Abstract
N,N'‐dimethyl‐4,4'bipyridinium dichloride (Paraquat) is a potent herbicide used widely in agriculture. We report the effects of an ingestion of paraquat by a 28 year old male with cystic fibrosis and the diagnostic and management challenges this posed in both the acute and longer term setting. We describe the effects of direct paraquat toxicity on the lung tissue secondary to aspiration and review the long‐term sequelae of paraquat, namely osteonecrosis. Our case is the first to describe osteonecrosis of the knee in the context of paraquat toxicity. Survival following ingestion remains poor with a high associated mortality. However, timely treatment with NAC and immunosuppression may impact on survival. In those patients who do survive the acute phase post ingestion, follow‐up over years may be required to detect the long‐term effects of paraquat on bone health.
Keywords: cystic fibrosis, orthopaedics, respiratory medicine, toxicology
N,N'‐dimethyl‐4,4'bipyridinium dichloride (Paraquat) is a potent herbicide used widely in agriculture. We report the effects of an ingestion of paraquat by a 28 year old male with cystic fibrosis and the diagnostic and management challenges this posed in both the acute and longer term setting.
INTRODUCTION
N,N′‐dimethyl‐4,4′bipyridinium dichloride (Paraquat) is a potent herbicide used widely in agriculture. Globally, in 2007, there were an estimated 250,000 to 370,000 deaths associated with pesticide poisoning with over 90% being intentionally consumed. 1 More recent data suggests an estimated 300,000 deaths per year in the Asia‐Pacific region alone. 2 Paraquat is rapidly but incompletely absorbed and predominantly distributed to the lungs, liver, kidney and muscle. 1 Pulmonary injury has 2 phases including acute alveolitis and a subsequent proliferative/fibrotic phase. 3 Inherent toxicity and the lack of effective treatments contribute to high mortality.
CASE REPORT
We report the effects of an ingestion of paraquat by a 28‐year‐old male with cystic fibrosis (F508del/G551D). He had moderate radiological lung disease with well‐preserved lung function FEV1 3.55 L (88% predicted) at baseline. Two millilitres of paraquat, consumed alongside alcohol, followed by immediate vomiting and subsequent presentation to a regional centre occurred 24 h after initial ingestion.
Following presentation, around 36 h after ingestion, he developed tachypnoea, tachycardia and fevers. He was transferred to a metropolitan tertiary‐referral hospital in Brisbane and initial management was instigated with an N‐acetyl cysteine (NAC) infusion and high dose dexamethasone (6 mg three times daily). Discharge occurred after 7 days and high dose dexamethasone continued for 4 weeks, tapered to cessation by the end of week five following ingestion. He was admitted to our Adult CF Centre 5.5 weeks after ingestion with large volumes of purulent sputum, fevers, hypoxia, and upper respiratory tract viral symptoms. Incidentally, human parainfluenza type 2 was detected on nasopharyngeal swab. Large cystic cavities with fluid levels (largest 10 cm) were noted in the upper lobes bilaterally and smaller cavities seen throughout both lungs, Figure 1A,B. Ground glass opacities were present diffusely. Given a history of chronic Pseudomonas aeruginosa lung infection and concerns about atypical organisms given recent immunosuppression, a broad‐spectrum antimicrobial regimen including intravenous Ceftazidime, Meropenem, Tobramycin, Voriconazole, Vancomycin and oral Co‐trimoxazole was instituted. This resulted in clinical improvement with eventual complete resolution of the cavitatory disease seen on subsequent long‐term follow‐up CXR imaging over the subsequent 6 years extending up to July 2023.
FIGURE 1.
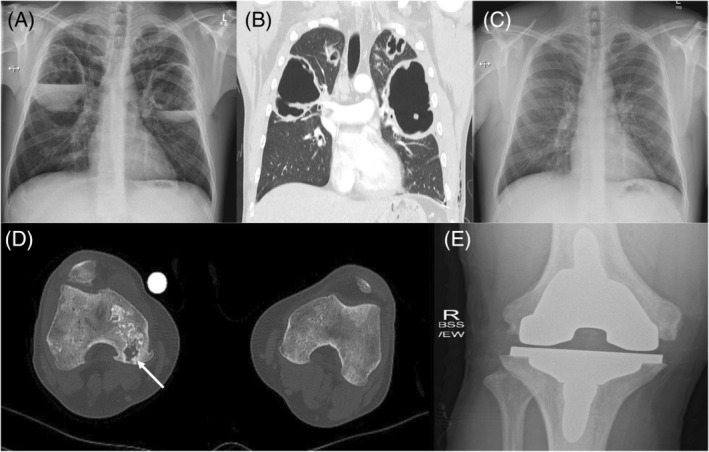
(A) Admission chest x‐ray (CXR) with evidence of bilateral cavitating lesions in both lungs with associated air fluid levels, (B) corresponding computed tomography (CT) chest images highlighting bilateral large volume cavities with associated smaller bi‐apical cavities, (C) follow‐up CXR at 5 years post ingestion showing complete resolution of the previously seen cavitating lesions and no evidence of associated interstitial lung disease or pulmonary fibrosis, (D) CT of lower extremities showing osteonecrosis of the right knee with evidence of bone destruction and avascular necrosis of the medical femoral condylar articular margin (highlighted by the arrow), (E) x‐ray of right knee post total knee replacement.
Subsequent follow‐up with regular monitoring of pulmonary function showed no evidence of deterioration over time. His FEV1 has remained unchanged at 3.66 L (94.8% predicted). This is in part due to the commencement of the CFTR modulator Elexacaftor/Tevacaftor/Ivacaftor (Trikafta®, Vertex Pharmaceuticals) in July 2022, with CFTR modulator therapy associated with reduced morbidity and improving life expectancy. 4 Preceding this he was treated with Ivacaftor (Vertex Pharmaceuticals), initially commenced in March 2014.
Approximately 4 years after ingestion of paraquat, he presented to his local hospital with severe worsening pain affecting both knees, right worse than left, causing significantly impaired mobility. An initial x‐ray of the knee revealed erosions in the medial condyle and slightly heterogenous density of the distal femur with no definite lesions. Subsequent magnetic resonance imaging (MRI) of the right knee demonstrated structural changes consistent with multiple bone infarcts, complex meniscal injury, degenerative changes, muscle oedema and moderate effusion of the knee joint with associated synovitis. MRI of the left knee also demonstrated evidence of chronic bone infarcts affecting the lateral and medial femoral condyles, the meniscus and joint appeared preserved. Over the course of 2 months, he reported increasing right knee pain and a subsequent computed tomography (CT) revealed a marked progression of bony abnormalities in the right distal femur and proximal tibia with fracture fragmentation and extensive avascular necrosis of the medial femoral condyle.
Two years after the initial diagnosis of osteonecrosis of the right knee, a pre‐operative planning CT demonstrated marked reduction in bone mineral density bilaterally at the knee and large medullary infarcts in the distal femurs and proximal tibias. Progression of the insufficiency fracture was also visualized. Structural changes concerning medullary infarcts bilaterally in the distal tibia and talus were also noted with accompanying features concerning joint instability. Our patient underwent an uncomplicated right total knee replacement, 6 years after paraquat ingestion.
Dual x‐ray absorptiometry (DEXA) around the time of presentation with knee pain was within normal limits with a Z‐score of +0.6 and bone mineral density of 1.144 gram per square cm in the total proximal femur with non‐significant decline compared to previous DEXA results 2 years prior. Follow‐up DEXA at time of operative management demonstrated a further non‐significant decline in the femoral bone mineral density.
DISCUSSION
Lung injury
Paraquat causes direct cellular damage by the production of superoxide and nitrite radicals. The clinical course is largely dependent on the dose ingested and the severity of local tissue injury and systemic toxicity. Recognized complications from ingestion include direct injury to the oropharyngeal and oesophageal tracts including erosions and ulcerations. In our case we propose that the most likely cause of the extensive pulmonary cavitatory disease may have been the result direct toxic effects of paraquat because of pulmonary aspiration secondary to vomiting at initial presentation. This adds a further potential way that pulmonary complications may occur following paraquat ingestion, alongside systemic absorption with resultant pneumonitis and rapidly progressive pulmonary fibrosis.
It has been proposed that the magnitude of adverse effects after ingestion of paraquat is dose dependent with serum levels as follows: <20 mg/dL—oral mucosa/oesophageal corrosive injury; 20‐50 mg/dL—renal tubular necrosis, hepatic necrosis and pulmonary fibrosis (preceded by an initial pneumonitis in the acute phase); >50 mg/dL—fulminant disease with associated shock and multiorgan failure. The mortality associated with ingestion above 20 mg/dL is high and usually seen within 3 days. 5
The management of paraquat poisoning is mainly supportive in nature given the absence of a known antidote. In the setting of acute ingestion with early presentation the use of absorbents is indicated, such as activated charcoal (1–2 g/kg). The use of systemic NAC as a free radical scavenger agent has been indicated, whilst the use of immunosuppression has been evaluated in a meta‐analysis of three papers showing that the use glucocorticoid therapy, in addition to cyclophosphamide, was associated with a lower risk of death. 6 Both NAC and high dose immunosuppression, in the form of dexamethasone, were utilized in our patient with an ultimately positive outcome and survival.
One of the complicating factors in our case was the coexistence of CF and its associated structural lung damage, namely established bronchiectasis. The delayed presentation with cavitatory lung disease offered a particular challenge necessitating the use of multiple broad spectrum antimicrobial agents to cover for pre‐existing respiratory pathogens and super‐added infection secondary to probable aspiration and recent immunosuppression. Imaging at the time also showed evidence of ground glass changes, possibly representative of paraquat associated pneumonitis. The most striking feature of this case is the degree to which his lungs recovered with little to no evidence of the previous cavities remaining or paraquat associated pulmonary fibrosis.
Osteonecrosis
Osteonecrosis of the knee can be spontaneous, secondary or arthroscopy‐related. Spontaneous osteonecrosis of the knee (SONK) comprising most cases, is idiopathic in nature and typically affects adults over the age of 60. In contrast, secondary osteonecrosis of the knee affects younger adults and has been linked to conditions associated with heighted inflammation including obesity and systematic lupus erythematosus. Exposure to high dose glucocorticoids, solid‐organ transplantation and alcohol abuse also appear to be important risk factors. 7
Osteonecrosis of the knee affecting the femoral condyles, similar to our case, has previously been described in an adult with CF post‐lung transplantation. 8 Delayed development of osteonecrosis has also been reported with paraquat ingestion. However, all cases in the literature have involved the femoral head. 9 There is also substantial evidence linking glucocorticoid use to osteonecrosis. Genetic factors impacting blood supply, glucocorticoid metabolism, immunity and osteoblast function may also present important risk factors for developing this condition. 10
Presentation with osteonecrosis can occur anywhere between 3 months and over 6 years after exposure to either paraquat or glucocorticoids with considerable variability in individual response depending on cumulative dose and duration of therapy. 9 This is in alignment with our case which presented approximately 4 years post‐paraquat ingestion.
The mechanisms underlying osteonecrosis remain incompletely understood with interruption of vascular supply to the bone postulated to occur due to mechanical disruption, injury, thrombosis or embolism. 11 Additionally, direct osteocyte damage secondary to toxic exposure may also play a role. 12 Subchondral insufficiency fractures may also be an inciting event. 7 The final resultant histological and radiological appearances are stereotypical consisting of medullary infarcts and structural collapse. 13 The clinical trajectory of our case aligns with radiological progression captured by multiple modalities including early changes on x‐ray and MRI followed by extensive structural abnormalities on subsequent CT. Early radiological changes noted in his distal tibia and talar are concerning, with plans to monitor this closely and seek expert opinion regarding management options.
No studies to our knowledge have reported osteonecrosis of the knee with paraquat ingestion in a person with CF. We postulate osteonecrosis in our case was secondary to a combination of glucocorticoid exposure and paraquat ingestion. The impact of inflammatory and genetic risks associated with CF remain unclear, though they are factors in the development of other bone pathology, such as the development of osteoporosis.
In conclusion, direct toxicity and opportunistic infection following paraquat ingestion precipitated catastrophic pulmonary effects in this individual with CF and presented unique management challenges in the setting of pre‐existing pulmonary disease. Although difficult to ascertain, timely treatment with NAC and immunosuppression with high dose corticosteroids was likely a factor in the patient's survival. The onset of progressive cavitating lung lesions as a complication of paraquat ingestion has not been reported previously and whether the underlying CF played a role in this unique complication is worth considering. The need for continued follow‐up for the long‐term sequalae of complications associated with ingestion of paraquat is highlighted by this case, particularly for osteonecrosis, which may occur several years after initial exposure.
AUTHOR CONTRIBUTIONS
David Reid, Ieuan E. S. Evans, Scott C. Bell, and Shanal Kumar were directly involved in conceptualizing the case report with IE writing the article with contributions from David Reid and Shanal Kumar. David Reid, Ieuan E. S. Evans, Scott C. Bell, Daniel Henderson, Daniel Smith, George Tay, Phil Masel, and Megan France were involved in the inpatient care over the duration of his hospital admissions. All authors involved in critically reviewing and editing the report and all have provided final approval for publication.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
Evans IES, Kumar S, France M, Smith D, Masel P, Tay G, et al. Paraquat ingestion in an adult with cystic fibrosis (CF): Diagnostic and management dilemmas. Respirology Case Reports. 2023;11:e01235. 10.1002/rcr2.1235
Associate Editor: Arata Azuma
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Gunnell D, Eddleston M, Phillips MR, Konradsen F. The global distribution of fatal pesticide self‐poisoning: systematic review. BMC Public Health. 2007;7:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. BJCP. 2011;72(5):745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun B, Chen Y‐G. Advances in the mechanism of paraquat‐induced pulmonary injury. Euro Rev Med Pharmacol Sci. 2016;20:1597–1602. [PubMed] [Google Scholar]
- 4. Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, et al. The lancet respiratory medicine commission on the future of care of cystic fibrosis. Lancet Respir Med. 2022;8(1):65–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Janeela MA, Oomen A, Misra AK, Ramya I. Paraquat poisoning: case report of a survivor. J Family Med Prim Care. 2017;6(3):672–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li LR, Sydenham E, Chaudhary B, Beecher D, You C. Glucocorticoid with cyclophosphamide for paraquat‐induced lung fibrosis. Cochrane Database Syst Rev. 2014;7(8):CD008084. [DOI] [PubMed] [Google Scholar]
- 7. Karim AR, Cherian JJ, Jauregui JJ, Pierce T, Mont MA. Osteonecrosis of the knee: review. Ann Transl Med. 2015;3(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schoch OD, Speich R, Schmid C, Tschopp O, Russi EW, Weder W, et al. Osteonecrosis after lung transplantation: cystic fibrosis as a potential risk factor. Transplantation. 2000;69(8):1629–1632. [DOI] [PubMed] [Google Scholar]
- 9. Chan MJ, Huang CC, Hu CC, Huang WH, Hsu CW, Yen TH, et al. Osteonecrosis of femoral head, an overlooked long‐term complication after paraquat intoxication: a retrospective cohort study. Sci Rep. 2020;10:8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang T, Azeddine B, Mah W, Harvey EJ, Rosenblatt D, Séguin C. Osteonecrosis of the femoral head: genetic basis. Int Orthop. 2019;43(3):519–530. [DOI] [PubMed] [Google Scholar]
- 11. Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med. 1992. May 28;326(22):1473–1479. [DOI] [PubMed] [Google Scholar]
- 12. Motta F, Timisina S, Gershwin ME, Selmi C. Steroid‐induced osteonecrosis. J Trans Autoimmun. 2022;5:100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


