Abstract
The study aimed to analyze the mortality and leading causes of death associated with Sjögren’s syndrome (SS) in the United States (US) between 1999 and 2020 using a multicause approach. We analyzed mortality based on SS as the cause-of-death. Using mortality rates, number of deaths, and historical trends, we examined sex, age of death, comparisons of SS- and polymyalgia rheumatica-related deaths (multiple cause-of-death) in the last 20 years, changes in the ranking of causes of death when SS was the underlying cause-of-death (UCD) in the first and last 5 years of the last 20 years, and the number of deaths and standardized mortality (per 100,000 people) when SS combined with interstitial lung disease (ILD) or tumor was the multiple cause-of-death. An SS-standardized mortality trend chart and a trend line were created. In 22 years, the total number of SS-related deaths in the US was 7,817, including 7,016 women. When SS was the UCD and non-UCD, the standardized ratios of female-to-male deaths (per 100,000 people) were approximately 4.6–13:1 and 6.8–19.6:1, respectively. SS-related deaths were more common in people aged >60 years and concentrated in patients aged 60–79. In cases where SS and polymyalgia rheumatica were the multiple cause-of-death, the number of deaths and age-standardized mortality of SS and polymyalgia rheumatica increased, although lower in SS than in polymyalgia rheumatica. Regarding SS as the UCD, heart disease ranks first. Concerning the number of deaths and standardized mortality in the first (1999–2003) and second (2016–2020) 5 years, when SS-ILD and SS combined with tumors were the multiple causes of death, the number increased in the second 5 years compared to that in the first 5 years. When SS combined with COVID-19 was the multiple cause-of-death, 73 deaths occurred, comprising 64 females and 9 males. Death predominance was observed among women and patients aged 60–79 years with SS. Although the SS-standardized mortality rate was low, an increasing trend was observed. When SS was the primary cause-of-death, heart disease remained primarily involved, followed by malignant neoplasms. The number of patients with SS-ILD and SS combined with tumors in the past 22 years and the standardized mortality rate after 5 years increased compared with those of the previous 5 years. Concurrent SS and COVID-19 may be related to the increased SS deaths.
Keywords: Sjögren’s syndrome, polymyalgia rheumatica, interstitial lung disease, tumor, heart diseases
1. Introduction
Sjögren’s syndrome (SS) is a chronic autoimmune disease characterized by dryness of the eyes and mouth caused by lacrimal and salivary gland dysfunction. It primarily affects women aged 4–60 years, with an average male-to-female ratio of approximately 1:10 [1]. The estimated incidence and prevalence of SS were 6.92/100,000 and 60.82/100,000 persons/year, respectively [1]. The estimated 5- and 10-year survival rates after SS diagnosis are 95 and 90%, respectively [2], and the overall mortality within 7 years is twice that of the general population [3]. Research shows that the standardized mortality rate is 0.85–1.38 [4,5]. Similarly, polymyalgia rheumatica is a rheumatic disorder associated with moderate-to-severe musculoskeletal pain and neck, shoulder, and hip stiffness. Its incidence increases with age [6]. In different populations, the prevalence rate is 12–60/100,000 people, and the standardized mortality rate is approximately 0.70 [7,8], similar to that of SS; however, relevant research on comparing the two is lacking.
SS can involve extraglandular organs (including the skin, lungs, and blood system) and can be complicated by interstitial lung disease (ILD) and tumors. SS-ILD is considered mild and noninvasive; the average survival time after its diagnosis is 9.0 (range: 6.8–13.0) years [9]. However, during 2- to 8-year follow-up, reported mortality rates range from 7.1 to 39% [9]. In addition, SS can be associated with tumor occurrence, which is a risk factor for SS mortality. Combined tumors include hematological (including lymphoma, leukemia, and multiple myeloma) and solid (including thyroid and breast cancers) tumors [10]. A study published in 2010 investigated tumors in SS and found that lymphoma was the most common, with an incidence rate of 5% [11].
Furthermore, a meta-analysis showed that cardiovascular diseases, tumors, and infections were the primary cause-of-death in patients with SS [4]. Studies have shown that death risk owing to cardiovascular or hematological malignancies is comparable between patients with SS and the general population [12]. However, whether the ranking of the cause-of-death mentioned in the SS has changed over the past 22 years is unclear.
Coronavirus disease 2019 (COVID-19) was first reported in Wuhan, Hubei Province, China, in December 2019. A study in the United Kingdom found that patients with rheumatism had a higher death risk owing to COVID-19 [13]. SS has specific characteristics associated with an increased risk of developing severe COVID-19 (autoimmune damage to the lungs, use of immunosuppressants, and lymphoma) [14,15,16]. Therefore, SS combined with COVID-19 can increase the proportion of patients with a poor prognosis, which is relatable to the increased SS-related deaths.
Therefore, this study aimed to describe SS-related deaths and their leading causes using a multicause approach based on data from death certificates obtained from the Centers for Disease Control and Prevention (CDC) over the last 22 years.
2. Methods
Using the United States (US) CDC database, we obtained the number of deaths per 100,000 people and the standardized mortality rate between 1999 and 2020. According to the 10th Revision of the International Classification of Diseases 10 (ICD-10) [17], the following are the category codes: SS (M35.0), polymyalgia rheumatica (M35.3), neoplasms (C00-D48), ILD (J84), and COVID-19 (U07.1). Using mortality rates, number of deaths, and historical trends, we considered the following variables: sex, age of death (<40, 40–59, 60–79 years), mortality of SS and polymyalgia rheumatica as multiple cause-of-death in the last 22 years, changes in the cause-of-death ranking when SS was mentioned as the underlying cause-of-death (UCD) in the first and last 5 years when SS was mentioned as the UCD, and the number of deaths and standardized mortality when SS was combined with ILD or tumors as multiple cause-of-death. The ratios of female-to-male deaths when SS was listed as the underlying and non-underlying causes were calculated separately. SS and polymyalgia rheumatica deaths and standardized mortality (per 100,000 people) were plotted using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA), and trend lines were added. Ethical approval: the conducted research is not related to either human or animal use.
3. Results
The total number of SS-related deaths in the US between 1999 and 2020 was 7,817, including 801 males (10.2%) and 7,016 females (89.8%) (Table 1), with a male-to-female ratio of approximately 1:9. When SS was listed as the UCD, the number of deaths was 2,149 (27.5%) during the period 1999–2020 and showed an increasing trend, with the number of male and female deaths increasing independently a decreasing standardized ratio of female to male deaths (per 100,000 people) of approximately 4.6–13:1, and SS deaths occurring more frequently in persons aged >60 years. It was concentrated in patients aged 60–79 years (Table 1). When SS was classified as a non-fundamental cause-of-death, 5,668 deaths (72.5%) occurred during the period 1999–2020, with an annual increase in the number of male and female deaths and a standardized ratio of approximately 6.8–19.6 deaths per 100,000 people for females and males. SS-related deaths were more common in patients aged 60–79 years (Table 1).
Table 1.
Number of SS-related deaths in the US from 1999 to 2020 stratified by year
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All SS-related deaths, no. | 267 | 273 | 269 | 276 | 272 | 254 | 274 | 267 | 270 | 299 | 258 | 331 |
| SS listed as the UCD | 70 | 75 | 70 | 80 | 84 | 66 | 90 | 85 | 78 | 100 | 72 | 94 |
| Men, no. | 9 | 7 | 5 | 7 | 8 | 7 | 9 | 11 | 9 | 10 | 7 | 9 |
| Women, no. | 61 | 68 | 65 | 73 | 76 | 59 | 81 | 74 | 69 | 90 | 65 | 85 |
| Ratio of women to men | 6.8 | 9.7 | 13 | 10.4 | 9.5 | 8.4 | 9 | 6.7 | 7.6 | 9 | 9.3 | 9.4 |
| Age, no. | ||||||||||||
| <40 years | 1 | 0 | 1 | 0 | 0 | 0 | 4 | 1 | 2 | 3 | 1 | 1 |
| 40–59 years | 6 | 7 | 7 | 6 | 10 | 11 | 8 | 7 | 6 | 11 | 6 | 12 |
| 60–79 years | 42 | 42 | 41 | 32 | 40 | 27 | 34 | 37 | 35 | 41 | 34 | 38 |
| ≥80 years | 21 | 26 | 21 | 42 | 34 | 28 | 44 | 40 | 35 | 45 | 31 | 43 |
| SS listed as the NUCD | 197 | 198 | 199 | 196 | 188 | 188 | 184 | 182 | 192 | 199 | 186 | 237 |
| Men, no. | 24 | 15 | 14 | 16 | 17 | 15 | 16 | 14 | 21 | 14 | 24 | 24 |
| Women, no. | 173 | 183 | 185 | 180 | 171 | 173 | 168 | 168 | 171 | 185 | 162 | 213 |
| Ratio of women to men | 7.2 | 12.2 | 13.2 | 11.3 | 10.1 | 11.5 | 10.5 | 12 | 8.1 | 13.2 | 6.8 | 8.9 |
| Age, no | ||||||||||||
| <40 years | 2 | 3 | 4 | 3 | 3 | 1 | 4 | 2 | 2 | 3 | 1 | 2 |
| 40–59 years | 17 | 19 | 20 | 25 | 23 | 28 | 20 | 22 | 23 | 23 | 19 | 23 |
| 60–79 years | 105 | 92 | 99 | 105 | 95 | 87 | 83 | 83 | 76 | 94 | 81 | 108 |
| >80 years | 82 | 84 | 76 | 63 | 68 | 72 | 77 | 74 | 91 | 79 | 85 | 104 |
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | All | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All SS-related deaths, no. | 350 | 348 | 405 | 405 | 400 | 427 | 412 | 489 | 582 | 689 | 7,817 |
| SS listed as the UCD | 103 | 96 | 112 | 120 | 111 | 117 | 107 | 136 | 146 | 137 | 2,149 |
| Men, no. | 10 | 10 | 11 | 12 | 16 | 13 | 15 | 22 | 26 | 21 | 254 |
| Women, no. | 93 | 86 | 101 | 108 | 95 | 104 | 92 | 114 | 120 | 116 | 1,895 |
| Ratio of women to men | 9.3 | 8.6 | 9.1 | 9 | 5.9 | 8 | 6.1 | 5.1 | 4.6 | 5.5 | 7.4 |
| Age, no. | |||||||||||
| <40 years | 0 | 3 | 2 | 3 | 2 | 1 | 1 | 2 | 0 | 1 | 29 |
| 40–59 years | 9 | 5 | 14 | 7 | 10 | 19 | 18 | 14 | 11 | 20 | 224 |
| 60–79 years | 49 | 49 | 50 | 54 | 48 | 47 | 55 | 77 | 85 | 68 | 1,025 |
| >80 years | 45 | 39 | 46 | 56 | 51 | 50 | 33 | 43 | 50 | 48 | 871 |
| SS listed as the NUCD | 247 | 252 | 293 | 285 | 289 | 310 | 305 | 353 | 436 | 552 | 5,668 |
| Men, no. | 12 | 14 | 28 | 27 | 32 | 32 | 29 | 39 | 52 | 48 | 547 |
| Women, no. | 235 | 238 | 265 | 258 | 257 | 278 | 276 | 314 | 384 | 504 | 5,121 |
| Ratio of women to men | 19.6 | 17 | 9.5 | 9.6 | 8.0 | 8.7 | 9.5 | 8.1 | 7.4 | 10.5 | 9.4 |
| Age, no. | |||||||||||
| <40 years | 2 | 1 | 5 | 3 | 6 | 3 | 2 | 0 | 3 | 9 | 62 |
| 40–59 years | 27 | 34 | 28 | 31 | 39 | 40 | 34 | 43 | 44 | 48 | 630 |
| 60–79 years | 113 | 118 | 145 | 126 | 131 | 157 | 160 | 157 | 198 | 286 | 2,699 |
| >80 years | 105 | 100 | 115 | 125 | 113 | 110 | 109 | 153 | 191 | 209 | 2,277 |
Abbreviations: UCD: underlying cause of death; NUCD: non-underlying cause of death; SS: Sjögren’s syndrome.
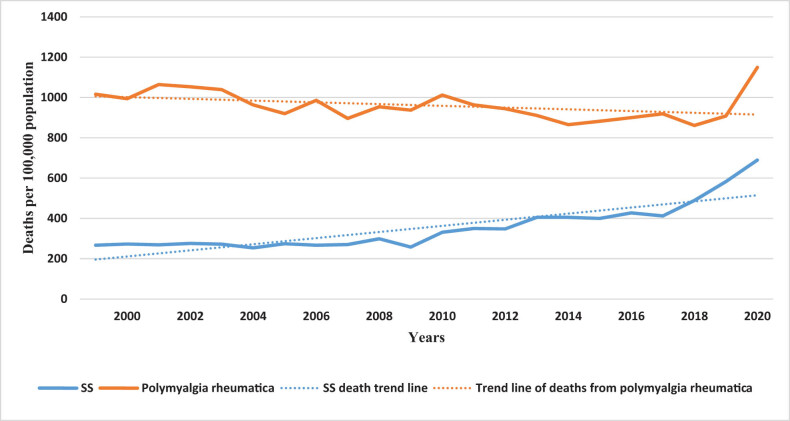
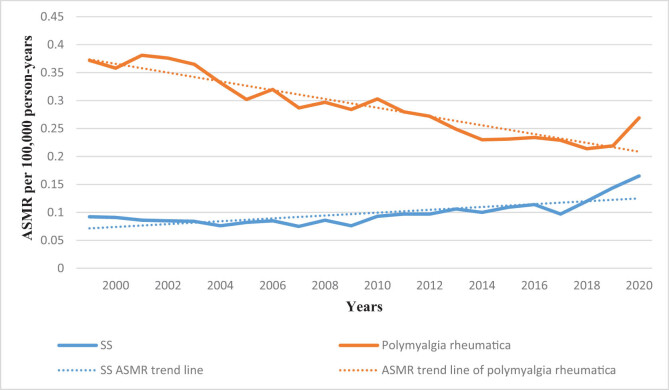
We compared the period 1999–2020 SS and polymyalgia rheumatica as the deaths of multiple cause-of-death and found that the death toll in SS and polymyalgia rheumatica and age-standardized mortality rates (ASMRs) (per thousand population) were increasing, and the death toll from SS and standardized mortality rates (per thousand population) are lower than those of polymyalgia rheumatica (Figures 1 and 2).
Figure 1.
Trends in SS and polymyalgia rheumatica death toll per 100,000 in the US from 1999 to 2020. Abbreviations: SS: Sjögren’s syndrome.
Figure 2.
Trends in SS and polymyalgia rheumatica age-adjusted mortality rates per 100,000 in the US from 1999 to 2020. Abbreviations: SS: Sjögren’s syndrome; ASMR: age-standardized mortality rate.
Similarly, we investigated the ranking of the top 10 cause-of-death in the first (1999–2003) and last (2016–2020) 5 years over the past 22 years by mentioning SS as the root cause-of-death (Tables 2 and 3) and found that compared with the first 5 years, heart diseases and malignant neoplasms remained ranked at first and second, respectively, and diabetes mellitus was ranked seventh. Some diseases were newly discovered or changed in rankings, with COVID-19 and accidents (unintentional injuries) ranking fifth and ninth, respectively. Chronic lower respiratory diseases were ranked from fourth to third, and cerebrovascular diseases dropped from third to fourth place. Alzheimer’s disease ranked sixth from 10th, influenza and pneumonia fell to seventh place from 5th, and chronic liver disease and narrowing dropped from 6th to 10th place.
Table 2.
Ranking of UCD in SS mentioned from 1999 to 2003
| UCD – ICD-10 113 cause list | Deaths |
|---|---|
| #SS | 1,357 |
| #Diseases of heart (I00–I09, I11, I13, I20–I51) | 216 |
| #Malignant neoplasms (C00–C97) | 157 |
| #Cerebrovascular diseases (I60–I69) | 68 |
| #Chronic lower respiratory diseases (J40–J47) | 51 |
| #Influenza and pneumonia (J09–J18) | 37 |
| #Septicemia (A40–A41) | 27 |
| #Chronic liver disease and cirrhosis (K70, K73–K74) | 22 |
| #Diabetes mellitus (E10–E14) | 17 |
| #Nephritis, nephrotic syndrome and nephrosis (N00–N07, N17–N19, N25–N27) | 14 |
| #Alzheimer disease (G30) | 13 |
Abbreviations: UCD: underlying cause of death; ICD-10: International Classification of Diseases 10.
Table 3.
Ranking of UCD in SS mentioned from 2016 to 2020
| UCD – ICD-10 113 cause list | Deaths |
|---|---|
| #SS | 2,599 |
| #Diseases of heart (I00–I09, I11, I13, I20–I51) | 348 |
| #Malignant neoplasms (C00–C97) | 303 |
| #Chronic lower respiratory diseases (J40–J47) | 98 |
| #Cerebrovascular diseases (I60–I69) | 81 |
| #COVID-19 (U07.1) | 66 |
| #Alzheimer disease (G30) | 53 |
| #Influenza and pneumonia (J09–J18) | 47 |
| #Diabetes mellitus (E10–E14) | 43 |
| #Accidents (unintentional injuries) (V01–X59, Y85–Y86) | 37 |
| #Chronic liver disease and cirrhosis (K70, K73–K74) | 35 |
Abbreviations: UCD: underlying cause of death; ICD-10: International Classification of Diseases 10.
In addition, we compared the number of deaths and standardized mortality rates in the first (1999–2003) and second (2016–2020) 5 years when SS was combined as a multiple cause-of-death. We found that the total number of deaths in the first 5 years when SS-ILD was used as a multiple cause-of-death was 182. The standardized mortality rate was 0.013, the total number of deaths in the last 5 years was 419, the standardized mortality rate was 0.026, the number of deaths in the last 5 years was higher than that in the previous 5 years, and the standardized mortality rate increased (Tables 4 and 5).
Table 4.
ILD deaths occurred in SS from 1999 to 2003
| Year | Deaths | Crude rate |
|---|---|---|
| 1999 | 33 | 0.012 |
| 2000 | 38 | 0.014 |
| 2001 | 36 | 0.013 |
| 2002 | 33 | 0.011 |
| 2003 | 42 | 0.014 |
| 1999–2003 | 182 | 0.013 |
Table 5.
ILD deaths occurred in SS from 2016 to 2020
| Year | Deaths | Crude rate |
|---|---|---|
| 2016 | 81 | 0.025 |
| 2017 | 57 | 0.017 |
| 2018 | 70 | 0.021 |
| 2019 | 111 | 0.034 |
| 2020 | 100 | 0.03 |
| 2016–2020 | 419 | 0.026 |
When SS was combined with tumors as a multiple cause-of-death, the total number of deaths and the standardized mortality rates in the first and second 5 years were 241 and 0.017 and 451 and 0.028, respectively, with an increased number of deaths and standardized mortality rate in the second 5 years compared with those of the first 5 years (Tables 6 and 7). Furthermore, we found that when SS was combined with COVID-19 as a multiple cause-of-death, 73 deaths occurred in 2020, including 64 women (87.7%) and 9 men (12.3%). This represents 9% of the total SS-related deaths in the last 22 years, including 8% in women and 1% in men.
Table 6.
Tumor deaths occurred in SS from 1999 to 2003
| Year | Deaths | Crude rate |
|---|---|---|
| 1999 | 56 | 0.02 |
| 2000 | 43 | 0.015 |
| 2001 | 52 | 0.018 |
| 2002 | 49 | 0.017 |
| 2003 | 41 | 0.014 |
| 1999–2003 | 241 | 0.017 |
Table 7.
Tumor deaths occurred in SS from 2016 to 2020
| Year | Deaths | Crude Rate |
|---|---|---|
| 2016 | 80 | 0.025 |
| 2017 | 77 | 0.024 |
| 2018 | 85 | 0.026 |
| 2019 | 107 | 0.033 |
| 2020 | 102 | 0.031 |
| 2016–2020 | 451 | 0.028 |
4. Discussion
Our study showed that between 1999 and 2020, the standardized mortality rate of women with SS was consistently higher than that of men, with a mortality ratio of approximately 9:1. Higher SS-related mortality in women was associated with a higher prevalence in women. When SS was listed as the UCD and non-underlying cause-of-death (NUCD), the number of deaths increased, with a slight fluctuation in the number of deaths in males and an increasing trend in females, which may be related to the higher incidence in females [1] and the increase in the population base. In addition, studies have found that tolerable pathological syndromes are more common in women at the onset of SS [18]. Therefore, the delay in seeking medical treatment may involve multiple organs in the body, exacerbating the disease, possibly related to the increase in female mortality. SS becomes complex in patients from diagnosis with extraglandular involvement or related diseases and is characterized by significantly reduced survival rates, such as Raynaud’s phenomenon in women [18]. In men, extraglandular involvement may be the first symptom; therefore, early diagnosis can be determined, and timely treatment can be obtained [18] to control disease progression, which may result in a low mortality rate in men. Furthermore, we found that when SS was listed as the UCD or NUCD, the number of deaths increased in patients aged 40–59, 60–79, and >80 years; however, it primarily occurred in patients aged >60 years, particularly in patients aged 60–79 years, which may be related to the age at diagnosis. The average age at the first diagnosis of SS is reportedly 56 years [19]. A prospective study in Greece reported a higher death risk in patients with SS than in the general population [20]; however, the risks were similar in another study [21].
SS is a chronic, slow-developing, non-life-threatening disease in many patients, with a 10-year cumulative survival rate of >90%. The mortality rate of patients with SS is insignificantly higher than that of the general population [4]. Despite the seemingly favorable prognosis of SS, the number of deaths per 100,000 people and standardized mortality rate (fluctuating between 0.075 and 0.165) of SS in our study increased over the last 22 years (Figures 1 and 2). A previous meta-analysis reported a quasi-mortality rate of 1.38 in patients with SS [4]. Polymyalgia rheumatica has a good prognosis, and its prevalence rate is similar to that of SS. The number of deaths and age-standardized mortality of polymyalgia rheumatica in the past 22 years was higher than that of SS; however, the overall trend was downward, associated with better disease control in patients with polymyalgia. Although the standardized mortality rates of these diseases are lower than that of rheumatoid arthritis (1.65) [22], early diagnosis and treatment, disease activity control, and mortality reduction remain essential.
Tables 2 and 3 comprise the rankings of SS mentioned as the UCD in 1999–2003 and 2016–2020. Age-related diseases including heart diseases, malignant neoplasms, cerebrovascular diseases, and chronic lower respiratory diseases top the list. Previous studies believed that cardiovascular diseases and tumors were the primary cause-of-death [4]. The current addition, COVID-19, is related to the recent epidemic and the use of immunosuppressants in patients with SS, although hydroxychloroquine reportedly affects COVID-19 [23]. Accidents (unintentional injuries) are related to improving living standards and the widespread use of cars as transportation means. Influenza, pneumonia, chronic liver disease, and cirrhosis dropped from the top 5, and septicemia dropped out of the top 10 in the second 5 years. It is related to the recent upgrade of antibiotics, antiviral drugs, liver protection drugs, and the application of artificial livers [24,25,26].
As the disease progresses, patients with SS may develop comorbidities. The comorbidities may be related to the SS process and/or its treatment or completely independent. These comorbidities are associated with increased mortality rates in women and men. We primarily studied the more common comorbidities of SS, that is, the cases of SS-ILD and SS combined with tumors in the last and the first 5 years in the past 22 years and the number of deaths caused by SS combined with COVID-19 in 2020. Different comorbidities act differently in females with SS, and progressive dyspnea and respiratory failure may occur when combined with lung lesions (such as ILD and pulmonary hypertension) [27]. When combined with infection [28], sepsis and septic shock may occur in severe cases. When combined with malignant tumors [5], cachexia may occur, and when it metastasizes to vital organs (intracranially), cerebral herniation may occur. These complications might cause higher mortality rate in females with SS.
Pulmonary involvement is a common extraglandular complication, with a recently reported prevalence of 16%, and is reportedly associated with poor prognosis and higher mortality in patients with SS [29]. Studies have reported that ILD is associated with decreased functional status in patients with SS, and 36.8% of patients with SS-ILD have significantly reduced activity owing to worsening ILD, causing respiratory failure in 11% of cases [30]. Palm et al. [31] reported a four-fold increased risk of death after 10 years in patients with SS-ILD. Although the 10-year survival rate for patients with SS-ILD was 81.7% [9], our results showed that deaths from SS-ILD as a multiple cause-of-death increased, and the standardized mortality rate increased in 2016–2020 compared with that of 1999–2003 (Tables 4 and 5). This finding may be related to patient age. Age is a risk factor for death, and ILD deterioration is more common in older aged patients with SS at ILD diagnosis and slightly related to inflammatory status and autoantibodies [30]. Therefore, clinicians should assess and monitor lung involvement, particularly in patients without pulmonary manifestations.
SS is strongly associated with an increased risk of developing tumors, particularly lymphoma, which is 10–44 times higher than that in healthy individuals and higher than that reported for systemic lupus erythematosus (seven times) and rheumatoid arthritis (four times) [32]. The standardized incidence (SIR) estimates for all tumors were 1.91 (95% CI, 1.60–2.28). The SIR for solid tumors and blood cancers was 1.13 (95% CI, 0.88–1.46) and 11.02 (95% CI, 8.35–14.54), respectively [10]. Our results showed that when SS combined with tumors was a multiple cause-of-death, deaths increased amongst patients, and the standardized mortality increased in the last 5 years compared with that of the first 5 years in the past 20 years; this may be related to age. Studies have found that the mean age at diagnosis of SS combined with tumor was 55.1 (SD 15.4) (range, 15.3–92.9) years [10]. Additionally, studies have confirmed that severe parotid involvement, purpura, leukopenia, anti-LA antibodies, elevated levels of β2 microglobulin, cryoglobulin, and hypocomplementemia are risk factors for cancer [10,33,34,35]. Therefore, physicians should observe the clinical manifestations of SS in patients aged >50 years and periodically review the relevant indicators. When changes occur, observing whether the tumor is combined is essential.
Furthermore, when SS combined with COVID-19 was a multiple cause-of-death, 73 deaths occurred in 2020 alone, 9% of the total SS-related deaths in the last 22 years. This may be related to the potential abnormal immune response and frequent use of immunosuppressants in patients with SS, causing a high hospitalization rate and poor prognosis [36], including respiratory failure and esophageal lymphocytosis, leading to patient death. Studies have shown increased risks of hospitalization and death, particularly in patients treated with corticosteroids [37].
We re-examined SS-related mortality over the past 22 years. Using multiple cause-of-death analysis is a primary feature and strength of this study. Multiple cause-of-death analyses have been used to determine the maximum possible number of deaths associated with autoimmune diseases, often referred to as related cause-of-death [38]. In this study, SS was classified as a related cause of 72.5% of deaths. The underestimation of rheumatic diseases in mortality statistics is attributed to identifying one of their complications, such as infection or cardiovascular disease, which often become the UCD.
This study has certain limitations. First, despite the automated processing of mortality data used in the US, including the cause of death, the study was conducted by trained disease logists who might have committed errors and introduced incorrect ICD-10 codes. Second, there were no search terms for adherence to therapy and marital status in the system, which could not be further analyzed. At the same time, the number of deaths was relatively small, and subgroup analysis of patients with various complications associated with SS could not be performed.
5. Conclusion
In this study, we used multiple cause-of-death analyses to characterize SS-related mortality in the US over nearly 22 years. We observed death predominance among women and older adults. Similarly, the standardized mortality rate for SS increases and that only 27.5% of deaths were identified as the underlying cause of SS. This suggests that the SS mortality statistics may be underestimated. In contrast, the age-standardized mortality in patients with polymyalgia rheumatica showed a downward trend. When SS was mentioned as the primary cause-of-death, heart disease remained the primary cause, followed by malignant neoplasms. The number of patients with SS-ILD and SS combined with tumors in the past 20 years and the standardized mortality rate after 5 years was higher than that in the first 5 years. Although our results showed that the overall mortality rate of SS was low, an upward trend suggested that clinicians should actively regulate and rationally treat the disease and closely monitor patients with risk factors to improve their prognosis and further reduce the mortality rate.
Footnotes
Funding information: None.
Author contributions: RXH performed and wrote the manuscript; XXH collected the references and designed the table and drew the figures; and JYL modified the manuscript. All authors read and approved the final manuscript.
Conflict of interest: we have no any competing interests.
Data availability statement: Data can be obtained from the website. https://wonder.cdc.gov/.
References
- [1].Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of primary Sjögren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(11):1983–9. 10.1136/annrheumdis-2014-205375. [DOI] [PubMed]
- [2].Brito-Zerón P, Kostov B, Solans R, Fraile G, Suárez-Cuervo C, Casanovas A, et al. Systemic activity and mortality in primary Sjögren syndrome: predicting survival using the EULAR-SS disease activity index (ESSDAI) in 1045 patients. Ann Rheum Dis. 2016;75(2):348–55. 10.1136/annrheumdis-2014-206418. [DOI] [PubMed]
- [3].Thomas E, Symmons DP, Brewster DH, Black RJ, Macfarlane GJ. National study of cause-specific mortality in rheumatoid arthritis, juvenile chronic arthritis, and other rheumatic conditions: a 20 year followup study. J Rheumatol. 2003;30(5):958–65. [PubMed]
- [4].Nannini C, Jebakumar AJ, Crowson CS, Ryu JH, Matteson EL. Primary Sjogren’s syndrome 1976-2005 and associated interstitial lung disease: a population-based study of incidence and mortality. BMJ Open. 2013;3(11):e003569. 10.1136/bmjopen-2013-003569. [DOI] [PMC free article] [PubMed]
- [5].Singh AG, Singh S, Matteson EL. Rate, risk factors and causes of mortality in patients with Sjögren’s syndrome: a systematic review and meta-analysis of cohort studies. Rheumatol (Oxf). 2016;55(3):450–60. 10.1093/rheumatology/kev354. [DOI] [PMC free article] [PubMed]
- [6].Mahmood SB, Nelson E, Padniewski J, Nasr R. Polymyalgia rheumatica: an updated review. Cleve Clin J Med. 2020;87(9):549–56. 10.3949/ccjm.87a.20008. [DOI] [PubMed]
- [7].Raheel S, Shbeeb I, Crowson CS, Matteson EL. Epidemiology of polymyalgia Rheumatica 2000-2014 and examination of incidence and survival trends over 45 years: a population-based study. Arthritis Care Res (Hoboken). 2017;69(8):1282–5. 10.1002/acr.23132. [DOI] [PMC free article] [PubMed]
- [8].Salvarani C, Macchioni P, Zizzi F, Mantovani W, Rossi F, Castri C, et al. Epidemiologic and immunogenetic aspects of polymyalgia rheumatica and giant cell arteritis in northern Italy. Arthritis Rheum. 1991;34(3):351–6. 10.1002/art.1780340313. [DOI] [PubMed]
- [9].Gao H, Sun Y, Zhang XY, Xie L, Zhang XW, Zhong YC, et al. Characteristics and mortality in primary Sjögren syndrome-related interstitial lung disease. Med (Baltim). 2021;100(35):e26777. 10.1097/MD.0000000000026777. [DOI] [PMC free article] [PubMed]
- [10].Brito-Zerón P, Kostov B, Fraile G, Caravia-Durán D, Maure B, Rascón FJ, et al. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J Hematol Oncol. 2017;10(1):90. 10.1186/s13045-017-0464-5. [DOI] [PMC free article] [PubMed]
- [11].Kovács L, Szodoray P, Kiss E. Secondary tumours in Sjögren’s syndrome. Autoimmun Rev. 2010;9(4):203–6. 10.1016/j.autrev.2009.07.002. [DOI] [PubMed]
- [12].Theander E, Manthorpe R, Jacobsson LT. Mortality and causes of death in primary Sjögren’s syndrome: a prospective cohort study. Arthritis Rheum. 2004;50(4):1262–9. 10.1002/art.20176. [DOI] [PubMed]
- [13].Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology alliance physician-reported registry. Ann Rheum Dis. 2021;80(7):930–42. 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed]
- [14].Brito-Zerón P, Kostov B, Fraile G, Caravia-Durán D, Maure B, Rascón FJ, et al. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J Hematol Oncol. 2017;10(1):90. 10.1186/s13045-017-0464-5. [DOI] [PMC free article] [PubMed]
- [15].Ramos-Casals M, Brito-Zerón P, Bombardieri S, Bootsma H, De Vita S, Dörner T, et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann Rheum Dis. 2020;79(1):3–18. 10.1136/annrheumdis-2019-216114. [DOI] [PubMed]
- [16].Sambataro G, Ferro F, Orlandi M, Sambataro D, Torrisi SE, Quartuccio L, et al. Clinical, morphological features and prognostic factors associated with interstitial lung disease in primary Sjӧgren’s syndrome: A systematic review from the Italian Society of Rheumatology. Autoimmun Rev. 2020;19(2):102447. 10.1016/j.autrev.2019.102447. [DOI] [PubMed]
- [17].World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries and Causes of Death. Vol. 1. Geneva: World Health Organization; 1997. Ninth Revision.
- [18].Horvath IF, Szanto A, Papp G, Zeher M. Clinical course, prognosis, and cause of death in primary Sjögren’s syndrome. J Immunol Res. 2014;2014:647507. 10.1155/2014/647507. [DOI] [PMC free article] [PubMed]
- [19].Westhoff G, Zink A. Epidemiologie des primären Sjögren-syndroms [Epidemiology of primary Sjörgren’s syndrome]. Z Rheumatol. 2010;69(1):41–9. 10.1007/s00393-009-0518-3. [DOI] [PubMed]
- [20].Alamanos Y, Tsifetaki N, Voulgari PV, Venetsanopoulou AI, Siozos C, Drosos AA. Epidemiology of primary Sjögren’s syndrome in north-west Greece, 1982-2003. Rheumatol (Oxf). 2006;45(2):187–91. 10.1093/rheumatology/kei107. [DOI] [PubMed]
- [21].Nannini C, Jebakumar AJ, Crowson CS, Ryu JH, Matteson EL. Primary Sjogren’s syndrome 1976-2005 and associated interstitial lung disease: a population-based study of incidence and mortality. BMJ Open. 2013;3(11):e003569. 10.1136/bmjopen-2013-003569. [DOI] [PMC free article] [PubMed]
- [22].Lee YK, Ahn GY, Lee J, Shin JM, Lee TH, Park DJ, et al. Excess mortality persists in patients with rheumatoid arthritis. Int J Rheum Dis. 2021;24(3):364–72. 10.1111/1756-185X.14058. [DOI] [PubMed]
- [23].Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(8):4539–47. 10.26355/eurrev_202004_21038. [DOI] [PubMed]
- [24].Hutchings MI, Truman AW, Wilkinson B. Antibiotics: past, present and future. Curr Opin Microbiol. 2019;51:72–80. 10.1016/j.mib.2019.10.008. [DOI] [PubMed]
- [25].Totura AL, Bavari S. Broad-spectrum coronavirus antiviral drug discovery. Expert Opin Drug Discov. 2019;14(4):397–412. 10.1080/17460441.2019.1581171. [DOI] [PMC free article] [PubMed]
- [26].Larsen FS. Artificial liver support in acute and acute-on-chronic liver failure. Curr Opin Crit Care. 2019;25(2):187–91. 10.1097/MCC.0000000000000584. [DOI] [PubMed]
- [27].Flament T, Bigot A, Chaigne B, Henique H, Diot E, Marchand-Adam S. Pulmonary manifestations of Sjögren’s syndrome. Eur Respir Rev. 2016;25(140):110–23. 10.1183/16000617.0011-2016. [DOI] [PMC free article] [PubMed]
- [28].Sun G, Fosbøl EL, Yafasova A, Faurschou M, Lindhardsen J, Torp-Pedersen C, et al. Long-term risk of heart failure and other adverse cardiovascular outcomes in primary Sjögren’s syndrome. J Intern Med. 2023 Apr;293(4):457–69. 10.1111/joim.13595. [DOI] [PubMed]
- [29].Kim YJ, Choe J, Kim HJ, Song JW. Long-term clinical course and outcome in patients with primary Sjögren syndrome-associated interstitial lung disease. Sci Rep. 2021;11(1):12827. 10.1038/s41598-021-92024-2. [DOI] [PMC free article] [PubMed]
- [30].Roca F, Dominique S, Schmidt J, Smail A, Duhaut P, Lévesque H, et al. Interstitial lung disease in primary Sjögren’s syndrome. Autoimmun Rev. 2017;16(1):48–54. 10.1016/j.autrev.2016.09.017. [DOI] [PubMed]
- [31].Palm O, Garen T, Berge Enger T, Jensen JL, Lund MB, Aaløkken TM, et al. Clinical pulmonary involvement in primary Sjogren’s syndrome: prevalence, quality of life and mortality--a retrospective study based on registry data. Rheumatol (Oxf). 2013;52(1):173–9. 10.1093/rheumatology/kes311. [DOI] [PubMed]
- [32].Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165(20):2337–44. 10.1001/archinte.165.20.2337. [DOI] [PubMed]
- [33].Quartuccio L, Isola M, Baldini C, Priori R, Bartoloni Bocci E, Carubbi F, et al. Biomarkers of lymphoma in Sjögren’s syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: results of a multicenter study. J Autoimmun. 2014;51:75–80. 10.1016/j.jaut.2013.10.002. [DOI] [PubMed]
- [34].Retamozo S, Gheitasi H, Quartuccio L, Kostov B, Corazza L, Bové A, et al. Cryoglobulinaemic vasculitis at diagnosis predicts mortality in primary Sjögren syndrome: analysis of 515 patients. Rheumatol (Oxf). 2016;55(8):1443–51. 10.1093/rheumatology/kew194. [DOI] [PubMed]
- [35].Gottenberg JE, Seror R, Miceli-Richard C, Benessiano J, Devauchelle-Pensec V, Dieude P, et al. Serum levels of beta2-microglobulin and free light chains of immunoglobulins are associated with systemic disease activity in primary Sjögren’s syndrome. Data at enrollment in the prospective ASSESS cohort. PLoS One. 2013;8(5):e59868. 10.1371/journal.pone.0059868. [DOI] [PMC free article] [PubMed]
- [36].Brito-Zerón P, Melchor S, Seror R, Priori R, Solans R, Kostov B, et al. SARS-CoV-2 infection in patients with primary Sjögren syndrome: characterization and outcomes of 51 patients. Rheumatol (Oxf). 2021;60(6):2946–57. 10.1093/rheumatology/keaa748. [DOI] [PMC free article] [PubMed]
- [37].Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–66. 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed]
- [38].Walsh SJ, DeChello LM. Excess autoimmune disease mortality among school teachers. J Rheumatol. 2001;28(7):1537–45. [PubMed]