Abstract
Cryptococcosis typically manifests as pulmonary lesions, with endobronchial lesions occurring rarely. Inhaled corticosteroids (ICS) may be a risk factor for cryptococcosis of the larynx but not of the bronchi. Here, we report a case involving a 73‐year‐old Japanese man who developed endobronchial cryptococcosis during ICS treatment for asthma. Chest computed tomography showed right mainstem bronchial stenosis and asthma control worsening when he received adequate asthma treatments. Bronchoscopy revealed multiple elevated lesions with white slough from the trachea to the right mainstem bronchus and the right mainstem bronchus lumen entrance narrowing. Bronchial lavage culture revealed Cryptococcus neoformans. Combination treatment with the antifungal agent, mepolizumab, and bronchodilation surgery successfully controlled cryptococcosis and asthma. Attention should be paid to central airway lesions during ICS treatment for uncontrolled asthma.
Keywords: asthma, bronchial stenosis, cryptococcosis, endobronchial cryptococcosis, inhaled corticosteroid
Cryptococcosis typically manifests as pulmonary lesions, with endobronchial lesions occurring rarely. Inhaled corticosteroids (ICS) may be a risk factor for cryptococcosis of the larynx but not of the bronchi. We report a case of endobronchial cryptococcosis during ICS treatment for uncontrolled asthma, highlighting the importance of paying attention to central airway lesions during ICS treatment for asthma.
INTRODUCTION
Cryptococcosis typically manifests as pulmonary lesions caused by fungal spores or poorly encapsulated yeast inhalation and deposition on pulmonary alveoli, 1 and rarely presents as endobronchial lesions. 2 Important risk factors for cryptococcosis include cellular immunodeficiency, including human immunodeficiency virus (HIV) infection and/or acquired immunodeficiency syndrome, hematologic malignancies, and systemic immunosuppressive therapy, including corticosteroids. 1 Nevertheless, immunocompetent patients can develop cryptococcosis. Inhaled corticosteroids (ICS) are known causes of occasional cryptococcosis of the larynx but not the bronchi. 2 , 3 Here, we report a case of endobronchial cryptococcosis with bronchial stenosis in a patient with severe asthma treated with ICS.
CASE REPORT
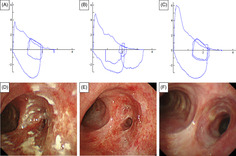
A 73‐year‐old Japanese man with asthma, eosinophilic sinusitis, and otitis media was treated with ICS/long‐acting beta‐agonist (LABA) (fluticasone propionate (500 μg)/formoterol twice daily) inhalation therapy and oral leukotriene receptor antagonists, and theophylline for 15 years. The patient had not received systemic steroids during the previous year. He was a former 15 pack‐year smoker with no immunodeficiency status. He presented to our hospital after wheezing had developed for a year. His chest auscultation of the anterior chest revealed wheezing, but no abnormal findings were found via other physical examinations. Laboratory data showed eosinophilia (560/mm3), although his white blood cell (5900/mm3), lymphocyte (1333/mm3), C‐reactive protein (0.12 mg/dL), albumin (4.3 g/dL), with a normal ratio of albumin/immunoglobulin (1.39), and total immunoglobulin E (IgE) levels (14 IU/mL) were in the normal range. HIV antigen and antibodies, and antineutrophil cytoplasmic antibodies were negative. His fractional exhaled nitric oxide level was elevated (113 ppb). Pulmonary function tests revealed an obstructive ventilatory impairment pattern (forced expiratory volume in 1 second [FEV1.0], 1.77 L; percent predicted FEV1.0 of normal, 62.1%; FEV1.0/forced vital capacity [FVC] ratio, 57.47%) (Figure 1A). Chest computed tomography (CT) revealed a thickened right mainstem bronchial wall with a narrow lumen (Figure 2) but showed normal findings in the bilateral lung fields. Fiberoptic‐bronchoscopy showed multiple lesions with white slough from the trachea to the right mainstem bronchus and right mainstem bronchus lumen narrowing (Figure 1D). A biopsy specimen obtained from a lesion showed inflammatory granulation tissue and yeast‐like fungi with a theca. Bronchial lavage fluid culture revealed Cryptococcus neoformans without other microorganisms. However, a serum Cryptococcus antigen test was negative. No evidence of cryptococcal infections was observed in other organs. Therefore, we made a definitive diagnosis of endobronchial cryptococcosis. We stopped ICS and empirically started oral 200 mg of itraconazole (ITCZ) as a broad‐spectrum anti‐fungal agent before obtaining evidence of endobronchial cryptococcosis because we suspected other common fungal infections such as candidiasis or aspergillosis. However, we restarted ICS/LABA with long‐acting muscarinic antagonists and continuous anti‐interleukin‐5 biologics in addition to oral ITCZ because his asthma control level worsened. He reached clinical remission 1 month after intensive treatment. After 8 months, his flow‐volume curve slightly improved (Figure 1B) and the bronchial findings of white slough lesions had nearly disappeared. Nonetheless, right mainstem bronchus narrowing persisted (Figure 1E). Therefore, bronchoplasty with thickened bronchial wall removal using argon plasma coagulation (APC), and bronchial balloon dilatation were performed. The patient's flow–volume curve improved (Figure 1C), with right mainstem bronchus dilation (Figure 1F), 6 months after bronchoplasty and bronchial balloon dilatation. ITCZ therapy was discontinued after 16 months because the patient's wheezing remained well controlled.
FIGURE 1.
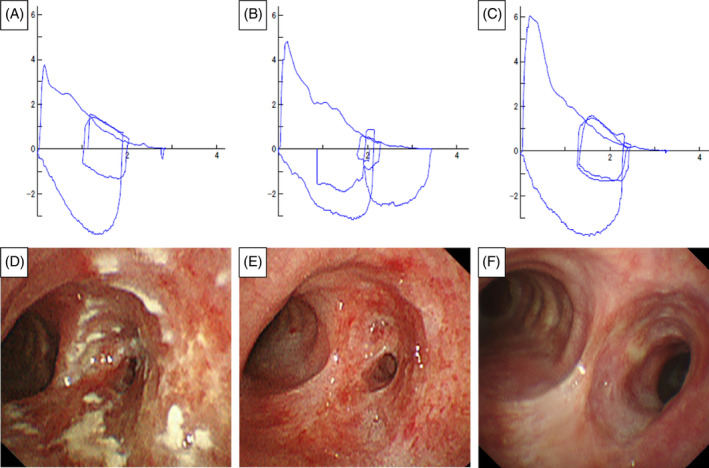
Flow‐volume curve (A–C) and bronchoscopic findings (D–F). Images showing slight flattening of the expiratory limb at the patient's initial visit (A) and 8 months after itraconazole administration (B) are shown. Images revealing improvement of expiratory limb flattening after bronchodilation surgery (C), multiple elevated white lesions from the trachea to the narrowed right mainstem bronchus at the initial visit (D), white lesion improvement and what remained of the narrowed right mainstem bronchus after 8 months of itraconazole administration (E), and bronchial stenosis reduction after bronchodilation surgery (F) are shown.
FIGURE 2.
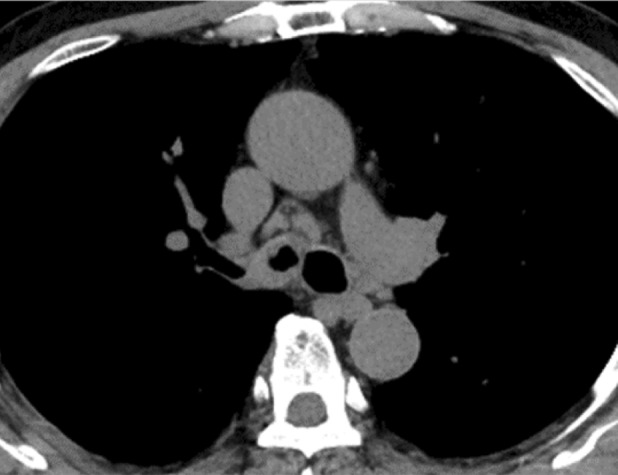
Chest computed tomography (CT) image captured at the initial visit. The image shows the presence of a thickened right mainstem bronchus with a narrowing lumen.
DISCUSSION
To our knowledge, only one prior case of endobronchial cryptococcosis without pulmonary lesions has been reported. 1 , 2 , 3 We suspected that ICS put the patient at risk of endobronchial cryptococcosis because his status was not defined as systemically immunocompromised, although cryptococcosis can occur in immunocompetent patients. The patient had no other risk factors including exposure to Cryptococcus spp., haematological malignancy, diabetes mellitus, or systemic immunosuppressive therapy. Bergeron et al. 3 reviewed 17 cases of laryngeal cryptococcosis, including five treated with ICS without other risk factors. Yoshimine et al. 2 reported the first case of endobronchial cryptococcosis without pulmonary lesions. The interaction between ICS and lower respiratory tract cryptococcosis remains unclear in our patient. His respiratory symptoms worsened despite stopping ICS for suspected cryptococcosis and starting ITCZ. Stopping ICS may have affected asthma control level worsening. Intensive asthma treatments with antifungal therapy successfully control asthma and mediate the removal of cryptococcus from the bronchial mucosa. We selected ITCZ, a broad‐spectrum fungal therapy, because we did not initially expect cryptococcosis due to its rarity. Fluconazole (FLCZ) is recommended as a first choice for non‐immunosuppressed pulmonary cryptococcosis. 4 ITCZ is an acceptable alternative for FLCZ. 4 In our case the drug was used successfully to treat cryptococcosis. However, the patient's right mainstem bronchus lumen had persistent stenosis after the removal of cryptococcus. The remodelling bronchial wall with remaining inflammation and granulomatous formation with cryptococcal infection was suspected to cause his bronchial wall thickening with lumen stenosis. We confirmed right mainstem bronchus wall thickness by chest CT, although histopathological findings were unknown. Successful additional bronchoplasty with the removal of the thickened bronchial wall by APC and bronchial balloon dilatation completely controlled the patient's respiratory symptoms and improved his flow‐volume curves. We believe that other possible causes of respiratory symptom worsening must be assessed, including respiratory tract impairment and infections, and adequate treatments for these causes may be effective in patients with asthma. Spirometry, chest CT, and fiberoptic‐bronchoscopy helped inform the diagnosis of our patient. This multidisciplinary treatment successfully controlled cryptococcosis and asthma. In conclusion, endobronchial cryptococcosis may occur due to ICS, particularly in those with bronchial stenosis. Attention should be paid to central airway lesions during ICS treatment for uncontrolled asthma.
AUTHOR CONTRIBUTIONS
Conceptualization: Takashi Kinoshita. Interpretation of imaging findings: Misa Sudou, Tomoaki Hoshino, Takayuki Horii and Reiko Takaki. Writing of the manuscript: Jun Sasaki. Editing of the manuscript: Masahiro Mitsuoka, Masaki Tominaga, and Tomotaka Kawayama. Supervision: Tomoaki Hoshino. All the authors have read and agreed to the published version of the manuscript.
FUNDING INFORMATION
This work was supported by JSPS KAKENHI Grant Number JP 21K16152, Japan (J. S.).
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and the accompanying images.
ACKNOWLEDGMENTS
This case report was presented at The Japanese Respiratory Society 90th Kyushu Branch Regional Meeting, Kumamoto City, 2023.
Sasaki J, Kinoshita T, Sudou M, Horii T, Takaki R, Mitsuoka M, et al. Endobronchial cryptococcosis with bronchial stenosis in a patient with severe asthma treated with inhaled corticosteroids: A case report. Respirology Case Reports. 2023;11:e01245. 10.1002/rcr2.1245
Associate Editor: Lucy Morgan
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Setianingrum F, Rautemaa‐Richardson R, Denning DW. Pulmonary cryptococcosis: a review of pathobiology and clinical aspects. Med Mycol. 2019;57:133–150. 10.1093/mmy/myy086 [DOI] [PubMed] [Google Scholar]
- 2. Yoshimine K, Tobino K, Sakabe M, Ooi R. Cryptococcosis in the vocal code, trachea, and bronchi. Intern Med. 2021;60:3003–3008. 10.2169/internalmedicine.6559-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergeron M, Gagne AA, Cote M, Chenevert J, Dube R, Pelletier R. Primary larynx cryptococcus neoformans infection: a distinctive clinical entity. Open Forum Infect Dis. 2015;2:ofv160. 10.1093/ofid/ofv160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291–322. 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


