Abstract
N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD)-quinone (6PPD-Q), a transformation byproduct of 6PPD used in tires as an antiozonant and antioxidant, was recently discovered as the chemical primarily responsible for the acute lethal toxicity of urban storm runoff to coho salmon. The asphalt concrete (AC) surface layer is the primary medium to contact 6PPD-Q immediately upon its release from tires, and the addition of recycled tire rubber (RTR) to the asphalt binder and mixture is a widely accepted practice in asphalt production. Therefore, it is urgent to understand the fate of 6PPD-Q at the asphalt concrete surface layer–water interface. This study analyzed the sorption and desorption of 6PPD-Q by compacted and crushed loose (loose particles, ∼5 mm) rubberized asphalt mixtures and their mobilization from compacted asphalt mixtures during simulated rainfall events. It should be noted that the crushed loose asphalt mixtures demonstrated the physicochemical properties of the asphalt materials, while the compacted asphalt mixtures represent in-service AC layers. Sorption of 6PPD-Q by crushed loose and compacted asphalt mixtures reached equilibrium within 12 days, with a sorption coefficient of 151.57–257.51 L/kg for compacted asphalt mixtures. Within 12 days, desorption of 6PPD-Q from crushed loose and compacted rubberized asphalt mixtures (20 g particles/L) to the double deionized (DDI) water and synthetic stormwater was 0.01–0.09 and 0.025–0.05 μg/L, respectively. Through the rainfall simulation experiments, 0.0015–0.0049 μg/L 6PPD-Q was detected in the runoff water, much lower than the lethal concentration (LC50) of 6PPD-Q of 0.095 μg/L and 308.67 μg/L for coho salmon and zebrafish larvae. Our results indicate that, while the release of 6PPD-Q from compacted rubberized asphalt mixtures is minor, the mixtures can serve as sorbents for tire-derived 6PPD-Q and retain this emerging contaminant.
Keywords: 6PPD-quinone, rubberized asphalt concrete, leachate, sorption, desorption, runoff
Introduction
N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD) is used ubiquitously as an antioxidant amendment for tires.1−5 Recently, an ozonation byproduct of 6PPD, namely, 6PPD-quinone (6PPD-Q), was determined as the toxicant responsible for “urban stream syndrome,” i.e., the rapid mortality of adult coho salmon caused by urban stormwater.6,7 Up to date, the lethal concentration (LC50) of 6PPD-Q was determined for a range of species (coho salmon,6 zebrafish larvae,8 brook trout,9 rainbow trout,9 white sturgeon,9 water flea,10 scud,10 and Salvelinus leucomaenis pluvis),10 ranging from 0.095 μg/L (updated using HPC standard versus originally reported 0.95 μg/L) for coho salmon to up to 308.67 μg/L for zebrafish larvae. The acute toxicity and ubiquitous occurrence of 6PPD-Q have triggered a lot of concerns from the academic community and administrator entities regarding its impact on the ecosystem and human health.6−18
As a known carrier and source for 6PPD-Q, the life cycles of tire rubber have attracted a good deal of attention.11−13 Every year, 75% of the 250 million scrap tires (4 billion tons) produced in the United States are recycled.19 The addition of recycled tire rubber (RTR) to asphalt binders to produce rubberized asphalt mixtures is a widely used practice in asphalt production that consumes about 17% of the total RTR today.19
Asphalt modified by the incorporation of crumb rubber through wet (mixing into hot asphalt) or dry processes (mixing with hot aggregate before the addition of bitumen) can provide benefits, including reduced noise, increased road lifespan, improved traction for safety, and others.20−23 Addressing the fate of 6PPD and 6PPD-quinone derived from RTR-modified asphalt is crucial for the sustainable reuse of RTR for pavement. 6PPD-Q release from rubberized asphalt mixtures is a complex process, depending on the chemical compositions and physical properties (air voids/surface area) of asphalt materials and the stormwater chemistry. No existing research has investigated the release of 6PPD-Q from rubberized asphalt mixtures.
Furthermore, asphalt pavement is the primary medium to directly contact 6PPD-Q upon its release from tires,24 and the fate of 6PPD-Q at the asphalt concrete (AC)–water interface is a critical knowledge gap for understanding the environmental processes of 6PPD-Q. While previous studies have examined the sorption of metals onto asphalt mixtures and porous AC pavements, limited published results are available for the sorption of organic pollutants by asphalt mixtures. The sorption coefficients of a pyrethroid insecticide (permethrin) on cement concrete were determined to be 5.11 ± 0.29 L/kg (calculated based on the reported values in the paper).25 The addition of RTR to an asphalt mixture may influence the sorption of organic compounds, as it adds organic carbon to the asphalt binder. At the AC–water interface, the sorption and desorption of 6PPD-Q, a relatively hydrophobic and less water-soluble compound, are still unknown.
Therefore, this study investigated the retention and release of 6PPD-Q by the rubberized AC: sorption and desorption of 6PPD-Q by compacted and crushed loose (loose particle, ∼5 mm) rubberized asphalt mixtures and the release of 6PPD-Q during the simulated rainfall events. The compacted asphalt mixtures represent in-service AC layers, while the crushed loose asphalt mixtures in the loose particle form were generated in the laboratory to demonstrate the physicochemical properties of asphalt materials.
Materials and Methods
Materials
Solid and solution standard materials of 6PPD-Q (solid standard purity = 97.26% g/g) were purchased from HPC Standards Inc. (Atlanta, GA, USA). The stock solution of 50 mg/L of 6PPD-Q was made by dissolving the solid particles in acetonitrile immediately after receiving the materials following the protocol: 5.2 mg of 6PPD-Q powder was mixed with 10.12 mL of acetonitrile, shaken in the dark overnight, and sonicated further for 3 h until no particles could be observed. The stock solution was stored at 4 °C before use. C18 solid-phase extraction cartridges (Millipore Sigma Supelco Supelclean ENVI-18 SPE 6 mL-0.5 g Tube) were obtained from Sigma-Aldrich (Bellefonte, PA, USA). Acetonitrile, methanol, hexane, and dichloromethane used for extractions and mass spectrometry (MS) analysis were analytical and MS grade, respectively (Fisher Scientific, Hampton, NH, USA). Double deionized (DDI) water (18.2 MΩ·cm–1) was used for all experiments. Three typical RTR-modified plant-produced asphalt mixtures from different geographical locations were used and assigned as RMM1, RMM2, and RMM3, meeting the California Department of Transportation (Caltrans) Standard Specifications Section 39.26 The asphalt mixture has a nominal maximum aggregate size (NMAS) of 12.5 mm with a design asphalt binder content of 7.5% by the total weight of the mix. The crumb rubber percentage was 18% by weight of asphalt binder. The loose plant-produced asphalt mixture was heated to 148 °C and compacted using a Superpave gyratory compactor to make cylindrical samples (150 mm diameter by 100 mm height). Four small columns were cut out of the 150 mm diameter cylindrical specimen using a 38-mm-diameter core bit (internal diameter) to obtain columns (38 mm diameter × 100 mm height). Then, the top and bottom portions of each of the small columns were cut using a masonry saw to get the columns (38 mm diameter by 38 mm height). For analysis of the total 6PPD-Q in asphalt mixtures, crushed loose mixtures (loose particles, ∼5 mm) were obtained by heating the loose plant-produced asphalt mixture to 110 °C and sieving through a no. 4 sieve (4.75 mm). The sieved particles were then frozen using liquid nitrogen, powdered with a mortar and pestle, and sieved. The material passing the no. 50 sieve (0.297 mm) was obtained for testing and is referred to as powdered crushed loose mixtures (Supporting Information (SI), Figure S1). The crushed loose and compacted mixtures were used for the sorption/desorption experiments. Only the compacted asphalt mixture was used for the rainfall simulation experiments, while the powdered crushed loose asphalt mixtures were used to measure the total 6PPD-Q contained in all of the asphalt mixtures by solvent extraction (SI, Figures S2 and S3).
Sorption and Desorption
Sorption kinetics were determined for rubberized crushed loose (loose particle, ∼ 5 mm) and compacted rubberized asphalt mixtures. Crushed loose rubberized asphalt mixtures were mixed with 6PPD-Q (100 μg/L)-spiked DDI water in a ratio of 100 g of solid/1 L of water. At different intervals (0, 1, 2, 4, 8, and 12 days), the supernatant was filtered with 0.45 μm glass fiber filters and analyzed for 6PPD-Q. Compacted rubberized asphalt mixtures (38 mm diameter × 38 mm height, 7% air voids) were first immersed in DDI water in 50 mL beakers for 20 days to achieve saturation with water. Glass beads (0.5 mm diameter) were used to fill the side space to minimize sorption by the side walls of asphalt mixtures (SI, Figure S4). After water-saturated conditions were achieved, the compacted rubberized asphalt mixtures were overlaid with 25 mL of 6PPD-Q (100 μg/L)-spiked DDI water. At different intervals within 12 days, the overlay water was filtered with 0.45 μm glass fiber filters and analyzed for 6PPD-Q (SI, Figure S3).
After the sorption kinetics were determined, the sorption isotherm analysis involved mixing loose rubberized asphalt (100 g/L) with 6PPD-Q (100–500 μg/L)-spiked deionized water (DDI). Similarly, compacted rubberized mixtures (38 mm diameter × 38 mm height, 7% air voids) were mixed with 6PPD-Q (0.1–400 μg/L)-spiked DDI water for the isotherm analysis. The background release of 6PPD-Q was found to be negligible compared with the spiked 6PPD-Q concentrations. The use of lower concentrations (0.1 and 10 μg/L) in the sorption isotherm analysis with compacted asphalt mixtures was chosen to represent environmentally relevant concentrations of 6PPD-Q. After the sorption of 6PPD-Q reached equilibrium, the supernatant was filtered with 0.45 μm glass fiber filters and analyzed for the residual concentration of 6PPD-Q. The control without rubberized asphalt mixtures was used for the same analysis to demonstrate the loss of 6PPD-Q during the experiment. For sorption kinetic and isotherm analyses, triplicate experiments were conducted for crushed loose rubberized asphalt mixtures, while duplicates were used for compacted rubberized asphalt mixtures.
Desorption of 6PPD-Q from rubberized asphalt particles was analyzed by mixing loose (20 g) or compacted (38 mm diameter × 38 mm height, 93–96 g, 7% air voids) rubberized asphalt particles with 1 L of DDI water. At different intervals within 12 days, the supernatant was filtered (0.45 μm, glass fiber filters) for analysis of 6PPD-Q. Furthermore, we analyzed the desorption of 6PPD-Q from crushed loose rubberized asphalt mixtures following a 12-day shaking period of 20 g of crushed loose mixtures with 1 L of double deionized water (DDI). All three materials (RMM1, RMM2, and RMM3) were used for the sorption and desorption experiments (SI, Figure S2).
Rainfall Simulation
A 3D-printed apparatus was fabricated for the rainfall simulation (SI, Figure S5). For a typical rainfall event simulation, DDI water and synthetic stormwater27 (SI, Table S1) were run through the simulator at a flow rate of 1 mL/s for 16–32 min (1 and 2 L—total precipitation, pH adjusted to 7 and 5). Duplicated rainfall simulation experiments were conducted.
Chemical Analysis
Chemical analysis of 6PPD-Q is detailed in the SI, Text S1 and Figures S6–S8. In brief, solution samples in sorption kinetics and isotherm analysis were analyzed by high-performance liquid chromatography (HPLC) with a diode-array detector (DAD) for ultraviolet (UV) analysis. For desorption kinetics and rainfall simulation, 6PPD-Q in solution samples was concentrated through the solid phase extraction (SPE) process to 100 μL of acetonitrile and analyzed by HPLC–time of the flight-mass spectrometry (TOF-MS). Total 6PPD-Q in asphalt mixtures was analyzed through solvent extraction of powdered crushed loose mixtures and analysis with HPLC-TOF-MS.
Data Analysis
Statistical analyses were performed with IBM SPSS Statistics (ver. 26, IBM Corp., Armonk, NY, USA). MS analysis was done with MestReNova (14.2, Mestrelab Research, Santiago de Compostela, Spain). Molecular formulas were assigned using Formularity (1.0.8045, PNNL, Washington, USA).
Results and Discussion
HPLC-TOF-MS Analysis of 6PPD-Q
6PPD-Q was analyzed by HPLC-TOF-MS with an instrument detection limit of 2 μg/L (n = 3, S/N = 3). With the C18 column, the retention time of 6PPD-Q was 22.1 ± 0.03 min; under the positive mode, the MS peak with m/z = 299.176 was detected for [C18H22O2N2 + H]+. A good calibration curve (R2 = 0.99) was established for 6PPD-Q in the range 10–1000 μg/L (SI, Figure S8). Solid-phase extraction (SPE) was performed for the DDI water and synthetic stormwater without and with spiked 6PPD-Q. Following the EPA guidelines,28 using replicate measurement (n = 7) of DDI water and synthetic stormwater through SPE-HPLC-TOF-MS, the method detection limit was determined as 8.0 × 10–4 and 7.0 × 10–4 μg/L (S/N = 3), respectively. The recovery of 6PPD-Q through the process was 58%.
Sorption Kinetics and Isotherm
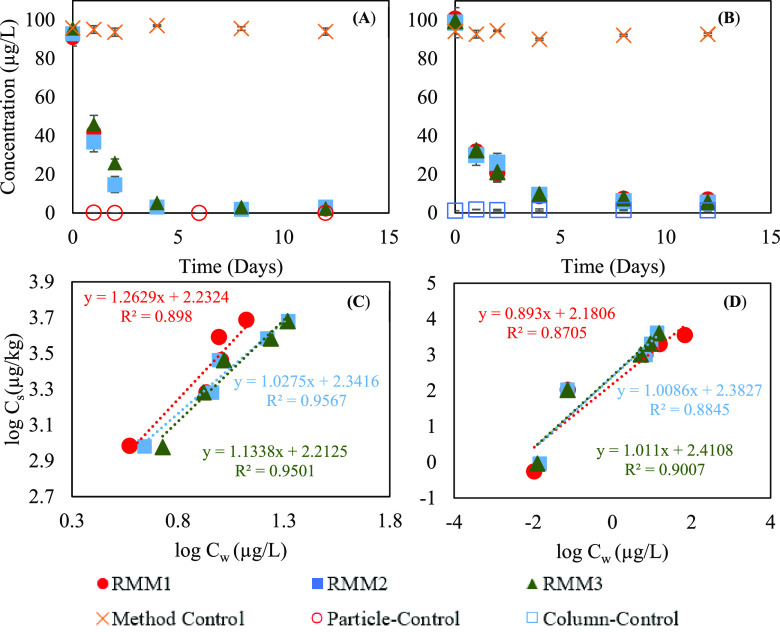
The sorption of 6PPD-Q on crushed loose rubberized asphalt mixtures (loose particles, ∼5 mm) and compacted asphalt mixtures reached equilibrium within 12 days (Figure 1A and B). For the crushed loose mixtures, the sorption isotherm can be fitted with the Freundlich sorption equation with a nonlinear index of 1.03–1.26 (R2 = 0.90–0.96; Figure 1C). The sorption of 6PPD-Q on presaturated rubberized compacted asphalt mixtures reached equilibrium within 12 days (Figure 1B; SI, Figure S9) and can also be fitted well with the Freundlich sorption equation (R2 = 0.87–0.9; Figure 1D; SI, Table S3). With the aqueous concentration of 6PPD-Q at 0.01 μg/L, the sorption coefficient of 6PPD-Q on the compacted columns ranged 151.57–257.51 L/kg. Based on the work of Jiang et al.,25 the sorption coefficient of permethrin (log Kow = 6.5) on cement concrete was 5.11 ± 0.29 L/kg. Hu et al.29 measured the Kow of 6PPD-Q to be 104.3±0.02. Tian et al.6 reported the Kow to be between 105 and 105.5 when the U.S. EPA’s EPI Suite prediction software predicts a Kow of 103.98.30 The sorption coefficient measured for 6PPD-Q was higher than that of permethrin, although the Kow of 6PPD-Q was lower, noting that cement concrete was used in the study for the sorption of permethrin when asphalt concrete was used in this study for the sorption of 6PPD-Q. Assuming field runoff conditions similar to that used in the experiment (25 mL (2.0 cm height) of overlaid water versus 3.8 cm of asphalt concrete surface layer), over 90% of 6PPD-Q would be sorbed by the asphalt concrete. These results indicate the potential for asphalt mixtures to act as a reservoir for 6PPD-Q upon its release into the stormwater.
Figure 1.
Sorption kinetics (A,B) and isotherm (C,D) of 6PPD-quinone on crushed loose (loose particles, ∼5 cm) rubberized asphalt mixtures and compacted rubberized asphalt mixtures, respectively. RMM1, RMM2, and RMM3 represent the three different crushed loose rubberized asphalt mixtures used; Particle-Control and Column-Control indicate experiments with crushed loose asphalt mixture (RMM1) and compacted asphalt mixtures (RMM2), respectively, without spiking 6PPD-quinone. Method Control represents samples with 6PPD-quinone spiked but without crushed loose/compacted asphalt mixtures. Cs and Cw are the concentrations of 6PPD-Q in the solid and in the aqueous phase, respectively. Error bars for A represent standard deviation derived from triplicate experiments, while error bars for B represent standard deviation derived from duplicate experiments, which is too small to be visualized for most data points.
Desorption Kinetics
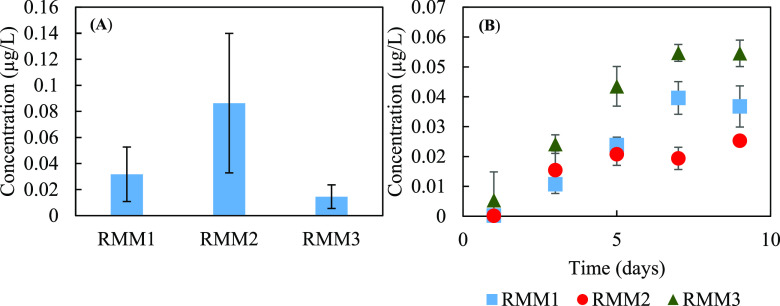
The desorption of 6PPD-Q from crushed loose (loose particle, ∼5 cm) rubberized asphalt mixtures was minimal. While the use of RTR material in the asphalt mixture (<1% by weight) contributed to 333–1530 μg/kg of 6PPD-Q (based on acetonitrile extraction using powdered crushed loose asphalt mixture; SI, Text S1), only 0.26–0.45% of mixture-bound 6PPD-Q was released to the aqueous phase (with a concentration of 0.01 ± 0.009–0.09 ± 0.05 μg/L) within 12 days (Figure 2A). The release of 6PPD-Q from the compacted rubberized asphalt mixture to the solution phase approached steady-state stage within 9 days and ranged from 0.025 ± 0.001– to 0.05 ± 0.004 μg/L (Figure 2B), equivalent to 0.064–0.35% of column-bound 6PPD-Q released, more representative of real-world application conditions and lower than that from the crushed loose mixtures.
Figure 2.
(A) Desorption of 6PPD-quinone from 20 g/L crushed loose (loose particles, ∼5 cm) rubberized asphalt mixtures to solution-phase during 12 days of experimentation. RMM1, RMM2, and RMM3 represent the different crushed loose rubberized asphalt mixtures. (B) Desorption of 6PPD-quinone from rubberized compacted asphalt mixtures. Error bars represent standard deviation derived from duplicate experiments.
In addition to 6PPD-Q, a range of chemicals was captured by HPLC-TOF-MS for the desorption from RMM1 crushed loose rubberized asphalt mixtures. There are several significant peaks with 25.0 and 27.0 min retention time (SI, Figure S10). Based on the molecular formula assignment, the desorption from rubberized asphalt mixtures was dominated by molecules with molecular weights ranging from 400 to 500 Da (SI, Figure S11).
Mobilization during Rainfall Simulation
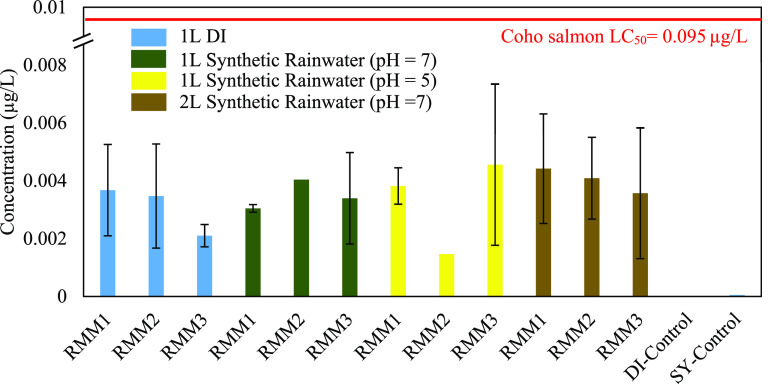
The release of 6PPD-Q during simulated rainfall experiments was minimal and well below the reported LC50 concentration.6−8,10 The influences of the rainfall conditions on the release of 6PPD-Q were minimal. Mobilization of 6PPD-Q during the rainfall simulations with compacted asphalt mixtures under all conditions ranged from 0.0015 ± 0.0002 to 0.0046 ± 0.003 μg/L (Figure 3), which is well below the reported LC50 for coho salmon (0.095 μg/L). Hu et al.29 reported an estimated 60–130 ng/L release of 6PPD-Q from tire wear particles on roadways in the U.S., much lower than the release from rubberized asphalt. The total organic carbon detected was 2.2–2.8 mg C/L for the runoff from compacted rubberized asphalt mixtures (SI, Figure S12). A full MS scan of the simulated rainfall runoff samples for RMM1 rubberized asphalt mixtures with 1 L of DDI water revealed over 6000 compounds. Based on the modified aromaticity index (AImod),31 the aliphatic compounds dominated the overall captured compounds, while in the aromatic components (AImod < 0.5), condensed aromatic compounds (AImod > 0.67) dominated (SI, Figure S13). The captured compounds had an average molecular weight of 929.8 Da and a formula of C48H65O15N1.
Figure 3.
Comparison of 6PPD-quinone concentration in different simulations with compacted rubberized asphalt mixtures. DI-Control corresponds to double deionized water run through the entire setup without the compacted asphalt mixture, and SY-Control corresponds to synthetic rainwater run through the setup without a compacted asphalt mixture. Error bars represent standard deviation derived from duplicate experiments.
Environmental Implication
Our study demonstrated that rubberized asphalt concrete materials released a minimal amount of 6PPD-Q when <1% by weight of RTR was added to the asphalt materials. A limited release can be the result for multiple reasons: 6PPD-Q was relatively less soluble and hydrophobic, with the release of 6PPD-Q lower than the release of organic carbon in rubberized asphalt materials. Second, the release of 6PPD-Q can be hindered by incorporating tire rubber in the asphalt matrix and binder with limited materials exposed on the exterior surface. Based on Tian et al.,7,32 the release of 6PPD-Q can be as high as 13.9 g of 6PPD-Q/kg of tire rubber, depending on how the release and leachate were conducted. Based on the 1% by weight addition of RTR into rubberized asphalt mixtures, the 6PPD-Q release was below 0.139 g of 6PPD-Q/kg of rubberized asphalt mixtures—assuming no loss of 6PPD-Q during production. Third, due to the heterogeneous porous materials of rubberized asphalt, 6PPD-Q can also be sorbed by other components used in asphalt, as our study demonstrated. It warrants further studies to fully understand the whole life cycle of 6PPD-Q during the production of rubberized asphalt materials and its exposure to the environment. The HPLC-TOF-MS analysis detected a wide range of compounds potentially released from rubberized asphalt materials, indicating the potential that 6PPD-Q or its transformation products may be released when the pavement is exposed to stormwater. Our current simulator targeted runoff collection from the surface of asphalt concrete columns. Still, stormwater can also infiltrate asphalt columns with air voids of 7%, as determined by the water infiltration experiment. The infiltrated water might contain higher 6PPD-Q, and more extensive interactions with the asphalt matrix, regulated by the permeability of asphalt materials, are possible, which are part of our next-step studies.
In addition, we determined that rubberized asphalt materials can sorb 6PPD-Q and serve as a sink for this emerging toxicant from stormwater. The sorption of 6PPD-Q in stormwater by the asphalt concrete will depend on the contact time and infiltration/penetration depth. Due to the higher surface area, loose asphalt mixtures have much higher sorption than compacted asphalt mixtures. More comprehensive investigation into the sorption of 6PPD-Q by the compacted asphalt mixtures and their major components responsible for the sorption of 6PPD-Q will help mitigate the environmental risk. Investigation of the release of 6PPD-Q from weathered asphalt can be of great value for understanding the long-term fate of 6PPD-Q at the asphalt–water interface.
Acknowledgments
This project was financially supported by Granite Construction Inc.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsenvironau.3c00023.
Additional experimental details, materials and methods, and results, including photographs of experimental setup (PDF)
The authors declare the following competing financial interest(s): This study was financially supported by Granite Constructions Inc (Watsonville, CA, USA).
Supplementary Material
References
- Kruger R. H.; Boissiere C.; Klein-Hartwig K.; Kretzschmar H. -J. New Phenylenediamine Antiozonants for Commodities Based on Natural and Synthetic Rubber. Food Addit. Contam. 2005, 22 (10), 968–974. 10.1080/02652030500098177. [DOI] [PubMed] [Google Scholar]
- Sheridan M.The Vanderbilt Rubber Handbook; RT Vanderbilt Company, Inc.: Norwalk, CT, 2010. [Google Scholar]
- Poldushova G. A.; Kandyrin K. L.; Reznichenko S. V. The Effect of the Structure of P-Phenylenediamine Antiagers on the Physicomechanical and Hysteresis Properties of Filled Rubber Compounds. Int. Polym. Sci. Technol. 2016, 43 (2), 19–22. 10.1177/0307174X1604300205. [DOI] [Google Scholar]
- Dorofeev A. N.; Zemskii D. N. Oxypropylated Aromatic Diamines–Stabilisers for Tyre Rubbers. Int. Polym. Sci. Technol. 2017, 44 (6), 27–30. 10.1177/0307174X1704400604. [DOI] [Google Scholar]
- Zheng W.; Jia Z.; Zhang Z.; Yang W.; Zhang L.; Wu S. Improvements of Lanthanum Complex on the Thermal-Oxidative Stability of Natural Rubber. J. Mater. Sci. 2016, 51 (19), 9043–9056. 10.1007/s10853-016-0157-4. [DOI] [Google Scholar]
- Tian Z.; Zhao H.; Peter K. T.; Gonzalez M.; Wetzel J.; Wu C.; Hu X.; Prat J.; Mudrock E.; Hettinger R.; Cortina A. E.; Biswas R. G.; Kock F. V. C.; Soong R.; Jenne A.; Du B.; Hou F.; He H.; Lundeen R.; Gilbreath A.; Sutton R.; Scholz N. L.; Davis J. W.; Dodd M. C.; Simpson A.; McIntyre J. K.; Kolodziej E. P. A Ubiquitous Tire Rubber–Derived Chemical Induces Acute Mortality in Coho Salmon. Science 2021, 371 (6525), 185–189. 10.1126/science.abd6951. [DOI] [PubMed] [Google Scholar]
- Tian Z.; Gonzalez M.; Rideout C. A.; Zhao H. N.; Hu X.; Wetzel J.; Mudrock E.; James C. A.; McIntyre J. K.; Kolodziej E. P. 6PPD-Quinone: Revised Toxicity Assessment and Quantification with a Commercial Standard. Environ. Sci. Technol. Lett. 2022, 9 (2), 140–146. 10.1021/acs.estlett.1c00910. [DOI] [Google Scholar]
- Varshney S.; Gora A. H.; Siriyappagouder P.; Kiron V.; Olsvik P. A. Toxicological Effects of 6PPD and 6PPD Quinone in Zebrafish Larvae. J. Hazard. Mater. 2022, 424, 127623. 10.1016/j.jhazmat.2021.127623. [DOI] [PubMed] [Google Scholar]
- Brinkmann M.; Montgomery D.; Selinger S.; Miller J. G. P.; Stock E.; Alcaraz A. J.; Challis J. K.; Weber L.; Janz D.; Hecker M.; Wiseman S. Acute Toxicity of the Tire Rubber-Derived Chemical 6PPD-Quinone to Four Fishes of Commercial, Cultural, and Ecological Importance. Environ. Sci. Technol. Lett. 2022, 9 (4), 333–338. 10.1021/acs.estlett.2c00050. [DOI] [Google Scholar]
- Hiki K.; Asahina K.; Kato K.; Yamagishi T.; Omagari R.; Iwasaki Y.; Watanabe H.; Yamamoto H. Acute Toxicity of a Tire Rubber-Derived Chemical, 6PPD Quinone, to Freshwater Fish and Crustacean Species. Environ. Sci. Technol. Lett. 2021, 8 (9), 779–784. 10.1021/acs.estlett.1c00453. [DOI] [Google Scholar]
- McIntyre J. K.; Prat J.; Cameron J.; Wetzel J.; Mudrock E.; Peter K. T.; Tian Z.; Mackenzie C.; Lundin J.; Stark J. D.; King K.; Davis J. W.; Kolodziej E. P.; Scholz N. L. Treading Water: Tire Wear Particle Leachate Recreates an Urban Runoff Mortality Syndrome in Coho but Not Chum Salmon. Environ. Sci. Technol. 2021, 55 (17), 11767–11774. 10.1021/acs.est.1c03569. [DOI] [PubMed] [Google Scholar]
- Rauert C.; Charlton N.; Okoffo E. D.; Stanton R. S.; Agua A. R.; Pirrung M. C.; Thomas K. V. Concentrations of Tire Additive Chemicals and Tire Road Wear Particles in an Australian Urban Tributary. Environ. Sci. Technol. 2022, 56 (4), 2421–2431. 10.1021/acs.est.1c07451. [DOI] [PubMed] [Google Scholar]
- Johannessen C.; Helm P.; Lashuk B.; Yargeau V.; Metcalfe C. D. The Tire Wear Compounds 6PPD-Quinone and 1, 3-Diphenylguanidine in an Urban Watershed. Arch. Environ. Contam. Toxicol. 2022, 82 (2), 171–179. 10.1007/s00244-021-00878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokstad E. Why Were Salmon Dying? The Answer Washed off the Road. Science 2020, 370 (6521), 1145. 10.1126/science.370.6521.1145. [DOI] [PubMed] [Google Scholar]
- Popick H. J.Assessment of Stormwater and Snowmelt Quality and Quantity Discharging from a Cold-Climate City to a Freshwater River. Thesis, University of Saskatchewan, 2022. [Google Scholar]
- Cao G.; Wang W.; Zhang J.; Wu P.; Zhao X.; Yang Z.; Hu D.; Cai Z. New Evidence of Rubber-Derived Quinones in Water, Air, and Soil. Environ. Sci. Technol. 2022, 56 (7), 4142–4150. 10.1021/acs.est.1c07376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masset T.; Ferrari B. J.; Oldham D.; Dudefoi W.; Minghetti M.; Schirmer K.; Bergmann A.; Vermeirssen E.; Breider F. In vitro digestion of tire particles in a fish model (Oncorhynchus mykiss): solubilization kinetics of heavy metals and effects of food coingestion. Environ. Sci. Technol. 2021, 55 (23), 15788–15796. 10.1021/acs.est.1c04385. [DOI] [PubMed] [Google Scholar]
- Johannessen C.; Helm P.; Lashuk B.; Yargeau V.; Metcalfe C. D. The Tire Wear Compounds 6PPD-Quinone and 1, 3-Diphenylguanidine in an Urban Watershed. Arch. Environ. Contam. Toxicol. 2022, 82 (2), 171–179. 10.1007/s00244-021-00878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PowerPoint Presentation: 2019 U.S. Scrap Tire Management Summary. ustires.org/sites/default/files/2019%20USTMA%20Scrap%20Tire%20Management%20Summary%20Report.pdf (accessed July 7, 2023).
- Anburuvel A. Utilizing Scrap Tyre in Unbound Pavement Layers: A State-of-the-Art Review. Int. J. Pavement Res. Technol. 2022, 1–18. 10.1007/s42947-022-00203-9. [DOI] [Google Scholar]
- Dhoska K.; Sakaj E.; Moezzi R. Enhancement of Road Construction by Using Recycled Tire Rubber Modified Bitumen. Journal of Transactions in Systems Engineering 2023, 1 (1), 1–9. [Google Scholar]
- Alfayez S. A.; Suleiman A. R.; Nehdi M. L. Recycling Tire Rubber in Asphalt Pavements: State of the Art. Sustainability 2020, 12 (21), 9076. 10.3390/su12219076. [DOI] [Google Scholar]
- Resource Responsible Use of Recycled Tire Rubber in Asphalt Pavements. https://www.fhwa.dot.gov/pavement/asphalt/hif20043.pdf (accessed July 7, 2023).
- Wagner S.; Klöckner P.; Reemtsma T. Aging of Tire and Road Wear Particles in Terrestrial and Freshwater Environments–a Review on Processes, Testing, Analysis and Impact. Chemosphere 2022, 288, 132467. 10.1016/j.chemosphere.2021.132467. [DOI] [PubMed] [Google Scholar]
- Jiang W. Y.; Gan J.; Haver D. Sorption and desorption of pyrethroid insecticide permethrin on concrete Environ. Sci. Technol. 2011, 45, 602–607. 10.1021/es1030323. [DOI] [PubMed] [Google Scholar]
- Caltrans Asphalt Rubber Usage Guide; State of California Department of Transportation, Materials Engineering and Testing Services: Sacramento, CA, 2006; 95819-4612. [Google Scholar]
- Ostrom T. K.; Davis A. P. Evaluation of an Enhanced Treatment Media and Permeable Pavement Base to Remove Stormwater Nitrogen, Phosphorus, and Metals under Simulated Rainfall. Water Res. 2019, 166, 115071. 10.1016/j.watres.2019.115071. [DOI] [PubMed] [Google Scholar]
- Definition and Procedure for the Determination of the Method Detection Limit, Revision 2; EPA 821-R-16-00; Environmental Protection Agency, 2016.
- Hu X.; Zhao H.; Tian Z.; Peter K. T.; Dodd M. C.; Kolodziej E. P. Chemical Characteristics, Leaching, and Stability of the Ubiquitous Tire Rubber-Derived Toxicant 6PPD-Quinone. Environ. Sci.: Processes Impacts 2023, 25 (5), 901–911. 10.1039/D3EM00047H. [DOI] [PubMed] [Google Scholar]
- Seung L. J. EPI Suite: A Fascinate Predictive Tool for Estimating the Fates of Organic Contaminants. J. Biorem. Biodegrad. 2016, 7, e171. [Google Scholar]
- Koch B. P.; Dittmar T. From Mass to Structure: An Aromaticity Index for High-Resolution Mass Data of Natural Organic Matter. Rapid Commun. Mass Spectrom. 2006, 20 (5), 926–932. 10.1002/rcm.2386. [DOI] [Google Scholar]
- Hu X.; Zhao H. N.; Tian Z.; Peter K. T.; Dodd M. C.; Kolodziej E. P. Transformation Product Formation upon Heterogeneous Ozonation of the Tire Rubber Antioxidant 6PPD (N-(1,3-Dimethylbutyl)-N′-Phenyl-p-Phenylenediamine). Environ. Sci. Technol. Lett. 2022, 9 (5), 413–419. 10.1021/acs.estlett.2c00187. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.