Abstract
Context
Prader-Willi syndrome (PWS) is a complex disorder combining hypothalamic dysfunction, neurodevelopmental delay, hypotonia, and hyperphagia with risk of obesity and its complications. PWS is caused by the loss of expression of the PWS critical region, a cluster of paternally expressed genes on chromosome 15q11.2-q13. As life expectancy of patients with PWS increases, age-related diseases like malignancies might pose a new threat to health.
Objective
To investigate the prevalence and risk factors of malignancies in patients with PWS and to provide clinical recommendations for cancer screening.
Methods
We included 706 patients with PWS (160 children, 546 adults). We retrospectively collected data from medical records on past or current malignancies, the type of malignancy, and risk factors for malignancy. Additionally, we searched the literature for information about the relationship between genes on chromosome 15q11.2-q13 and malignancies.
Results
Seven adults (age range, 18-55 years) had been diagnosed with a malignancy (acute lymphoblastic leukemia, intracranial hemangiopericytoma, melanoma, stomach adenocarcinoma, biliary cancer, parotid adenocarcinoma, and colon cancer). All patients with a malignancy had a paternal 15q11-13 deletion. The literature review showed that several genes on chromosome 15q11.2-q13 are related to malignancies.
Conclusion
Malignancies are rare in patients with PWS. Therefore, screening for malignancies is only indicated when clinically relevant symptoms are present, such as unexplained weight loss, loss of appetite, symptoms suggestive of paraneoplastic syndrome, or localizing symptoms. Given the increased cancer risk associated with obesity, which is common in PWS, participation in national screening programs should be encouraged.
Keywords: Prader-Willi syndrome, neoplasms, hypothalamo-hypophyseal system, comorbidity
Prader-Willi syndrome (PWS) is a rare genetic, multisystem disorder characterized by hypothalamic dysfunction, developmental delay, hypotonia, increased pain threshold, and typical dysmorphic features. Hypothalamic dysfunction may lead to several clinical features, including hyperphagia and pituitary hormone deficiencies (1–3). Hyperphagia in combination with a decreased basal metabolic rate and reduced physical activity results in a high prevalence of obesity (1, 4, 5).
PWS is caused by the absence of expression of a cluster of paternally expressed, maternally imprinted genes on chromosome 15q11.2-q13, also called the “PWS critical region.” In 65% to 75% of the patients, the underlying genotype is a type I (40%) or type II (60%) paternal deletion. Maternal uniparental disomy 15 (mUPD) occurs in 20% to 30% and 1% to 3% have an imprinting center defect (ICD). Balanced translocations (0.1%) and individual gene mutations (<0.1%) are rare (6).
As a result of earlier diagnosis, multidisciplinary care, and better weight management, the life expectancy of patients with PWS has substantially increased (7, 8). As patients with PWS become older, the development of age-related diseases is increasingly relevant. Additionally, adults with PWS have shorter leukocyte telomere lengths, premature symptoms of aging, an early functional decline, and higher brain age, all suggesting accelerated aging (9, 10). This highlights the importance of knowledge about the occurrence of age-related diseases, such as malignancies, in adults with PWS.
Previous studies investigating malignancies in PWS are limited by low numbers, lack of older patients, and results that were based on questionnaires only. Questionnaire studies could underestimate the occurrence of malignancies, as underdiagnosis of diseases in general is a common problem in patients with PWS (11). Underdiagnosis is common for several reasons, including their high pain threshold, specific behavioral phenotype, and the high prevalence of intellectual disability (1, 12).
In vitro studies, animal studies, and studies in non-PWS participants suggest that multiple genes in the 15q11.2-q13 chromosomal region may be involved in the development of malignancies (13–19). However, the relationship between genetic subtype and the development of malignancies has, to our knowledge, never been investigated.
To investigate the need to screen for malignancies in patients with PWS, we assessed the prevalence of malignancies in a large international cohort of adults and children with PWS. To understand the pathogenesis of malignancies in patients with PWS, we provide a literature overview of the relationship between the genes on chromosome 15q11.2-q13 and different types of malignancies.
Methods
All participating centers obtained approval from ethics committees and/or individual patients to retrospectively collect data on patients with PWS.
We collected data from patient records of 706 individuals (160 children and 546 adults) with PWS that were visiting or had previously been under the care of one of the centers participating in the INfoRMEd-PWS network in: Netherlands (115), United Kingdom (45), France (92), Spain (94), Italy (290), or Australia (70). The local investigators collected data from patients on: 1) past or current malignancies, and if applicable, which type; 2) growth hormone (GH) treatment during childhood and adulthood; 3) treatment with testosterone or estrogen replacement therapy; 4) for males, history of cryptorchidism and 5) measurements of prostate specific antigen (PSA); 6) type 2 diabetes mellitus; 7) family history of malignancy; 8) alcohol use; 9) smoking; 10) other substance abuse; and 11) baseline characteristics, including anthropometric measurements, current age, gender, genotype, and whether patients were still alive at the time of data collection. Data on height and weight was used to calculate body mass index (BMI). As measurements of fat mass were not available for all patients, obesity was defined as a BMI >30 kg/m2 for adults and a BMI > +2 standard deviation score (SDS) for children.
Literature Review
In collaboration with the Medical Library of the Erasmus University Medical Center, we performed a literature search on Embase, Medline, the Web of Science Core Collection, Cochrane Central Register of Controlled Trials, and Google Scholar. The search was last updated in September 2022. We reviewed studies that reported on the relationship between the expression of genes on chromosome 15q11.2-q13 and malignancies. Inclusion criteria were clinical trials, basic or translational research, and case reports or case series that researched the expression or methylation of one or more genes on chromosome 15q11.2-q13 in malignancies compared to normal cells/tissue. Exclusion criteria were meeting reports, workshop summaries, reviews, conference abstracts, guidelines, articles that were not available online, and articles that were not available in English. Articles that only reported on the relationship between gene expression and the prognosis or survival of patients with malignancies were also excluded. The full search strategy is included in Table S1 (20). As most genes were associated with both up- and downregulation, we concluded that a gene was mainly upregulated, when it was upregulated in ≥ 80% of studies and mainly downregulated, when it was downregulated in ≥ 80% of studies.
Data Analysis
Descriptive statistics for continuous variables are reported as median (interquartile range [IQR]). For dichotomous variables, the number and the percentage of people, n (%), are displayed. To investigate the relationship between malignancies and nominal variables, the Fisher exact test was used. To investigate the relationship between malignancies and genotype, genotype was dichotomized into deletion or no deletion. For the relationship between malignancies and continuous variables, the Wilcoxon rank sum test was used. The relationship between malignancies and anthropometric measurements (height, weight, and BMI) was investigated in adults only.
Results
Baseline characteristics are shown in Table 1. We included 160 children and 546 adults. The median age was 25 years (IQR, 18-33 years). Thirty-seven patients were 50 years old or older. Of the patients included, 326 (46%) were males. Obesity was prevalent (53%), with a median BMI of 32 kg/m2 (IQR 25-42 kg/m2). Deletion was the most common genotype (58%). Patients from 6 countries were included in this study. Most patients had received GH treatment at some point in their life (65%) and 227 (32%) received GH treatment at the time of data collection.
Table 1.
Baseline characteristics of 706 children and adults participating in this study
| Number of observations | Total N = 706 | Children N = 160 | Adults N = 546 | |
|---|---|---|---|---|
| Agea | ||||
| Median [IQR] | 706 | 25 [18-33] | 9 [5-14] | 28 [22-38] |
| Range | 0.4-73 | 0.4-18 | 18-73 | |
| Male gender | 706 | 326 (46%) | 75 (47%) | 251 (46%) |
| Anthropometric measurements | ||||
| Height, cm, median [IQR] | 690 | 153 [144-163] | 135 [105-151] | 156 [149-164] |
| Height, SDS, median [IQR] | 120 | −1.0 [−1.9; 0.16] | ||
| Weight, kg, median [IQR] | 690 | 78 [60-98] | 38 [18-59] | 83 [68-102] |
| Weight, SDS, median [IQR] | 28 | −0.5 [−1.3-1.6] | ||
| BMI, kg/m2, median [IQR] | 690 | 32 [25-42] | 21 [17-27] | 34 [27-44] |
| BMI, SDS, median [IQR] | 96 | 1.2 [0.01-1.9] | ||
| BMI, range | 690 | 13-80 | 13-80 | 17-73 |
| Obesity | 642 | 376 (53%) | 22 (23%) | 354 (65%) |
| Genotype | ||||
| Deletion | 706 | 410 (58%) | 78 (49%) | 332 (61%) |
| mUPD | 236 (33%) | 74 (46%) | 162 (30%) | |
| ICD | 13 (2%) | 4 (3%) | 9 (2%) | |
| mUPD or ICD | 20 (3%) | 0 (0%) | 20 (4%) | |
| Translocation | 1 (0%) | 1 (1%) | 0 (0%) | |
| Other | 8 (1%) | 0 (0%) | 8 (2%) | |
| Unknown | 18 (3%) | 3 (2%) | 15 (3%) | |
| Country | ||||
| Netherlands | 706 | 115 (16%) | 0 (0%) | 115 (21%) |
| United Kingdom | 45 (6%) | 1 (1%) | 44 (8%) | |
| France | 92 (13%) | 4 (3%) | 88 (16%) | |
| Spain | 94 (13%) | 54 (34%) | 40 (7%) | |
| Italy | 290 (41%) | 96 (60%) | 194 (36%) | |
| Australia | 70 (10%) | 5 (3%) | 65 (12%) | |
| GH treatment | ||||
| During childhood | 706 | 420 (60%) | 145 (91%) | 275 (50%) |
| During adulthood | 704 | 156 (22%) | NA | 156 (29%) |
| Childhood and/or adulthood | 706 | 462 (65%) | 145 (91%) | 317 (58%) |
| Current | 693 | 227 (32%) | 110 (69%) | 117 (21%) |
| Duration, median [IQR] | 396 | 8 [4-12] | 7 [3-10] | 8 [4-13] |
Data are displayed as n (%).
Abbreviations: ICD, imprinting center defect; IQR, interquartile range; mUPD, maternal uniparental disomy; SDS, standard deviation score.
a Current age or, for deceased patients, age of death.
Of 706 patients, 7 adults (4 male and 3 female), had been diagnosed with a malignancy, as seen in Table 2. Patients with a malignancy were significantly older, with a median age of 39 years (IQR, 22-46 years) compared to 24 years (IQR, 18-34 years) in the control group. All patients with a malignancy had a paternal deletion, compared with 58% in patients without a malignancy (P = .045). There was no relation between malignancies and gender, country, GH treatment, anthropometric measurements, use of alcohol or tobacco, sex hormone replacement, cryptorchidism, family history, or type 2 diabetes (T2DM).
Table 2.
Patient characteristics according to history of malignancies
| Number of observations | Malignancy absent N = 699 | Malignancy present N = 7 | P value | |
|---|---|---|---|---|
| Age | ||||
| Median [IQR] | 706 | 24 [18-34] | 39 [22-46] | .04 |
| Range | 0.4-73 | 18-55 | ||
| Male gender | 706 | 322 (46%) | 4 (57%) | .7 |
| Genotype | ||||
| Deletion | 706 | 403 (58%) | 7 (100%) | .045a |
| mUPD | 236 (34%) | 0 (0%) | ||
| ICD | 13 (2%) | 0 (0%) | ||
| mUPD or ICD | 20 (3%) | 0 (0%) | ||
| Other | 8 (1%) | 0 (0%) | ||
| Unknown | 18 (3%) | 0 (0%) | ||
| Country | ||||
| The Netherlands | 706 | 115 (17%) | 0 (0%) | |
| United Kingdom | 45 (6%) | 0 (0%) | ||
| France | 91 (13%) | 1 (14%) | ||
| Spain | 93 (13%) | 1 (14%) | ||
| Italy | 287 (41%) | 3 (43%) | ||
| Australia | 68 (10%) | 2 (29%) | .5 | |
| GH treatment | ||||
| During childhood | 706 | 418 (60%) | 2 (29%) | .1 |
| During adulthood | 704 | 155 (22%) | 2 (29%) | 1 |
| Childhood and/or adulthood | 706 | 458 (66%) | 4 (57%) | .7 |
| Current | 693 | 226 (32%) | 1 (14%) | 1 |
| Duration, median [IQR] | 396 | 8 [4-12] | 1 [0.6-10] | |
| Anthropometric measurements | ||||
| Height, cm, median [IQR] | 16 | 153 [144-163] | 155 [152-162] | .8b |
| Weight, kg, median [IQR] | 16 | 78 [60-98] | 100 [69-127] | .5b |
| BMI, kg/m2, median [IQR] | 16 | 32 [25-42] | 36 [28-55] | .4b |
| Obesity | 16 | 374 (55%) | 5 (71%) | .7b |
| Intoxications | ||||
| Alcohol | 593 | 12 (2%) | 1 (14%)c | .1 |
| Glasses per week, median [IQR]d | 13 | 2 [1-4] | 1c | |
| Smoking | 598 | 36 (5%) | 2 (29%)c | .07 |
| Cigarettes per week, median | 38 | 70 [42-113] | 35 and 49c | |
| [IQR]d | 592 | 0 (0%) | ||
| Drugs | 0 (0%) | |||
| Sex hormone replacement therapy | ||||
| Males, n (% of males) | 313 | 149 (48%) | 3 (75%) | .4 |
| Median age at start [IQR] | 313 | 18 [16-25] | 23, 30, & 30c | |
| Females, n (% of females) | 366 | 186 (51%) | 1 (33%)c | .6 |
| Median age at start [IQR] | 355 | 17 [15-20] | 13c | |
| Cryptorchidism, n (% of males) | 310 | 250 (81%) | 2 (67%) | .5 |
| Surgery for cryptorchidism, n (% of cryptorchidism) | 252 | 232 (93%) | 2 (100%) | |
| Known family history of malignancy in first degree relatives | 567 | 88 (16%) | 1 (20%) | .6 |
| Mortality | 706 | 25 (4%) | 4 (57%) | <.001 |
| Age of death | 706 | 33 [26-49] | 39 [24-46] | |
| Type 2 diabetes mellitus (T2DM) | 648 | 111 (17%) | 2 (29%) | .4 |
| Only non-insulin antidiabetics, n (% | 59 (60%) | 0 (0%) | ||
| of T2DM)e | 100 | 3 (3%) | 0 (0%) | |
| Only insulin, n (% of T2DM) | 35 (35%) | 1 (100%) | ||
| Both, n (% of T2DM) | 2 (2%) | 0 (0%) | ||
| None, n (% of T2DM) | ||||
Data are displayed as n (%).
Abbreviations: BMI, body mass index; ICD, imprinting center defect; IQR, interquartile range; mUPD, maternal uniparental disomy; T2DM, type 2 diabetes mellitus.
a P value calculated for deletion vs non-deletion.
b For adults only.
c Individual patient data as there were three or less patients in this category.
d In patients that smoke/drink alcohol only.
e Either oral antidiabetics or GLP-1 analogues.
Of 706 patients, 7 adults (4 male and 3 female), had been diagnosed with a malignancy, as seen in Table 2. Patients with a malignancy were significantly older, with a median age of 39 years (IQR, 22-46 years) compared to 24 years (IQR, 18-34 years) in the control group. All patients with a malignancy had a paternal deletion, compared with 58% in patients without a malignancy (P = .045). There was no relation between malignancies and gender, country, GH treatment, anthropometric measurements, use of alcohol or tobacco, sex hormone replacement, cryptorchidism, family history, or type 2 diabetes (T2DM).
Four patients with malignancies had died, of whom 3 had died as a result of their malignancy and 1 from an infection 2 years after being diagnosed with acute lymphoblastic leukemia. Table 3 shows the prevalence of malignancies for different age groups, demonstrating that the prevalence increased with age: 0-9 years 0.0%; 10-19 years 0.8%; 20-29 years 0.4%; 30-39 years 1.6%; 40-49 years 2.6%; and 50-74 years 2.7%. All patients had different types of malignancies, namely acute lymphoblastic leukemia, intracranial hemangiopericytoma, melanoma, adenocarcinoma of the stomach, biliary cancer, adenocarcinoma of the parotid gland, and colon cancer. One patient with a malignancy had a family history of malignancies.
Table 3.
Prevalence of malignancies for different age groups
| Age | Patients with malignancies/total (%) | Age at diagnosis | Current age | Type of malignancy | Genotype | Family history of malignancy in first degree relatives | WHO 1-year cancer prevalencea |
|---|---|---|---|---|---|---|---|
| 0-9 years | 0/85 (0%) | .014% | |||||
| 10-19 years | 1/131 (0.8%) | 18 | b | Acute lymphoblastic leukemia | Deletion, unspecified | None | 0.015% |
| 20-29 years | 1/247 (0.4%) | 22 | 22 | Intracranial hemangiopericytoma | Deletion, unspecified | None | 0.040% |
| 30-39 years | 2/129 (1.6%) | 39 | 39 | Melanoma in neck | Type 1 deletion | None | 0.093% |
| 33 | b | Adenocarcinoma of stomach | Type 2 deletion | None | |||
| 40-49 years | 2/77 (2.6%) | 44 | b | Biliary cancer | Deletion, unspecified | None | 0.22% |
| 46 | b | Adenocarcinoma parotid gland (metastasized) | Deletion, unspecified | None | |||
| 50-74 years | 1/37 (2.7%) | 55 | 55 | Colon cancer (metastasized) | Type 1 deletion | Pancreatic cancer (father) | 0.87% |
a The WHO 1-year cancer prevalence for Europe for both sexes in 2020 (21). It should be noted that these numbers are not directly comparable to our results, as we do not report a 1-year prevalence.
b Deceased.
Literature Review
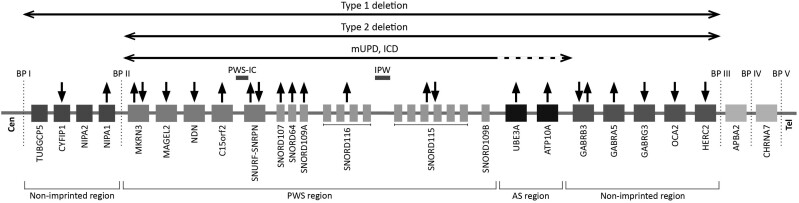
Table S2 (20) shows a literature-based overview of the genes on chromosome 15q11.2-q13 and their relation to malignancies. Genes that were (mainly) upregulated in malignant tumors were: NIPA1, C15orf2, SNORD107, SNORD64, SNORD109A, SNORD116, UBE3A, ATP10A, and GABRA5. Conversely, CYFIP1, MAGEL2, NDN, GABRG3, OCA2, and HERC2 were downregulated in malignant tumors. MKRN3, SNURF-SNRPN, SNORD115, and GABRB3 were associated with both up- and downregulation in malignant cells. These data are graphically summarized in Fig. 1.
Figure 1.
Genes on chromosome 15q11.2-q13 in relation to malignancies. Abbreviations: AS, Angelman syndrome; BP, breakpoint; Cen, centromere; IC, imprinting center; PWS, Prader–Willi syndrome; Tel, telomere. Figure based on Cheon et al (6). Chromosome 15q11.2-q13 can be divided into different regions: a proximal non-imprinted region, the Prader-Willi syndrome (PWS) region that is maternally imprinted, the paternally imprinted region that is also known as the Angelman syndrome region, and a distal non-imprinted region. Legends: Horizontal arrows indicate regions of chromosome 15q11.2-q13 affected by the different genotypes of PWS. Solid horizontal arrows indicate diminished or loss of expression, dotted arrows indicate increased expression. ↑: the gene is upregulated in one or multiple different malignancies in ≥ 80% of studies, ↓: the gene is downregulated in one or multiple different malignancies in ≥ 80% of studies, ↑↓: the gene is more often upregulated than downregulated in one or multiple different malignancies, ↓↑: the gene is more often downregulated than upregulated in one or multiple different malignancies, no symbol: we did not find any information about the relationship between this gene and the development of malignancies.
Discussion
Malignancies were rare in our cohort of 706 patients with PWS. Our cohort included 546 adults, of whom 37 were aged over 50 years. Only 7 adults had a malignancy. The malignancies that occurred were all of different origin. This suggests a multifactorial etiology of the malignancies. Therefore, we do not recommend to screen routinely for a particular type of cancer.
Although scarce, there are some studies that have previously investigated the occurrence of cancer in PWS. Patja et al reported 3 malignancies (acute lymphatic leukemia, testicular tumor, and breast cancer), in a cohort of 56 children and adults with PWS, while the expected number was 1.5 patients. They concluded that there is “a possibility of increased risk of malignancies among persons with PWS” (22). A questionnaire-based study performed in the United States in patients with PWS aged 0 to 63 years (with only 2 being older than 50 years) found that 3 children and 5 adults had a malignancy, while 4.8 cases were expected based on the prevalence in the general USA population (difference not significant). Three patients had leukemia, which was significantly more than expected based on the general population (0.075 cases expected) (23). Several case reports describe patients with PWS and cancer, including acute lymphoblastic leukemia (24), acute and chronic myeloid leukemia (25), hepatoblastoma (26), medulloblastoma (27), pulmonary carcinoid tumor (28), Wilms tumor (29), intratubular germ cell neoplasia (30) and testicular seminoma (31–33). In 1 male patient with PWS and testicular seminoma, loss of methylation of the Prader-Willi syndrome imprinting center (PWS-IC) was found during histological examination, suggesting involvement of genes in the PWS critical region (31).
All 7 patients with malignancies had a deletion of the paternal copy of the PWS region, which was also the most common genotype. No malignancies were found in patients with the genotypes mUPD or ICD. We performed a literature review to explain this finding.
Literature Review
Our literature review revealed that various genes on chromosome 15q11.2-13 are up- or downregulated in different types of cancer. However, this relationship appears to be complex, with several genes being both up- and downregulated in different types of malignancies.
The proximal non-imprinted region contains TUBGCP5, CYFIP1, NIPA2, and NIPA1. They are expressed from both the maternal and the paternal allele. While this region is not affected in patients with a type 2 deletion or a mUPD, one copy of these genes is deleted in patients with a type 1 deletion, leading to a decreased expression (34). CYFIP1 shows reduced expression in various types of human cancers as it acts as an invasion suppressor (15). Therefore, patients with a type 1 deletion might have an increased risk of malignancies. However, as the type of deletion was unknown for most patients, we were unable to investigate whether this was true in our cohort. Of the other genes in the proximal non-imprinted region, NIPA1 is upregulated in acute myeloid leukemia. We did not find any studies relating TUBGCP5 or NIPA2 to malignancies.
Apart from the proximal non-imprinted region, we also studied literature about the genes on the PWS critical region itself. The genes in this region are not expressed in patients with PWS. In patients with a deletion, the paternal allele is absent, and the maternal allele is present but not expressed. In patients with an mUPD or ICD, there are 2 maternal alleles, which are not expressed. According to our literature review, several genes in the PWS region have been associated with malignancies:
MKRN3 inactivation leads to proliferation and progression of non-small cell lung cancers (35). However, upregulation of MKRN3 has been found in osteosarcoma and squamous cell carcinoma of the head and neck (36, 37).
NDN , also known as necdin, is a tumor suppressor gene that represses cell-cycle-promoting proteins, interacts with p53 and inhibits cell growth (38–41). NDN is downregulated in many types of cancer. Lack of expression of this tumor suppressor gene in PWS might therefore, in theory, lead to an increased risk of cancer.
Little is known about the relation between MAGEL2 and C15orf2 and malignancies. MAGEL2 has been associated with down regulation in hepatocellular carcinoma (42) and C15orf2 was upregulated in acute myeloid leukemia in one study (43), but other types of malignancies have not been investigated.
SNURF-SNRPN , due to its relation with the PWS imprinting center (44), has been extensively investigated in order to understand the relationship between epigenetic imprinting and cancer development. Both up- and downregulation of SNURF-SNRPN have been reported in different types of malignancies. SNRPN might affect cancer development through regulation of the cell cycle, tumor proliferation, and apoptosis (45, 46).
Small nucleolar RNAs (snoRNAs) are a class of non-coding RNAs (ncRNAs). Some snoRNAs demonstrate the capability to affect tumorigenesis and metastasis (47). Although evidence is scarce, studies suggest a role of the snoRNAs located on the PWS region in the tumorigenesis of different types of cancer. Most studies report the upregulation of these snoRNAs, in particular SNORD116 and SNORD115, in malignancies. As these genes are not expressed in PWS, this might protect against cancer.
Downstream of the PWS region lies the Angelman syndrome region. This region contains UBE3A and ATP10A. Patients with an mUPD have increased expression of these genes compared to patients with a deletion or healthy controls (34).
UBE3A encodes E3 ligase E6-associated protein (E6AP), which is involved in viral oncogenesis (ie, human papillomavirus, hepatitis C virus, and Epstein-Bar virus-associated malignancies). UBE3A is also involved in the nonviral oncogenesis of multiple types of cancer by degradation of the tumor suppressor promyelocytic leukemia protein (PML) and p27Kip1. Thus, upregulation of UBE3A is likely related to tumorigenesis (48). This might indicate that patients with an mUPD could have an increased risk of malignancies, which was not confirmed in our cohort. Little is known about the relationship between ATP10A and malignancies.
Next to the Angelman region lies the distal non-imprinted region. The genes in the distal non-imprinted region are deleted on one allele in patients with a paternal deletion, but not affected in patients with an mUPD or ICD.
GABRB3, GABRA5, and GABRG3 all encode one of the 19 GABAA receptor subunits (49). The GABA pathway is involved in embryonic stem cell and peripheral neural crest cell proliferation, blunting rapid proliferation, resulting in a more tempered proliferation. This enhances genome integrity (50–52). Multiple studies reported loss of expression or decreased expression of GABRB3 in malignancies, while some reported increased expression. GABRA5 was upregulated in several malignancies and GABRG3 was downregulated in colon adenocarcinoma.
OCA2 is involved in pigmentation and eye color. Therefore, alterations in the OCA2 gene have been associated with melanoma (53–55). Mutations in OCA2 result in oculocutaneous albinism (56), which is associated with an increased risk of skin cancer (51, 57). Additionally, it is downregulated in thyroid carcinoma.
HERC2 is a member of the HERC family. HERCs play a role in replication stress and DNA damage, cell proliferation, and migration and immune response (58). HERC2 is associated with eye color and pigmentation. Genetic variants in this gene have been associated with an increased risk of melanoma (54, 59). Additionally, depletion of HERC2 leads to inhibition of the tumor suppressor p53 (60). Mutations in and downregulation of HERC2 have been associated with multiple types of malignancies (58). As patients with a deletion have only one copy of HERC2, this might lead to an increased risk of malignancies.
Hypopigmentation, which is common in patients with PWS with a deletion (61), is a risk factor for the development of skin cancers (51, 57). We report one patient with melanoma, who had a type 1 deletion.
We found several relatively rare types of malignancies in our population such as hemangiopericytoma, parotid gland cancer, and biliary cancer. Research regarding the relationship between these rare types of malignancy and the genes on chromosome 15q11.2-13 was scarce and therefore we could not explain this finding.
Besides the direct effects of altered gene expression, various clinical features of PWS may increase or decrease the risk of malignancies, including GH and sex hormone treatment, obesity, and use of alcohol and tobacco.
Growth Hormone Treatment
Nowadays, most children with PWS are treated with growth hormone (GH). Multiple observational studies in non-PWS populations did not indicate an increased risk of malignancies later in life after treatment with GH during childhood (62, 63). However, the Safety and Appropriateness of Growth Hormone Treatments in Europe (SAGHe) study showed increased incidence and mortality risks for several cancer sites, largely related to second primary malignancies in patients who received GH treatment after cancer treatment. Only the incidence of bone and bladder cancer was also significantly increased in patients without previous cancer who received GH therapy. Additionally, there was a significant increase in incidence of Hodgkin lymphoma with longer follow-up, also in patients without previous malignancies (64). However, these outcomes might reflect the effect of the underlying condition leading to GH treatment, rather than the effect of GH treatment itself. Therefore, GH treatment is still considered safe with regard to risk of malignancies.
Sex Hormone Replacement Therapy
Many patients with PWS have hypogonadism and are treated with estrogen or testosterone replacement therapy (65–67). In our cohort, 49% of males and 51% of females were receiving sex steroid replacement. In the general population, estrogen replacement therapy is associated with an increased risk of malignancies, especially breast cancer (68, 69). However, little is known about the risk of estrogen replacement therapy in patients with congenital hypogonadism.
The relationship between testosterone replacement therapy and prostate cancer remains complex. However, testosterone replacement therapy seems to be safe and might even be used to help control prostate cancer through normalization of testosterone concentrations (70). We recommend yearly measurement of prostate specific antigen in males with PWS who receive testosterone replacement therapy, according to the guidelines for the general population (71).
Obesity
Obesity was prevalent in our cohort (55%), especially among adults (65%). However, our definition of obesity was based on BMI only, which might lead to an underestimation of adiposity, due to abnormal body composition with low fat free mass compared to fat mass in patients with PWS (4). There is a clear correlation between obesity and many types of malignancies, with relative risks (RR) ranging from 1 to 3 per 10 kg/m2 increase in BMI (72). However, obese patients with PWS have reduced visceral adiposity (73) and are more insulin sensitive (73–75) compared to non-PWS obese adults. This may partly protect adults with PWS from the increased risk of malignancies caused by obesity (76).
In obese individuals with PWS, serum leptin concentrations are increased, as is expected in obesity (75, 77). Leptin is associated with a higher risk of malignancies, ie, breast cancer (78), colorectal cancer (79), thyroid cancer (80), and endometrial cancer (81), also after adjustment for obesity. However, while obesity usually suppresses plasma ghrelin, plasma ghrelin concentrations are increased in PWS (82–85). The relation between circulating ghrelin and the risk of malignancies is still controversial (86). Furthermore, obesity is also associated with chronic low-grade systemic inflammation and oxidative stress (87, 88), which plays a role in the development of malignancies (89, 90). However, there are contradictory reports as to whether peripheral inflammatory markers and adipocytokines are lower, appropriate, or raised for their obesity in patients with PWS (74, 91–93).
Use of Tobacco and Alcohol
Adults with PWS smoke and drink alcohol less often than non-PWS adults. In the general population, tobacco use is associated with lung, laryngeal, pharyngeal, upper digestive tract, and oral cancers (94). Alcohol use leads to an increased risk of cancers of the oral cavity, pharynx, esophagus, colon, rectum, liver, larynx, and breast (95). While 25% of the general European population are cigarette smokers (96), only 5% of our PWS cohort were cigarette smokers. While almost three-quarters of the European population drinks alcohol, only 2% of our PWS cohort drank alcohol (97). Based on these numbers, tobacco and alcohol-associated malignancies are expected to be less prevalent in patients with PWS.
Population Screening for Malignancies
Studies have reported a lower participation in population screening programs for breast, cervical, and colorectal cancer in adults with an intellectual disability (ID) compared to the general population (98–102). Additionally, the consumption of cancer-related healthcare is also lower in adults with an ID (101), while the prevalence of cancer seems to be higher than in the non-ID population (102, 103). This could be due to underdiagnosis and undertreatment in this patient population (101, 102). In our clinical experience, participation in cancer screening programs is also low for patients with PWS, especially for the cervical cancer screening. It is often assumed that cervical cancer screening is not indicated in patients with an ID as they are not sexually active. However, assumption is not always correct, as these patients can be sexually active as well (67). On the other hand, cervical cancer screening could be traumatic for some patients, depending on their sexual history. Therefore, the decision to screen for cervical cancer should be carefully made for each individual patient. We do recommend participation in national screening programs for breast and colon cancer for all PWS adults, due to the increased cancer risk associated with obesity.
Cancer Treatment and Intellectual Disability
The diagnosis and treatment of malignancies is especially complicated in patients with PWS and ID (104). First, their inability to express their physical complaints could lead to underdiagnosis (102). Second, when a malignancy is diagnosed, it is more difficult to convey this information in an effective way to the patient. Information material designed for patients with ID is often unavailable (105). Physicians for IDs, who are experts in communication with and management of patients with ID, are often unfamiliar with the details of cancer diagnosis and cancer treatment. On the other hand, oncologists often lack the specific background and education needed for communication with individuals with ID. Therefore, it is important that these specialists work together, to make sure that both effective communication and accurate information is provided to both patients and their parents/caregivers.
Strengths and Limitations
Strengths of this study include that we report on malignancies in a large international cohort of patients with PWS, that clinical assessments of patients with PWS were performed by experienced physicians and that we report an elaborate literature review. One limitation is the relatively young age of the participants. Although we were able to collect data on a very large cohort of patients with this rare disease, only 37 subjects were older than 50 years, while most malignancies often occur later in life. This lack of older adults with PWS is related to their limited life expectancy (7). The second limitation is the possibility of underdiagnoses. All patients were subject to a yearly follow-up including medical interview, physical examination, and blood measurements. This reduces the risk of underdiagnosis compared to questionnaire studies that only assess self-reported malignancies. However, underdiagnosis cannot be completely ruled out as we did not perform any specific screening for malignancies. Furthermore, national screening programs for malignancies (eg, cervical, breast, colon) vary between countries and data on participation in these screening programs was largely unavailable. The third limitation is the risk of survival bias. We collected data on patients that visited or had visited the PWS reference centers in the past. However, it is possible that patients had already died as a result of cancer before visiting one of the PWS reference centers. The fourth limitation is the lack of a control population. We performed a cross-sectional study where we reported whether patients had a past or current diagnosis of a malignancy. We did not have access to similar data in a control population. However, even without comparing our findings to a control population, we believe that it is unlikely that the risk of a certain type of malignancy is increased, as all types of cancer only occurred once. Lastly, our literature review addresses the potential effect of the genes on chromosome 15q11.1-13 on cancer risk. However, most of the literature did not provide insight into the causal relation between the up- or downregulation of these genes and the development of malignancies. Therefore, a causal relationship cannot be proven.
In conclusion, cancer is rare in our cohort of 706 patients with PWS. The 7 patients with malignancies all had different types of cancer, which suggests a multifactorial etiology. All patients with a malignancy had a paternal deletion. However, the relationship between the PWS genes and cancer risk is complex. Due to the increased cancer risk associated with obesity, we recommend participation in national screening programs for breast and colon cancer for all adults with PWS. The decision to screen for cervical cancer should be carefully made for each individual patient, depending on sexual history and degree of intellectual disability. In males who receive testosterone replacement therapy, we recommend measurement of prostate specific antigen (PSA) according to the general guidelines for testosterone therapy (71). Additional screening for malignancies is only indicated in case of a clinical suspicion based on unexplained weight loss, loss of appetite, paraneoplastic symptoms, or localizing symptoms.
Acknowledgments
The authors wish to thank Maarten F.M. Engel and Wichor Bramer from the Erasmus MC Medical Library for developing and updating the literature search strategies.
Abbreviations
- BMI
body mass index
- GH
growth hormone
- ICD
imprinting center defect
- mUPD
maternal uniparental disomy 15
- PWS
Prader-Willi syndrome
Contributor Information
Karlijn Pellikaan, Department of Internal Medicine, Division of Endocrinology, Erasmus Medical Center, University Medical Centre Rotterdam, 3015 GD Rotterdam, The Netherlands; Center for Adults with Rare Genetic Syndromes, Department of Internal Medicine, Division of Endocrinology, Erasmus Medical Center, University Medical Center Rotterdam, 3015 GD Rotterdam, The Netherlands; Dutch Center of Reference for Prader–Willi Syndrome, 3015 GD Rotterdam, The Netherlands; Academic Center for Growth Disorders, Erasmus Medical Center, University Medical Centre Rotterdam, 3015 GD Rotterdam, The Netherlands.
Naomi Q C Nguyen, Department of Internal Medicine, Division of Endocrinology, Erasmus Medical Center, University Medical Centre Rotterdam, 3015 GD Rotterdam, The Netherlands.
Anna G W Rosenberg, Department of Internal Medicine, Division of Endocrinology, Erasmus Medical Center, University Medical Centre Rotterdam, 3015 GD Rotterdam, The Netherlands; Center for Adults with Rare Genetic Syndromes, Department of Internal Medicine, Division of Endocrinology, Erasmus Medical Center, University Medical Center Rotterdam, 3015 GD Rotterdam, The Netherlands; Dutch Center of Reference for Prader–Willi Syndrome, 3015 GD Rotterdam, The Netherlands; Academic Center for Growth Disorders, Erasmus Medical Center, University Medical Centre Rotterdam, 3015 GD Rotterdam, The Netherlands.
Muriel Coupaye, Assistance Publique-Hôpitaux de Paris, Rare Diseases Center of Reference ‘Prader-Willi Syndrome and Obesity with Eating Disorders’ (PRADORT), Nutrition Department, Institute of Cardiometabolism and Nutrition, ICAN, Pitié-Salpêtrière Hospital, Sorbonne Université, INSERM, Nutriomics, F75013 Paris, France; International Network for Research, Management & Education on adults with Prader-Willi Syndrome (INfoRMEd-PWS).
Anthony P Goldstone, International Network for Research, Management & Education on adults with Prader-Willi Syndrome (INfoRMEd-PWS); PsychoNeuroEndocrinology Research Group, Division of Psychiatry, Department of Brain Sciences, Faculty of Medicine, Imperial College London, London SW7 2AZ, UK; Imperial Centre for Endocrinology, Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0NN, UK.
Charlotte Høybye, International Network for Research, Management & Education on adults with Prader-Willi Syndrome (INfoRMEd-PWS); ENDO-ERN (European Reference Network); Department of Molecular Medicine and Surgery and Department of Endocrinology, Karolinska Institute and Karolinska University Hospital, 17176 Stockholm, Sweden.
Tania Markovic, International Network for Research, Management & Education on adults with Prader-Willi Syndrome (INfoRMEd-PWS); Metabolism & Obesity Services, Royal Prince Alfred Hospital, Camperdown, NSW 2050, Australia; Boden Initiative, Charles Perkins Centre, University of Sydney, Camperdown, NSW 2006, Australia.
Graziano Grugni, International Network for Research, Management & Education on adults with Prader-Willi Syndrome (INfoRMEd-PWS); ENDO-ERN (European Reference Network); Division of Auxology, Istituto Auxologico Italiano, IRCCS, 20095 Piancavallo VB, Italy.
Antonino Crinò, International Network for Research, Management & Education on adults with Prader-Willi Syndrome (INfoRMEd-PWS); Reference Center for Prader-Willi syndrome, Bambino Gesù Hospital, Research Institute, 00165 Palidoro (Rome), Italy.
Assumpta Caixàs, International Network for Research, Management & Education on adults with Prader-Willi Syndrome (INfoRMEd-PWS); Department of Endocrinology and Nutrition, Hospital Universitari Parc Taulí, Institut d’Investigació i Innovació Parc Taulí (I3PT) and Department of Medicine, Universitat Autònoma de Barcelona, 08208 Sabadell, Spain.
Christine Poitou, Assistance Publique-Hôpitaux de Paris, Rare Diseases Center of Reference ‘Prader-Willi Syndrome and Obesity with Eating Disorders’ (PRADORT), Nutrition Department, Institute of Cardiometabolism and Nutrition, ICAN, Pitié-Salpêtrière Hospital, Sorbonne Université, INSERM, Nutriomics, F75013 Paris, France; International Network for Research, Management & Education on adults with Prader-Willi Syndrome (INfoRMEd-PWS); ENDO-ERN (European Reference Network).
Raquel Corripio, Department of Pediatric Endocrinology, Parc Taulí Hospital Universitari, Research and Innovation Institute Parc Taulí I3PT, Autonomous University of Barcelona, 08208 Sabadell, Spain.
Rosa M Nieuwenhuize, Department of Medical Oncology, Erasmus MC, University Medical Center Rotterdam, 3015 GD Rotterdam, The Netherlands.
Aart J van der Lely, Department of Internal Medicine, Division of Endocrinology, Erasmus Medical Center, University Medical Centre Rotterdam, 3015 GD Rotterdam, The Netherlands; ENDO-ERN (European Reference Network).
Laura C G de Graaff, Department of Internal Medicine, Division of Endocrinology, Erasmus Medical Center, University Medical Centre Rotterdam, 3015 GD Rotterdam, The Netherlands; Center for Adults with Rare Genetic Syndromes, Department of Internal Medicine, Division of Endocrinology, Erasmus Medical Center, University Medical Center Rotterdam, 3015 GD Rotterdam, The Netherlands; Dutch Center of Reference for Prader–Willi Syndrome, 3015 GD Rotterdam, The Netherlands; Academic Center for Growth Disorders, Erasmus Medical Center, University Medical Centre Rotterdam, 3015 GD Rotterdam, The Netherlands; International Network for Research, Management & Education on adults with Prader-Willi Syndrome (INfoRMEd-PWS); ENDO-ERN (European Reference Network).
Funding
None.
Disclosures
K.P., N.N., A.R., M.C., A.G., C.H., T.M., G.G., An.C., As.C., C.P., R.C., R.N., A.v.d.L., and L.d.G. have nothing to declare.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10‐26. [DOI] [PubMed] [Google Scholar]
- 2. Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest. 2015;38(12):1249‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M; Speakers contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS . Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93(11):4183‐4197. [DOI] [PubMed] [Google Scholar]
- 4. Schoeller DA, Levitsky LL, Bandini LG, Dietz WW, Walczak A. Energy expenditure and body composition in Prader-Willi syndrome. Metab Clin Exp. 1988;37(2):115‐120. [DOI] [PubMed] [Google Scholar]
- 5. Butler MG, Theodoro MF, Bittel DC, Donnelly JE. Energy expenditure and physical activity in Prader-Willi syndrome: comparison with obese subjects. Am J Med Genet A. 2007;143A(5):449‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheon CK. Genetics of Prader-Willi syndrome and Prader-Will-like syndrome. Ann Pediatr Endocrinol Metab. 2016;21(3):126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet Med. 2017;19(6):635‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manzardo AM, Loker J, Heinemann J, Loker C, Butler MG. Survival trends from the Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet Med. 2018;20(1):24‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donze SH, Codd V, Damen L, et al. Evidence for accelerated biological aging in young Adults with Prader–Willi syndrome. J Clin Endocrinol Metab. 2020;105(6):2053‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Azor AM, Cole JH, Holland AJ, et al. Increased brain age in adults with Prader-Willi syndrome. Neuroimage Clin. 2019;21:101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pellikaan K, Rosenberg AGW, Kattentidt-Mouravieva AA, et al. Missed diagnoses and health problems in adults with Prader-Willi syndrome: recommendations for screening and treatment. J Clin Endocrinol Metab. 2020;105(12):e4671‐e4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holm VA, Cassidy SB, Butler MG, et al. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91(2):398‐402. [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, Qin L, Li P, Mo W. Cyfip1 is downregulated in acute lymphoblastic leukemia and may be a potential biomarker in acute lymphoblastic leukemia. Tumor Biol. 2016;37(7):9285‐9288. [DOI] [PubMed] [Google Scholar]
- 14. Liu X, Xu KM, Wang J, Wang YF, Fu L, Ke XY. CYFIP1 Is a potential tumor suppressor in human diffuse large B-cell lymphoma. Int J Clin Exp Pathol. 2017;10(6):6405‐6414. [Google Scholar]
- 15. Silva JM, Ezhkova E, Silva J, et al. Cyfip1 is a putative invasion suppressor in epithelial cancers. Cell. 2009;137(6):1047‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang IW, Wang YH, Wu WJ, et al. Necdin overexpression predicts poor prognosis in patients with urothelial carcinomas of the upper urinary tract and urinary bladder. J Cancer. 2016;7(3):304‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barrow TM, Barault L, Ellsworth RE, et al. Aberrant methylation of imprinted genes is associated with negative hormone receptor status in invasive breast cancer. Int J Cancer. 2015;137(3):537‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Devaney JM, Wang S, Furbert-Harris P, et al. Genome-wide differentially methylated genes in prostate cancer tissues from African-American and Caucasian men. Epigenetics. 2015;10(4):319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang QL, Chen X, Zhang MH, Shen QH, Qin ZM. Identification of hub genes and pathways associated with retinoblastoma based on co-expression network analysis. Genet Mol Res. 2015;14(4):16151‐16161. [DOI] [PubMed] [Google Scholar]
- 20. Pellikaan K, Nguyen NQC, Rosenberg AGW, et al. Malignancies in Prader-Willi syndrome: results from a large international cohort and literature review. 2023. Repository: Pure, EUR & Erasmus MC Research Portal. Deposited April 21, 2023. https://pure.eur.nl/en/publications/malignancies-in-prader-willi-syndrome-results-from-a-large-intern [DOI] [PMC free article] [PubMed]
- 21. Cancer Today. Vol 2021: World Health Organization.
- 22. Patja K, Sund R, Kaski M, Pukkala E. Cancer incidence among persons with Prader-Willi syndrome in Finland. Int J Disabil Hum De. 2008;7(1):69‐72. [Google Scholar]
- 23. Davies HD, Leusink GL, McConnell A, et al. Myeloid leukemia in Prader-Willi syndrome. J Pediatr. 2003;142(2):174‐178. [DOI] [PubMed] [Google Scholar]
- 24. Kato M, Mugishima H, Chin M, Urakami T, Harada K. Acute lymphoblastic leukemia in a patient with Prader-Willi syndrome under growth hormone therapy. Pediatr Int. 2005;47(3):336‐337. [DOI] [PubMed] [Google Scholar]
- 25. Hall BD. Leukaemia and the Prader-Willi syndrome. Lancet. 1985;325(8419):46. [DOI] [PubMed] [Google Scholar]
- 26. Hashizume K, Nakajo T, Kawarasaki H, et al. Prader-Willi syndrome with del(15)(q11, q13) associated with hepatoblastoma. Acta Paediatr Jpn. 1991;33(6):718‐722. [DOI] [PubMed] [Google Scholar]
- 27. Panagopoulou P, Sattar S, Aquilina K, Jan W, Jacques T, Slater O. Challenges in the diagnosis of medulloblastoma recurrence at an unusual site in a patient with Prader-Willi syndrome. J Pediatr Hematol Oncol. 2019;42(5):e381‐e384. [DOI] [PubMed] [Google Scholar]
- 28. Nenekidis I, Stathopoulos GT, Anagnostakou V, et al. Atypical pulmonary carcinoid tumour in a 28-year-old nonsmoker with Prader-Willi syndrome. Eur Respir J. 2011;38(5):1230‐1233. [DOI] [PubMed] [Google Scholar]
- 29. Coppes MJ, Sohl H, Teshima IE, Mutirangura A, Ledbetter DH, Weksberg R. Wilms tumor in a patient with Prader-Willi syndrome. J Pediatr. 1993;122(5):730‐733. [DOI] [PubMed] [Google Scholar]
- 30. Jaffray B, Moore L, Dickson AP. Prader-Willi syndrome and intratubular germ cell neoplasia. Med Pediatr Oncol. 1999;32(1):73‐74. [DOI] [PubMed] [Google Scholar]
- 31. Eldar-Geva T, Gross-Tsur V, Hirsch HJ, et al. Incomplete methylation of a germ cell tumor (seminoma) in a Prader-Willi male. Mol Genet Genomic Med. 2018;6(5):811‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robinson AC, Jones WG. Prader Willi syndrome and testicular tumour. Clin Oncol (R Coll Radiol). 1990;2(2):117. [DOI] [PubMed] [Google Scholar]
- 33. Coppes MJ, Rose S, Cassidy S, McConnell A, Deyell M, Davies D. The risk of testicular cancer in males with Prader-Willi syndrome (PWS) registered with the US PWS association. Pediatr Res. 1999;45(4, Part 2 of 2):122a. [Google Scholar]
- 34. Bittel DC, Kibiryeva N, Butler MG. Expression of 4 genes between chromosome 15 breakpoints 1 and 2 and behavioral outcomes in Prader-Willi syndrome. Pediatrics. 2006;118(4):e1276‐e1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li K, Zheng X, Tang H, et al. E3 ligase MKRN3 is a tumor suppressor regulating PABPC1 ubiquitination in non-small cell lung cancer. J Exp Med. 2021;218(8):e20210151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Y, Meng G, Qn G. Changes in genomic imprinting and gene expression associated with transformation in a model of human osteosarcoma. Exp Mol Pathol. 2008;84(3):234‐239. [DOI] [PubMed] [Google Scholar]
- 37. Zhang S, Liu C, Li G, Liu Y, Wang X, Qiu Y. Elevated expression of MKRN3 in squamous cell carcinoma of the head and neck and its clinical significance. Cancer Cell Int. 2021;21(1):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Faveri LE, Hurst CD, Platt FM, et al. Putative tumour suppressor gene necdin is hypermethylated and mutated in human cancer. Br J Cancer. 2013;108(6):1368‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee M, Beggs SM, Gildea D, et al. Necdin is a breast cancer metastasis suppressor that regulates the transcription of c-Myc. Oncotarget. 2015;6(31):31557‐31568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hasegawa K, Yoshikawa K. Necdin regulates p53 acetylation via Sirtuin1 to modulate DNA damage response in cortical neurons. J Neurosci. 2008;28(35):8772‐8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang H, Das P, Yu Y, et al. NDN Is an imprinted tumor suppressor gene that is downregulated in ovarian cancers through genetic and epigenetic mechanisms. Oncotarget. 2016;7(3):3018‐3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li R, Gong J, Xiao C, et al. A comprehensive analysis of the MAGE family as prognostic and diagnostic markers for hepatocellular carcinoma. Genomics. 2020;112(6):5101‐5114. [DOI] [PubMed] [Google Scholar]
- 43. Yang MY, Lin PM, Yang CH, et al. Loss of ZNF215 imprinting is associated with poor five-year survival in patients with cytogenetically abnormal-acute myeloid leukemia. Blood Cells Mol Dis. 2021;90:102577. [DOI] [PubMed] [Google Scholar]
- 44. Perk J, Makedonski K, Lande L, Cedar H, Razin A, Shemer R. The imprinting mechanism of the Prader-Willi/Angelman regional control center. Embo J. 2002;21(21):5807‐5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ma J, Zhang Z, Wang J. Small nuclear ribonucleoprotein associated polypeptide N accelerates cell proliferation in pancreatic adenocarcinoma. Mol Med Rep. 2015;12(4):6060‐6064. [DOI] [PubMed] [Google Scholar]
- 46. Ji M, Ren L, Lv Y, et al. Small nuclear ribonucleoprotein polypeptide N accelerates malignant progression and poor prognosis in colorectal cancer transcriptionally regulated by E2F8. Front Oncol. 2020;10:561287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mannoor K, Liao J, Jiang F. Small nucleolar RNAs in cancer. Biochim Biophys Acta. 2012;1826(1):121‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Owais A, Mishra RK, Kiyokawa H. The HECT E3 ligase E6AP/UBE3A as a therapeutic target in cancer and neurological disorders. Cancers (Basel). 2020;12(8):2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yan L, Gong YZ, Shao MN, et al. Distinct diagnostic and prognostic values of γ-aminobutyric acid type A receptor family genes in patients with colon adenocarcinoma. Oncol Lett. 2020;20(1):275‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andäng M, Hjerling-Leffler J, Moliner A, et al. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature. 2008;451(7177):460‐464. [DOI] [PubMed] [Google Scholar]
- 51. Fiaschetti G, Castelletti D, Zoller S, et al. Bone morphogenetic protein-7 is a MYC target with prosurvival functions in childhood medulloblastoma. Oncogene. 2011;30(25):2823‐2835. [DOI] [PubMed] [Google Scholar]
- 52. Sengupta S, Weeraratne SD, Sun H, et al. α5-GABAA receptors negatively regulate MYC-amplified medulloblastoma growth. Acta Neuropathol. 2014;127(4):593‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Summa S, Lasorella A, Strippoli S, et al. The genetic germline background of single and multiple primary melanomas. Front Mol Biosci. 2020;7:555630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Amos CI, Wang LE, Lee JE, et al. Genome-wide association study identifies novel loci predisposing to cutaneous melanoma. Hum Mol Genet. 2011;20(24):5012‐5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Duffy DL, Zhao ZZ, Sturm RA, Hayward NK, Martin NG, Montgomery GW. Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. J Invest Dermatol. 2010;130(2):520‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grønskov K, Ek J, Brondum-Nielsen K. Oculocutaneous albinism. Orphanet J Rare Dis. 2007;2(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kromberg JG, Castle D, Zwane EM, Jenkins T. Albinism and skin cancer in Southern Africa. Clin Genet. 1989;36(1):43‐52. [DOI] [PubMed] [Google Scholar]
- 58. Sala-Gaston J, Martinez-Martinez A, Pedrazza L, et al. HERC Ubiquitin ligases in cancer. Cancers (Basel). 2020;12(6):1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Calbet-Llopart N, Combalia M, Kiroglu A, et al. Common genetic variants associated with melanoma risk or naevus count in patients with wildtype MC1R melanoma. Br J Dermatol. 2022;187(5):753‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cubillos-Rojas M, Amair-Pinedo F, Peiró-Jordán R, Bartrons R, Ventura F, Rosa JL. The E3 ubiquitin protein ligase HERC2 modulates the activity of tumor protein p53 by regulating its oligomerization. J Biol Chem. 2014;289(21):14782‐14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet. 1990;35(3):319‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cianfarani S. Risk of cancer in patients treated with recombinant human growth hormone in childhood. Ann Pediatr Endocrinol Metab. 2019;24(2):92‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Diez JJ, Sangiao-Alvarellos S, Cordido F. Treatment with growth hormone for adults with growth hormone deficiency syndrome: benefits and risks. Int J Mol Sci. 2018;19(3):893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Swerdlow AJ, Cooke R, Beckers D, et al. Cancer risks in patients treated with growth hormone in childhood: the SAGhE European Cohort Study. J Clin Endocrinol Metab. 2017;102(5):1661‐1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Emerick JE, Vogt KS. Endocrine manifestations and management of Prader-Willi syndrome. Int J Pediatr Endocrinol. 2013;2013(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pellikaan K, Ben Brahim Y, Rosenberg AGW, et al. Hypogonadism in adult males with Prader-Willi syndrome-clinical recommendations based on a Dutch cohort study, review of the literature and an international expert panel discussion. J Clin Med. 2021;10(19):4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pellikaan K, Ben Brahim Y, Rosenberg AGW, et al. Hypogonadism in women with Prader-Willi syndrome-clinical recommendations based on a Dutch cohort study, review of the literature and an international expert panel discussion. J Clin Med. 2021;10(24):5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem. 2006;102(1-5):89‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yue W, Wang JP, Li YB, et al. Effects of estrogen on breast cancer development: role of estrogen receptor independent mechanisms. Int J Cancer. 2010;127(8):1748‐1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yassin A, AlRumaihi K, Alzubaidi R, Alkadhi S, Ansari AA. Testosterone, testosterone therapy and prostate cancer. Aging Male. 2019;22(4):219‐227. [DOI] [PubMed] [Google Scholar]
- 71. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715‐1744. [DOI] [PubMed] [Google Scholar]
- 72. Kazan M, Karalti I. The association between obesity and cancer. Endocrinol Metab Syndrome. 2015;4(4):196. [Google Scholar]
- 73. Goldstone AP, Thomas EL, Brynes AE, et al. Visceral adipose tissue and metabolic complications of obesity are reduced in Prader-Willi syndrome female adults: evidence for novel influences on body fat distribution. J Clin Endocrinol Metab. 2001;86(9):4330‐4338. [DOI] [PubMed] [Google Scholar]
- 74. Haqq AM, Muehlbauer MJ, Newgard CB, Grambow S, Freemark M. The metabolic phenotype of Prader-Willi syndrome (PWS) in childhood: heightened insulin sensitivity relative to body mass Index. J Clin Endocr Metab. 2011;96(1):E225‐E232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lacroix D, Moutel S, Coupaye M, et al. Metabolic and adipose tissue signatures in adults with Prader-Willi syndrome: A model of extreme adiposity. J Clin Endocr Metab. 2015;100(3):850‐859. [DOI] [PubMed] [Google Scholar]
- 76. Cowey S, Hardy RW. The metabolic syndrome. A high-risk state for cancer? Am J Pathol. 2006;169(5):1505‐1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Goldstone AP, Brynes AE, Thomas EL, et al. Resting metabolic rate, plasma leptin concentrations, leptin receptor expression, and adipose tissue measured by whole-body magnetic resonance imaging in women with Prader-Willi syndrome. Am J Clin Nutr. 2002;75(3):468‐475. [DOI] [PubMed] [Google Scholar]
- 78. Wu MH, Chou YC, Chou WY, et al. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100(4):578‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Paik SS, Jang SM, Jang KS, Lee KH, Choi D, Jang SJ. Leptin expression correlates with favorable clinicopathologic phenotype and better prognosis in colorectal adenocarcinoma. Ann Surg Oncol. 2009;16(2):297‐303. [DOI] [PubMed] [Google Scholar]
- 80. Akinci M, Kosova F, Cetin B, Aslan S, Ari Z, Cetin A. Leptin levels in thyroid cancer. Asian J Surg. 2009;32(4):216‐223. [DOI] [PubMed] [Google Scholar]
- 81. Petridou E, Belechri M, Dessypris N, et al. Leptin and body mass index in relation to endometrial cancer risk. Ann Nutr Metab. 2002;46(3-4):147‐151. [DOI] [PubMed] [Google Scholar]
- 82. Cummings DE, Clement K, Purnell JQ, et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8(7):643‐644. [DOI] [PubMed] [Google Scholar]
- 83. Goldstone AP, Thomas EL, Brynes AE, et al. Elevated fasting plasma ghrelin in Prader-Willi syndrome adults is not solely explained by their reduced visceral adiposity and insulin resistance. J Clin Endocrinol Metab. 2004;89(4):1718‐1726. [DOI] [PubMed] [Google Scholar]
- 84. Goldstone AP, Patterson M, Kalingag N, et al. Fasting and postprandial hyperghrelinemia in Prader-Willi syndrome is partially explained by hypoinsulinemia, and is not due to peptide YY3-36 deficiency or seen in hypothalamic obesity due to craniopharyngioma. J Clin Endocrinol Metab. 2005;90(5):2681‐2690. [DOI] [PubMed] [Google Scholar]
- 85. Kweh FA, Miller JL, Sulsona CR, et al. Hyperghrelinemia in Prader-Willi syndrome begins in early infancy long before the onset of hyperphagia. Am J Med Genet A. 2015;167(1):69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sever S, White DL, Garcia JM. Is there an effect of ghrelin/ghrelin analogs on cancer? A systematic review. Endocr Relat Cancer. 2016;23(9):R393‐R409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rodriguez-Hernandez H, Simental-Mendia LE, Rodriguez-Ramirez G, Reyes-Romero MA. Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol. 2013;2013:678159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Marseglia L, Manti S, D'Angelo G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16(1):378‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H MELL. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12(1):376‐390. [DOI] [PubMed] [Google Scholar]
- 91. Caixàs A, Giménez-Palop O, Broch M, et al. Adult subjects with Prader-Willi syndrome show more low-grade systemic inflammation than matched obese subjects. J Endocrinol Invest. 2008;31(2):169‐175. [DOI] [PubMed] [Google Scholar]
- 92. Viardot A, Sze L, Purtell L, et al. Prader-Willi syndrome is associated with activation of the innate immune system independently of central adiposity and insulin resistance. J Clin Endocrinol Metab. 2010;95(7):3392‐3399. [DOI] [PubMed] [Google Scholar]
- 93. McAlister KL, Fisher KL, Dumont-Driscoll MC, Rubin DA. The relationship between metabolic syndrome, cytokines and physical activity in obese youth with and without Prader-Willi syndrome. J Pediatr Endocrinol Metab. 2018;31(8):837‐845. [DOI] [PubMed] [Google Scholar]
- 94. Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122(1):155‐164. [DOI] [PubMed] [Google Scholar]
- 95. Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112(3):580‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gallus S, Lugo A, Liu X, et al. Who smokes in Europe? Data from 12 European countries in the TackSHS survey (2017-2018). J Epidemiol. 2021;31(2):145‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Global status Report on Alcohol and Health 2014. World Health Organization; 2014. [Google Scholar]
- 98. Horner-Johnson W, Dobbertin K, Andresen EM, Iezzoni LI. Breast and cervical cancer screening disparities associated with disability severity. Womens Health Issues. 2014;24(1):e147‐e153. [DOI] [PubMed] [Google Scholar]
- 99. Parish SL, Swaine JG, Son E, Luken K. Determinants of cervical cancer screening among women with intellectual disabilities: evidence from medical records. Public Health Rep. 2013;128(6):519‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bates C, Triantafyllopoulou P. Exploring the impact of mental capacity on breast screening for women with intellectual disabilities. Health Soc Care Community. 2019;27(4):880‐888. [DOI] [PubMed] [Google Scholar]
- 101. Cuypers M, Tobi H, Huijsmans CAA, et al. Disparities in cancer-related healthcare among people with intellectual disabilities: a population-based cohort study with health insurance claims data. Cancer Med. 2020;9(18):6888‐6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Willis D, Samalin E, Satgé D. Colorectal cancer in people with intellectual disabilities. Oncology. 2018;95(6):323‐336. [DOI] [PubMed] [Google Scholar]
- 103. Liu Q, Adami HO, Reichenberg A, Kolevzon A, Fang F, Sandin S. Cancer risk in individuals with intellectual disability in Sweden: a population-based cohort study. PLoS Med. 2021;18(10):e1003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. O'Regan P, Drummond E. Cancer information needs of people with intellectual disability: a review of the literature. Eur J Oncol Nurs. 2008;12(2):142‐147. [DOI] [PubMed] [Google Scholar]
- 105. Tuffrey-Wijne I, Bernal J, Jones A, Butler G, Hollins S. People with intellectual disabilities and their need for cancer information. Eur J Oncol Nurs. 2006;10(2):106‐116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.