Abstract
Context
Inflammation has been associated with atherosclerosis and metabolic disorders in youth. Preventing inflammation through exposure to different accelerometer-based movement behaviors has not been longitudinally examined.
Objective
This work aimed to examine the mediating role of fat mass, lipids, and insulin resistance on the associations of cumulative sedentary time (ST), light physical activity (LPA), and moderate-to-vigorous physical activity (MVPA) with inflammation.
Methods
From the Avon Longitudinal Study of Parents and Children, United Kingdom, 792 children with data on at least 2 time-point measures of accelerometer-based ST, LPA, and MVPA during age 11, 15, and 24 years follow-up clinic visits with complete high-sensitivity C-reactive protein (hsCRP) measures at age 15, 17, and 24 years were studied. Mediating associations were examined using structural equation models. When the magnitude of the association between the exposure and outcome is increased after including a third variable, suppression occurred but mediation if decreased.
Results
Among 792 (58% female; mean [SD] age at baseline, 11.7 [0.2] years), ST increased, LPA decreased, and MVPA had a U-shaped increase while hsCRP increased during 13-year follow-up. Insulin resistance partly suppressed (23.5% suppression) the positive associations of ST with hsCRP among participants who were overweight/obese. Fat mass partly mediated (30% mediation) the negative associations of LPA with hsCRP. Fat mass had a 77% mediation effect on the negative associations of MVPA with hsCRP.
Conclusion
ST worsens inflammation, but increased LPA had a 2-fold inflammatory-lowering effect and was more resistant to the attenuating effect of fat mass compared with MVPA, and hence should be targeted in future interventions.
Keywords: pediatrics, mediation, longitudinal study, movement behavior, adiposity, insulin resistance
Inflammation has been associated with cardiovascular diseases and obesity in adults (1–5). Recent evidence suggests that higher inflammation measured using high-sensitivity C-reactive protein (hsCRP) may causally be associated with subclinical atherosclerosis and arteriosclerosis among adolescents and young adults (6). Moreover, a proinflammatory diet during childhood has been associated with worsening cardiometabolic health in late adolescence and early adulthood (7). Recent physical activity (PA) guidelines have recommended decreasing sedentary time (ST) and increasing moderate-to-vigorous PA (MVPA) in children and adolescents for the prevention of cardiometabolic diseases (8, 9). Since inflammation causally associate with cardiovascular and metabolic disease, it is of public health importance to investigate whether exposure to MVPA reduces inflammation (3, 6, 8). Long-term longitudinal evidence on the associations of accelerometer-measured ST, light PA (LPA), and MVPA with hsCRP in the pediatric population are limited (8, 10–14). The longitudinal mediating roles of body composition, insulin resistance, lipids, and systolic blood pressure in these associations are unknown (10, 11, 15–17).
The present study examined the longitudinal associations of cumulative accelerometer-measured ST, LPA, and MVPA with repeated measures of fasting plasma hsCRP in 11-year-old children followed up for 13 years with the mediating role of cumulative total fat mass, lean mass, insulin resistance, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), triglycerides, and systolic blood pressure using data from the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort, England, United Kingdom.
Materials and Methods
Study Cohort
Data were from the ALSPAC birth cohort, which investigates factors that influence childhood development and growth. Pregnant women resident in Avon, United Kingdom, with expected dates of delivery between April 1, 1991 and December 31, 1992, were invited to take part in the study. A total of 20 248 pregnancies have been identified as being eligible and the initial number of pregnancies enrolled was 14 541. Of the initial pregnancies, there was a total of 14 676 fetuses, resulting in 14 062 live births and 13 988 children who were alive at age 1 year. When the oldest children were approximately age 7 years, an attempt was made to bolster the initial sample with eligible cases who had failed to join the study originally. As a result, when considering variables collected from the age of 7 onward (and potentially abstracted from obstetric notes) there are data available for more than the 14 541 pregnancies mentioned earlier. The number of new pregnancies not in the initial sample (known as phase 1 enrollment) that are currently represented in the released data and reflecting enrollment status at age 24 is 906, resulting in an additional 913 children being enrolled (456, 262, and 195 recruited during phases 2, 3, and 4, respectively). The total sample size for analyses using any data collected after age 7 is therefore 15 447 pregnancies, resulting in 15 658 fetuses. Of these, 14 901 children were alive at age 1 year. Regular clinic visits of the children commenced at age 7 years and are still ongoing into adulthood. Study data at age 24 years were collected and managed using REDCap electronic data capture tools (18). In this study, of 2040 participants with at least 2 time-point valid ST, LPA, and MVPA measurements at age 11, 15, or 24 years clinic visit, only 792 participants with complete fasting plasma samples at age 15, 17, and 24 years clinic visits were eligible for analyses (Supplementary Fig. S1) (19). The excluded participants who had one or no time-point measure of ST and PA during the 13-year-long follow-up study had similar baseline characteristics to those included in the study (Supplementary Table S1) (19). Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time (20–22). Consent for biological samples has been collected in accordance with the Human Tissue Act (2004). The study website contains details of all the data that are available through a fully searchable data dictionary and variable search tool (http://www.bristol.ac.uk/alspac/researchers/our-data/).
Sedentary Time and Physical Activity Assessment
ST, LPA, and MVPA were assessed with an ActiGraph accelerometer worn for 7 consecutive days at 11- and 15-year clinic visits (23), whereas at age 24 years movement behavior was assessed using an ActiGraph GT3X+ accelerometer device worn for 4 consecutive days. A valid day was defined as providing data for at least 10 hours per day (excluding sequences of ≥10 minutes with consecutive zero counts) and children were included in the analyses only if they provided at least 3 valid days of recording. The devices capture movement in terms of acceleration as a combined function of frequency and intensity. Data are recorded as counts that result from summing postfiltered accelerometer values (raw data at 30 Hz) into 60-second epoch units. Data were processed using Kinesoft software, version 3.3.75 (Kinesoft), according to established protocol (24). Activity counts per minute threshold validated in young people were used to calculate the amount of time spent; MVPA, more than 2296 counts per minute (cpm); for LPA, 100 to 2296 cpm; and for ST, 0 to less than 100 cpm at ages 11 and 15 years, but 2020 cpm for the 24-year MVPA assessment (24–26). The Evenson cut point used in stratifying activity threshold has shown the best overall performance across all intensity levels and was suggested as the most appropriate cut point for youth (27, 28).
Inflammation
There were no measures of fasting plasma hsCRP at age 11 years. Plasma hsCRP was assessed at age 15-, 17-, and 24-year clinic visits and a detailed assessment has been reported earlier (coefficient of variation <5%) (6, 29, 30). Using standard protocols, fasting plasma samples at ages 15, 17, and 24 years were collected, spun, and frozen at −80 °C. An automated particle-enhanced immunoturbidimetric assay (Roche UK) was used in analyzing hsCRP.
Anthropometry, Body Composition, Cardiometabolic, and Lifestyle Factors
Anthropometry (height and weight) at ages 11, 15, and 24 years were assessed in line with standard protocols, and body mass index was computed as weight in kilograms per height in meters squared (26, 29). Body composition (total fat mass and lean mass) was assessed using a dual-energy x-ray absorptiometry scanner at 11-, 15-, and 24-year clinic visits as previously described (26, 29, 30).
Heart rate and systolic and diastolic blood pressure were measured with an Omron monitor at ages 11, 15, and 24 years as previously detailed (26, 29). Fasting insulin was assessed using an ultrasensitive automated microparticle enzyme immunoassay (Mercodia), which does not cross-react with proinsulin, and the sensitivity of the immunoassay was 0.07 mU/L (31). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated from (fasting plasma insulin × fasting plasma glucose/22.5) (32). Fasting plasma glucose, HDL-c, LDL-c, triglycerides, and total cholesterol were assessed at age 15-, 17-, and 24-year clinic visits (26, 29, 30).
At the 17-year clinic visit, participants were briefly asked about their personal and family (mother, father, and siblings) medical history such as a history of hypertension, diabetes, high cholesterol, and vascular disease. All participants had attained puberty at the 17-year clinic visit using a time (years) to age at peak height velocity objective assessment derived from Superimposition by Translation And Rotation mixed-effects growth curve analysis (25, 26, 33). Each participant's mother's socioeconomic status was grouped according to the 1991 British Office of Population and Census Statistics classification (34). Questionnaires to assess smoking behavior were administered at the 13-, 15-, and 24-year clinic visits. A specific question regarding whether participants smoked in the last 30 days was used as an indicator of current smoking status.
Statistical Analysis
Cohort descriptive characteristics were summarized as means and SD, medians, and interquartile ranges, or frequencies and percentages. Sex differences were explored using independent t tests, Mann Whitney-U tests, or chi-square tests for normally distributed, skewed, or dichotomous variables, respectively. Multicategory variables were analyzed using a one-way analysis of variance. Normality was assessed by histogram curve, quantile-quantile plot, and Kolmogorov-Smirnov tests. Logarithmic transformation of skewed variables was conducted and normality was confirmed prior to further analysis.
Mediation path analyses
Using structural equation models, we separately examined the mediating role of cumulative fat mass, lean mass, insulin resistance, HDL-c, LDL-c, triglycerides, and systolic blood pressure on the longitudinal associations of cumulative ST, LPA, or MVPA with cumulative hsCRP. Analyses were adjusted for age, sex, insulin resistance, family history of hypertension and cardiovascular diseases, smoking status, socioeconomic status, heart rate, total fat mass, lean mass, HDL-c, LDL-c, triglycerides, ST, LPA, and MVPA, depending on the mediator or predictor. The path models had 3 equations per regression analysis: the longitudinal associations of cumulative ST, LPA, or MVPA with cumulative fat mass, lean mass, insulin resistance, HDL-c, LDL-c, triglycerides, or systolic blood pressure (equation 1); the longitudinal associations of cumulative fat mass, lean mass, insulin resistance, HDL-c, LDL-c, triglycerides, or systolic blood pressure with cumulative hsCRP (equation 2); and the longitudinal associations of cumulative ST, LPA, and MVPA with cumulative hsCRP (equation 3, total effect), and equation 3' (direct effect) accounted for the mediating role of fat mass, lean mass, insulin resistance, HDL-c, LDL-c, triglycerides, or systolic blood pressure on the longitudinal associations of cumulative ST, LPA, and MVPA with cumulative hsCRP. The proportion of mediating or suppressing roles was estimated as the ratio of the difference between equation 3 and equation 3' or the multiplication of equations 1 and 2 divided by equation 3 and expressed in percentage. A mediating or indirect role is confirmed when there are statistically significant associations between (a) the predictor and mediator, (b) the predictor and outcome, (c) the mediator and outcome, and when (d) the longitudinal associations between the predictor and outcome variable were attenuated on inclusion of the mediator (35). However, when the magnitude of the longitudinal association between the predictor and outcome is increased on inclusion of a third variable, a suppression is confirmed (35). Path analyses were conducted with 1000 bootstrapped samples. Mediation analyses were repeated based on participant's body mass index obesity category and presented in the supplemental appendix (19). Covariates were selected based on previous studies (6, 8, 10, 11, 17, 26, 36, 37). Analyses involving 800 ALSPAC children at 0.8 statistical power, 0.05 α, and 2-sided P value would show a minimum detectable effect size of 0.09 SDs if they had relevant exposure for a normally distributed quantitative variable (38). All statistical analyses were performed using SPSS statistics software, version 27.0 (IBM Corp), and mediation analyses structural equation modeling was conducted using IBM AMOS version 27.0.
Results
Altogether, 792 participants who had at least 2 time-point measures of ST, LPA, and MVPA during age 11-, 15-, and 24-year clinic visits with complete fasting hsCRP at ages 15, 17, and 24 years were included (Supplementary Fig. S1) (19). ST increased, LPA decreased, and MVPA had a U-shaped from ages 11 through 24 years both in males and females (Table 1 and Fig. 1). From ages 15 to 24 years, hsCRP steadily increased both in males and females (see Table 1 and Fig. 1). Other characteristics are described in Table 1.
Table 1.
Descriptive characteristics of cohort participants
| Age at clinic visits/follow-up, y | 11 | 15 | 24 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Male (n = 337) | Female (n = 455) | P | Male (n = 337) | Female (n = 455) | P | Male (n = 337) | Female (n = 455) | P |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Anthropometry | |||||||||
| Age at clinic visit, y | 11.70 (0.20) | 11.71 (0.21) | .738 | 15.38 (0.22) | 15.39 (0.24) | .634 | 24.57 (0.79) | 24.45 (0.77) | .033 |
| Height, m | 1.51 (0.07) | 1.52 (0.07) | .139 | 1.75 (0.07) | 1.65 (0.06) | <.0001 | 1.80 (0.07) | 1.67 (0.06) | <.0001 |
| Weighta, kg | 41.20 (11.9) | 42.0 (12.0) | .208 | 63.0 (13.1) | 56.80 (10.9) | <.0001 | 79.30 (17.32) | 64.15 (16.55) | <.0001 |
| Ethnicity, White (n, %) | 303 (96.2) | 410 (97.9) | .188 | NA | NA | ||||
| Body composition | |||||||||
| Total fat massa, kg | 8.41 (8.40) | 10.76 (7.52) | <.0001 | 8.59 (7.51) | 16.80 (8.79) | <.0001 | 18.53 (11.60) | 21.16 (11.12) | <.0001 |
| Lean massa, kg | 29.86 (5.29) | 28.69 (6.39) | <.0001 | 50.0 (8.59) | 36.89 (4.81) | <.0001 | 56.90 (10.99) | 40.93 (6.34) | <.0001 |
| Body mass indexa | 18.07 (4.27) | 18.15 (3.95) | .579 | 20.31 (3.73) | 20.83 (4.08) | .009 | 24.38 (5.10) | 23.09 (5.57) | <.0001 |
| Vascular measures | |||||||||
| Heart rate, beat/min | 74 (11) | 78 (11) | <.0001 | 72 (12) | 77 (11) | <.0001 | 65 (11) | 68 (9) | <.0001 |
| Systolic blood pressure, mm Hg | 105 (9) | 105 (10) | .891 | 127 (10) | 120 (10) | <.0001 | 123 (10) | 111 (9) | <.0001 |
| Diastolic blood pressure, mm Hg | 58 (6) | 58 (6) | .919 | 68 (8) | 66 (9) | .012 | 67 (8) | 66 (8) | .009 |
| Lifestyle factors | |||||||||
| Smoked in the last 30 d (n, %) | <5 (0.3) | 8 (1.8) | .086 | 24 (7.3) | 55 (12.2) | .030 | 82 (24.6) | 105 (23.2) | .672 |
| Family history of H-D-C-V (n, %) | 94 (31.6) | 118 (28.1) | .319 | NA | NA | ||||
| Sedentary time, min/d | 364 (66) | 366 (70) | .807 | 464 (85) | 486 (78) | .001 | 524 (75) | 531 (92) | .615 |
| Light physical activity, min/d | 364 (55) | 368 (56) | .317 | 290 (65) | 271 (64) | <.0001 | 143 (46) | 152 (54) | .246 |
| MVPA, min/d | 66 (41) | 47 (20) | <.0001 | 56 (31) | 39 (21) | <.0001 | 62 (39) | 46 (26) | .001 |
| Maternal social economic status (n, %) | .230 | NA | NA | ||||||
| Professional | 17 (9.9) | 13 (6.0) | |||||||
| Managerial and technical | 74 (43) | 83 (38.4) | |||||||
| Skilled nonmanual | 49 (28.5) | 76 (35.2) | |||||||
| Skilled manual | <8 (1.2) | <8 (2.3) | |||||||
| Partly skilled | 25 (14.5) | 31 (14.4) | |||||||
| Unskilled | <8 (2.9) | 8 (3.7) | |||||||
| Fasting plasma metabolic indices | 15 y | 17 y | 24 y | ||||||
| Total cholesterol, mmol/L | 3.57 (0.60) | 3.90 (0.62) | <.0001 | 3.58 (0.60) | 3.91 (0.69) | <.0001 | 4.36 (0.82) | 4.49 (0.83) | .028 |
| High-density lipoprotein, mmol/L | 1.21 (0.27) | 1.35 (0.28) | <.0001 | 1.18 (0.27) | 1.33 (0.32) | <.0001 | 1.39 (0.35) | 1.65 (0.41) | <.0001 |
| Low-density lipoprotein, mmol/L | 2.00 (0.54) | 1.65 (0.41) | <.0001 | 2.02 (0.56) | 2.16 (0.54) | <.0001 | 2.49 (0.76) | 2.43 (0.75) | .310 |
| Triglyceridesa, mmol/L | 0.73 (0.34) | 0.75 (0.40) | .106 | 0.76 (0.36) | 0.73 (0.36) | .489 | 0.88 (0.56) | 0.79 (0.42) | <.0001 |
| Glucose, mmol/L | 5.30 (0.36) | 5.14 (0.34) | <.0001 | 5.14 (0.36) | 4.90 (0.33) | <.0001 | 5.49 (1.03) | 5.21 (0.52) | <.0001 |
| Insulina, mU/L | 8.09 (4.78) | 9.74 (5.54) | <.0001 | 5.80 (4.31) | 7.51 (4.14) | <.0001 | 7.38 (5.32) | 7.68 (5.93) | .413 |
| High-sensitivity C-reactive proteina, mg/L | 0.34 (0.60) | 0.34 (0.59) | .240 | 0.45 (0.59) | 0.60 (1.30) | .005 | 0.64 (1.41) | 1.00 (1.95) | <.0001 |
Values are means (SDs) and amedian (interquartile range) except for lifestyle factors and ethnicity. Differences between sexes were tested using t test for normally distributed continuous variables, Mann-Whitney U test for skewed continuous variables, chi-square test for dichotomous variables, and analysis of covariance for the multicategory variable. A 2-sided P value less than .05 is considered statistically significant.
Abbreviations: H-D-C-V, hypertension, diabetes, high cholesterol, and vascular disease; MVPA, moderate-to-vigorous physical activity; NA, not available/applicable; P value for sex differences.
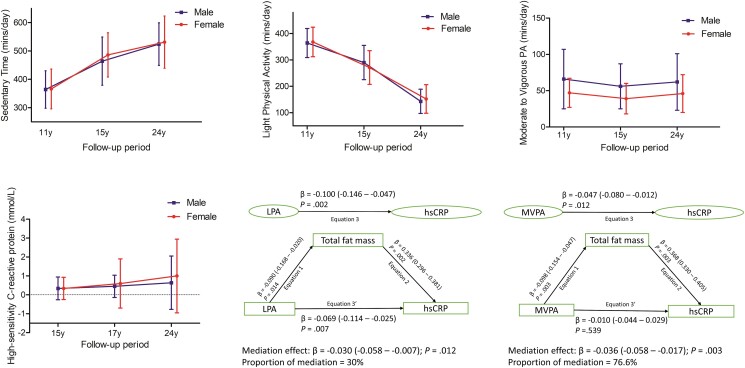
Figure 1.
The average time spent sedentary, in light physical activity, and moderate to vigorous physical activity from ages 11 through 24 years. The median distribution of high-sensitivity C-reactive protein in male and female participants aged 15 through 24 years. The longitudinal mediation analyses of total body fat mass in the relationships of light physical activity or moderate to vigorous physical activity with high-sensitivity C-reactive protein. Mediation structural equation model was adjusted for sex, family history of hypertension/diabetes/high cholesterol/vascular disease, socioeconomic status, and time-varying covariates measured both at baseline and follow-up such as age, heart rate, systolic blood pressure, smoking status, and fat mass, lean mass, insulin resistance, sedentary time, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides in addition to light physical activity or moderate-to-vigorous physical activity depending on the mediator and outcome. β is standardized regression coefficient. P value less than .05 was considered statistically significant.
Mediating or Suppressing Effects of Body Composition, Insulin Resistance, Lipids, and Systolic Blood Pressure in the Associations of Sedentary Time, Light Physical Activity, and Moderate-to-Vigorous Physical Activity With High-Sensitivity C-Reactive Protein
Cumulative ST was positively associated with cumulative hsCRP, but cumulative insulin resistance, LDL-c, and HDL-c partly suppressed the relationship (Table 2). Cumulative increase in systolic blood pressure and lean mass partly mediated the positive associations of ST with hsCRP (see Table 2). Fat mass and triglycerides had no statistically significant mediating or suppressing effect on the associations between ST and hsCRP (see Table 2). Among normal-weight participants, cumulative insulin resistance partly suppressed (2.8% suppression) the positive associations of cumulative ST with cumulative hsCRP (Supplementary Table S2 and Supplementary Fig. S2) (19). Among participants who were overweight/obese, cumulative total body fat mass (28.9 mediation) and lean mass (7% mediation) partly mediated while insulin resistance partly suppressed (23.5% suppression) the positive associations of cumulative ST with cumulative hsCRP (Supplementary Table S2 and Supplementary Fig. S2) (19).
Table 2.
Mediating or suppressing role of cumulative body composition, fasting insulin resistance and lipids, and systolic blood pressure on the longitudinal associations of cumulative sedentary time with cumulative inflammation
| Cumulative sedentary time for ages 11-24 y N = 792 | Total effect | Direct effect | Indirect effect | Mediation or suppression, % | |||
|---|---|---|---|---|---|---|---|
| Mediators | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Total fat mass | 0.068 (0.016 to 0.115) | .014 | 0.063 (0.015 to 0.107) | .009 | 0.005 (−0.014 to 0.028) | .616 | 7.4 |
| Lean mass | 0.173 (0.123 to 0.216) | .003 | 0.164 (0.112 to 0.209) | .003 | 0.009 (0.004 to 0.015) | .002 | 5.2 mediation |
| Insulin resistance | 0.204 (0.159 to 0.242) | .003 | 0.230 (0.187 to 0.268) | .003 | −0.026 (−0.042 to −0.015) | .001 | 12.8 suppression |
| LDL cholesterol | 0.183 (0.138 to 0.222) | .003 | 0.193 (0.150 to 0.232) | .003 | −0.010 (−0.021 to −0.002) | .015 | 5.5 suppression |
| HDL cholesterol | 0.238 (0.194 to 0.278) | .003 | 0.256 (0.209 to 0.294) | .003 | −0.018 (−0.029 to −0.008) | .002 | 7.6 suppression |
| Triglycerides | 0.199 (0.155 to 0.240) | .003 | 0.191 (0.149 to 0.231) | .002 | 0.008 (−0.006 to 0.019) | .244 | 4.0 |
| Systolic blood pressure | 0.198 (0.156 to 0.237) | .002 | 0.183 (0.138 to 0.223) | .003 | 0.015 (0.008 to 0.024) | .001 | 7.6 mediation |
Mediation structural equation model was adjusted for sex, family history of hypertension/diabetes/high cholesterol/vascular disease, socioeconomic status, and time-varying covariates measured both at baseline and follow-up such as age, heart rate, systolic blood pressure, smoking status, and fat mass, lean mass, insulin resistance, light physical activity, and moderate-to-vigorous physical activity, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, or triglycerides depending on the mediator and outcome. β is standardized regression coefficient. P value less than .05 was considered statistically significant and are bolded. When the magnitude of the association between the exposure and outcome is increased on inclusion of a third variable, suppression occurred but mediation if decreased.
Cumulative LPA was negatively associated with cumulative hsCRP with total body fat mass partly mediated the relationship (Table 3 and Fig. 1). Lean mass, insulin resistance, LDL-c, HDL-c, triglycerides, and systolic blood pressure had no statistically significant mediating or suppressing effect on the associations between LPA and hsCRP (see Table 3). Among normal-weight participants, cumulative total body fat mass partly mediated (20.6% mediation) the negative associations of cumulative LPA with cumulative hsCRP but no statistically significant mediating effect was observed in participants who were overweight/obese (Supplementary Table S2) (19).
Table 3.
Mediating or suppressing role of cumulative body composition, fasting insulin resistance and lipids, and systolic blood pressure on the longitudinal associations of cumulative light physical activity with cumulative inflammation
| Cumulative LPA for ages 11-24 y N = 792 | Total effect | Direct effect | Indirect effect | Mediation or suppression, % | |||
|---|---|---|---|---|---|---|---|
| Mediators | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Total fat mass | −0.100 (−0.146 to −0.047) | .002 | −0.069 (−0.114 to −0.025) | .007 | −0.030 (−0.058 to −0.007) | .012 | 30.0 mediation |
| Lean mass | −0.197 (−0.250 to −0.149) | .002 | −0.198 (−0.251 to −0.151) | .001 | 0.001 (−0.002 to 0.006) | .441 | 0.5 |
| Insulin resistance | −0.239 (−0.278 to −0.198) | .002 | −0.244 (−0.282 to −0.205) | .002 | 0.005 (−0.014 to 0.002) | .501 | 2.1 |
| LDL cholesterol | −0.212 (−0.252 to −0.167) | .002 | −0.209 (−0.249 to −0.165) | .002 | −0.003 (−0.013 to 0.010) | .646 | 1.4 |
| HDL cholesterol | −0.287 (−0.329 to −0.244) | .002 | −0.283 (−0.322 to −0.239) | .002 | −0.004 (−0.017 to 0.007) | .452 | 1.4 |
| Triglycerides | −0.207 (−0.247 to −0.164) | .002 | −0.207 (−0.246 to −0.167) | .002 | 0.000 (−0.016 to 0.017) | .949 | 0 |
| Systolic blood pressure | −0.214 (−0.255 to −0.171) | .002 | −0.209 (−0.248 to −0.168) | .002 | −0.005 (−0.015 to 0.003) | .223 | 2.3 |
Mediation structural equation model was adjusted for sex, family history of hypertension/diabetes/high cholesterol/vascular disease, socioeconomic status, and time-varying covariates measured at both baseline and follow-up such as age, heart rate, systolic blood pressure, smoking status, and fat mass, lean mass, insulin resistance, sedentary time, and moderate-to-vigorous physical activity, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, or triglycerides depending on the mediator and outcome. β is standardized regression coefficient. P value less than 05 was considered statistically significant and are bolded. When the magnitude of the association between the exposure and outcome is increased on inclusion of a third variable, suppression occurred but mediation if decreased.
Abbreviation: LPA, light physical activity.
Cumulative MVPA was negatively associated with cumulative hsCRP with total body fat mass significantly mediating the relationship (Table 4 and Fig. 1). Cumulative HDL-c and systolic blood pressure partly mediated the negative associations of MVPA with hsCRP (see Table 4). Lean mass partially suppressed the negative associations of MVPA with hsCRP (see Table 4). Insulin resistance, LDL-c, and triglycerides had no statistically significant mediating or suppressing effect on the associations between MVPA and hsCRP (see Table 4). Among normal-weight participants, cumulative total body fat mass (53.5% mediation) and lean mass (7.0% mediation) partly mediated the negative associations of cumulative MVPA with cumulative hsCRP but no statistically significant mediating effect was observed in participants who were overweight/obese (Supplementary Table S2) (19).
Table 4.
Mediating or suppressing role of cumulative body composition, fasting insulin resistance and lipids, and systolic blood pressure on the longitudinal associations of cumulative moderate to vigorous physical activity with cumulative inflammation
| Cumulative MVPA for ages 11-24 y N = 792 |
Total effect | Direct effect | Indirect effect | Mediation or suppression, % | |||
|---|---|---|---|---|---|---|---|
| Mediators | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Total fat mass | −0.047 (−0.080 to −0.012) | .012 | −0.010 (−0.044 to 0.029) | .539 | −0.036 (−0.058 to −0.017) | .003 | 76.6 mediation |
| Lean mass | −0.112 (−0.145 to −0.079) | .002 | −0.122 (−0.157 to −0.087) | .003 | 0.020 (0.005 to 0.016) | .005 | 17.9 suppression |
| Insulin resistance | −0.098 (−0.130 to −0.065) | .002 | −0.101 (−0.135 to −0.068) | .002 | 0.002 (−0.004 to 0.009) | .447 | 2.1 |
| LDL cholesterol | −0.100 (−0.134 to −0.067) | .002 | −0.094 (−0.128 to −0.060) | .003 | −0.006 (−0.013 to 0.001) | .064 | 6.0 |
| HDL cholesterol | −0.118 (−0.153 to −0.084) | .002 | −0.114 (−0.150 to −0.082) | .002 | −0.003 (−0.008 to −0.001) | .008 | 2.5 mediation |
| Triglycerides | −0.100 (−0.135 to −0.068) | .002 | −0.099 (−0.134 to −0.066) | .002 | −0.001 (−0.009 to 0.007) | .830 | 1.0 |
| Systolic blood pressure | −0.108 (−0.142 to −0.076) | .002 | −0.097 (−0.129 to −0.063) | .002 | −0.012 (−0.018 to 0.007) | .004 | 11.1 mediation |
Mediation structural equation model was adjusted for sex, family history of hypertension/diabetes/high cholesterol/vascular disease, socioeconomic status, and time-varying covariates measured at both baseline and follow-up such as age, heart rate, systolic blood pressure, smoking status, and fat mass, lean mass, insulin resistance, sedentary time, and light physical activity, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, or triglycerides depending on the mediator and outcome. β is standardized regression coefficient. P value less than .05 was considered statistically significant and are bolded. When the magnitude of the association between the exposure and outcome is increased on inclusion of a third variable, suppression occurred but mediation if decreased.
Abbreviation: moderate to vigorous physical activity.
Discussion
This longitudinal study is the first to demonstrate that a cumulative increase in objectively measured ST from childhood through young adulthood was independently associated with cumulative increase in inflammation during mid-adolescence through young adulthood. Both cumulative LPA and MVPA were associated with cumulative decrease in inflammation. Cumulative total fat mass from childhood through young adulthood attenuated the negative associations of LPA and MVPA with inflammation, with a 2-fold decrease in MVPA effect compared to LPA.
Sedentary Time and Inflammation
There is a paucity of longitudinal evidence on the associations of objectively measured ST with inflammation in the pediatric population (10, 11, 13, 14). In the present study, cumulative ST was positively associated with cumulatively increased inflammation even after adjustments for LPA and MVPA. ST has been associated with cardiovascular diseases in youth and adults (13, 39–41). It was postulated that higher ST increases the risk of dyslipidemia, hypertension, obesity, and metabolic disorders, which may in turn lead to a cascade of inflammatory events (1, 13, 41, 42). On the contrary, the present study suggests that increased ST from childhood is independently associated with increased inflammation and that increased LDL-c and insulin resistance may paradoxically suppress the adverse effect of ST on inflammation in youth.
Participants who were normal weight spent on average 7 hours/day sedentary during the 13-year follow-up compared to approximately 8 hours/day of ST among those overweight/obese. The cumulative median value of homeostatic HOMA-IR was 1.67, hsCRP median value was 0.44 mg/L, and the total body fat mass median value was 13 kg among normal-weight participants. However, the cumulative median value of HOMA-IR was 2.67, hsCRP median value was 1.42 mg/L, and the total body fat mass median value was 29.8 kg among participants who were overweight/obese. This showed that participants who are overweight/obese accumulated more ST and doubled the concentration of hsCRP, insulin resistance, and fat mass compared to normal-weight participants.
When insulin resistance was added to the regression of ST on inflammation, the magnitude of the effect increased. Insulin resistance suppressed the associations of ST with inflammation both in normal-weight and overweight/obese participants. The suppressing effect of insulin resistance was 10 times larger in overweight/obese participants when compared to normal-weight participants. This increase may occur because insulin resistance explained variability in inflammation; that is, increased inflammation is enhanced by increased insulin resistance. Importantly, increased insulin resistance in participants who were overweight and obese had a 3-fold effect on increased inflammation when compared with that among normal-weight participants. In a mice model, insulin resistance in adipocytes resulted in the production of the chemokine monocyte chemoattractant protein 1, which recruited monocytes and activated proinflammatory macrophages, creating a phenomenon in which insulin resistance temporally and casually preceded inflammation rather than vice versa (43, 44). Inflammation is increased in patients with type 2 diabetes, while physical inactivity increases fat accumulation, resulting in a vicious cycle of ST, obesity, and inflammation, which could enhance insulin resistance (44, 45).
In this study while fat mass and insulin resistance independently contributed to increased inflammation, an increased ST was paradoxically associated with reduced insulin resistance both in normal-weight and overweight/obese participants. The major consensus seems to be that ST increases inflammation, which in turn increases insulin resistance (45). However, the present study revealed a contrasting result in which insulin resistance independently increased inflammation (43, 44), while increased ST was associated with higher inflammation and reduced insulin resistance. Despite the complexity and unclear pathophysiological mechanisms associating ST with insulin resistance, several pathways have been highlighted such as increased β-cell insufficiency, dyslipidemia, oxidative damage, mitochondrial dysfunction, ceramide synthesis, genetic modulations, and decreased capillarization (45). The partial suppressing effect of LDL-c in the association of ST with inflammation was present only in the total cohort but not among normal-weight and overweight participants. ST leads to lower energy expenditure, dysregulation of lipid homeostasis, and enhanced lipid storage; however, among endurance-trained athletes higher intramuscular lipids may have no adverse effects on insulin sensitivity, but further mechanistic studies are warranted (45–47).
Of note, an increase in total body fat mass from childhood through young adulthood had no statistically significant mediating or suppressing effect on the positive relationship between ST and inflammation in the total cohort and among normal-weight participants. However, among participants who were overweight and or obese, total body fat mass explained nearly 30% of the relationship between ST and inflammation. Previous studies have reported that objectively measured ST had little or no effect on adiposity in children and adolescents (13), and evidence among adults suggests that inflammation is associated with physical inactivity, independent of obesity (14, 48, 49). The direct relationship of ST with inflammation appears to be partly mediated by lean mass and systolic blood pressure. Increased lean mass and systolic blood pressure have been established as drivers of physiologic arterial remodeling during growth from childhood through young adulthood (26). Hence, the partial mediating effect of less than 8% of either lean mass or systolic blood pressure may suggest a nondeleterious contribution to the ST-inflammation pathway in a general population of children, adolescents, and young adults; however further experimental studies are warranted.
Light Physical Activity and Inflammation
The World Health Organization's recent physical activity guideline lacked information on LPA recommendations for children and adolescents (8). Longitudinal evidence on accelerometer-measured LPA in association with inflammation is scarce in the pediatric population (8, 10, 12, 14). In the present study, cumulative LPA was negatively associated with cumulative increase in inflammation and the relationship was partly mediated by total body fat mass. This suggests that among children and adolescents with obesity or high body fat mass, the effect of LPA in decreasing inflammation may decrease by 30%. Neither insulin resistance, lipids, lean mass, nor systolic blood pressure mediated or suppressed this relationship. Suggested mechanisms supporting the effect of PA on inflammation include visceral fat mass reduction, increased production of anti-inflammatory cytokines from contracting skeletal muscle, and reduced expression of toll-like receptors on monocytes and macrophages (50). Taken together, this present finding supports LPA as a pragmatic target for future interventions and public health guidelines in the pediatric population since it is more feasible and accessible, does not require a high level of exercise skill or prior fitness, is incidental to daily living, and requires less motivation (10, 12, 14, 23, 25, 51).
Moderate-to-Vigorous Physical Activity and Inflammation
Evidence on the associations of MVPA with inflammation in adults is contrasting; while some report beneficial effects, others report deleterious or no effects (50, 52–56). Similarly, in the pediatric population experimental and observational studies investigating objectively measured MVPA relations with inflammation are contrasting and largely cross-sectional or small sample sized (10, 12, 14). The observed MVPA effects on decreasing inflammation tend to significantly attenuate after accounting for adiposity (12, 49, 50, 56). In the present study with a long observation period (13 years), cumulative MVPA from childhood through young adulthood was associated with cumulatively decreased inflammation that was significantly mediated by increased total body fat mass. This mediating ability of total body fat mass could diminish the effect of MVPA on lowering inflammation by approximately 80%. This finding suggests that accumulating excess fat before or after exposure to MVPA may increase the risk of inflammation by dampening the MVPA inflammation-lowering effect as confirmed in observational and experimental studies (8, 10, 12, 49, 50, 56). Importantly, MVPA effect on inflammation was twice less than the effect of LPA on inflammation before especially after accounting for the mediating effect of fat mass. This finding fills the knowledge gap on the intensities of exercise required to optimize the anti-inflammatory effects of exercise and clarifies the magnitude of exercise-induced reduction in inflammation that total body fat mass could attenuate (50, 56). It was also observed that cumulative MVPA was positively associated with lean mass, which in turn partly suppressed the decrease in inflammation. Among adults, physical inactivity has been associated with muscle atrophy and a higher inflammatory state (14, 49, 50).
Strength and Limitation
The extensive array of gold-standard and repeated longitudinal measures of movement behaviors, body composition, and covariates throughout the follow-up period in the ALSPAC cohort offered the possibility of estimating longitudinal mediation pathways. The findings fill knowledge gaps that might be useful in updating future PA guidelines (8, 10–12). Some limitations are that the study participants were mostly White, thus generalization of findings to other racial and ethnic groups is limited. Moreover, residual confounding, such as the unavailability of hsCRP assessment at age 11 years, could bias the findings; however, hsCRP at age 17 years was included in the analysis. Hormonal changes during the menstrual cycle phases have been associated with variations in CRP values in postpubertal females (57), but it was not possible to control for this factor because of the nonavailability of specific hormonal concentration. Cohort attrition could lead to bias in observational studies, which may be negligible since participants who lacked certain movement behavior and inflammation variables had similar characteristics to those included in the analyses.
Conclusion
Objectively measured ST from childhood through young adulthood was associated with worsening inflammation. Cumulative LPA was associated with decreased inflammation with total body fat mass mediating the relationship by 30%. Increased total fat mass may attenuate the effect of increased MVPA on decreased inflammation by 80% and hence should be targeted in intervention studies to improve and sustain the inflammatory-lowering effect of MVPA. Promoting LPA and MVPA while decreasing ST may be considered crucial intervention targets to attenuate the risk of increased inflammation and their cardiovascular and metabolic sequelae in the pediatric population.
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Abbreviations
- ALSPAC
Avon Longitudinal Study of Parents and Children
- cpm
counts per minute
- HDL-c
high-density lipoprotein cholesterol
- HOMA-IR
homeostatic model assessment of insulin resistance
- hsCRP
high-sensitivity C-reactive protein
- LDL-c
low-density lipoprotein cholesterol
- LPA
light physical activity
- MVPA
moderate-to-vigorous physical activity
- PA
physical activity
- ST
sedentary time
Funding
The UK Medical Research Council and Wellcome (grant No. 217065/Z/19/Z) and the University of Bristol provide core support for Avon Longitudinal Study and Parents and Children (ALSPAC). The British Heart Foundation (grant No. CS/15/6/31468) funded blood pressure and Actigraph activity monitoring device measurement at age 24 years. The Medical Research Council (grant No. MR/M006727/1) supported smoking data collection. A comprehensive list of grant funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf); A.O.A.'s research group (UndeRstanding FITness and Cardiometabolic Health In Little Darlings: urFIT-child) was funded by the Jenny and Antti Wihuri Foundation (grant No. 00180006); the North Savo regional and central Finnish Cultural Foundation (grant Nos. 65191835, 00200150, and 00230190); the Orion Research Foundation sr; Aarne Koskelo Foundation; Antti and Tyyne Soininen Foundation; Paulo Foundation; Paavo Nurmi Foundation; Yrjö Jahnsson Foundation (grant No. 20217390); and the Finnish Foundation for Cardiovascular Research (grant Nos. 220021 and 230012). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosures
The author has nothing to disclose.
Data Availability
The informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party–maintained public repository. However, data used for this submission can be made available on request to the ALSPAC executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. The ALSPAC study website contains details of all the data that are available (http://www.bristol.ac.uk/alspac/researchers/our-data/).
References
- 1. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685‐1695. [DOI] [PubMed] [Google Scholar]
- 2. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131‐2135. [DOI] [PubMed] [Google Scholar]
- 3. Danesh J, Pepys MB. Editorial: C-reactive protein and coronary disease: is there a causal link? Circulation. 2009;120(21):2036‐2039. [DOI] [PubMed] [Google Scholar]
- 4. Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128(7):864‐886. [DOI] [PubMed] [Google Scholar]
- 5. Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321(7255):199‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agbaje AO, Barmi S, Sansum KM, Baynard T, Barker AR, Tuomainen TP. Temporal longitudinal associations of carotid-femoral pulse wave velocity and carotid intima-media thickness with resting heart rate and inflammation in youth. J Appl Physiol (1985). 2023;134(3):657‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buckland G, Northstone K, Emmett PM, Taylor CM. The inflammatory potential of the diet in childhood is associated with cardiometabolic risk in adolescence/young adulthood in the ALSPAC birth cohort. Eur J Nutr. 2022;61(7):3471‐3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wijndaele K, White T, Andersen LB, et al. International Children’s Accelerometry Database (ICAD) Collaborators . Substituting prolonged sedentary time and cardiovascular risk in children and youth: a meta-analysis within the International Children's Accelerometry database (ICAD). Int J Behav Nutr Phys Act. 2019;16(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DiPietro L, Al-Ansari SS, Biddle SJH, et al. Advancing the global physical activity agenda: recommendations for future research by the 2020 WHO Physical Activity and Sedentary Behavior Guidelines Development Group. Int J Behav Nutr Phys Act. 2020;17(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carson V, Hunter S, Kuzik N, et al. Systematic review of sedentary behaviour and health indicators in school-aged children and youth: an update. Appl Physiol Nutr Metab. 2016;41(6 Suppl 3):S240‐S265. [DOI] [PubMed] [Google Scholar]
- 12. Poitras VJ, Gray CE, Borghese MM, et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Appl Physiol Nutr Metab. 2016;41(6 Suppl 3):S197‐S239. [DOI] [PubMed] [Google Scholar]
- 13. Barnett TA, Kelly AS, Young DR, et al. American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; and Stroke Council . Sedentary behaviors in today's youth: approaches to the prevention and management of childhood obesity: a scientific statement from the American Heart Association. Circulation. 2018;138(11):e142‐e159. [DOI] [PubMed] [Google Scholar]
- 14. Thomas NE, Williams DRR. Inflammatory factors, physical activity, and physical fitness in young people. Scand J Med Sci Sports. 2008;18(5):543‐556. [DOI] [PubMed] [Google Scholar]
- 15. Agbaje AO. Arterial stiffness preceding metabolic syndrome in 3862 adolescents: a mediation and temporal causal longitudinal birth cohort study. Am J Physiol Heart Circ Physiol. 2023;324(6):H905‐H911. [DOI] [PubMed] [Google Scholar]
- 16. Agbaje AO. Mediating role of body composition and insulin resistance on the association of arterial stiffness with blood pressure among adolescents: the ALSPAC study. Front Cardiovasc Med. 2022;9:939125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agbaje AO, Zachariah JP, Bamsa O, Odili AN, Tuomainen TP. Cumulative insulin resistance and hyperglycaemia with arterial stiffness and carotid IMT progression in 1,779 adolescents: a 9-year longitudinal cohort study. Am J Physiol Endocrinol Metab. 2023;324(3):E268‐E278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agbaje AO. Supplementary material for “Longitudinal mediating effect of fat mass and lipids on sedentary time, light PA, and MVPA with inflammation in youth.” Figshare. Date of deposit May 19, 2023. doi: 10.6084/m9.figshare.22962044.v1 [DOI] [PMC free article] [PubMed]
- 20. Boyd A, Golding J, Macleod J, et al. Cohort profile: the ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42(1):111‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Northstone K, Lewcock M, Groom A, et al. The Avon Longitudinal Study of Parents and Children (ALSPAC): an update on the enrolled sample of index children in 2019. Wellcome Open Res. 2019;4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agbaje AO. Associations of accelerometer-based sedentary time, light physical activity and moderate-to-vigorous physical activity with resting cardiac structure and function in adolescents according to sex, fat mass, lean mass, BMI, and hypertensive status. Scand J Med Sci Sports. Published online April 10, 2023. doi: 10.1111/sms.14365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181‐188. [DOI] [PubMed] [Google Scholar]
- 25. Agbaje AO, Lloyd-Jones DM, Magnussen CG, Tuomainen TP. Cumulative dyslipidemia with arterial stiffness and carotid IMT progression in asymptomatic adolescents: a simulated intervention longitudinal study using temporal inverse allocation model. Atherosclerosis. 2023;364:39‐48. [DOI] [PubMed] [Google Scholar]
- 26. Agbaje AO, Barker AR, Tuomainen TP. Cumulative muscle mass and blood pressure but not fat mass drives arterial stiffness and carotid intima-media thickness progression in the young population and is unrelated to vascular organ damage. Hypertens Res. 2023;46(4):984‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trost SG, Loprinzi PD, Moore R, Pfeiffer KA. Comparison of accelerometer cut points for predicting activity intensity in youth. Med Sci Sports Exerc. 2011;43(7):1360‐1368. [DOI] [PubMed] [Google Scholar]
- 28. Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sport Med. 2017;47(9):1821‐1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agbaje AO, Barker AR, Tuomainen TP. Effects of arterial stiffness and carotid intima-media thickness progression on the risk of overweight/obesity and elevated blood pressure/hypertension: a cross-lagged cohort study. Hypertension. 2022;79(1):159‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agbaje AO, Barker AR, Mitchell GF, Tuomainen TP. Effect of arterial stiffness and carotid intima-media thickness progression on the risk of dysglycemia, insulin resistance, and dyslipidaemia: a temporal causal longitudinal study. Hypertension. 2022;79(3):667‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Falaschetti E, Hingorani AD, Jones A, et al. Adiposity and cardiovascular risk factors in a large contemporary population of pre-pubertal children. Eur Heart J. 2010;31(24):3063‐3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487‐1495. [DOI] [PubMed] [Google Scholar]
- 33. Frysz M, Howe LD, Tobias JH, Paternoster L. Using SITAR (Superimposition by Translation and Rotation) to estimate age at peak height velocity in Avon Longitudinal Study of Parents and Children. Wellcome Open Res. 2018;3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agbaje AO. Elevated blood pressure and worsening cardiac damage during adolescence. J Pediatr. Published online March 3, 2023. doi: 10.1016/j.jpeds.2023.02.018 [DOI] [PubMed] [Google Scholar]
- 35. MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1(4):173‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Agbaje AO. Arterial stiffness precedes hypertension and metabolic risks in youth: a review. J Hypertens. 2022;40(10):1887‐1896. [DOI] [PubMed] [Google Scholar]
- 37. Agbaje AO, Haapala EA, Lintu N, et al. Associations of cardiorespiratory fitness and adiposity with arterial stiffness and arterial dilatation capacity in response to a bout of exercise in children. Pediatr Exerc Sci. 2019;31(2):238‐247. [DOI] [PubMed] [Google Scholar]
- 38. Golding G, Pembrey P, Jones J; ALSPAC Study Team . ALSPAC—The Avon Longitudinal Study of Parents and Children I. Study methodology. Paediatr Perinat Epidemiol. 2001;15(1):74‐87. [DOI] [PubMed] [Google Scholar]
- 39. Latouche C, Jowett JBM, Carey AL, et al. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J Appl Physiol (1985). 2013;114(4):453‐460. [DOI] [PubMed] [Google Scholar]
- 40. Saunders TJ, Chaput JP, Tremblay MS. Sedentary behaviour as an emerging risk factor for cardiometabolic diseases in children and youth. Can J Diabetes. 2014;38(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 41. Young DR, Hivert MF, Alhassan S, et al. Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Stroke Council . Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation. 2016;134(13):e262-e279. [DOI] [PubMed] [Google Scholar]
- 42. Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124(5):799‐815. [DOI] [PubMed] [Google Scholar]
- 43. Shimobayashi M, Albert V, Woelnerhanssen B, et al. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018;128(4):1538‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. 2020;126(11):1549‐1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yaribeygi H, Maleki M, Sathyapalan T, Jamialahmadi T, Sahebkar A. Pathophysiology of physical inactivity-dependent insulin resistance: a theoretical mechanistic review emphasizing clinical evidence. J Diabetes Res. 2021;2021:7796727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Rev Endocr Metab Disord. 2011;12(3):163‐172. [DOI] [PubMed] [Google Scholar]
- 47. Pesta D, Anadol-Schmitz E, Gancheva S, et al. Unraveling the athlete's paradox—higher insulin sensitivity and lower PKC? Activation despite higher bioactive lipids in endurance-trained athletes. Diabetes. 2018;67(Suppl 1):267-LB. [Google Scholar]
- 48. Fischer CP, Berntsen A, Perstrup LB, Eskildsen P, Pedersen BK. Plasma levels of interleukin-6 and C-reactive protein are associated with physical inactivity independent of obesity. Scand J Med Sci Sports. 2007;17(5):580‐587. [DOI] [PubMed] [Google Scholar]
- 49. Burini RC, Anderson E, Durstine JL, Carson JA. Inflammation, physical activity, and chronic disease: an evolutionary perspective. Sport Med Heal Sci. 2020;2(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607‐615. [DOI] [PubMed] [Google Scholar]
- 51. Chastin SFM, De Craemer M, De Cocker K, et al. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br J Sports Med. 2019;53(6):370‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Franklin BA, Thompson PD, Al-Zaiti SS, et al. American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Stroke Council . Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective-an update: a scientific statement from the American Heart Association. Circulation. 2020;141(13):e705‐e736. [DOI] [PubMed] [Google Scholar]
- 53. Aengevaeren VL, Mosterd A, Sharma S, et al. Exercise and coronary atherosclerosis: observations, explanations, relevance, and clinical management. Circulation. 2020;141(16):1338‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ. 2019;366:l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lin X, Zhang X, Guo J, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4(7):e002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fedewa MV, Hathaway ED, Ward-Ritacco CL. Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br J Sports Med. 2017;51(8):670‐676. [DOI] [PubMed] [Google Scholar]
- 57. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138‐146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party–maintained public repository. However, data used for this submission can be made available on request to the ALSPAC executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. The ALSPAC study website contains details of all the data that are available (http://www.bristol.ac.uk/alspac/researchers/our-data/).