Abstract
Metformin is the first-line medication for treatment of type 2 diabetes and has been shown to reduce heart damage and death. However, mechanisms by which metformin protects human heart remain debated. The aim of the study was to evaluate the cardioprotective effect of metformin on cardiomyocytes derived from human-induced pluripotent stem cells (hiPSC-CMs) and mitochondria isolated from human cardiac tissue. At concentrations ≤ 2.5 mM, metformin significantly increased oxygen consumption rate (OCR) in the hiPSC-CMs by activating AMPK-dependent signaling and enhancing mitochondrial biogenesis. This effect was abrogated by compound C, an inhibitor of AMPK. At concentrations > 5 mM, metformin inhibited the cellular OCR and triggered metabolic reprogramming by enhancing glycolysis and glutaminolysis in the cardiomyocytes. In isolated cardiac mitochondria, metformin did not increase the OCR at any concentrations but inhibited the OCR starting at 1 mM through direct inhibition of electron-transport chain (ETC) complex I. This was associated with reduction of superoxide production and attenuation of Ca2+-induced mitochondrial permeability transition pore (mPTP) opening in the mitochondria. Thus, in human heart, metformin might improve cardioprotection due to its biphasic effect on mitochondria: at low concentrations, it activates mitochondrial biogenesis via AMPK signaling and increases the OCR; at high concentrations, it inhibits the respiration by directly affecting the activity of complex I, reduces oxidative stress and delays mPTP formation. Moreover, metformin at high concentrations causes metabolic reprogramming by enhancing glycolysis and glutaminolysis. These effects can be a beneficial adjunct to patients with impaired endogenous cardioprotective responses.
Keywords: Cardiac mitochondria, metformin, oxidative stress, mitochondrial permeability transition pore, metabolic reprogramming
INTRODUCTION
Metformin is an oral hypoglycemic agent widely used to treat type 2 diabetes. The primary action of the drug is to lower hepatic glucose synthesis and enhance peripheral glucose uptake through the activation of the insulin receptor.1 It has also been demonstrated to lessen the risk and extent of myocardial infarction, reduce cardiovascular mortality, and improve clinical outcomes in patients with diabetes and heart failure.2–5 Recently, the American Diabetes clinical practice guideline has recommended using metformin as a first-line therapy in diabetic patients with stable chronic heart failure.6 In the preclinical studies, metformin has been demonstrated to reduce myocardial ischemia/reperfusion injury7–9 and adverse myocardial remodeling, thus attenuating the development of heart failure.10–12
The primary targets of metformin action in the heart remain elusive despite metformin’s effect on intracellular signaling through AMP-activated protein kinase A (AMPK)-dependent and AMPK-independent pathways.10,13,14 Whittington and colleagues showed that chronic metformin treatment of diabetic rats activated AMPK and PGC-1 α resulting in myocardial resistance to ischemia-reperfusion injury.15 AMPK activation by metformin was observed in vivo mice models of myocardial infarction11 and non-diabetic Sprague-Dawley rats.16 Although metformin activates AMPK, this may not explain all the therapeutic effects of the drug. In liver and primary hepatocytes from AMPK knockout mice, metformin has been demonstrated to inhibit hepatic glucose production via a decrease in hepatic energy charge indicating that neither AMPK nor upstream activation of kinase LKB1 are important for the drug action.17 The most accepted scientific view is that metformin targets mitochondria directly by transiently inhibiting the OXPHOS complex I. This, in turn, leads to a significant drop in cellular energetics.18–20 In addition, inhibition of complex I reduces superoxide production and delays mitochondrial permeability transition pore (mPTP) opening.9
It has been proposed that many preclinical studies use supratherapeutic concentrations of metformin than described in clinical studies.21 In in vitro and in vivo studies, inhibition of complex I activity requires concentration of metformin in millimolar range.9,18,22,23 Of note, there is no clear information about the plasma concentration of metformin in patients. It has been generally assumed that it is less than 100 μM.24 However, a recent systematic review of the literature by Kajbaf et al. has revealed a huge variation in metformin plasma concentrations with average range between 0.1–40 mg/L21 due to alterations of pharmacodynamics and pharmacokinetics of metformin in patients.25,26
Up to date, the effect of metformin on mitochondrial functions described in animal models has not been extensively explored in human myocardium. This is important because cardiac complications in humans are associated with complex phenotype that cannot be completely replicated in animal models as key determinants of myocardial dysfunction, responsiveness to medication, and cardioprotection are more complex and can be missed in animal models. In addition, understanding of the cardioprotective actions of metformin on energy metabolism, particularly on mitochondrial function, is important in the context of interest in repurposing for possible application in cardiac diseases.
Therefore, the purpose of this study was to assess the therapeutic effect of metformin on human cardiac mitochondria in vitro and in vivo with the cell environment. We have proposed that metformin at low doses enhances the cellular respiration as a consequence of activated mitochondrial biogenesis via AMPK signaling and inhibits the respiration at high doses by directly affecting the activity of complex I that results in reduction of superoxide production, delay in mPTP opening, and metabolic reprogramming.
MATERIAL AND METHODS
Patient recruitment.
Cardiac tissue from left atrial appendage of middle age and elderly patients (n=23) undergoing elective open-heart surgery at Aurora St. Luke’s Medical Center in Milwaukee, Wisconsin, was harvested. Patients undergoing emergency bypass surgery, requiring inotropic support or mitral valve replacement and patients with congenital heart disease, New York Heart Association class III and IV heart failure, systemic disorders such as infection or severe left ventricular (LV) dysfunction (LV ejection fraction <35%) were excluded from the study to avoid confounding effect of the disease on myocardial function. Patients who were under metformin treatment also were excluded in order to eliminate the drug’s interference with our results. The study was approved by the Aurora Institutional Review Board and adhered to the Health Insurance Portability and Accountability Act (HIPAA) and Aurora Health Care patient privacy and security guidelines, written informed consent was obtained from subjects, and all patients’ privacy rights were observed. The research study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Mitochondrial isolation.
Mitochondria were isolated from human left atrial appendage tissue. Within 5–10 minutes after removal from patients, the atrial appendage tissue was immediately transferred into ice-cold Dulbecco’s phosphate-buffered saline (DPBS, Thermo Fisher Scientific, Waltham, MA). Fat and connective tissue was trimmed off and the atrial tissue was used for mitochondrial isolation. Mitochondria were isolated by differential centrifugation.27 Briefly, after trimming, the tissue was transferred into the media containing (in mM) 200 mannitol, 50 sucrose, 5 KH2PO4, 1 EGTA, 5 MOPS, pH 7.3, 0.5% BSA and homogenized using OMNI GLH-115 Polytron homogenizer (OMNI International, Kennesaw, GA). Mitochondrial pellet was washed in the medium containing no BSA and EGTA. Protein concentration was determined using Pierce BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA). The purity of the mitochondrial pellet was determined by measuring citrate synthase activity.
Citrate Synthase Activity.
The activity of citrate synthase was measured in isolated mitochondria at room temperature according to the manufacturer’s protocol (Sigma-Aldrich, St. Louis, MO). The activity was determined by recording color development of 5-thio-2-nitrobenzoic acid (TNB), which is generated in the reaction of citrate synthesis, at λ = 412 nm. The citrate synthase functional activity was equal to 76.94 ± 25.96 nmol TNB/min/mg of mitochondrial protein in isolated human cardiac mitochondria. The relative enrichment of the mitochondrial protein in the mitochondrial pellet was also supported by data on citrate synthase activity in left atrial tissue homogenates (84.12 ± 22.64 nmol TNB/min/mg non-collagen protein in homogenate). The non-collagen protein was determined using Pierce BCA protein assay kit in the homogenates after treatment with 50 mM NaOH and pelleting the insoluble collagen protein by centrifugation at 12,000 g for 10 min.28,29 This approach excludes the collagen protein when normalizing the citrate synthase activity by protein in tissue homogenates.
Human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) culture and characterization using immunofluorescence staining.
One hiPSC line (1013) was generated from dermal fibroblasts isolated from a 36-year-old healthy donor (male) in the laboratory of Dr. Douglas Melton (Department of Stem Cell and Regenerative Biology, Harvard University). All data were generated using the 1013 hiPSC line. The hiPSCs were cultured in Matrigel (Corning Inc, Corning, NY, USA)-coated petri dishes with mTeSR1 medium (STEMCELL Technologies, Vancouver, BC, Canada) supplemented with 1% penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA, USA) in a hypoxic incubator (4% O2, 5% CO2) at 37°C as described previously.30,31 The procedure of hiPSC-CMs generation and purification with lactate was performed as previuosely described.30 This approach of purification is based on the remarkable biochemical differences in lactate and glucose metabolism between cardiomyocytes and non-cardiomyocytes.32 The cardiomyocytes purification medium was glucose free DMEM (Thermo Fisher Scientific) supplemented with 4 mM Sodium L-lactate (Sigma-Aldrich, St. Louis, MO, USA) and 1% penicillin/streptomycin.30,31 The purity was assessed using immunofluorescence staining of cardiac troponin T, a specific marker of cardiomyocytes. The percentage of cardiac troponin T-positive cells was calculated by the ratio of troponin T-positive CMs/Hoechst 33342-positive total cells (representing cell nuclei) on 8 different areas taken randomly per sample slide. The hiPSC-CMs were cultured in Matrigel (Corning Inc, Corning, NY, USA)-coated petri dishes with Roswell Park Memorial Institute (RPMI) medium (Thermo Fisher Scientific, Waltham, MA, USA) including B27 Supplement (Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). The hiPSC-CMs were characterized by the expresson of cardiomyocyte-specific markers troponin T and NKX2.5 using immunofluorescence staining.31 Brieflly, the hiPSC-CMs were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA), permeabilized with 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA), and blocked with 10% donkey serum (Millipore, Billerica, MA, USA). The cells were then stained with the following primary antibodies: mouse anti-cardiac troponin T (Thermo Fisher Scientific, Waltham, MA, USA) and rabbit anti-NKX2.5 (Cell Signaling Technology, Danvers, MA, USA). After washings with PBS, the cells were incubated with secondary antibodies: Alexa Fluor 488 goat anti-mouse IgG (1:1000, Thermo Fisher Scientific, Waltham, MA, USA) and Alexa Fluor 594 goat anti-mouse IgG (Thermo Fisher Scientific, Waltham, MA, USA). NucBlue Fixed Cell ReadyProbes Reagent (Thermo Fisher Scientific, Waltham, MA, USA) was used to stain nuclei and mount the coverslips onto the slides. Cells were observed under a fluorescence microscope (Olympus, Shinjuku, Tokyo, Japan).
For experiments, the hiPSC-CMs were cultured in the Seahorse XF96 cell culture microplate (Agilent Technologies, Santa Clara, CA, USA) coated with 50 μg/ml fibronectin (Sigma-Aldrich, St. Louis, MO,USA) at a density 10,000 cells/well, 6-well cell culure plates (Thermo Fisher Scientific, Waltham, MA) at density 1 mln cells/well, 60 mm tissue culture dish (Midsci, St. Louis, MO, USA) at density 2.5 mln cells/dish in RPMI/B27 medium (Thermo Fisher Scientific, Waltham, MA) without insulin supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA). On day 2 after plating cells, the medium was replaced by RPMI/B27 without insulin with 1% penicillin/streptomycin no FBS. On day 3, the cells were treated with different concentrations (0–20 mM) of metformin (Sigma-Aldrich, St. Louis, MO) with and without 2.5 μM compound C, a cell-permeable AMPK inhibitor, (Sigma-Aldrich, St. Louis, MO, USA) in humidified chamber at 37°C for 24h.
Seahorse assay.
The oxygen consumption rate (OCR) was measured in isolated cardiac mitochondria and human iPSC-CMs using XF96 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA, USA) in the Cancer Center Redox and Bioenergetics Shared Resources (CCRBSR) at Medical College of Wisconsin.
To measure the OCR and extracellular acidification rate (ECAR) in the human cardiomyocytes, the hiPSC-CMs were plated at density of 10,000 cells/well in the fibronnectin-coated Seahorse XF96 cell culture microplate 96-well plate and treated with different concentrations (0–20 mM) of metformin (Sigma-Aldrich, St. Louis, MO, USA) with and without 2.5 μM compound C (Sigma-Aldrich, St. Louis, MO) in humidified chamber at 37°C for 24h as described earlier. Before the Seahorse analysis, the hiPSC-CMs were incubated for 1h in 200 μl unbuffered DMEM containing 11 mM glucose in humidified chamber at 37°C. The OCR and ECAR were measured under baseline condition (basal OCR) and in response to 10 μM oligomycin A (Sigma-Aldrich, St. Louis, MO), 2 μM fluoro-carbonyl cyanide phenylhydrazone (FCCP, Sigma-Aldrich, St. Louis, MO), and 10μM antimycin A (Sigma-Aldrich, St. Louis, MO).33 Of note, treatment with oligomycin A is important to estimate the ATP-linked OCR, which is associated with ATP synthesis. The maximal OCR, a maximum capacity for mitochondrial substrate oxidation, was determined after uncoupling of mitochondria with FCCP. We also measured spare capacity OCR that indicates the ability of cells to meet increased ATP demand and resist periods of stress.33 All values of the OCR and ECAR were normalized to protein content.
After isolation, mitochondria were diluted to the needed concentration required for plating (3 μg/well) in the ice-cold incubation media containing (in mM) 70 sucrose, 220 mannitol, 10 KH2PO4, 5 MgCl2, 2 HEPES, pH 7.2, 1 EGTA, 0.2% BSA, 10 glutamate and 5 malate or 10 succinate and 0.002 rotenone.34 Next, 40 μl of the diluted mitochondrial suspension was delivered to each well, except of wells for background correction, while the plate was on ice. The plate was centrifuged at 2,000 × g for 20 min at 4°C. After centrifugation, 100 μl of the incubation media with corresponding substrates was added into each well and the plate was incubated at 37°C for 10 min. The plate was transferred to the XF96 Extracellular Analyzer and experiment was initiated at 37°C. The OCR was measured in the presence of the substrates (basal OCR), 4 mM ADP (State 3 OCR), 2.5 μg/ml oligomycin A (State 4o OCR), 3 μM FCCP (uncoupled OCR).
Cytotoxicity assay.
To monitor compound C cytotoxicity, the hiPSC-CMs were incubated for 24 h in the absence and presence of different concentrations of compound C (0.25 – 25 μM). The cytotoxicity was measured using the WST-1 assay for cell viability according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO). Changes in the number of viable cells result in an alteration in the overall activity of mitochondrial dehydrogenases in the sample, which in turn results in the amount of formazan dye formed. Quantification of the formazan dye produced by metabolically active cells has been done at λ = 420–480 nm.
Mitochondrial DNA isolation.
Mitochondrial DNA (mtDNA) was isolated from the hiPSC-CMs following the mitochondrial DNA isolation kit (BioVision, Inc., Milpitas, CA, USA) protocol. After 24 hours treatment with metformin, the hiPSC-CMs (5 mln cells) were trypsinized using 0.05% (w/v) trypsin-EDTA and washed two times in phosphate-saline buffer by centrifugation at 600 × g for 5 min at 4°C. The cardiomyocytes pellet was then resuspended in a cytosol extraction buffer and homogenized in an ice-cold Dounce tissue grinder (80 passes). Cell homogenates were centrifuged twice at 10,000 × g for 30 min at 4°C. The mitochondrial pellet was lysed in the mitochondrial lysis buffer with enzyme mix and centrifuged at top speed for 5 min at RT. The final pellet of mtDNA was washed with 70% ethanol and dried. The mtDNA was resuspended in TE buffer and measured using NanoQuant on the Tecan Infinite M200 plate reader (Tecan Group, Mannedorf, Switzerland) at λ = 260 nm.
RT2 Profiler PCR Array of mitochondrial energy metabolism.
After treatment with 0.5 mM and 10 mM metformin for 24h, total RNA was isolated from the hiPSC-CMs using TRIzol® Reagent (Thermo Fisher Scientific, Waltham, MA) and miRNeasy Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocol. RNA concentrations were evaluated spectrophotometrically using the NanoQuant on the Tecan Infinite M200 plate reader (Tecan Group, Mannedorf, Switzerland). Total RNA (500 ng) was used for reverse transcription using the RT2 First Strand Kit (Qiagen, Germantown, MD) on the Techne Thermal Cycler TC-312 (Techne, Stone, Staffordshire, United Kingdom). Gene expression profiling of 84 genes involved in mitochondrial respiration, including genes encoding components of the ETC and OXPHOS complexes was performed using the Human Mitochondrial Energy Metabolisdm RT2 Profiler PCR Array (Qiagen, Germantown, MD) according to the manufacturer’s instructions. qPCR was performed on the ABI 7300 Real-time PCR System (Applied Biosystems, Foster City, CA, USA), using the RT2 Sybr PCR qPCR MasterMix (Qiagen, Germantown, MD). Results were normalized to a pre-designed array endogenous control gene HPRT1 (hypoxanthine-guanine phosphoribosyltransferase).
Assessment of protein expression level by western blot.
The hiPSC-CMs were cultured at density 1 mln cells/well and exposed to 0.5 mM and 10 mM metformin with and without 2.5 μM compound C for 24h. After tripsinization, the cells were washed twice using phosphate-buffered saline and centrifuged rifuged at 200 × g 5 min at 4°C. Pellets were resuspended by RIPA lysis buffer with a protease/phosphatase inhibitor in it and centrifuged at 12,000 × g 10 min at 4°C. The cell extracts were separated by gel electrophoresis with NuPAGE 4–12% Bis-Tris gel and proteins were electrophoretically transferred to polyvinylidene fluoride (PVDF) membrane using the iBlot dry blotting system (Thermo Fisher Scientific, Waltham, MA). The membranes were blocked in 5% bovine serum albumin dissolved in Tris buffered saline containing 0.1% Tween-20. Primary antibodies against AMP-activated protein kinase (AMPKα, Cell Signaling Technologies, Danvers, MA) and phospho-AMPKα (Cell Signaling Technologies, Danvers, MA) were applied to the membrane. Respective horseradish peroxidase (HRP) conjugated secondary antibodies were used against the primary antibodies. Proteins were visualized by SuperSignal™ West Pico chemiluminescent substrate and monitored using UltraQuant® v6.0 software in molecular imaging systems (UltraLum, Claremont, CA). Densitometric evaluation of protein bands was performed using ImageJ software National Institutes of Health (NIH) (http://rsb.info.nih.gov/ij/).
Metabolites profiling.
Metabolites were determined in the hiPSC-CMs using a single normal-phase hydrophilic interaction liquid chromatography (HILIC) coupled to tandem mass spectrometry (Shimadzu Nexera 2 coupled to LC-MS 8030 detector) following a protocol published by Yuan et al35,36 in CCRBSR at Medical College of Wisconsin. Metabolites were separated using an Acquity UPLC BEH Amide column (1.7 μm, 2.1 mm i.d. × 150 mm length, Waters, Milford, MA, USA) over 18 min targeted acquisition. A gradient elution profile was employed using buffers A and B. The buffer A consisted of 95% (vol/vol) water (Honeywell Research Chemicals, Muskegon, MI, USA), 5 % (vol/vol) acetonitrile (Honeywell Research Chemicals, Muskegon, MI), 20 mM ammonium hydroxide (Sigma-Aldrich, Milwaukee, WI), and 20 mM ammonium acetate (Sigma-Aldrich, Milwaukee, WI), pH 9.0. The buffer B was 100% (vol/vol) acetonitrile. Before analyses, the column was equilibrated with the mixture of 90% mobile phase B and 10% mobile phase A, at the flow rate of 0.3 mL/min. After sample injection, the metabolites were eluted by an increase in the fraction of mobile phase A from 10% to 70% over 12 min, followed by further increase to 98% of mobile phase A over the next 2 min and kept at this value for the following 3 min (until 17 min post-injection). Between 17- and 18-min post-injection the mobile phase A fraction was brought down to 10% and the column re-equilibrated for 7 min before the next injection. The eluate was directed into the mass detector using a diverting valve between 1.9 min and 18 min post-injection. The metabolites were detected using dual ESI and APCI ionization source, with the nebulizing N2 flow of 2 L/min and drying gas (N2) flow of 20 L/min. MRM transitions of the glycolytic and Kreb’s cycle metabolites were as described elsewhere.35,36
Metabolites were extracted from 2 million adherent cells using an ice-cold extraction solution containing 80% (vol/vol) methanol (Thermo Fisher Scientific, Waltham, MA) as previously described.35,36 After extraction, a supernatant was separated, concentrated by a SpeedVac and lyophilized. Dried metabolite samples were stored at −80°C until analysis. Immediately before LC-MS analysis, the samples were resuspended in 20–25 μl of LC-MS grade water containing 10 μM pyruvate-13C3 as an internal standard.
The samples were analyzed in blinded randomized order. For quality measures, all peaks were compared to known standards (ATP13C,15N and Br-cAMP) to confirm the metabolite identity. Shimadzu LabSolution software was used for manual review of chromatograms and peak area integration. The relative quantification of identified metabolites was accomplished by comparing the peak area between the groups.
Superoxide measurement.
Mitochondria were isolated from human cardiac tissue and resuspended at concentration 0.1 mg protein/ml in buffer containing (in mM) 120 KCl, 10 MOPS, pH 7.3, 1 KH2PO4, 0.001 EGTA, 2 μg/ml oligomycin A, 5 glutamate and 2 malate or 5 succinate and 0.002 rotenone at RT and loaded with 2 μM dihydroethidium. Next, mitochondrial suspension was aliquoted into the 96 well plates and measurement was initiated. Superoxide formation was inferred from increased red fluorescence of ethidium cation (λex/λem=485/610 nm), a product of dihydroethidium (DHE) oxidation.37
Mitochondrial permeability transition pore opening.
The mPTP was induced by sequentially exposing isolated mitochondria from cardiac tissue to 10 μM of Ca2+ pulses at 3 min intervals and monitoring abrupt mitochondrial Ca2+ release using Fluo-5N (λex/λem=490/520 nm), mitochondrial depolarization by Safranine O (λex/λem=485/590 nm), and swelling of mitochondrial matrix (λabs=520 nm) in Infinite 200 Pro Plate Reader microplate reader (Tecan US, Research Triangle Park, NC) at 30°C. Mitochondria (0.1 mg protein/ml) were incubated in the buffer containing (in mM) 120 KCl, 10 MOPS, pH 7.3, 1 KH2PO4, 0.001 EGTA, 2 μg/ml oligomycin A, 5 glutamate and 2 malate or 5 succinate and 0.002 rotenone, 0.001 Fluo-5N, 0.002 Safranin O and metformin (0–10 mM) or cyclosporine A (2 μM), an inhibitor of mPTP, at RT.
Statistical Analysis.
Data were presented as the mean ± standard deviation (SD). All experiments were performed independently at least three times. SigmaPlot (version 12.3 Systat Software, Inc., San Jose, CA) and GraphPad Prism (version 7.04 GraphPad Software, La Jolla, CA) softwares were used for statistical analyses and all graphs. Tukey’s pairwise comparison and one-way ANOVA were applied for comparison between groups. Differences between the two groups were determined by using the t-test for normally distributed variables or the nonparametric Kruskal-Wallis test for the variables that were not following the normal distribution. P < 0.05 was considered significant.
RESULTS
Clinical characteristics of patients.
The clinical characteristics of patients’ samples used for the study are summarized in Table 1. In this respect, 74% of the patients had hypertension, 52% of the patients had hyperlipidemia, 48% of the patients had atrial fibrillation and coronary artery disease, and to lesser extent, heart failure and history of previous myocardial infarction (35% and 9%, respectively). The average age was 71 ± 13, 61% of the population were males. The echocardiographic parameters demonstrate that LVEF was 57 ± 14%. Patients were on medications such as beta blockers (57%), ACE/ARB inhibitors and statins (21%), calcium channel blockers (4%). In addition, 44% of the patients underwent valve repair surgery 9% had CABG, and 26% had CABG plus valve repair surgery.
Table 1.
Clinical characteristics.
| Number of patients, n | 23 |
|
| |
| Age, yrs | 71 ± 13 (25–90) |
| Sex (Male/Female) | 14/9 |
| Comorbidities, n (%) | |
| Coronary Artery Disease | 11 (48) |
| History of MI | 2 (9) |
| Hypertension | 17 (74) |
| Hyperlipidemia | 12 (52) |
| Diabetes Mellitus | 0 |
| Atrial fibrillation | 11 (48) |
| Heart failure | 8 (35) |
| Echocardiography parameters | |
| LVEF, % | 57 ± 14 |
| LVDD, cm | 5.2 ± 0.9 |
| LVSD, cm | 4.0 ± 1.2 |
| IV septum thickness, cm | 1.3 ± 0.4 |
| LV posterior wall thickness, cm | 1.1 ± 0.4 |
| Medications, n (%) | |
| Beta blockers | 13 (57) |
| ACE/ARB inhibitors | 5 (22) |
| Calcium blockers | 1 (4) |
| Metformin | 0 |
| Insulin | 3 (13) |
| Statins | 5 (22) |
| Cardiac surgeries, n (%) | |
| CABG alone | 2 (9) |
| Valve repair alone | 10 (44) |
| CABG and valve repair | 6 (26) |
Data shown as number of patients (n) and percent of total number in parenthesis (%). Age, left ventricular ejection fraction (LVEF), left ventricular diastolic dimension (LVDD), left ventricular systolic dimension (LVSD), intraventricular (IV) septum thickness, left ventricular (LV) posterior wall thickness are presented as the mean ± standard deviation (SD). MI, myocardial infarction; ACE I, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor antagonists; CABG, coronary artery bypass grafting.
Characterization of hiPSC-CMs.
The hiPSC-CMs started spontaneous contraction after 8 days of differentiation. The differentiated cells were positively stained by cardiomyocyte-specific markers troponin T and NKX2.5 (Supplemental Figure 1). The purities of in troponin T-positive cardiomyocyte were 98%.
Concentration-dependent effect of metformin on cellular oxygen consumption rate.
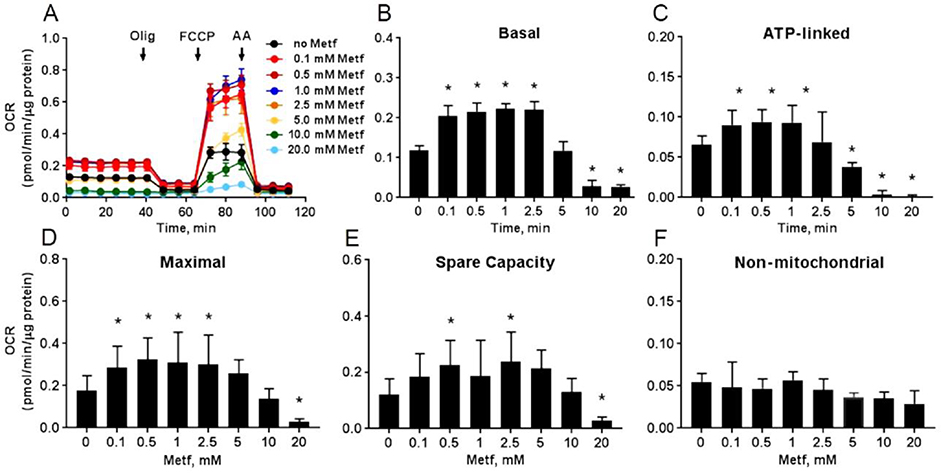
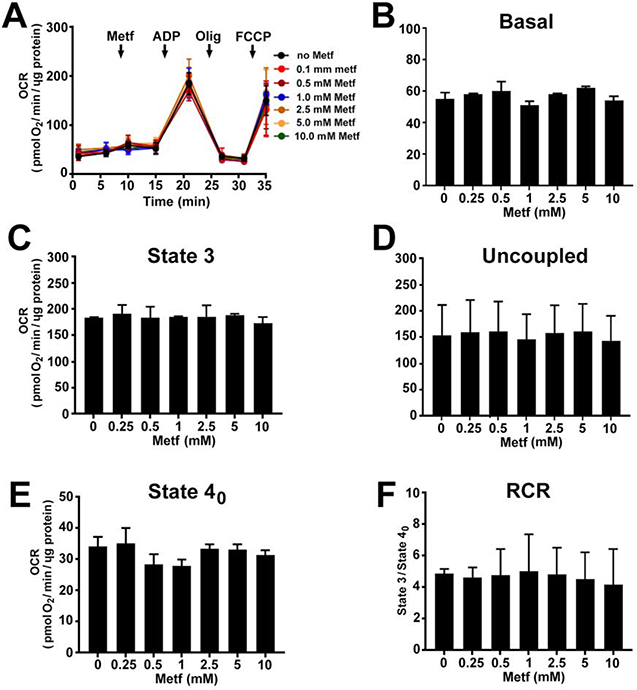
To validate effect of metformin on cellular respiration, hiPSC-CMs were treated with different concentrations of metformin (0–20 mM) for 24 hours (Figure 1). Metformin at concentrations ≤ 2.5 mM significantly increased the basal (Figure 1B, P<0.05), ATP-linked (Figure 1C, P<0.05), maximal (Figure 1D, P<0.05), and spare capacity (Figure 1E, P<0.05) OCR compared to the respiration in the control cells. However, treatment with concentrations ≥ 5 mM significantly reduced the OCR in cardiomyocytes. Metformin at any experimental concentrations did not alter the non-mitochondrial OCR (Figure 1F, P ≥ 0.05) proving that it selectively targeted mitochondrial respiration.
Fig. 1. Metformin dose-dependently alters respiration of cardiomyocytes.
(A) Raw traces of hiPSC-CMs oxygen consumption rate (OCR) recorded by XF96 Extracellular Flux Analyzer. The cardiomyocytes were exposed to different concentrations of metformin (Metf, 0 – 20 mM) for 24 h before the experiment. A basal measurement was recorded in the unbuffered DMEM supplemented with 11 mM glucose, followed by addition of 10 μM oligomycin A (Olig), 2 μM carbonyl cyanide p-triflouromethoxyphenylhydrazone (FCCP), and 10 μM antimycin A (AA). Treatment with Metf of ≤ 2.5 mM increased and ≥ 5 mM inhibited (B) basal, (C) ATP-linked, (D) maximal, (E) spare capacity OCR. There was no change in non-mitochondrial OCR (E) in the cardiomyocytes. Data are presented as mean ± SD; * p < 0.05 compared to no Metf (t-test for normally distributed variables and Kruskal-Wallis test for the variables that were not following the normal distribution); n=3.
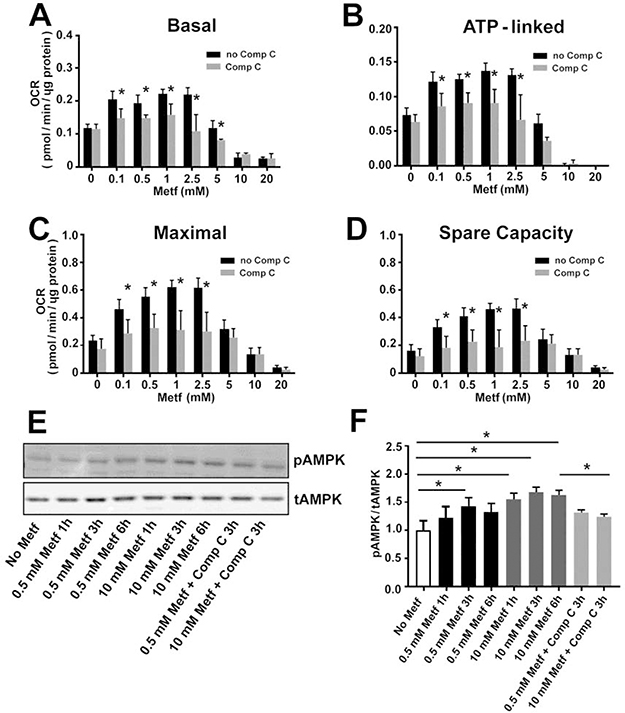
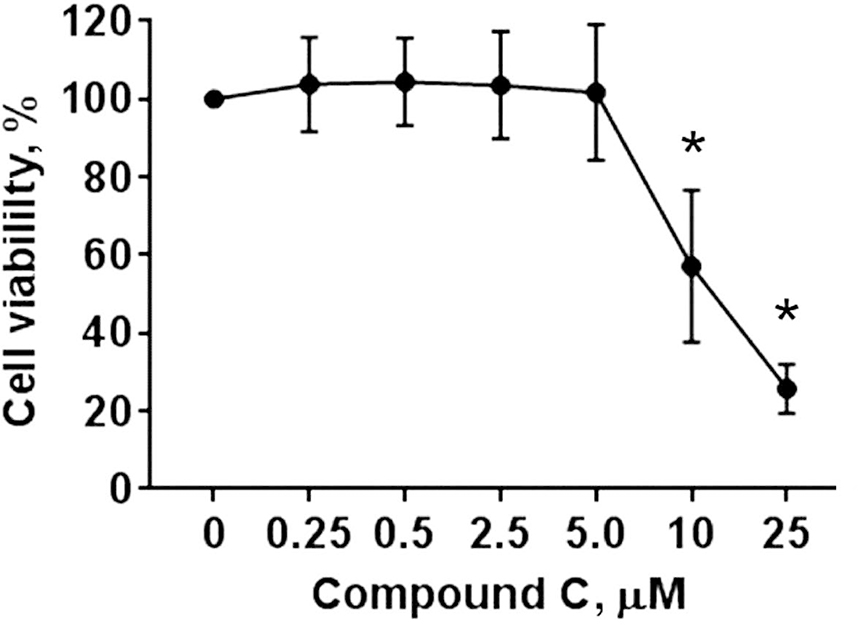
Interestingly, compound C, a specific inhibitor of AMPK, countered the effect of the metformin’s low concentrations (≤ 2.5 mM) on the cellular respiration by reducing the basal (Figure 2A, P<0.05), ATP-linked (Figure 2B, P<0.05), maximal (Figure 2C, P<0.05), and spare capacity (Figure 2D, P<0.05) OCR to similar rates observed in the control non-treated cells. The inhibition of the respiration by compound C at concentration (2.5 μM) used in our experiments was not associated with cytotoxicity (Figure 3). Therefore, our data indicate that increase in the cellular respiration after treatment of the cells with ≤ 2.5 mM metformin is mediated through activation of AMPK signaling pathway.
Fig. 2. Low concentrations of metformin increase cellular respiration in an AMPK-dependent manner.
Exposure to 2.5 μM compound C (Comp C) for 24 h eliminated stimulatory effect of low concentrations (≤ 2.5 mM) of metformin (Metf) on (A) basal, (B) ATP-linked, (C) maximal, and (D) spare capacity oxygen consumption rate (OCR). (E) Effect of metformin (0.5 mM and 10 mM) on phosphorylated AMPK (pAMPK) and total AMPK (tAMPK) expression. (F) Quantitative analysis of pAMPK to tAMPK ratio (one-way ANOVA and post-hoc Tukey test). Data are expressed as mean ± SD; * p < 0.05 compared to non-compound c (no Comp C); n=3.
Fig. 3. Dose-dependent effect of compound C on hiPSC-CMs cytotoxicity.
Cells were treated with different concentrations of compound C (0 – 25 μM) for 24 hours and assessed for the cytotoxicity using the WST-1 assay for cell viability. The cell viability was quantified based on the absorbance (λ = 420–480 nm) of the formazan dye produced by metabolically active cells. Data are expressed as mean ± SD; * p < 0.05 compared to 0 μM compound C.
Indeed, metformin treatment elevated activity of AMPK by increasing phosphorylation. Exposure of the cardiomyocytes to 0.5 mM and 10 mM metformin for 3h augmented phosphorylation level of AMPK by 1.4 (P=0.017) and 1.7-fold (P<0.001), respectively, compared to the control cells (Figure 2EF). Compound C significantly decreased AMPK phosphorylation (P=0.013), suggesting it inhibited AMPK activity.
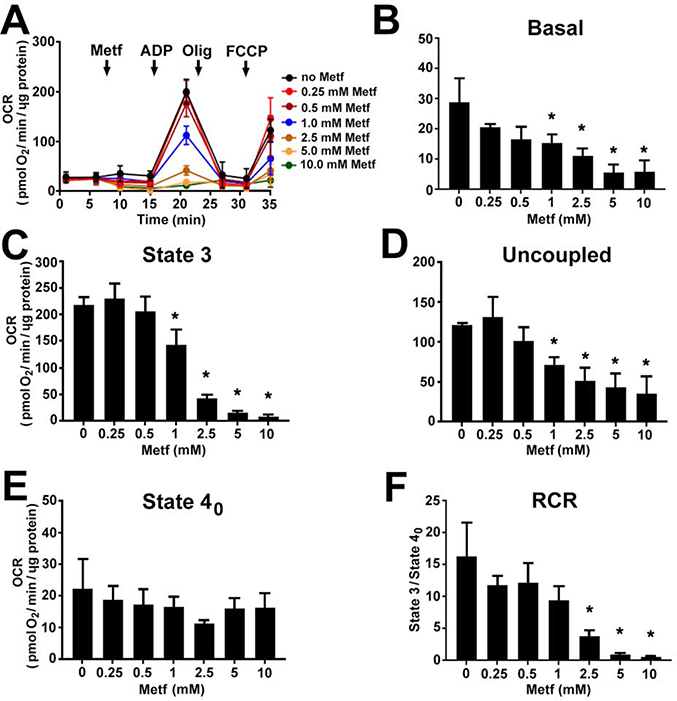
Moreover, to clarify whether the increase in the cellular OCR is associated with AMPK activation, we assessed the effect of metformin on the OCR in isolated cardiac mitochondria in the absence of cytosolic AMPK signaling. Metformin did not increase the respiration of the isolated mitochondria at any concentrations (Figure 4A). Starting at 1 mM, it caused a dose-dependent decrease in the basal (Figure 4B, P<0.05), state 3 (Figure 4C, P<0.05), uncoupled (Figure 4D, P<0.05) OCR and RCR (Figure 4F, P<0.05) when mitochondria were oxidizing glutamate and malate. At 10 mM, metformin had the strongest inhibitory effect on respiration. There was no significant effect on respiration when mitochondria were respiring succinate in the presence of rotenone (Figure 5A–F).
Fig. 4. Metformin dose-dependently decreases complex I-mediated respiration in isolated cardiac mitochondria.
(A) Traces of oxygen consumption rate (OCR) recorded by XF96 Extracellular Flux Analyzer in isolated mitochondria oxidizing 5 mM glutamate and 2 mM malate after addition of metformin (Metf, 0.25 – 10 mM), followed by injection of 4 mM ADP (State 3), 2.5 μg/ml oligomycin A (Olig, State 4o), and 3 μM FCCP (Uncoupled). Metformin dose-dependently reduced (B) basal, (C) state 3, (D) uncoupled OCR, and respiratory control ratio (RCR, State 3/State 4o). Metformin exposure did not change (E) state 4o OCR. Data are expressed as mean ± SD; * p < 0.05 compared to no Metf (t-test for normally distributed variables and Kruskal-Wallis test for the variables that were not following the normal distribution); n=3.
Fig. 5. Metformin has no effect on complex II-mediated respiration in isolated cardiac mitochondria.
(A) Traces of oxygen consumption rate (OCR) recorded by XF96 Extracellular Flux Analyzer in isolated mitochondria oxidizing 5 mM succinate in the presence of 4 μM rotenone after addition of metformin (Metf, 0.25 – 10 mM), followed by injection of 4 mM ADP (State 3), 2.5 μg/ml oligomycin A (Olig, State 4o), and 3 μM FCCP (Uncoupled). Acute treatment of mitochondria with metformin did not affect complex II respiration: (B) basal, (C) state 3, (D) uncoupled OCR, (E) state 4o OCR, and (F) respiratory control ratio (RCR, State 3/State 4o). Data are expressed as mean ± SD; * p < 0.05 compared to no Metf; n=3.
Altogether, these results suggested that the effect of metformin on the cellular respiration was mediated through its direct effect on mitochondria and indirectly via AMPK-dependent cell signaling. In the cardiomyocytes, metformin at lower concentrations enhanced the mitochondrial respiration by activating AMPK-dependent signaling and reduced the respiration at higher concentrations by directly suppressing complex I activity independently of AMPK signaling pathways.
Metformin alters expression of genes regulating OXPHOS.
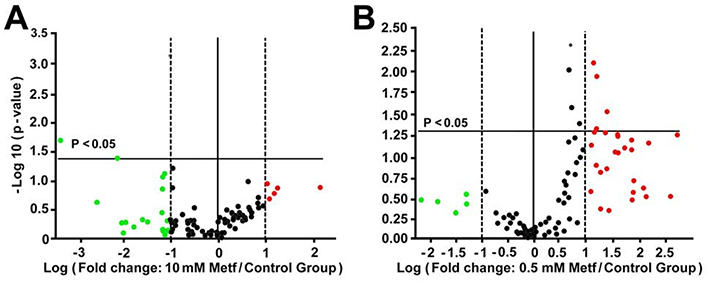
To test whether changes in the cellular OCR are related to alterations in the gene expression, we next studied the effect of metformin on the expression of 84 genes regulating the mitochondrial respiration. The functional activity of the OXPHOS has been primarily linked to changes in the expression of genes coding for the OXPHOS complexes I-V(27).27 The cardiomyocytes were treated with 0.5 and 10 mM metformin for 24h, concentrations selected based on the strongest stimulatory and inhibitory effects on mitochondrial respiration (Figures 1 and 4). Differences in expression of genes in the metformin-treated cells compared to the control cells were plotted as a volcano plot according to their statistical significance and fold change on their y- and x-axes, respectively (Figure 6AB). Surprisingly, metformin at 0.5 mM significantly downregulated NDUFA5 (P=0.049), NDUFB5 (P=0.001) genes coding for complex I and ARRDC3 (P=0.025) coding for arrestin domain containing 3 proteins without significant effect on other genes (Figure 6A, supplemental Table 1). Metformin at 10 mM significantly upregulated the expression of 8 genes out of 84 (Figure 6B, supplemental Table 1). These genes included NDUFA11 (P=0.026), NDUFB4 (P=0.005), and NDUFS5 (P=0.009) coding for complex I, SDHC (P=0.028) for complex II, COX5A (P=0.046) and COX5B (P=0.007) for complex IV, ATP5G1 (P=0.040) for complex V, and HSPA1B (P=0.011) encoding heat shock 70kDa protein 1B. Other genes that were upregulated but did not reach statistical significance included COX6A2 (P=0.053), ATP5C1 (P=0.060), ATP5G2 (P=0.055), ATP5O (P=0.056), CYC1 (P=0.055), EDN1 (endothelin 1, P=0.051), which is known as a survival factor against apoptosis in cardiomyocytes.38 These findings suggested that decrease in the mitochondrial OCR was associated with compensatory upregulation of the genes regulating mitochondrial respiration in the cardiomyocytes exposed to 10 mM metformin compared to the control cells and cells treated with 0.5 mM metformin in order to compensate energetic inefficiency associated with inhibition of complex I.
Fig. 6. Effect of metformin on expression of genes involved in mitochondrial respiration.
The volcano plot demonstrates statistical significance (p < 0.05) versus magnitude of change on the y- and x-axes, respectively. The horizontal solid line specifies the significance threshold where p < 0.05; all points above the line are considered statistically significant. The vertical central line indicates unchanged gene expression. The vertical dotted lines indicate the selective fold regulation threshold. Data points beyond the dotted lines in the right (red, upregulated) and left (green, downregulated) sections meet the 2-fold regulation threshold; n=3 for each group.
Metformin increases mitochondria biogenesis.
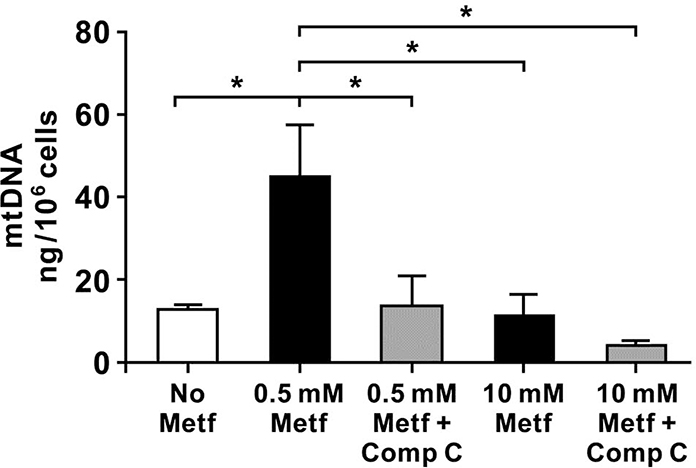
To determine whether alterations in the cellular OCR after exposure to metformin are associated with changes in the mitochondrial biogenesis, we analyzed the level of mtDNA in the hiPSC-CMs treated with 0.5 mM and 10 mM metformin in the presence or absence of compound C (2.5 μM). The overall content of mtDNA was significantly increased in the cells treated with 0.5 mM metformin compared to the untreated cells and cells exposed to 10 mM metformin (Figure 7). Surprisingly, compound C decreased the mtDNA content in the cells exposed to 0.5 mM metformin. There was no difference in the mtDNA between the untreated control cells and cells exposed to 10 mM metformin. In summary, these results revealed that metformin at the low concentration promoted mitochondrial biogenesis through AMPK activation.
Fig. 7. Metformin treatment increases mitochondrial DNA in the hiPSC-CMs.
Quantification of mtDNA in the control (no Metf) cells and cells exposed to metformin (Metf, 0.5 and 10 mM) with and without compound c (Comp C, 2.5 μM) for 24h. Metformin at 0.5 mM significantly increased mtDNA content in the cardiomyocytes that was abrogated by treatment with 2.5 μM compound c. Data are expressed as mean ± SD, * p < 0.05 compared to no Metf and 0.5 mM Metf (one-way ANOVA and post-hoc Tukey test); n=3 for each group.
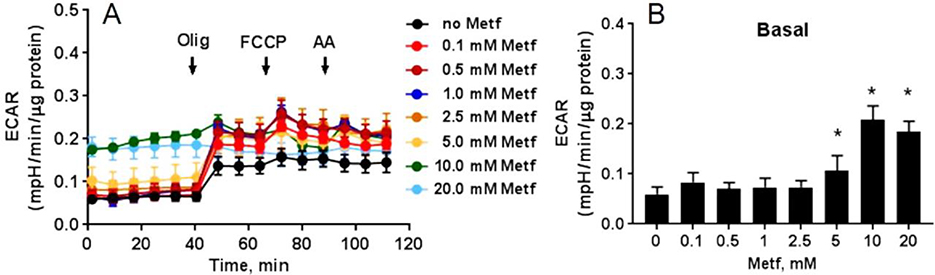
Metformin increases extracellular acidification rate (ECAR).
In the cardiomyocytes, metformin dose-dependently augmented ECAR that linked to the lactate production and changes in the extracellular pH (Figure 8A). Starting at 5 mM, metformin dose-dependently changed the basal ECAR (Figure 8B). Acute increase in the ECAR in response to oligomycin A correlated with lactate accumulation in the extracellular media as oligomycin A inhibited mitochondrial ATP synthesis and shifted the mitochondrial energy production to glycolysis, with the subsequent increase in ECAR revealing the cellular maximum glycolytic capacity.
Fig. 8. Metformin dose-dependently increases extracellular acidification rate (ECAR) in hiPSC-CMs.
(A) Raw traces of ECAR recorded by XF96 Extracellular Flux Analyzer. The cardiomyocytes were treated with different concentrations of metformin (Metf, 0–20 mM) for 24 h. A basal measurement was performed in DMEM supplemented with 11 mM glucose, followed by addition of 10 μM oligomycin A (Olig), 2 μM carbonyl cyanide p-triflouromethoxyphenylhydrazone (FCCP), and 10 μM antimycin A (AA). Metformin dose-dependently increased ECAR (B) at basal OCR. Data are presented as mean ± SD; * p < 0.05 compared to no Metf (t-test for normally distributed variables and Kruskal-Wallis test for the variables that were not following the normal distribution); n=3.
Metformin promotes metabolic reprograming.
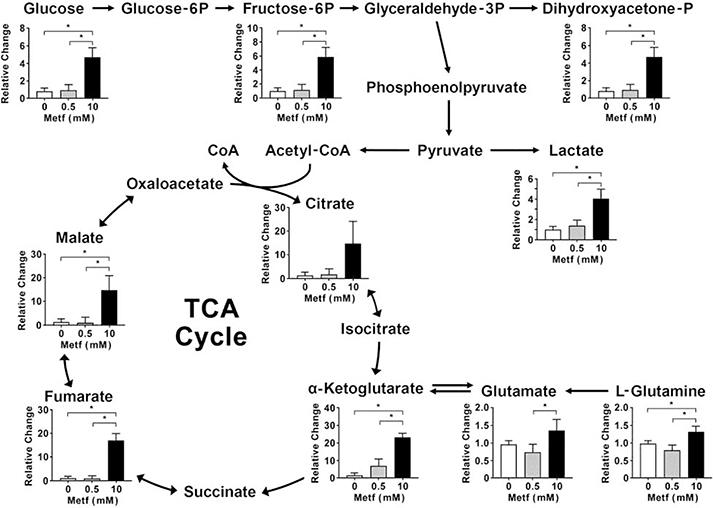
At high concentration, metformin caused metabolic alteration in the hiPSC-CMs. We analyzed metabolites of glycolysis and TCA cycle in the cardiomyocytes pretreated with 0.5 mM and 10 mM metformin for 24 hours. Treatment of the cardiomyocytes with 0.5 mM metformin did not alter metabolites’ level compared to the control cells. However, 10 mM metformin significantly elevated glycolytic metabolites including glucose (P<0.001), fructose-6-phosphate (P=0.001), dihydroxyacetone phosphate (P=0.006), and lactate (P<0.001) (Figure 9). In addition, the cells exposed to 10 mM metformin increased the level of TCA cycle intermediates particularly citrate (P=0.06), α-ketoglutarate (P<0.001), fumarate (P<0.001), and malate (P<0.001) compared to the control cells and cells treated with 0.5 mM metformin. Similarly, the proportion of glutamate (P=0.04) and L-glutamine (P=0.006) were also increased in the cardiomyocytes treated with 10 mM metformin. This suggested that the high concentration of metformin trigger upregulation of glycolysis and glutaminolysis as a compensatory mechanism in response to inhibition of the mitochondrial OXPHOS by metformin.
Fig. 9. Metformin at high concentrations triggers metabolic reprograming towards glycolysis and glutaminolysis.
The hiPSC-CMs were treated with 0.5 mM and 10 mM metformin (Metf) for 24 hours, then subjected to extraction of metabolites and LC-MS analysis. Data are expressed as mean ± SD, * p < 0.05 compared to 0 mM and 0.5 mM Metf (one-way ANOVA and post hoc Tukey test); n=3 for each group.
Metformin protects against oxidative stress.
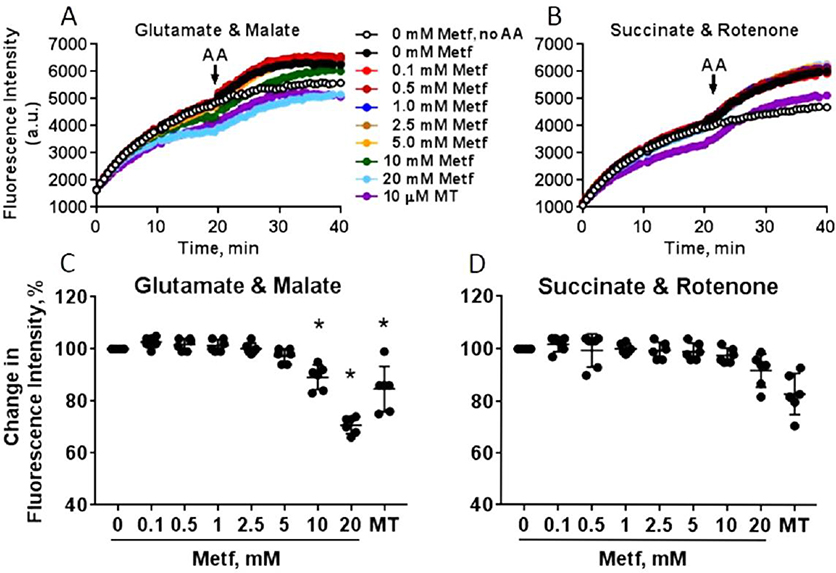
We further tested the effect of metformin on superoxide production in the isolated cardiac mitochondria oxidizing either complex I or II substrates using DHE that reacts with superoxide and forms fluorescent ethidium cation (Figure 10A–D). The increase in ethidium fluorescence indicates increase in superoxide production in mitochondria. Antimycin A, an inhibitor of complex III was added in the middle of the experiment to increase mitochondrial superoxide production by complex III. Metformin significantly decreased superoxide production in mitochondria oxidizing complex I substrates at concentrations of 10 mM (89 ± 4%, P<0.001) and 20 mM (71 ± 3%, P<0.001) similar to MitoTEMPO (76 ± 1%, P<0.001), a mitochondria-specific superoxide scavenger. Metformin did not reduce superoxide production in mitochondria oxidizing complex II substrates. Moreover, it did not change superoxide formation after inhibition of complex III with antimycin A. All these findings consistent with the conclusion that metformin reduces superoxide production in mitochondria by selectively targeting complex I.
Fig. 10. Metformin inhibits superoxide production in cardiac mitochondria.
Representative traces of superoxide production in mitochondria energized with (A) 5 mM glutamate and 2 mM malate or (B) 5 mM succinate in the presence of 2 μM rotenone. Mitochondria were incubated with different concentrations (0–20 mM) metformin (Metf) or 10 μM MitoTEMPO (MT) for 5 min at room temperature before the experiment. Antimycin A (AA) at 5 μM was added at 20 min to promote superoxide production by complex III. The change in the level of superoxide production (%) was quantified as difference in ethidium fluorescence intensity between non-treated and treated with metformin mitochondria oxidizing (C) glutamate and malate or (D) succinate when intensity reached plateau (at 20 min). Data are mean ± SE; * p < 0.05 compared to 0 mM Metf (t-test for normally distributed variables and Kruskal-Wallis test for the variables that were not following the normal distribution); n=6.
Metformin attenuates mPTP formation.
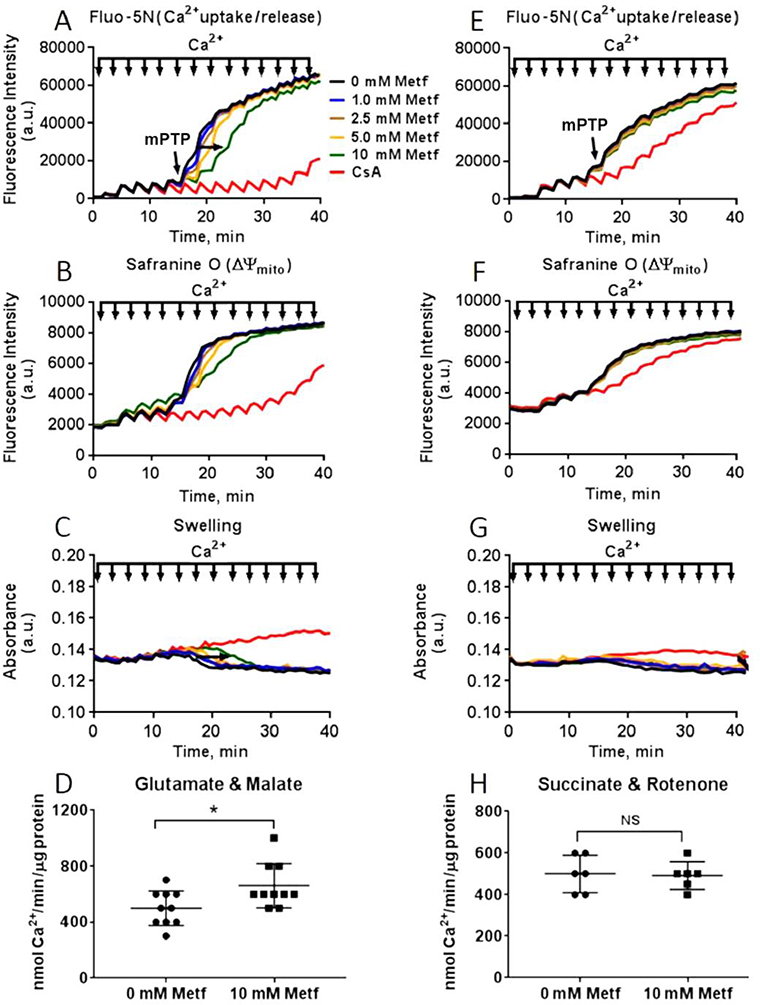
The extent of mPTP opening in the cardiac mitochondria was determined by measuring calcium retention capacity (CRC) simultaneously with membrane potential and swelling of mitochondrial matrix (Figure 11A–G). The addition of Ca2+ (10 μM) led to a rapid depolarization due to Ca2+ entry into the mitochondrial matrix followed by a small repolarization of membrane potential. The opening of mPTP was accompanied with rapid release of Ca2+ from matrix (Figure 11A–E) coincident with a rapid loss of membrane potential (Figure 11B–F) and decrease in light scattering (Figure 11C–G). As expected, cyclosporin A, an inhibitor of mPTP, significantly reduced the sensitivity of the mitochondria to mPTP opening by increasing the CRC. In mitochondria energized with complex I substrates, metformin increased tolerance of mitochondria towards Ca2+ - induced mPTP opening in a dose-dependent manner. At concentration of 10 mM, it increased the threshold for Ca2+ release from 500 ± 125 to 660 ± 158 nmol Ca2+/mg protein (P=0.02) (Figure 11D). Metformin did not have a protective effect against mPTP formation in mitochondria energized with complex II substrate (500 ± 100 vs. 490 ± 74 nmol Ca2+/mg protein, P=0.86) (Figure 11H). These results indicate that metformin delays Ca2+-induced mPTP opening in the cardiac mitochondria through its direct effect on the OXPHOS complex I but not the other downstream complexes.
Fig. 11. Metformin decreases susceptibility of cardiac mitochondria to mPTP opening.
Dose-dependent effect of metformin (Metf) on Ca2+ uptake/release (Fluo-5N), membrane potential (ΔΨmito, Safranin O), swelling of matrix (absorbance) in mitochondria oxidizing (A-C) 5 mM glutamate and 2 mM malate or (E-G) 5 mM succinate in the presence of 2 μM rotenone. The mPTP was induced by sequentially treating isolated mitochondria with 10 μM of Ca2+ pulses at 3 min intervals and monitoring abrupt mitochondrial Ca2+ release, mitochondrial depolarization, and swelling of mitochondrial matrix (decrease in light scattering). Metformin at 10 mM significantly improved resistance to Ca2+-induced mPTP opening in the mitochondria oxidizing (D) glutamate and malate without significant protective effect on mitochondria oxidizing (H) succinate. CsA – cyclosporine A (2 μM). Black arrow indicates 10 μM Ca2+ pulse, red arrow indicates threshold for mPTP opening. Data are mean ±SE; * p < 0.05 compared to 0 mM Metf (t-test for normally distributed variables and Kruskal-Wallis test for the variables that were not following the normal distribution); n=10 for glutamate and malate, n=5 for succinate and rotenone.
DISCUSSION
Our study demonstrated that metformin had a biphasic effect on the human cardiac mitochondria. At low doses, it caused activation of the respiration due to mitochondrial biogenesis via activation of AMPK signaling. At high doses, metformin triggered metabolic reprogramming, reduced the mitochondrial respiration by directly affecting the activity of complex I, decreasing oxidative stress and delaying mPTP opening.
Metformin is a known activator of the AMPK, a highly conserved cellular energy sensor.13 AMPK has been known to maintain cellular energetics by increasing catabolic pathways such as glycolysis and fatty acid oxidation, which increase ATP production.39,40 In 2001, Zhou et al. demonstrated that activation of AMPK by metformin was associated with increase in fatty acid oxidation and inhibition of glucose production in hepatocytes.41 Later, it was revealed that activation of AMPK by metformin required LKB1, an upstream kinase of AMPK, in the liver.42 Multiple studies demonstrated a cardioprotective effect of metformin in animal models that required activation of AMPK.10–12,15 In line with these studies, our results on the human cardiomyocytes demonstrated that treatment with metformin increased phosphorylation of AMPK. The phosphorylation level of AMPK was reduced when the cells were exposed to compound C, a potent inhibitor of AMPK.
At concentrations ≤ 2.5 mM, metformin elevated the cellular OCR. The increase in the OCR was inhibited by compound C, demonstrating that enhancement on the cellular respiration requires AMPK activation. In contrast, higher concentrations of metformin (> 5 mM) reduced the cellular OCR and compound C did not inhibit the OCR further, supporting AMPK – independent modulation of mitochondrial respiration. In isolated cardiac mitochondria, metformin did not enhance the OCR at any doses. It dose-dependently reduced the basal, state 3, uncoupled OCR as well as RCR, when mitochondria respiring the complex I substrates, but had no significant effect in the presence of the complex II substrate. Overall, these results indicate that complex I is a main target for metformin.
In the cardiomyocytes that are highly dependent on the OXPHOS, inhibition of the mitochondrial complex I results in energy inefficiency. To compensate for the limitation of ATP generation, the cells enhance aerobic glycolysis. A byproduct of the increased glycolytic turnover has been known to be a concomitant rise in lactate production from glucose that leads to release of protons following acidification of the extracellular medium.33 The ECAR associated with glycolytic metabolism is routinely measured by Seahorse XF Extracellular Analyzer. Our findings revealed that metformin at concentrations ≥ 2.5 mM dose-dependently increased ECAR that corresponded to decrease in the OCR. This is in agreement with another study by the Andrzejewski group which has shown that metformin has a direct effect on mitochondria by limiting complex I–mediated mitochondrial respiration that seems to shift mitochondrial metabolism towards aerobic glycolysis.20 Both in isolated mitochondria and intact cells, metformin inhibited the TCA cycle and favored lactic acid production.20 Another study by Hou et al. has revealed that complex I inhibition by amobarbital, a complex I inhibitor, and NDUFA13, a complex I subunit, silencing promoted glycolysis in HepG2 and C2C12 cells and suppressed gluconeogenesis in primary cultured hepatocytes.43 These results indicate that metformin causes a metabolic reprogramming related to glycolysis and mitochondrial respiration. Moreover, our LC-MS data have demonstrated more conclusive information on changes in glycolysis and TCA cycle intermediates in response to treatment of the cardiomyocytes with metformin. Metformin at high concentration (10 mM) caused a significant elevation in glucose, glucose-6-phosphate, dihydroxyacetone-phosphate and lactate. In addition, measurement of the TCA cycle metabolites demonstrated increase in citrate, α-ketoglutarate, fumarate and malate levels. Elevation in the TCA intermediates’ substrates also indicate that glutamine is likely a substrate contributing to the TCA cycle. To meet the energetic needs, the cardiomyocytes change their substrate preference, including increase in glutamine. Glutamine is the most abundant amino acid and may serve as an energy source for the heart.44 Its metabolism might be another pathway to derive nutrients and energy in the cells treated with metformin. Glutaminolysis provides precursors for nucleotides, protein, and amino-acid biosynthesis and substrates for the TCA cycle. Glutamine converts to α-ketoglutarate, a substrate of the TCA cycle, through glutamate.
We further investigated the effect of metformin on genes involved in mitochondrial function. Surprisingly, treatment of the cardiomyocytes with the high concentration of metformin (10 mM) resulted in upregulation of a few genes encoding for OXPHOS complexes compared to the non-treated cells and the cells exposed to the low concentration of metformin (0.5 mM). These genes included NDUFA11, NDUFB4, NDUFS5 for complex I, SDHC for complex II, COX5A, COX5B for complex IV, and ATP5G1 for complex V. As discussed previously, metformin at high doses makes mitochondrial metabolism energetically inefficient by directly inhibiting complex I activity. Thereby, cardiomyocytes exert compensatory mechanisms like upregulation of genes coding structural subunits of OXPHOS to compensate for the limitation in the mitochondrial respiration and ATP production. It has been also proposed that activation of AMPK increases both mtDNA copies and expression of proteins related to the OXPHOS complexes including NDUFS1 (complex I), SDHA (complex II), UQCRC1 (complex III), COX4 (complex IV) and ATP5A1 (complex V) in HEPG2 cells and in mice heart, liver and muscle tissue.45 Inhibition of AMPK by compound C significantly reduced mtDNA and expression of the OXPHOS subunits.45 Surprisingly we have observed that treatment of the cells with 0.5 mM metformin downregulated NDUFA5 and NDUFB5 genes coding complex I without affecting the mitochondrial complex I activity. NDUFA5 subunit is required for assembly and stability of the matrix arm of complex I in mitochondria.46 NDUFB5 subunit has a NADH dehydrogenase and oxidoreductase enzymatic activity. Considering that mammalian complex I is composed of 45 subunits, the downregulation of two subunits may not affect significantly the complex I activity. In our experiments, the increase in the cellular respiration in the hiPSC-CMs pretreated with ≤ 2.5 mM metformin might be a consequence of increased mitochondrial biogenesis promoted by activation of AMPK signaling. This is also supported by other studies. In vascular smooth muscle cells, metformin supplementation restored the β-glycerophosphate–mediated impairment of mitochondrial biogenesis by increasing mtDNA content and upregulating mitochondrial biogenesis-related gene expression, whereas compound C suppressed these effects.47 Also, metformin enhanced markers of mitochondrial biogenesis (Nrf1, Cpt1β, Tfam) in mice brown adipocytes.48 Findings by Wang et al., demonstrated that metformin at low concentrations stimulated mitochondrial fission and respiration via activation of AMPK signaling in primary hepatocytes.49
Little is known about interaction between metformin and complex I since the drug does not structurally resemble substrates or classical inhibitors of complex I. It has been considered that metformin does not alter the structural integrity of complex I50 but inhibits NADH oxidation51 reducing cellular NAD+/NADH ratio. In addition, transition between active and de-active forms of complex I plays an important role in interaction of the enzyme with metformin.9 A study by Bridges and colleagues has revealed that metformin binds complex I by “trapping” the enzyme in a de-active state.50 Transition of complex I from active to de-active state is a spontaneous process that is sensitive to temperature52 and oxygen level.53,54 Therefore, deactivation of complex I is expected to increase under hypoxic condition such as global ischemia.55 From the standpoint of clinical benefit, hypoxic deactivation of complex I may be protective, due to reduction of ROS overproduction during reoxygenation55 and subsequent cardiac damage. Therefore, binding of metformin to the de-active state of complex I during hypoxia/ischemia may be relevant therapeutic strategy because of inhibition of enzymatic catalysis and superoxide production by possibly both forward and reverse electron flux.56 This means that metformin is beneficial for patients with type 2 diabetes who exhibit increased risk for myocardial injury in the setting of increasing preload, or diabetic and non-diabetic heart during global ischemia and reperfusion9,57 when a burst of ROS production directly causes oxidative damage to mitochondria initiating myocardial damage. Indeed, there are clinical evidences that metformin significantly lessens the risk and extent of myocardial infarction, reduces cardiovascular mortality, and improves clinical outcomes in patients with type 2 diabetes and heart failure.2–5
Assessment of ROS production in our study demonstrated that metformin significantly reduced superoxide production in isolated mitochondria in the presence of the complex I substrates. The decrease in ROS production was not observed in mitochondria oxidizing the complex II substrate, indicating that metformin does not affect ROS generation by downstream complexes. In support, treatment of isolated mitochondria with antimycin A, an inhibitor of complex III, enhanced superoxide production by complex III that was not reduced by acute metformin treatment. These findings are in agreement with another study by Leverve group in mitochondria isolated from rat liver that metformin pretreatment did not reduce ROS production by complex III.56 Overall, these findings support that metformin diminishes ROS generation by inhibiting complex I.
Excessive generation of ROS results in cell death triggered by the opening of mPTP in mitochondria.58 In our study, metformin dose-dependently delayed Ca2+- induced mPTP opening in human cardiac mitochondria, consistent with findings reported by others.9,59 Lesnefsky and colleagues demonstrated that the acute treatment with high doses of metformin (2 mM) during early reperfusion significantly reduced the ROS production through partial inhibition of complex I in the C57BL/6 mice hearts.9 This, in turn, enhanced the mitochondrial calcium retention capacity and delayed mPTP formation, suggesting that reduced sensitivity to mPTP opening was a potential cardioprotective mechanism resulting from the metformin-induced stabilization of the de-active form of complex I.9 In addition, the same group observed that the long-term feeding of C57BL/6 mice with metformin decreased ROS production and delayed mPTP opening in cardiac mitochondria during thapsigargin-induced endoplasmic reticulum stress. The cardioprotection was executed through activation of AMPK by metformin that prevented CHOP (C/EBP homologous protein) expression and ROS generation from the mitochondrial ETC.59 Incubation with metformin for 15 min significantly delayed time to induce depolarization of mitochondrial membrane and opening of mPTP in isolated cardiomyocytes from Wistar and Goto-Kakizaki rats that were subjected to ischemia and reoxygenation.8 Similarly, in isolated human cardiac mitochondria, we observed that acute treatment with high concentrations of metformin protected mitochondria against superoxide production and mPTP opening. In agreement with other in vitro and in vivo studies, we assume that much higher concentrations of metformin are required for complex I inhibition in order to reduce ROS and delay in mPTP opening in isolated mitochondria.9,22
However, concerns for cytotoxicity at supratherapeutic concentrations of metformin has been raised. Metformin at concentrations > 5 mM reduced the proliferative activity and caused morphological, ultrastructural and apoptotic changes in mice adipose-derived multipotent mesenchymal stromal cells.60 It also induced apoptosis and cell death in breast cancer cells61 and adenoma carcinoma cells through mitochondria-dependent pathway62 indicating that metformin had a selective cytotoxic potential against different types of cancers.
A limitation of our study is the use of relatively high doses of metformin in the experiments that raises concern whether in vitro findings are relevant to those observed in clinical practice with lower plasma concentration.63 However, a clear level of metformin therapeutic concentrations is not available. A recent systematic review by Kajbaf et al. reported an average range between 0.1 to 4 mg/L with the lowest and highest boundaries between 0.000225–1800 mg/L.21 This variation results from many factors, including procedural errors, measurement after single rather than multi-dosage administration during long-term metformin therapy, neglecting compartmentalization effect and tissue and mitochondrial accumulation as plasma values do not necessarily reflect local concentrations, different dosage used in the clinical practice and variation introduced by metformin transporter gene polymorphism that ultimately alters pharmacodynamics and pharmacokinetics of metformin with up to 40-fold variation in plasma concentration.18,21,25,26 Discrepancy between clinical and in vitro conditions also could be explained by considering biophysical properties of metformin.18 As a positively charged ion, metformin has been expected to accumulate in the mitochondrial matrix by 1000-fold in response to the proton motive force across the inner mitochondrial membrane that may increase intramitochondrial concentrations far above plasma concentrations observed in vivo.18,50 In fact, it has been reported that metformin accumulates in the tissues where the concentrations of the drug could be several folds higher than in plasma.63,64 In addition, in vitro and in vivo studies generally employ high metformin concentrations than used in clinical research in order to study mechanisms of metformin action with acute administration.21
In conclusion, our results on the human iPSC-CMs and isolated human cardiac mitochondria confirm that the effect of metformin on mitochondria is a consequence both of the indirect effect of the drug via activation of AMPK65,66 and direct inhibitory effect on the mitochondrial complex I.56,67 The novelty of the current study is that metformin has a biphasic effect on human cardiac mitochondrial function that has been described in animal models but not in freshly isolated human cardiac mitochondria. At low concentrations, it increases the cellular respiration via activation of AMPK signaling that induces mitochondrial biogenesis in the cardiomyocytes. At high concentrations, metformin inhibits the mitochondrial complex I, reduces oxidative stress and attenuates mPTP opening regardless of preexisting activation of AMPK that supports AMPK-independent mechanism of cardioprotective effect. Moreover, metformin treatment at high concentrations enhances glycolysis and glutaminolysis to meet the energetic needs when the mitochondrial OXPHOS is suppressed. Thus, it is likely that metformin can be a beneficial adjunct to patients with impaired endogenous cardioprotective responses by reducing susceptibility to energetic failure and cell death during stress.
Supplementary Material
Supplemental figure 1. Characterization of human induced pluripotent stem cell (iPSCs)-derived cardiomyocytes (hiPSC-CMs). Confocal images of iPSC-CMs, cardiomyocytes differentiated from iPSCs expressed cardiomyocyte-specific marker troponin T (green signals in cytosol) and NKX2.5 (red signals in nuclei). Blue are cell nuclei stained with Hoechst 33342.
Brief commentary.
Background:
Metformin is an oral hypoglycemic agent used to treat type 2 diabetes. It also reduces the risk and extent of myocardial infarction, cardiovascular mortality. However, the exact molecular mechanisms of metformin’s therapeutic action in human heart remain obscure.
Translational Significance:
Findings on animal models demonstrated cardioprotective effect, however, translation to the clinical settings has been disappointing. Our data demonstrated metformin’s ability to elicit cardiac benefits independent of its diabetic effect via activation of AMPK and inhibition of complex I in human cardiomyocytes and isolated cardiac mitochondria. This can be a beneficial alternative to patients with impaired endogenous cardioprotective responses.
Acknowledgments
The authors are aware of the journal’s authorship statement and declare no conflict of interest. All authors have read and approved the final version of the manuscript.
This work was supported by National Heart, Lung, and Blood Institute Grant R01HL101240, 5P01 GM066730-15 and intramural Cardiac Research Awards AHC 570-3657 and 570-5015 form the Aurora Health Care Foundation.
The authors gratefully acknowledge the Advocate Aurora Health Care Biorepository and Specimen Resource Center (BSRC) for providing human cardiac specimens and Cancer Center Redox and Bioenergetics Shared Resources (CCRBSR) at Medical College of Wisconsin for Seahorse and LC-MS services. Also, the authors are grateful for Susan Nord and Jennifer Pfaff for editorial preparation of the manuscript and Brian Miller and Brian Schurrer for the help with the figures from Aurora Cardiovascular and Thoracic Services.
Abbreviations:
- AMPK
adenosine monophosphate activated protein kinase
- ANOVA
analysis of variance
- CCRBSR
Cancer Center Redox and Bioenergetics Shared Resources
- DHE
dihydroethidium
- ECAR
extracellular acidification rate
- ETC
electron transport chain
- FBS
fetal bovine serum
- HILIC
hydrophilic interaction liquid chromatography
- HPRT1
hypoxanthine-guanine phosphoribosyltransferase
- IF
immunofluorescence staining
- LV
left ventricular
- mDNA
mitochondrial DNA
- mPTP
mitochondrial permeability transition pore
- mTorc1
mammalian target of rapamycin complex 1
- OCR
oxygen consumption rate
- PRMI
Roswell Park Memorial Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 2014;10:143–56. [DOI] [PubMed] [Google Scholar]
- 2.[No authors listed]. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854–65. Erratum in: Lancet 1998;352: 1558. [PubMed] [Google Scholar]
- 3.Johnson JA, Majumdar SR, Simpson SH, Toth EL. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 2002;25:2244–8. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ Heart Fail 2011;4:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norwood DK, Chilipko AA, Amin SM, Macharia D, Still KL. Evaluating the potential benefits of metformin in patients with cardiovascular disease and heart failure. Consult Pharm 2013;28: 579–83. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care 2012;35: S11–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legtenberg RJ, Houston RJ, Oeseburg B, Smits P. Metformin improves cardiac functional recovery after ischemia in rats. Horm Metab Res 2002;34182–5. [DOI] [PubMed] [Google Scholar]
- 8.Bhamra GS, Hausenloy DJ, Davidson SM, et al. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol 2008;103: 274–84. [DOI] [PubMed] [Google Scholar]
- 9.Mohsin AA, Chen Q, Quan N, et al. Mitochondrial Complex I Inhibition by Metformin Limits Reperfusion Injury. J Pharmacol Exp Ther 2019;369:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paiva MA, Gonçalves LM, Providência LA, Davidson SM, Yellon DM, Mocanu MM. Transitory activation of AMPK at reperfusion protects the ischaemic-reperfused rat myocardium against infarction. Cardiovasc Drugs Ther 2010;24:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gundewar S, Calvert JW, Jha S, et al. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res 2009;104:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki H, Asanuma H, Fujita M, et al. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation 2009;119:2568–77. [DOI] [PubMed] [Google Scholar]
- 13.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia 2017;60:1577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Messaoudi S, Rongen GA, de Boer RA, Riksen NP. The cardioprotective effects of metformin. Curr Opin Lipidol 2011;22:445–53. [DOI] [PubMed] [Google Scholar]
- 15.Whittington HJ, Hall AR, McLaughlin CP, Hausenloy DJ, Yellon DM, Mocanu MM. Chronic metformin associated cardioprotection against infarction: not just a glucose lowering phenomenon. Cardiovasc Drugs Ther 2013;27:5–16. [DOI] [PubMed] [Google Scholar]
- 16.Yin M, van der Horst IC, van Melle JP, et al. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am J Physiol Heart Circ Physiol 2011;301:H459–68. [DOI] [PubMed] [Google Scholar]
- 17.Foretz M, Hébrard S, Leclerc J, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 2010;120:2355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 2000;348:607–14. [PMC free article] [PubMed] [Google Scholar]
- 19.Miller RA, Birnbaum MJ. An energetic tale of AMPK-independent effects of metformin. J Clin Invest 2010;120:2267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab 2014;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajbaf F, De Broe ME, Lalau JD. Therapeutic Concentrations of Metformin: A Systematic Review. Clin Pharmacokinet 2016;55:439–59. [DOI] [PubMed] [Google Scholar]
- 22.El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 2000;275:223–8. [DOI] [PubMed] [Google Scholar]
- 23.Piel S EJ, Elmér E, Hansson MJ. Metformin induces lactate production in peripheral blood mononuclear cells and platelets through specific mitochondrial complex I inhibition. Acta Physiol (Oxf) 2014;213:171–80. [DOI] [PubMed] [Google Scholar]
- 24.Wilcock C, Bailey CJ. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica 1994;24:49–57. [DOI] [PubMed] [Google Scholar]
- 25.Christensen MM, Brasch-Andersen C, Green H, et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics 2011;21:837–50. [DOI] [PubMed] [Google Scholar]
- 26.Goswami S, Yee SW, Stocker S, et al. Genetic variants in transcription factors are associated with the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther 2014;96:370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emelyanova L, Preston C, Gupta A, et al. Effect of Aging on Mitochondrial Energetics in the Human Atria. J Gerontol A Biol Sci Med Sci 2018;73:608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trumbeckaite S, Opalka JR, Neuhof C, et al. Different Sensitivity of Rabbit Heart and Skeletal Muscle to Endotoxin-induced Impairment of Mitochondrial Function. Eur J Biochem 2001;268:1422–9. [DOI] [PubMed] [Google Scholar]
- 29.Gellerich FN, Deschauer M, Chen Y, et al. Mitochondrial Respiratory Rates and Activities of Respiratory Chain Complexes Correlate Linearly with Heteroplasmy of Deleted DNA without Threshold and Independently of Deletion Size. Biochim Biophys Acta. 2002;1556:41–52. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi C, Bienengraeber M, Canfield S, et al. Comparison of Cardiomyocyte Differentiation Potential Between Type 1 Diabetic Donor- and Nondiabetic Donor-Derived Induced Pluripotent Stem Cells. Cell Transplant 2015;24:2491–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horikoshi Y, Yan Y, Terashvili M, et al. Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes. Cells 2019;8:pii: E1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ban K, Bae S, Yoon YS. Current Strategies and Challenges for Purification of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Theranostics 2017;7:2067–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Divakaruni AS, Paradyse A, Ferrick DA, Murphy AN, Jastroch M. Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods Enzymol 2014;547:309–54. [DOI] [PubMed] [Google Scholar]
- 34.Rogers GW, Brand MD, Petrosyan S. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria, PLoS One 2011;6:e21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc 2012;7:872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan M, Kremer DM, Huang H. Ex vivo and in vivo stable isotope labelling of central carbon metabolism and related pathways with analysis by LC-MS/MS. Nat Protoc 2019;14:313–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tikunov A, Johnson CB, Pediaditakis P, et al. Closure of VDAC causes oxidative stress and accelerates the Ca(2+)-induced mitochondrial permeability transition in rat liver mitochondria. Arch Biochem Biophys 2010;495:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwai-Kanai E, Hasegawa K, Adachi S, et al. Effects of endothelin-1 on mitochondrial function during the protection against myocardial cell apoptosis. Biochem Biophys Res Commun 2003;305:898–903. [DOI] [PubMed] [Google Scholar]
- 39.Driver C, Bamitale KDS, Kazi A, Olla M, Nyane NA, Owira PMO. Cardioprotective Effects of Metformin. J Cardiovasc Pharmacol 2018;72:121–27. [DOI] [PubMed] [Google Scholar]
- 40.Garcia D, Shaw RJ. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol Cell 2017;66:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005;310:1642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou WL, Yin J, Alimujiang M, et al. Inhibition of mitochondrial complex I improves glucose metabolism independently of AMPK activation. J Cell Mol Med 2018;22:1316–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curi R, Lagranha CJ, Doi SQ, et al. Molecular mechanisms of glutamine action. J Cell Physiol 2005;204:392–401. [DOI] [PubMed] [Google Scholar]
- 45.Deng XH, Liu JJ, Sun XJ, Dong JC, Huang JH. Benzoylaconine induces mitochondrial biogenesis in mice via activating AMPK signaling cascade. Acta Pharmacol Sin 2019;40:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rak M, Rustin P: Supernumerary subunits NDUFA3, NDUFA5 and NDUFA12 are required for the formation of the extramembrane arm of human mitochondrial complex I. FEBS Lett 2014;588:1832–8. [DOI] [PubMed] [Google Scholar]
- 47.Ma WQ, Sun XJ, Wang Y, Zhu Y, Han XQ, Liu NF. Restoring mitochondrial biogenesis with metformin attenuates β-GP-induced phenotypic transformation of VSMCs into an osteogenic phenotype via inhibition of PDK4/oxidative stress-mediated apoptosis. Mol Cell Endocrinol 2019;479:39–53. [DOI] [PubMed] [Google Scholar]
- 48.Karise I, Bargut TC, Del Sol M, Aguila MB, Mandarim-de-Lacerda CA. Metformin enhances mitochondrial biogenesis and thermogenesis in brown adipocytes of mice. Biomed Pharmacother 2019;111:1156–65. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, An H, Liu T, et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep 2019;29:1511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bridges HR, Jones AJ, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J 2014;462:475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vial G, Detaille D, Guigas B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front Endocrinol (Lausanne) 2019;10:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vinogradov AD. Catalytic properties of the mitochondrial NADH-ubiquinone oxidoreductase (complex I) and the pseudo-reversible active/inactive enzyme transition. Biochim Biophys Acta 1998;1364:169–85. [DOI] [PubMed] [Google Scholar]
- 53.Maklashina E, Sher Y, Zhou HZ, Gray MO, Karliner JS, Cecchini G. Effect of anoxia/reperfusion on the reversible active/de-active transition of NADH-ubiquinone oxidoreductase (complex I) in rat heart. Biochim Biophys Acta 2002;1556:6–12. [DOI] [PubMed] [Google Scholar]
- 54.Maklashina E, Kotlyar AB, Karliner JS, Cecchini G. Effect of oxygen on activation state of complex I and lack of oxaloacetate inhibition of complex II in Langendorff perfused rat heart. FEBS Lett 2004;556:64–8. [DOI] [PubMed] [Google Scholar]
- 55.Galkin A, Abramov AY, Frakich N, Duchen MR, Moncada S. Lack of oxygen deactivates mitochondrial complex I: implications for ischemic injury? J Biol Chem 2009;284:36055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batandier C, Guigas B, Detaille D, et al. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr 2006;38:33–42. [DOI] [PubMed] [Google Scholar]
- 57.Varjabedian L, Bourji M, Pourafkari L, Nader ND. Cardioprotection by Metformin: Beneficial Effects Beyond Glucose Reduction. Am J Cardiovasc Drugs 2018;18:181–93. [DOI] [PubMed] [Google Scholar]
- 58.Briston T, Roberts M, Lewis S, et al. Mitochondrial permeability transition pore: sensitivity to opening and mechanistic dependence on substrate availability. Sci Rep 2017;7:10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Q, Thompson J, Hu Y, Das A, Lesnefsky EJ. Metformin attenuates ER stress-induced mitochondrial dysfunction. Transl Res 2017;190:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Śmieszek A BK, Chrząstek K, Marycz K. In Vitro and In Vivo Effects of Metformin on Osteopontin Expression in Mice Adipose-Derived Multipotent Stromal Cells and Adipose Tissue J Diabetes Res 2015;2015:814896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marinello PC dST, Panis C, Neves AF, et al. Mechanism of metformin action in MCF-7 and MDA-MB-231 human breast cancer cells involves oxidative stress generation, DNA damage, and transforming growth factor β1 induction Tumour Biol 2016;37:5337–46. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Gao Q, Wang D, Wang Z, Hu C. Metformin inhibits growth of lung adenocarcinoma cells by inducing apoptosis via the mitochondria-mediated pathway. Oncol Lett 2015;10:1343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bailey CJ, Turner RC. Metformin. N Engl J Med 1996;334:574–9. [DOI] [PubMed] [Google Scholar]
- 64.Jensen JB, Sundelin EI, Jakobsen S, et al. [11C]-Labeled Metformin Distribution in the Liver and Small Intestine Using Dynamic Positron Emission Tomography in Mice Demonstrates Tissue-Specific Transporter Dependency. Diabetes 2016;65:1724–30. [DOI] [PubMed] [Google Scholar]
- 65.Ouyang J, Parakhia RA, Ochs RS. Metformin activates AMP kinase through inhibition of AMP deaminase. J Biol Chem 2011;286:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stephenne X, Foretz M, Taleux N, et al. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia 2011;54:3101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuzaki S, Humphries KM. Selective inhibition of deactivated mitochondrial complex I by biguanides. Biochemistry 2015;54:2011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Characterization of human induced pluripotent stem cell (iPSCs)-derived cardiomyocytes (hiPSC-CMs). Confocal images of iPSC-CMs, cardiomyocytes differentiated from iPSCs expressed cardiomyocyte-specific marker troponin T (green signals in cytosol) and NKX2.5 (red signals in nuclei). Blue are cell nuclei stained with Hoechst 33342.