Abstract
A quantitative analysis of the Cl− dependence of growth of Halobacillus halophilus was performed. Optimal growth rates were obtained at Cl− concentrations of between 0.5 and 2.0 M, and the final yield was also strictly dependent on the Cl− concentration. Br− but not I−, SO42−, NO2−, SO2−, OCN−, SCN−, BO2−, or BrO3− could substitute for Cl−. To analyze the function of chloride, chloride concentration was determined. At low external Cl− (Cle−) concentrations, the growth rate was low and Cl− was excluded from the cytoplasm; increasing the Cle− concentration led to an increase in the growth rate and an energy-dependent uptake of Cl−, thus decreasing the Cle−/internal Cli− gradient from ≥10 at 0.1 M Cle− to a nearly constant value of 2 at Cle− concentrations which allowed optimal growth. Two membrane proteins with apparent molecular masses of 31 and 16 kDa which were identified to be specific for Cl−-grown cultures are possible candidates for a chloride uptake system.
The North Sea coast of Germany is characterized by intertidal flats (Wadden Sea) that are flooded regularly with seawater. The foreland or salt marshes are established by slowly sedimenting material and subsequent colonialization by salt-tolerating plants. Salt marshes, due to their higher elevation, are flooded only 100 to 200 times a year. One of the major factors in this environment which undergoes frequent changes is the salt concentration. Due to a more or less constant influx of salt via the sea wind, as well as floodings with subsequent evaporation of seawater, the salt concentration in the salt marshes can be as high as 10%, whereas the average salt concentration in the North Sea is approximately 3.5%. On the other hand, after heavy rainfalls it can decrease to freshwater concentrations.
Seawater contains 550 mM Cl−, which is equivalent to 95% of the anions present in seawater, and, therefore, chloride concentration is one of the chemical factors in salt marshes which undergo drastic changes. However, whether chloride is important for the physiology of marine or salt marsh bacteria or whether it is even involved in signaling and adaptation processes remains almost unexplored. Already in 1956, MacLeod and coworkers reported the isolation of Cl−-dependent organisms from seawater (9, 10); however, the organisms were not further characterized with respect to the nature of chloride dependence, and, unfortunately, they are no longer available in culture collections. Claus et al. reported the isolation of an aerobic, endospore-forming bacterium, Halobacillus halophilus (formerly Sporosarcina halophila), from salt marshes. In their original description, they described that H. halophilus grows optimally at approximately 0.5 to 0.9 M NaCl and that “chloride cannot be replaced by sulfate” (2). Unfortunately, it was not determined whether growth failure was due to a lack of chloride or to a growth inhibition by sulfate, quantitative measurements were not done, and the physiology of the Cl− dependence was not analyzed. To unequivocally identify a chloride dependence of the salt marsh bacterium H. halophilus, we performed a detailed physiological characterization. This study unequivocally identifies chloride as an obligatory ion for H. halophilus, quantifies the requirements for chloride, reveals Br− and NO3− as a substitute for Cl−, indicates the presence of a chloride uptake system, and identifies two membrane-bound polypeptides which are possible candidates for the Cl− pump.
MATERIALS AND METHODS
Organism, cultivation, and growth experiments.
H. halophilus (DSM 2266) was maintained on nutrient broth (NB) (Difco) supplemented with 0.05 M MgSO4 and 0.5 M NaCl or 0.5 M NaNO3 (in case the NO3− dependence of growth was to be determined). Growth experiments were done in 15-ml tubes filled with 5 ml of the indicated medium. After inoculation (1%), the cultures were incubated on a rotary shaker at 30°C. The optical density at 600 nm (OD600) was determined in a Bausch and Lomb photometer. To determine the pH optimum, 0.05 M Tris (pH 7 to 10.5) or 0.05 M succinate (pH 5 to 6.5) was added to the medium, and the pH was adjusted as indicated in the experiments by the addition of NaOH or H2SO4. All data points given reflect the means of duplicate tubes from one experiment, and experiments were performed at least three times.
Preparation of cell suspensions and determination of chloride concentrations and internal volume.
For preparation of cell suspensions, NB with the indicated NaCl concentrations was inoculated (1%) from cultures maintained at 0.5 M NaCl. Fresh cell suspensions were prepared for each experiment. Cells in the late logarithmic growth phase were harvested by centrifugation and washed once with 0.05 M Tris containing 0.05 M MgSO4 and NaCl as indicated. The cell pellet was resuspended in the same buffer to a concentration of 2 to 5 mg/ml and stored on ice until use. The protein concentration of the cell suspension was determined by the method described by Schmidt et al. (20), with bovine serum albumin as a standard.
The experiments were carried out in Eppendorf tubes filled with 0.5 ml of 0.05 M Tris (pH 7.8), 50 μl of the concentrated cell suspension (15 to 25 mg of protein/ml), NaCl concentrations as indicated, and 10 μl of Na36Cl (specific activity, 50 μCi/mmol and 0.45 μCi). After incubation at room temperature on a rotary shaker for 15 min, seven samples of 50 μl each were taken and used to calculate internal Cl− (Cli−) concentrations. The cell suspension then received valinomycin (final concentration, 100 μM) and K2SO4 (final concentration, 0.125 M) and was incubated for 10 min. This treatment dissipated the membrane potential and led in any case to a collapse of the external Cl− (Cle−)/Cli− gradient, indicating that only freely permeable and not cell-surface-bound Cl− was measured. Samples were filtered through nitrocellulose filters (25 mm in diameter, pore size, 0.45 μm; Sartorius, Göttingen, Germany) and washed three times with 0.05 M Tris. Sampling and washing were done in less than 15 s. Nonspecific binding of 36Cl− was reduced by incubation of the filters overnight in 0.05 M Tris (pH 7.8) containing 0.5 M NaCl; all values were corrected for nonspecific binding of Cl− to the filters. The filters were air dried, and radioactivity was determined in a liquid scintillation counter type PW4700 (Philipps, Hamburg, Germany) by using ultima gold (Packard, Dreieich, Germany) as the scintillation cocktail.
The Cli− concentration was calculated by using the internal volume determined by silicone oil centrifugation as described elsewhere (17). 3H2O and [14C]dextrane were used as markers for the total and external water spaces, respectively. A 10-μCi amount of 3H2O and 1 μCi of [14C]dextrane were added to 10 ml of cell suspension. It was confirmed that the sugars were not taken up by the cells.
Preparation of cell extracts and SDS-PAGE.
Precultures were grown for at least two transfers with the anion at the concentration indicated and then used to inoculate 200 ml of NB containing 50 mM MgSO4 and the anion as sodium salt. The cells were harvested at an OD600 of 0.5, washed twice with 0.05 M Tris (pH 7.8), resuspended to a final concentration of 2.5 to 4 mg/ml in 0.05 M Tris (pH 7.8), and broken up in a French pressure cell. Cell debris was removed by low-speed centrifugation, and the resulting cell extract was then centrifuged at 110,000 × g and 4°C for 1 h. The pellet was resuspended in 2 ml of 0.05 M Tris (pH 7.8) to a final protein concentration of 1.5 to 2.5 mg/ml. The protein content was determined by the method described by Lowry et al. (8), and 10 μg of protein was applied to a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel according to the method described by Schägger and von Jagow (19) with 12.5% gels. Silver staining was done as described elsewhere (1).
RESULTS
Dependence of growth on NaCl, Na+, and Cl−.
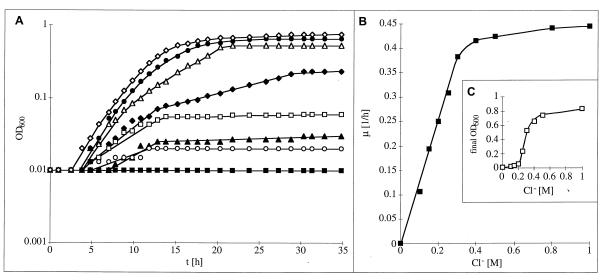
To perform a quantitative analysis of the dependence of the growth of H. halophilus on salt concentration, NB was supplemented with different concentrations of NaCl. Growth was impaired in the absence of salt, but the growth rate increased sharply with increasing NaCl concentrations. The highest growth rates were obtained between 0.5 and 2.0 M NaCl, but even concentrations of as much as 2.5 and 3.0 M were tolerated with growth rates of 50 and 38%, respectively. In addition, the final yield was dependent on the NaCl concentration (data not shown). To determine the dependence on salt in more detail, single components of the medium were substituted, while the ion concentration in the medium was kept constant. First, the Na+ concentration was varied while the total salt concentration was kept at least at 0.5 M by the appropriate addition of KCl. Growth was strictly dependent on Na+, and maximal growth rates were obtained at concentrations of between 0.5 and 2.0 M (data not shown). Na+ could not be substituted by Li+ or K+. Of particular interest was the effect of Cl− on the growth of H. halophilus. To determine Cl− dependence, the NaCl concentration was varied from 0 to 1.0 M, while the total salt concentration was kept at least at 0.5 M by the appropriate addition of NaNO3. As can be seen from Fig. 1, growth was impaired in the absence of Cl−, but increasing Cl− concentrations led to increasing growth rates. Saturation was obtained at 0.5 to 1.0 M Cl−. Interestingly, the final yield was also strictly dependent on the Cl− concentration, but the dependence showed a sigmoidal character; at 0.2 M Cl−, the final yield was only 1/10 of the maximal yield obtained at 0.5 M Cl− (Fig. 1).
FIG. 1.
Cl− dependence of growth of H. halophilus. (A) Growth determined in NB (pH 7.8), containing 0.05 M MgSO4 and 0 (■), 0.1 (○), 0.15 (▴), 0.2 (□), 0.25 (⧫), 0.3 (▵), 0.4 (•), or 0.5 (◊) M NaCl. Salt concentration was maintained at least at 0.5 M by appropriate addition of NaNO3. (B) Growth rates plotted against Cl− concentration. (C) Cell yields (final OD600) plotted against the Cl− concentration. Precultures were maintained in NB containing 0.5 M NaCl.
To confirm that the observed dependence was indeed due to a stimulation by chloride and not an inhibition by nitrate (which was used to keep the osmolarity constant), the following controls were performed. First, addition of 0.5 M NaCl to a culture containing 0.5 M NaNO3 led to an immediate onset of growth indistinguishable from that of cultures maintained at 0.5 M NaCl. Second, when the total anion concentration was kept constant at 1.0 M by the appropriate addition of Na2SO4, the same chloride dependence was observed. Again, addition of NaCl to a culture containing Na2SO4 led to growth indistinguishable from that of cultures maintained at 0.5 M NaCl. These experiments unequivocally demonstrate a dependence on chloride for growth and biomass production of H. halophilus. The amounts of chloride, NaCl, and Na+ that were required for growth as well as that were tolerated were in the same range and reflect the chemical composition of the salt marshes.
To investigate the dependence on chloride in more detail, we analyzed whether other halides or anions could support growth. Of the compounds tested, only Br− and Cl− but not I−, SO42−, NO2−, SO2−, OCN−, SCN−, BO2−, or BrO3− supported growth of H. halophilus. The growth rates and final yields obtained with chloride or bromide were alike. Therefore, H. halophilus is the first aerobic bacterium for which a chloride dependence has been shown unequivocally, and, most interestingly, chloride can be substituted by another halide, bromide.
Determination of Cli− concentrations.
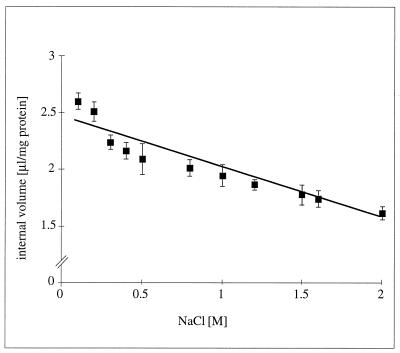
To analyze the function of chloride in H. halophilus, Cli− concentrations at different Cle− concentrations were determined. In order to calculate intracellular concentrations accurately, the intracellular volumes of the cells at different osmolarities were determined. As can be seen from Fig. 2, the intracellular volumes of H. halophilus varied with the external salt concentration by 40%, from 2.6 to 1.6 μl/mg of protein. To determine the Cli− concentration in H. halophilus, cell suspensions were incubated with Na36Cl and allowed to equilibrate, and the Cli− concentration was then determined during the steady state of endergonic respiration. In the presence of 0.5 M Na36Cl, the Cli− concentration was determined to be 0.08 M; upon dissipation of the membrane potential by the addition of K2SO4 plus valinomycin, Cl− entered the cells immediately within the time resolution of the experiment until Cle− and Cli− equilibrated (Cli− = 0.472 M [mean of seven experiments]). These experiments demonstrate that Cl− was freely permeable across the cytoplasmic membrane, and the low Cli− concentrations measured are therefore not due to a restricted permeability of the 36Cl− tracer used.
FIG. 2.
Internal volume of H. halophilus at increasing external NaCl concentrations. For explanations, see Materials and Methods. The data points reflect the means of three independent experiments.
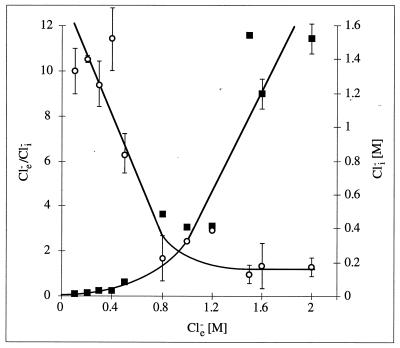
It is evident from Fig. 3 that at low Cl− concentrations which do not support growth (0.1 and 0.2 M), Cl− is excluded from the cytoplasm (Cle−/Cli− = 10). The Cle−/Cli− gradient of 10 has to be considered minimal, since the values determined for Cli− at low Cle− are very close to unspecific binding and, therefore, might be less than those determined. However, increasing the Cle− concentration from 0.1 to 0.5 M led to growth stimulation and, concomitantly, to an uptake of Cl− into the cytoplasm, thereby decreasing Cle−/Cli− continuously up to a value of 2 at a Cle− of ca. 1.2 M. A further increase of Cle− to as much as 2.0 M led to a nearly proportional uptake of Cl−, thereby keeping Cle−/Cli− fairly constant at 2. These experiments demonstrate that chloride is excluded from the cytoplasm at low Cle−. Increasing the Cle− concentration resulted not only in a stimulation of growth but, concomitantly, in an uptake of chloride which, based on thermodynamic calculations, was energy dependent (see Discussion).
FIG. 3.
Cli− concentrations (■) and Cle−/Cli− gradients (○) at increasing external NaCl concentrations. For explanations, see Materials and Methods.
Adaptation of H. halophilus to chloride-free media.
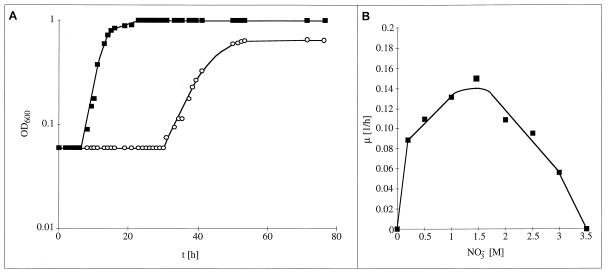
After heavy rainfalls, the salt concentration in salt marshes can decrease to freshwater concentrations and, therefore, we tested whether H. halophilus is able to adapt to salt- or chloride-free conditions. Even after incubations for as much as 4 weeks, H. halophilus did not grow in the absence of NaCl, indicating an obligate requirement for salt. However, when NaCl was replaced by NaNO3, approximately 90% of the cultures did grow after a variable adaptation time of approximately 30 h (Fig. 4). The protein compositions of 10 cultures examined were identical, as judged by SDS-PAGE, and microscopic examination of spores embedded in agar revealed that growth in nitrate was not restricted to few cells but rather was a common event (4a), indicating adaptation rather than mutation and selection as a basis for this effect. Growth of NO3−-adapted cells was still strictly dependent on the salt concentration, but at any given NaNO3 concentration the growth rate and the final yield were smaller in NaNO3- than in NaCl-containing medium (Fig. 4). Upon retransfer of nitrate-adapted cells into Cl−-containing medium, cells grew without any lag phase and the growth rate was less than that in Cl−-maintained cells but greater than that in NO3−-adapted cells (data not shown). Under the same conditions, the cultures did not adapt to sulfate. These results demonstrate the potential of H. halophilus to adapt to changing environmental conditions, although the nitrate concentration in the salt marshes might be too low to sustain growth.
FIG. 4.
Adaptation of H. halophilus to Cl−-free but NO3−-containing media. (A) Cells grown in NB (pH 7.8) containing 0.05 M MgSO4 and 0.5 M NaCl (squares) or NaNO3 (circles); (B) growth rates of NO3−-maintained cultures plotted against NO3− concentrations.
Cl−-induced proteins in H. halophilus.
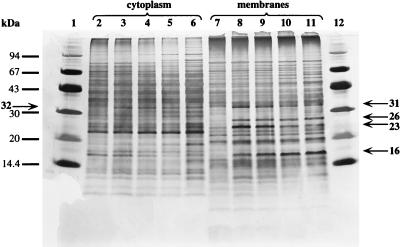
To determine chloride-induced proteins, cell extracts were prepared from cultures grown in Cl− or NO3− media, separated into cytoplasm and membrane fractions, and subjected to SDS-PAGE. As can be seen from Fig. 5, Cl−-grown cultures exhibited a polypeptide pattern different from those of NO3−-grown cultures. The cytoplasm of Cl−-grown cultures contained a 32-kDa polypeptide apparently absent in NO3−-grown cells. In addition, membranes of Cl−-grown cells exclusively contained two polypeptides with molecular masses of 31 and 16 kDa. The 26- and 23-kDa polypeptides did not appear consistently in Cl−-grown cells, indicating the necessity of other induction factors; however, if present, synthesis of at least the 23-kDa polypeptide was regulated by Cl−. Apparently, there are no indications for polypeptides exclusively found in NO3−-grown cells, as revealed by SDS-PAGE. An increase in the Cle− concentration led to a steady increase in the membrane-bound 16-kDa polypeptide, whereas the concentration of the 32-kDa cytoplasmic polypeptide reached a constant level at 1.0 M Cl−. On the other hand, the membrane-bound 31-kDa polypeptide was maximal at 0.25 and 0.5 M Cl− but steadily decreased with further increasing Cl− concentrations. These results clearly show the presence of chloride-induced proteins (two membrane bound and one cytoplasmic) whose synthesis is regulated by the Cle− concentration.
FIG. 5.
SDS-PAGE of cytoplasm and membranes of H. halophilus. Cells were grown in NB plus 0.05 M MgSO4 (pH 7.8) containing 0.5 M NaNO3 (lanes 2 and 7), or 0.25 (lanes 3 and 8), 0.5 (lanes 4 and 9), 1.0 (lanes 5 and 10), or 2.0 (lanes 6 and 11) M NaCl. Cultures were grown for at least two transfers in the medium indicated. Standards are shown in lanes 1 and 12. Chloride-induced proteins and their apparent molecular masses are indicated by arrows.
DISCUSSION
Living organisms depend on a variety of ions for growth, metabolism, and energy generation, and a requirement for a special ion might result from the adaptation of a microorganism to its environment. NaCl is present in almost all environments, and a biological role of Na+ is well documented in all kingdoms of life (4, 13). Chloride, on the other hand, is essential for eucaryotes, in which it is required for solute transport, osmoregulation, and maintaining electrical balances (11), but there are only a few examples showing that chloride is involved in cellular functions in procaryotes. Microorganisms growing at elevated salt concentrations are faced with the problem of keeping the water activity of their cytoplasm high (7, 15), and two types of osmoadaptation are known (6). First, compatible solutes which do not interfere with the central metabolism are synthesized. Second, salts are accumulated from the medium until the internal salt concentration equals the external one (the so called salt-in-cytoplasm mode of osmoadaptation). The major cations taken up are K+ and Na+, along with Cl− as the major anion. Uptake of the negatively charged chloride against the electrical field (inside negative) is energy dependent, and halobacteria employ two modes for Cl− uptake, i.e., the light-driven Cl− pump halorhodopsin (14, 21) and a light-independent Na+/Cl− symporter (5). Since H. halophilus is also slightly halophilic, one could speculate that chloride is taken up in the course of osmoadaptation by the salt-in-cytoplasm type. However, studies by Trüper, Galinski and coworkers revealed that H. halophilus, grown at 10% salinity (1.7 M NaCl) in synthetic or complex medium, synthesizes different compatible solutes at concentrations of 0.8 μmol/mg (dry weight) (6, 22). Taking into account the determined internal volume, this would correspond to an internal concentration of about 1.0 M. Although a quantitative discussion is always difficult in view of the inherent problems with the measurements, this calculation makes a major role of chloride in osmoadaptation highly unlikely. However, a contribution of chloride to the mechanism of osmoadaptation cannot be excluded, and it is noteworthy in this connection that certain halophiles such as Pseudomonas halosaccharolytica and Bacillus haloalkaliphilus, which produce compatible solutes, also take up chloride. P. halosaccharolytica keeps its internal chloride concentration fairly constant at 0.7 to 1.0 M at Cle− values of 1 to 3 M (12). On the other hand, B. haloalkaliphilus, like H. halophilus, excludes chloride from the cytoplasm at low Cle− concentrations but takes it up at higher Cle− concentrations, thereby decreasing Cle−/Cli− from 5.8 at 0.85 M Cle− to 1.3 at 3.4 M Cle− (23).
The observation that cell yield is also dependent on chloride concentration indicates a structural role of Cl−. A possible function could be a stabilization of enzymes or protein complexes, as seen, for example, with the photosystem II of plants or cyanobacteria (3). It is noteworthy that photosystem II is stabilized not only by chloride but also by bromide or nitrate, a striking similarity to the anion dependence of H. halophilus. In addition, it was reported that photosynthesis in cyanobacteria (and plants as well) is dependent on chloride (3, 16), and an active, ATP-dependent uptake of Cl− was demonstrated (24).
It is evident from the data presented that chloride is taken up upon increase of the growth rate. This uptake is energy dependent. The magnitude of the membrane potential of H. halophilus is approximately −180 mV (16a), and if chloride is in equilibrium with the membrane potential, it would sustain a Cle−/Cli− gradient of 1,000. However, we determined a Cle−/Cli− gradient of approximately 2 under optimal growth conditions; therefore, this is clear evidence that chloride is taken up against the electrical field in an energy-dependent fashion. The nature of the driving force is unknown, but N-terminal sequencing of the membrane proteins found specifically in halide-grown cells will facilitate identification of the transporters.
Whether chloride dependence is a striking feature of salt marsh or other halotolerant bacteria and whether chloride is involved in signaling of and adaptation to environmental changes such as salt concentration remain open questions. Recently, chloride-inducible genes in Lactococcus lactis were identified; however, the functions of the gene products are unknown and it is not known whether chloride is essential for growth of L. lactis (18). Future studies are aimed at identifying the role of chloride in the physiology of H. halophilus in light of its environment, i.e., salt marshes.
ACKNOWLEDGMENTS
We are indebted to D. Claus, Göttingen, Germany, for drawing our attention to H. halophilus, for fruitful and stimulating discussions, and for advice. The hospitality and helpful advice with the 36Cl− experiments of the staff of the Zentrales Isotopenlabor der Universität Göttingen are gratefully acknowledged.
This work was supported by a grant from the Fonds der Chemischen Industrie.
REFERENCES
- 1.Blum H, Beier H, Gross H J. Improved silver staining of plant proteins, RNA, and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–98. [Google Scholar]
- 2.Claus D, Fahmy F, Rolf H J, Tosunoglu N. Sporosarcina halophila sp. nov., an obligate, slightly halophilic bacterium from salt marsh soils. Syst Appl Microbiol. 1983;4:496–506. doi: 10.1016/S0723-2020(83)80007-1. [DOI] [PubMed] [Google Scholar]
- 3.Critchley C. The role of chloride in photosystem II. Biochim Biophys Acta. 1985;811:33–46. [Google Scholar]
- 4.Dimroth P. Primary sodium ion translocating enzymes. Biochim Biophys Acta. 1997;1318:11–51. doi: 10.1016/s0005-2728(96)00127-2. [DOI] [PubMed] [Google Scholar]
- 4a.Dohrmann, A.-B., and V. Müller. Unpublished data.
- 5.Duschl A, Wagner G. Primary and secondary chloride transport in Halobacterium halobium. J Bacteriol. 1986;168:548–552. doi: 10.1128/jb.168.2.548-552.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galinski E A, Trüper H G. Microbial behaviour in salt-stressed ecosystems. FEMS Microbiol Rev. 1994;15:95–108. [Google Scholar]
- 7.Lanyi J K. Light-driven primary ionic pumps. In: Krulwich T A, editor. Bacterial energetics. San Diego, Calif: Academic Press; 1990. pp. 55–86. [Google Scholar]
- 8.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 9.MacLeod R A, Onofrey E. Nutrition and metabolism of marine bacteria II. Observations on the relation of sea water to the growth of marine bacteria. J Bacteriol. 1956;71:661–667. doi: 10.1128/jb.71.6.661-667.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLeod R A, Onofrey E. Nutrition and metabolism of marine bacteria VI. Quantitative requirements for halides, magnesium, calcium, and iron. Can J Microbiol. 1957;3:753–759. doi: 10.1139/m57-085. [DOI] [PubMed] [Google Scholar]
- 11.Mannino J. Human biology. St. Louis, Mo: Mosby; 1995. [Google Scholar]
- 12.Masui M, Wada S. Intracellular concentrations of Na+, K+, and Cl− of a moderately halophilic bacterium. Can J Microbiol. 1973;10:1181–1186. doi: 10.1139/m73-191. [DOI] [PubMed] [Google Scholar]
- 13.Müller V, Blaut M, Heise R, Winner C, Gottschalk G. Sodium bioenergetics in methanogens and acetogens. FEMS Microbiol Rev. 1990;87:373–377. [Google Scholar]
- 14.Oesterhelt D. Structure and function of halorhodopsin. Isr J Chem. 1995;35:475–494. [Google Scholar]
- 15.Oren A. The ecology of the extremely halophilic archaea. FEMS Microbiol Rev. 1994;13:415–439. doi: 10.1111/j.1574-6941.2002.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 16.Putnam-Evans C, Bricker T M. Site-directed mutagenesis of the basic residues 321K to 321G in the CP47 protein of photosystem II alters the chloride requirement for growth and oxygen-evolving activity in Synechocystis 6803. Plant Mol Biol. 1997;34:455–463. doi: 10.1023/a:1005826411702. [DOI] [PubMed] [Google Scholar]
- 16a.Roeßler, M., and V. Müller. Unpublished data.
- 17.Rottenberg H. The measurement of membrane potential and ΔpH in cells, organelles and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- 18.Sanders J W, Leenhouts K, Burghoorn J, Brands J R, Venema G, Kok J. A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol Microbiol. 1998;27:299–310. doi: 10.1046/j.1365-2958.1998.00676.x. [DOI] [PubMed] [Google Scholar]
- 19.Schägger H, von Jagow G. Tricine-sodium dodecylsulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:369–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt K, Liaanen-Jensen S, Schlegel H G. Die Carotinoide der Thiorodaceae. Arch Mikrobiol. 1963;46:117–126. [PubMed] [Google Scholar]
- 21.Schobert B, Lanyi J K. Halorhodopsin is a light-driven chloride pump. J Biol Chem. 1982;257:10306–10313. [PubMed] [Google Scholar]
- 22.Severin J. Kompatible Solute und Wachstumskinetik bei halophilen aeroben heterotrophen Eubakterien. Ph.D. thesis. Germany: University of Bonn; 1993. [Google Scholar]
- 23.Weisser J, Trüper H G. Osmoregulation in a new haloalkaliphilic Bacillus from the Wadi Natrun (Egypt) Syst Appl Microbiol. 1985;6:7–11. [Google Scholar]
- 24.Zdrou I, Tromballa H W. Active transport of chloride by Anacystis nidulans. Arch Microbiol. 1981;129:325–330. [Google Scholar]