Abstract
Context
Total thyroidectomy in pediatric papillary thyroid carcinoma (PTC) is recommended in national guidelines because of the high incidence of multifocal disease (MFD).
Objective
To determine the incidence of MFD in childhood and adolescent vs adult PTC and whether MFD is a predictor for poorer outcomes in childhood and adolescent PTC.
Methods
We conducted an institutional review board-approved review of patients with PTC undergoing surgery (1986-2021) at Memorial Sloan Kettering Cancer Center. Clinical and pathological characteristics in patients with unifocal disease (UFD) and MFD were compared using Pearson's χ2 test. Survival outcomes were analyzed using the Kaplan-Meier method and log-rank test. Multivariate analysis assessed the impact of MFD on outcome.
Results
MFD was less common in childhood and adolescent patients with PTC (45%; 127/283) than in adults (54%; 3023/5564; P = .002). Childhood and adolescent patients with UFD and MFD had similar tumor stage and PTC subtype at presentation, with no significant difference in histopathologic features. Median follow-up was 68 months. There was no significant difference in 5-year recurrence-free probability and overall survival was 100% in both groups. There was no significant difference in 5-year contralateral lobe PTC-free probability between patients with UFD and MFD treated with lobectomy. Multivariate analysis showed MFD was not a predictor for recurrence.
Conclusion
MFD was less common in childhood and adolescent patients with PTC than adults and was not a predictor of poor outcome on multivariate analysis, with excellent long-term outcomes in all patients with PTC. MFD does not appear to warrant completion thyroidectomy in childhood and adolescent patients selected for lobectomy.
Keywords: pediatric thyroid carcinoma, papillary thyroid carcinoma, multifocality
Papillary thyroid carcinoma (PTC) accounts for approximately 90% of pediatric thyroid cancer (1). PTC is the most common form of thyroid cancer in children, and over recent decades, there has been great interest in determining the optimal treatment guidelines for this disease. In 2015, the American Thyroid Association (ATA) provided several recommendations for managing pediatric differentiated thyroid carcinoma, including PTC. Recommendation 11 specifically cited several factors to justify performing total thyroidectomy for most children with PTC (2). One such factor was the high rate of multifocal disease (MFD) reported in pediatric PTC. Multifocality may present clinically as part of macroscopic bilateral disease or, more commonly, occur incidentally as microscopic foci detected only on histological examination. Interestingly, a thorough review of the literature revealed a paucity of data on the true incidence of multifocality in pediatric patients and its subsequent effect on oncologic outcomes. For example, MFD was found in 46% of pediatric patients with PTC in a recent multicenter study but the association between MFD and long-term outcomes was not assessed (3). Although the ATA guidelines recommend total thyroidectomy in the majority of pediatric patients with PTC, the management of MFD remains controversial, with some authors advocating for a more conservative surgical approach (4, 5). A review of National Cancer Database statistics also demonstrates the increasing use of thyroid lobectomy in the pediatric population (6). In adult patients, multifocality is not a mandatory indication for completion thyroidectomy because there is no difference in risk of contralateral lobe PTC, regional recurrence, or survival in comparison with unifocal disease (UFD) (7). Further studies are necessary to assess the impact of MFD on pediatric patients with PTC. The objectives of our study were to report the incidence of MFD in childhood and adolescent patients compared with adults with PTC at a tertiary cancer center. We also sought to determine (1) if MFD is a predictor for higher recurrence and poorer outcome in childhood and adolescent PTC and (2) the rate of contralateral lobe PTC in patients selected to have thyroid lobectomy.
Materials and Methods
Patient Selection
We obtained institutional review board approval and a waiver of informed consent from Memorial Sloan Kettering Cancer Center (institutional review board number: 16-160). Our research was completed in accordance with the Declaration of Helsinki as revised in 2013. We performed a retrospective review of a prospectively maintained database of patients undergoing surgery for thyroid malignancy between 1986 and 2021 at Memorial Sloan Kettering Cancer Center. A total of 299 childhood and adolescent patients and 5775 adult patients with a diagnosis of PTC were identified. Childhood and adolescent patients were defined using the American Academy of Pediatrics definition as patients 21 years of age or younger at the time of surgery (8). For our subgroup analysis of thyroid lobectomy patients, immediate completion thyroidectomies, defined as being performed within 12 months of thyroid lobectomy, were excluded.
Data Collection
Data were collected on clinical (sex, age) pathological (TNM stage, lymphovascular invasion, extrathyroidal extension, surgical margin, focality, extranodal extension, tumor encapsulation), and treatment characteristics.
Clinical Endpoints
Regional recurrence-free probability, disease-specific survival (DSS), and overall survival were calculated in months from the date of initial surgery. The follow-up interval was until the most recent office visit with a member of the institution's disease management team. Regional recurrence-free probability was calculated to the first date indicating a contralateral lobe PTC or structural recurrence. A recurrence was recorded if identified as (1) cytologically confirmed recurrence, (2) imaging “consistent with,” “suspicious for,” or “probably” a PTC or recurrence, or (3) biochemical evidence of disease. The use of these keywords on imaging reports at our institution aims to give a level of diagnostic certainty of >75%. For patients managed by thyroid lobectomy, we also calculated the contralateral lobe PTC-free probability (CLPFP).
Statistical Analysis
We performed statistical analysis using R version 4.2.0 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2022). Median and interquartile range (IQR) were used for averages in the case of unequal distribution. Pearson χ2 test and Fisher exact test were used to compare categorical variables between groups, the latter if number of events was less than 5. Wilcoxon rank sum test was used to compare continuous variables between groups. CLPFP, overall survival, DSS, and recurrence-free survival (RFS) were calculated using the Kaplan-Meier method. Differences in survival were assessed using the log-rank test. Unadjusted and adjusted hazard ratios (HR) were calculated using the Cox proportional hazards regression model. Factors found to be significant (P value <.05) in univariate analysis were included in multivariate analysis.
Results
Comparison of Pathological Characteristics Between Childhood and Adolescent and Adult PTC
Table 1 compares childhood and adolescent against adult PTC patient and tumor characteristics. Childhood and adolescent patients with PTC had a significantly higher T stage at presentation (P < .001). There was a significant difference in N stage between childhood and adolescent, and adult populations (P < .001); childhood and adolescent patients were more likely to present with N1a (childhood and adolescent 28% vs adult 18%) or N1b disease (childhood and adolescent 34% vs adult 13%). A total of 4.7% of childhood and adolescent patients presented with distant metastases, compared with 1.1% of adults (P < .001)
Table 1.
. Patient and tumor characteristics in childhood and adolescent and adult PTC
| Variable | CAA n = 299a | % | Adult n = 5775a | % | P valueb |
|---|---|---|---|---|---|
| Age (y) a | 19 [16-20] | — | 48 [38-58] | — | <.001* |
| Sex | |||||
| Male | 69 | 23% | 1613 | 28% | .067 |
| Female | 230 | 77% | 4162 | 72% | |
| pT stage | |||||
| T1 | 172 | 58% | 4125 | 72% | <.001* |
| T2 | 74 | 25% | 1004 | 17% | |
| T3 | 33 | 11% | 420 | 7.3% | |
| T4 | 17 | 5.7% | 213 | 3.7% | |
| pN stage | |||||
| N0 | 113 | 38% | 3956 | 69% | <.001* |
| N1a | 84 | 28% | 1036 | 18% | |
| N1b | 102 | 34% | 777 | 13% | |
| M stage at diagnosis | |||||
| M0 | 285 | 95% | 5713 | 99% | <.001* |
| M1 | 14 | 4.7% | 62 | 1.1% | |
| Gross ETE | |||||
| No | 254 | 86% | 5179 | 90% | <.001* |
| Yes | 43 | 14% | 596 | 10% | |
| Microscopic ETE | |||||
| No | 193 | 65% | 4068 | 71% | .050 |
| Yes | 103 | 35% | 1699 | 29% | |
| Margins | |||||
| Negative | 221 | 82% | 4964 | 91% | <.001* |
| Positive | 48 | 18% | 521 | 9.5% | |
| Multifocality | |||||
| No | 156 | 55% | 2541 | 46% | .002* |
| Yes | 127 | 45% | 3023 | 54% | |
| Lymphovascular invasion | |||||
| No | 197 | 74% | 4698 | 88% | <.001* |
| Yes | 71 | 26% | 665 | 12% | |
| Tumor encapsulation | |||||
| None | 119 | 46% | 2037 | 40% | <.001* |
| Partial | 86 | 33% | 1413 | 28% | |
| Complete | 55 | 21% | 1660 | 32% | |
Abbreviations: ETE, extrathyroidal extension; M, metastasis; pN, pathological node; PTC, papillary thyroid carcinoma.
a Median (interquartile range); n (%).
b Wilcoxon rank sum test; Pearson χ2; Fisher exact test. Missing observations excluded from counts. Percents rounded to 2 significant figures.
*Denotes significant P value.
Of the 283 childhood and adolescent patients that had focality reported on histopathology, 45% (127/283) had MFD and 55% (156/283) had UFD. In comparison, 54% (3023/5564) of adult patients with PTC had MFD and 46% (2541/5564) had UFD. The greater incidence of MFD in adults compared with childhood and adolescents was statistically significant (P = .002).
Lymphovascular invasion (26% childhood and adolescent vs 12% adult; P < .001) and positive margins (18% childhood and adolescent vs 9.5% adult; P < .001) were more common in childhood and adolescent PTC. Complete tumor encapsulation was more common in adult PTC (21% pediatric vs 32% adult; P < .001). There was no significant difference in microscopic extrathyroidal extension (35% pediatric vs 29% adult; P = .050).
Impact of MFD on Recurrence in Pediatric PTC
Median follow-up was 68 months (IQR, 36-119 months) in childhood and adolescent patients with PTC. Univariate and multivariate analysis for RFS in childhood and adolescent PTC is shown in Table 2. pN stage (HR, 6.73; P < .001), extranodal extension (HR, 5.08; P < .001), pT stage (HR, 3.28; P < .001), positive margins (HR, 2.97; P < .001), gross extrathyroidal extension (HR, 2.96; P < .001), diffuse sclerosing PTC (HR, 2.52; P = .032), and multifocality (HR, 2.31; P = .009) conferred worse RFS. However, no factors were significant after multivariate analysis. Multifocality was not an independent risk factor for recurrence (HR, 1.15; P = .7).
Table 2.
. Univariate and multivariate analysis of recurrence-free survival among childhood and adolescent PTC, n = 299
| Factor | Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age | (Continuous) | 0.96 | 0.88-1.05 | .354 | NA | ||
| Sex | Male | REF | NA | ||||
| Female | 0.59 | 0.32-1.08 | .089 | ||||
| Total thyroidectomy | No | REF | REF | ||||
| Yes | 1.51 | 0.75-3.05 | .245 | 0.65 | 0.21-2.04 | .5 | |
| pT stage | T1/2 | REF | REF | ||||
| T3/4 | 3.28 | 1.80-5.97 | <.001* | 2.17 | 0.95-4.97 | .067 | |
| pN stage | N0 | REF | REF | ||||
| N1a/N1b | 6.73 | 2.41-18.8 | <.001* | 3.00 | 0.96-14.4 | .057 | |
| Gross ETE | No | REF | |||||
| Yes | 2.96 | 1.58-5.53 | <.001* | ||||
| Microscopic ETE | No | REF | NA | ||||
| Yes | 2.39 | 1.33-4.29 | .003* | ||||
| Margins | Negative | REF | NA | ||||
| Positive | 2.97 | 1.56-5.66 | <.001* | ||||
| Multifocality | No | REF | REF | ||||
| Yes | 2.31 | 1.23-4.36 | .009* | 1.15 | 0.56-2.38 | .7 | |
| LVI | No | REF | NA | ||||
| Yes | 1.52 | .80-2.88 | .197 | ||||
| ENE | No | REF | REF | ||||
| Yes | 5.08 | 2.56-10.1 | <.001* | 2.13 | 0.90-5.04 | .084 | |
| Histologic subtype | Classical PTC | REF | NA | ||||
| DSPTC | 2.52 | 1.08-5.88 | .032* | ||||
| FPTC | 0.44 | 0.15-1.27 | .128 | ||||
| Unknown | 1.55 | 0.71-3.35 | .269 | ||||
| Solid/Trabecular | 1.53 | 0.21-11.3 | .680 | ||||
| TCV | 0.62 | 0.15-2.63 | .516 | ||||
Abbreviations: DSPTC, diffuse sclerosing PTC; ETE, extrathyroidal extension; ENE, extranodal extension; FPTC, follicular PTC; HR, hazard ratio; LVI, lymphovascular invasion; pN, pathological node; PTC, papillary thyroid carcinoma; pT, pathological tumor; TCPTC, tall cell PTC.
*Denotes significant P value.
Clinical and Pathology Characteristics of Thyroid Lobectomy Patients
Sixty-six (22%) childhood and adolescent patients with PTC were managed by thyroid lobectomy after excluding immediate completion thyroidectomies (defined as within 12 months of lobectomy). Of these, 24% (16/66 patients) had MFD and 76% (50/66) had UFD. Table 3 describes the patient and tumor characteristics of the 2 groups (UFD vs MFD). Patients with UFD and MFD were similar in age, sex, T stage, M stage, and subtype. No patients had distant metastases in either group, so all were classified as stage I according to the American Joint Committee on Cancer 8th Edition TNM staging system (9). There was no difference in presence of lymphovascular invasion, microscopic extrathyroidal extension, and margin status.
Table 3.
. Patient and tumor characteristics in UFD and MFD childhood and adolescent lobectomy patients, n = 66
| Variable | UFD n = 50 | % | MFD n = 16 | % | P valueb |
|---|---|---|---|---|---|
| Age (y) | 18.4 (15.2, 20.2)a | — | 19.0 (17.6, 21.0)a | — | .2 |
| Sex | |||||
| Male | 9 | 18 | 2 | 13 | >.9 |
| Female | 41 | 82 | 14 | 88 | |
| pT stage | |||||
| T1 | 36 | 72 | 12 | 80 | .595 |
| T2 | 12 | 24 | 2 | 13 | |
| T3 | 2 | 4 | 1 | 6.7 | |
| T4 | 0 | 0 | |||
| pN stage | |||||
| N0 | 39 | 78 | 9 | 56 | .017* |
| N1a | 10 | 20 | 3 | 19 | |
| N1b | 1 | 2.0 | 4 | 25 | |
| M stage | |||||
| M0 | 50 | 100 | 16 | 100 | NA |
| M1 | 0 | 0 | |||
| ATA pediatric risk | |||||
| Low | 46 | 92 | 12 | 75 | .023* |
| Intermediate | 3 | 6.0 | 0 | ||
| High | 1 | 2.0 | 4 | 25 | |
| PTC subtype | |||||
| Classical | 26 | 53 | 8 | 80 | .5 |
| Follicular | 15 | 31 | 1 | 10 | |
| Tall cell | 7 | 14 | 1 | 10 | |
| Solid/trabecular | 1 | 2.0 | 0 | ||
| LVI | |||||
| No | 46 | 92 | 11 | 92 | >.9 |
| Yes | 4 | 8.0 | 1 | 8.3 | |
| Microscopic ETE | |||||
| No | 45 | 90 | 11 | 73 | .2 |
| Yes | 5 | 10 | 4 | 27 | |
| Margins | |||||
| Negative | 46 | 98 | 10 | 91 | .3 |
| Positive | 1 | 2.1 | 1 | 9.1 | |
Missing observations excluded from counts. Percents rounded to 2 significant figures.
Abbreviations: ATA, American Thyroid Association; ETE, extrathyroidal extension; LVI, lymphovascular invasion; M, metastasis; MFD, multifocal disease; pN, pathological node; pT, pathological tumor; PTC, papillary thyroid carcinoma; UFD, unifocal disease.
a Median (interquartile range); n (%).
b Wilcoxon rank sum test; Pearson χ2 test; Fisher exact test.
*Denotes significant P value.
Among the lobectomy patients who did not have completion thyroidectomy, those with MFD were more likely to have N1b disease (1/50 UFD vs 4/16 MFD; P = .008), which translates to high risk under the ATA Pediatric Risk Stratification (2). The 4 patients in our MFD cohort who had lobectomy and N1b disease would have received total thyroidectomy under our current institutional practice. All 4 were operated on between 1986 and 1993 and had lobectomy and modified radical neck dissection despite a preoperative diagnosis of lateral lymph node disease. Nevertheless, none of these patients recurred or developed contralateral lobe PTC. Two patients (2/16) in the MFD group had contralateral lobe ablation with radioactive iodine (RAI) in years 1986 and 1993, 29 mCi and 52 mCi, respectively. Neither of these had recurrence of disease. No patients (0/50) in the unifocal disease group had RAI. The use of RAI was not statistically significant (12.5% in MFD vs 0% in UFD; P = .12)
Incidence of Contralateral Lobe Disease in Child and Adolescent Lobectomy Patients With MFD and UFD
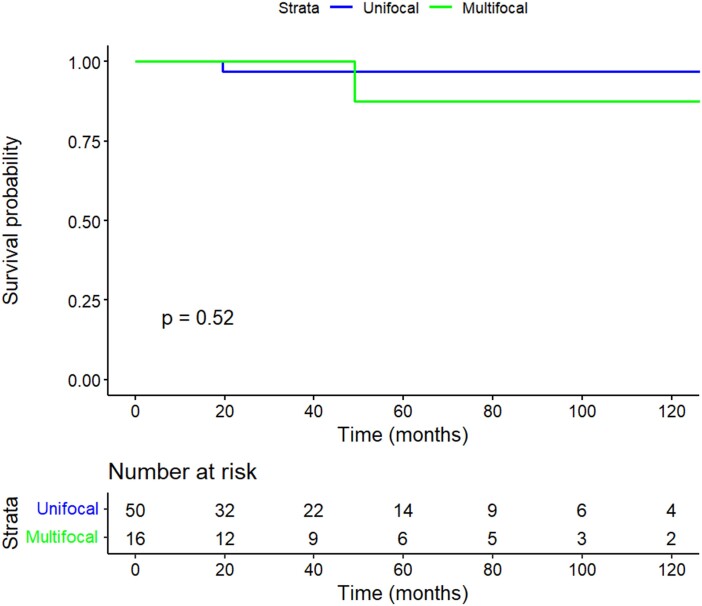
With a median follow-up of 41 months (IQR, 24-87), contralateral lobe carcinoma was detected in 1 patient with UFD and 1 patient with MFD during the study period. There was no significant difference in 5- and 10-year CLPFP rates between the UFD and MFD groups, as shown in Fig. 1 (5- and 10-year CLPFP: 97.0% UFD vs 87.5% MFD; P = .5). The patient with UFD had contralateral lobe PTC 19.6 months after lobectomy and the patient with MFD had contralateral lobe PTC 49 months after lobectomy. Table 4 shows the 2 patients who had contralateral lobe PTC, with details on the time of diagnosis, management, and outcome.
Figure 1.
Kaplan-Meier of 10-year contralateral lobe PTC-free probability by focality.
Table 4.
Details and outcomes of patients with contralateral lobe PTC
| Patient | Focality status | Time to contralateral lobe PTC (months) | Management of contralateral lobe PTC | Outcome—ATA response to treatment |
|---|---|---|---|---|
| 1 | UFD | 19.6 | Surgery | NED—excellent |
| 2 | MFD | 49 | Surgery | ALD—structural incomplete |
Abbreviations: ALD, alive with locoregional disease; ATA, American Thyroid Association; MFD, multifocal disease; NED, no evidence of disease; PTC, papillary thyroid carcinoma; UFD, unifocal disease.
Oncologic Outcome of MFD and UFD Child and Adolescent Lobectomy Patients
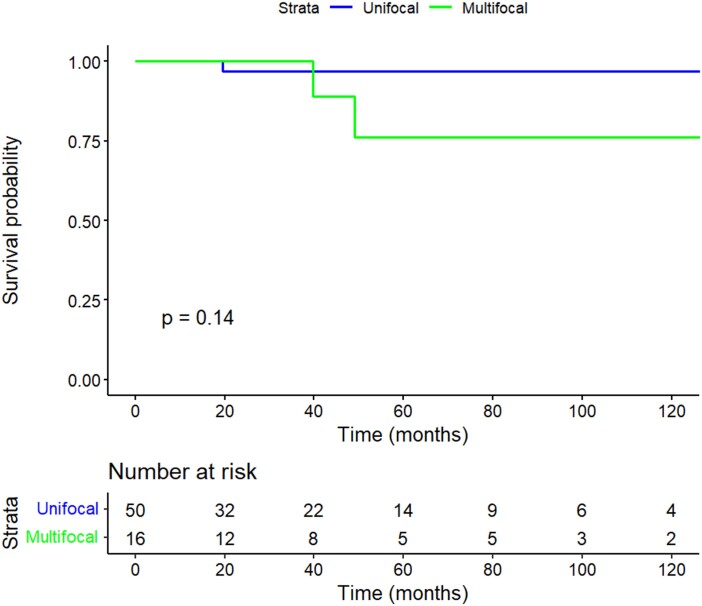
Of the patients who had lobectomy, those with MFD had rates of recurrence comparable with patients with UFD. During the follow-up period, there were no distant recurrences in either group. In the UFD group, 1 patient developed local and regional recurrence. In the MFD group, 1 patient developed local and regional recurrence and 1 patient developed regional recurrence. There was no significant difference in 5- and 10-year RFS between the UFD and MFD groups, as shown in Fig. 2 (5- and 10-year RFS: 97.0% UFD vs 76.2% MFD; P = .14)
Figure 2.
Kaplan-Meier plot of 10-year recurrence-free survival stratified by focality.
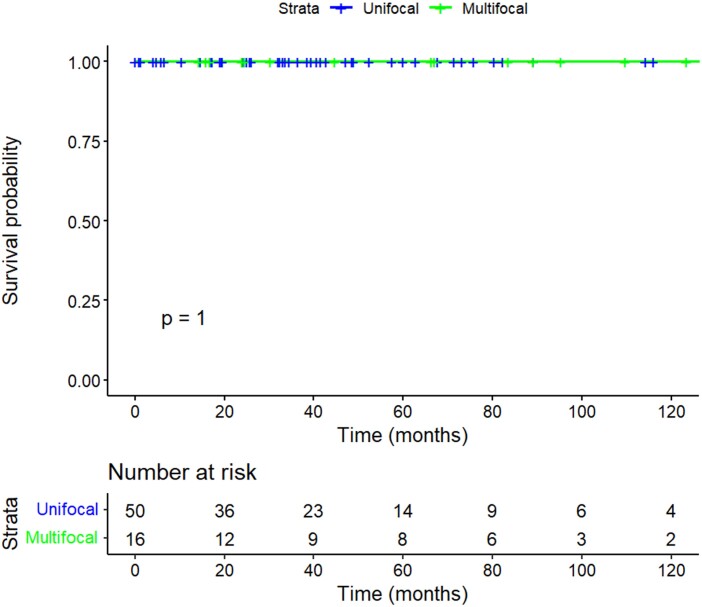
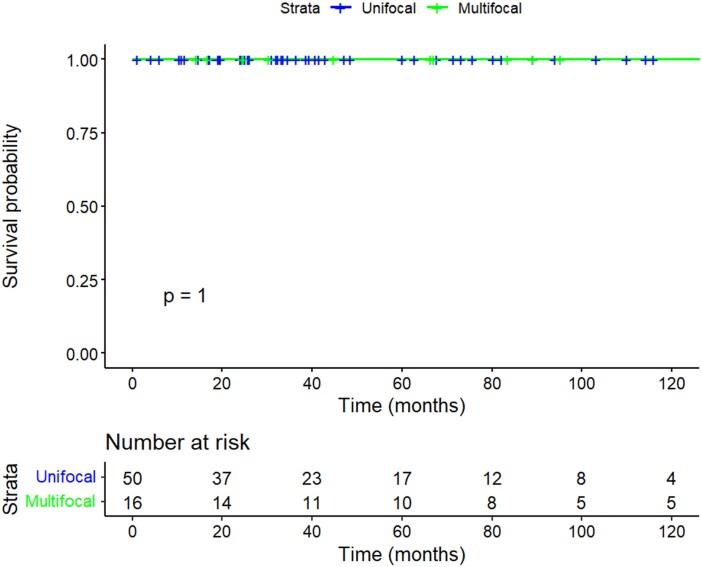
Of the 3 patients who developed locoregional recurrence, 2 underwent surgery and had no evidence of disease at last follow-up, both 22 months after surgery. One patient underwent surgery and had evidence of further recurrence in level 6 that did not progress under active surveillance. Table 5 shows the 3 patients who developed local or regional recurrence, with details on time to recurrence, management, and outcome. There were no disease-specific deaths in either group (Fig. 3; 5 and 10-year DSS 100% UFD vs 100% MFD, P = 1). The 5- and 10-year overall survival was 100% in both groups (Fig. 4; P = 1). One lobectomy patient with UFD had a biochemically incomplete response to treatment and biochemical evidence of disease at the last follow-up 115 months postsurgery, without structural evidence of disease.
Table 5.
Details and outcomes of patients with locoregional recurrence
| Patient | Focality status | Histological details | Time to recurrence (months) | Location of recurrence | Management of recurrence | Outcome—ATA response to treatment |
|---|---|---|---|---|---|---|
| 1 | UFD | Tall cell PTC, 1.6 cm, no VI, no ETE, negative margins | 19.6 | Ipsilateral level 4; ipsilateral thyroid bed; contralateral lobe | CT + CND + MRND | NED—excellent |
| 2 | MFD | cPTC, 1.7 cm, no VI, no ETE, negative margins | 49.0 | Ipsilateral level 4 and 6; contralateral lobe | CT + CND + MRND | ALD—structural incomplete |
| 3 | MFD | cPTC with tall cell features, 1 cm, no VI, no ETE, negative margins | 39.7 | Ipsilateral level 3 | MRND | NED—excellent |
Abbreviations: ALD, alive with locoregional disease; ATA, American Thyroid Association; CND, central neck dissection; cPTC, classical PTC; CT, completion thyroidectomy; ETE, extrathyroidal extension; MFD, multifocal disease; MRND, modified radical neck dissection; NED, no evidence of disease; UFD, unifocal disease; VI, vascular invasion.
Figure 3.
Kaplan-Meier plot of 10-year disease-specific survival stratified by focality.
Figure 4.
Kaplan-Meier plot of 10-year overall survival stratified by focality.
Discussion
In this study, we have used multivariate analysis of RFS to show that multifocality is not an independent prognostic factor for recurrence in childhood and adolescent PTC. Patients with MFD and UFD had 100% survival rates, with no disease-specific deaths. Our findings of the impact of multifocality on outcome are supported by 2 other studies that have reported multifocality to be unassociated with persistent disease in pediatric patients treated with total thyroidectomy and RAI (10, 11). All patients in these studies were treated with total thyroidectomy, whereas our study is novel because it compares outcomes of patients with UFD and MFD treated with lobectomy, including a comparison of contralateral lobe PTC-free probability. We found that those with MFD were not more likely to develop recurrence or contralateral lobe PTC. The lack of effect of multifocality on long-term outcome echoes findings in adult PTC and suggests MFD does not justify completion thyroidectomy in patients treated with thyroid lobectomy (7, 12). Our finding of lobectomy being an effective treatment for patients with microscopic MFD is supported by 1 other study of 276 pediatric multifocal PTC patients, which showed no difference in RFS between lobectomy and total thyroidectomy patients (5).
The presumed high incidence of multifocality in pediatric PTC is 1 of the key reasons for the recommendation of total thyroidectomy in pediatric patients (2). However, before our current study, robust data regarding the incidence of MFD in the pediatric vs adult population were lacking, alongside its effect on contralateral lobe PTC, recurrence, and disease-specific survival. Our data from 283 childhood and adolescent and 5564 adult patients suggest that MFD is more common in the adult population (childhood and adolescent MFD 45% vs adult MFD 54%; P = .002). The MFD incidence of 45% is equivalent to the 46% found in a multicenter study of 212 pediatric patients, but that study lacked comparison with an adult cohort and did not assess long-term outcomes (3). Other studies have reported similar or increased rates of MFD in pediatric patients with PTC. For example, Alzahrani et al (2016) did not find any significant difference in the incidence of multifocality in their analysis of 97 pediatric and 213 adult patients (43.3 vs 34.4; P = .26) (13). Kim et al (2012) suggested MFD was more common in children (31%; aged 10-18 years) than young adults (15.6%; aged 19-24 years), although this was not statistically significant (P = .224) (14). Our study uses a very large cohort of adult patients undergoing surgery at a single institution and therefore gives a high degree of accuracy in the true incidence of MFD in both the adult and childhood and adolescent populations. Because of the size of the cohorts and the expert pathological review by pathologists who specialize in thyroid cancer, our data likely represent the true figures for MFD in the childhood and adolescent and adult populations and show clearly that MFD is less common in childhood and adolescent PTC.
In the current study, MFD was not associated with other potentially aggressive pathological features such as lymphovascular invasion, extrathyroidal extension, and positive margins. The 4 MFD lobectomy patients who had N1b disease were managed between 1986 and 1993 when lobectomy for N1b was practiced at our institution. If these patients had been managed more recently, they would have undergone total thyroidectomy because of the presence of lateral cervical nodal metastases and thus excluded from the thyroid lobectomy analysis.
Our study is not designed to assess the impact of histological subtypes, such as diffuse sclerosing PTC or tall cell PTC, on outcomes. All patients with diffuse sclerosing PTC underwent total or completion thyroidectomy. The 3 locoregional recurrences in lobectomy patients occurred in tall cell PTC, classical PTC, and classical PTC with tall cell features. These numbers are too low to draw conclusions on the impact of subtypes.
The complications associated with total thyroidectomy in pediatric patients have been well documented. LaQuaglia et al (1988) reported in their retrospective review of 93 patients over a 35-year period that total thyroidectomy increased the risk of major complications compared with lobectomy (P < .01) (4). The same study reported no deaths from disease and recommended the avoidance of total or subtotal thyroidectomy. These conclusions were supported by the Children's Cancer Group, which cited a temporary and permanent hypocalcemia rate of 29% and 12%, respectively, associated with the use of subtotal or total thyroidectomy (P = .001) (15). Postoperative recurrent laryngeal nerve paralysis was seen temporarily in 12% and permanently in 2% of patients. Routine total or subtotal thyroidectomy did not improve patient outcomes in localized tumors; there were 2 deaths from disease of 329 patients. The risk of complications and lack of clear oncological benefit in patients with low-risk childhood and adolescent PTC suggest any recommendation for total thyroidectomy should be supported by other adverse patient and tumor characteristics. The lack of clear oncologic benefit of total thyroidectomy is likely to be 1 of the factors underlying the findings of a National Cancer Database review of 4776 pediatric patients with differentiated thyroid cancer that showed hemithyroidectomy utilization has increased significantly since 2015 (6).
Our study has several limitations to consider. First, this is a retrospective review of a single center's clinical experience. Though our center is a high-volume institution and a tertiary cancer center, future multi-institutional studies will likely be necessary to confirm these findings. Second, the management of childhood and adolescent PTC has evolved over time, with our center favoring a more conservative approach to low-risk PTC treatment in recent years. Third, the use of serum thyroglobulin to monitor for recurrence was not universal until 2000 and, therefore, we have no data before 2000. However, reports have found using serum thyroglobulin to monitor for biochemical recurrence during follow-up of lobectomy patients to be of limited utility (16). In addition, the only patients with biochemical evidence of recurrence in our study also had structural recurrence. Last, there is inconsistency regarding the defined age cutoff for pediatric patients (2, 5). For this reason, it may be considered preferable to refer to our patient group as children and adolescents. In our study, we chose to use the American Academy of Pediatrics definition (8) as patients ≤ 21 years of age because recent studies on growth rate have confirmed the indolent nature of PTC, meaning it is highly likely the disease existed for years before presentation (17). This is particularly true in patients who present with palpable disease, which was the reason for presentation in 84% (249/296) of our patients. This is echoed by Sugino et al (2022), who assessed age cutoffs of 18 and 14 years and found that age was not significantly related to clinical outcome, although clinical characteristics may differ (18). They also concluded that age at presentation does not necessarily represent the age of occurrence because of the indolent behavior of DTC.
Conclusions
Our study has shown that multifocality in childhood and adolescent PTC is less common than the 65% quoted in national guidelines and is significantly less common than in adult patients. We found that MFD was not a predictor of poor outcomes on multivariate analysis, and long-term outcomes are excellent in childhood and adolescent PTC regardless of focality. This highlights the need for further debate regarding the indications for total thyroidectomy in childhood and adolescent patients and provides evidence to support the use of lobectomy in low-risk PTC with microscopic MFD.
Acknowledgments
D.W.S. was supported by The Dowager Countess Eleanor Peel Trust and The Colledge Family Memorial Fellowship Fund to undertake a Research Fellowship for 12 months.
Abbreviations
- ATA
American Thyroid Association
- CLPFP
contralateral lobe papillary thyroid carcinoma-free probability
- DSS
disease-specific survival
- HR
hazard ratio
- IQR
interquartile range
- MFD
multifocal disease
- PTC
papillary thyroid carcinoma
- RAI
radioactive iodine
- RFS
recurrence-free survival
- UFD
unifocal disease
Contributor Information
Daniel W Scholfield, Department of Surgery, Head and Neck Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Joseph Lopez, Division of Pediatric Head & Neck Surgery, AdventHealth for Children, Orlando, FL 32803, USA.
Alana Eagan, Department of Surgery, Head and Neck Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Zoltan Antal, Department of Pediatrics, Endocrinology Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
R Michael Tuttle, Department of Medicine, Endocrinology Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Ronald Ghossein, Department of Pathology and Laboratory Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Michael LaQuaglia, Department of Surgery, Pediatric Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Ashok R Shaha, Department of Surgery, Head and Neck Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Jatin P Shah, Department of Surgery, Head and Neck Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Richard J Wong, Department of Surgery, Head and Neck Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Snehal G Patel, Department of Surgery, Head and Neck Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Ian Ganly, Department of Surgery, Head and Neck Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Funding
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748.
Author Contributions
D.W.S.: Conceptualization (supporting); data collection (lead); methodology (equal); formal analysis (equal); writing—original draft (equal); and writing—review and editing (equal). J.L.: Conceptualization (supporting); methodology (supporting); formal analysis (supporting); writing—original draft (equal); and writing—review and editing (equal). A.E.: Formal analysis (equal) and writing—review and editing (equal). Z.A.: Formal analysis (supporting) and writing—review and editing (equal). R.M.T.: Formal analysis (supporting) and writing—review and editing (equal). R.G.: Histopathology review and analysis (lead); formal analysis (supporting); and writing—review and editing (equal). M.L.: Formal analysis (supporting) and writing—review and editing (equal). A.R.S.: Formal analysis (supporting) and writing—review and editing (equal). J.P.S.: Formal analysis (supporting) and writing—review and editing (equal). R.J.W.: Formal analysis (supporting) and writing—review and editing (equal). S.G.P.: Methodology (equal); formal analysis (supporting); and writing—review and editing (equal). I.G.: Conceptualization (lead); methodology (lead); formal analysis (lead); writing—original draft (equal); and writing—review and editing (lead).
Disclosures
The authors have no conflicts of interest to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Research Ethics Statement
This research meets the ethics guidelines, including adherence to the legal requirements of the country where the study was performed.
References
- 1. Paulson VA, Rudzinski ER, Hawkins DS. Thyroid cancer in the pediatric population. Genes (Basel). 2019; 10(9):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Francis GL, Waguespack SG, Bauer AJ, et al. American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banik GL, Shindo ML, Kraimer KL, et al. Prevalence and risk factors for multifocality in pediatric thyroid cancer. JAMA Otolaryngol Head Neck Surg. 2021; 147(12):1100‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. La Quaglia MP, Corbally MT, Heller G, Exelby PR, Brennan MF. Recurrence and morbidity in differentiated thyroid carcinoma in children. Surgery. 1988;104(6):1149‐1156. [PubMed] [Google Scholar]
- 5. Chen J, Huang N, Ji Q, Wang Y, Zhu Y, Li D. Multifocal papillary thyroid cancer in children and adolescents: 12-year experience in a single center. Gland Surg. 2019;8(5):507‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stein E, Raval MV, Hazkani I, et al. The 2015 American Thyroid Association guidelines and trends in hemithyroidectomy utilization for pediatric thyroid cancer. Head Neck. 2022;44(8):1833‐1841. [DOI] [PubMed] [Google Scholar]
- 7. Harries V, Wang LY, McGill M, et al. Should multifocality be an indication for completion thyroidectomy in papillary thyroid carcinoma? Surgery. 2020;167(1):10‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hardin AP, Hackell JM, COMMITTEE ON PRACTICE AND AMBULATORY MEDICINE . Age limit of pediatrics. Pediatrics. 2017;140(3):e20172151. [DOI] [PubMed] [Google Scholar]
- 9. Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual, 8th ed. American College of Surgeons; 2018. [Google Scholar]
- 10. Cistaro A, Quartuccio N, Garganese MC, et al. Prognostic factors in children and adolescents with differentiated thyroid carcinoma treated with total thyroidectomy and RAI: a real-life multicentric study. Eur J Nucl Med Mol Imaging. 2022;49(4):1374‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zanella AB, Scheffel RS, Nava CF, et al. Dynamic risk stratification in the follow-up of children and adolescents with differentiated thyroid cancer. Thyroid. 2018; 28(10):1285‐1292. [DOI] [PubMed] [Google Scholar]
- 12. Kim H, Kwon H, Moon BI. Association of multifocality with prognosis of papillary thyroid carcinoma: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021;147(10):847‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alzahrani AS, Alkhafaji D, Tuli M, Al-Hindi H, Sadiq BB. Comparison of differentiated thyroid cancer in children and adolescents (≤20 years) with young adults. Clin Endocrinol. 2016;84(4):571‐577. [DOI] [PubMed] [Google Scholar]
- 14. Kim SS, Kim SJ, Kim IJ, Kim BH, Jeon YK, Kim YK. Comparison of clinical outcomes in differentiated thyroid carcinoma between children and young adult patients. Clin Nucl Med. 2012;37(9):850‐853. [DOI] [PubMed] [Google Scholar]
- 15. Newman KD, Black T, Heller G, et al. Differentiated thyroid cancer: determinants of disease progression in patients < 21 years of age at diagnosis: a report from the Surgical Discipline Committee of the Children's Cancer Group. Ann Surg. 1998;227(4):533‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chou R, Dana T, Brent GA, et al. Serum thyroglobulin measurement following surgery without radioactive iodine for differentiated thyroid cancer: a systematic review. Thyroid. 2022;32(6):613‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tuttle RM, Fagin J, Minkowitz G, et al. Active surveillance of papillary thyroid cancer: frequency and time course of the six most common tumor volume kinetic patterns. Thyroid. 2022;32(11):1337‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sugino K, Nagahama M, Kitagawa W, et al. Cutoff age between pediatric and adult thyroid differentiated cancer: is 18 years old appropriate? Thyroid. 2022 Feb;32(2):145‐152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.