The motility of microorganisms through the environment is driven by chemical gradients: They move towards nutrients and away from signals that indicate unfavorable conditions. This chemotaxis is mediated by transmembrane chemoreceptors that recognize one or many target molecules. In most cases, the encounter with a ligand is recorded by a periplasmic sensor domain, which in turn transmits a signal through the membrane to a cytoplasmic signaling domain (1). Under conditions of environmental stress, these signaling cascades may induce profound lifestyle changes from planktonic cells to a biofilm or from active to inactive cells (2). While it is relatively straightforward to annotate most prokaryotic chemoreceptors from the ever-increasing number of sequenced genomes and environmental samples, the identity of their binding partners is often not clear from protein sequence. As the specificity of downstream signaling events is determined by the sensor domain, it is critical to learn about novel pairings between ligands and their receptors. Using a range of computational and experimental approaches, Cerna-Vargas et al. show in PNAS that a subset of a wide-spread group of dCache_1 receptors evolved to recognize various types of biological amines (3).
The double Cache domain (dCache_1) (4) represents the largest family within the Cache superfamily of proteins (5). These extracellular sensors are predominantly found in prokaryotes as components of all major signal transduction systems. A typical member of this protein family, with two Cache domains next to each other and bookended by two trans-membrane helices, is shown in Fig. 1B. It has been reported that many chemically unrelated ligands could be accommodated by changing the binding pocket of one or both Cache domains (4, 6–9). A recent report demonstrated that dCache_1AA, a variant of the dCache_1 domain, is present in all major lineages of life and that it recognizes amino acids and related ligands, including γ-aminobutyric acid (GABA) (6). It is notable that dCache_1AA is found in eukaryotic α2δ subunits of voltage-gated calcium channels that are targeted by GABA-related compounds, thus opening new ways to potentially create custom drugs for pain relief.
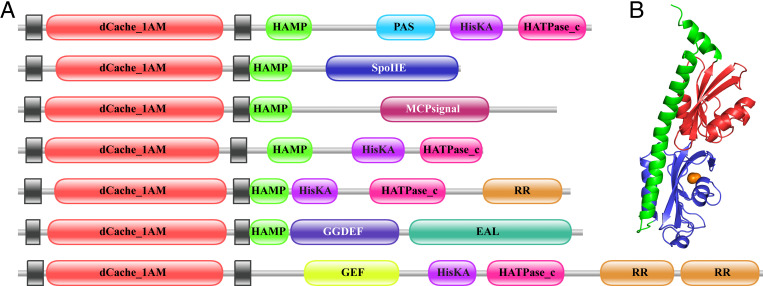
Fig. 1.
(A) Representative domain architectures of dCache_1AM proteins. A schematic representation of several protein architectures that contain dCache_1AM domains. Transmembrane helices are present at both ends of dCache_1AM (black boxes). The remaining domains are an α-helical domain, HAMP; a sensor domain, PAS; a dimerization, and phospho-acceptor domain of histidine kinases, HisKA; a C-terminal ATPase domain, HATPase_c; a serine/threonine phosphatase, SpoIIE; a methyl-accepting chemotaxis domain, MCPsignal; a response regulator receiver domain, RR; a diguanylate cyclase, GGDEF; a diguanylate phosphodiesterase, EAL; a cyclic guanosine monophosphate (cGMP) receptor, GAF. From top to bottom, protein architectures in this figure correspond to the following accession numbers from the non-redundant protein database: WP_011034866.1, WP_027185430.1, WP_014528895.1, WP_091710416.1, WP_015707342.1, WP_056043800.1, WP_052635864.1. (B) A typical structure of the dCache_1 domain. The figure shows protein database (PDB) structure ID 3LIB (10). The two Cache domains are colored blue and red, while the ligand position in the proximal Cache domain is shown as an orange ball.
Biogenic amines are decarboxylation products of amino acids. They have a variety of biological roles related to membrane function, neurotransmission, signaling, and post-translational modifications (11). It is known that both amino acids and small biogenic amines signal via G protein-coupled receptors (12, 13). Isolated cases have been reported for polyamine receptors in bacteria (7, 9), yet those receptors are poorly characterized and most likely do not have a wide distribution. Cerna-Vargas et al. observed that in several dCache_1 crystal structures the biogenic amine molecules are found in the same binding pocket, but in different orientations. Further inspection revealed aromatic residues that make cation–π bonds with amine ligands regardless of their orientation and a pair of charged residues not in direct contact with the ligand that most likely maintain a correct geometry of the binding pocket (see figure 2 in ref. 3 for details). By performing careful profile searches and after aligning thousands of resulting protein matches, the authors identified conserved ligand-binding residues. Knowing a signature motif within the dCache_1AM domain that recognizes biogenic amines enabled the authors to explore two major research avenues: 1) to detect other family members from a large cohort of dCache_1 proteins and explore their domain organizations and 2) to select residues for mutagenesis studies.
“Using a range of computational and experimental approaches, Cerna-Vargas et al. show in PNAS that a subset of a wide-spread group of dCache_1 receptors evolved to recognize various types of biological amines.”
Proteins with dCache_1AM domains belong to all major classes of prokaryotic transmembrane receptors: chemoreceptors, sensor histidine kinases, serine/threonine phosphatases, and diguanylate cyclases/phosphodiesterases (3). Domain neighborhood analysis identified the usual suspects; dCache_1AM is almost always in the company of other domains found in signal transduction proteins (Fig. 1A), and only in rare cases functions as a stand-alone domain. In terms of phylogenetic distribution, dCache_1AM is found in many bacterial phyla and predominantly as a sensor domain of chemoreceptors. Conversely, Halobacteriota are the only archaeal phylum employing dCache_1AM domains, and mostly in histidine kinases (10). It is most likely that dCache_1AM was horizontally transferred to archaea from their bacterial cohabitants (3).
In the final verification step the authors selected eight diverse proteins with dCache_1AM domains and created two binding pocket mutants. Nine of these proteins were soluble and were tested for binding to an array of biogenic amines using isothermal titration calorimetry. Notwithstanding individual differences in binding affinities and in the broadness of ligand specificity, there were two main conclusions: 1) all but one of the expressed proteins were found to bind at least one biogenic amine, and about half of wild-type proteins interacted strongly with multiple ligands; 2) binding pocket mutants did not bind any of the tested amines. Given that the experiment was done on a limited number of proteins that were selected in order to represent diverse receptor classes and phylogenetic groups rather than to target different ligand classes, it appears likely that there are dCache_1AM domains among the thousands of untested proteins that recognize a wide variety of biogenic amines.
The results by Cerna-Vargas et al. indicate that relatively modest changes in the dCache_1AA amino acid receptor—a small insertion in the binding pocket and mutations of key ligand-interacting residues—are enough to switch its recognition from amino acids to biogenic amines. This principle of divergence has been proven many times: it is more expeditious in evolutionary terms to develop related functionalities by modifying existing protein domains rather than inventing them from scratch. Even as we recognize the prevalence of divergent evolution, it must be mentioned that there are known examples of convergent evolution of receptors where structurally unrelated domains recognize the same ligand (14).
As is the case with any early discovery, there are outstanding questions, both for the dCache_1AM domain and for regulation via biogenic amines in general. While the tested members of dCache_1AM family bind a variety of shorter bioamines with different affinities, they showed little to no affinity for polyamines. This could be due to a limited testing sample, as there are known dCache_1 proteins that bind polyamines (7, 9). Indirect evidence suggests that polyamines could also be recognized by other Cache members, as the spike in intracellular spermidine concentration after bacteriophage infection signals through GacS histidine kinase that contains a single Cache domain (15). Similarly, spermidine and GacS factor together in the regulation of type III secretion system (16). Yet another open question is why dCache_1AM has not proliferated more broadly in archaeal phyla after its acquisition by Halobacteriota. Covalent modifications of proteins and tRNA by polyamines are known to occur in archaea, while archaeal thermophiles specifically use polyamines for growth at high temperatures (17). It is notable that polyamines are not present in high concentration in halophiles (18), which could be the reason for their use as signaling molecules only in this group.
The work by Cerna-Vargas et al. (3) adds to the rising number of sensory domains with known ligands and expands our knowledge of the events that govern signal reception and processing. This analysis continued the path of functional characterization the authors have reported for the dCache_1AA domain (6) and provides a recipe for future studies aimed at characterizing novel receptor-ligand interactions. Given that we are better at classifying proteins as receptors than at identifying their ligands, this broad research area awaits novel and high-throughput solutions.
Acknowledgments
Author contributions
M.D. wrote the paper.
Competing interests
The author declares no competing interest.
Footnotes
See companion article, “Amine-recognizing domain in diverse receptors from bacteria and archaea evolved from the universal amino acid sensor,” 10.1073/pnas.2305837120.
References
- 1.Galperin M. Y., What bacteria want. Environ. Microbiol. 20, 4221–4229 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagar E., Schwarz R., To be or not to be planktonic? Self-inhibition of biofilm development. Environ. Microbiol. 17, 1477–1486 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Cerna-Vargas J. P., Gumerov V. M., Krell T., Zhulin I. B., Amine-recognizing domain in diverse receptors from bacteria and archaea evolved from the universal amino acid sensor. Proc. Natl. Acad. Sci. U.S.A. 120, e2305837120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upadhyay A. A., Fleetwood A. D., Adebali O., Finn R. D., Zhulin I. B., Cache domains that are homologous to, but different from PAS domains comprise the largest superfamily of extracellular sensors in prokaryotes. PLoS Comput. Biol. 12, e1004862 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anantharaman V., Aravind L., Cache - a signaling domain common to animal Ca2+-channel subunits and a class of prokaryotic chemotaxis receptors. Trends Biochem. Sci. 25, 535–537 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Gumerov V. M., et al. , Amino acid sensor conserved from bacteria to humans. Proc. Natl Acad. Sci. U.S.A. 119, e2110415119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavira J. A., et al. , Structural basis for polyamine binding at the dCACHE domain of the McpU chemoreceptor from Pseudomonas putida. J. Mol. Biol. 430, 1950–1963 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Zhang L., et al. , Sensing of autoinducer-2 by functionally distinct receptors in prokaryotes. Nat. Commun. 11, 5371 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matilla M. A., et al. , Chemotaxis of the human pathogen Pseudomonas aeruginosa to the neurotransmitter acetylcholine. mBio 13, e0345821 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung J., Hendrickson W. A., Sensor domains of two-component regulatory systems. Curr. Opin. Microbiol. 13, 116–123 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagar N. A., Tarafdar S., Agarwal S., Tarafdar A., Sharma S., Polyamines: Functions, metabolism, and role in human disease management. Med. Sci. (Basel) 9, 44 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K. H., Manning J. J., Javitch J., Shi L., A novel “activation switch” motif common to all aminergic receptors. J. Chem. Inf. Model. 63, 5001–5017 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellaithy A., Gonzalez-Maeso J., Logothetis D. A., Levitz J., Structural and biophysical mechanisms of class C G protein-coupled receptor function. Trends Biochem. Sci. 45, 1049–1064 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavira J. A., et al. , How bacterial chemoreceptors evolve novel ligand specificities. mBio 11, e03066-19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Mattos C. D., et al. , Polyamines and linear DNA mediate bacterial threat assessment of bacteriophage infection. Proc. Natl. Acad. Sci. U.S.A. 120, e2216430120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Q., et al. , Spermidine is an intercellular signal modulating T3SS expression in Pseudomonas aeruginosa. Microbiol. Spectr. 10, e0064422 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael A. J., Polyamine function in archaea and bacteria. J. Biol. Chem. 293, 18693–18701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamana K., Hosoya R., Itoh T., Polyamine analysis of methanogens, thermophiles and extreme halophiles belonging to the domain Archaea. J. Japanese Soc. Extremophiles 6, 25–31 (2007). [Google Scholar]