Abstract
Context
Obesity is associated with nonalcoholic fatty liver disease (NAFLD). Sleeve gastrectomy (SG) is an effective means of weight loss and improvement of NAFLD in adults; however, data regarding the efficacy of SG in the early stages of pediatric NAFLD are sparse.
Objective
To assess the impact of SG on hepatic fat content 1 year after SG in youth with obesity compared with nonsurgical controls with obesity (NS).
Design
A 12-month prospective study in 52 participants (mean age, 18.2 ± .36 years) with obesity, comprising 25 subjects who underwent SG (84% female; median body mass index [BMI], 44.6 [42.1-47.9] kg/m2) and 27 who were NS (70% female; median BMI, 42.2 [38.7-47.0] kg/m2).
Main outcome measures
Hepatic fat content by computed tomography (liver/spleen ratio), abdominal fat by magnetic resonance imaging.
Results
Mean 12-month decrease in BMI was greater in SG vs NS (−12.5 ± .8 vs −.2 ± .5 kg/m2, P < .0001). There was a within-group increase in the liver-to-spleen (L/S) ratio in SG (.13 ± .05, P = .014) but not NS with a trend for a difference between groups (P = .055). All SG participants with an L/S ratio <1.0 (threshold for the diagnosis of NAFLD) before surgery had a ratio of >1.0 a year after surgery, consistent with resolution of NAFLD. Within SG, the 12-month change in L/S ratio was negatively associated with 12-month change in visceral fat (ρ = −.51 P = .016).
Conclusions
Hepatic fat content as assessed by noncontrast computed tomography improved after SG over 1 year in youth with obesity with resolution of NAFLD in all subjects. This was associated with decreases in visceral adiposity.
Keywords: nonalcoholic fatty liver disease (NAFLD), obesity, metabolic and bariatric surgery (MBS), sleeve gastrectomy, adolescents, computed tomography (CT)
Nonalcoholic fatty liver disease (NAFLD) is a progressive disease characterized by the accumulation of fat in the liver parenchyma, which can lead to steatohepatitis and eventually irreversible fibrosis or cirrhosis of the liver (1). Severe obesity is associated with an increased risk of developing NAFLD, now one of the most common liver diseases in children (2).
The North American Society for Pediatric Gastroenterology Hepatology and Nutrition recommends screening for NAFLD using alanine transaminase (ALT) levels in all children aged 9 to 11 years with obesity and in overweight children who have additional risk factors (2), with liver biopsy being the gold standard for the diagnosis of NAFLD. However, because of associated complications and the invasive nature of the procedure, noninvasive imaging modalities such as ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) are increasingly used to aid in the diagnosis, with MRI being the reference standard of choice (3, 4). Recent studies have established strong correlations between MRI- and CT-based hepatic fat quantification, allowing for the latter to accurately quantify hepatic fat (5, 6).
With the rising prevalence of NAFLD in children and adolescents and its possible link with cardiovascular complications, there is an urgent need for therapies that successfully manage this condition. Various pharmacological therapies continue to be under investigation, but there are currently no US Food and Drug Administration–approved treatments for NAFLD (2). Sleeve gastrectomy (SG) is the most commonly performed metabolic and bariatric surgery (MBS) in children with moderate to severe obesity and results in substantial weight loss associated with metabolic improvements (7). Studies in adults have shown that it also leads to the improvement of NAFLD (8). However, there are limited data regarding the impact of SG on the early stages of NAFLD in adolescents and young adults with obesity and the possible mechanisms that underlie these effects.
Our objective was to determine the impact of SG on hepatic fat content using CT in adolescents and young adults with obesity and to determine whether reductions in hepatic fat content are associated with alterations in markers of metabolic risk such as liver enzymes, body composition, and lipid levels. We hypothesized that SG would lead to significant reductions in hepatic fat content associated with reductions in liver enzymes and improvement in abdominal fat depots and lipid levels.
Methods
Study Design and Participant Selection
We enrolled 52 participants between the ages of 13 and 25 years with moderate-severe obesity (body mass index [BMI] ≥ 35 kg/m2 or ≥120% of the 95th percentile BMI for age and sex with ≥1 obesity-related comorbidity or a BMI ≥ 40 kg/m2 or ≥140% of the 95th percentile BMI for age and sex) in this study. Participants were recruited from obesity clinics in Boston and surrounding areas as well as institutional advertisements and college job boards and followed for 12 months. Twenty-five participants underwent SG and 27 were followed with routine nonsurgical care (NS).
Exclusion criteria for the study were (1) pregnancy or breastfeeding at the time of the study; (2) intake of any medication affecting bone, such as oral glucocorticoids, phenytoin, and phenobarbitone within 8 weeks of baseline visit (as the protocol included assessment of bone endpoints, not being reported in this manuscript); (3) intake of antipsychotic drugs known to cause weight gain if treated for less than 6 months or if the dose was not stable over the past 2 months, (4) untreated thyroid dysfunction, (5) smoking, and (6) contraindications for MRI.
Study Protocol
Participants undergoing SG were matched by age to NS controls. The baseline visit occurred before surgery, and a second visit occurred 12 months after surgery. Subjects in the NS group were evaluated at 2 time points 12 months apart. Written informed consent was obtained from all subjects 18 years and older. For subjects aged <18 years, assent was obtained from the subject and consent from the legal guardian. Self-reported race and ethnicity were documented. Approval was obtained from our institutional review board, and the study was conducted in compliance with Health Insurance Portability and Accountability regulations.
Study Measurements and Biochemical Analysis
At each visit, medical history was obtained and a physical examination was performed. Height was measured with a wall-mounted stadiometer as the mean of 3 measurements and weight was measured to the nearest .1 kg with an electronic scale.
Imaging
Participants underwent noncontrast CT of the upper abdomen (GE Healthcare, Waukesha, WI, USA) in the supine position with the following scan parameters: tube voltage of 120 kV, tube current of 100 mA, slice thickness of 2.5 mm, field of view of 500 mm, and table height of 144 mm. All scans were performed with a calibration phantom (Mindways Software, Inc., Austin, TX, USA). Liver and spleen attenuation was measured by placing a circular region of interest (ROI) in the periphery of the right hepatic lobe, avoiding vessels or artifacts, and in the caudal pole of the spleen, respectively. The size of the ROI was held constant at 200 mm2. Hepatic fat content was quantified by taking the attenuation measurement in Hounsfield units of the right liver lobe ROI and dividing it by the spleen Hounsfield units measurement and calculating the liver-to-spleen ratio (L/S ratio), where a lower L/S ratio indicates higher hepatic fat. Further, an L/S ratio <1 is indicative of hepatic steatosis (9, 10).
Single-slice MRI of the abdomen at 3T (Siemens Trio; Siemens Medical Systems, Erlangen, Germany) was performed using a T1-weighted fast spin-echo pulse sequence (slice thickness, 10 mm; repetition time, 300 ms; echo time, 12 ms; echo train, 4; matrix, 512 × 512) at the level of L4, and cross-sectional areas (cm2) of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT), were determined with commercial software (VITRAK, Merge/eFilm, Milwaukee, WI, USA).
Biochemical Analysis
Fasting blood samples were drawn to obtain a standard lipid panel (total cholesterol [TC], high-density lipoprotein [HDL], low-density lipoprotein [LDL], and triglycerides [TG]) and liver enzymes (ALT and aspartate transaminase [AST]). All assays were processed using spectrophotometry by Quest Diagnostics. Reported coefficients of variation (CVs) are 2.3%, 2.4%, <5%, and 2.6% for TC, HDL, LDL, and TG, respectively; reported sensitivity is 1 mg/dL for TC, HDL, and LDL, and 5 mg/dL for TG. The assay manufacturer, Beckman Coulter, reports a sensitivity of 5 IU/L for both AST and ALT, an intra-assay CV of 2% for AST and 2.1% for ALT, and total precision of less than 10% CV for both.
Statistical Analysis
JMP v16.0 was used for analysis. The normality of distribution was assessed using the Shapiro-Wilk test. Data are reported as mean ± standard error of the mean for normally distributed data or median (first quartile, third quartile) for data not normally distributed. For between-group comparisons, the Student t test or the Wilcoxon rank sum test was used depending on data distribution. For within-group comparison, we used either the paired t test or Wilcoxon signed rank test, depending on data distribution. Spearman correlation analysis was performed to determine associations between the 12-month change in L/S ratio on CT and the 12-month change in body composition, liver enzymes, and lipid levels. P < .05 was used to determine statistical significance and P < .1 was used to determine a trend.
Results
Baseline Characteristics and Changes Over 12 Months in Anthropometric Measures
Participants had a mean age of 18.2 ± .4 years and a median BMI of 42.8 kg/m2 (40.1, 48.0). Seventy-seven percent of the participants were females. Participants in the SG and NS groups did not differ for age or weight. BMI (but not BMI Z-score) trended higher in SG vs NS at baseline (Table 1). A positive history of alcohol intake was noted in 6 and 7 participants in the SG and NS groups, respectively, at baseline (P = .532) and in 9 and 7 participants in the SG vs NS groups at 12 months (P = .405). As expected, weight and BMI decreased significantly in the SG vs NS group, with a significant within-group decrease in weight and BMI following SG (Table 2).
Table 1.
Baseline characteristics of participants with obesity undergoing sleeve gastrectomy and nonsurgical controls with obesity
| Baseline characteristics | Sleeve gastrectomy (n = 25) | Nonsurgical controls (n = 27) | P |
|---|---|---|---|
| Age | 18.1 ± .5 | 18.2 ± .1 | .986 |
| Ethnicity, n (%) | .554 | ||
| Hispanic | 10 (40) | 13 (48.1) | |
| Non-Hispanic | 15 (60) | 14 (51.9) | |
| Race, n (%) | .621 | ||
| Black | 5 (20) | 4 (14.8) | |
| Non-Black | 20 (80) | 23 (85.2) | |
| Sex, n (%) | .240 | ||
| Male | 4 (16) | 8 (29.6) | |
| Female | 21 (84) | 19 (70.4) | |
| Weight, kg | 125.0 (111.2, 141.3) |
121.0 (101.3, 133.5) |
.153 |
| Height, cm | 167.0 ± 2.0 | 166.5 ± 1.6 | .830 |
| BMI, kg/m2 | 44.6 (42.1, 47.9) |
42.2 (38.7, 47.0) |
.071 |
| BMI Z score | 2.53 ± .06 | 2.49 ± .06 | .699 |
Abbreviation: BMI, body mass index.
Data represented as mean ± standard error of the mean or median (first quartile, third quartile) depending on data distribution.
Table 2.
Baseline and 12-month data, and change over 12 months in body composition, liver fat on CT, and biochemical parameters
| Baseline measures | P | 12M measures | P | Change over 12 months | Within group change P | Between-group change P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgical (n = 25) | Nonsurgical (n = 27) | Surgical (N = 25) | Nonsurgical (N = 27) | Surgical (N = 25) | Nonsurgical (N = 27) | ||||||
| Body composition and liver fat | |||||||||||
| BMI (kg/m2) | 44.2(41.3, 53.7) | 42.2(38.7, 47.0) | .073 | 31.5(27.8, 36.4) | 41.1(39.0, 47.0) | <.0001 | −12.5 ± .8 | −.2 ± .5 | <.0001 | .963 | <.0001 |
| Weight (kg) | 125.0(111.2, 141.3) | 121.0(102.4, 135.5) | .156 | 89.7(77.1, 108.5) | 122.4(101.9, 136.7) | <.0001 | −34.6 ± 2.4 | .8 ± 1.4 | <.0001 | .474 | <.0001 |
| SAT (cm2) | 700.4 ± 31.2(n = 21) | 695.0 ± 23.9(n = 27) | .892 | 382.6(260.9, 544.4)(n = 22) | 733.6(592.4, 788.4)(n = 26) | <.0001 | −291.4 ± 26.8(n = 21) | 13.6 ± 15.7(n = 26) | <.0001 | .378 | <.0001 |
| VAT (cm2) | 102.0(73.5, 125.6)(n = 21) | 97.1(57.5, 144.6)(n = 27) | .693 | 45.9(31.4, 61.6)(n = 22) | 112.0(74.7, 138.7)(n = 26) | <.0001 | −50.8(−61.3, −34.1)(n = 21) | 13.6(−10.3, 34.8)(n = 26) | <.0001 | .060 | <.0001 |
| Liver/spleen ratio | 1.04(.88, 1.19) | 1.07(.89, 1.22) | .784 | 1.14(1.06, 1.19) | 1.08(.86, 1.18) | .133 | .13 ± .05 | .02 ± .03 | .014 | .861 | .055 |
| Biochemical parameters | |||||||||||
| AST (U/L) | 18.0(13.0, 24.0)(n = 23) | 16.5(14.3, 22.5)(n = 24) | .941 | 13.0(11.3, 16.5)(n = 24) | 17.0(14.5, 22.0)(n = 25) | .010 | −2.0(−9.0; .0)(n = 23) | −1.0(−4.0; 2.0)(n = 23) | .002 | .626 | .082 |
| ALT (U/L) | 12.0(10.0, 22.0)(n = 23) | 13.0(9.5, 17.0)(n = 25) | .555 | 7.0(5.0, 12.3)(n = 24) | 11.5(9.0, 18.3)(n = 26) | .0008 | −5.0(−13.0; −1.0)(n = 23) | −1.0(−2.0; 2.5)(n = 25) | .0003 | .979 | .003 |
| TC (mg/dL) | 151.9 ± 4.7(n = 23) | 147.4 ± 5.9(n = 25) | .560 | 145.7 ± 5.5(n = 24) | 151.4 ± 6.1(n = 26) | .490 | −6.8 ± 4.2(n = 23) | 3.2 ± 4.8(n = 25) | .115 | .515 | .123 |
| HDL (mg/dL) | 44.1 ± 2.3(n = 23) | 44.9 ± 2.1(n = 25) | .788 | 49.9 ± 2.5(n = 24) | 46.1 ± 1.8(n = 26) | .220 | 6.2 ± 1.7(n = 23) | .8 ± 1.4(n = 25) | .001 | .581 | .019 |
| LDL (mg/dL) | 89.7 ± 4.4(n = 23) | 84.0 ± 4.4(n = 25) | .367 | 78.0(62.8, 92.8)(n = 24) | 86.0(65.3, 97.3)(n = 26) | .415 | −11.1 ± 3.3(n = 23) | 2.3 ± 4.2(n = 25) | .002 | .876 | .017 |
| TG (mg/dL) | 91.7 ± 7.7(n = 23) | 95.6 ± 6.6(n = 25) | .708 | 67.5(47.5, 99.0)(n = 24) | 91.0(70.0, 125.0)(n = 26) | .074 | −12.2 ± 5.7(n = 23) | 1.4 ± 6.3(n = 25) | .064 | .886 | .120 |
Abbreviations: ALT, alanine aminotransferase; AST; aspartate aminotransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SAT, subcutaneous adipose tissue; TC, total cholesterol; TG: triglyceride; VAT, visceral adipose tissue.
Data are represented as mean ± standard error of the mean or median (first quartile, third quartile) depending on data distribution. Significant P values are bolded.
Baseline Hepatic fat and Body Composition and Changes Over 12 Months
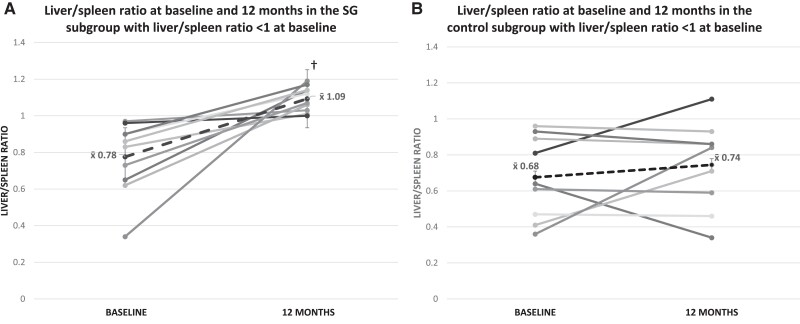
Baseline hepatic fat content did not differ between SG vs NS groups (Table 2). A significant increase in the L/S ratio, indicating a decrease in hepatic fat content, was noted within the SG group (P = .014) 12 months post-SG, and there was a trend for a significant difference between groups for changes in hepatic fat over 12 months (P = .055) (Table 2). These findings persisted after controlling for alcohol intake at baseline and follow-up. Of the 52 study participants, 19 (10 SG, 9 NS) had a baseline L/S ratio <1, indicating the presence of hepatic steatosis. In the SG group, in the 10 participants with a baseline L/S ratio of <1, the L/S ratio increased over 12 months to >1 in all participants (P = .002), indicating resolution of NAFLD (Fig. 1). Figure 2 shows the change in hepatic fat in a representative participant with an L/S ratio of <1 at baseline and changes 12 months following SG. In contrast, only 1 of the 9 NS participants with a baseline L/S ratio <1 had resolution of NAFLD over 12 months (P = .43). In the 19 participants with baseline L/S ratio <1, the change in the L/S ratio over 12 months differed significantly in the SG vs NS groups (P = .03) (Fig. 1). Conversely, in youth with a baseline L/S ratio of ≥1, there were no significant within- or between-group changes in the L/S ratio over 12 months.
Figure 1.
(A) Liver/spleen ratio (L/S) at baseline and 12 months in participants undergoing sleeve gastrectomy in the subgroup with L/S ratio <1 at baseline, †P < .05 for within-group change. A low L/S ratio indicates fatty infiltration. (B) Change in L/S ratio over 12 months in nonsurgical controls in the subgroup with L/S ratio <1 at baseline. Although all participants in the sleeve gastrectomy group showed resolution of hepatic steatosis 12 months after surgery, only 1 participant in the control group showed resolution of hepatic steatosis.
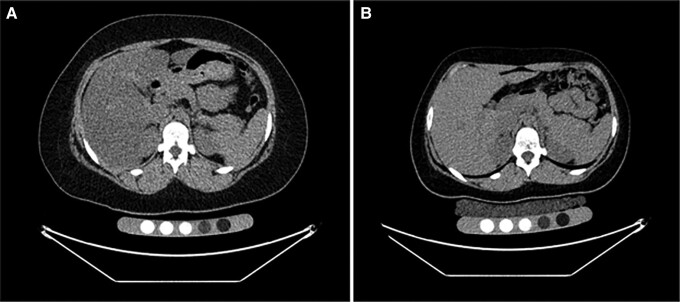
Figure 2.
Computed tomography of the abdomen for assessment of liver fat in a 16-year-old female study participant with severe obesity (BMI, 42 kg/m2) before sleeve gastrectomy (A) and 12 months after surgery (BMI, 27 kg/m2) (B). There is an increase in liver attenuation corresponding to a decrease in liver fat after surgery (liver/spleen ratio .4 presurgery vs 1.2 postsurgery). BMI, body mass index.
At baseline, SAT and VAT were higher in SG compared with NS. After 12 months, SAT and VAT decreased within the SG group and in SG vs NS (Table 2).
Baseline Biochemical Parameters and Changes Over 12 Months
Liver enzymes and lipid levels did not differ between SG and NS groups at baseline (Table 2). Levels of AST and ALT decreased within the SG group (P = .002 and .0003, respectively) over 12 months, and the SG vs NS groups differed significantly for changes in ALT (but not AST) levels over 12 months (P = .003). The SG group demonstrated a significant within-group increase in HDL (P = .001) and a reduction in LDL (P = .002) 12 months after surgery. SG and NS groups differed significantly for the 12-month change in HDL and LDL levels (P = .019, .017, respectively).
Associations Between Changes in Body Composition and Changes in Hepatic fat
In the SG group, reductions in VAT correlated with increases in the L/S ratio over 12 months (ρ = −.51, P = .016). There were also significant inverse associations between the 12-month change in VAT and the 12-month change in the L/S ratio in the SG subgroup with baseline L/S ratio <1 (ρ = −.78, P = .01).
Associations Between Changes in Hepatic fat and Changes in Biochemical Parameters
Significant inverse associations at baseline were found in the whole group between liver enzymes (ALT, AST) and L/S (ρ = −.46, P = .001; ρ = −.43, P = .003, respectively). However, there were no significant associations between changes in the L/S ratio and changes in levels of liver enzymes or lipids in the entire SG group and in those with L/S ratio <1.
Discussion
Our study identified improvement in hepatic fat content in youth 12 months after SG. Furthermore, in individuals with CT findings of NAFLD before surgery, there was resolution of NAFLD in all individuals 12 months after SG. Our findings are consistent with a prospective study by Manco et al, in which 20 adolescents with obesity and NAFLD diagnosed by biopsy undergoing SG showed improvement of NAFLD, with grade 2 fibrosis reverting in 90% of patients over a year (11). Further, VAT decreased following SG and the decrease in VAT correlated with improvement in hepatic fat content. ALT levels decreased significantly in the SG group, but this decrease did not correlate with improvement in hepatic fat content. Our study is the first to demonstrate an improvement in NAFLD in this age range using noninvasive CT measures of hepatic steatosis.
The impact of SG on body composition is well established (12), and consistent with this, we found reductions in SAT and VAT following SG in our participants. Further, studies in adults with obesity have demonstrated positive associations of BMI and VAT with hepatic fat content, suggesting that the accumulation of VAT may play a role in NAFLD pathophysiology (13, 14). A study by Jang et al, evaluating 306 adults with obesity, reported significant positive correlations of VAT with CT measures of hepatic steatosis (13). The Framingham Heart Study has similarly reported positive associations between VAT and intrahepatic lipid content (15). VAT has been linked to the secretion of inflammatory cytokines and free fatty acids and induces changes in adipokines, which together are believed to lead to NAFLD (1). Our findings of an inverse association between changes in VAT and L/S ratio demonstrate that this relationship between the 2 ectopic fat depots holds in adolescents and young adults with obesity undergoing SG. To our knowledge, this relationship in youth undergoing SG has not been previously reported.
Our finding of reductions in ALT following SG are consistent with findings from a previous retrospective study by our group that showed a decrease in ALT following MBS, with a greater reduction in ALT among those with the most severe NAFLD by biopsy (16). Reductions in ALT have also been reported in the study by Manco et al of adolescents with obesity and NAFLD diagnosed by biopsy undergoing SG (11). We found inverse associations between liver enzymes and the L/S ratio at baseline in the entire group. However, although liver enzymes decreased following SG, there were no associations between changes in liver enzymes and changes in hepatic fat, indicating that changes in liver enzymes in youth following SG do not necessarily reflect concurrent changes in hepatic fat and vice versa. These findings may be explained by the possibility that rapid changes in liver fat content are not reflected by changes in liver enzymes and by the fact that significant elevations in ALT occur only in the later stages of NAFLD (2, 17, 18). These findings highlight the critical need to standardize accurate screening tools for NAFLD and to consider incorporating imaging methods in the evaluation of comorbidities of obesity in youth.
We found an improvement in serum lipids following SG, with a significant increase in HDL and a decrease in LDL. This is consistent with similar findings reported by our group in a retrospective study of 22 youth undergoing SG (19). Similarly, the TEEN-LABS cohort showed a 66% remission of dyslipidemia following MBS (20). However, we did not find any association between changes in hepatic fat and changes in serum lipids over 12 months. In contrast to our findings, Shorr et al reported a negative association between hepatic fat content and HDL in men but not in women (21). Similarly, the Framingham Heart Study has reported a negative association between the CT L/S ratio and HDL (22), and the biochemical parameter that showed the strongest correlation with all ectopic fat depots was TG (15). These discrepancies between our results and others may reflect differences in interactions of cardiometabolic markers and ectopic fat deposits across the lifespan.
Limitations of our study include a relatively small sample size and the lack of liver biopsies in study participants. However, CT measures of hepatic fat have been shown to accurately reflect hepatic fat content confirmed by biopsy (10). In addition, our control group underwent routine nonsurgical care rather than a pharmacological intervention such as administration of GLP-1 agonists that have been shown to improve NAFLD in adults (23, 24). Future studies are necessary that compare the impact of a potent GLP-1 analogue (such as liraglutide or semaglutide) vs metabolic and bariatric surgery on hepatic fat in adolescents and young adults. Strengths of our study include its prospective design, the thorough phenotyping of study participants, and the use of abdominal cross-sectional imaging to assess hepatic fat and body composition. Prospective, larger, and longer term studies are needed to confirm our findings and to determine the long-term impact of SG on NAFLD, especially in youth at the early stages of the condition.
In summary, our study demonstrates a significant reduction in hepatic fat following SG in adolescents and young adults with obesity, associated with reductions in VAT. In our cohort, change in ALT was not a good marker of change in hepatic fat over 12 months.
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- BMI
body mass index
- CT
computed tomography
- CV
coefficient of variation
- HDL
high-density lipoprotein
- L/S ratio
liver-to-spleen ratio
- LDL
low-density lipoprotein
- MBS
metabolic and bariatric surgery
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- NS
nonsurgical care
- ROI
region of interest
- SAT
subcutaneous adipose tissue
- SG
sleeve gastrectomy
- TC
total cholesterol
- TG
triglycerides
- VAT
visceral adipose tissue
Contributor Information
Ana Paola López López, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Shubhangi Tuli, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Meghan Lauze, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Imen Becetti, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA; Division of Pediatric Endocrinology, Massachusetts General Hospital for Children and Harvard Medical School, Boston, MA 02114, USA.
Clarissa C Pedreira, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Florian A Huber, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA; Institute of Diagnostic and Interventional Radiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Emre Omeroglu, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Vibha Singhal, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA; Division of Pediatric Endocrinology, Massachusetts General Hospital for Children and Harvard Medical School, Boston, MA 02114, USA; Pediatric Program MGH Weight Center, Massachusetts General Hospital, Boston, MA 02114, USA.
Madhusmita Misra, Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA; Division of Pediatric Endocrinology, Massachusetts General Hospital for Children and Harvard Medical School, Boston, MA 02114, USA.
Miriam A Bredella, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Funding
This work was supported by the following grants: National Institutes of Health (NIH) K24DK109940 (M.A.B.), NIH R01 DK103946 (M.M., M.A.B.), NIH K23DK110419 (V.S.), K24HD071843 (M.M.), and NIH P30DK057521 (V.S.).
Disclosures
The authors do not have any conflicts of interest to disclose relevant to the content of this manuscript.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
Clinical Trial Information
ClinicalTrials.gov Identifier: NCT02557438 (registered September 23, 2015).
References
- 1. Polyzos S, Kountouras J, Matzoros CH. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82‐97. [DOI] [PubMed] [Google Scholar]
- 2. Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Xanthakos SA. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64(2):319‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(8):484‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21(1):87‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyce CJ, Pickhardt PJ, Kim DH, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. Am J Roentgenol. 2010;194(3):623‐628. [DOI] [PubMed] [Google Scholar]
- 6. Pickhardt PJ, Graffy PM, Reeder SB, Hernando D, Li K. Quantification of liver fat content with unenhanced MDCT: phantom and clinical correlation with MRI proton density fat fraction. Am J Roentgenol. 2018;211(3):W151‐W157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murakami E, Nakahara T, Hiramatsu A, et al. Therapeutic effects of sleeve gastrectomy for non-alcoholic steatohepatitis estimated by paired liver biopsy in morbidly obese Japanese patients. Medicine (Baltimore). 2021;100(26):e26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lassailly G, Caiazzo R, Ntandja-Wandji LC, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159(4):1290‐1301.e5. [DOI] [PubMed] [Google Scholar]
- 9. Kawata R, Sakata K, Kunieda T, Saji S, Doi H, Nozawa Y. Quantitative evaluation of fatty liver by computed tomography in rabbits. Am J Roentgenol. 1984;142(4):741‐746. [DOI] [PubMed] [Google Scholar]
- 10. Bydder GM, Chapman RW, Harry D, Bassan L, Sherlock S, Kreel L. Computed tomography attenuation values in fatty liver. J Comput Tomogr. 1981;5(1):33‐35. [DOI] [PubMed] [Google Scholar]
- 11. Manco M, Mosca A, De Peppo F, Caccamo R, Cutrera R, Caccamo R. The benefit of sleeve gastrectomy in obese adolescents on nonalcoholic steatohepatitis and hepatic fibrosis. J Pediatr. 2017;180:31‐37.e2. [DOI] [PubMed] [Google Scholar]
- 12. Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jang S, Lee CH, Choi KM, et al. Correlation of fatty liver and abdominal fat distribution using a simple fat computed tomography protocol. World J Gastroenterol. 2011;17(28):3335‐3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Church TS, Kuk JL, Ross R, et al. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130(7):2023‐2030. [DOI] [PubMed] [Google Scholar]
- 15. Lee JJ, Pedley A, Hoffmann U, Massaro JM, Levy D, Long MT. Visceral and intrahepatic fat are associated with cardiometabolic risk factors above other ectopic fat depots: the Framingham Heart Study. Am J Med. 2018;131(6):684‐692.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stanford FC, Mushannen T, Cortez P, Bredella MA, Misra M, Singhal V. Comparison of short and long-term outcomes of metabolic and bariatric surgery in adolescents and adults. Front Endocrinol (Lausanne). 2020;11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinou E, Pericleous M, Stefanova I, Kaur V, Angelidi AM. Diagnostic modalities of non-alcoholic fatty liver disease: from biochemical biomarkers to multi-omics non-invasive approaches. Diagnostics (Basel). 2022;12(2):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwimmer JB, Newton KP, Awai HI, et al. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38(10):1267‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maffazioli GD, Stanford FC, Stanley TL, Singhal V, Bredella MA, Misra M. Comparing outcomes of two types of bariatric surgery in an adolescent obese population: Roux-en-Y gastric bypass vs. sleeve gastrectomy. Front Pediatr. 2016;4:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michalsky MP, Inge TH, Jenkins TM, et al. Cardiovascular risk factors after adolescent bariatric surgery. Pediatrics. 2018;141(2):e20172485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schorr M, Dichtel LE, Gerweck AV, et al. Sex differences in body composition and association with cardiometabolic risk. Biol Sex Differ. 2018;9(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51(6):1979‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dutta D, Kumar M, Shivaprasad K, Kumar A, Sharma M. Impact of semaglutide on biochemical and radiologic measures of metabolic-dysfunction associated fatty liver disease across the spectrum of glycaemia: a meta-analysis. Diabetes Metab Syndr. 2022;16(6):102539. [DOI] [PubMed] [Google Scholar]
- 24. Flint A, Andersen G, Hockings P, et al. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2021;54(9):1150‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.