Abstract
Context
Childhood overweight has been linked to earlier development of adrenarche and puberty, but it remains unknown if lifestyle interventions influence sexual maturation in general populations.
Objective
To investigate if a 2-year lifestyle intervention influences circulating androgen concentrations and sexual maturation in a general population of children.
Methods
We conducted a 2-year physical activity and dietary intervention study in which 421 prepubertal and mostly normal-weight 6- to 9-year-old children were allocated either to a lifestyle intervention group (119 girls, 132 boys) or a control group (84 girls, 86 boys). The main outcome measures were serum dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS), androstenedione (A4), and testosterone concentrations, and clinical adrenarchal and pubertal signs.
Results
The intervention and control groups had no differences in body size and composition, clinical signs of androgen action, and serum androgens at baseline. The intervention attenuated the increase of DHEA (P = .032), DHEAS (P = .001), A4 (P = .003), and testosterone (P = .007) and delayed pubarche (P = .038) in boys but it only attenuated the increase of DHEA (P = .013) and DHEAS (P = .003) in girls. These effects of lifestyle intervention on androgens and the development of pubarche were independent of changes in body size and composition, but the effects of intervention on androgens were partly explained by changes in fasting serum insulin.
Conclusion
A combined physical activity and dietary intervention attenuates the increase of serum androgen concentrations and sexual maturation in a general population of prepubertal and mostly normal-weight children, independently of changes in body size and composition.
Keywords: adrenarche, puberty, DHEAS, lifestyle intervention, physical activity, diet
Sexual maturation from childhood to adulthood includes 2 main events, adrenarche and puberty (1, 2). Adrenarche refers to the maturation of the adrenocortical zona reticularis, leading to increased production of adrenal androgens, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS), androstenedione (A4), and 11-hydroxy-A4 (11OHA4) (1). Increased exposure to adrenal androgens in adrenarche eventually leads to clinical signs, which include adult-type body odor, oily hair and skin, microcomedonal acne, and the development of axillary and pubic hair. Adrenocorticotropic hormone (ACTH) stimulation from the hypothalamic-pituitary-adrenal (HPA) axis is necessary for zona reticularis development and the onset of adrenarche, but adrenal androgen production is not similarly dependent and regulated by the hypothalamic-pituitary-adrenal axis as puberty is by pulsatile secretion of gonadotropins from the hypothalamic-pituitary-gonadal (HPG) axis (1). The activation of the hypothalamic-pituitary-gonadal axis and the onset of puberty are usually seen a few years after adrenarche with testicular enlargement in boys and breast development in girls being their first clinical signs (2).
Although adrenarche and puberty are independent events with different regulatory mechanisms, they seem to be linked, as pubertal development is advanced in girls with premature timing of adrenarche (3) and in girls with higher circulating DHEAS concentrations in mid-childhood (4). Also, early timing of adrenarche and puberty are associated with adiposity at the age of these events (5, 6) and weight gain in early childhood (7-9). Secular trends of the increased prevalence of childhood obesity and earlier timing of puberty have been shown to share similar patterns, and early weight gain leading to increased fat mass and childhood obesity has been suggested to be one factor behind a trend of earlier sexual maturation, especially in girls (6, 10). Other possible factors behind earlier timing of sexual maturation may be insulin and insulin-like growth factors (IGFs), as it is known that children with premature adrenarche have signs of insulin resistance together with increased circulating IGF-1 concentrations (5, 7) and that there is a physiological decrease in insulin sensitivity during puberty (11).
Physical activity and dietary interventions have been found to decrease adiposity and improve glucose tolerance in overweight and obese children (12). Given that adipose tissue is somehow regulating the timing of maturation, as suggested by the association between early timing of puberty and adiposity (6, 10), it could be hypothesized that lifestyle interventions affecting body fat content and insulin sensitivity also influence adrenarchal and pubertal development. Supporting this hypothesis, earlier studies have found that weight loss lowered circulating A4 and testosterone levels in obese children (13) and that lifestyle modifications had beneficial effects on hormonal status in adolescent girls with the polycystic ovary syndrome (PCOS) (14), a condition characterized by hyperandrogenism. Exercise has also been found to improve insulin sensitivity in adult women with PCOS (15). Little is known, however, whether lifestyle interventions influence adrenarchal and pubertal development in general populations of children with mostly normal weight.
We have reported earlier that a 2-year combined physical activity and dietary intervention attenuated the increase of insulin resistance in a general population of mostly normal-weight primary school children participating in the Physical Activity and Nutrition in Children (PANIC) study (16). It is not well known how lifestyle interventions affect sexual maturation in general populations of mostly normal-weight children, and we therefore report here the effects of the same 2-year lifestyle intervention on serum androgen concentrations and on adrenarchal and pubertal development in these children.
Methods
Participants and Design
We investigated a cohort of children who had participated in the Finnish PANIC study. The PANIC study is a nonrandomized controlled trial on the effects of a combined physical activity and dietary intervention on cardiometabolic risk factors and other health outcomes in a general population of children from the city of Kuopio, Finland (17, 18). The city of Kuopio has approximately 120 000 inhabitants and includes a central urban area and a few surrounding rural villages. The study protocol has been approved by the Research Ethics Committee of the Hospital District of Northern Savo (statement 69/2006), and the study has been carried out in accordance with the principles of the Declaration of Helsinki. The parents or caregivers of children provided their written consent, and the children gave their assent to participation. The PANIC study has been registered at www.clinicaltrials.gov (No. NCT01803776).
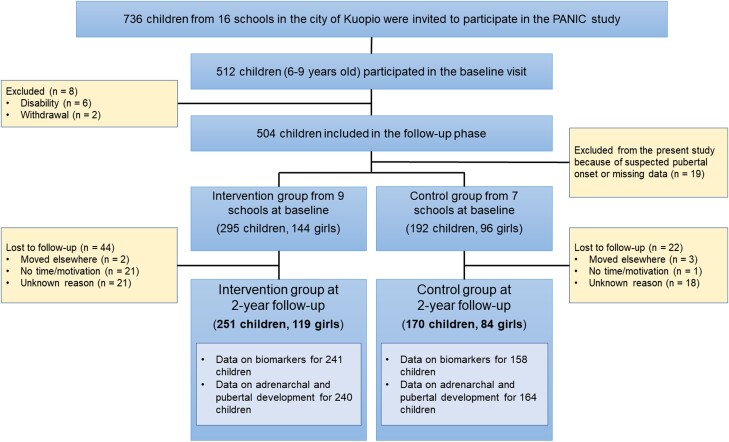
A flow chart of this study is presented in Fig. 1. A total of 736 children, aged 6 to 9 years, who started the first grade in 16 primary schools of the city of Kuopio between 2007 and 2009, were invited to participate. Altogether, 512 (70%) children accepted the invitation and participated in the baseline examination between October 2007 and December 2009. The participants did not differ in sex, age, height standard deviation score (SDS), or body mass index (BMI) SDS from all children who started the first grade in the city of Kuopio between 2007 and 2009. A total of 8 children were excluded at baseline either due to disabilities that could hamper participation in the intervention study or because of withdrawal of the families. Thus, 504 children participated in the 2-year intervention study.
Figure 1.
Flow chart of the study.
Children from 9 schools were allocated to an intervention group and children from 7 other schools to a control group to avoid contamination in the control group by local or national health promotion programs that could have been initiated in the study region over the 2-year intervention study. The intervention and control groups were proportionally matched according to the location of the schools (urban vs rural) to minimize sociodemographic differences between the groups. More children were included in the intervention group as a larger number of dropouts were expected in this group. The children, their parents, or caregivers, or people carrying out the examination visits or doing the measurements were not blinded to the group assignment. A total of 66 children were lost to follow-up over 2 years because of moving elsewhere, lack of time or motivation, or unknown reasons.
We excluded 19 children from the present analyses because of suspected early signs of pubertal development or missing pubertal data at baseline, and altogether 251 children (119 girls, 131 boys) from the intervention group and 170 children (84 girls, 86 boys) from the control group completed the 2-year follow-up period and were included in the present analyses (Fig. 1). Data on biomarkers at baseline and 2-year follow-up examinations were available for 241 children in the intervention group and for 158 children in the control group. Data on clinical adrenarchal and pubertal signs were available for 240 children in the intervention group and for 164 children in the control group. The partly incomplete data on biomarkers were due either to missing blood samples or to hemolysis interfering with the analyses.
Physical Activity and Dietary Intervention
The main goals for the individualized and family-based physical activity and dietary intervention were to (i) decrease the consumption of significant sources of saturated fat and particularly high-fat dairy and meat products; (ii) increase the consumption of significant sources of unsaturated fat and particularly high-fat vegetable oil-based margarines, vegetable oils, and fish; (iii) increase the consumption of vegetables, fruits, and berries; (iv) increase the consumption of significant sources of fiber and particularly whole grain products; (v) decrease the consumption of significant sources of sugar and particularly sugar-sweetened beverages and dairy products, and candies; (vi) decrease the consumption of significant sources of salt and the use of salt in cooking; (vii) increase total physical activity by emphasizing its diversity; (viii) decrease total and particularly screen-based sedentary behavior; and (ix) avoid excessive energy intake.
The 2-year intervention consisted of 6 intervention visits that occurred 0.5, 1.5, 3, 6, 12, and 18 months after baseline examinations. Each intervention visit included 30 to 45 minutes of physical activity counseling and 30 to 45 minutes of dietary counseling for the children and their parents or caregivers. The children and their parents or caregivers received individualized advice from a specialist in exercise medicine and a clinical nutritionist on how to increase physical activity, decrease sedentary time, and improve diet among the children in everyday conditions. Each visit had a specific topic of discussion (physical activity, sedentary time, diet) in accordance with the goals of the intervention and included practical tasks on these topics for the children. The children and their parents or caregivers also received fact sheets on physical activity, sedentary time, and diet, as well as verbal and written information on opportunities for exercising in the city of Kuopio. Some material support was also given for physical activity, such as exercise equipment and allowance for playing indoor sports. Of the children in the intervention group who attended the baseline examination, 87% participated in all 6 visits, 92% in at least 5 visits, and 96% in at least 4 visits. The children in the intervention group, particularly those who did not attend organized sports or exercise, were also encouraged to participate in after-school exercise clubs organized at the 9 schools by trained exercise instructors of the PANIC study. The children in the control group were not allowed to attend these exercise clubs to avoid a nonintentional intervention in the control group. Altogether, 83% of the children in the intervention group participated in at least one of the after-school exercise clubs, and 41% attended these exercise clubs at least once a month.
In the control group, the children and their parents or caregivers received general verbal and written advice on health-improving physical activity and diet only at baseline with no further lifestyle counseling.
Assessment of Body Size and Composition, Physical Activity, and Dietary Factors
Anthropometric measurements were performed in the morning after a 12-hour fast. Height was measured thrice using a calibrated wall-mounted stadiometer to an accuracy of 0.1 cm with the children standing in the Frankfurt plane, and the average of the 2 closest values was used in the analyses. Weight was measured twice using a weight scale integrated into the InBody 720 bioelectrical impedance device (Biospace, Seoul, South Korea) to an accuracy of 0.1 kg with the children having emptied the bladder and wearing light underwear, and the mean of the 2 values was used in the analyses. Body lean and fat masses were measured using the same InBody 720 bioelectrical impedance device. BMI was calculated as body weight (kg) divided by body height (m) squared. Baseline growth velocity (cm/year) was calculated from height values between the age of 5 years and the age at baseline, and 2-year growth velocity from height values between the age at baseline and 2-year follow-up examinations. Age- and sex- standardized height SDS and BMI SDS were calculated using the Finnish national references (19). Physical activity was assessed using the PANIC Physical Activity Questionnaire and dietary factors using 4-day food records (17).
Assessment of Adrenarchal and Pubertal Status
A trained research physician assessed pubertal status according to the Tanner staging method (20, 21). For girls, breast development (Tanner B) was assessed by inspection and palpation and scored as 1 to 5. For boys, testicular development (Tanner G) was assessed with orchidometer and scored as 1 to 5. For girls and boys, development of pubic hair (Tanner P) was assessed with inspection and scored as 1 to 5. Pubertal onset was defined as breast development (Tanner B) ≥2 for girls and testicular volume ≥4 mL (Tanner G ≥2) for boys.
A trained research physician assessed also adrenarchal signs that included adult-type body odor, greasiness of the hair and skin, acne, and development of axillary or pubic hair. Adult-type body odor and greasiness of the hair and skin were assessed also by asking the parents. Clinical adrenarche was defined if one or more of these clinical signs were present.
Collection of Birth Data
Birth data were collected retrospectively from the national Medical Birth Register and the local Kuopio University Hospital register. Preterm birth was defined as if a child was born before 37.0 weeks of gestational age. Birth weight SDS and birth length SDS were calculated using Finnish national references (22). Being born small for gestational age was defined as birth weight and/or birth length ≤ −2 SDS.
Biochemical Analyses
All blood samples were collected in the morning after a 12-hour fast, and the serum samples were stored at −80 °C until used for biochemical analyses.
Serum DHEAS concentrations were measured using an enzyme-linked immunosorbent assay (ELISA) kit (cat # 1950, RRID:AB_2819763, Alpha Diagnostic International, San Antonio, TX, USA). The intra-assay and inter-assay coefficients of variation (CV) for this assay were 7.5% to 11.5% and 7.0% to 11.0%, respectively. The detection limit of the DHEAS immunoassay was 0.014 µmol/L. Biochemical adrenarche was defined as serum DHEAS concentration ≥1 µmol/L (37 ng/dL).
Serum concentrations of other androgens than DHEAS were measured using liquid chromatography—tandem mass spectrometry (LC-MS/MS), as described previously (23). At the time of measuring serum androgen concentrations at baseline and 2-year follow-up examination by LC-MS/MS, our in-house method did not include androgens in the so-called 11-oxygenated androgen pathway (24). Therefore, only androgens belonging to the classic pathway, including DHEA, DHEAS, A4, and testosterone, are used in the present study.
Absolute changes in serum androgen levels over 2 years (Δ) were calculated by the following formula: [steroid concentration at 2-year follow-up examination − steroid concentration at baseline]. Relative changes (%) in serum androgen levels were calculated with the following formula: [(androgen concentration at 2-year follow-up examination − androgen concentration at baseline examination)/androgen concentration at baseline examination) × 100].
Serum IGF-1 concentrations were measured using an ELISA kit (cat # E20, RRID:AB_2813791, Mediagnost, Reutlingen, Germany). The intra-assay and inter-assay CVs for this assay were 5.1% to 6.6% and 7.7% to 9.2%, respectively. Serum luteinizing hormone (LH) concentrations were measured using an electrochemiluminescence immunoassay (Cat # 11732234, RRID:AB_2800498, Roche Diagnostics Gmbh, Mannheim, Germany) with an inter-assay CV of 1.6% to 1.9% and an intra-assay CV of 1.4%. Biochemical evidence for pubertal onset at 2-year follow-up examination was defined as serum LH concentration of at least 0.3 U/L (25).
Serum insulin concentrations were analyzed using an electrochemiluminescence assay kit with the sandwich principle (Cat # 12017547, RRID:AB_2756877, Roche Diagnostics, Mannheim, Germany). The intra-assay and inter-assay CVs for this assay were 1.3% to 3.5% and 1.6% to 4.4%, respectively. A hexokinase method was used to analyze plasma glucose (Roche Diagnostics). The intra-assay and inter-assay CVs for this method were 0.7% to 0.9% and 1.5% to 1.8%, respectively. Homeostatic model assessment for insulin resistance (HOMA-IR) was calculated using the following formula: [(fasting insulin (mU/L) × fasting glucose (mmol/L))/22.5] (26).
Statistical Analyses
All statistical analyses were performed using the IBM SPSS statistics software, Version 25.0 (IBM Corp., Armonk, NY, USA). A P value less than .05 was used to indicate statistical significance. Distributions of the variables were analyzed by the Shapiro-Wilk test and visual observation of the histograms. The Mann-Whitney U test and the Student t test were used to compare differences in continuous variables between groups. For categorical variables, the Pearson χ2 test was used. Intervention effects on the changes of serum androgen concentrations during the follow-up period (Δ steroid concentration) were analyzed using linear regression models that were tested to meet assumptions of normality, linearity, homoscedasticity, and absence of multicollinearity. In the case of continuous variables with skewed distributions, logarithmic transformation was used before regression analyses.
Results
Characteristics of Children at Baseline
Children in the control group had slightly lower birth length SDS (Table 1) and slightly higher serum IGF-1 concentration at baseline (Table 2) than children in the physical activity and dietary intervention group. These differences between the groups reached statistical significance in boys but not in girls. Serum androgen concentrations were comparable between the groups at baseline (Table 2).
Table 1.
Clinical data at birth and at baseline and 2-year follow-up examinations among children in the 2-year combined physical activity and dietary intervention group and among children in the control group
| All children | Girls | Boys | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention (n = 251) |
Control (n = 170) |
P | Intervention (n = 119) |
Control (n = 84) |
P | Intervention (n = 132) |
Control (n = 86) |
P | |
| At birth | |||||||||
| Gestational age, weeks | 39.9 (1.5) | 39.8 (1.7) | .520 | 39.7 (1.6) | 39.7 (1.8) | .953 | 39.9 (1.3) | 39.8 (1.5) | .374 |
| Birth length SDS | −0.07 (1.03) | −0.36 (0.87) | .003 | −0.03 (1.05) | −0.28 (0.89) | .084 | −0.10 (1.02) | −0.43 (0.85) | .014 |
| Birth weight SDS | −0.02 (1.08) | −0.19 (0.92) | .090 | 0.06 (1.10) | −0.18 (0.99) | .123 | −0.09 (1.05) | −0.21 (0.85) | .390 |
| Small for gestational age, %a | 5 | 7 | .406 | 3 | 9 | .206 | 7 | 6 | .818 |
| Preterm, %b | 4 | 5 | .633 | 7 | 5 | .764 | 2 | 6 | .115 |
| At baseline examination | |||||||||
| Age, y | 7.6 (0.3) | 7.6 (0.4) | .733 | 7.6 (0.3) | 7.5 (0.4) | .095 | 7.6 (0.3) | 7.7 (0.4) | .269 |
| Height SDS | 0.17 (0.95) | 0.09 (1.03) | .388 | 0.10 (0.87) | 0.09 (1.10) | .937 | 0.24 (1.02) | 0.09 (0.95) | .274 |
| Growth velocity, cm/yc | 6.73 (0.89) | 6.71 (0.71) | .719 | 6.59 (0.64) | 6.69 (0.66) | .285 | 6.86 (1.06) | 6.72 (0.75) | .323 |
| Body mass index SDS | −0.20 (1.04) | −0.24 (1.14) | .736 | −0.22 (1.02) | −0.37 (1.07) | .333 | −0.18 (1.07) | −0.11 (1.19) | .651 |
| Waist circumference, cm | 56.4 (5.3) | 56.6 (6.2) | .742 | 55.5 (4.8) | 55.3 (6.0) | .711 | 57.2 (5.8) | 57.9 (6.2) | .383 |
| Body fat percentage | 19.5 (7.9) | 19.6 (8.0) | .958 | 21.9 (6.9) | 20.9 (7.6) | .337 | 17.3 (8.1) | 18.2 (8.2) | .447 |
| Lean mass percentage | 76.6 (8.4) | 76.6 (8.5) | .988 | 73.9 (7.3) | 75.1 (8.0) | .304 | 79.0 (8.6) | 78.0 (8.7) | .443 |
| VAT-to-body fat mass, % | 1.54 (1.25) | 1.63 (1.30) | .496 | 0.88 (0.65) | 0.97 (0.74) | .337 | 2.15 (1.36) | 2.29 (1.40) | .468 |
| Clinical adrenarche, %d | 16 | 18 | .502 | 24 | 23 | .816 | 8 | 14 | .171 |
| Biochemical adrenarche, %e | 19 | 18 | .869 | 20 | 17 | .611 | 17 | 19 | .794 |
| Clinical and/or biochemical adrenarche, %f | 31 | 31 | .989 | 36 | 32 | .570 | 26 | 29 | .585 |
| Pubarche, %g | 0 | 1 | .568 | 1 | 2 | .571 | 0 | 0 | .999 |
| At 2-year follow-up examination | |||||||||
| Age, y | 9.7 (0.4) | 9.7 (0.5) | .838 | 9.8 (0.4) | 9.7 (0.5) | .150 | 9.8 (0.4) | 9.8 (0.5) | .277 |
| Height SDS | 0.10 (0.95) | 0.04 (1.04) | .538 | 0.03 (0.86) | 0.02 (1.10) | .924 | 0.17 (1.02) | 0.07 (0.99) | .470 |
| Growth velocity, cm/yh | 5.43 (0.68) | 5.48 (0.71) | .514 | 5.56 (0.75) | 5.55 (0.74) | .970 | 5.32 (0.60) | 5.40 (0.67) | .341 |
| Body mass index SDS | −0.17 (1.04) | −0.13 (1.08) | .687 | −0.19 (1.00) | −0.23 (1.02) | .795 | −0.15 (1.07) | −0.03 (1.14) | .427 |
| Waist circumference, cm | 61.0 (6.9) | 61.2 (7.7) | .369 | 59.6 (6.1) | 59.5 (7.5) | .867 | 62.2 (7.4) | 63.0 (7.6) | .480 |
| Body fat percentage | 24.2 (9.6) | 24.4 (9.7) | .893 | 26.6 (8.7) | 25.1 (8.8) | .261 | 22.0 (10.0) | 23.7 (10.6) | .264 |
| Lean mass percentage | 72.7 (9.4) | 72.6 (9.6) | .904 | 70.4 (8.5) | 71.8 (8.6) | .252 | 74.9 (9.8) | 73.3 (10.4) | .263 |
| VAT-to-body fat mass, % | 1.39 (0.99) | 1.35 (0.94) | .679 | 0.90 (0.61) | 0.98 (0.68) | .420 | 1.85 (1.07) | 1.71 (1.01) | .346 |
| Clinical adrenarche, %d | 63 | 61 | .704 | 73 | 63 | .174 | 54 | 58 | .506 |
| Biochemical adrenarche, %e | 29 | 33 | .363 | 26 | 30 | .585 | 31 | 36 | .433 |
| Clinical and/or biochemical adrenarche, %f | 67 | 72 | .267 | 73 | 73 | .955 | 62 | 72 | .166 |
| Pubarche, %g | 8 | 10 | .318 | 15 | 15 | .989 | 1 | 6 | .038 |
| Puberty, only clinical signs, %i | 20 | 21 | .939 | 27 | 35 | .259 | 14 | 6 | .107 |
| Puberty, clinical signs and/or biochemical evidence, %j | 24 | 29 | .300 | 28 | 41 | .057 | 21 | 17 | .494 |
Continuous variables are expressed as means (SD) and categorical variables as percentages. Differences between the intervention and control groups were analyzed using the Student t test for continuous variables and the χ2 test for categorical variables, and P values < .05 indicating statistically significant differences between the groups are bolded.
Abbreviations: SDS, standard deviation score; VAT, visceral adipose tissue mass.
a Birth length and/or birth weight ≤ −2 SDS.
b Gestational age <37.0 weeks.
c Growth velocity between the age of 5 years and baseline examination.
d At least one of the following signs: adult-type body odor, greasiness of hair and skin, comedones/acne, and axillary/pubic hair.
e Serum DHEAS concentration ≥1 µmol/L.
f At least one clinical sign of adrenarche and/or serum DHEAS concentration ≥1 µmol/L.
g Tanner P ≥ 2.
h Growth velocity between baseline and follow-up examination.
i Tanner B for girls or G for boys ≥2.
j Tanner B for girls or G for boys ≥2 and/or serum luteinizing hormone ≥0.3 U/L.
Table 2.
Biochemical data at baseline and 2-year follow-up examinations among children in the 2-year combined physical activity and dietary intervention group and among children in the control group
| All children | Girls | Boys | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention (n = 250) |
Control (n = 170) |
P | Intervention (n = 119) |
Control (n = 84) |
P | Intervention (n = 131) |
Control (n = 86) |
P | |
| At baseline examination (6-9 y) | |||||||||
| DHEA, nmol/L | 1.70 (0.86-2.91) | 1.33 (0.81-2.57) | .313 | 1.92 (1.02-3.34) | 1.52 (0.84-2.72) | .382 | 1.40 (0.73-2.49) | 1.24 (0.67-2.07) | .443 |
| DHEAS, µmol/L | 0.59 (0.33-0.84) | 0.56 (0.31-0.86) | .505 | 0.57 (0.35-0.84) | 0.49 (0.31-0.85) | .457 | 0.60 (0.32-0.84) | 0.58 (0.32-0.88) | .844 |
| A4, nmol/L | 0.74 (0.47-1.07) | 0.67 (0.40-0.99) | .062 | 0.82 (0.47-1.21) | 0.74 (0.44-1.10) | .306 | 0.63 (0.46-0.98) | 0.62 (0.35-0.87) | .083 |
| Testosterone, pmol/L | 122 (83-168) | 117 (76-178) | .689 | 133 (92-194) | 124 (92-190) | .712 | 115 (79-154) | 107 (71-174) | .789 |
| Insulin, U/L | 4.2 (2.7-5.7) | 4.2 (2.9-6.0) | .466 | 4.7 (3.4-6.0) | 4.3 (3.3-6.1) | .736 | 3.9 (2.5-5.5) | 4.0 (2.6-6.0) | .234 |
| HOMA-IR | 0.91 (0.55-1.27) | 0.91 (0.64-1.34) | .525 | 1.00 (0.68-1.32) | 0.92 (0.70-1.29) | .630 | 0.85 (0.53-1.19) | 0.89 (0.55-1.36) | .233 |
| IGF-1, nmol/L | 21.7 (17.5-25.9) | 22.9 (18.8-28.3) | .013 | 22.5 (18.5-28.1) | 24.1 (19.4-30.1) | .264 | 20.2 (16.2-24.5) | 22.1 (18.4-27.4) | .017 |
| At 2-year follow-up examination (9-11 y) | |||||||||
| DHEA, nmol/L | 3.06 (1.71-4.75) | 3.99 (2.13-6.49) | .003 | 3.47 (1.93-5.23) | 4.22 (2.70-6.76) | .033 | 2.78 (1.57-4.42) | 3.27 (2.00-6.38) | .046 |
| DHEAS, µmol/L | 0.69 (0.38-1.05) | 0.67 (0.42-1.19) | .449 | 0.67 (0.36-1.02) | 0.63 (0.36-1.02) | .819 | 0.74 (0.40-1.10) | 0.74 (0.42-1.22) | .378 |
| A4, nmol/L | 1.20 (0.79-1.70) | 1.20 (0.80-1.84) | .613 | 1.36 (0.92-1.98) | 1.33 (0.92-2.06) | .991 | 1.07 (0.73-1.52) | 1.07 (0.70-1.70) | .633 |
| Testosterone, pmol/L | 202 (135-291) | 206 (148-313) | .383 | 250 (156-347) | 215 (154-328) | .540 | 185 (123-241) | 197 (127-277) | .128 |
| Insulin, U/L | 4.9 (3.5-7.2) | 5.9 (3.9-7.9) | .028 | 5.4 (4.0-8.2) | 6.3 (4.1-8.2) | .167 | 4.6 (3.1-6.6) | 5.1 (3.5-7.4) | .120 |
| HOMA-IR | 1.10 (0.77-1.61) | 1.27 (0.84-1.76) | .034 | 1.13 (0.88-1.81) | 1.38 (0.87-1.84) | .208 | 1.03 (0.68-1.50) | 1.16 (0.76-1.71) | .114 |
| IGF-1, nmol/L | 29.8 (24.1-35.8) | 29.7 (24.3-35.6) | .942 | 33.2 (26.8-40.3) | 32.1 (26.8-40.3) | .586 | 26.2 (22.9-33.5) | 28.2 (22.9-32.1) | .821 |
The values are medians (25th-75th percentile range). HOMA-IR was calculated using the following formula: [(insulin (mU/L)×glucose (mmol/L))/22.5]. Differences between the intervention and control groups were analyzed using the Mann-Whitney U test, and P values < .05 indicating statistically significant differences between the groups are bolded. To have values in conventional units, multiply DHEA by 0.2884 (ng/mL), DHEAS by 36.85 (µg/dL), A4 by 0.2864 (ng/mL), testosterone by 0.2885 (pg/mL), and IGF-1 by 7.65 (ng/mL).
Abbreviations: A4, androstenedione; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; HOMA-IR, homeostatic model assessment for insulin resistance; IGF-1, insulin-like growth factor 1.
Intervention Effects on Physical Activity and Dietary Factors
Total and unsupervised physical activity increased in the intervention group but decreased in the control group over 2 years (total physical activity, +7 vs −5 minutes/d, P = .003; unsupervised physical activity, +6 vs −9 minutes/d, P < .001). There were no differences in the changes of organized exercise between the groups (+4 vs +3 minutes/d, P = .444). The effects of intervention on dietary factors over 2 years have been published comprehensively elsewhere (17). In brief, the intervention improved diet quality; for example, it increased the consumption of vegetables, fruits, and berries and replaced the consumption of foods high in saturated fat with the consumption of foods high in unsaturated fat. There was no difference in the change of protein intake between the groups.
Intervention Effects on Height, Growth Velocity, and Body Composition
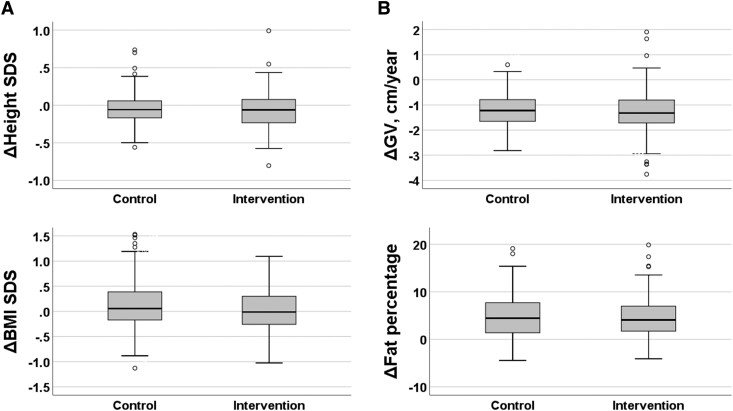
There were no differences in measures of body size or composition between the intervention and control groups at baseline or 2-year follow-up examinations (Table 1). The intervention had no effects on height SDS, growth velocity, BMI SDS, or body fat percentage over 2 years in the whole study population or in girls or in boys separately (Fig. 2 and Table 1).
Figure 2.
Absolute change (Δ) in height SDS (panel A), growth velocity (GV, panel B), body mass index SDS (BMI, panel C), and body fat percentage (panel D) over 2 years among children in the combined physical and dietary intervention group and among children in the control group. The mean age at baseline was 7.6 years. Medians and interquartile ranges (IQRs) in boxes, ranges (IQR*1.5) in whiskers, and outliers (circles) are provided in boxplot charts. No statistically significant differences were found between the groups in any of these parameters (Mann-Whitney U test, P values > .140), also when analyzing girls and boys separately.
Intervention Effects on Clinical Signs of Adrenarche and Puberty
The prevalence of pubarche at 2-year follow-up examination was lower among boys in the intervention group than among boys in the control group (Table 1). Otherwise, we did not find any statistically significant differences between the groups in the prevalence of clinical adrenarchal or pubertal signs. When pubertal onset was defined by Tanner B ≥2 and/or serum LH concentration ≥0.3 U/L, girls in the intervention group tended to have a lower prevalence of puberty at 2-year follow-up examination than girls in the control group, but this difference did not reach the level of statistical significance (P = .057).
Intervention Effects on Serum Androgens and Other Biomarkers
Serum DHEA concentration at 2-year follow-up examination was lower among children in the intervention group than among children in the control group (Table 2). No differences in serum DHEAS, A4, or testosterone concentrations were found between the groups at the 2-year follow-up examination.
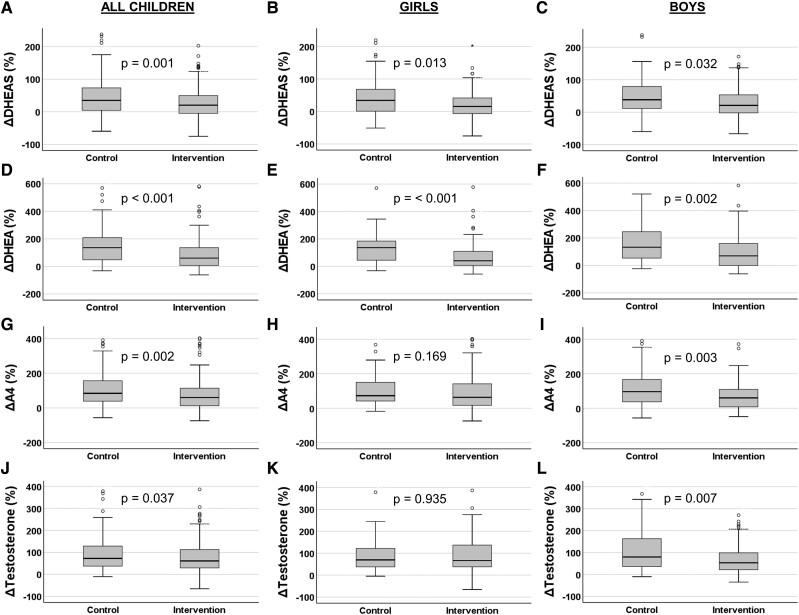
Serum DHEA, DHEAS, A4, and testosterone concentrations increased less over 2 years among children in the intervention group than among children in the control group (Fig. 3). In boys, the levels of all androgens increased less in the intervention group than in the control group, but in girls this was the case only for DHEA and DHEAS (Fig. 3).
Figure 3.
Relative unadjusted changes (%) in serum dehydroepiandrosterone sulfate (DHEAS; panels A-C), dehydroepiandrosterone (DHEA; D-F), androstenedione (A4; G-I), and testosterone (J-L) concentrations among children in the combined physical activity and dietary intervention group and among children in the control group over 2 years. The mean age at baseline was 7.6 years. Medians and interquartile ranges (IQRs) in boxes, ranges (IQR*1.5) in whiskers, and outliers (circles) are provided in boxplot charts. Differences between the groups were analyzed using the Mann-Whitney U test, and P values were used to indicate statistical significance of the differences.
The intervention attenuated the increase of serum DHEA concentration over 2 years after adjustment for gender, gestational age, birth length SDS, age, serum IGF-1 concentration, BMI SDS, and presence of adrenarchal signs at baseline, and presence of pubertal signs at 2-year follow-up (Table 3; model 1). The intervention also attenuated the increase of serum testosterone concentration over 2 years in boys but not in girls after these adjustments. Further adjustment for the change of fasting serum insulin concentration over 2 years had no statistically significant effect on these results (Table 3; model 2). However, the change of HOMA-IR correlated positively with and the changes of serum DHEA, A4, and testosterone concentrations over 2 years in girls (r = 0.212 and P < .001 for DHEA; r = 0.199 and P = .010 for A4; r = 0.153 and P = .049 for testosterone) but not in boys.
Table 3.
Effects of the 2-year combined physical activity and dietary intervention on changes (Δ) in serum DHEA, DHEAS, A4, and testosterone concentrations
| Outcome (dependent) variable | ||||||||
|---|---|---|---|---|---|---|---|---|
| ΔDHEA (nmol/L) | ΔDHEAS (µmol/L) | ΔA4 (nmol/L) | ΔTestosterone (pmol/L) | |||||
| β (95% CI) |
P | β (95% CI) |
P | β (95% CI) |
P | β (95% CI) |
P | |
| Model 1: | ||||||||
| All children (n = 421) | −1.23 (−1.85 to −0.62) |
<.001 | −0.05 (−0.14 to 0.04) |
.292 | −0.12 (−0.27 to 0.03) |
.111 | −121 (−237 to −5.27) |
.041 |
| Girls only (n = 203) | −1.27 (−2.11 to −0.43) |
.003 | −0.07 (−0.18 to 0.05) |
.244 | −0.02 (−0.26 to 0.21) |
.855 | 22.9 (−9.24 to 53.1) |
.167 |
| Boys only (n = 218) | −1.31 (−2.24 to −0.37) |
.007 | −0.05 (−0.19 to 0.09) |
.503 | −0.19 (−0.38 to 0.01) |
.063 | −253 (−476 to −29.5) |
.027 |
| Model 2: | ||||||||
| All children (n = 421) | −1.14 (−1.77 to −0.52) |
<.001 | −0.07 (−0.16 to 0.03) |
.169 | −0.11 (−0.26 to 0.04) |
.156 | −124 (−243 to −4.36) |
.042 |
| Girls only (n = 203) | −1.20 (−2.07 to −0.34) |
.007 | −0.07 (−0.19 to 0.04) |
.215 | −0.04 (−0.24 to 0.25) |
.972 | 24.0 (−8.35 to 56.4) |
.145 |
| Boys only (n = 218) | −1.15 (−2.09 to −0.22) |
.016 | −0.05 (−0.20 to 0.09) |
.467 | −0.19 (−0.39 to 0.01) |
.058 | −256 (−485 to −28.0) |
.028 |
Values are from linear regression models adjusted for gestational age, birth length SDS, age, serum IGF-1 concentration, BMI SDS, and presence of adrenarchal signs at baseline, and presence of pubertal signs at 2-year follow-up (Model 1) and additionally for the change of fasting serum insulin concentration over 2 years (Model 2). The mean age at baseline was 7.6 years. P values < .05 indicating statistical significance are bolded.
Abbreviations: A4, androstenedione; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate.
Discussion
This 2-year nonrandomized controlled trial showed that the physical activity and dietary intervention attenuated the increase of serum DHEAS, DHEA, A4, and testosterone concentrations in a general population of initially prepubertal and mostly normal-weight children. The intervention attenuated the increase of all these serum androgens in boys but only the increase of serum DHEA and DHEAS in girls. The intervention also decreased the development of pubarche in boys but not in girls. The effects of physical activity and dietary intervention on serum androgens and the development of pubarche were independent of changes in body size and composition, but the intervention effects might be partly explained by changes in fasting serum insulin.
Studies that have reported the effects of physical activity or dietary interventions on circulating concentrations of androgens or other steroid hormones in children are scarce, and to the best of our knowledge, there are no such studies in general populations of initially prepubertal and mostly normal-weight children. In German obese prepubertal 8-year-old children who had received physical activity, dietary, and other behavioral interventions, weight loss was associated with decreases in circulating concentrations of glucocorticoids, A4, and testosterone but not DHEAS (13). Children in our study differed from those in the German study by being mostly normal weight and showing no intervention effects on measures of body size or composition. Consistent with the results of the German study, however, we found that the intervention did not decrease but attenuated the gradual increase of serum androgen concentrations between the ages of 6 and 10 years. The same German group also reported that successful weight loss was associated with increased steroid sulfation capacity in obese children (27). In our study, DHEAS was the only measured sulfated steroid, and we did not detect major changes in the absolute serum DHEAS concentration after intervention, although its relative gradual increase was smaller in the intervention group than in the control group between the ages of 6 to 11 years.
A meta-analysis showed that physical activity, dietary, and other behavioral interventions improved the free androgen index and clinical manifestations, including the Ferriman-Gallwey score of hairiness and menstrual irregularities, in adolescent girls with PCOS (14), which is a condition characterized by hyperandrogenism. Although hyperandrogenic adolescent girls are metabolically different from the mostly normal-weight children participating in our study, the results of the meta-analysis also suggest that lifestyle interventions can modulate androgen production and metabolism in children. In another meta-analysis among PCOS women, lifestyle modifications were found to be associated with reduced fasting blood glucose and serum insulin levels (28). Insulin is an important biomarker of sexual maturation, and premature adrenarche and normal central puberty are known to be associated with decreased insulin sensitivity (5, 11). We have previously shown that the 2-year combined physical activity and dietary intervention attenuated the increase of insulin resistance in the present population of initially prepubertal and mostly normal-weight children (16). In the present study, we found that the same intervention attenuated the increase of serum androgen concentrations and the change in HOMA-IR was positively correlated with the changes in DHEA, A4, and testosterone concentrations during the follow-up in girls. These results together suggest that physical activity and dietary interventions may slow the development of adrenarche, and that this may be partly explained by enhanced insulin sensitivity, especially in girls.
Previous studies in children have taught us that the nutritional and weight status are drivers for the timing and intensity of adrenarche and puberty. For example, children with premature adrenarche are more likely to be overweight or obese than those with later timing of adrenarche (5), and childhood obesity is associated with earlier pubertal onset (6, 10). These 2 developmental events seem to be linked, as children with premature adrenarche have more advanced pubertal development at the age of 12 years than those with normal timing of adrenarche (3). We and other research groups have also provided evidence that some dietary factors, such as increased protein intake, are associated with circulating adrenal androgen concentrations in children (29, 30). Our finding that the lifestyle intervention attenuated the increase of serum androgens and decreased the prevalence of pubarche in boys at the age of adrenarche suggests that changes in environmental factors, such as physical activity and dietary factors, may have effects on the biological system regulating the timing and strength of the sexual development in children. Our lifestyle intervention mainly affected serum androgens but not the clinical signs of androgen action, except delaying pubarche in boys. The reason for this may be that the duration of intervention of 2 years may have been too short given that approximately one-fifth of the initially prepubertal and mostly normal-weight children had already entered adrenarche by baseline and less than one-fourth of the children entered puberty over the next 2 years. It is also well known that the onset of adrenarche and puberty is seen later in boys than in girls, which was also found in our study cohort. Therefore, another explanation for observing the effect of our lifestyle intervention only on pubarche in boys but not on other clinical signs of androgen action in either sex is that the intervention started when many girls had already reached adrenarche.
The strengths of our study include the relatively large general population of initially prepubertal and mostly normal-weight children examined, the long-term and controlled lifestyle intervention conducted, the sensitive LC-MS/MS method used to measure most serum androgens, and the comprehensive assessments of clinical androgenic and pubertal signs. Serum DHEAS was not measured by the LC-MS/MS method, but it was well measurable by an immunoassay since it is abundant in the circulation already in mid-childhood. Body size and composition in our cohort were comparable to those of the national reference population (19) making it possible to generalize the results to other children of the same age in Finland. We emphasized the individual needs of the families and parental involvement in our intervention, both of which have been observed to improve adherence of families to lifestyle interventions (31). Thus, only about 15% of the children in the intervention group dropped out during the 2-year follow-up, and almost 90% of the children and their parents or caregivers participated in all physical activity and dietary counseling sessions, indicating that the intervention was well accepted by the participants.
A limitation of the study is that we did not randomly allocate the participants to the intervention and control group, but instead allocated the children from 9 schools to the intervention group and the children from 7 other schools to the control group to avoid contamination in the control group by local or national health promotion programs that could have been initiated in the study region during the follow-up period. This type of allocation of the children to the study groups also enabled us to organize after-school exercise clubs as part of the intervention at the 9 school premises and thus avoid a nonintentional intervention in the control group. We matched the intervention and control groups according to the location of the schools so that children from urban and rural areas were included in both groups to minimize sociodemographic differences between the groups. There were only minor differences in baseline characteristics between the intervention and control groups, suggesting fair success in avoiding selection bias. Our individualized and family-based lifestyle intervention consisted mainly of physical activity counseling for the children and their parents or caregivers, whereas providing the children with supervised exercise played a much smaller role in our intervention. This kind of physical activity intervention could be seen as a limitation of our study. However, we found that total and unsupervised physical activity increased in the intervention group but decreased in the control group, showing that this kind of intervention was able to increase physical activity among these children. It is possible in an unblinded study design, which we used, that knowing the study group has an influence on participants when answering the questionnaire or on investigators when performing the assessments. Another limitation is the lack of data on circulating sex hormone-binding globulin concentrations that the lifestyle intervention could have altered and thereby affected the bioavailability of circulating androgens (32). One may question the reliability of the clinical assessment of adult-type body odor and greasiness of hair and skin. Although our experience indicates that these signs of androgen action are well detectable in clinical examinations and that parents usually recognize the onset of these signs, we do agree that they are weak indicators of clinical adrenarche. We also agree that defining biochemical adrenarche as a serum DHEAS concentration of ≥1 µmol/L, which is commonly used for this purpose, has its limitations because serum DHEAS concentrations are sexually dimorphic and do not necessarily correlate straightforwardly with clinical signs of androgen action (33, 34).
To conclude, we found that the 2-year combined physical activity and dietary intervention in mid-childhood attenuated the increase of serum androgen concentrations in a general population of initially prepubertal and mostly normal-weight children, and that the intervention delayed pubarche in boys. These intervention effects on serum androgens were independent of changes in body size and composition but may have been mediated through altered insulin sensitivity, especially in girls. Future studies should consider the limitations of our study and confirm its results, preferably with supervised exercise interventions starting even at younger ages. Our findings highlight the importance of targeting physical activity and dietary interventions at children with hyperandrogenic conditions, including those with a normal body weight. Our findings also emphasize that a healthy diet and a physically active lifestyle in childhood might prevent conditions like premature adrenarche, which, at least in some cases, may precede later unfavorable health outcomes (35, 36).
Acknowledgments
We are grateful to all children and their parents and caregivers who have participated in the PANIC study. We are also indebted to all members of the PANIC research team for their invaluable contribution in the acquisition of the data throughout the study. We also want to thank Biocenter Finland and Biocenter Kuopio for supporting their core LC-MS laboratory facility.
Abbreviations
- A4
androstenedione
- BMI
body mass index
- CV
coefficient of variation
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- ELISA
enzyme-linked immunosorbent assay
- HOMA-IR
homeostatic model assessment for insulin resistance
- IGF
insulin-like growth factor
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- LH
luteinizing hormone
- PANIC
Physical Activity and Nutrition in Children
- PCOS
polycystic ovary syndrome
- SDS
standard deviation score
Contributor Information
Jani Liimatta, Kuopio Pediatric Research Unit (KuPRU), University of Eastern Finland, 70029 Kuopio, Finland; Department of BioMedical Research (DBMR), University of Bern, 3008 Bern, Switzerland; Pediatric Endocrinology, Diabetology, and Metabolism, Inselspital, Bern University Hospital, 3010 Bern, Switzerland.
Christa E Flück, Department of BioMedical Research (DBMR), University of Bern, 3008 Bern, Switzerland; Pediatric Endocrinology, Diabetology, and Metabolism, Inselspital, Bern University Hospital, 3010 Bern, Switzerland.
Aino Mäntyselkä, Kuopio Pediatric Research Unit (KuPRU), University of Eastern Finland, 70029 Kuopio, Finland; Department of Pediatrics, Kuopio University Hospital, 70029 Kuopio, Finland.
Merja R Häkkinen, School of Pharmacy, University of Eastern Finland, 70211 Kuopio, Finland; Department of Health Security, Finnish Institute for Health and Welfare (THL), 70701 Kuopio, Finland.
Seppo Auriola, School of Pharmacy, University of Eastern Finland, 70211 Kuopio, Finland.
Raimo Voutilainen, Kuopio Pediatric Research Unit (KuPRU), University of Eastern Finland, 70029 Kuopio, Finland; Department of Pediatrics, Kuopio University Hospital, 70029 Kuopio, Finland.
Jarmo Jääskeläinen, Kuopio Pediatric Research Unit (KuPRU), University of Eastern Finland, 70029 Kuopio, Finland; Department of Pediatrics, Kuopio University Hospital, 70029 Kuopio, Finland.
Timo A Lakka, Institute of Biomedicine, School of Medicine, University of Eastern Finland, 70211 Kuopio, Finland; Department of Clinical Physiology and Nuclear Medicine, Kuopio University Hospital, 70210 Kuopio, Finland; Foundation for Research in Health Exercise and Nutrition, Kuopio Research Institute of Exercise Medicine, 70211 Kuopio, Finland.
Funding
Open access funding provided by University of Eastern Finland (UEF), including Kuopio University Hospital. The PANIC study has been supported financially by grants from Ministry of Education and Culture of Finland, Academy of Finland, Ministry of Social Affairs and Health of Finland, Research Committee of the Kuopio University Hospital Catchment Area (State Research Funding), Finnish Innovation Fund Sitra, Social Insurance Institution of Finland, Finnish Cultural Foundation, Foundation for Pediatric Research, Diabetes Research Foundation in Finland, Finnish Foundation for Cardiovascular Research, Juho Vainio Foundation, Paavo Nurmi Foundation, Yrjö Jahnsson Foundation, and the city of Kuopio.
This work was also supported by the Sigrid Jusélius Foundation (Helsinki, Finland; Research Fellowship to J.L.), the Foundation for Pediatric Research (Helsinki, Finland; Research Fellowship to J.L.).
The funding sources have not been involved in: 1) design and conduct of the study; 2) collection, management, analysis, and interpretation of data; 3) review or approval of the manuscript; or 4) decision to submit the manuscript for publication.
Author Contributions
T.A.L. and J.J. designed the study. T.A.L. and A.M. conducted the study. Steroid profiling with the LC-MS/MS method was performed by M.R.H., S.A., J.J., and R.V. J.L planned and performed the statistical analysis. J.L., C.E.F., J.J., and T.A.L. interpreted the results. J.L. drafted the manuscript. All authors critically revised the manuscript for its intellectual content and approved the final version of the manuscript. T.A.L. is the principal investigator of the PANIC study.
Disclosures
The authors have nothing to disclose.
Data Availability
Information about the PANIC study and the data used in the present paper are available at www.panicstudy.fi/en/etusivu. The data are not publicly available due to research ethical reasons and because the owner of the data is the University of Eastern Finland and not the research group. However, the corresponding author can provide further information on the PANIC study and the PANIC data on a reasonable request.
Clinical Trials Registration
The PANIC study has been registered at www.clinicaltrials.gov (No. NCT01803776).
References
- 1. Rosenfield RL. Normal and premature adrenarche. Endocr Rev. 2021;42(6):783‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Livadas S, Chrousos GP. Control of the onset of puberty. Curr Opin Pediatr. 2016;28(4):551‐558. [DOI] [PubMed] [Google Scholar]
- 3. Liimatta J, Utriainen P, Voutilainen R, Jääskeläinen J. Girls with a history of premature adrenarche have advanced growth and pubertal development at the age of 12 years. Front Endocrinol (Lausanne). 2017;8:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Santos-Silva R, Fontoura M, Severo M, Santos AC. Dehydroepiandrosterone sulphate levels at 7 years old are positively associated with more advanced pubertal development between 10 and 13 years old in girls. Clin Endocrinol (Oxf). 2022;97(6):747‐754. [DOI] [PubMed] [Google Scholar]
- 5. Utriainen P, Jääskeläinen J, Romppanen J, Voutilainen R. Childhood metabolic syndrome and its components in premature adrenarche. J Clin Endocrinol Metab. 2007;92(11):4282‐4285. [DOI] [PubMed] [Google Scholar]
- 6. Akslaege L, Juul A, Olsen LW, Sørensen TIA. Age at puberty and the emerging obesity epidemic. PLoS One. 2009;4(12):e8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Utriainen P, Voutilainen R, Jääskeläinen J. Girls with premature adrenarche have accelerated early childhood growth. J Pediatr. 2009;154(6):882‐887. [DOI] [PubMed] [Google Scholar]
- 8. Dunger BD, Ahmed ML, Ong KK. Early and late weight gain and the timing of puberty. Mol Cell Endocrinol. 2006;254-255:140‐145. [DOI] [PubMed] [Google Scholar]
- 9. Ong K, Potau N, Petry CJ, et al. Opposing influences of prenatal and postnatal weight gain on adrenarche in normal boys and girls. J Clin Endocrinol Metab. 2004;89(6):2647‐2651. [DOI] [PubMed] [Google Scholar]
- 10. Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between obesity and pubertal timing: a systematic review and meta-analysis. Int J Environ Res Public Health. 2017;14(10):1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444‐2450. [DOI] [PubMed] [Google Scholar]
- 12. Ho M, Garnett SP, Baur LA, et al. Impact of dietary and exercise interventions on weight change and metabolic outcomes in obese children and adolescents: a systematic review and meta-analysis of randomized trials. JAMA Pediatr. 2013;167(8):759‐768. [DOI] [PubMed] [Google Scholar]
- 13. Reinehr T, Kulle A, Wolters B, et al. Steroid hormone profiles in prepubertal obese children before and after weight loss. J Clin Endocrinol Metab. 2013;98(6):E1022‐E1030. [DOI] [PubMed] [Google Scholar]
- 14. Abdolahian S, Tehrani FR, Amiri M, et al. Effect of lifestyle modifications in anthropometric, clinical, and biochemical parameters in adolescent girls with polycystic ovary syndrome: a systematic review and meta-analysis. BMC Endocr Disord. 2020;20(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shele G, Genkil J, Speelman D. A systematic review of the effects of exercise on hormones in women with polycystic ovary syndrome. J Funct Morphol Kinesiol. 2020;5(2):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lakka T, Lintu N, Väistö J, et al. A 2 year physical activity and dietary intervention attenuates the increase in insulin resistance in a general population of children: the PANIC study. Diabetologia. 2020;63(11):2270‐2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Viitasalo A, Eloranta AM, Lintu N, et al. The effects of a 2-year individualized and family-based lifestyle intervention on physical activity, sedentary behavior and diet in children. Prev Med. 2016;87:81‐88. [DOI] [PubMed] [Google Scholar]
- 18. Venäläinen TM, Viitasalo AM, Schwab US, et al. Effect of a 2-y dietary and physical activity intervention on plasma fatty acid composition and estimated desaturase and elongase activities in children: the physical activity and nutrition in children study. Am J Clin Nutr. 2016;104(4):964‐972. [DOI] [PubMed] [Google Scholar]
- 19. Saari A, Sankilampi U, Hannila ML, Kiviniemi V, Kesseli K, Dunkel L. New Finnish growth references for children and adolescents aged 0 to 20 years: length/height-for-age, weight-for-length/height, and body mass index-for-age. Ann Med. 2011;43(3):235‐248. [DOI] [PubMed] [Google Scholar]
- 20. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sankilampi U, Hannila ML, Saari A, Gissler M, Dunkel L. New population-based reference for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med. 2013;45(5-6):446‐454. [DOI] [PubMed] [Google Scholar]
- 23. Häkkinen MR, Heinosalo T, Saarinen N, et al. Analysis by LC-MS/MS of endogenous steroids from human serum, plasma, endometrium and endometriotic tissue. J Pharm Biomed Anal. 2018;152:165‐172. [DOI] [PubMed] [Google Scholar]
- 24. Rege J, Turcu AF, Kasa-Vubu JZ, et al. 11-Ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J Clin Endocrinol Metab. 2018;103(12):4589‐4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harrington J, Palmert MR, Hamilton J. Use of local data to enhance uptake of published recommendations: an example from the diagnostic evaluation of precocious puberty. Arch Dis Child. 2014;99(1):15‐20. [DOI] [PubMed] [Google Scholar]
- 26. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412‐419. [DOI] [PubMed] [Google Scholar]
- 27. Reinehr T, Sánchez-Guijo A, Lass N, Wudy SA. Higher steroid sulfation is linked to successful weight loss in obese children. Endocr Connect. 2018;7(10):1020‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Domecq JP, Prutsky G, Mullan RJ, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98(12):4655‐4663. [DOI] [PubMed] [Google Scholar]
- 29. Shi L, Wudy SA, Buyken AE, Hartmann MF, Remer T. Body fat and animal protein intakes are associated with adrenal androgen secretion in children. Am J Clin Nutr. 2009;90(5):1321‐1328. [DOI] [PubMed] [Google Scholar]
- 30. Mäntyselkä A, Jääskeläinen J, Eloranta AM, et al. Associations of lifestyle factors with serum dehydroepiandrosterone sulphate and insulin-like growth factor-1 concentration in prepubertal children. Clin Endocrinol (Oxf). 2018;88(2):234‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Showel NN, Fawole O, Segal J, et al. A systematic review of home-based childhood obesity prevention studies. Pediatrics. 2013;132(1):193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aroda VR, Christophi CA, Edelstein SL, et al. Circulating sex hormone binding globulin levels are modified with intensive lifestyle intervention, but their changes did not independently predict diabetes risk in the diabetes prevention program. BMJ Open Diabetes Res Care. 2020;8(2):e001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mäntyselkä A, Jääskeläinen J, Lindi V, et al. The presentation of adrenarche is sexually dimorphic and modified by body adiposity. J Clin Endocrinol Metab. 2014;99(10):3889‐3894. [DOI] [PubMed] [Google Scholar]
- 34. Utriainen P, Voutilainen R, Jääskeläinen J. Continuum of phenotypes and sympathoadrenal function in premature adrenarche. Eur J Endocrinol. 2009;160(4):657‐665. [DOI] [PubMed] [Google Scholar]
- 35. Liimatta J, Utriainen P, Laitinen T, Voutilainen R, Jääskeläinen J. Cardiometabolic risk profile among young adult females with a history of premature adrenarche. J Endocr Soc. 2019;3(10):1771‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tennilä J, Jääskeläinen J, Utriainen P, et al. PCOS features and steroid profiles among young adult women with a history of premature adrenarche. J Clin Endocrinol Metab. 2021;106(9):e3335‐e3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Information about the PANIC study and the data used in the present paper are available at www.panicstudy.fi/en/etusivu. The data are not publicly available due to research ethical reasons and because the owner of the data is the University of Eastern Finland and not the research group. However, the corresponding author can provide further information on the PANIC study and the PANIC data on a reasonable request.