Abstract
Background
This study aimed to estimate the anti‐SARS‐CoV‐2 antibody seroprevalence in the general population of Bobo‐Dioulasso and Ouagadougou (Burkina Faso).
Methods
We collected from March to April 2021 blood samples from randomly selected residents in both main cities based on the World Health Organization (WHO) sero‐epidemiological investigations protocols and tested them with WANTAI SARS‐CoV‐2 total antibodies enzyme‐linked immunosorbent assay (ELISA) kits intended for qualitative assessment. We also recorded participants' socio‐demographic and clinical characteristics and information on exposure to SARS‐CoV‐2. Data were analysed with descriptive and comparative statistics.
Results
We tested 5240 blood samples collected between 03 March and 16 April 2021. The overall test‐adjusted seroprevalence for SARS‐CoV‐2 antibodies was (67.8% [95% CI 65.9–70.2]) (N = 3553/3982). Seroprevalence was highest among participants aged 15–18 years old (74.2% [95% CI 70.5–77.5]) (N = 465/627), compared with those aged 10–14 years old (62.6% [95% CI 58.7–66.4]) (N = 395/631), or those over 18 (67.6% [95% CI 66.2–69.1]) (N = 2693/3982). Approximately 71.0% (601/860) of participants aged 10–18 years old who tested positive for SARS‐CoV‐2 antibodies experienced no clinical COVID‐19 symptoms in the weeks before the survey, compared with 39.3% (1059/2693) among those aged over 18 years old.
Conclusion
This study reports the results of the first known large serological survey in the general population of Burkina Faso. It shows high circulation of SARS‐CoV‐2 in the two cities and a high proportion of asymptomatic adolescents. Further studies are needed to identify the SARS‐CoV‐2 variants and to elucidate the factors protecting some infected individuals from developing clinical COVID‐19.
Keywords: Burkina Faso, COVID‐19, infectious disease, population‐based seroprevalence, SARS‐CoV‐2, WHO UNITY study
1. INTRODUCTION
On 5 May 2023, the Director‐General of World Health Organization (WHO) announced COVID‐19 emergency over with the decreasing trend in COVID‐19 deaths, the decline in COVID‐19‐related hospitalizations and intensive care unit admissions the high levels of population immunity to SARS‐CoV‐2. 1 Many governments have adjusted their strategies to reflect the status of the pandemic. But the return to normalcy worldwide, after enforcing severe restrictions to contain COVID‐19, requires continuous efforts to monitor the dynamic of the pandemic and prevent new waves. Indeed, the persistent circulation of SARS‐CoV‐2 and its variants in many countries 2 call for global monitoring and research and to inform and adjust the COVID‐19 responses. To ensure better preparedness for future pandemic, many countries have embarked on activities to identify the key learnings from the COVID‐19 response to further strengthen health security and health system resilience. 3 This requires access to quality COVID‐19 epidemiological data 4 and a deep understanding of the measures developed and implemented by all stakeholders to contribute to control the pandemic.
When exploring the number of cases and deaths officially reported by health authorities, many sub‐Saharan African (SSA) countries appear to have defeated the odds of high morbidity and mortality‐related COVID‐19 and do not constitute a threat to global health security. 5 Indeed, compared with countries in America, Europe and Asia, the healthcare impact of COVID‐19 seems substantially lower in SSA. For example, in Burkina Faso, 22,056 cumulative confirmed cases of COVID‐19 and 396 cumulative deaths were reported as of 19 July 2023. 6 However, emerging results from research suggest that weak public health surveillance systems 7 and low access to healthcare in SSA may have led to shortfalls in the detection and reporting of COVID‐19 cases and deaths. New estimates reveal that the true number of COVID‐19 deaths in Africa may be more than three times higher than that officially reported. 8 , 9 This may have resulted in a significant underestimation of the spread of COVID‐19 in the region. 10 , 11 Indeed, several seroprevalence studies using non‐standardized methodology have reported conflicting findings regarding the circulation of SARS‐CoV‐2 on the continent, with estimates of seroprevalence ranging from 0% to 59%, depending on the population studied, the sampling strategy, the sample size, the serological test used and the time of the survey. 12 , 13 High‐quality, large‐scale seroprevalence studies are needed to more accurately estimate the true burden of COVID‐19 and to understand the dynamics and nature of immune responses to SARS‐CoV‐2 infection in the African epidemiological context. 14
The WHO developed early investigation protocols for SARS‐CoV‐2 antibodies seroprevalence in the general population and in healthcare settings, branded as the ‘UNITY studies’. 15 These protocols have been implemented in all WHO regions and half of all Humanitarian Response Plan countries, including Burkina Faso. They may generate standardized and robust local seroprevalence data, as well as key epidemiological, virological and clinical parameters that will improve our understanding of the important characteristics of the SARS‐CoV‐2 infection. 14 This could also provide insight to inform the design of effective COVID‐19 control programmes and potential similar pandemics, especially in the context of scarce resources, limited access and acceptance of COVID‐19 vaccines, as is the case in Burkina Faso. 16
The ANRS‐CoV‐13 ‘EMULCOVID’ research group reports here the results of the first known large SARS‐CoV‐2 seroprevalence study performed in the general population of Burkina Faso's two main cities (Ouagadougou and Bobo‐Dioulasso) as part of the WHO ‘UNITY studies’. 15
2. METHODS
2.1. Study design and setting
This was a cross‐sectional, population‐based, age‐stratified sero‐epidemiological investigation of COVID‐19 viral infection conducted in the two major cities of Burkina Faso, namely, Ouagadougou, the capital and largest city, and Bobo‐Dioulasso, the second largest city. Burkina Faso is a landlocked country in the Sahel of West Africa and has an estimated population of over 20 million inhabitants. 16 According to a recent general population housing survey, the cities of Ouagadougou and Bobo‐Dioulasso together account for more than 62% of the urban population, with 2.5 million and 903,000 inhabitants, respectively. 17 Since the beginning of the pandemic in March 2020, most data on COVID‐19 in BF have been reported from these two cities.
Ouagadougou is in the centre of the country and had 12 boroughs as of 2017 and 1276 enumeration areas (EAs) (Figure 1). The city of Bobo‐Dioulasso, located in the southwest of the country, had seven boroughs and 536 EAs (Figure 1). In both cities, each EA had an average of 220 households and each household comprised an average of 4.8 people. 17 Figure 1 presents the EAs of Ouagadougou and Bobo‐Dioulasso where the data were collected.
FIGURE 1.
SARS‐CoV‐2 seroprevalence sampling collection sites in Ouagadougou and Bobo‐Dioulasso (Burkina Faso, March 2021).
2.2. Participants
We use the sample size calculator available on OpenEpi 18 to estimate the sample size considering an expected SARS‐CoV‐2 seroprevalence of 10% (based on data reported at the study period), 19 an accuracy of ± 3%, an alpha risk = 5% and no design effect (Deff = 1). An estimated 384 persons in each gender and age stratum was required, and this number was rounded to 452 to account for approximately 85% usable data. Full details of the study sampling method have been described elsewhere. 20 We used a two‐stage sampling strategy: We sampled EA and then long‐term residents aged 10 years and older from each sampled household, stratifying by age group (10–18 years, 19–50 years, 60 years and older) and sex (male, female). We invited those selected to provide a blood sample and complete a questionnaire. In some households, more than one resident was sampled (up to five maximum), but they were from different age and sex groups.
2.3. Data sources/measurement
Qualified, accredited field staff collected blood and administered a questionnaire.
In total, 4 mL of venous blood was drawn into a 5 mL EDTA tube, triple‐packed in an isotherm container with an icebox and transferred to the either of the main reference laboratories of Burkina Faso within 6 h, namely, the Laboratory of the National Center for Malaria Research and Training in Ouagadougou or the Laboratory of the MURAZ Centre in Bobo‐Dioulasso. Plasma was centrifuged and aliquoted within 12 h of collection.
2.3.1. SARS‐CoV‐2 seroprevalence testing
We measured SARS‐CoV‐2 antibodies in the collected blood samples using the WANTAI SARS‐CoV‐2 Ab enzyme‐linked immunosorbent assay (ELISA), an ELISA intended for qualitative detection of total antibodies (including IgG and IgM) to SARS‐CoV‐2 in human serum and acid citrate dextrose (ACD) plasma. The WANTAI SARS‐CoV‐2 Ab ELISA is intended for use as an aid in identifying individuals with an adaptive immune response to SARS‐CoV‐2, indicating recent or prior infection. The WANTAI SARS‐CoV‐2 Ab ELISA cannot discriminate IgG and IgM.
The test results of the study participants were the main outcomes of the study. Data were recorded in an electronic case report form, along with the questionnaire data.
2.3.2. Questionnaire data
Each study participant completed a questionnaire as previously described by Traoré et al. 20 to collect socio‐demographic characteristics (age, profession, marital status, level of education and socio‐professional category), prior medical history (history of cardiovascular and thrombo‐embolic disease [arterial hypertension, rhythm disorders, cardiomyopathy, deep venous thrombosis and/or pulmonary embolism]), history of metabolic diseases (diabetes mellitus and kidney failure), history of pulmonary disease (tuberculosis, asthma and chronic obstructive pulmonary disease [COPD]) and other relevant medical history. The questionnaire also asked about lifestyle habits: tobacco and alcohol consumption, clinical signs of COVID‐19 (fever [temperature ≥38°C], cough, dyspnoea, etc.) and exposure to COVID‐19 up to 14 days prior to data collection (any contact with a confirmed case of COVID‐19).
2.4. Analyses
We described the socio‐economic characteristics of the study population. Quantitative variables were described as mean ± standard deviation (SD) and qualitative variables as number and percentage. The crude and adjusted SARS‐CoV‐2 seroprevalence were estimated with 95% confidence intervals in adolescents, overall and by age group and sex. We compared the characteristics of SARS‐CoV‐2 positive versus negative participants. We described COVID‐19 symptoms in the participants who tested positive for SARS‐CoV‐2 antibodies using numbers and percentage. All analyses were conducted separately for adults and adolescents and were presented separately by age and sex groups. SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used to perform all analyses.
People within the same household and EA are more likely to resemble each other than people in other areas and are more likely to share risk factors for infection (e.g., genetic, environmental and socio‐economic). Infectious disease tends to cluster in time and space. This dependence in the data was therefore accounted for in the statistical analysis because each person provides less information than if their response was independent of other people in the same area. To account for clustering at the household and EA level, all analyses were adjusted per EA using standard methods to obtain point estimates of seroprevalence with ‘robust’ standard errors that are calculated based on the observed variability in seroprevalence among clusters. The adjustment was done using SAS (PROC SURVEYFREQ).
3. RESULTS
3.1. Characteristics of the study population
The population had a good acceptance of the study despite the need for blood sampling. Only 29% (2138/7402) of the residents sampled either refused or were absent from home (repeatedly absent) during the data collection period.
A total of 5264 residents were sampled from 03 March to 16 April 2021. After blood sample collection and testing, data from 5240 individuals were validated and analysed: 67.6% (N = 3540) in Ouagadougou and 32.4% (N = 1700) in Bobo‐Dioulasso. Data from 12 individuals were duplicated with conflicting seroprevalence results and were excluded from the analysis.
In total, 32.1% of participants were aged 60 years and older (N = 1426), and 51.7% (N = 2000) were female. More than 26% (N = 1382) of the study participants had received zero schooling.
The most frequent comorbidities reported were in patients with high blood pressure 13.8% (N = 722), chronic neurological or neuromuscular disease 4.5% (N = 238) or diabetes 3.1% (N = 162). More than 89% (N = 4686) of participants had received the Bacille Calmette–Guérin (BCG) vaccination against tuberculosis.
The percentage of current smokers within the study population was 5.7% (N = 299), and 2.7% (N = 144) of the study population had received long‐term corticosteroid treatment. Prior to the study, 2.2% (N = 115) of the study population had nasopharyngeal swabs to test for COVID‐19 as part of the national response strategy, 7.3% (N = 8/115) of which were positive.
Overall, 52.9% (N = 2770) of all study participants reported having had at least one clinical symptom suggestive of COVID‐19 during the 2 weeks prior to the data collection. Students represented 15% of SARS‐CoV‐2 positive adults compared with 12.1% among those who tested negative for SARS‐CoV‐2 antibodies.
The full characteristics of the study participants are presented in Table 1.
TABLE 1.
Characteristics of the study population tested for SARS‐CoV‐2 in Ouagadougou and Bobo‐Dioulasso (Burkina Faso, March 2021).
| Variables | 10–18 years (N = 1258) | 19–59 years men (N = 1279) | 19–59 years women (N = 1277) | 60 years or over (N = 1426) |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Town | ||||
| Bobo‐Dioulasso | 409 (32.5) | 413 (32.3) | 413 (32.3) | 465 (32.6) |
| Ouagadougou | 849 (67.5) | 866 (67.7) | 864 (67.7) | 961 (67.4) |
| Sex | ||||
| Female | 710 (56.4) | – | – | 723 (50.7) |
| Male | 548 (43.6) | – | – | 703 (49.3) |
| Age group (years) | ||||
| 10–14 | 631 (50.2) | – | – | – |
| 15–18 | 627 (49.8) | – | – | – |
| Education level | ||||
| No school | 79 (6.6) | 127 (9.9) | 385 (30.1) | 791 (55.5) |
| Basic literacy | 22 (1.8) | 69 (5.4) | 62 (4.9) | 132 (9.2) |
| Junior high school | 464 (38.5) | 223 (17.4) | 294 (23.0) | 260 (18.2) |
| Senior high school | 635 (52.7) | 566 (44.3) | 435 (34.1) | 185 (13.0) |
| University | 5 (0.4) | 294 (23.0) | 101 (7.9) | 58 (4.1) |
| Unknown | 53 | – | – | – |
| Marital status | ||||
| Single | – | 647 (50.7) | 304 (23.8) | 15 (1.0) |
| Living martially | – | 32 (2.5) | 28 (2.2) | 1 (0.1) |
| Married | – | 585 (45.8) | 874 (68.5) | 905 (63.5) |
| Separated/divorced | – | 9 (0.7) | 12 (0.9) | 15 (1.0) |
| Widowed | – | 4 (0.3) | 58 (4.6) | 489 (34.4) |
| Unknown | – | 2 | 1 | 1 |
| Profession | ||||
| Civil servant | – | 78 (6.1) | 32 (2.5) | 13 (0.9) |
| Private‐sector worker | – | 123 (9.6) | 39 (3.1) | 26 (1.8) |
| Tradesman | – | 187 (14.6) | 264 (20.7) | 161 (11.4) |
| Volunteer | – | 32 (2.5) | 22 (1.7) | 10 (0.7) |
| Student | – | 369 (28.9) | 184 (14.4) | 4 (0.3) |
| Homemaker/housewife | – | 6 (0.5) | 550 (43.1) | 471 (33.3) |
| Informal other than trade | – | 245 (19.2) | 110 (8.6) | 92 (6.5) |
| Retired | – | 13 (1.0) | 5 (0.4) | 393 (27.8) |
| Jobseeker | – | 26 (2.0) | 15 (1.2) | 33 (2.3) |
| Invalidity | – | 5 (0.4) | 4 (0.3) | 104 (7.3) |
| Other | – | 194 (15.2) | 51 (4.0) | 109 (7.7) |
| Unknown | – | 1 | 1 | 10 |
| BMI (kg/m2) a | ||||
| <18.5 | – | 92 (7.2) | 66 (5.2) | 134 (9.4) |
| 18.5–24.9 | – | 804 (62.9) | 515 (40.3) | 698 (49.0) |
| 25–29.9 | – | 275 (21.5) | 355 (27.8) | 364 (25.5) |
| ≥30 | – | 108 (8.4) | 341 (26.7) | 230 (16.1) |
| Tobacco | ||||
| Current smoker | 8 (0.7) | 239 (18.7) | 0 (0) | 52 (3.7) |
| Former smoker | 0 (0) | 135 (10.6) | 0 (0) | 114 (8.0) |
| Never smoked | 1194 (99.3) | 905 (70.7) | 1274 (100) | 1258 (88.3) |
| Unknown | 56 | 0 | 3 | 2 |
| Alcohol | ||||
| No | 1245 (99.0) | 1001 (78.3) | 1146 (89.7) | 1251 (87.7) |
| Yes—misuse b | 13 (1.0) | 241 (18.8) | 123 (9.6) | 159 (11.2) |
| Yes—dependence c | 0 (0) | 37 (2.9) | 8 (0.6) | 16 (1.1) |
| Medical history | ||||
| Diabetes | 1 (0.1) | 15 (1.3) | 30 (2.6) | 117 (8.9) |
| Hypertension | – | 51 (4.2) | 132 (10.8) | 539 (39.2) |
| Chronic liver disease | 1 (0.1) | 7 (0.6) | 11 (0.9) | 2 (0.2) |
| Chronic heart disease | 5 (0.4) | 25 (2.0) | 40 (3.2) | 81 (5.8) |
| Chronic kidney disease | – | 9 (0.7) | 20 (1.6) | 55 (4.0) |
| Chronic neurological or neuromuscular disease | 2 (0.2) | 31 (2.5) | 35 (2.8) | 170 (12.1) |
| Chronic rheumatological disease | 3 (0.3) | 10 (0.8) | 37 (2.9) | 81 (5.7) |
| Chronic respiratory disease | 15 (1.3) | 29 (2.3) | 55 (4.3) | 39 (2.8) |
| HIV | – | 2 (0.2) | 9 (0.7) | 3 (0.2) |
| Tuberculosis | – | 0 (0) | 0 (0) | 6 (0.4) |
| Malignant tumour | – | 0 (0) | 1 (0.1) | 3 (0.2) |
| Other | 11 (0.9) | 82 (6.6) | 95 (7.7) | 143 (10.4) |
| BCG vaccine | ||||
| Yes | 1179 (98.0) | 1215 (95.1) | 1219 (95.7) | 1073 (75.3) |
| No | 24 (2.0) | 62 (4.9) | 55 (4.3) | 351 (24.7) |
| Unknown | 55 | 2 | 3 | 2 |
| Exposure to COVID‐19 | ||||
| Visiting COVID‐19 patients | 3 (0.3) | 18 (1.4) | 8 (0.6) | 10 (0.7) |
| Working with COVID‐19 patients | 1 (0.1) | 30 (2.6) | 8 (0.7) | 6 (0.4) |
| Contact (<1 m) of a COVID patient | 4 (0.3) | 29 (2.6) | 18 (1.6) | 16 (1.2) |
| Sharing the same environment with a COVID patient | 4 (0.3) | 36 (3.2) | 22 (1.9) | 17 (1.3) |
| Travel with a COVID patient | 3 (0.3) | 8 (0.7) | 5 (0.4) | 1 (0.1) |
| Providing direct care to a COVID patient | – | 8 (0.6) | 6 (0.5) | 4 (0.3) |
| Have you ever done a nasopharyngeal swabs test for COVID‐19? | ||||
| Yes | 5 (0.4) | 47 (3.7) | 29 (2.3) | 34 (2.4) |
| No | 1224 (99.6) | 1229 (96.3) | 1240 (97.7) | 1387 (97.6) |
| Unknown | 29 | 16 | ||
| Long‐term corticosteroid therapy (>10 days of treatment)? | ||||
| Yes | 15 (1.3) | 25 (2.0) | 67 (5.3) | 37 (2.6) |
| No | 1189 (98.7) | 1251(98.0) | 1210 (94.7) | 1380 (97.4) |
| Unknown | 54 | 3 | 0 | 9 |
| Had at least one COVID‐19 symptom d | 365 (29.7) | 711 (55.6) | 761 (59.6) | 933 (65.4) |
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus.
For BMI, height and weight were self‐reported.
Misuse: AUDIT‐c score ≥3 in women or ≥4 in men.
Dependence: AUDIT‐c score ≥10.
Participant who reported at least one common COVID‐19 symptoms.
3.2. Outcome data
3.2.1. Seroprevalence of SARS‐CoV‐2 antibodies
Overall, the seroprevalence of SARS‐CoV‐2 antibodies was 67.8% (95% CI 65.9–70.2) (N = 3553): 68.3% (N = 2419) in Ouagadougou and 66.7% (95% CI 63.8–69.5) (N = 1134) in Bobo‐Dioulasso (Figure 1). Table 2a,b presents the crude and adjusted seroprevalence of SARS‐CoV‐2 antibodies by sex and age groups.
TABLE 2.
Crude and adjusted seroprevalence estimates by age group in Ouagadougou and Bobo‐Dioulasso (Burkina Faso, March 2021).
| a: Crude and ZD‐adjusted a seroprevalence estimates for anti‐SARS‐CoV‐2 antibodies in adults (aged >18 years) | ||||
|---|---|---|---|---|
| Variables | All | SARS‐CoV‐2 seropositive N (%) | Seroprevalence (95% confidence interval) | |
| Crude | ZD‐adjusted a | |||
| Overall | 3982 | 2693 (67.6) | 67.6 (66.2–69.1) | 67.6 (65.9–69.3) |
| Sex | ||||
| Female | 2000 | 1351 (67.5) | 67.5 (65.5–69.6) | 67.5 (65.2–69.8) |
| Male | 1982 | 1342 (67.7) | 67.7 (65.6–69.8) | 67.7 (65.5–69.9) |
| Age groups | ||||
| 18–59 years old | 2556 | 1725 (67.5) | 67.5 (65.6–69.3) | 67.5 (65.5–69.4) |
| ≥60 years old | 1426 | 968 (67.9) | 67.9 (65.4–70.3) | 67.9 (65.2–70.5) |
| Age and sex | ||||
| 19–59‐year‐old men | 1279 | 867 (67.8) | 67.8 (65.2–70.3) | 67.8 (65.1–70.3) |
| 19–59‐year‐old women | 1277 | 858 (67.2) | 67.2 (64.5–69.8) | 67.2 (64.3–70.0) |
| 60 years old or over | 1426 | 968 (67.9) | 67.9 (65.4–70.3) | 67.9 (65.2–70.5) |
| City | ||||
| Bobo‐Dioulasso | 1291 | 861 (66.7) | 66.7 (64.1–69.3) | 66.7 (63.8–69.5) |
| Ouagadougou | 2691 | 1832 (68.1) | 68.1 (66.3–69.8) | 68.1 (65.9–70.2) |
| Symptoms | ||||
| Yes | 2405 | 1634 (67.9) | 67.9 (66.0–69.8) | 67.9 (66.0–69.8) |
| No | 1577 | 1059 (67.1) | 67.1 (64.8–69.5) | 67.1 (64.1–70.1) |
| b: Crude and ZD‐adjusted a seroprevalence estimates for anti‐SARS‐CoV‐2 antibodies in young participants (aged 10–18 years) | ||||
|---|---|---|---|---|
| Variables | All | SARS‐CoV‐2 seropositive N (%) | Seroprevalence (95% confidence interval) | |
| Crude | ZD‐adjusted a | |||
| Overall | 1258 | 860 (68.4) | 68.4 (65.7–70.9) | 68.4 (65.4–71.2) |
| Sex | ||||
| Female | 710 | 470 (66.2) | 66.2 (62.6–69.7) | 66.2 (62.6–69.7) |
| Male | 548 | 390 (71.2) | 71.2 (67.2–74.9) | 71.2 (66.6–75.4) |
| Age groups | ||||
| 10–14 years old | 631 | 395 (62.6) | 62.6 (58.7–66.4) | 62.6 (58.4–66.7) |
| 15–18 years old | 627 | 465 (74.2) | 74.2 (70.5–77.5) | 74.2 (70.5–77.6) |
| Age and sex | ||||
| Boys 10–14 years old | 308 | 204 (66.2) | 66.2 (60.6–71.5) | 66.2 (59.9–72.2) |
| Girls 10–14 years old | 323 | 191 (59.1) | 59.1 (53.5–64.5) | 59.1 (53.5–64.6) |
| Boys 15–18 years old | 240 | 186 (77.5) | 77.5 (71.7–82.6) | 77.5 (71.5–82.8) |
| Girls 15–18 years old | 387 | 279 (72.1) | 72.1 (67.3–76.5) | 72.1 (67.3–76.5) |
| City | ||||
| Bobo‐Dioulasso | 409 | 273 (66.7) | 66.7 (61.9–71.3) | 66.7 (61.3–71.9) |
| Ouagadougou | 849 | 587 (69.1) | 69.1 (65.9–72.2) | 69.1 (65.6–72.5) |
| Symptoms | ||||
| Yes | 365 | 246 (67.4) | 67.4 (62.3–72.2) | 67.4 (62.3–72.2) |
| No | 865 | 601 (69.5) | 69.5 (66.3–72.5) | 69.5 (65.9–72.9) |
The ZD‐adjusted uses standard methods to obtain point estimates of seroprevalence but with ‘robust’ standard errors that are calculated based on the observed variability in seroprevalence among clusters.
Participants aged 60 years or more had a seroprevalence of SARS‐CoV‐2 antibodies comparable with the overall study population, at 67.9% (95% CI 65.2–70.5). Seroprevalence was 62.6% (95% CI 58.4–66.7) among those aged 10–14 years, 74.2% (95% CI 70.5–77.6) in participants aged 15–18 years and 67.6% (95% CI 65.9–69.3) among those 18 years and older.
The seroprevalence was highest among boys: 66.2% (95% CI (59.9–72.2) in boys aged 10–14 years old and 77.5% (95% CI 71.7–82.8) in those aged 15–18 years old. In girls, it was 59.1% (95% CI 53.5–64.6) and 72.1% (95% CI 67.3–76.5), respectively.
3.2.2. Comparison of characteristics according to SARS‐CoV‐2 antibody status (Table 3a,b)
TABLE 3.
Comparison of the characteristics between adult study participants who tested positive for SARS‐CoV‐2 and those who tested negative in Ouagadougou and Bobo‐Dioulasso (Burkina Faso, March 2021).
| a: Comparison of the characteristics between adult study participants aged 18+ years old who tested positive for SARS‐CoV‐2 and those who tested negative in Ouagadougou and Bobo‐Dioulasso (Burkina Faso, March 2021) | |||
|---|---|---|---|
| Variables | Positive (N = 2693) | Negative (N = 1289) | p value a |
| N (%) | N (%) | ||
| City | 0.3815 | ||
| Bobo‐Dioulasso (N = 1291) | 861 (32.0) | 430 (33.4) | |
| Ouagadougou (N = 2691) | 1832 (68.0) | 859 (66.6) | |
| Sex | 0.9144 | ||
| Female (N = 2000) | 1351 (50.2) | 649 (50.4) | |
| Male (N = 1982) | 1342 (49.8) | 640 (49.6) | |
| Age groups | 0.7989 | ||
| <60 years old (N = 2556) | 1725 (64.0) | 831 (64.5) | |
| ≥60 years old (N = 1426) | 968 (36.0) | 458 (35.5) | |
| Education level | 0.6469 | ||
| No school (N = 1303) | 888 (33.0) | 415 (32.2) | |
| Basic literacy (N = 263) | 171 (6.3) | 92 (7.1) | |
| Primary (N = 777) | 518 (19.2) | 259 (20.1) | |
| Secondary (N = 1186) | 799 (29.7) | 387 (30.0) | |
| University (N = 453) | 317 (11.8) | 136 (10.6) | |
| Marital status | 0.0467 | ||
| Single (N = 966) | 689 (25.6) | 277 (21.5) | |
| Living maritally (N = 61) | 39 (1.5) | 22 (1.7) | |
| Married (N = 2364) | 1568 (58.3) | 796 (61.7) | |
| Separated/divorced (N = 36) | 21 (0.8) | 15 (1.2) | |
| Widowed (N = 551) | 372 (13.8) | 179 (13.9) | |
| Profession | 0.0405 | ||
| Civil servant (N = 123) | 76 (2.8) | 47 (3.6) | |
| Private‐sector worker (N = 188) | 138 (5.1) | 50 (3.9) | |
| Tradesman (N = 612) | 413 (15.4) | 199 (15.5) | |
| Volunteer (N = 64) | 41 (1.5) | 23 (1.8) | |
| Student (N = 557) | 401 (15.0) | 156 (12.1) | |
| Homemaker/housewife (N = 1027) | 694 (25.9) | 333 (25.9) | |
| Informal other than trade (N = 447) | 301 (11.2) | 146 (11.3) | |
| Retired (N = 411) | 283 (10.5) | 128 (9.9) | |
| Jobseeker (N = 74) | 42 (1.6) | 32 (2.5) | |
| Invalidity (N = 113) | 72 (2.7) | 41 (3.2) | |
| Other (N = 354) | 222 (8.3) | 132 (10.3) | |
| BMI (kg/m2) | 0.1151 | ||
| <18.5 (N = 292) | 182 (6.8) | 110 (8.5) | |
| 18.5 ≤ BMI < 25 (N = 2017) | 1360 (50.5) | 657 (51.0) | |
| 25 ≤ BMI < 30 (N = 994) | 674 (25.0) | 320 (24.8) | |
| ≥30 (N = 477) | 477 (17.7) | 202 (15.7) | |
| Tobacco | 0.0413 | ||
| Current smoker (N = 291) | 180 (6.7) | 111 (8.6) | |
| Former smoker (N = 249) | 161 (6.0) | 68 (6.9) | |
| Never smoked (N = 3437) | 2351 (87.3) | 1086 (84.5) | |
| Alcohol | 0.3517 | ||
| No (N = 3398) | 2313 (85.9) | 1085 (84.2) | |
| Yes—misuse (N = 523) | 341 (12.7) | 182 (14.1) | |
| Yes—dependence (N = 61) | 39 (1.4) | 22 (1.7) | |
| Long‐term corticosteroid therapy (>10 days of treatment)? | 0.5898 | ||
| Yes (N = 129) | 90 (3.4) | 39 (3.0) | |
| No (N = 3841) | 2593 (96.6) | 1248 (97.0) | |
| Symptoms | 0.6028 | ||
| Yes (N = 2405) | 1634 (60.7) | 771 (59.8) | |
| No (N = 1577) | 1059 (39.3) | 518 (40.2) | |
| b: Comparison of the characteristics between young study participants aged 10–18 years old who tested positive for SARS‐CoV‐2 and those who tested negative in Ouagadougou and Bobo‐Dioulasso (Burkina Faso, March 2021) | |||
|---|---|---|---|
| Variables | Positive (N = 860) | Negative (N = 398) | p value a |
| N (%) | N (%) | ||
| City | 0.3928 | ||
| Ouagadougou (N = 849) | 587 (68.3) | 262 (65.8) | |
| Bobo‐Dioulasso (N = 409) | 273 (31.7) | 136 (34.2) | |
| Sex | 0.0602 | ||
| Female (N = 710) | 470 (54.6) | 240 (60.3) | |
| Male (N = 548) | 390 (45.4) | 158 (39.7) | |
| Age | <0.0001 | ||
| 10–14 years (N = 631) | 395 (45.9) | 236 (59.3) | |
| 15–18 years (N = 627) | 465 (54.1) | 162 (40.7) | |
| Education level | 0.0006 | ||
| No school (N = 79) | 44 (5.3) | 35 (9.3) | |
| Basic literacy (N = 22) | 17 (2.1) | 5 (1.3) | |
| Junior high school (N = 464) | 298 (35.9) | 166 (44.1) | |
| Senior high school (N = 635) | 465 (56.1) | 170 (45.2) | |
| University (N = 5) | 5 (0.6) | 0 (0) | |
| Unknown | 31 | 22 | |
| Profession | 0.9641 a | ||
| Tradesman (N = 2) | 1 (0.3) | 1 (0.5) | |
| Student (N = 565) | 360 (94.2) | 205 (94.1) | |
| Homemaker/housewife (N = 9) | 5 (1.3) | 4 (1.8) | |
| Informal other than trade (N = 10) | 6 (1.6) | 4 (1.8) | |
| Invalidity (N = 1) | 1 (0.3) | 0 (0) | |
| Other (N = 13) | 9 (2.4) | 4 (1.8) | |
| Unknown | 478 | 180 | |
| Symptoms | 0.4712 | ||
| Yes (N = 365) | 246 (29.0) | 119 (31.1) | |
| No (N = 865) | 601 (71.0) | 264 (68.9) | |
| Unknown | 13 | 15 | |
| Alcohol | 0.5629 a | ||
| No (N = 1245) | 852 (99.1) | 393 (98.7) | |
| Yes—misuse (N = 13) | 8 (0.9) | 5 (1.3) | |
| Tobacco | 0.4472 a | ||
| Current smoker (N = 8) | 7 (0.9) | 1 (0.3) | |
| Never smoked (N = 1194) | 821 (99.1) | 373 (99.7) | |
| Unknown (N = 56) | |||
| Long‐term corticosteroid therapy (>10 days of treatment)? | 1.000 a | ||
| Yes (N = 15) | 11 (1.3) | 4 (1.1) | |
| No (N = 1189) | 818 (98.7) | 371 (98.9) | |
| Unknown | 31 | 23 | |
The p value was calculated using chi‐square, Freeman–Halton or Fischer tests.
Adults who tested positive for SARS‐CoV‐2 antibodies had similar characteristics to those who tested negative, except that they were more likely to be single (25.6% [N = 689] among adults who tested positive for SARS‐CoV‐2 antibodies, compared with 21.5% [N = 277] among those who tested negative). Students represented 15.0% (N = 401) of SARS‐CoV‐2 antibodies positive adults compared with 12.1% (N = 156) among those who tested negative for SARS‐CoV‐2 antibodies. Those who tested positive were also less likely to be current smokers (6.7%, N = 180), in comparison with 8.6% (N = 111) who tested negative.
Adolescents 15–18 years old were most likely to have positive SARS‐CoV‐2 antibodies, at 54.1% (N = 465) compared with the 40.7% (N = 162) who tested negative for SARS‐CoV‐2 antibodies. They were also more likely to be in secondary school (56.1% (N = 465), compared with 45.2% (N = 170) in those who tested negative).
Lifestyle, clinical history of disease and current treatment plans were similar among those who tested positive and negative for SARS‐CoV‐2 antibodies. Among participants who tested positive for SARS‐CoV‐2 antibodies, more than 39% had no current clinical symptoms related to COVID‐19.
3.2.3. History of COVID‐19 symptoms in participants who tested positive for SARS‐CoV‐2 antibodies, by age group (Figure 2)
FIGURE 2.
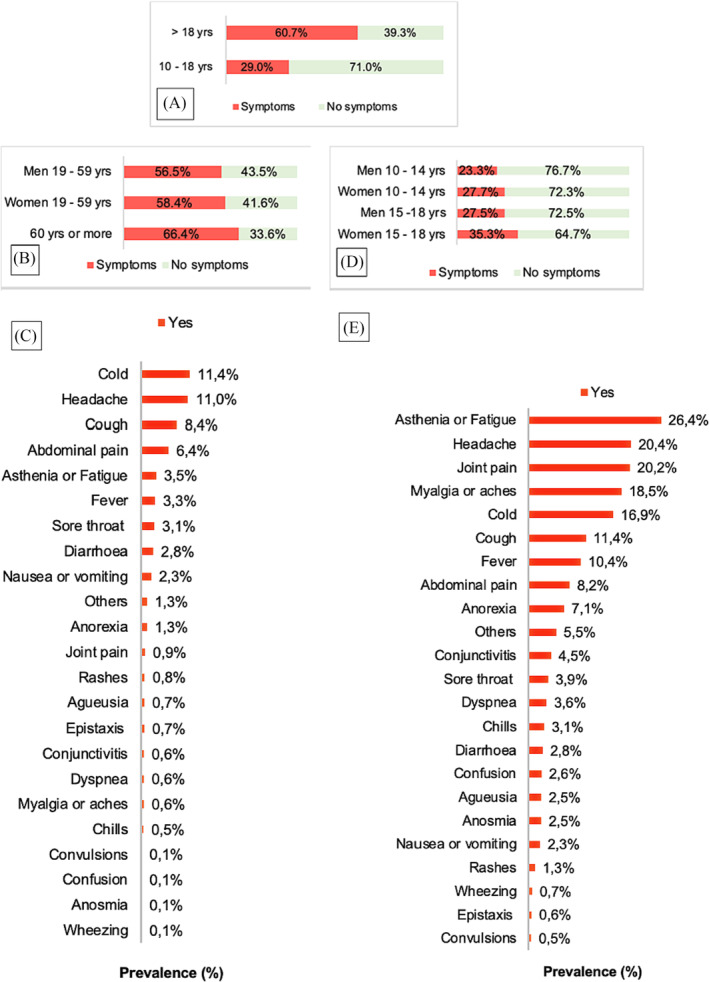
Description of symptoms suggestive of COVID‐19 in study participants who tested positive for SARS‐CoV‐2 antibodies by age group in Ouagadougou and Bobo‐Dioulasso (Burkina Faso, March 2021). (A) Prevalence of symptoms in participants who tested positive for anti‐SARS‐CoV‐2 antibodies according to age. (B) Prevalence of symptoms in participants who tested positive for anti‐SARS‐CoV‐2 antibodies according to age and sex in participants aged over 18 years old. (C) Description of symptoms suggestive of COVID‐19 in participants aged over 18 years old, (D) Prevalence of symptoms in participants who tested positive for anti‐SARS‐CoV‐2 antibodies according to age and sex in participants aged 10–18 years old. (E) Description of symptoms suggestive of COVID‐19 in participants aged 10–18 years old.
Adolescents who tested positive for SARS‐CoV‐2 antibodies reported fewer COVID‐19 clinical symptoms in the 2 weeks prior to the survey than adults. In fact, 71.0% of participants aged 10–18 years old who tested positive for SARS‐CoV‐2 antibodies reported experiencing no COVID‐19 clinical symptoms in the weeks before the survey, compared with the 39.3% symptomless among those aged 18 years and over. The most common symptoms reported primarily by adults who tested positive for SARS‐CoV‐2 antibodies were fatigue, headache, joint pain and myalgia. Adolescents primarily reported experiencing chills, headache, cough and abdominal pain.
4. DISCUSSION
Our study highlighted a high seroprevalence of total SARS‐CoV‐2 antibodies in the general population of adolescent and adult residents in the two major cities of Burkina Faso. To the best of our knowledge, as of 13 June 2022, these estimates are among the highest seroprevalence data reported from studies performed in the general population worldwide. Several studies conducted at the earliest stage of the pandemic that used different samples or analysis protocols reported lower SARS‐CoV‐2 antibodies seroprevalence in various groups of the population. 21 , 22 , 23 , 24 For instance, Nwosu et al. reported a test‐adjusted seroprevalence of 29.2% for anti‐SARS‐CoV‐2 IgG antibodies in a random sample of residents of a health district in Yaoundé, Cameroon, tested from 14 October to 26 November 2020. 23 Nkuba Ndaye et al. found a seropositivity rate between 13% and 36% in a cross‐sectional serological survey of staff working in healthcare facilities in Kinshasa, the capital of the Democratic Republic of the Congo. 25 Using data collected between February and June 2021, Struck et al. reported SARS‐CoV‐2 antibodies seroprevalence adjusted for test performance and population characteristics of 37.4% in Bobo‐Dioulasso and 41.5% in Ouagadougou. 26 However, their study sample was smaller than ours, which resulted in wider confidence intervals between their estimates.
Like our study, other research conducted in general populations using samples collected at least 1 year after the WHO declared COVID‐19 to be a public health emergency of international concern has also reported high anti‐SARS‐CoV‐2 antibody seroprevalence rates. A cross‐sectional population‐based study performed by Moreira‐Soto et al. in San Martin, a rural area of Peru, reported an overall seroprevalence of SARS‐CoV‐2 IgG antibodies of 59.0% (95% CI: 55% to 63%). 27 In a systematic review and meta‐analysis of standardized population‐based seroprevalence studies, Bergeri et al. reported the global epidemiology of SARS‐CoV‐2 infection from January 2020 to December 2021. 28 They show that in the first half of 2021, SARS‐CoV‐2 reached 70.1% in Africa due to infection mainly. In Mali, Sagara et al. reported an anti‐SARS‐CoV‐2 antibody seroprevalence of 73.4% in the urban community of Sotuba, 53.2% in the rural town of Bancoumana and 37.1% in the rural village of Donéguébougou. 29
In Burkina Faso, Peru and Mali, the low number of cases officially reported by health authorities may not reflect the reality of the disease's epidemiological pattern. Indeed, the high anti‐SARS‐CoV‐2 antibody seroprevalence rate reported in these areas supports the fact that PCR‐confirmed case counts are biased proxies for the true attack rate of SARS‐CoV‐2 and may underestimate the true burden of COVID‐19. 23 This can be explained by several factors, including the weakness of the health system and poor laboratory diagnostic capacity. 7 , 30 Although the situation has improved during the COVID‐19 pandemic, 31 the improvements have not bridged the gap in laboratory diagnostic capacity. Moreover, the concept of disease and health‐seeking behaviour in these countries, on top of the fear of being infected by COVID‐19 in healthcare facilities, reduces the likelihood that some citizens will request care when presented with COVID‐19 symptoms. 32 Moreover, because some COVID‐19 symptoms are similar to those of existing endemic diseases, such as malaria and dengue, some patients may defer to usual self‐medication. 33 This behaviour is exacerbated by the stigma and rejection faced by COVID‐19 patients and their families at the beginning of the pandemic. 34
This study was conducted in the most densely populated cities of Burkina Faso, in EAs and households with different socio‐economic conditions. Most of these households lacked adequate sanitation facilities and basic infrastructures and faced challenges in applying prevention measures, such as social distancing and good hygiene practices. This context may have facilitated wide circulation and high transmission of SARS‐CoV‐2. 26 Yet, there were minor differences in seroprevalence estimates after adjustment because to the low variability in seroprevalence among clusters. This may be due to the multiple control measures implemented at community level to prevent the spread of the SARS‐CoV‐2.
The young population age structure in Burkina Faso may also have contributed to the low case‐fatality rate when compared with the situation in Europe and North America. About 80% of the population in Burkina Faso is under 35 years of age. 17 Younger age is thought to be generally protective against both serious illness and death from COVID‐19. As reported by Diop et al., the large population of youths tends to lead to more infections in this group, but most of these infections are asymptomatic or mild and probably go undetected. 35
This study found the highest anti‐SARS‐CoV‐2 antibody seroprevalence rate among study participants aged 10–18 years; the majority of whom did not report any clinical symptoms related to COVID‐19 in the 14 days prior to the study. This may be the results of some of the study limitations. First, the estimation of seroprevalence may be biased. We may have failed to detect newly infected individuals, or those with a weak immunologic response or low viral load, 36 which may have led to an underestimation of the true seroprevalence rate. False positive results for the WANTAI SARS‐CoV‐2 Ab rapid test may have occurred due to cross‐reactivity from pre‐existing antibodies or other possible causes. 37 Tso et al. found significantly higher SARS‐CoV‐2 serological cross‐reactivity in samples from SSA where specific human seasonal coronaviruses may have induced partially protective responses against SARS‐CoV‐2, compared with samples from the United States. 38 Our seroprevalence estimation may have been overestimated due to this cross‐reactivity. However, the WANTAI SARS‐CoV‐2 Ab ELISA is recommended by WHO and has proven accurate in prior studies. An assessment of the clinical performance of four immunoassays for antibodies to SARS‐CoV‐2 in a real‐life routine care setting, conducted by Marlet et al., recommended first‐line serology testing for SARS‐CoV‐2 antibodies using WANTAI Ab because of its specificity and sensitivity that ranges from 98% to 100% and 98.0% to 99.1%, respectively, 14 days after the onset of COVID‐19 symptoms. 39 A further limitation of our study is that we used a test that provides information regarding the presence of anti‐SARS‐CoV‐2 total antibodies, without discriminating IgG and IgM, their titration or the virus variant. It does not provide any data on who was at the acute phase of infection and who was in a later phase. Antibodies to SARS‐CoV‐2 are generally detectable in the blood several days after the initial infection, but the length of time the antibodies persist post‐infection is not yet well known. 39 Finally, this study was limited to urban areas of Burkina Faso, and the assessment of past exposure using a questionnaire may have led to some recall bias.
The strengths of this study include the use of a rigorous methodology recommended by the WHO. This methodology was further strengthened by (1) estimating the sample size of the study based on the age and sex distribution of the population in the households and (2) performing a census in the EAs randomly selected for the study, to update the estimation of the residents before randomly sampling them for the data collection.
The high anti‐SARS‐CoV‐2 antibodies seroprevalence rate reported in our study could lead to the abandonment of COVID‐19 mitigation measures in Burkina Faso, because people may think that we have reached supposed collective immunity. However, an increase in population immunity could create selective pressure, which would favour variants that will infect people who have been immunized. 40
5. CONCLUSION
This study provides strong evidence of the rapid spread of infection among city dwellers in Burkina Faso and probably throughout similar SSA countries and suggests that previous reports may have substantially underestimated the spread of infection in the region. Further studies are needed to estimate the seroprevalence of anti‐ SARS‐CoV‐2 antibodies in rural areas, where almost three quarters of the country's population lives and to elucidate the factors protecting some infected individuals from developing clinical COVID‐19. Immunization against SARS‐CoV‐2 should remain a priority for health authorities worldwide. This is particularly important in Burkina Faso because we do not have an estimation of the anti‐SARS‐CoV‐2 seroprevalence in rural areas, where almost three quarters of the country's population lives.
AUTHOR CONTRIBUTIONS
Samiratou Ouedraogo, Isidore Tiandiogo Traoré, Dramane Kania, Blahima Konaté, Rachel Médah, Gilles Paradis, Halidou Tinto and Tienhan Sandrine Dabakuyo‐Yonli conceived the study design. Samiratou Ouedraogo, Isidore Tiandiogo Traoré, Dramane Kania, Nongodo Firmin Kaboré, Hermann Badolo, Mimbouré Yara, Blahima Konaté, Rachel Médah, Ariane Kamga Mamguem, Gilles Paradis, Halidou Tinto and Tienhan Sandrine Dabakuyo‐Yonli participated in the data collection, literature search and the preparation of the first draft of the paper. All authors participated in the writing of the manuscript, the analysis and interpretation of the results. All authors read and approved the final version of this manuscript.
CONFLICT OF INTEREST STATEMENT
No conflict of interest declared.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1111/irv.13216.
ETHICS STATEMENT
The study was approved by the Ministry of Health of Burkina Faso (Ref No. 2020‐00952/MS/CAB/INSP/CM) and the Burkina Faso National Ethics Committee for health research (Ref No. 2020‐8‐140).
STATEMENT OF PATIENT CONSENT
All data collected was confidential and written informed consent was obtained from all participants prior to participation.
ACKNOWLEDGEMENTS
We are grateful to the study participants and health authorities of the two cities. We would like to thank the members of the scientific council formed as part of the project implementation for their continuous scientific advice: Léon Savadogo, Philippe Van De Perre, Dominique Costagliola, Fernand Bationo, Alice Montoyo, Apolline Sondo and Christine Kafando. The authors thank Fiona Ecarnot, PhD (EA3920, University of Franche‐Comté, Besançon, France) for editorial assistance.
Ouedraogo S, Traoré IT, Kania D, et al. The burden of the coronavirus disease 2019 virus infection in Burkina Faso: Results from a World Health Organization UNITY population‐based, age‐stratified sero‐epidemiological investigation. Influenza Other Respi Viruses. 2023;17(11):e13216. doi: 10.1111/irv.13216
Funding information This project was funded by the ANRS (France Recherche Nord&Sud Sida‐HIV Hépatites) (ANRS‐COV13/EMuLCOVID‐19). The WANTAI SARS‐CoV‐2 Ab ELISA serological tests were donated by The World Health Organization. We also thank the following institutions for their financial support to Samiratou Ouedraogo as part of the Health System Impact Fellowship: (i) the Canadian Institutes of Health Research (CIHR), Ottawa, Ontario, Canada; (ii) Institut National de Santé Publique du Québec (INSPQ), Montreal, Quebec, Canada; and (iii) the Department of Epidemiology, Biostatistics and Occupational Health, Faculty of Medicine, McGill University, Montreal, Quebec, Canada. The funders of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
DATA AVAILABILITY STATEMENT
The data presented in the manuscript are available on request.
REFERENCES
- 1. World Health Organization . Statement on the fifteenth meeting of the IHR 2005. Emergency Committee on the COVID‐19 pandemic. Accessed July 25, 2023. https://apps.who.int/iris/rest/bitstreams/1500086/retrieve
- 2. World Health Organization . Tracking SARS‐CoV‐2 variants. 2022. Accessed September 20, 2022. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 3. World Health Organization . Lessons learned from COVID‐19 pandemic: virtual regional consultation with informal expert group. 2021. Accessed July 25, 2023. https://apps.who.int/iris/rest/bitstreams/1472676/retrieve
- 4. Badr HS, Zaitchik BF, Kerr GH, et al. Unified real‐time environmental‐epidemiological data for multiscale modeling of the COVID‐19 pandemic. Sci Data. 2023;10(1):367. doi: 10.1038/s41597-023-02276-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nordling L. ‘A ticking time bomb’: scientists worry about coronavirus spread in Africa|Science|AAAS. 2020. Accessed September 20, 2022. https://www.science.org/news/2020/03/ticking-time-bomb-scientists-worry-about-coronavirus-spread-africa
- 6. World Health Organization . WHO Coronavirus (COVID‐19) Dashboard: situation by region, country, territory & area. Accessed July 25, 2023. https://covid19.who.int/table
- 7. Mooketsane KS, Phirinyane MB. Health governance in Sub‐Saharan Africa. Glob Soc Policy. 2015;15(3):345‐348. doi: 10.1177/1468018115600123d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adams J, MacKenzie MJ, Amegah AK, et al. The conundrum of low COVID‐19 mortality burden in sub‐Saharan Africa: myth or reality? Glob Health Sci Pract. 2021;9(3):433‐443. doi: 10.9745/GHSP-D-21-00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mutapi F. COVID‐19 Shows Why African Data Is Key for the Continent's Response to Pandemics. The Conversation; 2021. Accessed September 20, 2022. http://theconversation.com/covid-19-shows-why-african-data-is-key-for-the-continents-response-to-pandemics-169197 [Google Scholar]
- 10. Wang H. Estimation of Total and Excess Mortality Due to COVID‐19. Institute for Health Metrics and Evaluation; 2021. Accessed September 20, 2022. http://www.healthdata.org/special‐analysis/estimation‐excess‐mortality‐due‐covid‐19‐and‐scalars‐reported‐covid‐19‐deaths [Google Scholar]
- 11. Institute for Health Metrics and Evaluation . COVID‐19 has caused 6.9 million deaths globally, more than double what official reports show. 2021. Accessed November 6, 2021. http://www.healthdata.org/news-release/covid-19-has-caused-69-million-deaths-globally-more-double-what-official-reports-show
- 12. Bobrovitz N, Arora RK, Cao C, et al. Global seroprevalence of SARS‐CoV‐2 antibodies: a systematic review and meta‐analysis. PLoS ONE. 2021;16:e0252617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tessema SK, Nkengasong JN. Understanding COVID‐19 in Africa. Nat Rev Immunol. 2021;21(4):69‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . Population‐Based Age‐Stratified Seroepidemiological Investigation Protocol for Coronavirus 2019 (COVID‐19) Infection, 26 May 2020, Version 2.0. World Health Organization; 2020. Accessed June 15, 2022. https://apps.who.int/iris/handle/10665/332188 [Google Scholar]
- 15. Bergeri I, Lewis HC, Subissi L, et al. Early epidemiological investigations: World Health Organization UNITY protocols provide a standardized and timely international investigation framework during the COVID‐19 pandemic. Influ Other Respir Viruses. 2022;16(1):7‐13. doi: 10.1111/irv.12915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Worldometer . Burkina Faso Demographics. Population of Burkina Faso. 2020. Accessed June 15, 2022. https://www.worldometers.info/demographics/burkina-faso-demographics/
- 17. Institut National de la démographie du Burkina Faso . Cinquième Recensement général de la population et de l'habitation. 2020. Accessed June 15, 2021. https://www.insd.bf/index.php/rgph-5
- 18. Dean AG, Sullivan KM, Soe MM. OpenEpi: open‐source epidemiologic statistics for public health. 2013. Accessed June 15, 2022. https://www.openepi.com/Menu/OE_Menu.htm
- 19. Pollán M, Pérez‐Gómez B, Pastor‐Barriuso R, et al. Prevalence of SARS‐CoV‐2 in Spain (ENE‐COVID): a nationwide, population‐based seroepidemiological study. Lancet. 2020;396(10250):535‐544. doi: 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Traoré IT, Ouedraogo S, Kania D, et al. COVID‐19 epidemiological, sociological and anthropological investigation: study protocol for multidisciplinary mixed methods research in Burkina Faso. BMC Infect Dis. 2021;21(1):896. doi: 10.1186/s12879-021-06543-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Azami M, Moradi Y, Moradkhani A, Aghaei A. SARS‐CoV‐2 seroprevalence around the world: an updated systematic review and meta‐analysis. Eur J Med Res. 2022;27(1):81. doi: 10.1186/s40001-022-00710-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsiao M, Davies M‐A, Kalk E, et al. SARS‐COV‐2 seroprevalence in the Cape Town metropolitan sub‐districts after the peak of infections. 2020. Accessed September 23, 2022. https://www.nicd.ac.za/wp‐content/uploads/2020/09/COVID‐19‐Special‐Public‐Health‐Surveillance‐Bulletin_Issue‐5.pdf
- 23. Nwosu K, Fokam J, Wanda F, et al. SARS‐CoV‐2 antibody seroprevalence and associated risk factors in an urban district in Cameroon. Nat Commun. 2021;12(1):5851. doi: 10.1038/s41467-021-25946-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ngere I, Dawa J, Hunsperger E, et al. High seroprevalence of SARS‐CoV‐2 but low infection fatality ratio eight months after introduction in Nairobi, Kenya. Int J Infect Dis. 2021;112:25‐34. doi: 10.1016/j.ijid.2021.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nkuba Ndaye A, Hoxha A, Madinga J, et al. Challenges in interpreting SARS‐CoV‐2 serological results in African countries. Lancet Glob Health. 2021;9(5):e588‐e589. doi: 10.1016/S2214-109X(21)00060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Struck NS, Lorenz E, Deschermeier C, et al. High seroprevalence of SARS‐CoV‐2 in Burkina‐Faso, Ghana and Madagascar in 2021: a population‐based study. BMC Public Health. 2022;5(1):1676. doi: 10.1186/s12889-022-13918-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moreira‐Soto A, Diaz JMP, González‐Auza L, et al. High SARS‐CoV‐2 seroprevalence in rural Peru, 2021; a cross‐sectional population‐based study. mSphere. 2021;22(6):e0068521. doi: 10.1128/mSphere.00685-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bergeri I, Whelan M, Harriet Ware Subissi L, et al. Global epidemiology of SARS‐CoV‐2 infection: a systematic review and meta‐analysis of standardized population‐based seroprevalence studies. medRxiv. 2021. doi: 10.1101/2021.12.14.21267791 [DOI] [Google Scholar]
- 29. Sagara I, Woodford J, Kone M, et al. Rapidly increasing severe acute respiratory syndrome coronavirus 2 seroprevalence and limited clinical disease in 3 Malian communities: a prospective cohort study. Clin Infect Dis. 2021;74(6):1030‐1038. doi: 10.1093/cid/ciab589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mulu A, Bekele A, Abdissa A, et al. The challenges of COVID‐19 testing in Africa: the Ethiopian experience. Pan Afr Med J. 2021;38:6. doi: 10.11604/pamj.2021.38.6.26902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. African Development Fund . Covid‐19: African countries win the race against time with testing laboratories. African Development Bank—Building today, a better Africa tomorrow. 2021. Accessed June 15, 2022. https://www.afdb.org/en/news-and-events/covid-19-african-countries-win-race-against-time-testing-laboratories-44228
- 32. Arthur‐Holmes F, Akaadom MKA, Agyemang‐Duah W, Abrefa Busia K, Peprah P. Healthcare concerns of older adults during the COVID‐19 outbreak in low‐ and middle‐income countries: lessons for health policy and social work. J Gerontol Soc Work. 2020;63(6–7):717‐723. doi: 10.1080/01634372.2020.1800883 [DOI] [PubMed] [Google Scholar]
- 33. Hussein MIH, Albashir AAD, Elawad OAMA, Homeida A. Malaria and COVID‐19: unmasking their ties. Malar J. 2020;19(1):457. doi: 10.1186/s12936-020-03541-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuan Y, Zhao Y‐J, Zhang Q‐E, et al. COVID‐19‐related stigma and its sociodemographic correlates: a comparative study. Glob Health. 2021;17(1):54. doi: 10.1186/s12992-021-00705-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diop BZ, Ngom M, Biyong CP, Biyong JNP. The relatively young and rural population may limit the spread and severity of COVID‐19 in Africa: a modelling study. BMJ Glob Health. 2020;5(5):e002699. doi: 10.1136/bmjgh-2020-002699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hou H, Wang T, Zhang B, et al. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Transl Immunol. 2020;9(5):e01136. doi: 10.1002/cti2.1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beijing Wantai Biological Pharmacy Enterprise . WANTAI SARS‐CoV‐2 Ab Rapid Test. 2021. Accessed June 15, 2022. https://www.fda.gov/media/140030/download
- 38. Tso FY, Lidenge SJ, Peña PB, et al. High prevalence of pre‐existing serological cross‐reactivity against severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) in sub‐Saharan Africa. Int J Infect Dis. 2021;102:577‐583. doi: 10.1016/j.ijid.2020.10.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marlet J, Petillon C, Ragot E, et al. Clinical performance of four immunoassays for antibodies to SARS‐CoV‐2, including a prospective analysis for the diagnosis of COVID‐19 in a real‐life routine care setting. J Clin Virol. 2020;132:104633. doi: 10.1016/j.jcv.2020.104633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aschwanden C. Five reasons why COVID herd immunity is probably impossible. Nature. 2021;591(7851):520‐522. doi: 10.1038/d41586-021-00728-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in the manuscript are available on request.


