Abstract
Context
Clinical guidelines have recommended a trial of liothyronine (LT3) with levothyroxine (LT4) in select patients with hypothyroidism. However, little is known about the real-world use of LT3 and desiccated thyroid extract (DTE) and the characteristics of patients treated with LT3 and DTE.
Objectives
(1) Determine national trends of new LT4, LT3, and DTE prescriptions in the United States; (2) determine whether sociodemographic, healthcare access, and dietary factors are associated with different thyroid hormone (TH) therapies.
Methods
Parallel cross-sectional studies were conducted using 2 datasets: (1) a national patient claims dataset (2010-2020) and (2) the National Health and Nutrition Examination Study (NHANES) dataset (1999-2016). Included participants had a diagnosis of primary or subclinical hypothyroidism. Study outcomes included the impact of demographics and healthcare access on differences in the proportion of TH therapies consisting of LT4, LT3, and DTE (patient claims) and differences in dietary behaviors between DTE-treated participants and LT4-treated matched controls (NHANES).
Results
On an average annual basis, 47 711 adults received at least 1 new TH prescription, with 88.3% receiving LT4 monotherapy, 2.0% receiving LT3 therapy, and 9.4% receiving DTE therapy. The proportion receiving DTE therapy increased from 5.4% in 2010 to 10.2% in 2020. In the analysis between states, high primary care and endocrinology physician densities were associated with increased use of LT4 monotherapy (odds ratio 2.51, P < .001 and odds ratio 2.71, P < .001). DTE-treated NHANES participants (n = 73) consumed more dietary supplements compared to LT4-treated participants (n = 146) (4.7 vs 2.1, P < .001).
Conclusions
The proportion of new TH therapies containing DTE for hypothyroidism doubled since 2010 while LT3 therapies remained stable. DTE treatment was associated with decreased physician density and increased dietary supplement use.
Keywords: hypothyroidism, levothyroxine, desiccated thyroid extract, geographic trends, dietary supplements
Hypothyroidism is a common endocrine disorder in which the thyroid fails to produce an adequate amount of thyroid hormone (TH). Primary and subclinical hypothyroidism collectively affect nearly 10% of the US population (1-3). Patients are treated with TH replacement to resolve hypothyroid symptoms and restore biochemical euthyroidism (4-6). Levothyroxine (LT4)—a synthetic form of T4—is the first-line therapy recommended and one of the most frequently prescribed medications in the United States annually (7, 8). However, a significant minority of patients treated with LT4 are dissatisfied with therapy (9), highlighted by persistent cognitive (10) and metabolic (11, 12) symptoms. These findings have caused some clinicians to reevaluate the current treatment approach of LT4 for patients with persistent symptoms. The American Thyroid Association has called for a more patient-centric treatment approach to hypothyroidism and further investigation of causes and potential treatments for persistent hypothyroid symptoms (13).
One approach to thyroid-related persistent symptoms in LT4-treated patients is to trial the addition of T3 in the form of liothyronine (LT3) (ie, combination therapy). One argument for adding T3 has been the relatively lower levels of T3 that have been observed in some LT4-treated patients (14), which may be a marker for incomplete restoration of normal thyroid hormone action throughout the body. Indeed, in the last decade US- and European-based clinical guidelines on hypothyroidism management have included recommendations for the use of combination therapy in select patients with persistent symptoms (4, 15). Survey studies have found physicians to be more open to prescribing T3-based therapies (16, 17). A recent randomized clinical trial revealed that T3-containing therapies [either combination therapy or desiccated thyroid extract (DTE)] minimized persistent thyroid-related symptoms (eg, memory loss, word-finding difficulty, fatigue, palpitations, etc.), without significant adverse reactions, at doses that normalized serum TSH (18).
Since the apparent shift in attitude toward treatment with T3, real-world trends in LT4 monotherapy and combination therapy prescribing for primary and subclinical hypothyroidism in the United States have not been described. Furthermore, despite not being recommended by the clinical guidelines (4), anecdotal observations suggest that many patients receive T4 + T3 therapy as DTE, which contains both T4 and T3 in an approximate 4:1 ratio. Although this ratio is higher than the 13:1 ratio observed in the human thyroid (19, 20), its effectiveness in normalizing serum TSH and maintaining normal T3 levels is explained by the relatively short T3 half-life in a single daily administration compared to the human thyroid secreting T3 continuously over 24 hours. Given the higher T3 content in DTE tablets, adverse reactions such as palpitations, fine tremor, and weight loss can be observed. However, these are usually associated with overtreatment as documented by suppressed serum TSH levels (21). In a recent phase 2 randomized clinical trial evaluating the safety and efficacy of DTE compared with LT4, ≥94% achieved a normal TSH after the titration period in both arms, and there were no significant differences in adverse reactions (22).
Here we sought to examine the volume of new prescriptions of LT4, LT3, and DTE for patients diagnosed with primary and subclinical hypothyroidism to allow us to identify differences in treatment practices throughout the United States and examine potential differentiating factors between those treated with LT4 and other TH therapies. From survey studies, we hypothesize that both healthcare system and individual (physician and patient) factors have an impact on TH treatment decisions that result in significant differences in the number and type of TH therapies prescribed (23, 24). We focused on sociodemographic, healthcare access, and dietary factors. We were particularly interested in dietary supplement use given patient interest in thyroid-specific supplements (25) and patient interest in dietary modification to treat thyroid-related symptoms, including brain fog (26, 27). In order to investigate the impact of these diverse factors on TH treatments, we chose to analyze 2 datasets in parallel: a national patient claims dataset to analyze national prescription trends and sociodemographic and healthcare access factors and the National Health and Nutrition Examination Study (NHANES) dataset to examine dietary behavior factors.
In summary, we have utilized national patient claims and NHANES datasets to examine national trends in new TH prescriptions and the sociodemographic, healthcare access, and dietary differences in TH-treated patients for primary and subclinical hypothyroidism. The primary objectives of this study were to (1) determine the number of new LT4, LT3, and DTE prescriptions on an annual basis over the study period (2010-2020); (2) examine the impact of state residence, urban-rural residence classification, and physician density by state on the likelihood of treatment with LT4, LT3, or DTE; and (3) compare nutrient and dietary supplement intake between LT4 and DTE-treated patients.
Materials and Methods
Analysis of a National Patient Claims Dataset
In the first component of this cross-sectional study, we examined the number of new thyroid hormone prescriptions nationally and by state and compared geographic and clinical characteristics of those patients being prescribed LT4 monotherapy, LT3-containing therapy, and DTE-containing therapy within 12 months of a hypothyroidism diagnosis. We analyzed outpatient and prescription insurance claims from the IBM MarketScan® Commercial Claims and Encounters and Medicare Supplemental and Coordinate of Benefits databases from 2009 to 2020. The databases include millions of patients, with annual enrollment from 25 to 60 million patients per year. The database includes granular geographic data, including state of residence and metropolitan statistical area (MSA). This study was deemed to meet criteria for exemption by the University of Chicago Biological Sciences Division's Institutional Review Board.
Identification of the study population
The prescription database for each year of the study period was examined to identify all patients aged 18 years and older who had received at least 1 new thyroid hormone prescription and had a diagnosis of hypothyroidism (ICD-9 244.x, ICD-10 E03.2-9) or chronic lymphocytic thyroiditis (ICD-9 245.2). It is important to note that due to the categories available in both ICD-9 and 10 coding structures, the study population includes those with primary and subclinical hypothyroidism. All thyroid hormone prescriptions were categorized as LT4, LT3, or DTE. A prescription was considered new if the patient had no prior thyroid hormone prescription and no diagnosis of hypothyroidism in the calendar year prior to the first recorded thyroid hormone prescription. Thus, all patients were required to have at least 24 consecutive months of enrollment in the MarketScan database to be included in the study. Patients were excluded from the study if they had a history of thyroid cancer, congenital hypothyroidism, a prior diagnosis of hyperthyroidism, iodine deficiency, thyroiditis, pregnancy during the study period, or received a prescription for methimazole, propylthiouracil, or cabergoline or bromocriptine (due to the possibility to pituitary dysfunction). This allowed for the selection of a specific patient population requiring treatment for primary or subclinical hypothyroidism because clinicians may consider thyroid hormone therapies differently for other indications, such as for TSH suppression in thyroid cancer, central hypothyroidism, or treatment after thyroid resection or ablation. Because a key part of the analysis required data on state of residence, patients residing in Washington, D.C., and Puerto Rico, or if the state of residence was not specified, were excluded, as well.
Thyroid hormone treatment classes
Because patients may have received more than 1 type of thyroid hormone prescription during the index year (ie, year during which the patient received his or her first thyroid hormone prescription), 4 treatment classes were created to capture the most common forms of thyroid hormone therapy. Treatment classes included (1) LT4 monotherapy (ie, the patient received only LT4 during the index year), (2) LT3 therapy with or without LT4, (3) DTE therapy with or without LT4, or (4) other (ie, LT3 + DTE or LT4 + LT3 + DTE). TH types were identified using the national drug codes associated with each pharmaceutical claims linked to the generic drug name via the Micromedex Red Book (updated yearly). Generic and brand name TH preparations were included. Brand versions of DTE included Armour Thyroid, NP Thyroid, Nature-Throid, and Westhroid.
Demographic and geographic covariates
Age at the time of first thyroid hormone prescription and sex were collected for each patient. Geographic data associated with each TH prescription claim included US region (eg, northeast, north central, south, west), state of residence, and MSA. Physician density was selected as a primary geographic covariate to account for healthcare access. Physician density is a key determinant of health outcomes (28), and, specifically, it offers an indirect measurement of a patient's ability to change physicians. Doctor switching is common among patients dissatisfied with hypothyroidism treatment (9). Access was limited to primary and endocrinology care because those specialties are responsible for >70% of all thyroid hormone therapies prescribed in the United States (29). Physician density was determined by estimating the number of primary care physicians (including internal medicine, family medicine, obstetrics and gynecology, and geriatrics specialties) and endocrinologists per 100 000 population in each state. Estimates of the number of active physicians for the year 2020 was collected from the Kaiser Family Foundation Providers & Service Use database.
To determine the association between TH prescription patterns and population density within states, MSA-level data was divided into county-level data using the National Bureau of Economic Research Census Core-Based Statistical Area to Federal Information Processing Series County Crosswalk according to a previously described method (30). Counties were assigned to 1 of 5 urban-rural classes (large central metro, large fringe metro, small metro/micropolitan, and non-core) using the National Center for Health Statistics Urban-Rural Classification Scheme for Counties. Each MSA was assigned the most common urban-rural classification among the counites within it. If the counties were split evenly between 2 classes, the MSA was assigned the more populous classification. Small metro and micropolitan classes were combined due to the small number of MSAs categorized as micropolitan within the MarketScan dataset.
Clinical covariates
Additional clinical data was collected from each study patient in an effort to account for clinical factors that may affect thyroid hormone metabolism and/or the decision to use non-LT4 therapy. Diagnosis and prescription data were collected on each study participant from the index year. Relevant comorbidities included ICD-9/10 diagnoses consistent with ischemic heart disease, congestive heart failure, atrial fibrillation, stroke, dementia, celiac disease, chronic kidney disease, osteoporosis, and diabetes type 1 and 2. Coprescribed medications were placed into 6 categories: corticosteroids (oral or inhaled), amiodarone, lithium, antiseizure medications, beta blockers, and statins.
Statistical methods
The number of patients with new TH prescriptions and the number of patients in each treatment class were determined for each calendar year of the study. The means and standard deviations were calculated across all years. US maps were constructed to visualize the distribution of LT4, LT3, and DTE therapies across the United States. Each state was assigned a TH prescription percentage category based on the proportion of new LT4 monotherapies (<85%, 85-90%, 90-95%, ≥95%), LT3 therapies (<1%, 1-2%, 2-3%, ≥3%) and the proportion of new DTE therapies (<5%, 5-10%, 10-15%, ≥15%). Multiple analyses were conducted to evaluate the role of primary care and endocrinology physician density on the proportion of LT4, LT3, and DTE therapies prescribed. First, US maps were constructed with the number of primary care physicians (PCPs) and endocrinologists (ENDOs) per 100 000 population per state represented by 4 categories (PCPs: <125, 125-150, 150-175, ≥175; ENDOs: <1.5, 1.5-2.0, 2.0-3.0, ≥3.0). Next, the proportion of LT3 and DTE therapies in each state were plotted by PCP and ENDO density. Linear regression curves were fit to the data and R2 and P-values were calculated for each curve. Finally, the proportion of LT4, LT3, and DTE therapies was modeled using multivariable beta regression to estimate the effect of PCP and ENDO density on the proportion of TH therapies in each state. Covariates included mean age, proportion female, and proportion of the state population residing in a non-core zone. PCP and ENDO density covariates were not modeled together due to collinearity (based on a variance inflation factor >5). To determine the effect of urban-rural classification on the likelihood of an individual receiving LT4, LT3, or DTE therapy, univariable and multivariable logistic regression analyses were used. The multivariable analyses included all other covariates (age, sex, US region of residence, prescription year, coprescribed medications, and comorbidities). Data manipulation and analyses were completed using statistical software SAS (version 9.4) and R (version 4.1.2). R packages used in the analysis included “usmap” for constructing US maps and “betareg” or beta regression modeling.
Nutrient and Dietary Supplement Intake Analysis Using the NHANES Dataset
The NHANES is a nationally representative survey of the US civilian, noninstitutionalized population. Researchers collect various types of health, demographic, and nutritional data in a standardized format in 2-year cycles. Data from the continuous NHANES dataset from 1999 to 2016 was included in this part of the study.
Study population and matching process
We filtered out all participants below 18 years of age. We found all patients taking DTE as a prescription drug and excluded those patients taking all other medications to treat hypothyroidism (in combination with DTE). We also selected all patients taking LT4 monotherapy to serve as the control group. To minimize the effects of confounding sociodemographic covariates, we identified a matched LT4 population in a 1:2 ratio based on age, sex, and ethnicity. To perform the matching, we used the “matchit” package, implementing a nearest neighbor matching algorithm.
Statistical methods
We imported all the available variables related to nutrient and supplement intake and serum/urine micronutrient levels, which were labeled as categorical or continuous. Categorical variables were compared using the Pearson's chi-squared Test or the Fisher's exact test, as appropriate. The cutoff for significance was P = .05. If the test yielded a significant result, a post hoc test of pairwise nominal independence was performed using the rcompanion package. For the continuous variables, we used a one-way type II ANOVA test. If significant, a post hoc Tukey-Kramer test using the “lsmeans’ package was conducted to perform pairwise comparisons. Data manipulation and analyses were completed using statistical software R (version 4.1.2).
Results
Patient Claims Dataset Analysis
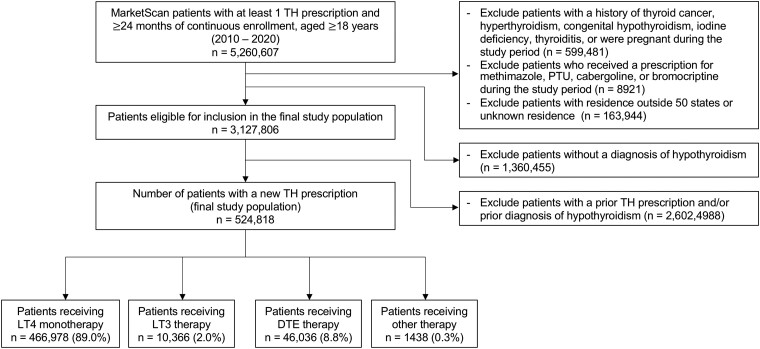
A total of 524 818 adult patients with a diagnosis of hypothyroidism received 537 594 new thyroid hormone prescriptions over the 11-year study period. Of the total population, 89.0% (n = 466 978) received LT4 monotherapy, 2.0% (n = 10 366) received LT3 therapy, and 8.8% (n = 46 036) received DTE therapy (Fig. 1). The mean population age was 50.8 14.9 years, and 72.1% of the total proportion were women. The majority of patients resided in either the north central (24.0%) or south (44.1%) regions of the United States. The number of new TH prescriptions and the proportion of each TH therapy class per study year are shown in Table 1. In 2010, 91.8% of new TH therapies were LT4 monotherapy, 2.4% were LT3 therapy, and 5.4% were DTE therapy. In 2020, 87.2% of new TH therapies were LT4 monotherapy, 2.2% were LT3 therapy, and 10.2% were DTE therapy. On average, the patient populations treated with LT3 and DTE therapies tended to be younger (mean age 47.0 12.8 years and 47.2 11.6 years, respectively, vs 51.3 15.1 years; P-value <.001) and have a higher proportion of women (83.8% and 83.2%, respectively, vs 70.7%; P-value <.001) (Table 2.)
Figure 1.
Patient claims dataset flowchart. Abbreviations: DTE, desiccated thyroid extract; LT3, liothyronine; LT4, levothyroxine; PTU, propylthiouracil; TH, thyroid hormone.
Table 1.
New thyroid hormone prescriptions in the US, 2010-2020
| TH therapy classes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Adults with new TH prescription | LT4 monotherapy | LT3 therapy (no DTE) | DTE therapy (no LT3) | Other | ||||
| n | n | % | n | % | n | % | n | % | |
| 2010 | 56 077 | 51 495 | 91.8 | 1371 | 2.4 | 3049 | 5.4 | 162 | 0.3 |
| 2011 | 69 907 | 63 572 | 90.9 | 1352 | 1.9 | 4841 | 6.9 | 142 | 0.2 |
| 2012 | 77 484 | 70 018 | 90.4 | 1422 | 1.8 | 5861 | 7.6 | 183 | 0.2 |
| 2013 | 61 508 | 55 471 | 90.2 | 1148 | 1.9 | 4742 | 7.7 | 147 | 0.2 |
| 2014 | 62 564 | 55 564 | 88.8 | 1119 | 1.8 | 5709 | 9.1 | 172 | 0.3 |
| 2015 | 49 520 | 43 142 | 87.1 | 905 | 1.8 | 5338 | 10.8 | 135 | 0.3 |
| 2016 | 44 204 | 38 577 | 87.3 | 722 | 1.6 | 4783 | 10.8 | 122 | 0.3 |
| 2017 | 34 889 | 29 866 | 85.6 | 719 | 2.1 | 4178 | 12.0 | 126 | 0.4 |
| 2018 | 25 574 | 21 911 | 85.7 | 609 | 2.4 | 2946 | 11.5 | 108 | 0.4 |
| 2019 | 24 910 | 21 502 | 86.3 | 590 | 2.4 | 2728 | 11.0 | 90 | 0.4 |
| 2020 | 18 181 | 15 860 | 87.2 | 409 | 2.2 | 1861 | 10.2 | 51 | 0.3 |
| Mean (SD) | 47 710.7 | 42 452.5 | 88.3 | 942.4 | 2.0 | 4185.1 | 9.4 | 130.7 | 0.3% |
Only patients with a diagnosis of primary or subclinical hypothyroidism from the patient claims dataset are included.
Abbreviations: DTE, desiccated thyroid extract; LT3, liothyronine; LT4, levothyroxine; TH, thyroid hormone.
Table 2.
Baseline demographic and healthcare access characteristics of patients with at least 1 new TH prescription
| LT4 monotherapy | LT3 therapy | DTE therapy | Other | ||
|---|---|---|---|---|---|
| Characteristics | (n = 466 978) | (n = 10 366) | (n = 46 036) | (n = 1438) | P-value |
| Age [mean (SD)] | 51.29 (15.13) | 46.98 (12.80) | 47.15 (11.63) | 45.32 (11.26) | <.001 |
| Female gender (%) | 330 082 (70.7) | 8691 (83.8) | 38 285 (83.2) | 1274 (88.6) | <.001 |
| US region (%) | <.001 | ||||
| Northeast | 67 364 (14.4) | 1009 (9.7) | 3040 (6.6) | 120 (8.3) | |
| North Central | 115 684 (24.8) | 1992 (19.2) | 7860 (17.1) | 240 (16.7) | |
| South | 201 796 (43.2) | 4460 (43.0) | 24 513 (53.2) | 678 (47.1) | |
| West | 82 129 (17.6) | 2905 (28.0) | 10 623 (23.1) | 400 (27.8) | |
| Urban-rural classification (%) | <.001 | ||||
| Large central metro | 52 685 (11.3) | 1672 (16.1) | 5571 (12.1) | 198 (13.8) | |
| Large fringe metro | 174 025 (37.3) | 3926 (37.9) | 19 127 (41.5) | 637 (44.3) | |
| Medium metro | 102 451 (21.9) | 2225 (21.5) | 9409 (20.4) | 254 (17.7) | |
| Small metro/micropolitan | 41 771 (8.9) | 839 (8.1) | 3930 (8.5) | 119 (8.3) | |
| Non-core | 96 046 (20.6) | 1704 (16.4) | 7999 (17.4) | 230 (16.0) | |
| PCP density (n/100 K) | <.001 | ||||
| <125 | 79 654 (17.1) | 2434 (23.5) | 14 537 (31.6) | 423 (29.4) | |
| 125-149 | 207 080 (44.3) | 4639 (44.8) | 20 425 (44.4) | 625 (43.5) | |
| 150-174 | 49 636 (10.6) | 1303 (12.6) | 3929 (8.5) | 171 (11.9) | |
| ≥175 | 130 604 (28.0) | 1990 (19.2) | 7145 (15.5) | 219 (15.2) | |
| ENDO density (n/100 K) | <.001 | ||||
| <1.5 | 34 249 (7.3) | 1070 (10.3) | 5790 (12.6) | 205 (14.3) | |
| 1.5-1.99 | 137 236 (29.4) | 3323 (32.1) | 18 231 (39.6) | 504 (35.0) | |
| 2.0-2.99 | 194 800 (41.7) | 4004 (38.6) | 16 293 (35.4) | 491 (34.1) | |
| 3.0 | 100 689 (21.6) | 1969 (19.0) | 5722 (12.4) | 238 (16.6) |
Five patients with LT4 monotherapy were classified as unknown for US region. Physician densities by state are presented as number of physicians per 100 000 residents. Of note, this table represents data from the patient claims cohort only.
Abbreviations: DTE, desiccated thyroid extract; ENDO, endocrinologist; LT3, liothyronine; LT4, levothyroxine; PCP, primary care physician; TH, thyroid hormone.
Differences in thyroid hormone prescribing by state
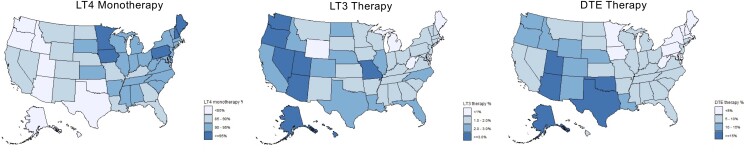
The proportion of each class of TH therapy in each state patient population were mapped (Fig. 2). It was observed that LT4 monotherapy made up a greater proportion of all new TH therapies prescribed in states in the Northeast and upper Midwest regions relative to the rest of the United States. Alternatively, LT3 and DTE therapies were more commonly prescribed in the west and southwest regions.
Figure 2.
Average proportion of each TH therapy class that made up all new TH therapies by state over the study period. Abbreviations: DTE, desiccated thyroid extract; LT3, liothyronine; LT4, levothyroxine; PTU, propylthiouracil; TH, thyroid hormone.
The numbers of each class of TH therapy per 1000 TH therapies per state were plotted against the number of PCPs and ENDOs per 100 000 residents (Supplementary Fig. S1A-F) (31). A trend line was estimated for each plot via linear regression. The number of LT4 monotherapies were positively correlated to the number of PCPs (R2 = 0.24, P > .001) and ENDOs (R2 = 0.19, P = .002). The number of DTE therapies were negatively correlated to the number of PCPs (R2 = 0.27, P < .001) and ENDOs (R2 = 0.20, P = .001). The number of LT3 therapies followed a similar pattern to DTE therapies; however, linear regression analyses were not significant.
Multivariable beta regression analysis examining the proportion of each TH class as a function of PCP and ENDO density by state found similar results (Table 3). When physician density was treated as a categorical variable, PCP density of ≥175/100 000 residents was associated with an increased likelihood of treatment with LT4 monotherapy [odds ratio (OR) 2.51, 95% confidence interval (CI) 1.63-3.86, P < .001). A similar association was seen with ENDO density of ≥3/100 000 residents (OR 2.71, 95% CI 1.84-4.00, P < .001). Alternatively, higher state PCP and ENDO densities were associated with decreased likelihood of treatment with LT3 and DTE therapies (Table 3).
Table 3.
Association between physician density by state and new TH therapy class
| LT4 monotherapy | LT3 therapy | DTE therapy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Physician density (continuous) | Coef | S.E. | P-value | Coef | S.E. | P-value | Coef | S.E. | P-value |
| PCP (n/1 K pop) | 0.954 | 0.208 | <.001 | −0.689 | 0.197 | <.001 | −0.972 | 0.206 | <.001 |
| ENDO (n/100 K pop) | 0.221 | 0.058 | <.001 | −0.145 | 0.053 | .007 | −0.225 | 0.058 | <.001 |
| Physician density (categorical) | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value |
| PCP (n/100 K pop) | |||||||||
| <125 | Ref | — | — | Ref | — | — | Ref | — | — |
| 125-149 | 1.34 | (0.96-1.88) | .089 | 0.83 | (0.59-1.16) | .278 | 0.74 | (0.54-1.03) | .076 |
| 150-174 | 1.84 | (1.26-2.69) | .002 | 0.74 | (0.51-1.07) | .110 | 0.51 | (0.35-0.74) | <.001 |
| ≥175 | 2.51 | (1.63-3.86) | <.001 | 0.49 | (0.32-0.74) | <.001 | 0.40 | (0.26-0.61) | <0.001 |
| ENDO (n/100 K pop) | |||||||||
| <1.5 | Ref | — | — | Ref | — | — | Ref | — | — |
| 1.5-2.0 | 1.73 | (1.25-2.39) | .001 | 0.66 | (0.48-0.92) | .015 | 0.59 | (0.43-0.81) | .001 |
| 2.0-3.0 | 1.74 | (1.25-2.44) | .001 | 0.57 | (0.41-0.80) | .001 | 0.61 | (0.44-0.85) | .004 |
| ≥3.0 | 2.71 | (1.84-4.00) | <.001 | 0.51 | (0.35-0.72) | <.001 | 0.37 | (0.25-0.54) | <.001 |
Adjusted for statewide mean age, proportion female, and proportion non-core (urban-rural classification). Physician densities are based on 2020 active physician census data. Of note, this table represents data from the patient claims cohort only.
Abbreviations: CI, confidence interval; Coef, coefficient; DTE, desiccated thyroid extract; LT3, liothyronine; LT4, levothyroxine; OR, odds ratio; Ref, reference; TH, thyroid hormone.
Differences in thyroid hormone prescribing by urban-rural classification
Each patient was assigned an urban-rural class based on the National Center for Health Statistics Classification Scheme through MarketScan MSA linkage. In the unadjusted analysis, LT3 and DTE therapies were associated with higher population density zones relative to the non-core (ie, rural) classified zone (large central metro: OR 1.75, 95% CI 1.63-1.87, P < .001; OR 1.25 95% CI 1.21-1.30, P < .001, respectively) (Table 4). Conversely, LT4 monotherapy was less likely to be prescribed in metro (ie, urban) zones relative to the non-core zone (large central metro: OR 0.73, 95% CI [0.71-0.76, P < .001). Results were similar after adjusting for covariates (eg, age, sex, US region, prescription year, coprescribed medications, and comorbidities).
Table 4.
Association between patient residence urban-rural classification and new TH therapy class
| LT4 monotherapy | LT3 therapy | DTE therapy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Urban-rural classification | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Unadjusted analysis | |||||||||
| Non-core | Ref | — | — | Ref | — | — | Ref | — | — |
| Small metro/micro | 0.88 | (0.85-0.92) | <.001 | 1.12 | (1.03-1.22) | .007 | 1.13 | (1.08-1.17) | <.001 |
| Medium metro | 0.89 | (0.87-0.92) | <.001 | 1.21 | (1.14-1.29) | <.001 | 1.10 | (1.06-1.13) | <.001 |
| Large fringe metro | 0.76 | (0.74-0.78) | <.001 | 1.24 | (1.17-1.31) | <.001 | 1.31 | (1.28-1.35) | <.001 |
| Large central metro | 0.73 | (0.71-0.76) | <.001 | 1.75 | (1.63-1.87) | <.001 | 1.25 | (1.21-1.30) | <.001 |
| Adjusted analysisa | |||||||||
| Non-core | Ref | — | — | Ref | — | — | Ref | — | — |
| Small metro/micro | 0.90 | (0.87 - 0.94) | <.001 | 1.06 | (0.98-1.16) | .143 | 1.11 | (1.07-1.16) | <.001 |
| Medium metro | 0.95 | (0.92-0.97) | <.001 | 1.13 | (1.06-1.21) | <.001 | 1.04 | (1.01-1.07) | .017 |
| Large fringe metro | 0.77 | (0.75-0.79) | <0001 | 1.21 | (1.15-1.29) | <.001 | 1.28 | (1.25-1.32) | <.001 |
| Large central metro | 0.90 | (0.87-0.93) | <.001 | 1.32 | (1.22-1.42) | <.001 | 1.05 | (1.01-1.10) | .008 |
Urban-rural classification based on the 2013 NCHS Classification Scheme. Of note, this table represents data from the patient claims cohort only.
Abbreviations: CI, confidence interval; DTE, desiccated thyroid extract; LT3, liothyronine; LT4, levothyroxine; micro, micropolitan; NCHS, National Center for Health Statistics; OR, odds ratio; Ref, reference; TH, thyroid hormone.
a Covariates include age, sex, US region, prescription year, coprescribed medications, and comorbidities.
Nutrient and Dietary Supplement Intake in LT4- and DTE-treated NHANES Participants
We identified 73 female participants treated with DTE for whom nutritional data were available. The mean age of the population was 59.4 years, and 70% were non-Hispanic White. A total of 146 LT4-treated female participants for whom nutritional data are available were identified after 2:1 matching to the DTE-treated group. No post-match differences were observed in waist circumference, pulse, systolic and diastolic blood pressures, or serum TSH between the 2 groups (Table 5).
Table 5.
Post-match sociodemographic and clinical characteristics of women treated with LT4 or DTE
| Characteristics | LT4-treated (n = 146) | DTE-treated (n = 73) | P-value |
|---|---|---|---|
| Age (mean, SD) | 59.8 ± 14.9 | 59.4 ± 15.2 | .85 |
| Race/ethnicity (n) | 1.0 | ||
| Non-Hispanic White | 102 | 51 | |
| Mexican American | 14 | 7 | |
| Non-Hispanic Black | 16 | 8 | |
| Other Hispanic | 8 | 4 | |
| Other race | 6 | 3 | |
| Education level (n) | 1.0 | ||
| Did not complete high school | 8 | 4 | |
| High school graduate/equivalent | 34 | 17 | |
| Some college or AA degree | 58 | 29 | |
| College graduate or above | 44 | 22 | |
| Poverty-to-income ratio (mean ± SD) | 3.1 ± 1.7 | 3.2 ± 1.6 | .81 |
| Clinical characteristics (mean ± SD) | |||
| BMI, kg/m2 | 25.0 ± 5.4 | 24.8 ± 6.4 | .84 |
| Waist circumference, cm | 85.3 ± 17.0 | 83.9 ± 17.9 | .60 |
| Pulse | 73 ± 13 | 74 ± 12 | .45 |
| Systolic blood pressure | 128 ± 21 | 125 ± 19 | .32 |
| Diastolic blood pressure | 69 ± 12 | 70 ± 16 | .89 |
| Serum TSH, mIU/L | 2.0 ± 3.3 | 1.6 ± 1.3 | .46 |
NHANES participants treated with LT4 were matched to DTE-treated group on age, sex, and race/ethnicity. No men were included either group.
Abbreviations: AA, associate; BMI, body mass index; DTE, desiccated thyroid extract; LT4, levothyroxine; NHANES, National Health and Nutrition Examination Survey.
Several differences in dietary intake of macronutrients, vitamins, and minerals were noted between the 2 groups (Supplementary Table S1) (31). The overall reported utilization of dietary supplements was high in both groups, with 86% of DTE-treated participants reporting dietary supplement use vs 73% of LT4-treated participants (P = .02) (Table 6). On average, participants on DTE reported taking 4.7 supplements vs 2.1 for the LT4 group (P < .001). The estimated daily intake of thiamin, riboflavin, Vitamin B12, magnesium, and selenium was significantly higher in the DTE-treated group vs the LT4 group. At the same time, the serum levels of Vitamins A, B6, C, and D were significantly higher in the participants taking DTE (Supplementary Table S2) (31). Of note, the mean urinary iodine level was not significantly different between the 2 groups.
Table 6.
Comparison of estimated daily intake of dietary supplements that contain vitamins and minerals
| Supplement intake | LT4-treated (n = 146) | DTE-treated (n = 73) | P-value |
|---|---|---|---|
| Takes at least 1 dietary supp per day (%) | 106 (72.6) | 63 (86.3) | .02 |
| Number of dietary supp taken per day (mean ± SD) | 2.1 ± 2.2 | 4.7 ± 4.4 | <.001 |
| Vitamin/mineral intake (mean ± SD) | |||
| Thiamin (mg) | 5.8 ± 17.0 | 22.2 ± 31.5 | .01 |
| Riboflavin (mg) | 3.7 ± 6.3 | 16.0 ± 21.6 | .002 |
| Niacin, (mg) | 90 ± 330 | 44 ± 54 | .48 |
| Vitamin B6 (mg) | 21 ± 54 | 21 ± 49 | .59 |
| Folic acid (mcg) | 441 ± 324 | 628 ± 664 | .13 |
| Vitamin B12 (mcg) | 200 ± 391 | 863 ± 1618 | .01 |
| Vitamin C (mg) | 272 ± 340 | 409 ± 533 | .18 |
| Vitamin K (mcg) | 38 ± 28 | 58 ± 50 | .07 |
| Vitamin D (mcg) | 39 ± 42 | 58 ± 54 | .06 |
| Calcium (mg) | 612 ± 522 | 571 ± 642 | .74 |
| Phosphorus (mg) | 68 ± 73 | 66 ± 44 | .93 |
| Magnesium (mg) | 86 ± 107 | 190 ± 216 | .01 |
| Iron (mg) | 13 ± 12 | 22 ± 21 | .11 |
| Zinc (mg) | 16 ± 19 | 15 ± 10 | .80 |
| Copper (mg) | 1.4 ± 1.0 | 1.2 ± 0.8 | .59 |
| Sodium (mg) | 12 ± 12 | 13 ± 12 | .85 |
| Potassium (mg) | 65 ± 28 | 94 ± 90 | .16 |
| Selenium (mcg) | 51 ± 46 | 93 ± 97 | .03 |
This table includes participants from the NHANES cohort only. Bold type in vitamin/mineral intake indicates significance of p-value <0.05.
Abbreviations: DTE, desiccated thyroid extract; LT4, levothyroxine; NHANES, National Health and Nutrition Examination Survey; supp, supplement.
Discussion
We identified 2 nationwide trends in new TH prescriptions for hypothyroidism in the United States from 2010 to 2020: (1) the proportion of DTE therapies nearly doubled over the 11-year study period, on average accounting for 9.4% of all new TH therapies, and (2) there was significant region-to-region and state-to-state variation in TH prescribing, with DTE and LT3 therapies making up a greater proportion of all TH therapies prescribed in the west and southwest US regions compared to the north and upper midwest region. State-by-state analyses found that higher densities of PCPs and ENDOs were associated with a greater proportion of prescribed LT4 therapies. Separately, residence in more urban zones, regardless of state, was associated with a greater likelihood of treatment with LT3 and DTE therapies relative to the most rural zone. In the dietary behavior analysis, DTE-treated participants took more supplements and had higher daily intake of several micronutrients. Overall, these results illustrate a complex process in which TH treatment decisions exist as a function of where patients live and individual physician and patient preferences that exist in the context of other health-related behaviors.
The increase in DTE prescriptions during the 2010 to 2020 study period coincides with recent physician surveys suggesting increased openness to prescribing T3-based therapies for select hypothyroid patients (16, 17). A standardized TH prescribing survey issued across several European countries found similar trends in some, but not all, countries (32-36). This shift in practice occurred at the same time that the European Thyroid Association published (in 2012) detailed guidelines on how to start symptomatic LT4-treated patients on combination therapy (15). Two years later, the American Thyroid Association agreed that combination therapy might be used on a trial basis to resolve persistent symptoms in LT4-treated patients, as long as it does not constitute a routine for all patients with hypothyroidism (4). However, it is important to note that combination therapy in this context refers to the addition of LT3 to LT4 therapy, not replacement of LT4 with DTE.
Currently, there is a consensus amongst experts that clinical trials examining the effectiveness of T3-inclusive therapies in patients with persistent symptoms on LT4 are needed (13). One possible explanation for the increase in DTE prescriptions is that patients are requesting it more frequently. Increasingly, patients are seeking out information on thyroid disease from sources other than their physicians (37). This may increase interest in T3-based therapies, especially in patients dissatisfied with their current treatment. Patient preference has been shown to have a strong influence on TH decision-making among physicians (16, 24). Along these lines, we found that female NHANES participants treated with DTE took more dietary supplements when compared to women treated with LT4, adding to evidence of increased awareness with health and quality of life in the patient population with hypothyroidism (38, 39). A previous NHANES study identified that many patients take supplements to “improve or maintain overall health,” despite most supplements not being recommended by physicians (26). Increased supplement use in the DTE-treated population may reflect a more health-conscious lifestyle or a preference for a more nutrient-centric approach to health management.
The patient claims analysis focused on 2 potential geographic determinants of TH class prescription patterns: (1) state-wide PCP and ENDO density and (2) urban-rural classification of patient residence. Patients living in states with higher densities of physicians (both PCPs and ENDOs), which were concentrated in the northeast and north central regions of the United States, were more likely to receive LT4 monotherapy. The reasons underlying the relationship between physician density and TH prescribing are likely multifactorial but probably relate to both physician and patient characteristics (16, 23, 24). At the same time, patients living in more urban zones were more likely to be treated with LT3 or DTE therapy relative to patients living in rural zones. Patients who live in urban zones and are dissatisfied with LT4 treatment (and therefore may seek alternate therapies) may have access to a larger local physician supply and are able to find providers who are willing to treat with LT3 or DTE. Ultimately, these sociodemographic, healthcare access, and dietary differences in patients taking different TH classes highlight the need for a more comprehensive understanding of how the features of our healthcare system and patient-physician level decisions interact to influence large-scale trends in hypothyroidism treatment.
Several important limitations of this study should be considered. First, the study population from the patient claims dataset was made up of a higher proportion of commercially insured patients under the age of 65 years than would be representative of the true US population with treated hypothyroidism. Given that the populations treated with T3-containing therapies tended to be younger, our findings may overestimate the proportion of new TH therapies containing T3 prescribed in the United States over the last decade. Also, due to the study exclusion criteria, the national trends presented in this study are limited to the patient population with primary or subclinical hypothyroidism. Treatment trends for other indications, such as thyroid cancer or congenital hypothyroidism, should not be inferred from these data. Second, the cross-sectional study design only provides a determination of whether or not an association exists between TH prescribing patterns and geographic characteristics. We cannot assume a cause-effect relationship or determine the true mechanism for why 1 class of TH is more likely to be prescribed in 1 location vs another. However, we have attempted to account for sociodemographic and clinical factors in multivariable analyses to manage the effects of confounding on our estimation of the effect size of physician density and urban-rural classification. Third, due to the limited granularity of the geographic data available in the pharmaceutical claims from all study years (eg, lack of ZIP codes), we were only able to estimate the urban-rural classification of individual patients based on MSA designation using the National Bureau of Economic Research Census Core-Based Statistical Area to Federal Information Processing Series County Crosswalk. This provided an estimate of urban-rural classification based on counties within MSAs, but some patients may be misclassified using this approach, especially in MSAs with a diverse urban-rural makeup. The estimated physician density by state was based on census data on active physicians in 2020, the last year of the study period. However, primary care and medical specialty physician density appeared to have remained relatively stable based on county-wide data over the study period (40). Finally, due to the relatively small number of NHANES participants taking DTE, weighting was not used in the analysis; thus, our findings are not representative of the US female population treated with LT4 and DTE at large.
To conclude, we found that the number of DTE prescriptions doubled in the United States over the study period. The proportions of LT4, LT3, and DTE of all TH therapies varied significantly across the United States, but there appears to be a general trend toward more LT3 and DTE prescriptions in the western US. The prescription claims component of this study demonstrated that higher physician density was positively correlated with the proportion of LT4 monotherapy, while residence in more urban zones was associated with a higher proportion of LT3 and DTE therapies. While the causes underlying the observed geographic variation in TH prescribing trends are likely multifactorial, it is important to recognize the use of DTE to treat hypothyroidism is increasing. In the NHANES component, dietary supplement use is high among TH-treated women, and DTE-treated women, in particular. Continued education on the safe and appropriate use of these treatments is crucial as more patients receive long-term DTE treatment.
Contributor Information
Matthew D Ettleson, Department of Medicine, Section of Endocrinology, Diabetes, and Metabolism, University of Chicago, Chicago, IL 60637, USA.
Sabrina Ibarra, Department of Medicine, Section of Endocrinology, Diabetes, and Metabolism, University of Chicago, Chicago, IL 60637, USA.
Wen Wan, Department of Medicine, Section of General Internal Medicine, University of Chicago, Chicago, IL 60637, USA.
Sarah Peterson, Department of Clinical Nutrition, Rush University Medical Center, Chicago, IL 60612, USA.
Neda Laiteerapong, Department of Medicine, Section of General Internal Medicine, University of Chicago, Chicago, IL 60637, USA.
Antonio C Bianco, Department of Medicine, Section of Endocrinology, Diabetes, and Metabolism, University of Chicago, Chicago, IL 60637, USA.
Funding
The study was supported by the National Institute of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under award 5T32DK007011-46 (M.D.E.).
Disclosures
M.D.E., S.I, W.W., and N.L. have nothing to disclose. A.C.B. reports consulting fees from AbbVie, Avionrx, Sention Therapeutics, Synthonics, and Thyron. These are not relevant to the content of this manuscript.
Data Availability
All data presented in this manuscript is contained within figures and tables presented in the manuscript.
References
- 1. Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17(12):1211‐1223. doi: 10.1089/thy.2006.0235 [DOI] [PubMed] [Google Scholar]
- 2. Hollowell JG, Staehling NW, Flanders WD, et al. T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489‐499. doi: 10.1210/jcem.87.2.8182 [DOI] [PubMed] [Google Scholar]
- 3. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado Thyroid Disease Prevalence Study. Arch Intern Med. 2000;160(4):526‐534. doi: 10.1001/archinte.160.4.526 [DOI] [PubMed] [Google Scholar]
- 4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24(12):1670‐1751. doi: 10.1089/thy.2014.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22(12):1200‐1235. doi: 10.1089/thy.2012.0205 [DOI] [PubMed] [Google Scholar]
- 6. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84(6):799‐808. doi: 10.1111/cen.12824 [DOI] [PubMed] [Google Scholar]
- 7. Johansen ME, Marcinek JP, Doo Young Yun J. Thyroid hormone use in the United States, 1997-2016. J Am Board Fam Med. 2020;33(2):284‐288. doi: 10.3122/jabfm.2020.02.190159 [DOI] [PubMed] [Google Scholar]
- 8. Rodriguez-Gutierrez R, Maraka S, Ospina NS, Montori VM, Brito JP. Levothyroxine overuse: time for an about face? Lancet Diabetes Endocrinol. 2017;5(4):246‐248. doi: 10.1016/S2213-8587(16)30276-5 [DOI] [PubMed] [Google Scholar]
- 9. Peterson SJ, Cappola AR, Castro MR, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid. 2018;28(6):707‐721. doi: 10.1089/thy.2017.0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):377‐383. doi: 10.1097/MED.0000000000000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McAninch EA, Rajan KB, Miller CH, Bianco AC. Systemic thyroid hormone status during levothyroxine therapy in hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(12):4533‐4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samuels M, Kolobova I, Smeraglio A, Peters D, Purnell J, Schuff K. Effects of levothyroxine replacement or suppressive therapy on energy expenditure and body composition. Thyroid. 2016;26(3):347‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31(2):156‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ito M, Miyauchi A, Morita S, et al. TSH-suppressive doses of levothyroxine are required to achieve preoperative native serum triiodothyronine levels in patients who have undergone total thyroidectomy. Eur J Endocrinol. 2012;167(3):373‐378. [DOI] [PubMed] [Google Scholar]
- 15. Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J. 2012;1(2):55‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jonklaas J, Tefera E, Shara N. Physician choice of hypothyroidism therapy: influence of patient characteristics. Thyroid. 2018;28(11):1416‐1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jonklaas J, Tefera E, Shara N. Short-term time trends in prescribing therapy for hypothyroidism: results of a survey of American Thyroid Association members. Front Endocrinol (Lausanne). 2019;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine + liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021;106(11):e4400‐e4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dayan C, Panicker V. Management of hypothyroidism with combination thyroxine (T4) and triiodothyronine (T3) hormone replacement in clinical practice: a review of suggested guidance. Thyroid Res. 2018;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mortoglou A, Candiloros H. The serum triiodothyronine to thyroxine (T3/T4) ratio in various thyroid disorders and after levothyroxine replacement therapy. Hormones (Athens). 2004;3(2):120‐126. [DOI] [PubMed] [Google Scholar]
- 21. Idrees T, Palmer S, Maciel RMB, Bianco AC. Liothyronine and desiccated thyroid extract in the treatment of hypothyroidism. Thyroid. 2020;30(10):1399‐1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meeting program and abstracts . Thyroid. 2022;32(Suppl 1): P-1-A-135. [Google Scholar]
- 23. Jonklaas J, Tefera E, Shara N. Prescribing therapy for hypothyroidism: influence of physician characteristics. Thyroid. 2019;29(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Esfandiari NH, Reyes-Gastelum D, Hawley ST, Haymart MR, Papaleontiou M. Patient requests for tests and treatments impact physician management of hypothyroidism. Thyroid. 2019;29(11):1536‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang GY, Parks JR, Fileta B, et al. Thyroxine and triiodothyronine content in commercially available thyroid health supplements. Thyroid. 2013;23(10):1233‐1237. [DOI] [PubMed] [Google Scholar]
- 26. Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173(5):355‐361. [DOI] [PubMed] [Google Scholar]
- 27. Ettleson MD, Raine A, Batistuzzo A, et al. Brain fog in hypothyroidism: understanding the patient's perspective. Endocr Pract. 2022;28(3):257‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Basu S, Berkowitz S, Phillips R, Bitton A, Landon B, Phillips R. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179(4):506‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brito JP, Ross JS, El Kawkgi OM, et al. Levothyroxine use in the United States, 2008-2018. JAMA Intern Med. 2021;181(10):1402‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park JY, Veenstra DL, Wallick CJ, Marcum ZA. Prescribing Alzheimer's disease treatments by provider type and geographic region: a comparison among physicians, nurse practitioners, and physician assistants. BMC Geriatr. 2022;22(1):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ettleson M, Ibarra S, Wan W. Supplemental material for: Demographic, healthcare access, and dietary factors associated with thyroid hormone treatments for hypothyroidism. Zenodo; 2023. [DOI] [PMC free article] [PubMed]
- 32. Burlacu MC, Attanasio R, Hegedüs L, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a THESIS* survey of Belgian specialists *THESIS: treatment of hypothyroidism in Europe by specialists: an international survey. Thyroid Res. 2022;15(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Galofré JC, Attanasio R, Hegedüs L, et al. Use of thyroid hormone in hypothyroid patients and euthyroid subjects in Spain: a THESIS* questionnaire survey. Endocrinol Diabetes Nutr (Engl Ed). 2022;69(7):520‐529. [DOI] [PubMed] [Google Scholar]
- 34. Jiskra J, Paleček J, Attanasio R, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a 2020 THESIS questionnaire survey of members of the Czech Society of Endocrinology. BMC Endocr Disord. 2022;22(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mustafa M, Ali E, McGowan A, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a THESIS questionnaire survey of members of the Irish Endocrine Society. Ir J Med Sci. Published online December 2022. doi: 10.1007/s11845-022-03235-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Younes YR, Perros P, Hegedüs L, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a THESIS questionnaire survey of UK endocrinologists. Clin Endocrinol (Oxf). 2023;98(2):238‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mitchell AL, Hegedüs L, Žarković M, Hickey JL, Perros P. Patient satisfaction and quality of life in hypothyroidism: an online survey by the British Thyroid Foundation. Clin Endocrinol (Oxf). 2021;94(3):513‐520. [DOI] [PubMed] [Google Scholar]
- 38. Woźniak D, Drzymała S, Przysławski J, Drzymała-Czyż S. Dietary supplements in hypothyroidism. Acta Sci Pol Technol Aliment. 2021;20(4):375‐381. [DOI] [PubMed] [Google Scholar]
- 39. McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D. 2016;16(1):53‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Machado S, Jayawardana S, Mossialos E, Vaduganathan M. Physician density by specialty type in urban and rural counties in the US, 2010 to 2017. JAMA Network Open. 2021;4(1):e2033994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in this manuscript is contained within figures and tables presented in the manuscript.