Abstract
Airborne actinomycete spores, important contaminants in occupational and residential environments, were studied with respect to their (i) release into the air, (ii) aerodynamic and physical size while airborne, and (iii) survival after collection onto agar with an impactor. Three actinomycete species were selected for the tests to exemplify the three main spore types: Streptomyces albus for arthrospores, Micromonospora halophytica for aleuriospores, and Thermoactinomyces vulgaris for endospores. The results show that the incubation conditions (temperature, time, and nutrients) needed for the development of spores for their release into air are different from the conditions that are needed for colony growth only. Additional drying of M. halophytica and T. vulgaris cultures was needed before spores could be released from the culture. The aerodynamic sizes of the spores, measured with an aerodynamic particle sizer, ranged from 0.57 (T. vulgaris) to 1.28 μm (M. halophytica). The physical sizes of the spores, when measured with a microscope and an image analysis system, were found to be smaller than previously reported in the literature. The relative recovery of the spores on agar media ranged from 0.5 (T. vulgaris) to 35% (S. albus). The results indicate that the culturability of the collected airborne actinomycete spores varies widely and is affected by several variables, such as the species and the sampling flow rate. Therefore, alternatives to commonly used cultivation methods need to be developed for the enumeration of actinomycete spores.
Actinomycetes are a diverse group of gram-positive bacteria. They resemble fungi because they are adapted to life on solid surfaces (8) and they can produce mycelium and dry spores like most fungi (15). Actinomycete spores are known to be important air contaminants in occupational environments, such as agriculture and waste composting facilities (18, 26), and have recently gained special attention as indicators of mold problems in buildings (31). They do not belong to the normal microbial flora in indoor air but have been found in buildings suffering from moisture and mold problems (4, 25). In addition, airborne spores of several actinomycete species (e.g., Saccharopolyspora rectivirgula, Micropolyspora faeni, Thermoactinomyces vulgaris, and Streptomyces albus) have been related to the incidence of allergic alveolitis and other severe health effects (14, 19, 21, 28, 33). The cellular mechanism of the health effects caused by actinomycete spores was recently studied by Hirvonen et al. (11). Their study shows that Streptomyces spp. are able to stimulate lung macrophage reactions, which can lead to inflammation and tissue injury.
Actinomycete spores are formed either by subdivision of existing hyphae by fragmentation or swelling or by endogenous spore formation. The hyphae that subdivide into spores can be sheathless or have a sheath, which partly remains in the spores after fragmentation (35). This leads to three main spore types: arthrospores (subdivision of sheathed hypha), aleuriospores (subdivision of sheathless hypha), and endospores. The significance of the differences in the spore structure is not known, but these differences are expected to cause differences in the survival and airborne behavior of these spores.
Although actinomycete spores have been detected in air samples, their release into the air is not well understood. In nature, actinomycete spores can become airborne by mechanical disturbance of the substance they are growing on, e.g., by operation of an agricultural implement or by exposure to gusty wind (22). Only a few laboratory studies have been performed using airborne actinomycete spores. Lacey and Dutkiewicz (20) released actinomycete spores from contaminated hay by mechanical handling, whereas Madelin and Johnson (24) released actinomycete spores from culture media by air currents. Actinomycete spores are more difficult to aerosolize than fungal spores because they are smaller than fungal spores (30). More information needs to be gained on the aerodynamic diameter (da), agglomeration, and hygroscopicity of airborne actinomycete spores because these properties affect actinomycete behavior in air, in the human respiratory tract, in air-purifying filters, and in aerosol samplers.
Actinomycetes have been sampled from the air with impactors and have been analyzed by culturing (4, 7, 25). However, it is not clear how many of the total number of viable spores can be enumerated by these techniques. Collection by impaction can cause stress, which may result in the decrease of microbial culturability. This has been demonstrated with vegetative cells of bacteria (32), but no information appears to have been available prior to this study on the collection stress of spores. The culturability of impacted microorganisms may also be decreased when they are insufficiently embedded in the agar (32). When the impaction velocity is low, the microorganisms may deposit on the top of the collection agar instead of penetrating into the agar medium. Thus, they may have limited ability to obtain nutrients and moisture from the agar. On the other hand, endospores usually need activation, e.g., by heating, before they are able to germinate and form colonies (15).
In the present study, we investigated the following properties of actinomycete spores: (i) their release into the air, (ii) their aerodynamic and physical sizes in the airborne state, and (iii) their survival after collection onto agar with an impactor. The release of actinomycete spores was studied semiquantitatively to find the growth and aerosolization conditions which ensure a sufficient amount of released spores for the experiments. Information on the properties of actinomycete spores can be used for the development of new detection and control methods for them.
MATERIALS AND METHODS
Actinomycete species and their preparation for aerosolization.
Three actinomycete species available from the American Type Culture Collection (ATCC, Rockville, Md) were selected to represent the three main spore types: S. albus (ATCC 3004) represented arthrospores, Micromonospora halophytica (ATCC 27596) represented aleuriospores, and T. vulgaris (ATCC 43649) represented endospores. S. albus spores are formed in chains and are slightly ellipsoidal in shape. They have been reported to be 0.7 to 1.0 μm in length and 0.7 μm in width (24). M. halophytica spores are formed as singlets and are spherical. Their physical size has been reported to be about 1.2 μm (16). T. vulgaris spores are produced as singlets, and they are spherical or slightly ellipsoidal. They have been reported to be 0.5 to 1.5 μm in physical size (18) and to have the same morphology as endospores of Bacillus and Clostridium spp. (5, 8). As is typical for endospores, Thermoactinomyces spores are normally dormant and need activation to enhance their germination. In this study, cold activation was used for T. vulgaris samples by keeping the samples at 20°C for 24 to 48 h before incubation, as suggested by Kalakoutski and Agre (15).
In the initial phase of this study, the incubation conditions recommended by the ATCC (1) were used (Table 1). Both NZA medium and tryptic soy agar (TSA) contained 1.5% agar, whereas ISP2 medium contained 2% agar (NZA medium contained the following: glucose, 10 g; soluble starch, 20 g; yeast extract, 5 g; N-Z amine type A, 5 g; CaCO3, 1 g; agar, 15 g; and distilled water, 1 liter; TSA was obtained from Becton Dickinson Microbiological System, Cockeysville, Md, and ISP2 medium was from Difco Laboratories, Detroit, Mich.).
TABLE 1.
Incubation conditions for actinomycete spores
| Actinomycete species | Incubation conditions recommended by the ATCC for growth
|
Incubation conditions recommended for spore releasea
|
|||||
|---|---|---|---|---|---|---|---|
| Medium | Incubation temp (°C) | Incubation time (wk) | Medium | Incubation temp (°C) | trelease (wk) | Drying time (h) before aerosolization | |
| S. albus | ISP-2 | 26 | Not indicated | ISP-2 | 37 | 1 | 0 |
| M. halophytica | NZA | 26 | Not indicated | NZA with 2% agar | 37 | 3 | 24 |
| T. vulgaris | TSA | 50 | Not indicated | TSA with 2% agar | 50 | 3 | 24 |
Recommendations are from the present study.
When the incubation conditions recommended by the ATCC were used, sufficient amounts of spores were not aerosolized for the experiments. Therefore, different nutritional conditions, incubation temperatures, and incubation times, ranging from 1 to 5 weeks, were tested to determine which conditions are most appropriate for each species to produce enough spores for the experiments. In this report, the incubation times needed for sufficient aerosolization of the spores are given as “trelease” to distinguish them from the incubation times needed for colony growth on Andersen samples (tAndersen) and agar slide samples (tagar slide) (samplers described below). In order to enhance the release of spores from the actinomycete culture, the following tests were conducted: irradiation by a UV lamp, irradiation by infrared (IR) lamp, humidification of the air flow over the actinomycete culture, and desiccation of that air flow. To test the effect of UV radiation, one culture was irradiated by an UV lamp (60 Hz; George W. Gates & Co. Inc., New York, N.Y.) right before aerosolization and another culture was irradiated 1 day before aerosolization. In another experiment, one culture was dried by IR radiation (250 W; Philips Lighting Company, Somerset, N.J.) right before aerosolization and another culture was dried by IR radiation 1 day before aerosolization. The distance from the radiation source to the actinomycete culture was 20 cm and the irradiation time was 1 h for both types of irradiation. To change the relative humidity (RH) of the air flow over the actinomycete culture, the aerosolizing air was humidified from an RH of 20% up to an RH of 60% by passage through a vertical glass tube filled with distilled water and Raschig rings (6-mm-diameter glass rings). The humidified air was applied for 30 min. In another experiment, a desiccated air flow (20%) was passed over the culture for 30 min. Only incubation conditions that resulted in an adequate release of spores (Table 1) were used when testing the aerodynamic size, hygroscopicity, and survival of the spores. The minimum concentration of spores needed in the experiments (ca. 0.04 spores cm−3) was related to the sensitivity of the aerodynamic particle sizer, which measured the total concentration of spores as described below.
Experimental setup for aerosolization of spores.
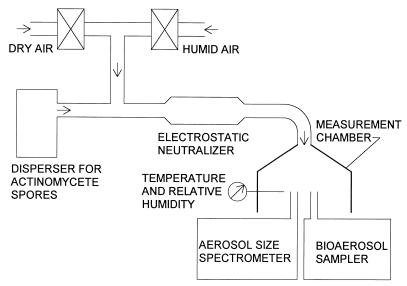
The physical and microbiological characteristics of actinomycete spores were determined by using the experimental setup schematized in Fig. 1. To prevent the release of spores outside the experimental system, all components were housed inside a biosafety cabinet (Model 6TX; Baker Company, Inc., Sanford, Maine). This setup has previously been used for testing different dispersion techniques for other microorganisms (30). Actinomycete spores were aerosolized by using our recently developed swirling-flow disperser (30). In this disperser, agar was placed on the inner walls of the dispersion vessel. The agar was inoculated, covered, and incubated as described above. After incubation, HEPA-filtered air was blown through two nozzles, which were directed tangentially toward the actinomycete growth on the inner wall of the vessel. This air flow (15 liters min−1) released actinomycete spores directly from the microbial growth. The spore aerosol was diluted with HEPA-filtered air ranging from 15 to 40 liters min−1, depending on the airborne spore concentration desired for the test. The desired concentration was calculated for the optimal colony surface density on impactor samples with insignificant masking effect (3). Unless indicated otherwise, the test aerosol flow was adjusted to 30% ± 5% RH by mixing appropriate portions of desiccated and humidified air.
FIG. 1.
Experimental setup.
During the hygroscopicity test with S. albus, the RH of the aerosol flow was increased up to ∼100% immediately after aerosolizing the spores. The residence time of the spores between humidification and measurement was approximately 2 s, which is a typical time for particle exposure to humid air during the human respiratory cycle. The air temperature ranged from 22 to 24°C during the hygroscopicity test.
Measurement of spores.
In the measurement chamber of the setup depicted in Fig. 1, the total concentration and the size distribution of aerosolized actinomycete spores were measured with an aerodynamic particle sizer (Aerosizer, API Mach II; Amherst Process Instruments, Inc., Hadley, Mass.). The Aerosizer measures the concentration of particles and the health-related aerodynamic equivalent diameters, which depend on the shape and density of the particles. We assume that the detection limit of the Aerosizer is 0.0004 airborne spores cm−3, which corresponds to the detection of one particle during a 1-min time interval at a flow rate of 2.5 liters min−1. However, at least 10 particles per size channel should be measured to achieve reliable size distributions (10). Assuming that the particle size distribution consists of 10 size channels and the aerodynamic particle sizer is operated for 1 min at 2.5 liters min−1, the minimum total spore concentration needed for statistically reliable data is 0.04 spores cm−3. The culturable count of actinomycete spores was measured with the N-6 Andersen impactor (Graseby Andersen, Smyrna, Ga.) (12) and with an agar slide impactor. The latter device is not commercially available but has been described in detail by Juozaitis et al. (13). The relative recovery of collected spores was determined by relating the culturable count of spores collected on the impactor substrate to the total count measured upstream and downstream of the impactor with the Aerosizer, as further described below.
Microscopic size measurements.
In order to determine the size distribution of actinomycete spores, a piece of actinomycete culture was taken from an agar plate with a sterilized knife, placed on a microscopic slide (Superfrost/Plus; Fisher Scientific, Pittsburgh, Pa.), and allowed to air dry. A drop of permount medium (Fisher Scientific) was added and then covered with a coverslip. The sample was then digitally imaged with a color video camera (DXC-760MD 3CCD; Sony Electronics, Inc., Tokyo, Japan) attached to the trinocular head of a phase-contrast microscope (Eclipse E800; Nikon Corp., Tokyo, Japan). The phase-contrast optical system consisted of a phase-contrast condenser (0.9 N.A.) and an oil immersion objective (1.25 N.A., CFI DL 100X; Plan Achromat). The video camera control unit was connected to a Matrox Millennium video board. The actinomycete culture sample was analyzed using the Image-1/MetaMorph imaging software system (Universal Imaging Corporation, West Chester, Pa.) running on a Pentium 166 MHz CPU platform. Interactive morphometric analysis of the samples with Image-1/MetaMorph system consisted of decoding the color image, configuring the decoded image “Lookup Table” as a monochrome, and then applying a threshold-based boundary detection algorithm. A calibration scale was then activated and the “Show Regional Statistics” tool was engaged to display the resulting morphological measurements.
Prior to measuring the size of the actinomycete spores, the microscope and image analysis system were calibrated with standard polystyrene latex particles (PSL) of two sizes: 1.02 and 2.43 μm (Bangs Laboratories, Inc., Carmel, Ind.). When determining the size of PSL particles and of each of the actinomycete species, at least 100 particles were measured per sample, from which the averages and standard deviations were calculated.
Assessment of the recovery of aerosolized spores.
The relative recoveries (see definition below) of different actinomycete species were compared with each other by use of an Andersen impactor, which is widely used in occupational and public health applications to collect actinomycete spores (4, 7, 25). Because the spore size distribution was measured with the Aerosizer, only the sixth stage of the Andersen sampler (N-6) was utilized for spore collection. The flow rate for the Andersen impactor was 28.3 liters min−1 (air velocity through each of the 400 impaction holes was 24 m s−1). To test the effect of impact stress on the relative recovery of S. albus spores, the spores were collected with the agar slide impactor at flow rates of 3.8, 6, 8, 10, 15, 20, 25, and 28 liters min−1. These flow rates correspond to air velocities through the single slit of this impactor of 24, 38, 50, 63, 94, 125, 156, and 175 m s−1, respectively. The velocity of spore impact on the agar surface is approximately equal to the velocity of the air jet coming from the slit or from the holes of the impaction plate above the agar surface.
All impactor samples were collected onto the agars recommended by the ATCC at incubation temperatures described above. No ATCC recommendations are available for the incubation times of actinomycetes, and therefore, preliminary experiments were conducted to determine sufficient incubation times. Initially, three incubation times (1, 2, and 3 weeks) were tested with the Andersen samples of all the tested actinomycetes. A tAndersen incubation time of 1 week was found to be sufficient for the colony growth of S. albus and M. halophytica; a tAndersen of 2 weeks was found to be sufficient for T. vulgaris. With T. vulgaris, incubation was first performed without activation, as traditionally done. Later, a cold activation at 20°C for 24 h before incubation was added to the procedure. For S. albus collected with the agar slide impactor, three incubation times, 18, 24, and 38 h, were tested to find the best incubation time for the growth of microcolonies. A tagar slide incubation time of 24 h was selected because it gave the highest number of colonies.
From the Andersen samples, the macrocolonies were counted and the results were corrected by the positive-hole correction method (23). From the agar slide samples, microcolonies were counted with a bright-field phase-contrast microscope (Labophot-2; Nikon Corp.) with a magnification of ×100 by the procedure of Stewart et al. (32). The concentrations in the air were determined and expressed as CFU m−3.
The percent relative recovery was determined as CCFU/(Ctotal × Ecoll) × 100, where CCFU is the concentration of spores obtained with the Andersen impactor or the agar slide impactor (CFU m−3), Ctotal is the total spore concentration obtained with the Aerosizer (spores m−3), and Ecoll is the collection efficiency of the bioaerosol impactor. The last parameter was determined for each actinomycete species and each impactor flow rate by measuring the spore concentrations up- and downstream of the bioaerosol impactor with the Aerosizer.
Assessment of the effect of spore embedding in agar.
The association between the relative recovery and different levels of spore embedding in agar during collection with the impactor was studied by conducting two experiments with S. albus spores. In both experiments, agar plates were first exposed to spores in the test chamber for 20 s by letting the spores sediment onto the agar. Gravity settling is not a recommended practice for the quantitative sampling of bioaerosols in field situations because it depends on particle size and is influenced strongly by air turbulence. Our experiments were done in the laboratory in a measurement chamber with laminar air flow and with monodisperse spore aerosols. The gravity settling was used in these experiments to minimize the impaction velocity and thus eliminate the embedding of spores during their retrieval on the agar.
In the first experiment, the spores were embedded in agar by manually pressing against each other two identical agar plates with spores deposited on them. The agar plates (5 cm in diameter) were prepared as contact plates so that the surface of the agar was higher than the walls of the agar plates. From 18 agar plates with spores on them, 6 randomly selected agar plates were incubated as controls, and the remaining 12 agar plates were randomly divided into six pairs of agar plates. The two agar plates of each pair were manually pressed against each other, thus embedding the spores deep into the agar.
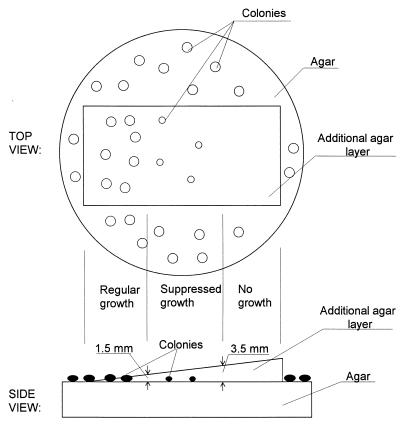
In the second experiment, the depth of embedding was studied. A wedge-shaped agar layer was deposited on top of the spores, as shown in Fig. 2. Thus, the spores were either fully exposed or covered by an agar layer that varied from a depth of 0.1 mm on the thin side of the wedge to a depth of 5 mm on the thick side of the agar wedge. About half of the surface area was left uncovered with agar to confirm the uniformity of the spore deposition.
FIG. 2.
Effect of excess embedding on the growth of S. albus spores.
Statistical analysis.
All statistical tests were conducted in accordance with the general linear model procedures of the Statistical Analysis System (SAS Institute, Inc., Gary, N.C.). The Scheffe’s test was used to identify the difference that the general linear model indicated.
RESULTS
Release of actinomycete spores into air.
The first set of experiments on the study of actinomycete spore release from surfaces was conducted by growing the actinomycete cultures under the conditions recommended by the ATCC (Table 1). It was found that these conditions are sufficient for colony formation and spore production but are not optimal for the efficient release of spores from the swirling-flow disperser. For instance, regular NZA and TSA media turned out to be too soft: the dispersing air flow produced holes on the agar layer. The amount of agar in the growth media was therefore increased from 1.5 to 2% (i.e., to the same level as in the ISP2 medium). Microscopic examination showed that this increase in agar concentration increased the amount of spores produced by the actinomycete culture. However, the amount of spores released from any of the three actinomycete cultures was still below the detection limit of the Aerosizer (0.0004 spores cm−3) when cultures were incubated for 1 to 5 weeks at the incubation temperatures recommended by the ATCC.
In the second set of experiments, attempts were made to enhance the release of spores from the incubated actinomycete cultures, which contained 2% agar and were incubated at the temperature recommended by the ATCC. The cultures were irradiated by a UV lamp and the RH was increased from 20 to 60%. Such treatments have been reported to enhance the release of fungal spores (9, 27). The RH was changed by drying the culture with an IR lamp before aerosolization and desiccating or humidifying the aerosolization air. While the spore release increased above the detection limit of the Aerosizer, the spore concentration was not yet sufficiently high for statistically significant enumeration by the aerodynamic particle sizer (the concentration was below 0.04 spores cm−3).
In the third set of experiments, the actinomycete cultures were again prepared with 2% agar but incubation was tested at three different temperatures: 26, 37, and 50°C. As expected, mesophilic S. albus and M. halophytica did not grow at 50°C, and thermophilic T. vulgaris did not grow at 26°C. Among these temperatures, 37°C turned out to be most suitable for growing S. albus and M. halophytica for aerosolization experiments. S. albus released an average concentration of 1.21 ± 0.43 spores cm−3 (n = 5) after 1 week of incubation at 37°C, which was sufficient for the experiments. When testing T. vulgaris and M. halophytica, it was found that drying the culture after incubation was necessary before the spores could be released from the culture. Incubation of M. halophytica for 3 weeks at 37°C and of T. vulgaris for 3 weeks at 50°C with subsequent drying were found to be suitable for sufficient spore release. In the subsequent experiments, the cultures were dried by keeping the culture vessels in the incubator uncovered for 24 h before aerosolization. The average concentrations of aerosolized M. halophytica and T. vulgaris spores were 0.13 ± 0.10 and 0.21 ± 0.10 spores cm−3, respectively (n = 5 for both species).
The conditions found for sufficient release of the three actinomycete spore species are summarized in Table 1.
Physical characteristics of airborne actinomycete spores.
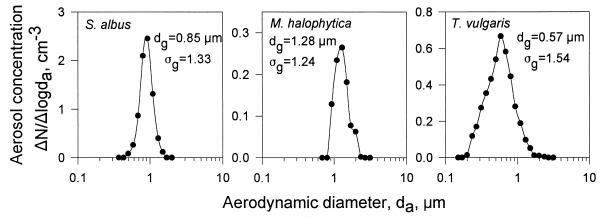
Typical size distributions of spores representing the three actinomycete species are presented in Fig. 3. The Aerosizer measured the number concentration, N, of spores in equal logarithmic intervals of particle size, with the size expressed as da (29, 30, 34). For a particle density of 1 g cm−3, the da equals the physical diameter of a spherical particle (2). As seen, the geometric mean of the da, dg, ranged from 0.57 μm for T. vulgaris to 1.28 μm for M. halophytica, and the geometric standard deviation, ςg, was fairly narrow, ranging from 1.24 for M. halophytica to 1.54 for T. vulgaris. The sizes for the three different actinomycete spores were all statistically significantly different from each other (P = 0.0001).
FIG. 3.
Size distributions of airborne actinomycete spores at an RH of 30%.
The physical sizes of the spores, measured with the microscope and image analysis system, are presented in Table 2. The spore sizes indicated by the image analysis system were confirmed by measuring PSL spheres. PSL spheres of 1.02 μm were measured to be 1.04 μm in diameter and 2.43 μm PSL spheres were measured to be 2.51 μm in diameter. Hygroscopicity tests with S. albus spores showed that the da of these spores increased from 0.85 ± 0.03 μm at RH = 30% to 1.07 ± 0.03 μm at RH ≈ 100% (n = 3), which was a statistically significant size increase (P = 0.0001).
TABLE 2.
Physical size of tested actinomycete spores measured by microscope and image analysis systema
| Actinomycete species | Width (μm) | Length (μm) |
|---|---|---|
| S. albus | 0.68 ± 0.13 | 0.84 ± 0.17 |
| M. halophytica | 0.55 ± 0.12 | 0.72 ± 0.17 |
| T. vulgaris | 0.66 ± 0.12 | 0.79 ± 0.12 |
A total of 100 spores were tested for each species.
Spore survival during sampling.
The relative recovery values for spores of the three different actinomycete species were as follows: 35.3% ± 11.6% for S. albus (n = 4), 7.4% ± 5.2% for M. halophytica (n = 3), 0.5% ± 0.1% for T. vulgaris without activation (n = 3), and 4.6% ± 1.0% for T. vulgaris (n = 5) with cold activation. The relative recovery of S. albus was statistically significantly higher than the recoveries of the two other actinomycetes (P = 0.0001).
The effect of the impaction process on the relative recovery of actinomycete spores was studied more closely with the agar slide impactor. The relative recovery of S. albus spores varied from 30 to 86%, depending on the flow rate (Table 3). The lowest air flow rate, 3.8 liters min−1 resulted in the lowest relative recovery, 30%. The recovery rate reached its maximum at an air flow rate of 20 liters min−1 and decreased with further increases in the flow rate.
TABLE 3.
Relative recovery of S. albus spores as a function of the collection flow rate measured with the agar slide impactor
| Flow rate (liters min−1) | Impaction velocity (m s−1) | n | Relative recovery (%)a |
|---|---|---|---|
| 3.8 | 24 | 5 | 30 ± 4* |
| 6 | 38 | 6 | 56 ± 20 |
| 8 | 50 | 6 | 68 ± 18 |
| 10 | 63 | 7 | 56 ± 20 |
| 15 | 94 | 7 | 54 ± 17 |
| 20 | 125 | 4 | 86 ± 11* |
| 25 | 156 | 5 | 75 ± 14* |
| 28 | 175 | 6 | 62 ± 12 |
Values marked with asterisks indicate statistically significant differences from recovery values for the other flow rates tested (P < 0.05 by the general linear model of the Statistical Analysis System followed by the Scheffe’s test). See text for more detail.
In the first set of experiments on the effect of embedding actinomycete spores into the agar, an agar plate with spores deposited on it was pressed against another agar plate. This embedding resulted in an average of 1.9 times higher microbial recovery than without embedding. However, this increase was not statistically significant (P = 0.3418). The second set of embedding experiments showed that actinomycete growth may be inhibited if the embedding is too deep, as shown in Fig. 2. When the thickness of the agar layer above the collected spores did not exceed 1.5 mm, the colony growth was the same as without that layer. When the agar layer was between 1.5 and 3.5 mm in thickness, the colony growth was slower and the resulting number of colonies was half or less. When the agar layer exceeded 3.5 mm, no colony growth was observed.
DISCUSSION
The experiments on the release of actinomycete spores showed that the incubation conditions needed for the development of spores for their release into air are different from the conditions needed for colony growth only. An increase in agar concentration from 1.5 to 2% was found to increase the production of spores such as M. halophytica and T. vulgaris. An increase in spore production with increasing agar concentration has also been observed by Kalakoutski and Agre (15) in their tests with Streptomyces spp. They showed that lower agar concentrations favor vegetative growth, whereas higher agar concentrations enhance the sporulation of Streptomyces spp. However, in the present experiments, spore release was not enhanced with increasing amounts of spores in the actinomycete culture. S. albus needed elevating the incubation temperature, T. vulgaris needed drying of the culture, and M. halophytica needed both of these treatments for sufficient spore release. One explanation for the need for these specific conditions to enhance spore release may be found in the spore maturation process. S. albus and M. halophytica are formed by the fragmentation of hyphae. If the fragmentation process is not totally completed, e.g., due to insufficient temperature, the spores may not be readily released even when they have grown into spore chains that are observable under the microscope. T. vulgaris is formed inside the hyphae and may also need specific conditions before the spores can be effectively released from the hyphae.
Only limited information on the concentrations of individual actinomycete species in air is available in the literature. Madelin and Johnson (24) aerosolized three different actinomycete species that were growing on a mixture of agar and hay and measured the airborne concentrations of their spores with an aerodynamic particle sizer. The concentrations of S. albus spores in their experiments were below 4.5 spores cm−3, while the concentrations of two thermophilic actinomycetes, Faenia rectivirgula and Saccharomonospora viridis, were as high as 170 spores cm−3. Nevalainen et al. (25) measured mesophilic actinomycetes in moldy buildings and found concentrations close to 106 CFU cm−3. In some occupational environments, thermophilic actinomycete concentrations have been reported to be as high as 108 CFU cm−3 (7, 17). In the present laboratory study, S. albus spores were aerosolized by the air currents in the swirling-flow disperser at concentrations of up to 1.8 spores cm−3; the concentrations of airborne M. halophytica and T. vulgaris were about 0.3 spores cm−3. We conclude that the latter two spore species were not tested at their optimal maturation conditions. One reason for the low concentrations of laboratory-generated spores in this study, relative to those found in the nature, is that the natural aerosolization of actinomycete spores is rather complex and involves other mechanisms in addition to mechanical release by air currents. In outdoor environments, actinomycete spores can be released from the soil by gusty winds or rain drops (22). In indoor environments, the release may be enhanced by mechanical disturbance of the actinomycete growth by people or animals. In this study, however, the laboratory-generated spore concentrations were considered sufficient for adequate measurements of the particle size distributions and the determination of the microbial recovery of actinomycete spores. More research is needed to better understand the release mechanisms and the optimum release conditions for actinomycete spores from biocontaminated building materials in indoor environments.
The aerodynamic spore size distributions of S. albus and M. halophytica were found to be close to monodisperse: the ςgs were 1.33 and 1.24, respectively (Fig. 3). The dg of the T. vulgaris spores was the smallest of the three tested species; these spores also showed the largest variation in spore size. All the physical sizes of the actinomycete spores measured in this study with the microscope and image analysis system were smaller than those reported by Madelin and Johnson for S. albus (24) by Kawamoto for M. halophytica (16), and by Lacey for T. vulgaris (18). By measuring monodisperse standard PSL particles with this system, we found that the measurement system used was suitable for determining the physical size of spores, as the error rate was ≤3%. The differences between the physical sizes of the spores determined in this study and those reported in the literature appear to be related to the preparation technique for microscopic analysis. Typically, slides are prepared by staining the spores with water-based stains. Water may be absorbed by the spores, causing them to extend to their maximum diameter. However, hygroscopicity tests with S. albus spores showed that large size increases of the spores are not likely to occur during the normal human breathing cycle. For a growth time of 2 s, typical for the residence time of spores in the humid air environment of the human respiratory tract, their size increased by no more than 26%. When spores are exposed to dry conditions, e.g., in normal indoor air environments, the water inside the spores evaporates and the spores shrink to their minimum size. Therefore, the size measurements of dry spores, as shown in this study, appear to be more appropriate for human health-related studies.
Comparison of the relative spore recoveries for the three actinomycete species obtained after collection from the air showed that S. albus spores have the highest survival level. The difference in survival level between S. albus and M. halophytica appears to be due to the different structure of the spore wall. S. albus spores have an outer sheath, which protects them against physical damage and drying, whereas M. halophytica spores do not have such a sheath. The low relative recovery of T. vulgaris spores is probably related to the dormant nature of these spores, i.e., they need activation before they can germinate. Although the conventionally used mechanism for bacterial spore activation is heating (15), we used cold activation in this study instead of heat activation because the heat would have melted the agar. If the spores had been collected on a filter, activation by heating could also have been used. However, heat activation was not tested in this study, and therefore, the change in relative recovery by heat activation remains to be determined. When T. vulgaris was activated at 20°C for 24 h before incubation, the relative recovery increased about 10-fold but was still lower than those of the other two tested actinomycete species.
The effect of impact velocity was studied with S. albus spores. The lowest relative recovery was measured at the lowest sampling air flow rate (3.8 liters min−1). Similar results have been found in an earlier study with vegetative bacterial cells (32) and can be explained as being due to insufficient embedding of the spores at low sampling flow rates. After reaching a maximum at about 20 liters min−1, the relative recovery decreased with higher air flow rates. We attribute this to mechanical injury of the inner structures of spores by their impaction onto the agar medium. At a flow rate of 28 liters min−1 the impaction velocity is very high, about 175 m s−1, resulting in injury or excess embedding or a combination of both. Our results showed that the growth of S. albus spores can be decreased or totally prevented if they are too deeply embedded in agar. This result appears to be related to the high oxygen need of actinomycetes (6). If the spores are embedded too deeply in the agar, they may not get the amount of oxygen needed for their germination and growth. At flow rates of 10 to 15 liters min−1, the relative recovery rate seems to decrease. If this observation is not due to measurement error, an explanation may be sought in the two competing processes of insufficient embedding and excessive embedding or injury of spores. A third competing process might be that of spore activation due to impaction. These competing processes deserve further study.
Our results show that the recovery of actinomycete spores varies considerably depending on the species, the sampling flow rate, and other sampling parameters. Surprisingly low recovery rates were measured for T. vulgaris. To increase the accuracy and precision of spore enumeration, new noncultivation methods need to be developed for the analysis of actinomycete spores. Among the conventional methods, direct microscopic counting with bright-field or epifluorescence microscopy is used for fungal spores in situations when the total (not culturable) count is of interest. This method is not appropriate for actinomycete spores due to their small size, which makes them difficult to distinguish if the sample also contains a high concentration of fungal spores or other particles.
ACKNOWLEDGMENTS
This work was initiated by T.A.R. during postdoctoral research supported by the U.S. Center for Indoor Air Research (CIAR) and was continued in collaboration with S.V.G., who was also supported by a postdoctoral fellowship through the CIAR.
The microscope and the image analysis facility for the measurement of the physical spore sizes were made available by Marshall Anderson, Jonathan Wiest, and Kenneth Conwell; helpful advice on actinomycete cultivation and spore production was given by Pamela Dulaney. We are thankful for their assistance.
REFERENCES
- 1.American Type Culture Collection. Catalogue of bacteria and & bacteriophages. Rockville, Md: American Type Culture Collection; 1992. [Google Scholar]
- 2.Baron P, Willeke K. Gas and particle motion. In: Willeke K, Baron P, editors. Aerosol measurement, principles, techniques, and applications. New York, N.Y: Van Nostrand Reinhold; 1993. pp. 23–40. [Google Scholar]
- 3.Chang C-W, Grinshpun S, Willeke K, Macher J, Donnelly J, Clark S, Juozaitis A. Factors affecting microbiological colony count accuracy for bioaerosol sampling and analysis. Am Ind Hyg Assoc J. 1994;56:979–986. doi: 10.1080/15428119591016403. [DOI] [PubMed] [Google Scholar]
- 4.Cole E C, Foarde K K, Leese K E, Green D A, Franke D L, Berry M A. Assessment of fungi in carpeted environments. In: Samson R A, Flannigan B, Flannigan M E, Verhoeff A P, Adan O C G, Hoekstra E S, editors. Health implications of fungi in indoor environments. Air quality monographs. Vol. 2. Amsterdam, The Netherlands: Elsevier; 1994. pp. 103–128. [Google Scholar]
- 5.Cross T. Thermophilic actinomycetes. J Appl Bacteriol. 1968;31:36–53. doi: 10.1111/j.1365-2672.1968.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 6.Cross T. Growth and examination of actinomycetes—some guidelines. In: Williams S T, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 4. Baltimore, Md: Williams & Wilkins; 1989. pp. 2340–2343. [Google Scholar]
- 7.Dawson M W, Scott J G, Cox L M. The medical and epidemiological effects on workers of the levels of airborne Thermoactinomyces spp. spores present in Australian raw sugar mills. Am Ind Hyg Assoc J. 1996;57:1002–1012. doi: 10.1080/15428119691014332. [DOI] [PubMed] [Google Scholar]
- 8.Ensign J C. Formation, properties and germination of actinomycete spores. Annu Rev Microbiol. 1978;32:185–219. doi: 10.1146/annurev.mi.32.100178.001153. [DOI] [PubMed] [Google Scholar]
- 9.Hawker L E. Environmental influences on reproduction. In: Ainsworth G C, Sussman A S, editors. The fungi II. New York, N.Y: Academic Press; 1966. pp. 435–469. [Google Scholar]
- 10.Hinds W C. Aerosol technology. New York, N.Y: John Wiley & Sons, Inc.; 1982. [Google Scholar]
- 11.Hirvonen M-R, Nevalainen A, Makkonen M, Mönkkönen J, Savolainen K. Streptomyces spores from mouldy houses induce nitric oxide, TNFx and IL-6 secretion from RAW264.7 macrophage cell line without causing subsequent cell death. Environ Toxicol Pharmacol. 1997;3:57–63. doi: 10.1016/s1382-6689(96)00140-8. [DOI] [PubMed] [Google Scholar]
- 12.Jones W, Morring K, Morey P, Sorenson W. Evaluation of the Andersen viable impactor for single stage sampling. Am Ind Hyg Assoc J. 1985;46:294–298. doi: 10.1080/15298668591394833. [DOI] [PubMed] [Google Scholar]
- 13.Juozaitis A, Willeke K, Grinshpun S A, Donnelly J. Impaction onto a glass slide or agar versus impingement into a liquid for the collection and recovery of airborne microorganisms. Appl Environ Microbiol. 1994;60:861–870. doi: 10.1128/aem.60.3.861-870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kagen S L, Fink J N, Schlueter D P, Kurup V P, Fruchtman R B. Streptomyces albus: a new cause of hypersensitivity pneumonitis. J Allergy Clin Immunol. 1981;68:295–299. doi: 10.1016/0091-6749(81)90155-x. [DOI] [PubMed] [Google Scholar]
- 15.Kalakoutski L V, Agre N S. Comparative aspects of development and differentiation in actinomycetes. Bacteriol Rev. 1976;40:469–524. doi: 10.1128/br.40.2.469-524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamoto I. Genus Micromonospora. In: Williams S T, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 4. Baltimore, Md: Williams & Wilkins; 1989. pp. 2442–2444. [Google Scholar]
- 17.Kotimaa M, Husman K, Terho E, Mustonen M. Airborne molds and actinomycetes in the work environment of farmer’s lung patients in Finland. Scand J Work Environ Health. 1984;10:115–119. doi: 10.5271/sjweh.2356. [DOI] [PubMed] [Google Scholar]
- 18.Lacey J. Thermoactinomycetes. In: Williams S T, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 4. Baltimore, Md: Williams & Wilkins; 1989. pp. 2573–2585. [Google Scholar]
- 19.Lacey J, Crook B. Fungal and actinomycete spores as pollutants of the workplace and occupational allergens. Ann Occup Hyg. 1988;32:515–533. doi: 10.1093/annhyg/32.4.515. [DOI] [PubMed] [Google Scholar]
- 20.Lacey J, Dutkiewicz J. Isolation of actinomycetes and fungi from mouldy hay using a sedimentation chamber. J Appl Bacteriol. 1976;41:315–319. doi: 10.1111/j.1365-2672.1976.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 21.Lacey J, Dutkiewicz J. Bioaerosols and occupational lung disease. J Aerosol Sci. 1994;25:1371–1404. [Google Scholar]
- 22.Lloyd A B. Dispersal of Streptomyces in air. J Gen Microbiol. 1969;57:35–40. doi: 10.1099/00221287-57-1-35. [DOI] [PubMed] [Google Scholar]
- 23.Macher J M. Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. Am Ind Hyg Assoc J. 1989;50:561–568. doi: 10.1080/15298668991375164. [DOI] [PubMed] [Google Scholar]
- 24.Madelin T M, Johnson H E. Fungal and actinomycete spore aerosols measured at different humidities with an aerodynamic particle sizer. J Appl Bacteriol. 1992;72:400–409. doi: 10.1111/j.1365-2672.1992.tb01853.x. [DOI] [PubMed] [Google Scholar]
- 25.Nevalainen A, Pasanen A-L, Niininen M, Reponen T, Jantunen M J, Kalliokoski P. The indoor air quality in Finnish homes with mold problems. Environ Int. 1991;17:299–1302. [Google Scholar]
- 26.Nielsen E M, Breum N O, Nielsen B H, Würtz H, Poulsen O M, Midtgaard U. Bioaerosol exposure in waste collection: a comparative study on the significance of collection equipment, type of waste and seasonal variation. Ann Occup Hyg. 1997;41:325–344. [Google Scholar]
- 27.Pasanen A-L, Pasanen P, Jantunen M J, Kalliokoski P. Significance of air humidity and air velocity for fungal spore release into the air. Atmos Environ. 1991;25(Part A):459–462. [Google Scholar]
- 28.Pepys J, Jenkins P A, Festenstein G N, Gregory P H, Lacey M E, Skinner F A. Farmer’s lung: thermophilic actinomycetes as a source of “farmer’s lung hay” antigen. Lancet. 1963;ii:607–611. doi: 10.1016/s0140-6736(63)90398-2. [DOI] [PubMed] [Google Scholar]
- 29.Qian Y, Willeke K, Ulevicius V, Grinshpun S A, Donnelly J. Dynamic size spectrometry of airborne microorganisms: laboratory evaluation and calibration. Atmos Environ. 1995;29:1123–1129. [Google Scholar]
- 30.Reponen T, Willeke K, Ulevicius V, Grinshpun S A, Donnelly J. Techniques for dispersion of microorganisms into air. Aerosol Sci Technol. 1997;27:405–421. [Google Scholar]
- 31.Samson R A, Flannigan B, Flannigan M E, Verhoeff A P, Adan O C G, Hoekstra E S, editors. Health implications of fungi in indoor environments. Air quality monographs. Vol. 2. Amsterdam, The Netherlands: Elsevier; 1994. [Google Scholar]
- 32.Stewart S L, Grinshpun S A, Willeke K, Terzieva S, Ulevicius V, Donnelly J. Effect of impact stress on microbial recovery on an agar surface. Appl Environ Microbiol. 1995;61:1232–1239. doi: 10.1128/aem.61.4.1232-1239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terho E, Lacey J. Microbiological and serological studies of farmer’s lung in Finland. Clin Allergy. 1979;9:43–52. doi: 10.1111/j.1365-2222.1979.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 34.Terzieva S, Donnelly J, Ulevicius V, Grinshpun S, Willeke K, Stelma G, Brenner K. Comparison of methods for detection and enumeration of airborne microorganisms collected by liquid impingement. Appl Environ Microbiol. 1996;62:2264–2272. doi: 10.1128/aem.62.7.2264-2272.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams S T, Sharples G P, Bradshaw R M. The fine structure of the Actinomycetales. In: Sykes G, Skinner F A, editors. Actinomycetales: characteristics and practical importance. New York, N.Y: Academic Press, Inc.; 1973. pp. 113–130. [Google Scholar]