Abstract
Aims
Atrial fibrillation (AF) haemodynamics is less well studied due to challenges explained by the nature of AF. Until now, no randomized data are available. This study evaluates haemodynamic variables after AF induction in a randomized setting.
Methods and results
Forty-two patients with AF who had been referred for ablation to the University Hospital, Linköping, Sweden, and had no arrhythmias during the 4-day screening period were randomized to AF induction vs. control (2:1). Atrial fibrillation was induced by burst pacing after baseline intracardiac pressure measurements. Pressure changes in the right and left atrium (RA and LA), right ventricle (RV), and systolic and diastolic blood pressures (SBP and DBP) were evaluated 30 min after AF induction compared with the control group. A total of 11 women and 31 men (median age 60) with similar baseline characteristics were included (intervention n = 27, control group n = 15). After 30 min in AF, the RV end-diastolic pressure (RVEDP) and RV systolic pressure (RVSP) significantly reduced compared with baseline and between randomization groups (RVEDP: P = 0.016; RVSP: P = 0.001). Atrial fibrillation induction increased DBP in the intervention group compared with the control group (P = 0.02), unlike reactions in SBP (P = 0.178). Right atrium and LA mean pressure (RAm and LAm) responses did not differ significantly between the groups (RAm: P = 0.307; LAm: P = 0.784).
Conclusion
Induced AF increased DBP and decreased RVEDP and RVSP. Our results allow us to understand some paroxysmal AF haemodynamics, which provides a haemodynamic rationale to support rhythm regulatory strategies to improve symptoms and outcomes.
Trial registration number (clinicaltrials.gov)
No NCT01553045. https://clinicaltrials.gov/ct2/show/NCT01553045?term=NCT01553045&rank=1
Keywords: Atrial fibrillation, Haemodynamics, Randomized controlled study, Radiofrequency ablation
Graphical Abstract
Graphical Abstract.
Introduction
Atrial fibrillation (AF) often results in haemodynamic consequences that cause subjective symptoms and consequently end up in emergency contact. This is of great importance, since AF, the cardiac flu, exists globally and impacts patients and healthcare systems worldwide.1,2
Over the decades, efforts have been made to clarify possible mechanisms that give clues to ‘symptomatic’ AF, in which different haemodynamics during AF probably participate. The difficulties in understanding cause and effect in the on-and-off AF setting can partly be explained by challenges in evaluating AF vs. sinus rhythm (SR) in a practical model and a heterogenic population in terms of symptoms, comorbidities, and outcomes over time.
The two likely mechanisms for detrimental effects on haemodynamics are fibrillatory atrial activation and irregular ventricular contractions. In normal SR, the atria contracts to optimize ventricular filling. In comparison, the disorganized electrical activity during AF results in very rapid (>300 b.p.m.) and often irregular atrial contractions. Consequently, loss of atrial-to-ventricular synchronization hampers ventricular filling through loss of atrial kick3 and decreases cardiac preload. Additionally, ventricular irregularity, increased left atrial (LA) and right atrial (RA) pressure, neurohumoral activation, and autonomic imbalance may all reduce cardiac output (CO).4–8
It is plausible that these mechanisms, at least partially, explain the development of ‘subjective symptoms’ such as shortness of breath, fatigue, and reduced exercise tolerance,5 which often impacts quality of life (QoL). Atrial fibrillation also usually affects blood pressure and systemic circulation, in which ventricular irregularity may cause fluctuations in blood pressure and promote hypertension and other cardiovascular comorbidities.9 Atrial fibrillation can also impact the function of the cardiac autonomic nervous system, further affecting the haemodynamics of the heart.10
Sinus rhythm restoration, if maintained, can reverse the flawed cardiac haemodynamics and may lead to symptom relief, enhanced QoL,11 and a more favourable outcome over time.
Clinical data/models empathizing with the haemodynamic effects of AF are few. This is probably explained by the difficulties of creating an ‘accurate model’ that allows measuring cardiac haemodynamics during AF. As a result, computer modelling has emerged as a tool to hypothesize haemodynamic outcomes in the setting of AF with or without different comorbidities.12
A better understanding of the haemodynamic changes caused by AF can have important clinical implications and provide clues for individualized therapeutic strategies.
In this randomized study, we aim to shed light on the acute impact of cardiac haemodynamics in patients eligible for radiofrequency ablation (RFA). We use a model that includes AF initiation and compare pressure changes in the RA and LA, right ventricle (RV), and systolic and diastolic arterial pressures (SBP and DBP), respectively.
Methods
Study design
This is a substudy of the SMURF (Symptom burden, Metabolic profile, Ultrasound findings, Rhythm, neurohormonal activation, haemodynamics, and health-related quality of life in patients with AF) study.5 The design has been presented previously.13
The setting was randomized, controlled, and interventional, with an allocation ratio of 2:1 in favour of the interventional group (AF initiation).
Participants
Between February 2012 and April 2014, patients with AF referred for RFA at the University Hospital in Linköping, Sweden, were eligible for participation in the current study. Patients with AF, age >18 years, with sufficient knowledge of the Swedish language, and referred for first-time RFA treatment were eligible for inclusion.
Exclusion criteria included previous catheter or surgical AF ablation, previous or planned heart surgery, left ventricular (LV) ejection fraction (EF) <35%, ascertained acute coronary syndrome during the past 3 months, and any arrhythmia episodes in the last 4 days before RFA.
Informed consent and ethical considerations
The Regional Ethical Review Board of the Faculty of Health Sciences, Linköping, Sweden (registration number: 2011/40-31), approved the study protocol. All participants gave their written consent to participate in the study. This study complies with the Declaration of Helsinki.14
Trans-telephonic electrocardiogram
We used the Zenicor device (Zenicor Medical Systems, Stockholm, Sweden) to monitor eligible patients, which is well-validated15,16 and widely used in clinical practice to screen for AF and other arrhythmias.17,18
After the screening, eligible patients received written and verbal information about the study and were equipped with trans-telephonic Zenicor electrocardiogram (ECG) devices. Registrations were made before randomization by placing the thumbs on the Zenicor’s two measuring plates. The rendered 30 s rhythm strip was classified into one of four groups: SR, AF, atrial tachycardia, or not specified rhythm, using a centralized digital ECG database accessed by authorized personnel. Recordings were sent twice daily, and patients were instructed to send additional recordings within 4 days of the planned RFA if subjective arrhythmia symptoms were experienced.
Randomization
Patients were randomized with a 2:1 allocation ratio at the time of catheterization in favour of the intervention group. Blinding was not feasible due to the nature of the intervention.13
Subject measurements
Those who agreed to participate and fulfilled inclusion and exclusion criteria signed the informed consent and were included in the SMURF study. The patients were equipped with Zenicor devices. Those with ‘no arrhythmia’ 4 days prior to the scheduled RFA were eligible for the haemodynamic substudy. Baseline evaluation included medical history, physical examination, ECG, trans-thoracic and trans-oesophageal echocardiogram, and a computed tomographic scan of the heart, according to the clinical routine. If no arrhythmia episodes were registered by trans-telephonic ECGs, the respective patient was randomized. Detailed subject measurements have been described previously.13,19 All patients fasted >6 h prior to the intervention. We evaluated the euvolemic state through history, physical examination, and basic laboratory tests. A 2.5% glucose infusion was started upon arrival. Catheterization and RFA were performed during mild conscious sedation (propofol and remifentanil). Femoral sheaths were inserted in the right femoral vein (one short, two long). The two long sheaths (trans-septal, SL1) were perfused by heparinized saline (3 mL/h). An initial bolus of heparin, 100 IE/kg, was given after the trans-septal puncture and, from there, monitored and adjusted to achieve an activated clotting time of >350 s.
The study was conducted after the trans-septal puncture. Pressure zeroing was done in the middle of the sagittal thoracic diameter in the second intercostal space. We used a Cordis (MR-A1, 5F) high-flow multipurpose catheter for invasive pressure measurements and measured the LA pressure followed by the RA and RV pressure during calm breathing and for at least 15 s. The measurements were analysed and stored using EP-workmate (St Jude Medical, Saint Paul, MN, USA). SBP and DBP were measured non-invasively throughout the procedure (Philips Easycare). Three repeated measurements presented as means were made at baseline vs. end of intervention, and blood samples were collected.
Intervention
After baseline intracardiac pressure measurements, the patients allocated to the intervention group were induced to AF with burst pacing using a cycle length of 170–300 ms. Atrial fibrillation had to be sustained in 30 min and, if necessary, immediately re-induced. Subsequently, new intracardiac measurements were performed.
After baseline measurements, the patients allocated to the control group were observed for 30 min while in SR. After that, we performed new intracardiac measurements.
RFA was performed after the measurements.13
Ablation procedure
All patients underwent antral pulmonary vein isolation with the ablation procedure described previously.13 Electrical disconnection of all pulmonary veins was verified by using standard pacing manoeuvres. The operator performed additional ablation when necessary.
Endpoint
The endpoint of this study was to investigate the changes in pressure of the RA, LA, RV, SBP, and DBP, respectively, 30 min after the initiation of AF compared with the control group in patients eligible for RFA.
Statistical methods
The sample size calculation was based on a pilot study that preceded this study.5,13
The results indicated that 45 patients were needed to show statistically significant results.
Continuous variables were expressed as means ± standard deviation (SD). Non-normally distributed variables were presented as medians with interquartile range (IQR) and categorical data as counts with percentages in brackets. Baseline characteristics between randomized groups were tested for possible differences (t-test—normally distributed data, Mann–Whitney U-test—non-parametric data, and χ2 for categorical data).
The primary endpoint was tested with repeated-measure analysis of variance (ANOVA). Time was used as a within-subject factor, the randomized groups were used as a between-subject factor, and changes in pressure measurements in the randomized groups over time were studied (time × randomization).
The repeated-measure ANOVA was corrected with the following co-variates: heart rate at baseline, age, hypertension, type of AF, and EF <50%.
Reported P-values were two-sided; a P < 0.05 was considered statistically significant. The analyses were performed using SPSS 25.0 (SPSS, Chicago, IL, USA).
Results
Patient inclusion flow and baseline characteristics
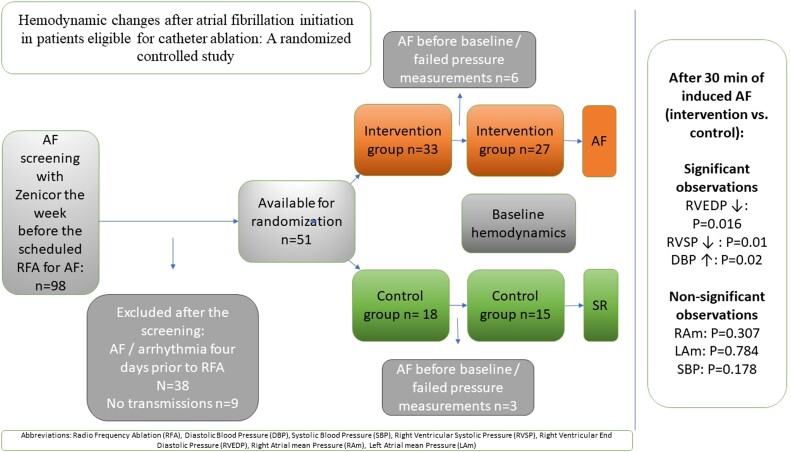
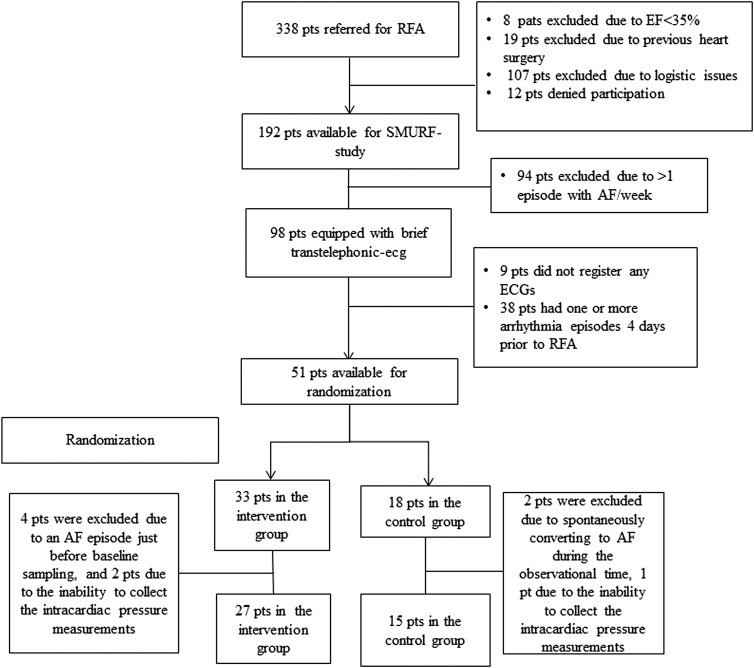
During the inclusion period, 338 patients were referred to the Department of Cardiology at Linköping University Hospital, Sweden, for first-time RFA of AF. Ninety-eight patients (29%) were eligible for the invasive part of the SMURF study and were screened with trans-telephonic ECG according to the study’s protocol. Of those, 42 patients were randomized and available for analysis (intervention n = 27 and control group n = 15). Figure 1 presents the inclusion flow chart.
Figure 1.
Inclusion and randomization flow chart. AF, atrial fibrillation; EF, ejection fraction; RFA, radiofrequency ablation; pts, patients.
In total, 11 women and 31 men (median age 60; IQR 14) years were included. Baseline characteristics, baseline intracardiac pressure, and non-invasive pressure were similar between the groups (Tables 1 and 2). No differences in intracardiac pressures were observed depending on the type of AF or diagnosis of hypertension at baseline. Approximately 25 mL of 2.5% glucose was infused from catheterization until the trans-septal puncture. The mean doses of propofol and remifentanil from the start of catheterization to the end of the last pressure measurements were 23 (control) vs. 25 mg (intervention) and 163 vs. 210 mg remifentanil, respectively. No patient received vasopressor drugs or fluid bolus infusion from catheterization until the last pressure measurement. The mean rate increased by 43 b.p.m. in patients induced to AF, whereas no change was observed in the control group.
Table 1.
Baseline characteristics of the two randomized groups (control and induction groups)
| Variables | Control group (n = 15) | Induction group (n = 27) | P-value |
|---|---|---|---|
| Age | 62 (IQR, 13) | 58 (IQR, 14) | NS |
| Female gender | 5 (33%) | 6 (22%) | NS |
| BMI (kg/m2) | 26.6 (IQR, 5.7) | 26 (IQR, 7.1) | NS |
| Paroxysmal AF | 6 (40%) | 12 (44%) | NS |
| Hypertension | 8 (53%) | 8 (30%) | NS |
| Diabetes mellitus | 1 (7%) | 1 (4%) | NS |
| Heart failure | 0 | 2 (7%) | NS |
| Atrial flutter | 1 (7%) | 2 (8%) | NS |
| CHA2DS2VASc | 2 (IQR, 3) | 1 (IQR, 2) | NS |
| TIA | 2 (13.3%) | 1 (3.7%) | NS |
| GFR < 60 mL/min/1.73 m2 | 4 (26.7%) | 3 (11.1%) | NS |
| Beta-blocker | 9 (60%) | 17 (63%) | NS |
| AAD | 4 (27%) | 12 (44%) | NS |
| ACEi or ARB | 6 (40%) | 6 (22%) | NS |
| Amilodipine/felodipine | 2 (13.3%) | 0 | NS |
| Potassium-sparing diuretics | 0 | 2 (7.4%) | NS |
| Furosemide | 3 (20%) | 4 (15%) | NS |
| Thiazide diuretics | 2 (13.3%) | 3 (11.1%) | NS |
| Statins | 3 (20%) | 9 (33.3%) | NS |
| HR baseline (b.p.m.) | 58 ± 11 | 63 ± 14 | NS |
| Procedural time | 188 ± 36 | 200 ± 45 | NS |
| Complications | 0 | 1 (4%) | NS |
| Propofol (mg) | 23 (IQR, 23) | 25 (IQR, 22) | NS |
| Remifentanil (mg) | 163 (IQR, 125) | 210 (IQR, 111) | NS |
| Heparin (IE) | 8770 ± 2230 | 8892 ± 1591 | NS |
| Volume received during the procedure (mL) | 412 ± 172 | 438 ± 189 | NS |
| NT-proBNP (pg/mL) | 92 (IQR, 144) | 66 (IQR, 90) | NS |
| MR-proANP (pmol/L) | 97 (IQR, 47) | 101 (IQR, 39) | NS |
| LAVmax/BSA (mL/m2) | 28.8 ± 8.4 | 25.9 ± 6.2 | NS |
| RAVmax/BSA (mL/m2) | 23 ± 10 | 19.8 ± 6.7 | NS |
| RV EDA/BSA (cm2/m2) | 8.9 ± 1.6 | 9 ± 1 | NS |
| EF (biplane) % | 63 ± 4 | 61 ± 5 | NS |
Normally distributed variables are presented as mean values ± SD, non-parametric variables as median values with IQR, and categorical data as counts and percentages. Results from t-tests for normally distributed variables, χ2 for categorical variables, and Mann–Whitney U-test for non-parametric variables are presented, and P-values <0.05 are considered statistically significant. The doses of remifentanil and propofol are the doses administrated from the time of catheterization until the last pressure measurement.
AAD, anti-arrhythmic drugs; ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin-2 receptor blocker; BMI, body mass index; BSA, body surface area; CHA2DS2VASc, congestive heart failure, hypertension, age >75 years, diabetes, stroke, vascular disease, female sex; EF, ejection fraction; HR, heart rate; GFR, glomerular filtration rate; IQR, interquartile range; LAV, left atrial volume; MR-proANP, mid-regional fragment of the N-terminal precursor of atrial natriuretic peptide; n, number of patients; NT-proBNP, N-terminal fragment of prodromal B-type natriuretic peptide; RAV, right atrial volume; RV EDA, right-ventricle end-diastolic area; SD, standard deviation; TIA, transient ischaemic attack.
Table 2.
Differences in reactions ofright atrium mean pressure, left atrium mean pressure, right ventricular systolic pressure, and right ventricular end-diastolic pressure in both randomized groups
| Baseline | 95% CI | 30 min after induction | 95% CI | P time×randomization | |
|---|---|---|---|---|---|
| RAm control n = 12 | 10.3 | 7.8–12.8 | 10.6 | 7.7–13.4 | 0.307 |
| RAm intervention n = 26 | 9.4 | 8–10.8 | 9.1 | 7.8–10.3 | |
| LAm control n = 13 | 15.2 | 12.5–17.8 | 14 | 10.9–17.1 | 0.784 |
| LAm intervention n = 26 | 14.8 | 13–16.6 | 13.9 | 12.5–15.2 | |
| RVSPcontrol n = 12 | 29.7 | 26.1–33.3 | 33.4 | 29.8–37.1 | 0.001 |
| RVSPintervention n = 25 | 32.6 | 30.4–35 | 28.9 | 26.8–31 | |
| RVEDPcontrol n = 12 | 8.1 | 5.9–10.3 | 8.7 | 5.6–11.7 | 0.016 |
| RVEDPintervention n = 25 | 8 | 6.6–9.4 | 6.8 | 5.6–8 | |
| SBPcontrol n = 15 | 135 | 125–146 | 132 | 124–141 | 0.178 |
| SBPintervention n = 27 | 129 | 124–135 | 132 | 124–139 | |
| DBPcontrol n = 15 | 75 | 70–80 | 74 | 70–78 | 0.02 |
| DBPintervention n = 27 | 75 | 71–80 | 82 | 77–88 |
Results from repeated-measure ANOVA within-subjects contrast tests. A P-value <0.05 was considered to be statistically significant. The intracardiac and systemic pressures are presented as means with 95% CI and measured in mmHg. Bold values denote statistical significance (P < 0.05).
ANOVA, analysis of variance; CI, confidence interval; DBP, diastolic blood pressure; LAm, left atrium mean pressure; RAm, right atrium mean pressure; RVEDP, right ventricular end-diastolic pressure; RVSP, right ventricular systolic pressure; SBP, systolic blood pressure.
The only reported complication was a case of cardiac tamponade needing pericardiocentesis. This complication occurred during the ablation procedure but was unrelated to the invasive part of this study.
Atrial fibrillation initiation and its effects on intracardiac and non-invasive pressures
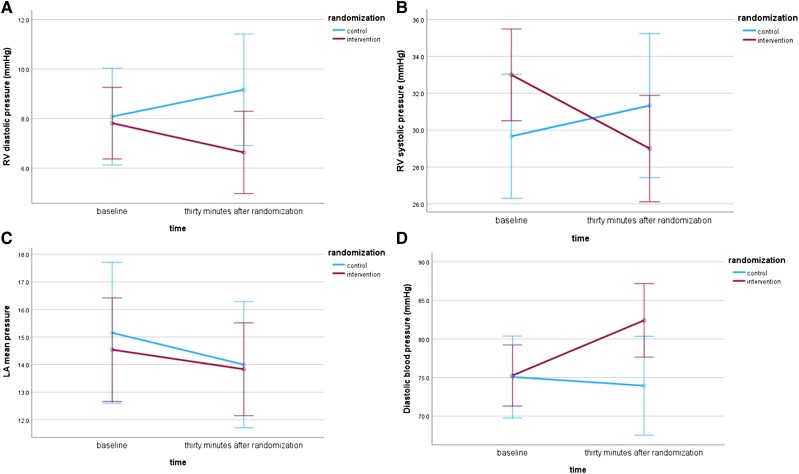
The RV end-diastolic pressure (RVEDP) and the RV systolic pressure (RVSP) significantly reduced after 30 min in AF compared with baseline, while they increased slightly in the control group. These reactions differed substantially between the randomization groups (RVEDP: P = 0.016; RVSP: P = 0.001; Figure 2 and Table 2). The RA and LA mean pressure (RAm and LAm, respectively) reactions did not differ significantly between the groups (RAm: P = 0.307; LAm: P = 0.784; Table 2. Moreover, the DBP increased in the intervention group after the induction of AF compared with the baseline. This reaction differed significantly from that of the control group (P = 0.02; Table 2 and Figure 2). The responses in SBP did not differ between the groups (P = 0.178; Table 2).
Figure 2.
The effect of atrial fibrillation initiation in (A) right ventricular end-diastolic pressure, (B) right ventricular systolic pressure, (C) left atrial mean pressure, and (D) diastolic blood pressure. AF, atrial fibrillation; RV, right ventricular; LA, left atrial.
Discussion
This randomized AF initiation model examined the haemodynamical impact of AF in the first 30 min after induction. The intracardiac pressures of the RA, LA, and RV and non-invasive blood pressures were measured. Interestingly, we observed a reduction of the RV pressures and an increase in DBP, unlike RA and LA pressures, which did not differ significantly.
Cardiac haemodynamics, in short
Normal SR implies a coordinated atrial contraction and facilitates optimal ventricular filling by contributing through the atrial contraction. The ‘atrial kick’ typically contributes to 20–30% of the ventricular filling volume and is pivotal in optimizing CO.3,4
In AF, the loss of coordinated atrial contraction disrupts the diastolic filling pattern. The ventricular filling becomes dependent on passive flow and may be further impaired or affected by age and other comorbidities exemplified by diabetes, valvular heart disease, heart failure, and ischaemic heart disease.
Consequently, AF can reduce ventricular preload, potentially compromising ventricular stroke volume and CO. A thorough understanding of these alterations is crucial for clinicians to manage AF effectively, that is, to better understand symptoms, optimize treatment strategies, and mitigate potential complications of AF itself.
Previous research within this field is surprisingly sparse, with conflicting results. The explanation for this is probably the nature of AF that makes it challenging to study. It reflects the heterogenic AF population in terms of symptoms and outcomes regardless of paroxysmal, persistent, or more longstanding AF.
Atrial fibrillation initiation and the effect on intracardiac pressures
In 1995, Alboni et al.7 reported findings from a cohort of 15 patients with no organic heart disease, in which they compared cardiac haemodynamics during SR to that of induced AF. Similar to our results, they observed significant reductions in RVEDP and RVSP. Two mechanisms probably explain these findings. First, the loss of atrial kick causes a decreased ventricular end-diastolic pressure, and second, the faster rate20 and irregular R-R intervals during AF, along with RV adaptability.
In contrast, no significant changes were observed in another study, i.e. RV pressures did not differ in SR vs. AF and RV apical pacing in a highly symptomatic group of patients scheduled for atrioventricular-junction ablation.4 These varied observations may be explained by changes in pulmonary blood flow dynamics during AF21 and different study populations.
Contrary to our findings, the Alboni group reported an increased LAm. We think our results are more ‘likely true’ based on the reduced RV pressures. An explanation for the opposite could be less atrial distensibility due to AF. However, this is more likely to occur over time and not in paroxysmal settings. Another explanation for the contradictory outcomes may be that changes in atrial pressures during AF initiation are highly dynamic and can vary depending on the phase and timing of the arrhythmia.5,10,17 Of note, all these observations are done in a laboratory setting with minimal influence from gravity in supine positions after 30 min of AF. Thus, it is unclear if these changes apply to activities during everyday life in an upright position. About RA pressures, our observations were concordant with the findings of Alboni.7
More recently, Dusik et al.22 presented the haemodynamical impact of AF and atrial tachycardia in terms of pulmonary hypertension (PH) in an observational cohort (controls n = 7, PH n = 10, LV heart failure n = 10). Their main observations were that patients with AF when restored to SR had an increased cardiac index within the AF group irrespective of PH and increased RV pressure in the LV heart failure group after arrhythmia termination. Compared with our data, similar observations were observed regarding RV mean pressure in the control and PH groups after arrhythmia termination. The difficulties in creating a real-life model to evaluate the haemodynamics in AF have formed the basis of computer modelling: One model,23 using parameters calibrated for a 25-year-old male (75 kg, height 175 cm), induced AF resulted in reduced CO, SV, EF and increased LV end-diastolic pressure, LA pressure, and pulmonary vein pressure, but the right chambers seemed less affected. In comparison, some of our results disagree (RAm, LAm, DBP, RVEDP, RVSP), suggesting difficulties in creating an accurate model in this setting. Additionally, the heterogeneity of real-life comorbidities clarifies the challenges of creating computer models.23,24
Atrial fibrillation initiation and the effect on blood pressure
Blood pressure and its fluctuations are thoroughly evaluated in SR and are generally an interplay between heart-rate variability, CO, respiration, total peripheral resistance, and the autonomic system. In our setting, we aimed to evaluate the impact of AF in terms of SBP and DBP.
Our results are consistent with those of previous findings, such that SBP is lower and DBP is higher in AF than in SR. However, we must consider that it is challenging to measure BP correctly in AF.25–27 In general, using the mean out of three repeated measures is recommended, as we did in our study. Blood pressure is usually measured by way of auscultation using the sphygmomanometric method or semi-automatically through oscillometric techniques. Even though accepted, their precision in AF has been questioned. In sphygmomanometric BP measurements, the irregular R-R intervals disrupt the correlation of the first and fourth or fifth Korotkoff sound representing SBP and DBP, respectively. The oscillometric method is based on the spindle-like oscillometric pressure pulse (OPP) during SR and is not validated in the AF population. Notably, the shape of the OPP is heart-rate dependent, and when it is >90 b.p.m., the OPP becomes more irregular and significantly underestimates SBP.28 So far, three systematic reviews reported reliable BP in AF29–31 and one the opposite32 in the AF population (auscultatory vs. oscillometric, the former reference).
An invasive evaluation of blood pressure and AF was recently published by Olbers et al.9 They evaluated the interplay between AF and BP in patients scheduled for a coronary angiogram (SR n = 12, AF n = 21) and assessed beat-to-beat systolic and diastolic blood pressures in different locations. They reported markedly evaluated blood pressure variability in AF patients, which was more pronounced in DBP (×6) vs. SBP (×2). Office BP came out lower when compared with corresponding invasive measurements and was more prominent in the AF group. In comparison, Sramko et al.33 reported decreased SBP in an AF simulation model.
When summarized, SBP after AF induction is trending to lower values contrasted with a rise in DBP. Further arguments for these findings could be sympathetic nerve activity that results in peripheral vasoconstriction and causes an increase in DBP in addition to the effect on heart rate.10,34 Other possible explanations for the observed rise in DBP may be increased systemic vascular resistance through decreased RV pressure35 and the limited effluence due to shorter diastole during the often faster and irregular heart cycles.7
Clinical and investigational importance
To the best of our knowledge, this is the first controlled randomized study using a model of AF initiation evaluating haemodynamic changes following 30 min of induced AF. Our study, showing no differences in LA and RA pressures, increased DBP, and reduced RV pressures in the first 30 min, contributes to a better understanding of some AF haemodynamics.
From a clinical perspective, our results support the haemodynamic rationale to support rhythm regulatory strategies such as catheter ablation or the use of anti-arrhythmic drugs, as also shown in other studies examining the haemodynamics of AF.36–38 However, more research is needed to confirm these hypotheses and determine the clinical significance of these findings.
Limitations
Our study is a single-centre randomized study with a relatively small sample size representing a healthy AF population in a design that probably excludes differential bias concerning baseline characteristics and a euvolemic state. However, extrapolation of our results to the general AF population must be done cautiously. The AF initiation model used denotes a simulation of the haemodynamic consequences of AF over a limited time interval. Examining haemodynamic parameters for more extended periods after AF initiation and a more rigorous invasive protocol would have provided valuable information. However, this was considered unethical and unsafe, and additional parameters were not included in the protocol.
Conclusions
In a model mimicking the onset of a paroxysm of AF, we observed a significant increase in heart rate, a decrease in RVEDP and RVSP, and an increase in DBP. This is the first human randomized study to examine cardiac haemodynamics after AF initiation. Our results concur with those of previous findings and allow us to understand the paroxysmal AF haemodynamics better, which provides the haemodynamic rationale to support rhythm regulatory strategies to control symptoms and improve outcomes.
Contributor Information
Henrik Almroth, Department of Cardiology, Linköping University Hospital, Garnisonsvägen 10, 581 85 Linköping, Sweden; Department of Health, Medicine and Caring Sciences, Linköping University, Garnisonsvägen 10, 581 85 Linköping, Sweden.
Lars O Karlsson, Department of Cardiology, Linköping University Hospital, Garnisonsvägen 10, 581 85 Linköping, Sweden; Department of Health, Medicine and Caring Sciences, Linköping University, Garnisonsvägen 10, 581 85 Linköping, Sweden.
Carl-Johan Carlhäll, Department of Health, Medicine and Caring Sciences, Linköping University, Garnisonsvägen 10, 581 85 Linköping, Sweden; Department of Clinical Physiology, Linköping University Hospital, Linköping, Sweden.
Emmanouil Charitakis, Department of Cardiology, Linköping University Hospital, Garnisonsvägen 10, 581 85 Linköping, Sweden; Department of Health, Medicine and Caring Sciences, Linköping University, Garnisonsvägen 10, 581 85 Linköping, Sweden.
Lead author biography
 Henrik Almroth is the first offspring of parents Elisabeth Almroth and Pappa Boe Hallberg; he graduated from Linkoping Health University in the year 2000. He became a cardiology resident in 2002 and started EP training guided by Docent Anders Englund at Örebro University Hospital around 2004. Response to Reviewers: In the year 2008, with tutors Anders Englund and Mårten Rosenqvist, research began within the field of arrhythmia, and Henrik received a PhD in 2012. His current position is as a senior EP consultant at Linköping University Hospital, with a particular focus on cardiac arrhythmias.
Henrik Almroth is the first offspring of parents Elisabeth Almroth and Pappa Boe Hallberg; he graduated from Linkoping Health University in the year 2000. He became a cardiology resident in 2002 and started EP training guided by Docent Anders Englund at Örebro University Hospital around 2004. Response to Reviewers: In the year 2008, with tutors Anders Englund and Mårten Rosenqvist, research began within the field of arrhythmia, and Henrik received a PhD in 2012. His current position is as a senior EP consultant at Linköping University Hospital, with a particular focus on cardiac arrhythmias.
Data availability
The data used for this study cannot be shared publicly with respect to participating subjects. Data can be shared after a request to the corresponding author.
Funding
This study has been supported by ALF grants (agreement on medical training and research grants between County Council of Östergötland and Linköping University), the Carldavid Jönsson Research Foundation, the Heart Foundation, Linköping University, and by unrestricted grants from Biosense Webster and Johnson & Johnson.
References
- 1. Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res 2020;127:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friberg L, Bergfeldt L. Atrial fibrillation prevalence revisited. J Intern Med 2013;274:461–468. [DOI] [PubMed] [Google Scholar]
- 3. Skinner NS Jr. The hemodynamic consequences of removing effective atrial function. Ala J Med Sci 1968;5:69–73. [PubMed] [Google Scholar]
- 4. Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol 1997;30:1039–1045. [DOI] [PubMed] [Google Scholar]
- 5. Charitakis E, Barmano N, Walfridsson U, Walfridsson H. Factors predicting arrhythmia-related symptoms and health-related quality of life in patients referred for radiofrequency ablation of atrial fibrillation: an observational study (the SMURF study). JACC Clin Electrophysiol 2017;3:494–502. [DOI] [PubMed] [Google Scholar]
- 6. Stojadinovic P, Deshraju A, Wichterle D, Fukunaga M, Peichl P, Kautzner J, Šramko M. The hemodynamic effect of simulated atrial fibrillation on left ventricular function. J Cardiovasc Electrophysiol 2022;33:2569–2577. [DOI] [PubMed] [Google Scholar]
- 7. Alboni P, Scarfo S, Fuca G, Paparella N, Yannacopulu P. Hemodynamics of idiopathic paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 1995;18:980–985. [DOI] [PubMed] [Google Scholar]
- 8. Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 2014;114:1483–1499. [DOI] [PubMed] [Google Scholar]
- 9. Olbers J, Gille A, Ljungman P, Rosenqvist M, Östergren J, Witt N. High beat-to-beat blood pressure variability in atrial fibrillation compared to sinus rhythm. Blood Press 2018;27:249–255. [DOI] [PubMed] [Google Scholar]
- 10. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 2014;114:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walfridsson U, Hassel Jönsson A, Karlsson LO, Liuba I, Almroth H, Sandgren E, Walfridsson H, Charitakis E. Symptoms and health-related quality of life 5 years after catheter ablation of atrial fibrillation. Clin Cardiol 2022;45:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anselmino M, Scarsoglio S, Ridolfi L, De Ferrari GM, Saglietto A. Insights from computational modeling on the potential hemodynamic effects of sinus rhythm versus atrial fibrillation. Front Cardiovasc Med 2022;9:844275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Charitakis E, Walfridsson U, Nyström F, Nylander E, Strömberg A, Alehagen U, Walfridsson H. Symptom burden, Metabolic profile, Ultrasound findings, Rhythm, neurohormonal activation, haemodynamics and health-related quality of life in patients with atrial fibrillation (SMURF): a protocol for an observational study with a randomised interventional component. BMJ Open 2015;5:e008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rickham PP. Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br Med J 1964;2:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hendrikx T, Rosenqvist M, Wester P, Sandstrom H, Hornsten R. Intermittent short ECG recording is more effective than 24-hour Holter ECG in detection of arrhythmias. BMC Cardiovasc Disord 2014;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghazal F, Theobald H, Rosenqvist M, Al-Khalili F. Validity of daily self-pulse palpation for atrial fibrillation screening in patients 65 years and older: a cross-sectional study. PLoS Med 2020;17:e1003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doliwa Sobocinski P, Anggardh Rooth E, Frykman Kull V, von Arbin M, Wallen H, Rosenqvist M. Improved screening for silent atrial fibrillation after ischaemic stroke. Europace 2012;14:1112–1116. [DOI] [PubMed] [Google Scholar]
- 18. Fredriksson T, Kemp Gudmundsdottir K, Frykman V, Friberg L, Al-Khalili F, Engdahl J, Svennberg E. Intermittent vs continuous electrocardiogram event recording for detection of atrial fibrillation-compliance and ease of use in an ambulatory elderly population. Clin Cardiol 2020;43:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Charitakis E, Walfridsson H, Nylander E, Alehagen U. Neurohormonal activation after atrial fibrillation initiation in patients eligible for catheter ablation: a randomized controlled study. J Am Heart Assoc 2016;5:e003957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parker JO, Khaja F, Case RB. Analysis of left ventricular function by atrial pacing. Circulation 1971;43:241–252. [DOI] [PubMed] [Google Scholar]
- 21. White CW, Kerber RE, Weiss HR, Marcus ML. The effects of atrial fibrillation on atrial pressure-volume and flow relationships. Circ Res 1982;51:205–215. [DOI] [PubMed] [Google Scholar]
- 22. Dusik M, Fingrova Z, Marek J, Dytrych V, Jansa P, Havranek S. The impact of atrial fibrillation and atrial tachycardias on the hemodynamic status of patients with pulmonary hypertension. Physiol Res 2022;71:791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scarsoglio S, Guala A, Camporeale C, Ridolfi L. Impact of atrial fibrillation on the cardiovascular system through a lumped-parameter approach. Med Biol Eng Comput 2014;52:905–920. [DOI] [PubMed] [Google Scholar]
- 24. Scarsoglio S, Camporeale C, Guala A, Ridolfi L. Fluid dynamics of heart valves during atrial fibrillation: a lumped parameter-based approach. Comput Methods Biomech Biomed Eng 2016;19:1060–1068. [DOI] [PubMed] [Google Scholar]
- 25. Ochiai H, Miyazaki N, Miyata T, Mitake A, Tochikubo O, Ishii M. Assessment of the accuracy of indirect blood pressure measurements. Jpn Heart J 1997;38:393–407. [DOI] [PubMed] [Google Scholar]
- 26. Kyriakoulis KG, Kollias A, Stergiou GS. Blood pressure and outcome in patients with atrial fibrillation: floating in uncharted waters. J Hypertens 2021;39:592–593. [DOI] [PubMed] [Google Scholar]
- 27. Posey JA, Geddes LA, Williams H, Moore AG. The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure. 1. Cardiovasc Res Cent Bull 1969;8:15–25. [PubMed] [Google Scholar]
- 28. Xie F, Xu J, Xia LL, Luo X, Jiang Z, Wu Y, Su H. The impact of atrial fibrillation on accuracy of oscillometric blood pressure measurement: effect of ventricular rate. Hypertens Res 2020;43:518–524. [DOI] [PubMed] [Google Scholar]
- 29. Stergiou GS, Kollias A, Destounis A, Tzamouranis D. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens 2012;30:2074–2082. [DOI] [PubMed] [Google Scholar]
- 30. Stergiou GS, Kyriakoulis KG, Stambolliu E, Destounis A, Karpettas N, Kalogeropoulos P, Kollias A. Blood pressure measurement in atrial fibrillation: review and meta-analysis of evidence on accuracy and clinical relevance. J Hypertens 2019;37:2430–2441. [DOI] [PubMed] [Google Scholar]
- 31. Park SH, Choi YK. Measurement reliability of automated oscillometric blood pressure monitor in the elderly with atrial fibrillation: a systematic review and meta-analysis. Blood Press Monit 2020;25:2–12. [DOI] [PubMed] [Google Scholar]
- 32. Clark CE, McDonagh STJ, McManus RJ. Accuracy of automated blood pressure measurements in the presence of atrial fibrillation: systematic review and meta-analysis. J Hum Hypertens 2019;33:352–364. [DOI] [PubMed] [Google Scholar]
- 33. Sramko M, Wichterle D, Kautzner J. Feasibility of in-vivo simulation of acute hemodynamics in human atrial fibrillation. PLoS One 2016;11:e0165241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Segerson NM, Sharma N, Smith ML, Wasmund SL, Kowal RC, Abedin M, MacGregor JF, Pai RK, Freedman RA, Klein RC, Wall TS, Stoddard G, Hamdan MH. The effects of rate and irregularity on sympathetic nerve activity in human subjects. Heart Rhythm 2007;4:20–26. [DOI] [PubMed] [Google Scholar]
- 35. Pinkerson AL, Kot PA. Systemic blood pressure response to changes in right ventricular function. Circ Res 1964;14:461–466. [DOI] [PubMed] [Google Scholar]
- 36. Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, Josephson RA, Kellen JC, Klein RC, Krahn AD, Mickel M, Mitchell LB, Nelson JD, Rosenberg Y, Schron E, Shemanski L, Waldo AL, Wyse DG; AFFIRM Investigators . Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004;109:1509–1513. [DOI] [PubMed] [Google Scholar]
- 37. Friberg L, Tabrizi F, Englund A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J 2016;37:2478–2487. [DOI] [PubMed] [Google Scholar]
- 38. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbüchel H, Hindricks G, Kautzner J, Kuck KH, Mont L, Ng GA, Rekosz J, Schoen N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns HJGM, Breithardt G. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this study cannot be shared publicly with respect to participating subjects. Data can be shared after a request to the corresponding author.