Abstract
Background:
A gap in research about the trajectories of function among men and women aging with functional limitations due to multiple sclerosis (MS) hinders ability to plan for future needs.
Objectives:
Using a biopsychosocial model, we characterize how men and women with MS report changes over time in their function and test how person-level differences in age, diagnosis duration, and sex influence perceived function.
Methods:
A longitudinal study with multiple waves of surveys was used to collect data on participant perceptions of function, as well as demographic and contextual variables. Self-reported functional limitation was measured over a decade. The study participants were community residing with physician diagnosed MS.
Results:
The people with MS had a diagnosis duration of about 13 years and were around 51 years of age, on average, at the start of the study. They were primarily women and non-Hispanic White. We analyzed the data using mixed-effects models. Subject-specific, functional limitation trajectories were described best with a quadratic growth model. Relative to men, women reported lower functional limitation and greater between-person variation and rates of acceleration in functional limitation scores.
Discussion:
Results suggest function progressed through two pathways for over a decade, particularly closer to diagnoses. Variability in trajectories between individuals based on sex and years since diagnosis of disease indicates that men and women with MS may experience perceptions of their function with age differently. This has implications for clinician advice to men and women with MS.
Keywords: aging, multiple sclerosis, sex differences
Variability in function over time with age and by sex has been studied (Kim & Won, 2022). Variations in disease pathology over time by sex leaves no doubt as to the importance of studying differences (McSweeney et al., 2003); however, few have studied functional limitation by sex in people aging with chronic functional limitation. People with multiple sclerosis (MS) over age 60 are increasing in numbers resulting in difficulties in medical and therapeutic management (Ostolaza et al., 2021). Research is needed on variations in function with age, including sex difference, to guide rehabilitation.
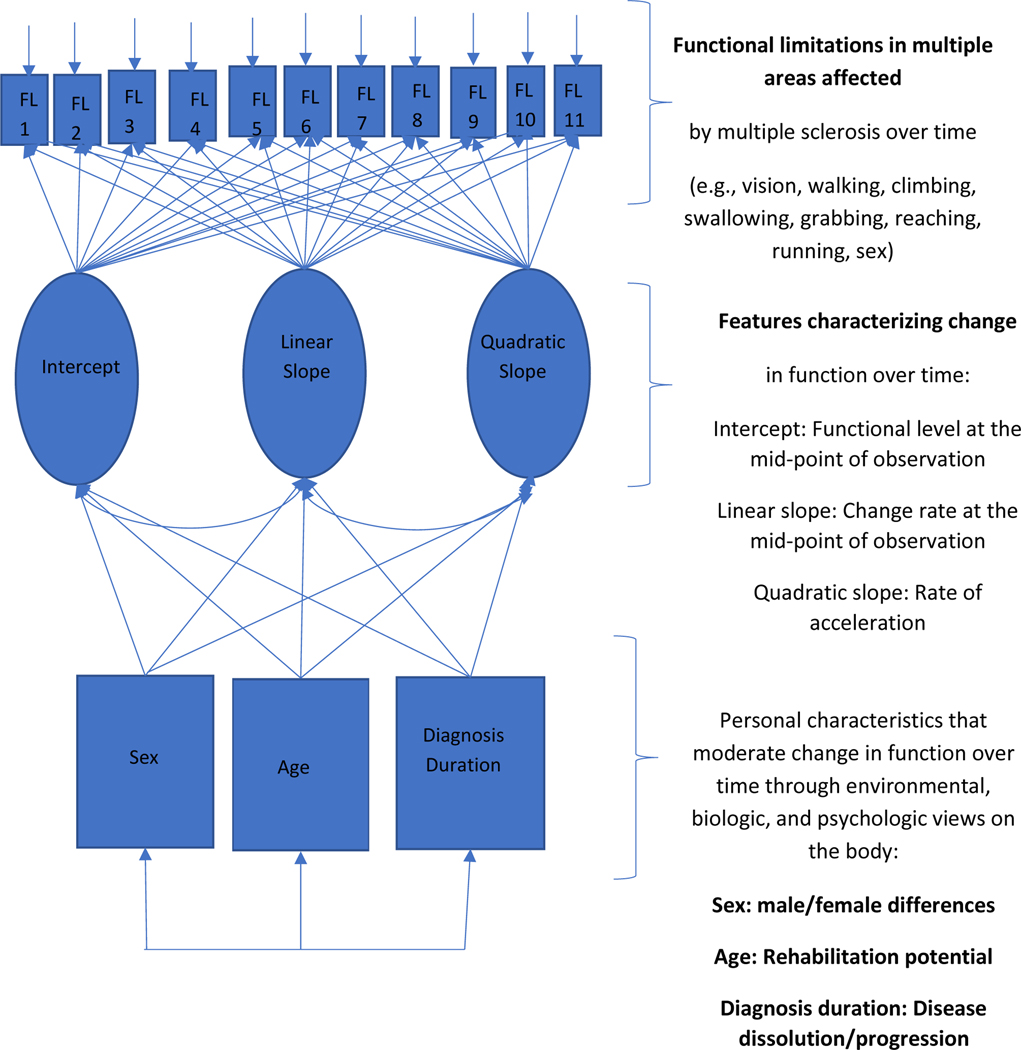
Rice et al.’s (2021) feminist-disability perspective on studying nonnormative body image, Yaghmaian and Miller Smedema’s (2019) definitions of psychosocial stigma/barriers, and disability theorists’ enabling/disabling environmental barriers perspective (Institute of Medicine [IOM], 1997) were used to posit and interpret how age, disease, and sex contribute to functional limitations with time for people with MS (Figure 1). While Rice’s feminist disability perspective challenges women to move past one-dimensional conceptualizations of the body to allow for change and reinterpretation of function with time, external attitudinal barriers from psychosocial factors affect function (IOM, 1997; Harrison et al., 2008) and continue to influence the perceptions of disease, age, and sex.
Figure 1.
Function with MS over 11 years as moderated by Sex, Age, and Diagnosis Duration
By also including the enabling–disabling theory of function that considers the body within an environment that supports or hinders function (IOM, 1997), we provide the possibility for a feminist biopsychosocial rehabilitation framework. Unlike the rehabilitation frameworks that view function “within biomedicine, [where] the aged body is increasingly constructed as a set of age-related diseases, as well as a site for continual restoration and improvement” (Joyce & Mamo, 2006, p. 99), our feminist rehabilitative approach assumes that function can change with the environment without negative implications for the individual. Nonaccommodating environments may create stress and trigger biological inflammatory illnesses that limit function but may also be corrected (Correale & Ysrraelit, 2022). Instead of a biomedical model of aging and disease decline, our feminist model posits a varied trajectory fluctuating over time. Here, we follow perceptions of change in function for over a decade and examine function within and between people with MS while examining predictive effects of age, diagnosis duration, and sex (Ram et al., 2010).
MS, a chronic inflammatory disease, is diagnostic of a nervous system disease, notable to the person after cells of the myelin sheath that coat nerves are modified (Backner & Levin, 2018). A T-cell-mediated autoimmune condition involving all cell types within the immune system, including B-cells (Bittner et al., 2017), manifestation may range from a sudden loss of vision to weakness in the leg. Impulses sent along the myelin sheath of high-capacity nerve cells are stopped, leaving the person gradually unable to move the innervated muscle (Grzegorski & Losy, 2017). Global prevalence of MS is estimated to be between 2 to 3 million people, with the majority diagnosed before 50 years (Vaughn, Jakimovski, Kavak, et al., 2019) and living longer (Ostolaza et al., 2021). Across the population with MS, the disease can manifest as a range of functional limitations, including an inability to walk (see Backner & Levin, 2018), control emotions, remember short-term tasks (Migliore et al., 2017), and control the bowels or bladder (Zecca et al., 2016). Because the inflammation of myelin can occur in many parts of the body, function in various areas is hindered (Grzegorski & Losy, 2017).
Age at diagnosis has been used as criterion for differentiating and studying MS (Tutuncu et al., 2013). The diagnosis of MS with presenting symptoms prior to age 19 is considered early, representing 5% of cases, while a diagnosis after age 50 years is considered late-onset, representing 5% to 10% of cases (Cossburn et al., 2012; Yeh et al., 2009). Most people are diagnosed before age 50 (Awad & Stüve, 2010). Of those diagnosed after age 50, 10%–34% may experience increased functional limitation despite studies suggesting there may be little to no worsening of disease pathology after age 75 (Tutuncu et al., 2013).
This may have been due to dissolution of MS pathology (Frischer et al., 2009; Tutuncu et al., 2013) or environmental adaptation (Roberts & Stuifbergen, 1998). Frischer et al. (2009) found that people deceased with MS (median age of 76 and median diagnosis duration of 31 years) had levels of inflammation and axonal injury like age-matched controls without MS. The MS symptoms and progression had seemingly stopped, and new functional limitation was attributed to comorbidity. MS inflammation and neurodegeneration may have just “die[d] out” (Frischer, 2009, p. 1175). This indicates an MS-disease dissolution hypothesis in what is otherwise known as an incurable chronic disease. For people over age 50, other autoimmune or aging-related diseases, such as dementia, heart disease, and Parkinson’s disease, make differentiating diagnoses more complex and contribute to functional limitations beyond the effects of MS (Chou et al., 2020). Further, by age 50, the reparative process between de-myelination/re-myelination tends to be unbalanced through intracellular and epigenetic mechanisms that are not fully understood within the complexity of the human with MS (see Correale & Ysrraelit, 2022 for an overview of aging cellular changes in MS with age), which has led to the need for longitudinal studies.
In the extant literature, two assertions for implications of aging with MS can be made. First, the age when first diagnosis of disease occurs may have as much significance for understanding the trajectory of functional limitation as does the length of the cellular pathology of the disease (Cottrell et al., 1999; Scalfari et al., 2010). Second, the length of diagnosis duration and the accumulation of comorbidity (Chou et al., 2020) combined with the person’s age may influence the possibility of MS disease dissolution (Scalfari et al., 2010) despite continued functional limitation.
Sex and MS
Several studies support an influence of sex on immune response (Giefing-Kröll et al., 2015). Whether due to environmental, social–behavioral, occupational exposure, or genetic and hormonal variation, differences in outcomes between men and women with MS influence disease susceptibility, disease progression, and mortality (Voskuhl et al., 2018). Klistorner et al. (2016) propose a biologic hypothesis of worsening functional limitation in men compared with women with MS on MRI due to men’s microstructural change compared with women’s diffuse change. Evidence supports both possible reasons for varying trajectories between men and women with MS (Klistorner et al., 2016; Voskuhl et al., 2018).
In this study, we describe the functional limitation trajectory of MS for men and women based on the effects of disease compared to aging and then discuss the implications. The operational model in Figure 1 is the basis for three research questions:
To what extent do sex, the number of years living with MS, and age influence perceived functional limitations?
Do individuals differ from each other in the year-to-year variation (intra-individual variability) in functional limitations after accounting for change in limitations attributable to time and measured covariates? Given individual differences in year-to-year variation, do sex, the number of years living with MS, or age account for between-subject differences in the year-to-year variation?
If there is evidence of individual differences in aspects of change in functional limitation, do sex, the number of years living with MS, or age account for between-person heterogeneity of the variances of the particular aspects of change in functional limitation?
Methods
Data Collection, Procedures, and Sample
Community-living individuals over age 18 with self-report of physician-diagnosed MS for at least 1 year were recruited. Following institutional review board approval, participants of a cross-sectional study were invited to participate. The data were collected in two ways. First, participants were recruited from an earlier cross-sectional study of 807 individuals with useable surveys from 936 people with MS who had been recruited from national MS Society chapters (see Stuifbergen et al., 2000 for a detailed review of the initial sample recruitment). Second, recruitment was initiated through newspaper advertisements in rural areas. A total of 749 persons were eligible for the longitudinal study after the cross-sectional study was complete, and 621 enrolled again (see Stuifbergen et al., 2006 for further details).
Measures
Functional Limitations
The Incapacity Status Scale (ISS; Kurtzke, 1984) was used as an indicator of function with MS. A self-report version of the ISS was used, each rated on a 5-point scale from 0 to 4 (higher scores indicate greater limitations), with a total score ranging from 0 to 64. Total scores on the ISS reflect total degrees of function, which is significantly related to ambulation performance (; Stuifbergen et al., 2016). Cronbach’s alpha ranged from .82 to .88 across the waves of measurement.
Diagnosis Duration
In the first wave, participants were asked the year they were diagnosed with MS, and from that date, the duration since disease diagnosis was calculated.
Age
Participants’ chronological age (in years) was reported at baseline.
Sex
Sex was coded as Sex = 1 if one self-identified as a woman and Sex = 0 if one self-identified as a man. The correlation between sex and age was r (604) = −.12 () and between sex and diagnosis duration was r (604) = −.14 ().
Data Analysis
Mixed-effects models were used to study individual differences in change in functional limitations. An introduction to this approach to analyzing longitudinal data is provided in Hedeker (2004). We started by fitting different growth models to characterize functional limitations across the 11-year period. Provisionally accepting the best-fitting model, we then tested the effects of sex, diagnosis duration, and age on aspects of change in functional limitation. Additionally, we studied individual differences in the within-person variability in responses about the individual’s fitted trajectories.
After identifying a growth model that described the individual’s central tendency of their functional limitation scores over time and accounting for the effects of sex, diagnosis duration, and age on aspects of change in the individual-level trajectories, the variance of the within-person residuals was studied according to sex, diagnosis duration, and age to test if these variables were related to the year-to-year variation in functional scores not captured by the fitted trajectory. Finally, we tested for heterogeneity of the variance in each random coefficient that defined the best-fitting growth model to test if sex, diagnosis duration, or age accounted for possible heterogeneity in aspects of change in functional limitation. Thus, whereas a typical application of a mixed-effects model is done to document the variances of the random coefficients to describe the extent to which individuals differ regarding aspects of change in a response variable, we tested whether the variances of the random coefficients were associated with sex, diagnosis duration, or age. The methods used to address these last two aspects of the longitudinal measures of functional limitation are described in Blozis (2022), Hedeker and Nordgren (2013), and Hedeker et al. (2008).
Growth Models
To study change, a decision was made on how to represent time. In studies of disease prognosis, disease duration is a common choice for the measure of time; conversely, age is often used in studies of disease incidence (Chubak et al., 2013). Diagnosis duration and age are naturally confounded because the two variables increase with time in parallel, so year of measurement was used to denote time, where year was within-person centered to the individual’s mean value of time (Blozis, 2008). Diagnosis duration and age, each centered on the sample means of 13.3 and 50.6, respectively, and sex was used as between-subject covariates to test their effects on aspects of change in functional limitation.
We applied four growth models representing different patterns of change: no change, linear change, quadratic change, and exponential change (equations are provided in Supplemental Table S1). The aim was to define a model that best described the individual-level trajectories as a function of the year of measurement. A general expression of a growth model can be given as
where, for individual , is the functional limitation score at Year , and denotes that the response is a function (e.g., a linear growth function) of and person-specific coefficients that are particular to a given growth function, plus the residual that is the difference between the individual’s observed score and the fitted response at Year . Across years and individuals, the within-person residuals were assumed to be independent and normally distributed with a mean of 0 and variance . The residual variance summarizes the variability in functional limitation scores about the individual-level fitted trajectory. Between individuals, the random coefficients were assumed to be independent and multivariate normal with means equal to 0 and variance–covariance matrix . As typically done when fitting a mixed-effects model, the matrix was unstructured to allow the variances and covariances of the random coefficients to be estimated without constraints. At this stage of the analyses, the within-person residual variance and the variance–covariance matrix of the random coefficients were assumed to be homogeneous across individuals. These assumptions are later relaxed. Using the Akaike information criterion (AIC) and Bayesian information criterion (BIC) to assess relative fit between models, the one with the smallest values was provisionally taken as best in describing change.
Testing the Effects of Sex, Diagnosis Duration, and Age on Functional Limitation
The research questions were tested by including sex, diagnosis duration, and age as predictors of the (a) random coefficients that defined growth in functional limitation, (b) within-person residual variance, and (c) variances of each random coefficient of the selected growth model.
Missing Data.
For some participants, functional limitation scores were missing in a monotonic pattern such that a participant missed a year of measurement and provided no data in subsequent years. For others, data were missing intermittently such that a measurement year was missed, but the individual provided data in one or more subsequent years. Inference from the models assumes that intermittently missing data are ignorable. To account for the timing of attrition in which a participant stopped their participation entirely, a variable—hereafter called maxyear—was created and was equal to the last year that the individual participated in the study (mean = 9 years, range = 1–11 years). Coefficients of the growth model were regressed on this variable.
Estimation.
Maximum likelihood (ML) estimation was carried out using SAS version 9.4 and the NLMIXED procedure for nonlinear mixed-effects models. Tests were two-tailed with α = .05. In fitting a nonlinear mixed-effects model, it is generally recommended that starting values for parameter estimates be provided. To do this, we started by fitting a model with initially fixed growth coefficients and used values that seemed reasonable based on descriptive statistics and plots of the functional limitation scores. We then added random effects, starting with one and incrementally adding one more as specified for a given model. Starting values for the effects of covariates were set equal to 0. Estimates were obtained using adaptive Gaussian quadrature.
Results
Sample
The analyses reported here are based on 606 persons’ data; 15 of the original 621 were misdiagnosed and excluded from analysis. Of these 606 persons, 83% identified as women, and 93% were non-Hispanic White. At the start of the longitudinal study, 72% were married, 85% completed high school, and 25% were employed full-time. On average, participants were 50.6 years old (SD = 10.3, minimum–maximum = 21–81 years) with a mean of 13.4 years since diagnosis (SD = 7.44, minimum–maximum = 1–50 years). Estimated correlation between age and years since diagnosis was r(604) = .48 (p < .001). Response rates were 63% in the 11th year.
Descriptive statistics of functional limitation scores by year of measurement are in Table 1. Supplemental Figure 1 displays functional limitation scores for all participants across the years. Because individual patterns of change are difficult to see in the plot that includes data for all individuals, Supplemental Figure 2 shows scores for a subset of nine arbitrarily selected individuals. Supplemental Figure 3 displays the fitted curves based on the four growth functions applied, with curves representing the fitted trajectories of the typical individual. Indices of fit for the four growth models considered are in Table 2. Relative to the other growth models, a quadratic growth model provided the best overall fit and was provisionally taken as the best-fitting model:
where, for individual , is the functional limitation score at Year , is the expected functional limitation level at Year , is the instantaneous rate of change at Year , and is the acceleration rate. As discussed earlier, Year was person-centered about the individual’s mean years in the study, so the model’s intercept and linear slope are interpreted as aspects of functional limitation at a person’s midpoint of participation in this observational study. If change in functional limitation follows a quadratic growth function, functional limitation scores tended to increase with time at a nonconstant rate of change.
Table 1.
Summary Statistics of Functional Limitation Scores, Diagnosis Duration and Age by sex
| Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Women | n | 502 | 455 | 438 | 418 | 397 | 395 | 371 | 367 | 365 | 348 | 325 |
| %1 | 100 | 91 | 87 | 83 | 79 | 79 | 74 | 73 | 73 | 69 | 65 | |
| Men | n | 104 | 88 | 81 | 80 | 79 | 77 | 68 | 71 | 69 | 64 | 59 |
| %1 | 100 | 85 | 78 | 77 | 76 | 74 | 65 | 68 | 66 | 62 | 57 | |
|
| ||||||||||||
| Functional Limitations | ||||||||||||
|
| ||||||||||||
| Women | Mean | 18.0 | 18.0 | 18.1 | 18.0 | 17.8 | 18.1 | 17.8 | 18.0 | 18.3 | 17.8 | 18.3 |
| Median | 17.0 | 17.0 | 16.0 | 17.0 | 16.0 | 17.0 | 17.0 | 17.0 | 17.0 | 17.0 | 17.0 | |
| SD | 9.9 | 9.8 | 9.5 | 9.5 | 9.7 | 9.8 | 9.6 | 9.4 | 9.8 | 9.6 | 10.0 | |
| Min | 1.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.0 | |
| Max | 54.0 | 49.0 | 46.0 | 45.9 | 45.9 | 51.4 | 53.7 | 43.7 | 48.0 | 50.1 | 49.1 | |
| Men | Mean | 20.1 | 20.2 | 19.3 | 20.0 | 18.9 | 20.2 | 19.6 | 20.8 | 19.9 | 20.3 | 20.8 |
| Median | 19.0 | 19.0 | 19.0 | 21.0 | 19.0 | 20.3 | 21.0 | 21.0 | 21.0 | 19.5 | 19.4 | |
| SD | 10.8 | 10.9 | 10.1 | 10.5 | 9.9 | 10.8 | 10.6 | 10.6 | 9.7 | 10.4 | 10.5 | |
| Min | 0 | 1.0 | 0 | 1.0 | 0 | 3.0 | 1.0 | 1.0 | 1.0 | 1.0 | 3.0 | |
| Max | 48.0 | 48.0 | 49.1 | 45.0 | 44.0 | 46.9 | 44.0 | 45.0 | 44.0 | 45.0 | 46.0 | |
|
| ||||||||||||
| Age | ||||||||||||
|
| ||||||||||||
| Women | Mean | 50.0 | ||||||||||
| Median | 50 | |||||||||||
| SD | 10.3 | |||||||||||
| Min | 21 | |||||||||||
| Max | 81 | |||||||||||
|
|
||||||||||||
| Men | Mean | 53.2 | ||||||||||
| Median | 52 | |||||||||||
| SD | 9.7 | |||||||||||
| Min | 29 | |||||||||||
| Max | 74 | |||||||||||
|
| ||||||||||||
| Diagnosis Duration | ||||||||||||
|
| ||||||||||||
| Women | Mean | 12.9 | ||||||||||
| Median | 12 | |||||||||||
| SD | 7.1 | |||||||||||
| Min | 1 | |||||||||||
| Max | 49 | |||||||||||
|
|
||||||||||||
| Men | Mean | 15.6 | ||||||||||
| Median | 14 | |||||||||||
| SD | 8.4 | |||||||||||
| Min | 5 | |||||||||||
| Max | 43 | |||||||||||
Note:
% is calculated within group (e.g., 91% of women responded in Year 2).
Table 2.
Indices of Model Fit and Comparisons between Growth Models (n = 606)
| Growth Function | q | −2lnL | AIC | BIC | Model comparison | p value | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 | No growth | 3 | 31099 | 31105 | 31118 | |||
| 2 | Linear | 6 | 30086 | 30098 | 30124 | 1 v. 2 | 1013(3) | <.001 |
| 3a | Quadratic | 10 | 30000 | 30020 | 30064 | 2 v. 3 | 86(4) | <.001 |
| 4 | Exponential | 10 | 30041 | 30059 | 30099 | |||
| 3b | Quadratic | 14 | 29880 | 29908 | 29970 | 3a v. 3b | 119.6(4) | <.001 |
Note. q = the number of model parameters; −2lnL = −2 times the log-likelihood; AIC = Akaike information criterion; BIC = Bayesian information criterion. Deviance tests are reported for nested models. AIC and BIC may be used to compare all models from each other, with lower values indicating relatively better fitting models. Models 1, 2, 3a and 4 exclude the effect of the last year of observation (maxyear). Model 3b includes the effect of the last year of observation as a predictor of all growth coefficients and the within-person residual variance. Maximum likelihood eestimation was carried out using adaptive Gaussian quadrature.
To account for the effects of attrition, the quadratic growth model was extended to include maxyear (centered at the sample mean of 9 years) as a predictor of the model’s intercept, the linear and quadratic slopes, and the within-person residual variance. For example, the person-level intercept was modeled as
where is the expected functional limitation level for individuals whose last year in the study was at the sample mean of 9 years, is the effect of the timing of attrition on the intercept, and is the residual of the intercept conditional on the timing of attrition. To model the within-person residual variance as a function of the timing of attrition, an exponential function was used (cf: Hedeker et al., 2008 and Hedeker & Nordgren, 2013):
where the subscript in indicates that the variance varies by individual. When exponentiated, is the residual variance for an individual whose last year of participation was equal to the sample mean, and is the effect of maxyear on the variance. A positive value of would indicate a greater degree of year-to-year variation in the residuals for those who remained enrolled longer in the study, and a negative value of would indicate a lower degree of variation in the residuals for those who remained enrolled for less time. Conditional on maxyear, individual differences in all three growth coefficients were evident (supported by indices of model fit and deviance tests that compared models with and without random coefficients), suggesting significant individual differences in aspects of change in functional limitation.
Estimates from the growth model are presented in two tables: Fixed effects estimates are in Table 3 (see Model 1), and the within-person residual variance and the variances of the random coefficients are in Table 4 (see Model 1). For the typical individual who participated in the mean number of study years, functional limitation was estimated to be 19.4 (95% CI: 18.6, 20.1) with an instantaneous rate of increase of 0.47 (95% CI: 0.38, 0.57) and acceleration rate of 0.06 (95% CI: 0.02, 0.11). Longer study enrollment corresponded to lower functional limitation levels, slower instantaneous rates of change, and reduced acceleration rates. This suggests that those who dropped from the study earlier had higher levels overall and increased their perceptions of functional limitation with time. The typical within-person residual variance was estimated to be 10.1, which varied according to maxyear (), indicating that longer study enrollment corresponded to lower year-to-year variability in scores about the fitted trajectory. In the next data analysis stage, the quadratic growth function was extended by including sex, diagnosis duration, and age as predictors in the three main parts of the model. Specifically, these variables were used as predictors of (a) each coefficient of the growth function to test their associations with functional limitation levels and change, (b) the within-person residual variance to test their associations with the year-to-year variation in observed functional limitation levels about everyone’s fitted trajectory, and (c) the between-person random coefficient variances. Estimates from fitting a model are in the second column of estimates (Model 2) in Tables 3 and 4.
Table 3.
Estimated Fixed Effects of the Quadratic Growth Models (n = 606)
| Model 1 | Model 2 | |||
|---|---|---|---|---|
|
| ||||
| Fixed Effect | MLE (SE) | 95% CI | MLE (SE) | 95% CI |
|
| ||||
| Intercept, | 19.4(0.39) | [18.6, 20.1] | 20.5(0.96) | [18.6, 22.3] |
| maxyear, | −0.95 | [−1.2, −0.71] | −0.91(0.14) | [−1.2, −0.64] |
| sex, | −1.3(1.1) | [−3.3, 0.78] | ||
| DD, | 0.15(0.06) | [0.03, 0.27] | ||
| age, | 0.09(0.04) | [0.01, 0.18] | ||
| Linear slope, | 0.69(0.09) | [0.52, 0.87] | ||
| maxyear, | 0.47(0.05) | [0.38, 0.57] | −0.12(0.03) | [−0.17, −0.06] |
| sex, | −0.27(0.08) | [−0.43, −0.10] | ||
| DD, | −0.001(0.005) | [−0.01, 0.01] | ||
| age, | 0.002(.004) | [−0.01, 0.01] | ||
| Quadratic slope, | 0.06(0.02) | [0.02, 0.11] | 0.066(0.025) | [0.016,0.12] |
| maxyear, | −0.03(0.01) | [−0.05, −0.01] | −0.024(0.011) | [−0.045,−0.003] |
| sex, | −0.012(0.018) | [−0.05,0.02] | ||
| DD, | −0.000(0.001) | [−0.002,0.002] | ||
| age, | 0.001(0.001) | [−0.001,0.002] | ||
|
| ||||
| −2lnL | 29880 | 29775 | ||
| AIC | 29908 | 29851 | ||
| BIC | 29970 | 30019 | ||
Note. DD= diagnosis duration; MLE = maximum likelihood estimates; SE = standard error. 95% CI = the estimated 95% confidence interval.
Table 4.
Estimated Within- and Between-Person Variances and Covariances of the Quadratic Growth Models (n = 606)
| Model 1 | Model 2 | |||
|---|---|---|---|---|
|
| ||||
| Within-person variance | MLE(SE) | 95% CI | MLE(SE) | 95% CI |
|
| ||||
| 2.31(0.03) | [2.25, 2.37] | 2.11(0.06) | [1.98, 2.23] | |
| maxyear, | −0.08(0.02) | [−0.11, −0.05] | −0.045(0.016) | [−0.076, −0.013] |
| sex, | 0.15(0.065) | [0.023, 0.28] | ||
| DD, | 0.009(0.004) | [0.001, 0.017] | ||
| age, | −0.001(0.003) | [−0.006, 0.004] | ||
|
| ||||
| Between-person variances | ||||
|
| ||||
| Intercept, | ||||
| 4.47(0.06) | [4.35, 4.59] | 4.5(0.14) | [4.2, 4.8] | |
| maxyear, | −0.069(0.019) | [−0.11, −0.03] | ||
| sex, | −0.099(0.15) | [−0.40, 0.20] | ||
| DD, | 0.02(0.01) | [−0.003, 0.035] | ||
| age, | −0.010.01 | [−0.025, 0.003] | ||
| Linear slope, | ||||
| −0.96(0.08) | [−1.13, −0.80] | −0.61(0.21) | [−1.02, −0.20] | |
| maxyear, | −0.24(0.05) | [−0.34, −0.14] | ||
| sex, | −0.004(0.22) | [−0.45, 0.44] | ||
| DD, | −0.02(0.01) | [−0.05, 0.01] | ||
| age, | 0.02(0.01) | [−0.004, 0.033] | ||
| Quadratic slope, | ||||
| −4.7(0.16) | [−5.0, −4.4] | −3.2(0.35) | [−3.9, −2.6] | |
| maxyear, | −0.61(0.10) | [−0.82, −0.41] | ||
| sex, | −0.50(0.35) | [−1.2, 0.18] | ||
| DD, | 0.002(0.028) | [−0.05, 0.06] | ||
| age, | −0.041(0.018) | [−0.078, −0.006] | ||
|
| ||||
| Calculated variance estimates | ||||
|
| ||||
| Within-person variance, | 10.1 | 8.2 | ||
| Intercept variance, | 87 | 91 | ||
| Linear slope variance, | 0.38 | 0.54 | ||
| Quadratic slope variance, | 0.009 | 0.039 | ||
|
| ||||
| −lnL | 5836.6 | 29775 | ||
| AIC | 5864.6 | 29851 | ||
| BIC | 5926.3 | 30019 | ||
Notes: AIC = Akaike information criterion; BIC = Bayesian information criterion; DD= diagnosis duration. The calculated variance estimates are obtained by exponentiating the intercept of the corresponding variance model. For example, .
Testing the Effects of Sex, Diagnosis Duration, and Age on Changes in Functional Limitation
Question 1 was answered by testing the effects of sex, diagnosis duration, and age on the intercept, instantaneous rate of change, and acceleration rate, adjusting for the effects of maxyear. For instance, the level-2 regression for the intercept was
Similar equations were specified for the random linear and quadratic slopes. As shown in Table 3, the estimated effects of diagnosis duration () and age () on the intercept were positive and significant, suggesting that functional limitation levels were higher for those with a relatively greater number of years since diagnosis and older ages. The effect of sex on the intercept was not significant, suggesting no difference in the expected functional limitation levels between men and women. The estimated effects of diagnosis duration and age on the instantaneous linear rate of change were not statistically significant; however, the effect of sex was (), indicating instantaneous rate of change tended to be lower for women than men. Sex, diagnosis duration, and age were not related to acceleration rate.
Testing the Effects of Sex, Diagnosis Duration, and Age on Year-to-Year Variability in Functional Limitation
Question 2 was answered by testing the effects of sex, diagnosis duration, and age on the within-person variance. Variance was modeled—with maxyear—using an exponential function with sex, diagnosis duration, and age as predictors:
When exponentiated, is the residual variance for a man whose last year of participation, diagnosis duration, and age are equal to the respective sample means. As shown in the upper part of Table 4, the effects of sex (]) and diagnosis duration () were significant, indicating that the degree of year-to-year variation in the residuals about the individual’s fitted trajectory tended to be greater for women and to correspond to a greater number of years since diagnosis.
Testing the Effects of Sex, Diagnosis Duration, and Age on Variation in the Random Growth Coefficients
Question 3 was answered by testing the effects of sex, diagnosis duration, and age on the variances of the random intercept and linear and quadratic slopes (after statistically adjusting for the effects of covariates on each coefficient, as described earlier in the report relating to Question 1). The variance of each random coefficient represents the extent to which individuals differ regarding that coefficient after adjusting for the effects of covariates. Heterogeneity of variance of each conditional coefficient was tested by including sex, diagnosis duration, and age as predictors of the corresponding variance—adjusting for the effect of maxyear. For instance, the variance of the random intercept was modeled as (cf: Hedeker & Nordgren, 2013):
where , when exponentiated, is the variance of the (conditional) random intercept for a man whose maxyear, diagnosis duration, and age are equal to their respective sample means. The variances of the random linear and quadratic slopes followed similar equations. As shown in the lower part of Table 4, only one effect of age—specifically as a predictor of the random quadratic slope (])—was significant, indicating a greater degree of between-person variation in the acceleration rates for older ages. Older individuals were increasingly different from each other in their acceleration rates, whereas younger individuals tended to be relatively similar in their rates of acceleration.
Discussion
The sample of people with MS studied for over a decade contributes to an understanding of trajectories of function for people with MS across cohorts, timing of onset, and sex, lending critical knowledge for men and women aging with MS, as well as both scientists and providers (Hirschmann, 2012; Yaghmaian & Miller Smedema, 2019). In general, if aging with MS were purely a process of functional decline, the variability found in the sample would not be significant within and between men and women, and the effects of diagnosis duration and age would not be variable. The biopsychosocial variables predicted distinct trajectories, as proposed in Figure 1, in precise and important ways.
Research questions were asked using advanced statistical analyses to control and predict time-dependent and -relevant measures, which will be further discussed before advancing the findings. Of importance, age and the diagnosis duration are time-dependent variables that progress together. To capture variation in progression of function, the variability in diagnosis duration of MS and age among individuals at the onset of the study was a statistical advantage. The variability in duration of MS and age as the individuals lived with their functional limitation provided the information for the study of time as indicators of between-subject change in function using mixed-effects models (Ram et al., 2010).
The three research questions led to knowledge of levels and change in function, which was variable over the 11-year period. It was not a trajectory of decline with age and diagnosis duration for men and women. More precisely, the quadratic model clarified changes in perceived functional limitation levels for individuals who differed in sex, age, and diagnosis duration. The typical individual with MS perceived that their function worsened with time at a nonconstant rate. In our analysis of between-individual differences, our biopsychosocial model included sex and diagnosis duration theorized to intertwine to influence function trajectories. The general findings support assertions.
Men in the study had a higher instantaneous rate of change in their scores, meaning they had incremental change scores estimated to be higher (e.g., worse function) than women’s incremental change scores. This instantaneous rate of change was estimated at each person’s midpoint of participation in the study. Sex was also predictive of the year-to-year variation in trajectories after accounting for differences in functional limitation trajectories due to sex, diagnosis duration, and age. Using a quadratic growth model, women in the study had greater individual variation in their residuals about their fitted trajectories.
The intercept of the growth model (i.e., function at one’s midpoint of participation in the study) was predicted by diagnosis duration. Functional limitation scores were significantly higher given a greater number of years since diagnosis. Diagnosis duration also predicted the individual variation in the year-to-year residuals about the fitted trajectories, indicating that after accounting for individual differences in aspects of change in functional limitation over time, individuals whose diagnosis duration was relatively high varied most in functional limitation from year to year.
The intercept of the growth model (i.e., function at one’s midpoint of participation in the study) was predicted by age. Functional limitation scores were higher for people who were older. Age predicted the random quadratic slope, indicating a greater degree of between-person variation in acceleration rates for older people compared with younger people with MS.
The variation in function is consistent with the extant literature suggesting that people should be valued for the variation in their ability to function in society with multiple biopsychosocial factors influencing their outcomes (Rice et al., 2021), including age (Neugarten, 1970), diagnosis duration (Chou et al., 2020), and sex (Yaghmaian & Miller Smedema, 2019). The hypothesized importance of accommodations in the environment (Harrison et al., 2010; IOM, 1997) and comorbidities/injuries were not directly measured but remained essential considerations. The within-person variability among women leads to questions about the potential fluctuating influences on function that need further investigation.
Two assertions based on the literature on age and diagnosis duration were crafted for consideration. These were based on the premise that age when first diagnosed and chronological age were important for predicting trajectories of function (Cottrell et al., 1999; Scalfari et al., 2010). Indeed, the trajectory of functional limitation was predicted by both age and diagnosis over the 11-year study. Variations in outcomes at later age did not follow pathways established earlier in the disease; this could be due to greater variability in inflammation with age (Chou et al., 2020), leading to disease dissolution (Scalfari et al., 2010; Tutuncu et al., 2013).
The between-person instantaneous rates of change in functional limitation according to the fitted quadratic model were similar for men and women and those with similar diagnosis duration. Between-person differences due to age were found, with an increase in differences for older ages. Variability in function increased among older individuals relative to younger individuals. The finding that age predicted a greater degree of between-person variation could be due to increased susceptibility to nondisease related perceptions of age (Levy, 2017) that ultimately change function quickly and reversibly (e.g., falls) as well as comorbidity (Chou et al., 2020).
This finding leads us to consider the importance of a feminist rehabilitation approach to the care of people with MS. Multiple risks for decline in function exists in the environment; there is not one best rehabilitation approach (Inouye et al., 2007) based upon sex that will improve intervention outcomes for MS. If disease dissolution were possible, it would not be due to diagnosis duration but most likely triggered at an older age, which requires aging. Ensuring the possibility for best function without decline while accepting the person at their current capacity is recommended. Differences in the capacity for variation in function might be due to inflammatory processes or environmental barriers that can have individual differences, especially in women. A longitudinal, biological study of inflammatory processes is yet to be done but is needed.
Limitations include few racial minorities and more women than men in the study. We cannot make inferences about the population of people with MS. Moreover, although these numbers reflect the demographic population of people with MS, our future goal is to make studies capture the nature of illness experience for all subgroups over time. Finally, the study was started a decade ago, and better measures and predictors may exist.
Conclusion
People with MS had a trajectory of function explained best by a quadratic model. Women had more variability in their individual scores, and men had worse changes in their individual scores with time. Age was predictive of the individual trajectories, specifically concerning the acceleration in functional limitation that was faster for those older. The pathways of function were best predicted along two pathways closer to diagnosis. The variability in function with increasing age supported that disease dissolution may occur, but not all people age into a stable or improved function. Feminist approaches to rehabilitation might be helpful for both sexes.
Supplementary Material
Supplemental Figure 1. A Spaghetti Plot of FL Scores Across Years of Measurement for All Participants (n=606)
Supplemental Figure 2. A Panel Display of FL Scores Across Years of Measurement for 9 Selected Participants
Supplemental Figure 3. Fitted Trajectories of the Typical Individual According to Different Growth Models
Acknowledgments
This work was supported by the National Institutes of Health, National Institute of Nursing Research (Grant No. R01NR003195).
The study was approved by The University of Texas at Austin Institutional Review Board (Protocol Number 2001-10-0059).
Footnotes
The authors have no conflicts of interest to report.
References
- Awad A, & Stüve O. (2010). Multiple sclerosis in the elderly patient. Drugs & Aging, 27, 283–294. 10.2165/11532120-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Backner Y, & Levin N. (2018). Keep your eyes wide open: On visual- and vision-related measurements to better understand multiple sclerosis pathophysiology. Journal of Neuro-Ophthalmology, 38, 85–90. 10.1097/WNO.0000000000000634 [DOI] [PubMed] [Google Scholar]
- Bittner S, Ruck T, Wiendl H, Grauer OM, & Meuth SG (2017). Targeting B cells in relapsing-remitting multiple sclerosis: From pathophysiology to optimal clinical management. Therapeutic Advances in Neurological Disorders, 10, 51–66. 10.1177/1756285616666741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blozis SA (2022). A latent variable mixed-effects location scale model with an application to daily diary data. Psychometrika, 87, 1548–1570. 10.1007/s11336-022-09864-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubak J, Yu O, Buist DSM, Wirtz HS, & Boudreau DM (2013). Time scale in follow-up studies: Considering disease prognosis. Epidemiology, 24, 628–629. 10.1097/EDE.0b013e3182961708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou IJ, Kuo CF, Tanasescu R, Tench CR, Tiley C, Constantinescu CS, & Whitehouse WP (2020). Comorbidity in multiple sclerosis: Its temporal relationships with disease onset and dose effect on mortality. European Journal of Neurology, 27, 105–112. 10.1111/ene.14040 [DOI] [PubMed] [Google Scholar]
- Correale J, & Ysrraelit MC (2022). Multiple sclerosis and aging: The dynamics of demyelination and remyelination. ASN neuro, 14, 17590914221118502. 10.1177/17590914221118502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossburn M, Ingram G, Hirst C. Ben-Shlomo Y, Pickersgill TP, & Robertson NP (2012). Age at onset as a determinant of presenting phenotype and initial relapse recovery in multiple sclerosis. Multiple Sclerosis Journal, 18, 45–54. 10.1177/1352458511417479 [DOI] [PubMed] [Google Scholar]
- Cottrell DA, Kremenchutzky M, Rice GPA, Koopman WJ, Hader W, Baskerville J, & Ebers GC (1999). The natural history of multiple sclerosis: A geographically based study: 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain, 122, 625–639. 10.1093/brain/122.4.625 [DOI] [PubMed] [Google Scholar]
- Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, & Lassmann H. (2009). The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain, 132, 1175–1189. 10.1093/brain/awp070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giefing-Kröll C, Berger P. Lepperdinger G, & Grubeck-Loebenstein B. (2015). How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell, 14, 309–321. 10.1111/acel.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzegorski T, & Losy J. (2017). Cognitive impairment in multiple sclerosis–a review of current knowledge and recent research. Reviews in the Neurosciences, 28, 845–860. 10.1515/revneuro-2017-0011 [DOI] [PubMed] [Google Scholar]
- Harrison T, Blozis S, & Stuifbergen A. (2008). Longitudinal predictors of attitudes toward aging among women with multiple sclerosis. Psychology and Aging, 23, 823–832. 10.1037/a0013802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TC, Umberson D, Lin L-C, & Cheng H-R (2010). Timing of impairment and health-promoting lifestyles in women with disabilities. Qualitative Health Research, 20, 816–829. 10.1177/1049732310362987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D. (2004). An introduction to growth modeling. In Kaplan D. (Ed.), The SAGE handbook of quantitative methodology for the social sciences (pp. 215–234). SAGE Publications. [Google Scholar]
- Hedeker D, Mermelstein RJ, & Demirtas H. (2008). An application of a mixed-effects location scale model for analysis of ecological momentary assessment (EMA) data. Biometrics, 64, 627–634. 10.1111/j.1541-0420.2007.00924.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, & Nordgren R. (2013). MIXREGLS: A program for mixed-effects location scale analysis. Journal of Statistical Software, 52, 1–38. 10.18637/jss.v052.i12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschmann NJ (2012). Disability as a new frontier for feminist intersectionality research. Politics & Gender, 8, 396–405. 10.1017/S1743923X12000384 [DOI] [Google Scholar]
- Institute of Medicine, Committee on Assessing Rehabilitation Science and Engineering. (1997). Enabling America: Assessing the role of rehabilitation science and engineering. National Academy Press. [Google Scholar]
- Inouye SK, Studenski S, Tinetti ME, Kuchel. (2007). Geriatric syndromes: Clinical, research, and policy implications of a core geriatric concept. Journal of the American Geriatrics Society, 55, 780–791. 10.1111/j.1532-5415.2007.01156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce K, & Mamo L. (2006). Graying the cyborg: New directions in feminist analyses of aging (pp. 99–121). In Calasanti TM & Slevin KF (Eds.), Age matters: Realigning feminist thinking. Routledge. [Google Scholar]
- Kim S, & Won CW (2022). Sex-different changes of body composition in aging: A systemic review. Archives of Gerontology and Geriatrics, 102, 104711. 10.1016/j.archger.2022.104711 [DOI] [PubMed] [Google Scholar]
- Klistorner A, Wang C, Yiannikas C, Graham SL, Parratt J. & Barnett MH (2016). Progressive injury in chronic multiple sclerosis lesions is gender-specific: A DTI study. PLoS ONE, 11, e0149245. 10.1371/journal.pone.0149245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke JF (1984). Disability rating scales in multiple sclerosis. In Scheinberg L. & Raine CS (Eds.), Annals of the New York Academy of Sciences: Multiple sclerosis. Experimental and clinical aspects, Vol. 436, (pp. 347–360). New York Academy of Sciences. 10.1111/j.1749-6632.1984.tb14805.x [DOI] [PubMed] [Google Scholar]
- Levy BR (2017). Age–stereotype paradox: Opportunity for social change. Gerontologist, 57, S118–S126. 10.1093/geront/gnx059 [DOI] [Google Scholar]
- McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK, & Garvin BJ (2003). Women’s early warning symptoms of acute myocardial infarction. Circulation, 108, 2619–2623. 10.1161/01.CIR.0000097116.29625.7C [DOI] [PubMed] [Google Scholar]
- Migliore S, Ghazaryan A, Simonelli I, Pasqualetti P, Squitieri F, Curcio G, Landi D, Palmieri MG, Moffa F, Filippi MM, & Vernieri F. (2017). Cognitive impairment in relapsing-remitting multiple sclerosis patients with very mild clinical disability. Behavioural Neurology, 2017, 7404289. 10.1155/2017/7404289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugarten BL (1970). Dynamics of transition of middle age to old age. Adaptation and the life cycle. Journal of Geriatric Psychiatry, 4, 71–100. [PubMed] [Google Scholar]
- Ostolaza A, Corroza J, & Ayuso T. (2021). Multiple sclerosis and aging: Comorbidity and treatment challenges. Multiple Sclerosis and Related Disorders, 50, 102815. 10.1016/j.msard.2021.102815 [DOI] [PubMed] [Google Scholar]
- Ram N, Gerstorf D, Fauth E, Zarit S, & Malmberg B. (2010). Aging, disablement, and dying: Using time-as-process and time-as-resources metrics to chart late-life change. Research in Human Development, 7, 27–44. 10.1080/15427600903578151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C, Riley S, LaMarre A, & Bailey KA (2021). What a body can do: Rethinking body functionality through a feminist materialist disability lens. Body Image, 38, 95–105. 10.1016/j.bodyim.2021.03.014 [DOI] [PubMed] [Google Scholar]
- Roberts G, & Stuifbergen AK (1998). Health appraisal models in multiple sclerosis. Social Science & Medicine, 47, 243–253. 10.1016/s0277-9536(98)00080-x [DOI] [PubMed] [Google Scholar]
- Scalfari A, Neuhaus A, Degenhardt A, Rice GP, Muraro PA, Daumer M, & Ebers GC (2010). The natural history of multiple sclerosis: A geographically based study 10: Relapses and long-term disability. Brain, 133, 1914–1929. 10.1093/brain/awq118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuifbergen AK, Blozis S, Becker H, Harrison T, & Kullberg V. (2016). Selected health behaviors moderate the progression of functional limitations in persons with multiple sclerosis: Eleven years of annual follow-up. Disability and Health Journal, 9, 472–478. 10.1016/j.dhjo.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuifbergen AK, Blozis SA, Harrison TC, & Becker HA (2006). Exercise, functional limitations, and quality of life: A longitudinal study of persons with multiple sclerosis. Archives of Physical Medicine and Rehabilitation, 87, 935–943. 10.1016/j.apmr.2006.04.003 [DOI] [PubMed] [Google Scholar]
- Stuifbergen AK, Seraphine A, & Roberts G. (2000). An explanatory model of health promotion and quality of life in chronic disabling conditions. Nursing Research, 49, 122–129. 10.1097/00006199-200005000-00002 [DOI] [PubMed] [Google Scholar]
- Tutuncu M, Tang J, Zeid NA, Kale N, Crusan DJ, Atkinson EJ, Siva A, Pittock SJ, Pirko I, Keegan BM, Lucchinetti CF, Noseworthy JH, Rodriguez M, Weinshenker BG, & Kantarci OH (2013). Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Multiple Sclerosis Journal, 19, 188–198. 10.1177/1352458512451510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn CB, Jakimovski D, Kavak KS, Ramanathan M, Benedict RHB, Zivadinov R, & Weinstock-Guttman B. (2019). Epidemiology and treatment of multiple sclerosis in elderly populations. Nature reviews. Neurology, 15(6), 329–342. 10.1038/s41582-019-0183-3 [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Sawalha AH & Itoh Y. (2018). Sex chromosome contributions to sex differences in multiple sclerosis susceptibility and progression. Multiple Sclerosis Journal, 24, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghmaian R, & Miller Smedema S. (2019). A feminist, biopsychosocial subjective well-being framework for women with fibromyalgia. Rehabilitation Psychology, 64, 154–166. 10.1037/rep0000226 [DOI] [PubMed] [Google Scholar]
- Yeh EA, Weinstock-Guttman B, Ramanathan M, Ramasamy DP, Willis L, Cox JL, & Zivadinov R. (2009). Magentic resonance imaging characteristics of children and adults with paediatric-onset multiple sclerosis. Brain, 132, 3392–3400. 10.1093/brain/awp278 [DOI] [PubMed] [Google Scholar]
- Zecca C, Riccitelli GC, Disanto G, Singh A, Digesu GA, Panicari L, Puccini F, Mattioli M, Tubaro A, & Gobbi C. (2016). Urinary incontinence in multiple sclerosis: Prevalence, severity and impact on patients’ quality of life. European Journal of Neurology, 23, 1228–1234. 10.1111/ene.13010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. A Spaghetti Plot of FL Scores Across Years of Measurement for All Participants (n=606)
Supplemental Figure 2. A Panel Display of FL Scores Across Years of Measurement for 9 Selected Participants
Supplemental Figure 3. Fitted Trajectories of the Typical Individual According to Different Growth Models