Abstract
Introduction:
Venous thromboembolism(VTE) remains a significant source of post-injury morbidity and mortality. HMG-CoA reductase inhibitors (rosuvastatin) significantly reduced pathologic clotting events in healthy populations in a prior trial. Furthermore, acetylsalicylic acid (ASA) has been shown to be non-inferior to prophylactic heparinoids for VTE prevention following orthopedic surgery. We hypothesized that a combination of rosuvastatin/ASA, in addition to standard VTE chemoprophylaxis, would reduce VTE in critically ill trauma patients.
Methods:
This was a double-blind, placebo-controlled, randomized trial, evaluating VTE rates in two groups: 1)ASA+statin (Experimental) and 2)Identical placebos (Control). Injured adults, 18–65 years old, admitted to the surgical intensive care unit without contraindications for VTE prophylaxis were eligible. Upon initiation of routine VTE chemoprophylaxis (i.e. heparin/heparin-derivatives), they were randomized to the Experimental or Control group. VTE was the primary outcome.
Results:
Of 112 potentially eligible patients, 33% (n=37, median new injury severity scale=27) were successfully randomized, of whom 11% had VTEs. The Experimental group had no VTEs, while the Control group had 6 VTEs (4 PEs and 2 DVTs) in 4 (22%) patients (p=0.046). The Experimental treatment was not associated with any serious adverse events. Due to the COVID-19 pandemic, the study was interrupted at the second interim analysis at <10% of the planned enrollment, with significance declared at p<0.012 at that stage.
Discussion:
The combination of ASA and rosuvastatin with standard VTE prophylaxis showed a favorable trend toward reducing VTEs with no serious adverse events. An appropriately powered phase III multicenter trial is needed to further investigate this therapeutic approach.
Level of Evidence:
Level II, Therapeutic
Keywords: venous thromboembolism; deep venous thrombosis; pulmonary embolism; trauma, statin
Introduction
Venous thromboembolism (VTE), which includes both deep venous thrombosis (DVT) and pulmonary embolism (PE), are acute threats to trauma patients following major injury and its ensuing critical illness (1–7). Administration of chemoprophylaxis with heparin agents and intermittent pneumatic compression devices of the lower extremities have reduced the incidence of VTE in trauma patients and other surgical and hospitalized populations (7–10). However, since the introductions of these preventive measures, there have been minimal further reductions. Interestingly, a recent seminal study by the CLOTT group showed that most macro-level pulmonary clots are not embolic but instead result from injury-induced inflammation, endotheliopathy, and hypercoagulability (2). At the microvascular level, thrombosis is also involved in the pathogenesis of postinjury organ dysfunction, principally of the lungs and kidneys, and remains a major cause of death and morbidity after trauma (11–14). For both of these macro- and microvascular thrombotic phenomena in trauma, a growing body of evidence has suggested that the persistence of inappropriate formation of pathologic clot after injury may be a result of resistance to fibrinolysis or fibrinolysis shutdown combined with endotheliopathy and other coagulation and inflammatory disturbances (12, 15–18).
Acute lung injury (ALI) after cardiac surgery is a demonstrative example, where it has been associated with increased coagulation activity and fibrin formation combined with insufficient fibrinolytic activity in the immediate postoperative period (19). Fibrin degradation is impaired by plasminogen-activator-inhibitor-1 (PAI-1) (12), where PAI-1 is the major inhibitor of two plasminogen activators; urokinase-type plasminogen activator (u-PA), which activates fibrinolysis at the tissue level, and tissue-type plasminogen activator (t-PA), which activates intravascular fibrinolysis (18). Trauma and most surgical patients have increased levels of PAI-1, which have been correlated with the subsequent development of organ failure including ALI (20). Platelets also play a role in both VTE and lung injury, thus anti-platelet agents (such as ASA) have been increasingly used in populations at-risk for thrombotic complications, including trauma patients (21, 22).
In 2009, the JUPITER randomized trial found that in 17,802 seemingly healthy patients who had mild elevations of the inflammatory marker C-reactive protein that administration of rosuvastatin dramatically reduced vascular thrombotic events and all-cause mortality (23). While the molecular mechanisms for these results were not explored in JUPITER, there are several properties of HMG-CoA reductase inhibitors (“statins”) that could contribute. One plausible explanation is that statins reduce tissue factor expression and thrombin generation while also enhancing thrombomodulin (endothelial anticoagulant-promoting surface protein) expression (24, 25). Additionally, statins have also been shown to reduce levels of plasminogen activator inhibitor-1 (PAI-1) in both circulation and at the endothelial level (26, 27), thereby releasing the brakes on fibrinolysis to maintain vascular patency. Indeed, PAI-1 elevation has been established in trauma patients and its level tracks closely with severity of injury (28), and PAI-1 is also known to be associated with both VTE (29, 30) and organ failure across multiple fields of medicine (31–34). Thus, it is possible that the reduction in thrombotic events observed in JUPITER was a direct result of reduced PAI-1 levels.
Another interesting observation is that trauma patients treated with anti-platelet agents prior to injury have a reduced incidence of organ failure, included ARDS, and improved overall survival (21, 22). Furthermore, in major orthopedic operations the administration of ASA has been shown to reduce the incidence of VTE and PE by approximately one-third (35), and a recent large RCT (36) indicated thromboprophylaxsis with ASA was non-inferior to low-molecular weight heparins in this population. Interestingly, one retrospective study showed that while statin use alone appears to attenuate ARDS, the effect seems more pronounced when combined with ASA (37). Building on the fact that statins can reduce PAI-1 levels through reduced endothelial transcription and thus allow endogenous fibrinolysis to maintain vascular patency, it is also well-known that platelets also serve as a major source of circulating PAI-1 levels through release from platelet alpha-granules upon platelet activation (38, 39). Thus, the combination of aspirin and statins may complement one another to reduce PAI-1, allowing for endogenous fibrinolysis to maintain vascular patency and thereby decrease the overall procoagulant state observed after injury.
Given their separate and complementary mechanisms of action to reduce the pro-thrombotic environment after injury, we hypothesized that rosuvastatin and aspirin administered in combination to severely injury trauma patients would reduce the incidence of VTE in the surgical intensive care unit (SICU) and performed a randomized controlled trial to evaluate the study question.
Methods
Trial Design:
The STAT trial was a single-center, phase II, pragmatic, randomized, double-blind, placebo controlled adaptive clinical trial, designed to evaluate whether daily rosuvastatin 20mg and aspirin 325mg delivered orally or via enteric tube could reduce the incidence of VTE in critically ill trauma patients. A secondary hypothesis was that this drug combination would reduce the incidence of acute lung injury and increase ventilator free days. The study enrollment was done in an urban, level 1 trauma center with a mature trauma system. The study was approved by our institutional review board (#16–0391) and registered prior to starting enrollment on clinicaltrials.gov (NCT02901067). An independent data and safety monitoring board (DSMB) was engaged to monitor the trial. The full study protocol is available upon request.
Participants:
Injured patients were eligible if they met the following entry criteria: 1) age 18–65 years; 2) admitted to the SICU for a traumatic injury with an expected hospital stay ≥3 days; 3) ICU admission <48 hours after their injury (initially this was limited to <24hours, but it was adapted to <48 hours to increase enrollment); 4) consent was obtained <72 hours post-injury. The exclusion criteria principally related to contraindications to anticoagulation or study medications: 1) known inherited bleeding disorder or coagulopathy, 2) known contraindication for pharmacologic anticoagulation, 3) spinal column fracture with epidural hematoma, 4) head trauma/central nervous system (CNS) injury (severe traumatic brain injury, defined as AIS head >3; intracranial hemorrhage; or other CNS injury with neurosurgery service objection to enrollment), 5) ongoing hemorrhage requiring blood product transfusion, 6) thrombocytopenia (platelet count < 50,000/mcl), 6) non-operatively managed liver or spleen injuries (American Association for the Surgery of Trauma AAST Grade III or above), 7) known chronic kidney disease (CKD) (Glomerular Filtration Rate < 15ml/min), rising blood creatinine > 1.5x baseline), 8) inclusion in other trials, 9) documented previous cerebral ischemic stroke, 11) known allergy or other contraindication to statins or ASA, and 12) pregnancy. In addition, we did not include known prisoners due to their inability to freely consent, and did not include patients who were already receiving statin or ASA therapy pre-injury as it would be unethical to place them in the control group.
Interventions, procedures and monitoring:
Upon consent and in tandem with the initiation of the routine SICU VTE chemoprophylaxis, the study medications (20mg rosuvastatin and 325mg aspirin) or identical placebos were administered once daily via enteric route. Patients assigned to the intervention arm received the standard of care prophylactic anticoagulation (40mg subcutaneous enoxaparin twice daily) plus the combination of experimental drugs (20mg of rosuvastatin daily and 325mg of aspirin) daily either orally or via feeding tube. These doses are consistent with those currently recommended in the postoperative period following cardiac surgery (40). The regimen was maintained during the entire ICU stay while patients were concomitantly receiving the standard of care VTE chemoprophylaxis (heparin or heparin-derivatives). Patients diagnosed with VTE were withdrawn from the study drugs and received the appropriate VTE treatment per institutional ICU protocols.
An ultrasonography was conducted by a radiologist blinded to treatment assignment to evaluate the central line sites and veins in the legs for blood clots on the third day of study drug. If the subject was discharged sooner than the third day, the ultrasonography was done at discharge. The results of the ultrasound were not available for patient clinical care as routine ultrasound surveillance for thrombotic events was not recommended for trauma patients (41). Pulmonary embolism (PE) was ascertained by pulmonary imaging in patients who developed suggestive clinical symptoms per the clinical treatment team’s discretion.
We determined hepatic and renal function as well as signs of myositis and rhabdomyolysis before initiation and upon the end of the therapy or as clinically necessary (i.e., any clinical indications of organ dysfunction). Stopping rules included: 1) bleeding (any ongoing bleeding requiring blood transfusion or operative procedure, unexplained drop in hemoglobin level >2 g/dl within 24 hours after the start of the study, or a drop in hemoglobin level greater than 1 g/dl daily for three consecutive days leading to VTE chemoprophylaxis); 2) Liver dysfunction (>3 times baseline aspartate aminotransferase, AST); 3) Renal dysfunction (> 2 times baseline serum creatinine); 4) Myositis or rhabdomyolysis (>10 times baseline serum creatine kinase concentration); 5) Study patient received aspirin, as prescribed by their SICU attending; and 6) Thrombocytopenia (<50,000/uL requiring transfusion of platelets while receiving the study drug). These criteria were monitored twice daily by trained professional research assistants. Any of the above stopping rules was reason for unmasking study group assignment and were reported as a Serious Adverse Event (SAE) to the DSMB for immediate review.
Outcomes:
All outcomes were pre-specified in the trial protocol. The primary outcome was VTE incidence (both DVT and PE) during the index hospitalization. Secondary outcomes included incidence of ALI within 2 weeks post-injury based on the Berlin Criteria (42), ventilator-free days (out of 28 days), incidence of arterial thrombotic complications (myocardial infarction, MI; and cerebrovascular accident, CVA), all-cause 30-day mortality, ICU free days, and organ failure (defined by the Denver MOF score (14)). Viscoelastic coagulation assays using thrombpelastography (TEG) were captured for exploratory analysis of relationships between fibrinolysis phenotypes, study intervention, and VTE outcomes.
Sample Size:
The sample size was limited by the trauma center volume. We expected to enroll 88 eligible patients/year during five years (total expected enrollment=440, annual trauma volume=245 patients, of whom about 50 patients were enrolled in other trials) assuming a 35% exclusion rate plus higher-than-usual 30% attrition/refusal rate. Trauma SICU series have reported variable VTE incidence under surveillance from 3–6% (4, 41, 43), in which case we could only detect reductions to 0–1% with 80% power, 95% confidence, and 2 interim looks at the data, with significance adjusted by the O’Brien-Fleming alpha spending function. However, certain at-risk patients, such as those deemed eligible for this study, have higher VTE rates of 11–28%, in which case, the study could detect 39–64% relative reductions (41).
Due to unforeseen circumstances, particularly the COVID-19 pandemic, an unpredicted high proportion of patients fulfilling exclusion criteria or refusing consent, and the change in anesthesia protocols to include ketorolac as an analgesia adjunct (which raised concerns about increased bleeding complications if given in conjunction to ASA and statin), we obtained permission from the DSMB and the IRB to produce the first interim analysis (significance declared at p<0.0001) at n=36 and a final second interim analysis (significance declared at p<0.012), when the study was terminated at n=37. The study lasted between February 2017 and August 2021, with the last patient being enrolled in March 2020.
Randomization:
Upon consent and receiving an order for prophylactic anticoagulation, the hospital pharmacy randomized the patient to the control or experimental arms in a 1:1 ratio according to a computer-generated randomization schedule.
Blinding:
Healthcare providers, research assistants and patients were blinded to study allocation (double-blind design). The hospital pharmacists were aware of the patient’s treatment arm so that rapid un-blinding was possible in the event of an adverse event possibly related to a study medication.
Statistical Methods:
All analyses were conducted as intent-to-treat and no patient crossed over. Effectiveness of randomization was assessed using standardized mean differences at baseline, and absolute values <0.20 were deemed as indicators of acceptable balance. Numerical variables are reported as median and interquartile range. Subgroup analyses were not possible due to the small sample size. Continuous variables were contrasted using the Wilcoxon-rank-sum non-parametric test. Dichotomous outcomes (mortality, adverse events, thromboembolic events) were contrasted between treatment arms with a Chi-squared test or Fisher Exact test if expected cell values were <5. Survival analysis was performed using Kaplan-Meier curves and Cox proportional hazards censoring for death as a competing risk and compared with the log-rank and Wilcoxon tests. The O’Brien-Fleming alpha-spending function determined that the p-value to declare significance at the second interim analysis was p<0.012.
Results
Overall, 2027 adult trauma patients were screened, of whom 1915 were determined to be ineligible (reasons for exclusions are included in Supplemental Table 1), and 112 patients were determined to meet eligibility requirements. Of these, 69 (62%) declined the study, a much higher proportion than anticipated based on previous trials. As illustrated in the CONSORT diagram in Figure 1, 43 (38%) elected to participate and signed the consent. Four patients (4/43, 9%) were withdrawn (1 was placed in police custody; 1 requested to be withdrawn from the study; 2 were found to have contraindications to study medications), and two of the participants (2/43, 5%) were not randomized, one due to early transfer from the ICU, and one received ASA prescribed by the clinical team. Of the remaining 37 patients, in this analysis 18 (49%) were randomized to the Control group and 19 (51%) to the Experimental group.
Figure 1:
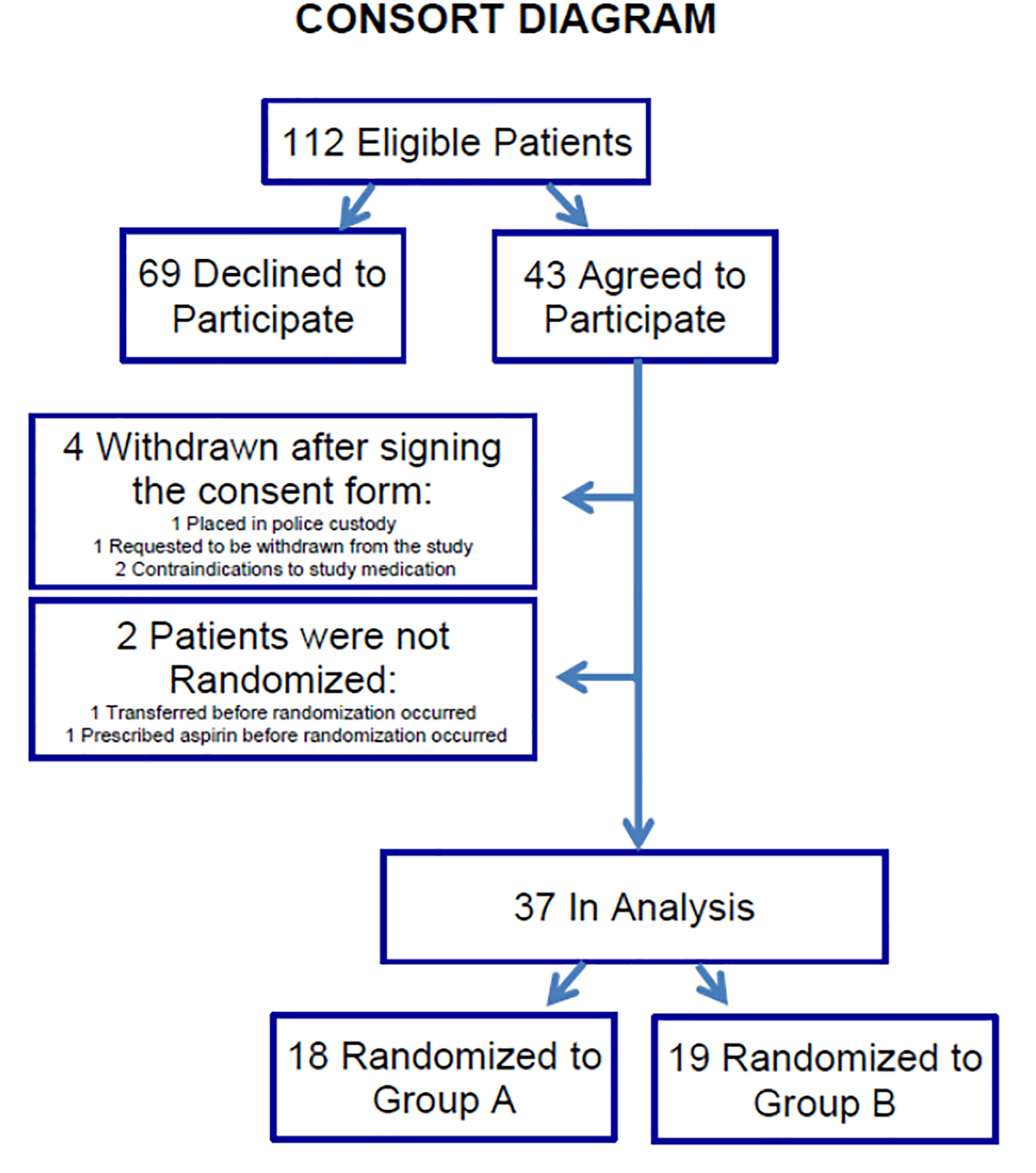
CONSORT diagram
Table 1 shows the characteristics of patients in each group. Per the CONSORT guidelines, we do not present p-values for the baseline characteristics. The study groups were mostly balanced in risk factors, except for a slight imbalance in blunt trauma, with the Experimental group having a higher proportion of blunt trauma than Control patients. However, as detailed below all 4 VTEs occurred in blunt trauma patients, which would have placed the Experimental group at higher risk of VTE. Thus, if anything, this imbalance would dampen any effect of the Experimental intervention. Nine patients (24.3%) required study medication discontinuation per the stopping criteria (4 in the Experimental group; and 5 in the Control group) as shown in Table 2.
Table 1:
Patient baseline characteristics (categorical variables are expressed in N (%) and numerical variables in median (interquartile range)). Standardized mean differences (SMD) assess group balance and effectiveness of randomization (SMD<=|0.20| indicates acceptable balance). AIS: Abbreviated Injury Scale
| Variables | Total | Control | Experimental | SMD |
|---|---|---|---|---|
| (N=37) | (N=18) | (N=19) | ||
| Sex | ||||
| Female | 9 (24.3) | 5 (27.8) | 4 (21.1) | 0.16 |
| Male | 28 (75.7) | 13 (72.2) | 15 (78.9) | |
| Ethnicity | ||||
| Hispanic | 15 (40.5) | 7 (38.9) | 8 (42.1) | 0.07 |
| Not Hispanic | 21 (56.8) | 11 (61.1) | 10 (52.6) | |
| Race | ||||
| Asian | 1 (2.7) | 1 (5.6) | 0.02 | |
| Black | 2 (5.4) | 1 (5.6) | 1 (5.3) | |
| Other | 2 (5.4) | 1 (5.6) | 1 (5.3) | |
| White | 31 (83.8) | 15 (83.3) | 16 (84.2) | |
| Age (years) | 34.9 (26.0–45.8) | 39.8 (27.4–55.0) | 31.8 (23.8–40.6) | 0.07 |
| BMI (kg/m 2 ) | 28.1 (24.8–33.4) | 26.4 (23.6–31.6) | 29.0 (25.0–35.1) | −0.02 |
| INJURY SEVERITY | ||||
| Blunt vs Penetrating | ||||
| Blunt | 29 (78.4) | 15 (83.3) | 14 (73.7) | 0.24 |
| Penetrating | 8 (21.6) | 3 (16.7) | 5 (26.3) | |
| New Injury Severity Score | 27.0 (17.0–34.0) | 26.5 (22.0–34.0) | 27.0 (17.0–34.0) | 0 |
| Max AIS head/neck | 0.0 (0.0–0.0) | 0.0 (0.0–2.0) | 0.0 (0.0–0.0) | 0 |
| Max AIS chest | 3.0 (0.0–3.0) | 3.0 (0.0–3.0) | 3.0 (0.0–3.0) | 0 |
| Max AIS abdomen/pelvis | 2.0 (0.0–3.0) | 2.0 (0.0–3.0) | 2.0 (2.0–4.0) | 0 |
| Max AIS extremities | 2.0 (0.0–3.0) | 2.0 (0.0–3.0) | 2.0 (0.0–3.0) | 0 |
| PHYSIOLOGY | ||||
| Field systolic blood pressure (mmHg) | 99.0 (82.0–122.0) | 90.0 (80.0–122.0) | 101.0 (96.0–118.0) | 0 |
| Field heart rate (bpm) | 100.0 (78.0–114.0) | 98.0 (77.0–109.0) | 100.0 (87.0–116.0) | −0.04 |
| Field shock index | 1.0 (0.7–1.1) | 0.9 (0.7–1.1) | 1.0 (0.8–1.2) | 0 |
| Field Glasgow coma scale | 14.0 (8.0–15.0) | 14.5 (9.0–15.0) | 14.0 (8.0–15.0) | 0 |
| Admission systolic blood pressure (mmHg) | 98.0 (80.0–113.0) | 96.0 (76.0–108.0) | 98.0 (82.0–118.0) | −0.04 |
| Admission heart rate (bpm) | 95.0 (73.0–112.0) | 96.0 (75.0–110.0) | 90.0 (71.0–117.0) | 0.02 |
| Admission shock index | 1.0 (0.8–1.2) | 1.0 (0.8–1.3) | 1.0 (0.8–1.1) | 0 |
| Admission Glasgow coma scale | 15.0 (9.0–15.0) | 15.0 (11.0–15.0) | 15.0 (8.0–15.0) | 0 |
| TRANSFUSIONS | ||||
| Red blood cells (RBC) units/6 hours | 1.0 (0.0–4.0) | 0.0 (0.0–6.0) | 1.0 (0.0–4.0) | 0 |
| Massive transfusion (>10 RBC units/6hrs or death<6 hours after >=1 RBC unit) | 8 (21.6) | 4 (22.2) | 4 (51.4) | 0.03 |
| Plasma units/ 6 hours | 0.0 (0.0–2.0) | 0.0 (0.0–5.0) | 0.0 (0.0–2.0) | 0 |
| Platelet units/ 6 hours | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0 |
| Cryoprecipitate units/6 hours | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0 |
| Tranexamic acid /3 hours | 2 (5.4) | 1 (5.6) | 1 (5.3) | 0.01 |
Table 2:
Reason for study medication discontinuation: 9 (24.3%) patients required study drug discontinuation: 5 (27.8%) patients in the Control group versus vs 4 (21.1%) in the Experimental group, p=0.71.
| group | Reason for discontinuation | Hospital day drug was discontinued | VTE |
|---|---|---|---|
| Control | Attending discretion | 4 | No |
| Control | Pulmonary embolism | 3 | Yes |
| Control | Received ASA per attending discretion | 7 | No |
| Control | Platelet count <50,000/mcl | 3 | Yes |
| Control | Suspicion of gastric bleeding, not confirmed | 19 | No |
| Experimental | Suspicion of pulmonary embolism, not confirmed | 2 | No |
| Experimental | Platelet count <50,000/mcl | 1 | No |
| Experimental | Pharmacy miscommunication | 2 | No |
| Experimental | Pharmacy miscommunication | 4 | No |
Outcomes: There were 2 DVTs (both common femoral vein) diagnosed by study screening ultrasound (1 of which was also detected clinically due to symptoms), and 4 PE diagnosed by pulmonary imaging (all segmental or larger, with no hemodynamic compromise), which occurred in 4 patients (2 patients had both DVT and PE). All VTEs occurred in the Control group (Table 3, p = 0.046, Hazard ratio Control vs Experimental: 1.20; 95% confidence interval: 0.62– 2.30), and all were treated the therapeutic anticoagulation upon diagnosis. PEs occurred on hospital days 2, 5, 8 and 12, while the DVTs were diagnosed on hospital days 3 and 7 (Figure 2, Log-rank test p=0.04; Wilcoxon test p=0.04). Two PEs (on hospital days 8 and 12) did not have an associated DVT diagnosed clinically or by the study surveillance, consistent with the results of the CLOTT study group (2). No patients developed ALI, MI, or stroke.
Table 3:
Patient outcomes by group (*note that significance is p < 0.012). (categorical variables are expressed in N (%) and numerical variables in median (interquartile range)).
| Control | Experimental | P-value | |
|---|---|---|---|
| (N=18) | (N=19) | ||
| Pulmonary Embolism | 3 (16.7) | 0 | 0.1050 |
| Pulmonary Embolism Occurrence (Hospital Day) | 2, 5, 8, 12 | ||
| Deep Venous Thrombosis | 2 (11.1) | 0 | 0.2297 |
| Overall VTE | 4 (22.2) | 0 | 0.0463 |
| Hospital days | 12.5 (6.0–33.0) | 14.0 (8.0–18.0) | 0.8314 |
| ICU days | 5.0 (2.0–13.0) | 4.0 (3.0–10.0) | 0.8785 |
| ICU free days | 23.0 (15.0–26.0) | 24.0 (15.0–25.0) | 1.0000 |
| Ventilation days | 2.0 (0.0–6.0) | 2.0 (0.0–5.0) | 0.8284 |
| Ventilator free days | 26.0 (22.0–28.0) | 26.0 (23.0–28.0) | 0.9014 |
| Death | 0 | 1 (5.6) Anoxic brain injury; deemed non-preventable | 1.0000 |
Figure 2:
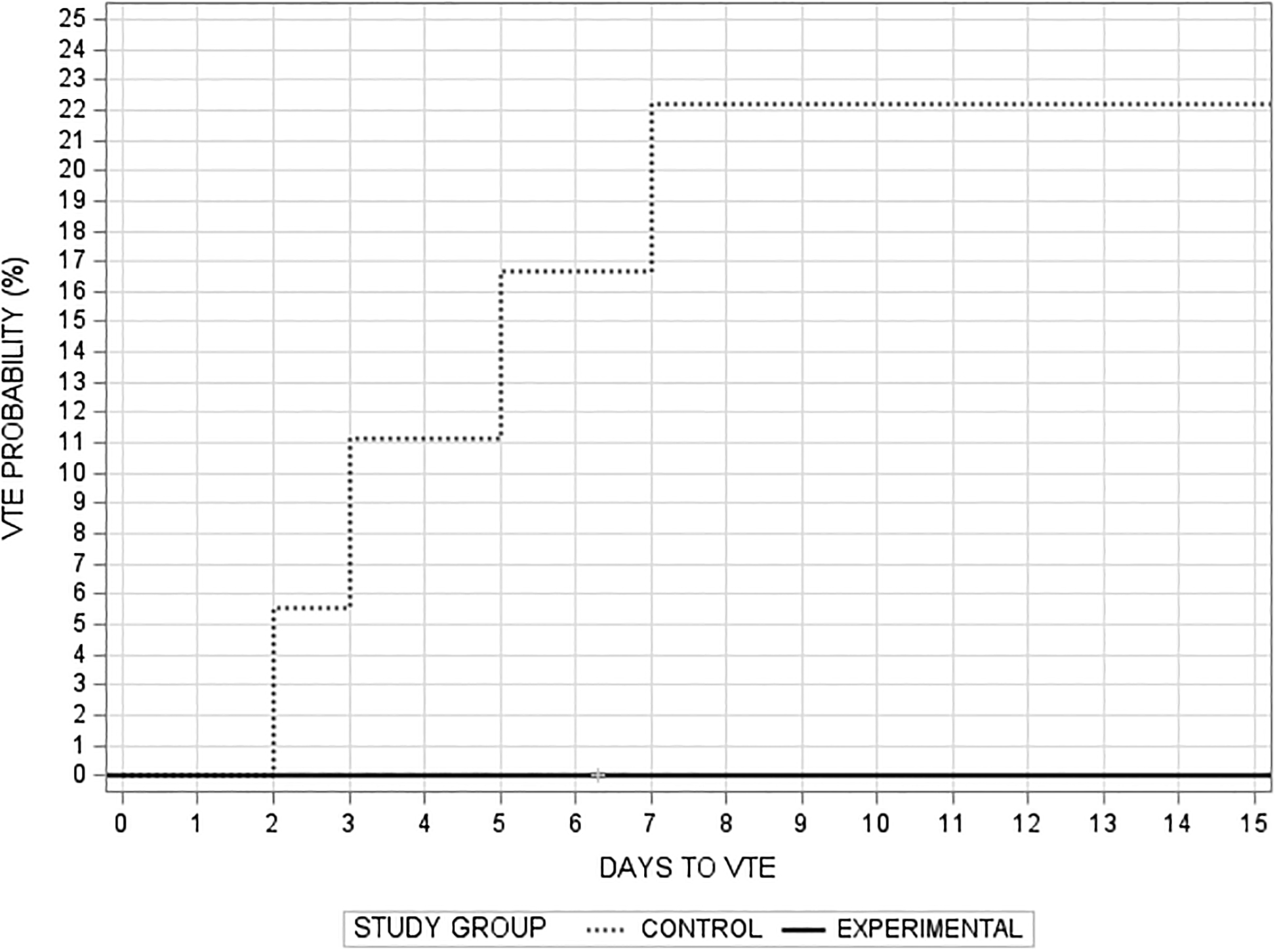
Probability of developing VTE by study group (Log-rank test p=0.04; Wilcoxon test p=0.04).
SAE and complications: One patient in the Control group developed an intra-abdominal abscess, and one patient in the Experimental group developed a pelvic fluid collection requiring drainage. There were no confirmed bleeding complications. Two SAEs in two patients were reported to the DSMB and IRB, for which, in accordance to protocol, the group assignment was unblinded and revealed to be the Control group: 1) severe, acute thrombocytopenia (<50,000/mcl) in a 31-year-old female with polytrauma due to motor vehicle crash; and 2) significant drop in hemoglobin in a 19-year-old male with polytrauma after a train crash; endoscopy revealed non-bleeding ulcers and no other source of bleeding were found. There were no SAEs in the Experimental group.
Discussion
Notwithstanding that the trial was underpowered due to slow enrollment and early termination in the setting of COVID-19, the results of this trial, while not statistically significant, are encouraging. The trial findings support the notion that targeting both endothelial and platelet-derived mediators (PAI-1) of macro- and microvascular thrombosis, maintained by suppressed fibrinolysis, may be an effective strategy to prevent VTE after trauma. Our study hypothesis was built, in part, on the results of the JUPITER trial that showed rosuvastatin administration markedly reduces thromboembolic complications in healthy outpatients (23), and also in part on multiple orthopedic surgery trials and medical patient trials that have shown benefit to ASA for preventing VTE (35, 44).
While our study did not have sufficient enrollment to observe an effect on organ failure, the observed reduction in macrovascular thrombotic events may offer promise for microvascular thrombotic burden in organ dysfunction, such as ALI and acute kidney injury. Acute respiratory distress syndrome (ARDS) directed trials using statins (simvastatin and rosuvastatin) 55, 56 have failed to demonstrate a benefit, however, these trials focused on treatment, while our proposed goal was prevention. In addition, trauma patients were under-represented in these studies. There were only 6 trauma patients in the “Rosuvastatin for Sepsis-Associated Acute Respiratory Distress Syndrome study” (45), while only 44 (8%) of the 540 patients were victims of trauma in the HARP-2 trial (46), which tested simvastatin to treat ARDS. In these ARDS treatment studies, the hypothesis was that statins inhibited the HMG-CoA reductase which itself would reduce systemic inflammatory mediators (TNF-α, IL-1ß, IL-6, and IL-8). However, statin inhibition of HMG-CoA results in a number of pleiotropic effects (25, 47–49) including downregulation of endothelial PAI-1 transcription, which is up-regulated post injury, resulting in suppressed fibrinolysis and in turn is associated with macro- and micro-thrombotic complications (50). One of the main mechanisms hypothesized to underly the benefit from the combination of ASA and statins in our cohort relied on their ability to individually reduce PAI-1 levels in circulation. Endothelial cells produce and secrete PAI-1 upon a sufficient stimulus of the endothelium (e.g. increased TNF-alpha, such as seen in trauma and sepsis) (51), while platelets store their PAI-1 in alpha-granules that extrude their contents upon platelet activation (38, 39). By targeting endothelial cell PAI-1 transcription with statins (26), and preventing platelet degranulation through ASA treatment (52), a lower threshold to achieve endogenous fibrinolysis capable of maintaining macro- and microvascular patency would logically follow. As a result, one would expect to observe less VTE and lower frequency and severity of organ failure through less fibrinolysis resistance, and thus greater ability to maintain vascular patency.
A major drawback in studies targeting VTE in trauma patients is the relatively low incidence of this outcome (albeit associated with devastating morbidity and mortality). This hampers our ability to conduct prevention and treatment trials, as the sample size to achieve enough numbers is large. Our recent publication of the predictive performance of the TEG tPA-challenge Ly30 showed that early fibrinolysis resistance at 12 hours postinjury was an independent predictor of VTE (53). Interestingly, initial fibrinolysis resistance was not associated with VTE, suggesting this may be an over-compensatory mechanism. Fibrinolysis activation with subsequent reduced fibrinolysis has been reported as early as 1964 (54), and subsequently named “fibrinolysis shutdown” after trauma (55). These investigators described a rapidly developing pattern of fast-acting inhibitor of tPA after trauma leading to fibrinolysis shutdown. To that end, higher levels of PAI-1 have also been detected in the plasma of patients who developed VTE after total hip surgery compared with those without VTE (56).
There are several limitations to this study. First and foremost, the trial was terminated early due to slow and difficult enrollment in the face of the unprecedented COVID-19 pandemic as well as higher than anticipated refusal rate. This led to a markedly underpowered study. Second, it was a single institution study, with limited generalizability. Finally, a large fraction of screened patients were not eligible for study, and while the enrollment criteria would potentially be less restrictive in future clinical practice it does limit applicability to a subset of the trauma patient population.
In summary, the combination of ASA and rosuvastatin in critically ill trauma patients was safe and may reduce the incidence of VTE, but the study was underpowered to detect a significant difference. These promising results warrant a larger Phase III study to determine if there is true benefit. The tPA challenge TEG LY30, already approved in Europe for clinical use, may prove to be an important tool to enable targeted enrollment.
Supplementary Material
Conflicts of Interest:
CDB has received research support from Instrumentation Laboratories. HBM and EEM are inventors on a patent related to the tPA-challenged TEG (US11137409B2). CDB, EEM, and HBM have a patent pending related to the plasmin TEG (U.S. Patent Application 62/505,021). EEM and HBM all receive research grant support from Haemonetics and Instrumentation Laboratories. CDB, EEM, HBM, and AS all have received research grant support from Genentech for other studies. All other authors have nothing to disclose.
Disclosures of Funding:
This work was supported by a grant from National Institutes of Health (P50 GM049222).
Footnotes
Meeting Presentations: This work was presented as a podium paper at the 52nd Annual Meeting of the Western Trauma Association, March 5–10, 2023 in Lake Louise, Alberta, Canada
Trial Registration: Registered on Clinicaltrials.gov on September 15, 2016 (NCT02901067).
Contributor Information
Angela Sauaia, School of Public Health, University of Colorado Denver, Aurora, CO.
Christopher D. Barrett, University of Nebraska Medical Center, Department of Surgery, Omaha, NE.
Ernest E. Moore, University of Colorado Denver, Department of Surgery, Denver, CO and Ernest E. Moore Shock and Trauma Center at Denver Health, Denver, CO.
James Chandler, University of Colorado Denver, Department of Surgery, Denver, CO and Ernest E. Moore Shock and Trauma Center at Denver Health, Denver, CO.
Hunter B. Moore, University of Colorado Denver, Department of Surgery, Denver, CO.
References
- 1.Ley EJ, Brown CVR, Moore EE, Sava JA, Peck K, Ciesla DJ, et al. Updated guidelines to reduce venous thromboembolism in trauma patients: A Western Trauma Association critical decisions algorithm. J Trauma Acute Care Surg. 2020;89(5):971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudson MM, Moore EE, Kornblith LZ, Shui AM, Brakenridge S, Bruns BR, et al. Challenging Traditional Paradigms in Posttraumatic Pulmonary Thromboembolism. JAMA Surg. 2022;157(2):e216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Gent JM, Calvo RY, Zander AL, Olson EJ, Sise CB, Sise MJ, et al. Risk factors for deep vein thrombosis and pulmonary embolism after traumatic injury: A competing risks analysis. J Trauma Acute Care Surg. 2017;83(6):1154–60. [DOI] [PubMed] [Google Scholar]
- 4.Shackford SR, Cipolle MD, Badiee J, Mosby DL, Knudson MM, Lewis PR, et al. Determining the magnitude of surveillance bias in the assessment of lower extremity deep venous thrombosis: A prospective observational study of two centers. J Trauma Acute Care Surg. 2016;80(5):734–9; discussion 40–1. [DOI] [PubMed] [Google Scholar]
- 5.Knudson MM, Gomez D, Haas B, Cohen MJ, Nathens AB. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann Surg. 2011;254(4):625–32. [DOI] [PubMed] [Google Scholar]
- 6.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240(3):490–6; discussion 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis JW, Menawat S, Von Thron J, Fallon WF Jr., Vinsant GO, Laneve LM, et al. Efficacy of deep venous thrombosis prophylaxis in trauma patients and identification of high-risk groups. J Trauma. 1993;35(1):132–8; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 8.Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335(10):701–7. [DOI] [PubMed] [Google Scholar]
- 9.Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003–20. [DOI] [PubMed] [Google Scholar]
- 10.Turpie AG, Bauer KA, Caprini JA, Comp PC, Gent M, Muntz JE, et al. Fondaparinux combined with intermittent pneumatic compression vs. intermittent pneumatic compression alone for prevention of venous thromboembolism after abdominal surgery: a randomized, double-blind comparison. J Thromb Haemost. 2007;5(9):1854–61. [DOI] [PubMed] [Google Scholar]
- 11.Ware LB, Camerer E, Welty-Wolf K, Schultz MJ, Matthay MA. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L307–11. [DOI] [PubMed] [Google Scholar]
- 12.Sebag SC, Bastarache JA, Ware LB. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. Curr Pharm Biotechnol. 2011;12(9):1481–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conhaim RL, Mangino MJ, Dovi WF, Watson KE, Warner TF, Harms BA. Microthrombus formation may trigger lung injury after acute blood loss. Shock. 2010;34(6):601–7. [DOI] [PubMed] [Google Scholar]
- 14.Sauaia A, Moore EE, Johnson JL, Chin TL, Banerjee A, Sperry JL, et al. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. J Trauma Acute Care Surg. 2014;76(3):582–92, discussion 92–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–7; discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. The journal of trauma and acute care surgery. 2012;73(1):60–6. [DOI] [PubMed] [Google Scholar]
- 17.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, et al. Acute Fibrinolysis Shutdown after Injury Occurs Frequently and Increases Mortality: A Multicenter Evaluation of 2,540 Severely Injured Patients. J Am Coll Surg. 2016;222(4):347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, et al. Trauma-induced coagulopathy. Nature Reviews Disease Primers. 2021;7(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen BS, Maltesen RG, Pedersen S, Kristensen SR. Early coagulation activation precedes the development of acute lung injury after cardiac surgery. Thrombosis Research. 2016;139:82–4. [DOI] [PubMed] [Google Scholar]
- 20.Moore HB, Moore EE. Temporal Changes in Fibrinolysis following Injury. Seminars in thrombosis and hemostasis. 2020;46(2):189–98. [DOI] [PubMed] [Google Scholar]
- 21.Harr JN, Moore EE, Johnson J, Chin TL, Wohlauer MV, Maier R, et al. Antiplatelet therapy is associated with decreased transfusion-associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients. Crit Care Med. 2013;41(2):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W, Janz DR, Bastarache JA, May AK, O’Neal HR Jr., Bernard GR, et al. Prehospital aspirin use is associated with reduced risk of acute respiratory distress syndrome in critically ill patients: a propensity-adjusted analysis. Crit Care Med. 2015;43(4):801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. [DOI] [PubMed] [Google Scholar]
- 24.Undas A, Brummel-Ziedins KE, Mann KG. Anticoagulant effects of statins and their clinical implications. Thromb Haemost. 2014;111(3):392–400. [DOI] [PubMed] [Google Scholar]
- 25.Krysiak R, Okopien B, Herman Z. Effects of HMG-CoA reductase inhibitors on coagulation and fibrinolysis processes. Drugs. 2003;63(17):1821–54. [DOI] [PubMed] [Google Scholar]
- 26.Swiatkowska M, Pawlowska Z, Szemraj J, Drzewoski J, Watala C, Cierniewski CS. Cerivastatin, a HMG-CoA reductase inhibitor, reduces plasminogen activator inhibitor-1 (PAI-1) expression in endothelial cells by down-regulation of cellular signaling and the inhibition of PAI-1 promoter activity. Jpn J Pharmacol. 2002;90(4):337–44. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Rockwood J, Zak D, Devaraj S, Jialal I. Simvastatin reduces circulating plasminogen activator inhibitor 1 activity in volunteers with the metabolic syndrome. Metab Syndr Relat Disord. 2008;6(2):149–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Condron M, Rowell S, Dewey E, Anderson T, Lealiiee L, Farrell D, et al. The procoagulant molecule plasminogen activator inhibitor-1 is associated with injury severity and shock in patients with and without traumatic brain injury. J Trauma Acute Care Surg. 2018;85(5):888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang J, Zhu W, Mei X, Zhang Z. Plasminogen activator inhibitor-1: a risk factor for deep vein thrombosis after total hip arthroplasty. J Orthop Surg Res. 2018;13(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frischmuth T, Hindberg K, Aukrust P, Ueland T, Braekkan SK, Hansen JB, et al. Elevated plasma levels of plasminogen activator inhibitor-1 are associated with risk of future incident venous thromboembolism. J Thromb Haemost. 2022;20(7):1618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Solh AA, Bhora M, Pineda L, Aquilina A, Abbetessa L, Berbary E. Alveolar plasminogen activator inhibitor-1 predicts ARDS in aspiration pneumonitis. Intensive Care Med. 2006;32(1):110–5. [DOI] [PubMed] [Google Scholar]
- 32.Sapru A, Curley MA, Brady S, Matthay MA, Flori H. Elevated PAI-1 is associated with poor clinical outcomes in pediatric patients with acute lung injury. Intensive Care Med. 2010;36(1):157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L20–8. [DOI] [PubMed] [Google Scholar]
- 34.Griemert EV, Schwarzmaier SM, Hummel R, Golz C, Yang D, Neuhaus W, et al. Plasminogen activator inhibitor-1 augments damage by impairing fibrinolysis after traumatic brain injury. Ann Neurol. 2019;85(5):667–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355(9212):1295–302. [PubMed] [Google Scholar]
- 36.Major Extremity Trauma Research C, O’Toole RV, Stein DM, O’Hara NN, Frey KP, Taylor TJ, et al. Aspirin or Low-Molecular-Weight Heparin for Thromboprophylaxis after a Fracture. N Engl J Med. 2023;388(3):203–13. [DOI] [PubMed] [Google Scholar]
- 37.O’Neal HR Jr., Koyama T, Koehler EA, Siew E, Curtis BR, Fremont RD, et al. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39(6):1343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Booth NA, Simpson AJ, Croll A, Bennett B, MacGregor IR. Plasminogen activator inhibitor (PAI-1) in plasma and platelets. Br J Haematol. 1988;70(3):327–33. [DOI] [PubMed] [Google Scholar]
- 39.Morrow GB, Whyte CS, Mutch NJ. Functional plasminogen activator inhibitor 1 is retained on the activated platelet membrane following platelet activation. Haematologica. 2020;105(12):2824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kulik A, Ruel M, Jneid H, Ferguson TB, Hiratzka LF, Ikonomidis JS, et al. Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation. 2015;131(10):927–64. [DOI] [PubMed] [Google Scholar]
- 41.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S–e77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Definition Task Force A, Ranieri V, Rubenfeld G, Thompson B, Ferguson N, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012;307(23):2526–33. [DOI] [PubMed] [Google Scholar]
- 43.Paffrath T, Wafaisade A, Lefering R, Simanski C, Bouillon B, Spanholtz T, et al. Venous thromboembolism after severe trauma: incidence, risk factors and outcome. Injury. 2010;41(1):97–101. [DOI] [PubMed] [Google Scholar]
- 44.Collaborative overview of randomised trials of antiplatelet therapy--III: Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308(6923):235–46. [PMC free article] [PubMed] [Google Scholar]
- 45.Rosuvastatin for Sepsis-Associated Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2014;370(23):2191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAuley DF, Laffey JG, O’Kane CM, Perkins GD, Mullan B, Trinder TJ, et al. Simvastatin in the Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2014;371(18):1695–703. [DOI] [PubMed] [Google Scholar]
- 47.Jansen JO, Lord JM, Thickett DR, Midwinter MJ, McAuley DF, Gao F. Clinical review: Statins and trauma--a systematic review. Crit Care. 2013;17(3):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iannuzzi JC, Rickles AS, Kelly KN, Rusheen AE, Dolan JG, Noyes K, et al. Perioperative pleiotropic statin effects in general surgery. Surgery. 2014;155(3):398–407. [DOI] [PubMed] [Google Scholar]
- 49.Skrlin S, Hou V. A review of perioperative statin therapy for noncardiac surgery. Semin Cardiothorac Vasc Anesth. 2010;14(4):283–90. [DOI] [PubMed] [Google Scholar]
- 50.Chapman MP, Moore EE, Moore HB, Gonzalez E, Chin T, Gamboni F, et al. Massive Plasminogen Activator Inhibitor-1 (PAI-1) Upregulation and Suppressed Fibrinolysis Is the Predominant Phenotype in Severely Injured Trauma Patients. Journal of the American College of Surgeons.219(3):S46. [Google Scholar]
- 51.Handt S, Jerome WG, Tietze L, Hantgan RR. Plasminogen activator inhibitor-1 secretion of endothelial cells increases fibrinolytic resistance of an in vitro fibrin clot: evidence for a key role of endothelial cells in thrombolytic resistance. Blood. 1996;87(10):4204–13. [PubMed] [Google Scholar]
- 52.Coppinger JA, O’Connor R, Wynne K, Flanagan M, Sullivan M, Maguire PB, et al. Moderation of the platelet releasate response by aspirin. Blood. 2007;109(11):4786–92. [DOI] [PubMed] [Google Scholar]
- 53.Knudson MM, Moore HB, Moore EE, Kornblith LZ, Kiraly LN, McNutt MK, et al. Tissue plasminogen activator resistance is an early predictor of posttraumatic venous thromboembolism: A prospective study from the CLOTT research group. The journal of trauma and acute care surgery. 2022;93(5):597–603. [DOI] [PubMed] [Google Scholar]
- 54.Innes D, Sevitt S. Coagulation and fibrinolysis in injured patients. Journal of clinical pathology. 1964;17(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kluft C, Verheijen JH, Jie AF, Rijken DC, Preston FE, Sue-Ling HM, et al. The postoperative fibrinolytic shutdown: a rapidly reverting acute phase pattern for the fast-acting inhibitor of tissue-type plasminogen activator after trauma. Scand J Clin Lab Invest. 1985;45(7):605–10. [DOI] [PubMed] [Google Scholar]
- 56.Yukizawa Y, Inaba Y, Watanabe S-i, Yajima S, Kobayashi N, Ishida, et al. Association between venous thromboembolism and plasma levels of both soluble fibrin and plasminogen-activator inhibitor 1 in 170 patients undergoing total hip arthroplasty. Acta Orthopaedica. 2012;83(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


