Abstract
Aims
This study aimed to investigate the association of testosterone replacement therapy (TTh) with risk of cardiovascular disease (CVD), and CVD-specific outcomes, in cisgender women and transgender population, and to determine whether this association varies by menopausal status.
Methods
In 25 796 cisgender women and 1580 transgender people (≥30 years old) who were enrolled in the Optum's deidentified Clinformatics Data Mart Database (2007-2021), we identified 6288 pre- and postmenopausal cisgender women and 262 transgender people diagnosed with incident composite of CVD (coronary artery disease [CAD], congestive heart failure, stroke, and myocardial infarction). Prediagnostic prescription of TTh was ascertained for this analysis. Multivariable adjusted Cox proportional hazards models were used to examine the independent association of TTh with incident CVD.
Results
We found a 24% increased risk of CVD (hazard ratio [HR] = 1.24; 95% CI, 1.15-1.34), 26% risk of CAD (HR = 1.26; 95% CI, 1.14-1.39), and a 29% risk of stroke (HR = 1.29; 95% CI, 1.14-1.45) after comparing cisgender women who used TTh with nonusers. Stratification by age group showed similar effects of TTh on CVD, CAD, and stroke. Among transgender people, TTh did not increase the risk of composite CVD, including by age stratification.
Conclusion
Use of TTh increased the risk of CVD, CAD, and stroke among cisgender women but not among transgender people. TTh is becoming more widely accepted in women, and it is the main medical treatment for transgender males. Therefore, use of TTh should be further investigated for the prevention of CVD.
Keywords: testosterone, cardiovascular, women, transgender
A body of literature suggests that low levels of serum testosterone increase the risk of cardiovascular disease (CVD) among men (1, 2). However, the effect of testosterone replacement therapy (TTh), which is the current treatment for low testosterone in men, on CVD remains conflicted (3-5). In parallel, much less is known about the effects of TTh on CVD among pre- and postmenopausal cisgender women (6-8). A wider research gap is observed among transgender people and their cardiovascular health in the presence of TTh (9, 10).
The use of TTh among women is becoming more acceptable, but to date, there is no Food and Drug Administration testosterone supplementation product approved for women (7, 11). The indication for TTh treatment for women is less specific (8), which is a public and clinical concern. Previous studies have addressed the gender bias related to the significant increasing use of TTh among men, and the potential hesitation for clinical recommendation for TTh use among cisgender women because of the lack of a Food and Drug Administration–approved testosterone supplementation for cisgender women (6-8). However, a recent global consensus position statement by 11 international societies endorsed hypoactive sexual desire dysfunction as the only evidence-based indication of TTh for postmenopausal women (12). Based on a systematic review and meta-analysis of randomized controlled trial (RCT) data (36 RCTs with 8480 participants), the task force reported that testosterone is effective for postmenopausal women with low sexual desire, and it was not associated with serious adverse events, but further research was warranted because of limited power sample size (8 RCTs, n = 107 adverse events), specifically for myocardial infarction (n = 4 events) and stroke (n = 4 events; see Islam et al appendix, p. 46) (8). Similarly, among premenopausal women, the data were insufficient (3 RCTs) to provide any recommendation (only 115 premenopausal women who used TTh; see Islam et al appendix, p. 20) (8).
Among transgender people, the burden of morbidity and mortality is largely unknown. However, a recent study reported that transgender people are at elevated risk for nearly all chronic conditions such as cardiovascular, neurological, weight, diabetes, thyroid conditions, mental health, substance use, and other chronic conditions (liver, renal, cancer, and AIDS/HIV) (13).
Transgender people can be treated with gender-affirming hormones to achieve the desired masculinization or feminization appearance (9, 14). TTh is considered the cornerstone medical treatment for transgender males (9, 10, 15). However, barriers to receiving culturally competent transgender care were previously reported in a 2015 representative survey of endocrinologists (16). Similar to cisgender women, there is a paucity of high-quality data because of a shortage of RCTs about the effects of TTh on CVD among transgender people, with 2 European studies reporting an increased risk of CVD and CVD mortality among them (14, 17), but not other studies (18, 19). Therefore, the objectives of this study are to investigate the effects of TTh on a composite CVD outcome, and its specific disease outcomes, in women and transgender people (≥30 years), and to determine whether these effects vary by age groups of pre- and postmenopausal status.
Patients and Methods
Data Source
We analyzed data from Optum's deidentified Clinformatics Data Mart Database 2007-2021 (Clinformatics), one of the largest commercial insurance databases containing patients’ demographics and clinical information such as prescription drugs dispensed and outpatient and inpatient claims. This study was reviewed and approved by the institutional review board of the University of Texas Medical Branch at Galveston, TX.
Study Cohort
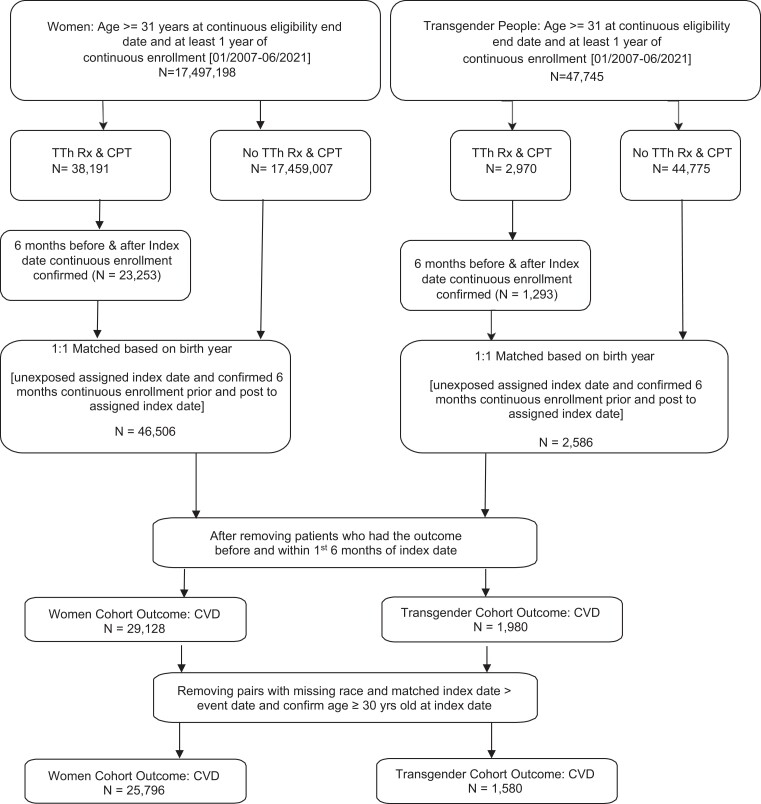
In this retrospective cohort study, we identified 2 cohorts between January 2007 and June 2021: 1 was of of cisgender women (N = 25 796) and the other of transgender people (N = 1580). All females and transgender people aged ≥ 30 years with at least 1 year of continuous enrollment and no previous history of CVD were included. Transgender information was obtained from International Classification of Diseases 9 (ICD-9), ICD-10, Current Procedural Terminology, and Healthcare Common Procedure codes (Supplementary Table 1) (13, 20). The first prescription date of TTh use (exposed group) was considered the index date. We then randomly matched on birth year and assigned the index date to the unexposed group while confirming continuous enrollment 6 months before and 6 months after the index date for each subject. We also confirmed there was no prior TTh use in the 6 months before the index date and that the composite CVD outcome did not occur within the first 6 months of the index date. Subjects were excluded if they were younger than 30 years at the index date or if they were missing race information (Fig. 1).
Figure 1.
Flowchart cohort derivation.
Prediagnostic use of Testosterone Replacement Therapy
Prescriptions of TTh use were established using National Drug Codes and Current Procedural Terminology codes (Supplemental Table 2) (20) for both cisgender women and transgender participants. TTh was dichotomized as “yes” or “no” (reference group). We further categorized only number of TTh injections, 1 vs 0 (reference group); in general, participants received 1 injection. The index date was defined as the date of the first prescription within the study period. For the composite CVD and CVD-specific outcome, we evaluated TTh use at any time during the study period and conducted a time-to-event analysis.
Composite CVD and CVD-specific Outcomes
The outcomes of interest of this study were the incident composite cardiovascular disease (CVD) (yes/no), which includes CVD specific outcomes, chronic coronary artery disease (CAD), stroke, congestive heart failure (CHF), peripheral arterial disease (PAD), and myocardial infarction (MI). CVD outcomes were identified using ICD-9 and ICD-10 codes (Supplemental Table 3) (20). We followed the patients from the index date (first TTh prescription date) until the outcome event occurred or until they lost insurance eligibility for 2 continuous months or until the end of study period (June 30, 2021).
Covariates
Patient characteristics including age, race, hyperlipidemia, hypertension, muscular wasting, malaise and fatigue, osteoporosis, depression, pituitary dysfunction, diabetes, Charlson comorbidity index, number of primary care physician visits, and insulin use. The Charlson comorbidities were summed for scoring after removing MI, CHF, CVD, and diabetes with and without complications because they were analyzed as outcomes or risk factors of interest (21).
Statistical Analysis
Patient characteristics, comorbidity, follow-up time, primary care physician (PCP) visits, and TTh injections were calculated and compared for both women (Table 1) and transgender cohorts (Table 2) by TTh use (yes/no) using χ2 (categorical-larger sample) or Fisher exact tests (categorical-smaller sample). The number of TTh injections by composite CVD status for women and transgender people are also reported (Supplemental Table 3) (20). We calculated the effect of TTh on the risk of composite CVD risk using multivariable Cox regression hazard models stratified by cisgender women and transgender people; for estimation, the SAS default “Breslow” method was used. Proportional hazard assumptions were assessed by scaled Schoenfeld residuals. We also conducted subgroup analysis for age groups (aged 30-51 and ≥ 52 years) under the hypothesis that natural menopause onset is at 52 years old. Further subgroup analysis was conducted among TTh injection recipients and by comparing the risk of CVD between transgender people who use TTh vs cisgender women non-TTh users. All the multivariable models were adjusted for the covariates identified at baseline (6 months before the index date [first TTh prescription date]). All tests of statistical significance were 2-sided, and analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
Table 1.
Baseline characteristics of 25 796 cisgender women 30+ years by testosterone replacement therapy (TTh) in Clinformatics 2007-2021
| Characteristics | Total | Testosterone therapy | Pa | |
|---|---|---|---|---|
| No N (%) | Yes N (%) | |||
| Total | 25 796 | 12 898 (50) | 12 898 (50) | |
| Cardiovascular disease (CVD) | ||||
| No | 19 508 | 10 059 (77.99) | 9449 (73.26) | .0001 |
| Yes | 6288 | 2839 (22.01) | 3449 (26.74) | |
| Age, y | ||||
| 30-40 | 4428 | 2214 (17.17) | 2214 (17.17) | 1 |
| 41-50 | 9298 | 4649 (36.04) | 4649 (36.04) | |
| 51-60 | 9100 | 4550 (35.28) | 4550 (35.28) | |
| 61-70 | 2388 | 1194 (9.26) | 1194 (9.26) | |
| ≥71 | 582 | 291 (2.26) | 291 (2.26) | |
| Race/ethnicity | ||||
| Asian | 1010 | 824 (6.39) | 186 (1.44) | .0001 |
| Black | 2774 | 1444 (11.2) | 1330 (10.31) | |
| Hispanic | 2805 | 1855 (14.38) | 950 (7.37) | |
| White | 19 207 | 8775 (68.03) | 10 432 (80.88) | |
| Follow-up time, y | ||||
| ≤1 | 5404 | 2543 (19.72) | 2861 (22.18) | .0001 |
| 2-3 | 11 637 | 5666 (43.93) | 5971 (46.29) | |
| ≥4 | 8755 | 4689 (36.35) | 4066 (31.52) | |
| Hyperlipidemia | ||||
| No | 22 727 | 11 684 (90.59) | 11 043 (85.62) | .0001 |
| Yes | 3069 | 1214 (9.41) | 1855 (14.38) | |
| Hypertension | ||||
| No | 21 443 | 10 859 (84.19) | 10 584 (82.06) | .0001 |
| Yes | 4353 | 2039 (15.81) | 2314 (17.94) | |
| Muscular wasting | ||||
| No | 25 609 | 12 847 (99.6) | 12 762 (98.95) | .0001 |
| Yes | 187 | 51 (0.4) | 136 (1.05) | |
| Malaise and fatigue | ||||
| No | 21 649 | 12 294 (95.32) | 9355 (72.53) | .0001 |
| Yes | 4147 | 604 (4.68) | 3543 (27.47) | |
| Osteoporosis | ||||
| No | 25 247 | 12 696 (98.43) | 12 551 (97.31) | .0001 |
| Yes | 549 | 202 (1.57) | 347 (2.69) | |
| Depression | ||||
| No | 23 903 | 12 313 (95.46) | 11 590 (89.86) | .0001 |
| Yes | 1893 | 585 (4.54) | 1308 (10.14) | |
| Pituitary dysfunction | ||||
| No | 25 760 | 12 888 (99.92) | 12 872 (99.8) | .0076 |
| Yes | 36 | 10 (0.08) | 26 (0.2) | |
| Diabetes | ||||
| No | 24 300 | 12 128 (94.03) | 12 172 (94.37) | .2412 |
| Yes | 1496 | 770 (5.97) | 726 (5.63) | |
| Number of Charlson comorbidity index | ||||
| 0 | 22 680 | 11 546 (89.52) | 11 134 (86.32) | .0001 |
| 1 | 2741 | 1186 (9.2) | 1555 (12.06) | |
| ≥2 | 375 | 166 (1.29) | 209 (1.62) | |
| Number of primary care physician visits | ||||
| 0 | 9694 | 6060 (46.98) | 3634 (28.17) | .0001 |
| 1 | 5791 | 2970 (23.03) | 2821 (21.87) | |
| 2 | 4066 | 1727 (13.39) | 2339 (18.13) | |
| ≥3 | 6245 | 2141 (16.6) | 4104 (31.82) | |
| Insulin use | ||||
| No | 25 512 | 12 741 (98.78) | 12 771 (99.02) | .0734 |
| Yes | 284 | 157 (1.22) | 127 (0.98) | |
a P values calculated from χ2 test.
Table 2.
Multivariable analysisa in the effects of TTh on the compositive cardiovascular disease (CVD), and its specific disease outcomes, among 25 796 cisgender women in Clinformatics 2007-2021
| Outcomes | Events/N | Hazard ratiob (95% CI) |
|---|---|---|
| Composite CVD | 6288/25 796c | 1.24 (1.15-1.34) |
| Coronary artery disease | 3342/25 796 | 1.26 (1.14-1.39) |
| Stroke | 2392/25 796 | 1.29 (1.14-1.45) |
| Congestive heart failure | 1001/25 796 | 0.91 (.76-1.11) |
| Peripheral arterial disease | 125/25 796 | 1.09 (.59-2.01) |
| Myocardial infarction | 42/25 796 | 1.16 (.29-4.70) |
Boldface indicates statistical significance.
Abbreviation: TTh, testosterone replacement therapy.
a Multivariable analysis adjusted for age, race/ethnicity, hyperlipidemia, hypertension, muscular wasting, malaise and fatigue, osteoporosis, pituitary dysfunction, diabetes, Charlson comorbidity index, number of primary care visits, and insulin use.
b Cox regression hazard models calculated hazard ratios. Asterisk (*) denoted for the models not converged because of low rates of incidence.
c TTh users, n = 12 898; non-TTh users, n = 12 898; TTh users with CVD, n = 3449; non-TTh users with CVD, n = 2839.
Results
Cisgender Women
We identified 6288 cisgender women with CVD in Clinformatics 2007-2021. Patient characteristics by TTh use (no/yes) among women are shown in Table 1. Patients were matched on birth year and, in general, the mean age was 52 years for cisgender women. The largest racial group was White (74%). More than 34% of cisgender women were followed for more than 4 years in the development of CVD. In Table 1, compared with no TTh users, cisgender women who used TTh were more likely to have hyperlipidemia, be hypertensive, and have muscular wasting, malaise and fatigue, osteoporosis, depression, pituitary disfunction, higher Charlson comorbidity index score, and a higher number of PCP visits. In further descriptions of TTh users (eg, injections, gels) among cisgender women, those who received 1 TTh injection had a smaller percentage of CVD than those who did not take an injection (30.85% vs 69.15; Supplemental Table 4) (20). Multivariable analyses of the effects of TTh use on the composite CVD and its specific diseases outcomes among cisgender women are shown in Table 2. Among cisgender women, TTh use increased the risk of the composite CVD 24% (hazard ratio [HR] = 1.24; 95% CI, 1.15-1.34) compared with nonusers (Table 2). Similarly, there was a 26% increased risk of CAD (HR = 1.26; 95% CI, 1.14-1.39) and 29% increased risk of stroke (HR = 1.29; 95% CI, 1.14-1.45) for cisgender women who used TTh compared with nonusers. No significant associations for CHF, PAD, and MI were found.
In the sensitivity analysis, we stratified the association of TTh use with composite CVD and its specific outcomes among cisgender women (Table 3) by age groups (aged 30-51 and ≥ 52 years). Among cisgender women within the age groups, the use of TTh increased the risk of composite CVD compared with those who did not use TTh (aged 30-51 years: HR = 1.29; 95% CI, 1.15-1.45; ≥ 52 years: HR = 1.20; 95% CI, 1.10-1.33). A similarly increased risk for chronic CAD and stroke was observed, but not for CHF and MI. There was a reduced risk for PAD among older cisgender women (≥52 years) who used TTh (HR = 0.40; 95% CI, .16-.96).
Table 3.
Age group stratificationa of the effects of TTh on the compositive cardiovascular disease (CVD), and its specific disease outcomes among 25 796 cisgender women in Clinformatics 2007-2021
| Cisgender women (age 30-51 years) N = 14 974 | Cisgender women (age ≥ 52 years) N = 10 822 | |||
|---|---|---|---|---|
| Outcome | Events/N | HR (95% CI)b | Events/N | HR (95% CI)b |
| Composite CVD | 2838/14 974 | 1.29 (1.15-1.45) | 3450/10 822 | 1.20 (1.10-1.33) |
| Coronary artery disease | 1453/14 974 | 1.33 (1.14-1.55) | 1889/10 822 | 1.21 (1.10-1.39) |
| Stroke | 1131/14 974 | 1.37 (1.15-1.65) | 1261/10 822 | 1.23 (1.05-1.45) |
| Congestive heart failure | 432/14 974 | 0.95 (0.69- 1.30) | 569/10 822 | 0.88 (0.69-1.13) |
| Peripheral arterial disease | 44/14 974 | * | 81/10 822 | 0.40 (0.16-0.96) |
| Myocardial infarction | 18/14 974 | * | 24/10 822 | * |
Boldface indicates statistical significance.
Abbreviations: HR, hazard ratio; TTh, testosterone replacement therapy.
a Multivariable analysis adjusted for race/ethnicity, hyperlipidemia, hypertension, muscular wasting, malaise and fatigue, osteoporosis, pituitary dysfunction, diabetes, Charlson comorbidity index, number of primary care visits, and insulin use.
b Cox regression hazard models calculated HRs. Asterisk (*) denoted for the models not converged because of low rates of incidence.
Transgender Population
We identified 262 transgender participants with CVD in Clinformatics 2007-2021. Patient characteristics by TTh use (no/yes) among transgender people are shown in Table 4. Patients were matched on birth year and, in general, mean age was 36 years for transgender people. The largest racial group was White for transgender people (67%). More than 28% of transgender people were followed for more than 4 years in the development of CVD. In Table 4, compared with no TTh use, transgender people who used TTh were more likely to have hyperlipidemia, be hypertensive, and have malaise and fatigue, depression, and a higher number of PCP visits. In further description of TTh use (eg, injections, gels) among transgender people who did not receive more than 1 TTh injection and among transgender people who received 1 TTh injection, there was no higher frequency of CVD (11.83%; Supplemental Table 4) (20).
Table 4.
Baseline characteristics of 1580 transgender people 30+ years by testosterone replacement therapy in Clinformatics 2007-2021
| Characteristics | Total | Testosterone therapy | Pa | |
|---|---|---|---|---|
| No N (%) | Yes N (%) | |||
| Total | 1580 | 790 (50) | 790 (50) | |
| Cardiovascular disease | ||||
| No | 1318 | 658 (83.29) | 660 (83.54) | .8924 |
| Yes | 262 | 132 (16.71) | 130 (16.46) | |
| Age, y | ||||
| 30-40 | 910 | 455 (57.59) | 455 (57.59) | 1 |
| 41-50 | 382 | 191 (24.18) | 191 (24.18) | |
| 51-60 | 216 | 108 (13.67) | 108 (13.67) | |
| 61-70 | 58 | 29 (3.67) | 29 (3.67) | |
| ≥71 | 14 | <11 (<11)c | <11 (<11)c | |
| Race/ethnicity | ||||
| Asian | 50 | 20 (2.53) | 30 (3.80) | .0153 |
| Black | 296 | 169 (21.39) | 127 (16.08) | |
| Hispanic | 167 | 89 (11.27) | 78 (9.87) | |
| White | 1067 | 512 (64.81) | 555 (70.25) | |
| Follow-up time, y | ||||
| ≤1 | 314 | 137 (17.34) | 177 (22.41) | .0042 |
| 2-3 | 809 | 399 (50.51) | 410 (51.9) | |
| ≥4 | 457 | 254 (32.15) | 203 (25.7) | |
| Hyperlipidemia | ||||
| No | 1450 | 741 (93.80) | 709 (89.75) | .0034 |
| Yes | 130 | 49 (6.20) | 81 (10.25) | |
| Hypertension | ||||
| No | 1337 | 683 (86.46) | 654 (82.78) | .0431 |
| Yes | 243 | 107 (13.54) | 136 (17.22) | |
| Muscular wasting | ||||
| No | 1572 | 789 (99.87) | 783 (99.11) | .0696b |
| Yes | <11c | <11 (<11)c | <11 (<11)c | |
| Malaise and fatigue | ||||
| No | 1502 | 765 (96.84) | 737 (93.29) | .0011 |
| Yes | 78 | 25 (3.16) | 53 (6.71) | |
| Osteoporosis | ||||
| No | 1567 | 781 (98.86) | 786 (99.49) | .1638 |
| Yes | 13 | <11 (<11)c | <11 (<11)c | |
| Depression | ||||
| No | 1358 | 712 (90.13) | 646 (81.77) | .0001 |
| Yes | 222 | 78 (9.87) | 144 (18.23) | |
| Pituitary dysfunction | ||||
| No | 1578 | 789 (99.87) | 789 (99.87) | 1 |
| Yes | <11c | <11 (<11)c | <11 (<11)c | |
| Diabetes | ||||
| No | 1481 | 747 (94.56) | 734 (92.91) | .1772 |
| Yes | 99 | 43 (5.44) | 56 (7.09) | |
| Number of Charlson comorbidity index | ||||
| 0 | 1324 | 653 (82.66) | 671 (84.94) | .0852 |
| 1 | 219 | 112 (14.18) | 107 (13.54) | |
| ≥2 | 37 | 25 (3.16) | 12 (1.52) | |
| Number of primary care physician visits | ||||
| 0 | 534 | 341 (43.16) | 193 (24.43) | .0001 |
| 1 | 377 | 173 (21.90) | 204 (25.82) | |
| 2 | 265 | 120 (15.19) | 145 (18.35) | |
| ≥3 | 404 | 156 (19.75) | 248 (31.39) | |
| Insulin use | ||||
| No | 1562 | 782 (98.99) | 780 (98.73) | .6354 |
| Yes | 18 | <11 (<11)c | <11 (<11)c | |
a P values calculated from χ2 test.
b Fisher exact test was calculated.
c Counts less than 11 have been suppressed.
Multivariable analyses of the effects of TTh use on the composite CVD and its specific diseases outcomes among transgender people are shown in Table 5. Among transgender people, there was no increased risk of composite CVD for those who used TTh compared with those who did not (HR = 0.99; 95% CI, .69-1.42), albeit this association did not reach statistical significance (Table 5). Similarly, none of the associations of TTh use with CAD (HR = 1.19; 95% CI, .59-2.37), stroke (HR = 0.97; 95% CI, .56-1.70), and CHF (HR = 0.41; 95% CI, .13-1.22) reached statistical significance. Because of the small sample size, analyses with PAD and MI could not be conducted.
Table 5.
Multivariable analysisa in the effects of TTh on the compositive cardiovascular disease (CVD), and its specific disease outcomes, among 1580 transgender people in Clinformatics 2007-2021
| Outcome | Events/N | HRb (95% CI) |
|---|---|---|
| Composite CVD | 262/1580c | 0.99 (0.69-1.42) |
| Coronary artery disease | 93/1580 | 1.19 (0.59-2.7) |
| Stroke | 114/1580 | 0.97 (0.56-1.70) |
| Congestive heart failure | 72/1580 | 0.41 (0.13-1.22) |
| Peripheral arterial disease | <11/1580d | * |
| Myocardial infarction | <11/1580d | * |
Abbreviations: HR, hazard ratio; TTh, testosterone replacement therapy.
a Multivariable analysis adjusted for age, race/ethnicity, hyperlipidemia, hypertension, muscular wasting, malaise and fatigue, osteoporosis, pituitary dysfunction, diabetes, Charlson comorbidity index, number of primary care visits, and insulin use.
b Cox regression hazard models calculated as HRs.
c TTh users, n = 790; non-TTh users, n = 790; TTh users with CVD, n = 130; non-TTh users with CVD, n = 132.
d Counts less than 11 have been suppressed. Asterisk (*) denotes the models not converged because of low rates of incidence.
In the sensitivity analysis, we stratified the association of TTh use with composite CVD, and its specific outcomes among transgender people (Table 6) by age groups (aged 30-51 and ≥ 52 years). Among transgender people within the 2 age groups, none of the associations of TTh use with composite CVD, CAD, stroke, and CHF reached statistical significance (Table 6). Because of the small sample size in MI, analyses were not conducted.
Table 6.
Age group stratificationa of the effects of TTh on the compositive cardiovascular disease (CVD), and its specific disease outcomes, among 1580 transgender people in Clinformatics 2007-2021
| Transgender people (age 30-51 years) N = 1318 | Transgender people (age ≥ 52 years) N = 262 | |||
|---|---|---|---|---|
| Outcome | Events/N | HR (95% CI)b | Events/N | HR (95% CI)b |
| Composite CVD | 173/1318 | 0.85 (.54-1.35) | 89/262 | 0.80 (.42-1.92) |
| Coronary artery disease | 60/1318 | 1.67 (.63-4.60) | 33/262 | 1.55 (.25-9.65) |
| Stroke | 74/1318 | 0.53 (.21-1.33) | 40/262 | 0.94 (.24-3.71) |
| Congestive heart failure | 51/1318 | 0.61 (.17-2.21) | 21/262 | 0.50 (.05-5.52) |
| Peripheral arterial disease | <11/1318c | * | <11/262c | * |
| Myocardial infarction | <11/1318c | * | <11/262c | * |
Abbreviation: HR, hazard ratio; TTh, testosterone replacement therapy.
a Multivariable analysis adjusted for race/ethnicity, hyperlipidemia, hypertension, muscular wasting, malaise and fatigue, osteoporosis, pituitary dysfunction, diabetes, Charlson comorbidity index, number of primary care visits, and insulin use.
b Cox regression hazard models calculated as HRs.
c Counts less than 11 have been suppressed. Asterisk (*) denoted for the models not converged because of low rates of incidence.
Further sensitivity analysis was conducted among cisgender women and transgender people who received only 1 TTh injection compared with nonusers and assess their effect on the risk of CVD and its specific outcomes (Supplemental Tables 4-6) (20). Similarly increased risks for composite CVD (HR = 1.23; 95% CI, 1.11-1.37), chronic CAD (HR = 1.29; 95% CI, 1.12-1.48), and stroke (HR = 1.28; 95% CI, 1.08-1.52) were observed among cisgender women, but no significant association were observed among transgender people (Supplemental Table 5) (20). We further compared the risk of CVD between aged-matched transgender TTh users vs cisgender women non-TTh users, but there were no significant associations observed (Supplemental Table 6) (20).
Discussion
Overall, we found that TTh increased the risk of composite CVD, chronic CAD, and stroke among cisgender women (≥30 years old). These effects persisted across age strata (aged 30-51 and ≥ 52 years). None of the associations of TTh use with CHF, PAD and MI reached a statistical significance among cisgender women. Among transgender people, none of the associations of TTh with composite CVD, and its specific outcomes, were statistically significant, overall or across the 2 age strata. Similar associations were observed with those who received only 1 TTh injection in cisgender women and transgender people.
TTh and CVD in Cisgender Women
The first studies conducted between 2000 and 2010 investigating the interplay between testosterone implant therapy, endogenous testosterone, and cardiovascular function among cisgender women found that parenteral testosterone improves arterial function by enhancing endothelium-dependent (flow-mediated) and endothelium-independent brachial artery vasodilation (22). Others have suggested that there was evidence that women with low testosterone levels have significantly smaller age-adjusted, flow-mediated endothelium-dependent vasodilation (23), and that low concentrations of testosterone were associated with cardiovascular events, independent of traditional risk factors (24).
Yet, a recent meta-analysis of 8 RCTs (Islam et al 2019) reported that among postmenopausal women, TTh was not associated with more frequent reporting of serious adverse events (relative risk [RR] = 0.97; 95% CI, .65-1.44; n = 107 events), MI (RR = 0.66; 95% CI, .09-4.69; n = 4 events), and stroke (RR = 0.66; 95% CI, .09-4.69; n = 4 events), but the power sample size of this meta-analysis to make conclusions was minimal (8). Similarly, in this meta-analysis, the data were insufficient (3 RCTs) to provide any recommendation among premenopausal women (only 115 premenopausal women who used TTh; see Islam et al appendix p. 20) (8). Our study findings do not seem to be in the same direction as previous studies, which is possible that our large CVD sample size (CVD cases n = 6327; stroke cases n = 2409; MI cases n = 43) and retrospective cohort study design are contributing to these differences. Menopausal status can be another contributing factor to these disparities among studies; however, we stratified by age groups under the hypothesis that older age is strongly associated with postmenopausal status, but our findings among women ≥ 52 years old remained the same, except for incident PAD. Our study found that there was a reduced risk of PAD in relation with TTh use, and this finding is different compared with a small study (n = 21 with PAD) of older women (mean age ≥75 years) that investigated serum testosterone levels, not TTh, and reported that high levels of serum testosterone increased PAD (25). Our study is focused on the effect of use of TTh on PAD but is limited with a small sample of PAD cases and lack of information about baseline serum testosterone levels of these women before and after the use of TTh. Future studies should focus on the interplay between serum testosterone, use of TTh, and incident PAD in younger and older women.
TTh and CVD in Transgender People
TTh is considered the cornerstone medical treatment for transgender males to achieve the masculinization appearance, but for transgender females to achieve the feminization appearance the medical treatment is estrogen/antiandrogen (9, 10, 15) Therefore, although in our study, we are only including transgender people and not distinguishing between transgender males and transgender females, there is a higher possibility that our study includes mainly transgender males because of the current use of TTh. We compared transgender TTh users with 2 groups (transgender non-TTh users [Table 5] and cisgender women non-TTh users [Supplemental Table 6]) (20) to minimize any confounding bias with comparison groups.
Compared with the large number of studies conducted among men who use TTh, there remains a paucity of complete and well-detailed data in relation to the association between testosterone replacement therapy and CVD among transgender people. Two early systematic review and meta-analysis studies (Elamin et al 2010, Maraka et al 2017) among transgender people reported that the data about important patient outcomes, such as MI and stroke, in relation with TTh, were sparse to derive insightful conclusions and recommendations (26, 27). Although our study has a large sample size of CVD cases among transgender people (n = 273), similar to the previous meta-analyses, we were still limited to provide a definite conclusion. Recently, 2 European retrospective cohort studies reported an increased risk of CVD (stroke observed cases, n = 35; MI observed cases, n = 41) (17) and CVD mortality (CVD deaths, n = 50 transgender women; CVD deaths, n = <10 transgender men) (14) among transgender people. We also conducted a retrospective cohort study, yet our findings with a transgender population were not significant, and it is possible that this is because we could not differentiate between the transgender population (transgender males and females). Therefore, the use of different comparison groups has the potential to affect an association between the use of a medication and a health outcome. Another study of transgender men (n = 50) who received TTh for an average of 10 treatment years reported no elevated incidence of CVD, such as MI or stroke (18). As with our study, this study included a long follow-up period, but both studies did not find significant associations. A cross-sectional study of 138 transgender males reported that incidence of MI and CVD were identical to the control male and female patients (19). Because we conducted a retrospective cohort study, not a cross-sectional one, it is possible that the different study designs influenced our findings. A general observation related to the different findings between our and previous studies is the study design, sample size, and comparison groups (eg, transgender people vs cisgender men vs cisgender women or general population) that by themselves have the potential to influence the associations between TTh and CVD in cisgender and transgender population.
Strengths and Limitations
Our study has strengths. This investigation included a large sample of women and transgender people with incident CVD, some of its specific disease outcomes, a large enough sample to be able to investigate the association of TTh use with the composite CVD, and to determine whether the association of TTh use with composite CVD varies by age groups in both women and transgender people. Yet, the present study has limitations as well, and our findings must be interpreted in view of these limitations. First, the observed inverse association between TTh and PAD among women (≥52 years), may be due, at least in part, to residual confounding from known and unknown confounders. Similarly, although we had the power sample size to investigate the association of TTh use with the composite CVD, chronic CAD, stroke, and CHF among transgender people, there were other specific analysis we could not conduct because of the small number of cases with PAD and MI. Second, our study could not differentiate between transgender males and transgender females. However, our transgender study population used TTh, which is the main and first line of treatment to achieve masculinization for transgender men; therefore, it is possible that our transgender population included a larger number of transgender males. To further minimize potential confounding bias related to comparison groups, we compared transgender TTh users with transgender non-TTh users and cisgender women non-TTh users, but findings remained null. Third, cisgender women and the transgender population may have obtained testosterone from clinicians not reimbursed by their insurance. Fourth, there are general limitations of retrospective analysis based on health care claims data, such as possible coding errors, omissions of claims (28), or use of medications before 2007 (earliest year of data available). However, this potentially inaccurate capture of information will be considered nondifferential misclassification because it was collected before the disease developed, which in general influences associations to the null (1.0). Fifth, limiting the preindex period to 6 months did not allow capture of full medical history; for instance, patients may have had the comorbidity of interest before the start of the preindex period. Finally, although we adjusted for several potential risk factors for CVD, we cannot rule out potential residual confounding by these factors or by those potential confounders not included in this study (eg, geographical location). Clinformatics does not include laboratory information from occupational, environmental, nutritional and/or several lifestyle factors, so we could not adjust for those. This includes no available data for obesity (body mass index ≥ 30 kg/m2), a strong risk factor for CVD. Although we adjusted for strong factors associated with obesity such as diabetes, hypertension, Charlson comorbidity index comorbidity score, and use of insulin, it is possible that some levels of obesity were captured and adjusted for (29), yet residual confounding may remain. Furthermore, in this study, we did not include measurements of estrogen and testosterone levels before TTh injection; however, future studies with larger numbers of sex steroid hormone measurements and TTh prescriptions in relation with CVD among women should be further investigated. A more in-depth analysis of menopause status with menstrual cycle information is also warranted.
Conclusion
In summary, in this large Clinformatics® 2007-2021 claims-based analysis, we found that prediagnostic use of TTh increased the risk of composite CVD and its specific outcomes such as chronic CAD and stroke among cisgender women. These associations remained in the same direction regardless of the age group analysis. No significant associations were observed among transgender people, including specific age group analysis. Further research is warranted to remove potential residual confounding and determine through which biological mechanism(s) TTh use has the potential to influence the risk of CVD among women, but not among transgender people.
Abbreviations
- CAD
coronary artery disease
- CHF
congestive heart failure
- CVC
cardiovascular disease
- HR
hazard ratio
- ICD
International Classification of Diseases
- MI
myocardial infarction
- PAD
peripheral artery disease
- PCP
primary care physician
- RCT
randomized controlled trial
- RR
relative risk
- TTh
testosterone replacement therapy
Contributor Information
David S Lopez, School of Public and Population Health, University of Texas Medical Branch, Galveston, TX 77555-0553, USA.
Juwairia S Mulla, Division of Cardiology, Internal Medicine, University of Texas Medical Branch, Galveston, TX 77555-0553, USA.
Danielle El Haddad, Division of Cardiology, Internal Medicine, University of Texas Medical Branch, Galveston, TX 77555-0553, USA.
Md Ibrahim Tahashilder, School of Public and Population Health, University of Texas Medical Branch, Galveston, TX 77555-0553, USA.
Efstathia Polychronopolou, School of Public and Population Health, University of Texas Medical Branch, Galveston, TX 77555-0553, USA.
Jacques Baillargeon, School of Public and Population Health, University of Texas Medical Branch, Galveston, TX 77555-0553, USA.
Yong-Fang Kuo, School of Public and Population Health, University of Texas Medical Branch, Galveston, TX 77555-0553, USA.
Syed Gilani, Division of Cardiology, Internal Medicine, University of Texas Medical Branch, Galveston, TX 77555-0553, USA.
Wissam I Khalife, Division of Cardiology, Internal Medicine, University of Texas Medical Branch, Galveston, TX 77555-0553, USA.
Funding
D.S.L. was supported by the National Institutes of Health (NIH) and National Institute on Aging, Grant #P30 AG059301; and Cancer Prevention and Research Institute of Texas (CPRIT), Grant #RP210130.
Author Contributions
Conception and design (DSL, WK, SG, EP, YFK). Writing original draft (DSL, JSM). Methodology and statistical analysis (DSL, MIT, EP, YFK). Interpretation of data, statistical analysis, biostatistics, and computational analysis (DSL, MIT, EP, YFK, WIK, SG). Interpretation and writing review and editing (all authors).
Disclosures
The authors declare no potential conflicts of interest.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request (Optum Clinformatics is not publicly available). The authors confirm that data used in developing this article are available on reasonable request to the corresponding author. This study used the linked database Optum's deidentified Clinformatics Data Mart Database (2007-2021). The interpretation and reporting of these data are the sole responsibility of the authors.
Ethics Approval and Consent to Participate
The secondary data analysis was approved by Institutional Review Board at the University of Texas Medical Branch, Galveston, TX.
References
- 1. Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J Sex Med. 2018;15(9):1260‐1271. [DOI] [PubMed] [Google Scholar]
- 2. Kloner RA, Carson C 3rd, Dobs A, Kopecky S, Mohler ER 3rd. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67(5):545‐557. [DOI] [PubMed] [Google Scholar]
- 3. Lopez DS, Canfield S, Wang R. Testosterone replacement therapy and the heart: friend, foe or bystander? Transl Androl Urol. 2016;5(6):898‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corona G, Rastrelli G, Maseroli E, Sforza A, Maggi M. Testosterone replacement therapy and cardiovascular risk: a review. World J Mens Health. 2015;33(3):130‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis SR, Wahlin-Jacobsen S. Testosterone in women–the clinical significance. Lancet Diabetes Endocrinol. 2015;3(12):980‐992. [DOI] [PubMed] [Google Scholar]
- 7. Donovitz GS. Testosterone supplementation and the gender divide. Neurourol Urodyn. 2021;40(3):938‐940. [DOI] [PubMed] [Google Scholar]
- 8. Islam RM, Bell RJ, Green S, Page MJ, Davis SR. Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. Lancet Diabetes Endocrinol. 2019;7(10):754‐766. [DOI] [PubMed] [Google Scholar]
- 9. Irwig MS. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol. 2017;5(4):301‐311. [DOI] [PubMed] [Google Scholar]
- 10. Irwig MS. Cardiovascular health in transgender people. Rev Endocr Metab Disord. 2018;19(3):243‐251. [DOI] [PubMed] [Google Scholar]
- 11. Baillargeon J, Urban RJ, Raji MA, et al. Testosterone prescribing among women in the USA, 2002-2017. J Gen Intern Med. 2020;35(6):1891‐1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Clin Endocrinol Metab. 2019;104(10):4660‐4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hughes L, Shireman TI, Hughto J. Privately insured transgender people are at elevated risk for chronic conditions compared with cisgender counterparts. Health Aff (Millwood). 2021;40(9):1440‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Blok CJ, Wiepjes CM, van Velzen DM, et al. Mortality trends over five decades in adult transgender people receiving hormone treatment: a report from the Amsterdam Cohort of Gender Dysphoria. Lancet Diabetes Endocrinol. 2021;9(10):663‐670. [DOI] [PubMed] [Google Scholar]
- 15. Korpaisarn S, Chiewchalermsri D, Arunakul J, Chinthakanan O, Poomthavorn P, Sriphrapradang C. Effects of testosterone treatment on transgender males: a single-institution study. SAGE Open Med. 2021. doi: 10.1177/20503121211051546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Irwig MS. Transgender care by endocrinologists in the United States. Endocr Pract. 2016;22(7):P832‐P836. [DOI] [PubMed] [Google Scholar]
- 17. Nota NM, Wiepjes CM, de Blok CJM, Gooren LJG, Kreukels BPC, den Heijer M. Occurrence of acute cardiovascular events in transgender individuals receiving hormone therapy. Circulation. 2019;139(11):1461‐1462. [DOI] [PubMed] [Google Scholar]
- 18. Wierckx K, Mueller S, Weyers S, et al. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med. 2012;9(10):2641‐2651. [DOI] [PubMed] [Google Scholar]
- 19. Wierckx K, Elaut E, Declercq E, et al. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol. 2013;169(4):471‐478. [DOI] [PubMed] [Google Scholar]
- 20. Lopez DS, Mulla JS, El Haddad D, et al. Testosterone replacement therapy in relation with cardiovascular disease in cisgender women and transgender people. DRAFT VERSION ed: Harvard dataverse; 2023; deposited June 5, 2023. 10.7910/DVN/DVEVWC [DOI] [PMC free article] [PubMed]
- 21. MCHP SAS macros. University of Manitoba . Accessed July 10, 2023. http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?conceptID=1048
- 22. Worboys S, Kotsopoulos D, Teede H, McGrath B, Davis SR. Evidence that parenteral testosterone therapy may improve endothelium-dependent and -independent vasodilation in postmenopausal women already receiving estrogen. J Clin Endocrinol Metab. 2001;86(1):158‐161. [DOI] [PubMed] [Google Scholar]
- 23. Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F, Pujia A. Endogenous testosterone and endothelial function in postmenopausal women. Coron Artery Dis. 2007;18(1):9‐13. [DOI] [PubMed] [Google Scholar]
- 24. Sievers C, Klotsche J, Pieper L, et al. Low testosterone levels predict all-cause mortality and cardiovascular events in women: a prospective cohort study in German primary care patients. Eur J Endocrinol. 2010;163(4):699‐708. [DOI] [PubMed] [Google Scholar]
- 25. Maggio M, Cattabiani C, Lauretani F, et al. The relationship between sex hormones, sex hormone binding globulin and peripheral artery disease in older persons. Atherosclerosis. 2012;225(2):469‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maraka S, Singh Ospina N, Rodriguez-Gutierrez R, et al. Sex steroids and cardiovascular outcomes in transgender individuals: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2017;102(11):3914‐3923. [DOI] [PubMed] [Google Scholar]
- 27. Elamin MB, Garcia MZ, Murad MH, Erwin PJ, Montori VM. Effect of sex steroid use on cardiovascular risk in transsexual individuals: a systematic review and meta-analyses. Clin Endocrinol (Oxf). 2010;72(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 28. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(Supplement):3-IV-18. [DOI] [PubMed] [Google Scholar]
- 29. Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176‐184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request (Optum Clinformatics is not publicly available). The authors confirm that data used in developing this article are available on reasonable request to the corresponding author. This study used the linked database Optum's deidentified Clinformatics Data Mart Database (2007-2021). The interpretation and reporting of these data are the sole responsibility of the authors.