotenoid concentrations or MPOD. Most studies demonstrate a correlation of 2–3 of the investigated SNPs and carotenoid concentrations of interest, highlighting the role that genetics play in interindividual response to a given intake level. Exploration of the role of BCO1 SNPs in health and disease (i.e. metabolic disease, AMD, cancer) have only recently begun, and many diseases and populations have not yet been investigated. Likewise, although the studies conducted on BCO2 SNPs in humans have observed little correlation with carotenoid concentrations, only a limited number have been conducted in primarily healthy populations.
Highlights-BBALIP-19-415.
Vertebrates have two carotenoid cleavage enzymes, a family widespread in other taxa
BCO1 catalyzes central cleavage of dietary provitamin A carotenoids to retinaldehyde
BCO2 catalyzes eccentric cleavage of a wider variety of carotenes and xanthophylls
BCO1/2 function in vitamin A, carotenoid, lipid, energy, and oxidative homeostasis
BCO1/2 SNPs in humans are associated with metabolic, eye, and neoplastic diseases
Abbreviations:
- AC
α-carotene
- AMD
age-related macular degeneration
- ATBC
α-Tocopherol, β-Carotene Cancer Prevention Study
- BC
β-carotene
- BCO1
β-carotene 15,15’-oxygenase
- BCO2
β-carotene 9’10’-oxygenase 2
- CAREDS
Carotenoids in Age-Related Eye Disease Study
- BCrypt
β-cryptoxanthin
- CRC
colorectal cancer
- LUT
lutein
- DDCH
Danish Diet, Cancer and Health
- LYC
lycopene
- MPOD
macular pigment optical density
- NCI BPC3
National Cancer Institute Breast and Prostate Cancer Cohort Consortium
- NHS
Nurses’ Health Study
- ROL
retinol
- SR-B1
scavenger receptor class B, type I
- SNPs
single nucleotide polymorphisms
- T2D
Type 2 diabetes
- TRL
triglyceride-rich lipoprotein fraction of blood
- ULSAM
Uppsala Longitudinal Study of Adult Men
- WHAS
Women’s Health and Aging Study I
- ZEA
zeaxanthin
Footnotes
Declaration of interests
The authors declare that they have no competing or conflicting interests in the publication of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Goodman DS, Huang HS, Biosynthesis of vitamin A with rat intestinal enzymes, Science, 149 (1965) 879–880. [DOI] [PubMed] [Google Scholar]
- [2].Olson JA, Hayaishi O, The enzymatic cleavage of β-carotene into vitamin A by soluble enzymes of rat liver and intestine, Proc. Natl. Acad. Sci. U.S.A, 54 (1965) 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].von Lintig J, Vogt K, Filling the gap in vitamin A research: Molecular identification of an enzyme cleaving β-carotene to retinal, J. Biol. Chem, 275 (2000) 11915–11920. [DOI] [PubMed] [Google Scholar]
- [4].Wyss A, Wirtz G, Woggon W, Brugger R, Wyss M, Friedlein A, Bachmann H, Hunziker W, Cloning and expression of β,β-carotene 15,15’-dioxygenase, Biochem. Biophys. Res. Commun, 271 (2000) 334–336. [DOI] [PubMed] [Google Scholar]
- [5].Lindqvist A, Andersson S, Biochemical properties of purified recombinant human β-carotene 15,15’-monooxygenase, J. Biol. Chem, 277 (2002) 23942–23948. [DOI] [PubMed] [Google Scholar]
- [6].Lindqvist A, Andersson S, Cell type-specific expression of β-carotene 15,15′-mono-oxygenase in human tissues, J. Histochem. Cytochem, 52 (2004) 491–499. [DOI] [PubMed] [Google Scholar]
- [7].Yan W, Jang GF, Haeseleer F, Esumi N, Chang J, Kerrigan M, Campochiaro M, Campochiaro P, Palczewski K, Zack DJ, Cloning and characterization of a human β,β-carotene-15,15’-dioxygenase that is highly expressed in the retinal pigment epithelium, Genomics, 72 (2001) 193–202. [DOI] [PubMed] [Google Scholar]
- [8].Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS, Expression and characterization of a murine enzyme able to cleave β-carotene The formation of retinoids, J. Biol. Chem, 276 (2001) 32160–32168. [DOI] [PubMed] [Google Scholar]
- [9].Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX, Identification, expression, and substrate specificity of a mammalian β-carotene 15,15’-dioxygenase, J. Biol. Chem, 276 (2001) 6560–6565. [DOI] [PubMed] [Google Scholar]
- [10].Takitani K, Zhu C-L, Inoue A, Tamai H, Molecular cloning of the rat β-carotene 15,15’-monooxygenase gene and its regulation by retinoic acid, Eur. J. Nutr, 45 (2006) 320–326. [DOI] [PubMed] [Google Scholar]
- [11].Morales A, Rosas A, González A, Antaramian A, Varela-Echavarria A, Shimada A, Mora O, Cloning of the bovine β-carotene-15,15’-oxygenase and expression in gonadal tissues, Int. J. Vitam. Nutr. Res, 76 (2006) 9–17. [DOI] [PubMed] [Google Scholar]
- [12].Lampert JM, Holzschuh J, Hessel S, Driever W, Vogt K, von Lintig J, Provitamin A conversion to retinal via the β, β-carotene-15,15’-oxygenase (BCOX) is essential for pattern formation and differentiation during zebrafish embryogenesis, Development, 130 (2003) 2173–2186. [DOI] [PubMed] [Google Scholar]
- [13].Cui Y, Freedman JH, Cadmium induces retinoic acid signaling by regulating retinoic acid metabolic gene expression, J. Biol. Chem, 284 (2009) 24925–24932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J, Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A, J. Biol. Chem, 276 (2001) 14110–14116. [DOI] [PubMed] [Google Scholar]
- [15].Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma J, RPE65 is the isomerohydrolase in the retinoid visual cycle, PNAS, 102 (2005) 12413–12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lobo GP, Amengual J, Palczewski G, Babino D, von Lintig J, Carotenoid-oxygenases: Key players for carotenoid function and homeostasis in mammalian biology, Biochim. Biophys. Acta, 1821 (2012) 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].von Lintig J, Provitamin A metabolism and functions in mammalian biology, Am. J. Clin. Nutr, 96 (2012) 1234S–1244S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lietz G, Lange J, Rimbach G, Molecular and dietary regulation of β,β-carotene 15,15’-monooxygenase 1 (BCMO1), Arch. Biochem. Biophys, 502 (2010) 8–16. [DOI] [PubMed] [Google Scholar]
- [19].Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J, ISX is a retinoic acid-sensitive gatekeeper that controls intestinal β,β-carotene absorption and vitamin A production, FASEB J, 24 (2010) 1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harrison EH, dela Seña C, Assimilation and Conversion of Dietary Vitamin A into Bioactive Retinoids, in: Dollé P, Neiderreither K (Eds.), The Retinoids, John Wiley & Sons, Inc, 2015: pp. 35–56. [Google Scholar]
- [21].Kowatz T, Babino D, Kiser P, Palczewski K, von Lintig J, Characterization of human β,β-carotene-15,15′-monooxygenase (BCMO1) as a soluble monomeric enzyme, Arch. Biochem. Biophys, 539 (2013) 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].dela Seña C, Narayanasamy S, Riedl KM, Curley RW Jr, Schwartz SJ, Harrison EH, Substrate specificity of purified recombinant human β-carotene 15,15’-oxygenase (BCO1), J. Biol. Chem, 288 (2013) 37094–37103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Amengual J, Widjaja-Adhi MAK, Rodriguez-Santiago S, Hessel S, Golczak M, Palczewski K, von Lintig J, Two carotenoid oxygenases contribute to mammalian provitamin A metabolism, J. Biol. Chem, 288 (2013) 34081–34096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kelly ME, Ramkumar S, Sun W, Colon Ortiz C, Kiser PD, Golczak M, von Lintig J, The biochemical basis of vitamin A production from the asymmetric carotenoid β-cryptoxanthin, ACS Chem. Biol, 13 (2018) 2121–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mein JR, Dolnikowski GG, Ernst H, Russell RM, Wang X-D, Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and β-cryptoxanthin by ferret carotene-9’,10’-monooxygenase, Arch. Biochem. Biophys, 506 (2011) 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Amengual J, Lobo GP, Golczak M, Li HNM, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J, A mitochondrial enzyme degrades carotenoids and protects against oxidative stress, FASEB J, 25 (2011) 948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dela Seña C, Sun J, Narayanasamy S, Riedl KM, Yuan Y, Curley RW, Schwartz SJ, Harrison EH, Substrate specificity of purified recombinant chicken β-carotene 9’,10’-oxygenase (BCO2), J. Biol. Chem, 291 (2016) 14609–14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Babino D, Palczewski G, Widjaja-Adhi MAK, Kiser PD, Golczak M, von Lintig J, Characterization of the role of β-carotene-9,10’-dioxygenase in macular pigment metabolism, J. Biol. Chem, 41 (2015) 24844–24857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W, Bernstein PS, Inactivity of human β,β-carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment, PNAS, 111 (2014) 10173–10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A, CMO1 deficiency abolishes vitamin A production from β-carotene and alters lipid metabolism in mice, J. Biol. Chem, 282 (2007) 33553–33561. [DOI] [PubMed] [Google Scholar]
- [31].Lobo GP, Amengual J, Li HNM, Golczak M, Bonet ML, Palczewski K, von Lintig J, β,β-Carotene decreases peroxisome proliferator receptor γ activity and reduces lipid storage capacity of adipocytes in a β,β-carotene oxygenase 1-dependent manner, J. Biol. Chem, 285 (2010) 27891–27899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shmarakov I, Fleshman MK, D’Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, Curley RW, von Lintig J, Rubin LP, Harrison EH, Blaner WS, Hepatic stellate cells are an important cellular site for β-carotene conversion to retinoid, Arch. Biochem. Biophys, 504 (2010) 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee S-A, Jiang H, Trent CM, Yuen JJ, Narayanasamy S, Curley RW, Harrison EH, Goldberg IJ, Maurer MS, Blaner WS, Cardiac dysfunction in β-carotene-15,15′-dioxygenase-deficient mice is associated with altered retinoid and lipid metabolism, Am. J. Physiol.-Heart C, 307 (2014) H1675–H1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW, Loss of carotene-9’,10’-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice, J. Nutr, 140 (2010) 2134–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD, The biochemical characterization of ferret carotene-9’,10’-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo, J. Biol. Chem, 281 (2006) 19327–19432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Maeda T, Perusek L, Amengual J, Babino D, Palczewski K, von Lintig J, Dietary 9-cis-β,β-carotene fails to rescue vision in mouse models of Leber congenital amaurosis, Mol. Pharmacol, 80 (2011) 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ford NA, Moran NE, Smith JW, Clinton SK, Erdman JW, An interaction between carotene-15,15’-monooxygenase expression and consumption of a tomato or lycopene-containing diet impacts serum and testicular testosterone, Int. J. Cancer, 131 (2012) E143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Palczewski G, Widjaja-Adhi MAK, Amengual J, Golczak M, von Lintig J, Genetic dissection in a mouse model reveals interactions between carotenoids and lipid metabolism, J. Lipid Res, 57 (2016) 1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lim JY, Liu C, Hu K-Q, Smith DE, Wang X-D, Ablation of carotenoid cleavage enzymes (BCO1 and BCO2) induced hepatic steatosis by altering the farnesoid X receptor/miR-34a/sirtuin 1 pathway, Arch. Biochem. Biophys, 654 (2018) 1–9. [DOI] [PubMed] [Google Scholar]
- [40].Lim JY, Liu C, Hu K-Q, Smith DE, Wu D, Lamon-Fava S, Ausman LM, Wang X-D, Dietary β-cryptoxanthin Inhibits high-refined carbohydrate diet–induced fatty liver via differential protective mechanisms depending on carotenoid cleavage enzymes in male mice, J. Nutr, 149 (2019) 1553–1564. [DOI] [PubMed] [Google Scholar]
- [41].Gong X, Marisiddaiah R, Zaripheh S, Wiener D, Rubin LP, Mitochondrial β-carotene 9′,10′ oxygenase modulates prostate cancer growth via NF-κB inhibition: A lycopene-independent function, Mol. Cancer Res, 14 (2016) 966–975. [DOI] [PubMed] [Google Scholar]
- [42].Sharvill DE, Familial hypercarotinaemia and hypovitaminosis A, Proc. R. Soc. Med, 63 (1970) 605–606. [PMC free article] [PubMed] [Google Scholar]
- [43].Frenk E, Etat kératodermique avec taux sérique abaissé de la vitamine A et hypercarotinémie, Dermatologica, 132 (1966) 96–98. [PubMed] [Google Scholar]
- [44].Cohen L, Observations on carotenemia, Ann. Intern. Med, 48 (1958) 219–227. [DOI] [PubMed] [Google Scholar]
- [45].McLaren DS, Zekian B, Failure of enzymic cleavage of β-carotene: The cause of vitamin A deficiency in a child, Am. J. Dis. Child, 121 (1971) 278–280. [DOI] [PubMed] [Google Scholar]
- [46].Monk BE, Metabolic carotenaemia, Br. J. Dermatol, 106 (1982) 485–488. [DOI] [PubMed] [Google Scholar]
- [47].Svensson A, Vahlquist A, Metabolic carotenemia and carotenoderma in a child, Acta Derm. Venereol, 75 (1995) 70–71. [DOI] [PubMed] [Google Scholar]
- [48].Lindqvist A, Sharvill J, Sharvill DE, Andersson S, Loss-of-function mutation in carotenoid 15,15’-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A, J. Nutr, 137 (2007) 2346–2350. [DOI] [PubMed] [Google Scholar]
- [49].Ferrucci L, Perry JRB, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, Corsi A-M, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba RD, et al. , Common variation in the β-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: A genome-wide association study, Am. J. Hum. Gen, 84 (2009) 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yabuta S, Urata M, Kun RYW, Masaki M, Shidoji Y, Common SNP rs6564851 in the BCO1 gene affects the circulating levels of β-carotene and the daily intake of carotenoids in healthy Japanese women, PLOS ONE, 11 (2016) e0168857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yonova-Doing E, Hysi PG, Venturini C, Williams KM, Nag A, Beatty S, Liew SHM, Gilbert CE, Hammond CJ, Candidate gene study of macular response to supplemental lutein and zeaxanthin, Exp Eye Res, 115 (2013) 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Meyers KJ, Mares JA, Igo RP, Truitt B, Liu Z, Millen AE, Klein M, Johnson EJ, Engelman CD, Karki CK, Blodi B, Gehrs K, Tinker L, Wallace R, Robinson J, LeBlanc ES, Sarto G, Bernstein PS, SanGiovanni JP, Iyengar SK, Genetic evidence for role of carotenoids in age-related macular degeneration in the carotenoids in age-related eye disease study (CAREDS), Invest. Ophthalmol. Vis. Sci, 55 (2014) 587–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hendrickson SJ, Hazra A, Chen C, Eliassen AH, Kraft P, Rosner BA, Willett WC, β-Carotene 15,15’-monooxygenase 1 single nucleotide polymorphisms in relation to plasma carotenoid and retinol concentrations in women of European descent, Am. J. Clin. Nutr, 96 (2012) 1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Borel P, Desmarchelier C, Nowicki M, Bott R, A combination of single-nucleotide polymorphisms is associated with interindividual variability in dietary β-carotene bioavailability in healthy men, J. Nutr, 145 (2015) 1740–1747. [DOI] [PubMed] [Google Scholar]
- [55].Borel P, Desmarchelier C, Nowicki M, Bott R, Morange S, Lesavre N, Interindividual variability of lutein bioavailability in healthy men: characterization, genetic variants involved, and relation with fasting plasma lutein concentration, Am. J. Clin. Nutr, 100 (2014) 168–175. [DOI] [PubMed] [Google Scholar]
- [56].Borel P, Desmarchelier C, Nowicki M, Bott R, Lycopene bioavailability is associated with a combination of genetic variants, Free Radic. Biol. Med, 83 (2015) 238–244. [DOI] [PubMed] [Google Scholar]
- [57].Leung WC, Hessel S, Méplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G, Two common single nucleotide polymorphisms in the gene encoding β-carotene 15,15′-monoxygenase alter β-carotene metabolism in female volunteers, FASEB J, 23 (2009) 1041–1053. [DOI] [PubMed] [Google Scholar]
- [58].Lietz G, Oxley A, Leung W, Hesketh J, Single nucleotide polymorphisms upstream from the β-carotene 15,15’-monoxygenase gene influence provitamin A conversion efficiency in female volunteers, J. Nutr, 142 (2012) 161S–165S. [DOI] [PubMed] [Google Scholar]
- [59].Oxley A, Warnke I, Wyss A, Schalch W, Lietz G, The BETASNP2 study: Effect of β-carotene formulation and rs6564851 on β-carotene plasma responses, FASEB J, 29 (2015) 605.7. [Google Scholar]
- [60].Moran NE, Thomas-Ahner JM, Fleming JL, McElroy JP, Mehl R, Grainger EM, Riedl KM, Toland AE, Schwartz SJ, Clinton SK, Single nucleotide polymorphisms in β-carotene oxygenase 1 are associated with plasma lycopene responses to a tomato-soy juice intervention in men with prostate cancer, J. Nutr, 149 (2019) 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].During A, Dawson HD, Harrison EH, Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe, J. Nutr, 135 (2005) 2305–2312. [DOI] [PubMed] [Google Scholar]
- [62].During A, Harrison E, Digestion and intestinal absorption of dietary carotenoids and vitamin A, in: Physiology of the Gastrointestinal Tract, 4th ed., Academic Press, 2006: pp. 1735–1752. [Google Scholar]
- [63].Feigl B, Morris CP, Voisey J, Kwan A, Zele AJ, The relationship between BCMO1 gene variants and macular pigment optical density in persons with and without age-related macular degeneration, PLOS ONE, 9 (2014) e89069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kyle-Little Z, Zele AJ, Morris CP, Feigl B, The effect of BCMO1 gene variants on macular pigment optical density in young healthy caucasians, Front. Nutr, 1 (2014) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, Karki CK, Liu Z, Igo RP, Truitt B, Klein ML, Snodderly DM, Blodi BA, Gehrs KM, Sarto GE, Wallace RB, Robinson J, LeBlanc ES, Hageman G, Tinker L, Mares JA, Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study, Invest. Ophthalmol. Vis. Sci, 54 (2013) 2333–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Borel P, de Edelenyi FS, Vincent-Baudry S, Malezet-Desmoulin C, Margotat A, Lyan B, Gorrand J-M, Meunier N, Drouault-Holowacz S, Bieuvelet S, Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans, Ann. Med, 43 (2011) 47–59. [DOI] [PubMed] [Google Scholar]
- [67].Hendrickson SJ, Lindström S, Eliassen AH, Rosner BA, Chen C, Barrdahl M, Brinton L, Buring J, Canzian F, Chanock S, Clavel-Chapelon F, Figueroa JD, Gapstur SM, Garcia-Closas M, Gaudet MM, Haiman CA, Hazra A, Henderson B, Hoover R, Hüsing A, et al. , Plasma carotenoid- and retinol-weighted multi-SNP scores and risk of breast cancer in the national cancer institute breast and prostate cancer cohort consortium, Cancer Epidemiol Biomarkers Prev, 22 (2013) 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Andersen V, Halekoh U, Bohn T, Tjønneland A, Vogel U, Kopp TI, No Interaction between Polymorphisms Related to Vitamin A Metabolism and Vitamin A Intake in Relation to Colorectal Cancer in a Prospective Danish Cohort, Nutrients, 11 (2019) 1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].He F, Xiao R, Lin T, Xiong W, Xu Q, Li X, Liu Z, He B, Hu Z, Cai L, Dietary patterns, BCMO1 polymorphisms, and primary lung cancer risk in a Han Chinese population: a case-control study in Southeast China, BMC Cancer, 18 (2018) 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Clifford AJ, Rincon G, Owens JE, Medrano JF, Moshfegh AJ, Baer DJ, Novotny JA, Single nucleotide polymorphisms in CETP, SLC46A1, SLC19A1, CD36, BCMO1, APOA5, and ABCA1 are significant predictors of plasma HDL in healthy adults, Lipids in Health and Disease, 12 (2013) 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Beydoun MA, Nalls MA, Canas JA, Evans MK, Zonderman AB, Gene polymorphisms and gene scores linked to low serum carotenoid status and their associations with metabolic disturbance and depressive symptoms in African-American adults, Br J Nutr, 112 (2014) 992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Perry JRB, Ferrucci L, Bandinelli S, Guralnik J, Semba RD, Rice N, Melzer D, Saxena R, Scott LJ, McCarthy MI, Hattersley AT, Zeggini E, Weedon MN, Frayling TM, the DIAGRAM Consortium, Circulating β-carotene levels and type 2 diabetes—cause or effect?, Diabetologia, 52 (2009) 2117–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Czeczuga-Semeniuk E, Galar M, Jarząbek K, Kozłowski P, Sarosiek NA, Wołczyński S, The preliminary association study of ADIPOQ, RBP4, and BCMO1 variants with polycystic ovary syndrome and with biochemical characteristics in a cohort of Polish women, Advances in Medical Sciences, 63 (2018) 242–248. [DOI] [PubMed] [Google Scholar]
- [74].Lindqvist A, He Y-G, Andersson S, Cell type-specific expression of β-carotene 9’,10’-monooxygenase in human tissues, J. Histochem. Cytochem, 53 (2005) 1403–1412. [DOI] [PubMed] [Google Scholar]
- [75].Våge DI, Boman IA, A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries), BMC Genetics, 11 (2010) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Strychalski J, Gugołek A, Brym P, Antoszkiewicz Z, Effect of the β-carotene oxygenase 2 genotype on the content of carotenoids, retinol and α-tocopherol in the liver, fat and milk of rabbit does, reproduction parameters and kitten growth, Journal of Animal Physiology and Animal Nutrition, 103 (2019) 1585–1593. [DOI] [PubMed] [Google Scholar]
- [77].Berry SD, Davis SR, Beattie EM, Thomas NL, Burrett AK, Ward HE, Stanfield AM, Biswas M, Ankersmit-Udy AE, Oxley PE, Barnett JL, Pearson JF, van der Does Y, MacGibbon AHK, Spelman RJ, Lehnert K, Snell RG, Mutation in Bovine β-Carotene Oxygenase 2 Affects Milk Color, Genetics, 182 (2009) 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Eriksson J, Larson G, Gunnarsson U, Bed’hom B, Tixier-Boichard M, Strömstedt L, Wright D, Jungerius A, Vereijken A, Randi E, Jensen P, Andersson L, Identification of the Yellow Skin Gene Reveals a Hybrid Origin of the Domestic Chicken, PLOS Genetics, 4 (2008) e1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]