Abstract
BACKGROUND:
Heart failure with preserved ejection fraction is associated with significant functional limitations, yet treatments for improving exercise performance have been elusive. We sought to explore the association between prespecified patient characteristics and changes in 6-minute walk distance that constitute a clinically significant response to dapagliflozin.
METHODS:
We performed a responder analysis to understand patient characteristics associated with clinically meaningful improvement in 6-minute walk test (6MWT) distance ≥15 m among patients randomized to 12 weeks of dapagliflozin versus placebo in the double-blind PRESERVED-HF trial (Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients With Preserved Ejection Fraction Heart Failure).
RESULTS:
A total of 289 randomized patients had 6MWT distance completed at baseline and 12 weeks. Patients randomized to dapagliflozin improved walking distance by ≥15 m more frequently than those on placebo (n=64, 44% versus n=48, 34%). After adjusting for baseline covariates, patients randomized to dapagliflozin were more likely to experience a clinically meaningful improvement in 6MWT distance compared with those that received placebo (adjusted odds ratio, 1.66 [95% CI, 1.00–2.75]; P=0.05). Dapagliflozin-treated patients were also less likely to have a ≥15 m reduction in 6MWT distance compared with placebo-treated patients (adjusted odds ratio, 0.56 [95% CI, 0.33–0.94]; P=0.03). These results were consistent across all prespecified subgroups (all P values for interaction were not significant).
CONCLUSIONS:
Compared with those on placebo, patients with heart failure with preserved ejection fraction randomized to dapagliflozin were more likely to experience a clinically meaningful improvement and less likely to experience a deterioration in physical function over 12 weeks as measured by 6MWT distance. Beneficial response to dapagliflozin was consistent across prespecified subgroups.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03030235.
Keywords: dapagliflozin, heart failure, preserved ejection fraction, sodium-glucose transporter 2 inhibitors, walk test
WHAT IS NEW?
Among patients with heart failure with mildly reduced ejection fraction or heart failure with preserved ejection fraction in the PRESERVED-HF trial (Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients With Preserved Ejection Fraction Heart Failure), dapagliflozin improved 6-minute walking distance, increased the proportion of patients with meaningful improvement, and reduced the proportion of patients with significant deterioration in walking distance compared with placebo.
Patients with a clinically meaningful (≥15 m) improvement in walking distance at 12 weeks had similar demographic characteristics, clinical comorbidities, NT-proBNP (N-terminal pro-B-type natriuretic peptide) levels, Kansas City Cardiomyopathy Questionnaire scores, and loop diuretic dosing as those who did not experience a meaningful increase in walking distance with dapagliflozin. However, patients with above-median body mass index and diabetes tended to have the greatest improvement in walking distance.
WHAT ARE THE CLINICAL IMPLICATIONS?
Exercise limitation is a cardinal manifestation of heart failure that has largely proven to be refractory to pharmacotherapies trialed in heart failure with preserved ejection fraction to date.
Our responder analysis in the PRESERVED-HF trial showed that patients with heart failure with mildly reduced ejection fraction or heart failure with preserved ejection fraction derive clinically meaningful walking distance benefits from dapagliflozin, providing additional rationale for its use.
The benefits of dapagliflozin on walking distance were consistent across patient subgroups examined. Future studies should attempt to explore the mechanisms of dapagliflozin’s benefit.
See Editorial by Banner and Silverman
Heart failure (HF) with preserved ejection fraction (HFpEF) is characterized by marked exercise limitation, even when congestion is absent at rest.1–3 The extent of exertional limitation in people with HFpEF frequently falls within a range lower than that required for performing activities of daily living.2 In the PRESERVED-HF study (Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients With Preserved Ejection Fraction Heart Failure) of the SGLT2 (sodium-glucose cotransporter-2) inhibitor dapagliflozin versus placebo, the mean baseline 6-minute walk test (6MWT) distance at study entry was severely impaired (249 m), substantially lower than that reported in patients with other chronic diseases that cause exercise limitation, including pulmonary arterial hypertension and HF with reduced ejection fraction.4–9 Finding pharmacotherapies to ameliorate exercise limitation in HFpEF has proven remarkably challenging; previously conducted trials have not convincingly demonstrated an impact on exercise limitation in HFpEF.10
Therefore, the finding that treatment with dapagliflozin in the PRESERVED-HF trial resulted in a statistically significant 20-m improvement in 6MWT compared with placebo represented a substantial advance in the field of HFpEF. However, it remains unclear which characteristics were associated with this novel and important benefit. To address this question, we performed a post hoc secondary analysis in PRESERVED-HF to understand the following: (1) the likelihood of patients treated with dapagliflozin (versus placebo) exceeding the clinically meaningful change in the 6MWT distance threshold; and (2) the relationship between prespecified patient characteristics and changes in exercise limitation that may represent a clinically meaningful response.
METHODS
The protocol was approved by the Institutional Review Board at each participating center. Written informed consent was obtained from every participant before study enrollment. PRESERVED-HF was designed primarily to evaluate the effects of dapagliflozin versus placebo on HF-related health status using the Kansas City Cardiomyopathy Questionnaire (KCCQ) clinical summary score measured at 12 weeks, which was the primary end point. The methods and results published previously prespecified and hierarchically tested key secondary objective—specifically to evaluate the effect of dapagliflozin versus placebo on 6MWT distance at 12 weeks.4 Included patients were adults aged >18 years, symptomatic from HF with ejection fraction ≥45%, elevated natriuretic peptide (NT-proBNP [N-terminal pro-B-type natriuretic peptide] ≥225 pg/mL, or BNP [B-type natriuretic peptide] ≥75 pg/mL), on stable medical therapy including a diuretic before enrollment and at least 1 of the following: (1) HF hospitalization/urgent HF visit with intravenous diuretic treatment in the past 12 months; (2) documented elevated filling pressures on right or left heart catheterization; or (3) echocardiographic evidence of structural heart abnormalities. Deidentified participant data will be made available on reasonable request 2 years after the date of publication. Requests should be directed to the corresponding author. Requestors will be required to sign a data access agreement to ensure the appropriate use of the study data.
Notable exclusion criteria were active HF decompensation, history of type 1 diabetes, estimated glomerular filtration rate (eGFR) <20 mL/min per 1.73 meter squared, recent acute coronary syndrome or percutaneous coronary intervention or cardiac surgery, and planned cardiovascular procedural intervention. Patients with known infiltrative cardiomyopathy, active myocarditis, hypertrophic cardiomyopathy, severe aortic or mitral valvular pathology, severe lung disease, isolated right HF, complex congenital heart disease, uncontrolled hypertension, or a history of ejection fraction <45% were also excluded.
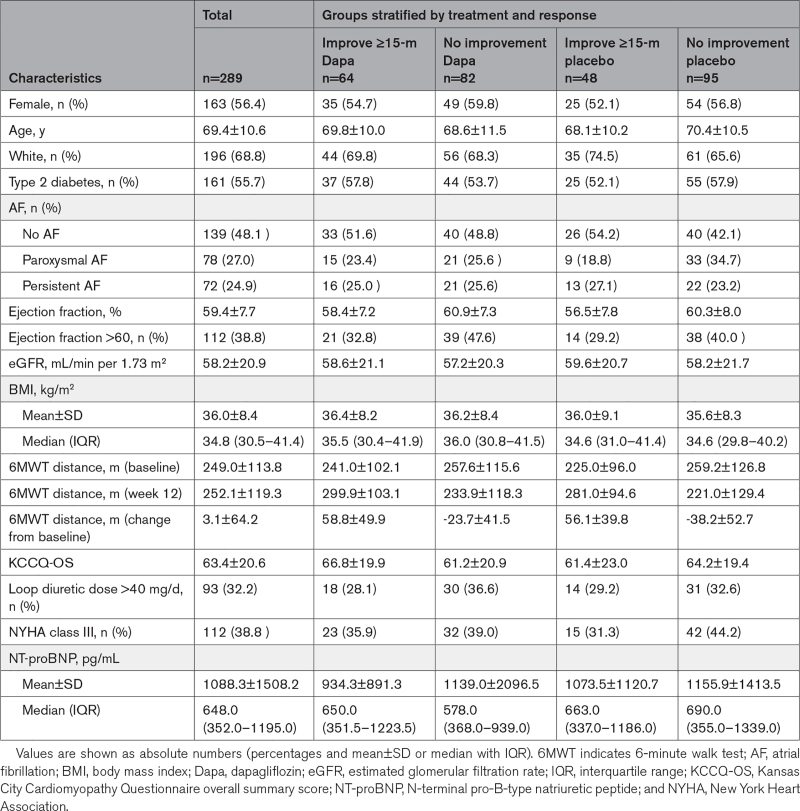
Patient demographics, clinical characteristics, medical history, and laboratory findings were described overall and stratified by treatment group and 6MWT distance response (≥15 m versus no response [<15 m, which included deterioration]). Continuous measures were summarized by mean±SD or median and interquartile range and compared using t tests or Wilcoxon rank-sum tests, respectively. Categorical variables were summarized by frequency and percent and compared using χ2 or Fisher exact tests.
Analysis of the 6MWT distance at 12 weeks was performed using the modified intention-to-treat data set, defined as all patients who were randomized to study treatment, received at least 1 dose of study medication, and had sufficient evaluable data for end point ascertainment during follow-up. Mean±SD was reported for the 6MWT distance at baseline, 12-week follow-up, and its change for the entire cohort and by treatment group. As previously reported, an ANCOVA model was used to estimate the effect of dapagliflozin on the 12-week 6MWT distance, adjusting for baseline measurements including sex, history of diabetes, eGFR, left ventricular ejection fraction (LVEF), history, and type of atrial fibrillation (AF), and baseline 6MWT distance. All continuous variables were included as restricted cubic splines to account for nonlinear effects. Prespecified subgroup analyses were also performed using similar models. They included age (<70 and ≥70 years), sex, race (White, non-White), history of diabetes, body mass index (BMI; < median, ≥ median), LVEF (<60% and ≥60%), history and type of AF (none, paroxysmal, and persistent/permanent), KCCQ-overall summary score (< median, ≥ median), eGFR (<60 and ≥60 mL/min per 1.72 meter squared), loop diuretic dose (furosemide equivalent mean daily dose ≤40 mg and >40 mg), New York Heart Association (NYHA) functional class (II and III–IV), NT-proBNP (< median and ≥ median), and baseline 6MWT distance (< median and ≥ median). Lastly, because previous studies have identified a potential SGLT2 inhibitor responder population of patients with higher BMI, a similarly adjusted model for 12-week 6MWT distance, which included a 3-way interaction between dapagliflozin, history of diabetes, and BMI (< median and ≥ median), was tested.11
The responder analysis calculated the unadjusted proportion of patients achieving a ≥15 m improvement, no change, and ≥15 m deterioration in 6MWT distance at 12 weeks for the treatment and placebo groups. A ≥15 m improvement was chosen as a clinically meaningful threshold, because it represents a >6% relative improvement in average baseline 6MWT distance in the PRESERVED trial population, a relative change shown to be associated with reduced clinical events and improved quality of life in other HF trials (and achieved through established interventions such as exercise training).12,13 Separate logistic regression models (1 for improvement and 1 for deterioration) were used to assess the treatment effect, adjusting for the same baseline measurements as the primary analysis. A supplemental responder analysis looking at the ≥20-m threshold for defining deterioration versus improvement was also performed.
All statistical tests were 2-tailed and were evaluated at a significance level of 0.05. All analyses were completed using SAS software version 9.4 (SAS Institute Inc, Cary, NC) and R software version 4.1.3 (FreeSoftwareFoundation, Boston, MA).
RESULTS
Among the overall study population of 598 patients screened, 324 qualified and were randomized (162 patients to dapagliflozin and 162 patients to placebo). The 6MWT distance results were available for 289 patients at baseline and 12 weeks and are described in the Table. The baseline characteristics of patients with incomplete 6MWT data (compared with those with complete pre- and post-6MWT distance data) are summarized in Table S1. Patients with missing data were more likely to have marked functional limitations as assessed by 6MWT distance and KCCQ at baseline and at 12 weeks. Among the patients with missing 6MWT data, treatment group proportions were similar (dapagliflozin [n=16] and placebo [n=19]).
Table.
Characteristics at Baseline and After 12 Weeks, Stratified by Treatment Group and Response
In the linear ANCOVA model adjusted for baseline 6MWT distance, sex, history of diabetes, eGFR, LVEF, and history and type of AF, patients treated with dapagliflozin walked an average of 20.1 m further than those not on treatment ([95% CI, 5.6–34.7]; P=0.007). When we further explored the interaction between dapagliflozin effect on 6MWT and continuous baseline 6MWT distance, the interaction was not significant (P=0.19). Patients observed to improve 6MWT distance by ≥15 m were not more likely to be women, older, or of White race than patients without improvement in 6MWT distance. Groups also had a similar frequency/distribution of clinical and laboratory covariates, including diabetes, AF, renal function, BMI, KCCQ-overall summary score, loop diuretic dose, the severity of symptoms of heart failure, and natriuretic peptide levels. While baseline 6MWT distance was nominally less for patients observed to experience improvement at 12 weeks, those with clinically meaningful walking improvement averaged a 57.7±45.7 m increase in 6MWT distance. An average deterioration of −31.5±48.2 m was observed among patients without improvement.
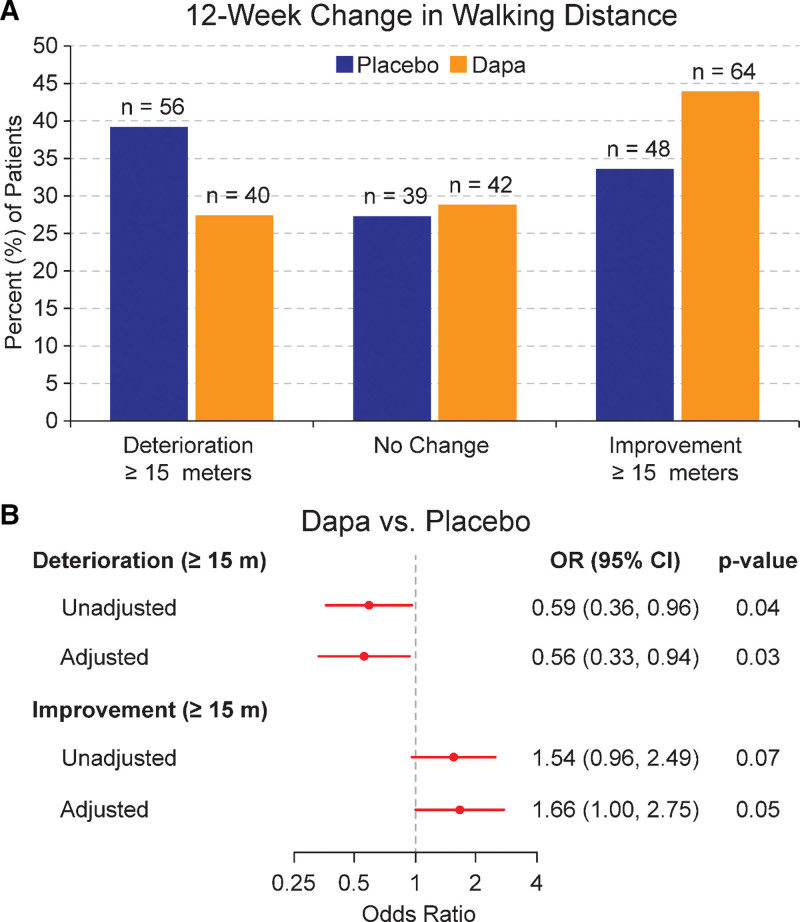
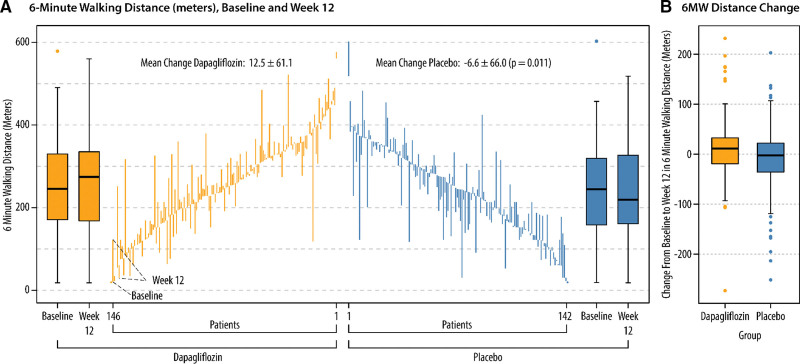
More patients treated with dapagliflozin (n=64; 44%) meaningfully improved walking distance compared with those on placebo (n=48; 34%). We also observed that more patients randomized to placebo (n=56; 35%) experienced walking distance deterioration (a reduction of ≥15 m) compared with those treated with dapagliflozin (n=40; 25%; Figure 1A). A similar frequency of patients experienced no change in 6MWT distance across the 2 treatment groups. After adjusting for baseline measurements including age, sex, race, history of diabetes, BMI, LVEF, history, and type of AF, KCCQ, eGFR, loop diuretic dose, NYHA functional class, NT-proBNP, and baseline 6MWT distance (Figure 1B), patients randomized to dapagliflozin were more likely to experience a clinically meaningful improvement in 6MWT distance at 12 weeks compared with those on placebo (adjusted odds ratio [OR], 1.66 [95% CI, 1.0–2.75]; P=0.05). Dapagliflozin-treated patients were also less likely than placebo-treated patients to experience a 15 m reduction in 6MWT distance (adjusted OR, 0.56 [95% CI, 0.33–0.94]; P=0.03). Patients receiving dapagliflozin were also more likely to experience a 20 m or greater improvement in 6MWT distance compared with those treated with placebo (adjusted OR, 1.83 [95% CI, 1.09–3.08]; Figure S1). Dapagliflozin-treated patients were also less likely (albeit not statistically significant) than placebo-treated patients to experience a 20 m reduction in 6MWT distance (adjusted OR, 0.60 [95% CI, 0.35–1.03]; P=0.06). Figure 2 visually demonstrates the raw data for baseline, change over 12 weeks, and final 6MWT distance measured at 12 weeks for each patient, stratified by treatment group.
Figure 1.
Responder analysis of effects of dapagliflozin (Dapa) on 6-minute walk test (6MWT) distance. A, Data are represented as proportion of patients experiencing 6MWT distance deterioration, no change, or improvement per treatment group. B, Likelihood of improvement ≥15 m and deterioration ≥15 m on Dapa (vs placebo) after adjusting for baseline measurements including age, sex, race, history of diabetes, body mass index (BMI), left ventricular ejection fraction history, and type of atrial fibrillation (AF), Kansas City Cardiomyopathy Questionnaire (KCCQ), estimated glomerular filtration rate (eGFR), loop diuretic dose, New York Heart Association (NYHA) functional class, NT-proBNP (N-terminal pro-B-type natriuretic peptide), and baseline 6MWT distance.
Figure 2.
Baseline values and changes in 6-minute walk test (6MWT) distance. Baseline 6MWT distance values and week 12 peak 6MWT distance values are shown for individual patients receiving dapagliflozin (gold) vs placebo (blue) in A. Changes in 6MWT distance (medians and interquartile ranges [IQRs]) in meters from baseline to week 12 are shown in B. Box edges indicate the IQRs. Horizontal lines between the edges indicate the medians. Whiskers extend to the upper and lower adjacent values, and dots represent outside values.
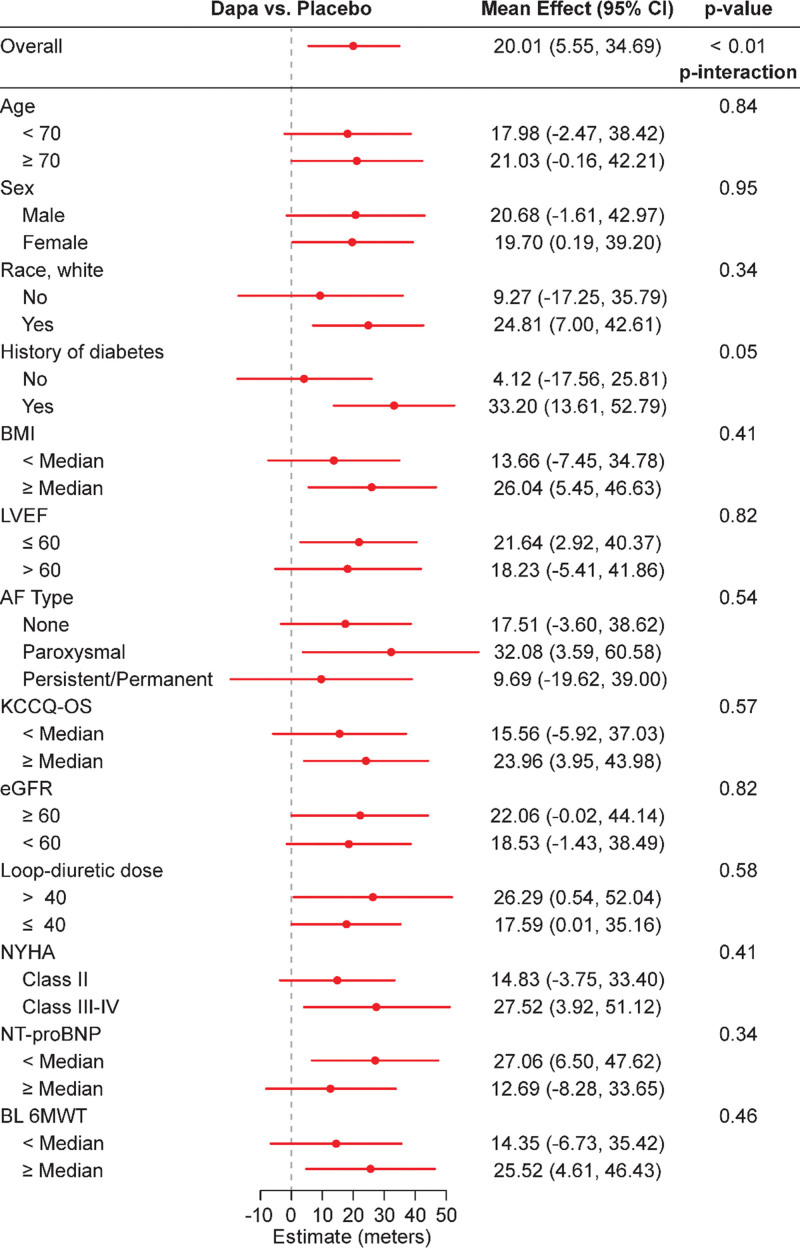
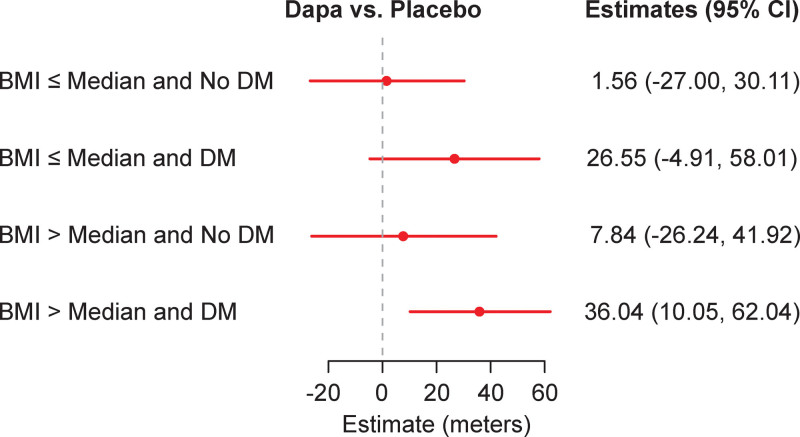
Figure 3 demonstrates the estimated treatment effect of dapagliflozin compared with placebo, stratified by prespecified subgroups. Improvements in 6MWT distance tended to be numerically larger among patients with diabetes, higher BMI, and more advanced symptoms (NYHA class III–IV) who received dapagliflozin compared with those who received placebo. The potential relationship in response to dapagliflozin between patients with diabetes and elevated BMI (greater than the median) was further explored by adding a 3-way interaction into the model. (Figure 4). Patients treated with dapagliflozin (compared with those treated with placebo) who had a combination of both diabetes and above-median BMI improved 6MWT distance by an average of 36 m (95% CI, 10–62 m). In contrast, patients without diabetes and below-median BMI experienced no change in 6MWT distance with dapagliflozin versus placebo (1.6 m [95% CI, −27 to 30 m]). However, the 3-way interaction between dapagliflozin, history of diabetes, and BMI (< median, ≥ median) was not significant (P=0.92 for 3-way interaction).
Figure 3.
Effect of dapagliflozin on 6-minute walk distance stratified by subgroups. Units for loop diuretic dose, mg furosemide equivalents. Data are presented as mean values with 95% CI. All P values are for interactions and are 2-sided with no adjustments made for multiple comparisons. AF indicates atrial fibrillation; BL 6MWT, baseline 6-minute walk test; BMI, body mass index; Dapa, dapagliflozin; eGFR, estimated glomerular filtration rate; KCCQ-OS, Kansas City Cardiomyopathy Questionnaire overall summary score; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; and NYHA, New York Heart Association.
Figure 4.
Treatment interaction estimates with diabetes and body mass index (BMI) as determinants. A similarly adjusted model for 12-week 6-minute walk test (6MWT) distance, which included a 3-way interaction between dapagliflozin (Dapa) treatment group, history of diabetes (DM), and BMI (< median and ≥ median) was tested (P=0.92 for 3-way interaction).
DISCUSSION
In the PRESERVED-HF multicenter, double-blind, randomized, placebo-controlled clinical trial of patients with HFpEF, those treated with dapagliflozin experienced a statistically significant and clinically meaningful improvement in 6MWT distance when compared with patients receiving placebo. This prespecified secondary end point analysis showed that patients treated with dapagliflozin had 66% higher odds of experiencing a 15 m or more 12-week improvement in 6MWT distance than patients on placebo. Responders (patients experiencing a 15 m or greater improvement) averaged ≈60 m increase in 6MWT distance. Our supplemental responder analysis also confirmed a consistent and significant 6MWT distance improvement of 20 m or greater for patients treated with dapagliflozin. Furthermore, compared with those treated with placebo, patients treated with dapagliflozin had 40% lower odds of experiencing a 15 m or greater deterioration in 6MWT distance at 12-week follow-up. These observations were consistent across all subgroups, although patients with diabetes and above-median BMI appeared to have a numerically larger increase in 6MWT distance in response to dapagliflozin.
We note several relevant clinical implications of our findings. Exercise limitations associated with HFpEF have proven largely to be refractory to pharmacotherapies. Our responder analysis in the PRESERVED trial confirms that meaningful walking distance improvements result from the treatment of patients with HFpEF with dapagliflozin over 12 weeks. Finally, we observed the benefits of dapagliflozin to be consistent across all examined patient subgroups with minimal heterogeneity; such clinical implications should provide additional rationale for SGLT2 inhibitor use in clinical practice for patients with HFpEF or heart failure with mildly reduced ejection fraction.
Although patients living with HF have indicated through surveys that they value improved physical function at least as much as avoiding death,14 numerous pharmacotherapy trials in HFpEF targeting improvement in 6MWT distance or cardiopulmonary exercise testing parameters have been neutral or showed worsening of exercise limitation.10 A 15 m or greater increase in 6MWT distance was determined to represent the threshold for clinical significance, because it reflects a >6% increase in exercise capacity, a value determined to independently predict improved clinical events in other HF populations.12 Notably, the 20 m overall increase in 6MWT distance observed at 12 weeks in PRESERVED-HF with dapagliflozin versus placebo, and the nearly 60 m increase among patients with meaningful improvement (>15 m at 12 weeks) is similar to the robust improvements in fitness observed in patients undergoing supervised exercise training interventions for heart failure.15,16 Importantly, 6MWT distance is more strongly tied to health status in patients with HFpEF than other surrogate markers of disease severity, potentially providing greater insight into the origins of the improvement in KCCQ scores in PRESERVED-HF.17 The potential signal for a greater increase in 6MWT distance among patients with diabetes and higher BMI in this trial is intriguing, considering the results of the DELIVER trial, which showed that patients with higher BMI were more likely to experience a symptomatic improvement with dapagliflozin versus placebo compared with patients with BMI <30 kg/m2.11 In addition, within PRESERVED-HF, the average BMI was 36 kg/m2, and >75% of participants had BMI >30 kg/m2, distinguishing this US-based HFpEF population from study populations in other international HF studies with SGLT2 inhibitors in which BMI levels were markedly lower.
Our results differ from those of a previous study of comparable size and duration of treatment. The EMPERIAL-Preserved (Effect of Empagliflozin on Exercise ability and HF symptoms in Patients with Chronic Heart Failure) trial assessed the effects of empagliflozin on the exercise limitation of patients with HFpEF and reported a modest, nonsignificant 4 m improvement in 6MWT distance with empagliflozin versus placebo.18 Compared with those in EMPERIAL-Preserved trial, PRESERVED-HF trial enrolled a US population with a greater proportion of women (57% versus 43%) and Black patients (30% versus 10%) with a significantly higher BMI (36.0 versus 29.6 kg/m2). Patients in PRESERVED-HF trial were also significantly more exercise limited, as measured by NYHA functional class and baseline 6MWT distance. With a 249 m mean baseline 6MWT distance, 42% of PRESERVED-HF patients reported exercise limitations consistent with NYHA Class III/IV (compared with a 299 m median baseline 6MWT distance and 22% functional class III observed at baseline in EMPERIAL-Preserved). Furthermore, patients enrolled in PRESERVED-HF trial were required to have elevated natriuretic peptides, and the use of diuretics was higher compared with EMPERIAL-Preserved.
The DETERMINE-Preserved (Dapagliflozin Effect on Exercise Capacity Using a 6-Minute Walk Test in Patients With Heart Failure With Preserved Ejection Fraction) trial randomized patients with HFpEF to dapagliflozin versus placebo and evaluated changes in KCCQ scores and 6MWT distance as coprimary outcomes with a 16-week follow-up. The results are unpublished, but public reporting on clinicaltrials.gov indicates no significant improvement in 6MWT distance or KCCQ. Limited demographic and baseline health status information is available to compare the study populations of DETERMINE-preserved with PRESERVED-HF adequately, but based on geographic considerations, patients were more likely to resemble the EMPERIAL-Preserved study population. In lower LVEF trials, nonuniform effects on exercise limitation and quality of life have also been observed.18–20 While 6MWT provides a real-life assessment of physical function that is directly relevant to daily activities and is generalizable and practical in a clinical trial setting, other fitness measures such as peak VO2 have lower variance than 6MWT distance. It is possible that, if measured, these measures also might have improved with dapagliflozin in the PRESERVED trial.21
Historically there has been a lack of concordance between pharmacotherapies that improve the clinically meaningful outcome of objectively measured exercise limitation in HF and those that improve longevity or hospitalization rates. For example, in HF with reduced ejection fraction, among established therapies such as beta-blockers, ACE inhibitors, angiotensin receptor neprilysin inhibitors, and spironolactone, none consistently impact exercise limitation. Conversely, some positive inotropes address exercise limitation but hasten mortality in HF with reduced ejection fraction.22,23 Hence, despite mounting evidence of SGLT2 inhibitor improving the outcome of cardiovascular death or HF hospitalizations in HFpEF, one cannot extrapolate such findings to ameliorating exercise limitation.
Modeling studies have suggested that improvement in exercise limitation in HFpEF is incomplete even with robust correction of cardiac output deficits (as measured by peak VO2) due to abnormalities in other components of the O2 pathway.24 Interventions must have large effect sizes on cardiac performance or have salutary effects on multiple components of the O2 pathway (such as exercise training) to improve exercise limitation. Hence, findings to date that pharmacotherapies trialed in HFpEF have largely failed to improve exercise limitation are not entirely surprising. While we can only speculate on how dapagliflozin improved 6MWT distance in PRESERVED-HF, the positive findings suggest that dapagliflozin may have improved multiple components of the O2 pathway in PRESERVED-HF 6MWT responders. SGLT2 inhibitor may improve exercise through numerous mechanisms, including increases in hemoglobin levels (via decongestion, erythropoiesis stimulation, and hepcidin reduction), increased ketone body oxidation in cardiac and skeletal muscle, lower oxidative stress and inflammation, reduced epicardial fat levels,25 improved microvascular circulation, cardiovascular hemodynamics, and improved myocardial and skeletal muscle energy metabolism.26,27 As measured by ambulatory pulmonary artery diastolic pressure monitoring, decongestion has been associated with improved symptom relief (as measured by KCCQ total symptom score) with a sustained effect of at least 1 week beyond discontinuation of an SGLT2 inhibitor.28 However, our subgroup analysis investigating potential markers of increased congestion at baseline (higher loop diuretic dose, NYHA class III–IV symptoms, or increased NT-proBNP) did not identify any interactions suggesting an increased likelihood to respond more favorably in 6MWT distance to dapagliflozin (Figure 3). Ultimately SGLT2 agents may have succeeded, where others have been unable to improve both 6MWT distance and clinical events in HFpEF, because they have broad, pleiotropic properties that may address the systemic impairments that seem to promote HFpEF and its adverse outcomes.29
The present study has multiple strengths, most notably its focus on the effects of dapagliflozin on 6MWT distance in an appropriately powered randomized clinical trial, the inclusion of many patients with elevated BMI (who are often excluded from HFpEF trials), and the large proportions of women and Black individuals enrolled in the trial. Important limitations include the potential for confounding associated with 6MWT (related to test administration variability by site), although this would be expected to be equally distributed between dapagliflozin- and placebo-treated patients by virtue of PRESERVED-HF being a randomized, double-blind trial. Other limitations include study duration, which limited our ability to examine the long-term impact of dapagliflozin on exertional capacity; the inclusion of only US-based patients in our study (thus limiting the generalizability of our results to other geographic regions); the reduced power for subgroup analyses and possible overestimation of risk when using ORs; and the lack of cardiopulmonary exercise testing, which could have provided greater mechanistic insights into our findings. Patients with missing 6MWT data represent 10.8% of the patient population and tended to be older (average +2.5 years) with worse kidney function and higher symptom burden. Based on the relative homogeneity of responsiveness to dapagliflozin (including across strata of age, kidney function, and NYHA class) and the fact that a similar proportion of patients randomized to dapagliflozin (n=16) and placebo (n=19) had missing 6MWT data, it is unlikely that this had a meaningful impact on our results.
In conclusion, in a secondary analysis of PRESERVED-HF, dapagliflozin, as compared with placebo, improved 6MWT distance among patients with HFpEF, and this treatment benefit was consistent across all prespecified subgroups. In addition, a greater proportion of dapagliflozin-treated patients experienced a clinically meaningful improvement, and fewer dapagliflozin-treated patients had a deterioration in 6MWT during 12 weeks of follow-up. Collectively, these findings provide further support for dapagliflozin use in patients with HFpEF.
ARTICLE INFORMATION
Sources of Funding
PRESERVED-HF (Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients With Preserved Ejection Fraction Heart Failure) was an investigator-initiated trial funded by AstraZeneca Pharmaceuticals LP (Wilmington, DE) and conducted by Saint Luke’s Mid America Heart Institute (Kansas City, MO), independent of the funding source.
Disclosures
Dr Lewis reports research funding from the National Institutes of Health (NIH) R01-HL 151841, R01-HL131029, R01-HL159514, U01-HL160278, Amgen, Cytokinetics, Applied Therapeutics, AstraZeneca, SoniVie, NXT, and Rivus. He has received honoraria for advisory boards outside of the current study from Pfizer, Merck, Boehringer-Ingelheim, NXT, American Regent, Cyclerion, Cytokinetics, Amgen, RIVUS, and NXT and receives royalties from UpToDate for scientific content authorship related to exercise physiology. Dr Nassif is a consultant to Vifor and has received research support from Cytokinetics. Dr Borlaug receives research support from the NIH (R01 HL128526, R01 HL162828, and U01 HL160226) and the US Department of Defense (W81XWH2210245), and research grant funding from AstraZeneca, Axon, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, and Tenax Therapeutics. Dr Borlaug has served as a consultant for Actelion, Amgen, Aria, Axon Therapies, BD, Boehringer-Ingelheim, Cytokinetics, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, NGM, NXT, and VADovations and is named inventor (US Patent no. 10,307,179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure. Dr Kitzman has received honoraria as a consultant for Boehringer-Ingelheim, Novo Nordisk, AstraZeneca, Corvia, Rivus, Keyto, Saint Luke’s Medical Center, and Novartis and grant funding from Novartis, Bayer, Novo Nordisk, Rivus, Saint Luke’s Medical Center, and AstraZeneca and stock ownership in Gilead Sciences. Dr Shah is supported by research grants from the NIH (U54 HL160273, R01 HL140731, and R01 HL149423), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer-Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, GSK, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Sardocor, Shifamed, Tenax, Tenaya, and United Therapeutics. Dr Umpierrez is partly supported by research grants from the NIH/National Center for Advancing Translational Sciences (UL 3UL1TR002378-05S2) from the Clinical and Translational Science Award program, and from the NIH and National Center for Research Resources (NIH/National Institute of Diabetes and Digestive and Kidney Diseases 2P30DK111024-06). He has also received research support (to Emory University) from Dexcom, Bayer, Abbott, and AstraZeneca. Dr Sharma is an advisory board member and consultant for Alleviant, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Cytokinetics, Janssen, Novartis, Novo Nordisk, and RIVUS and receives honoraria. Dr Khan receives funding from the American Heart Association (19TPA34890060) and NIH (1R01HL159250). Dr Kosiborod receives grant/research support from AstraZeneca, Boehringer-Ingelheim, and Pfizer; honoraria from AstraZeneca, Boehringer-Ingelheim, and Novo Nordisk; is a consultant for 35Pharma, Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer-Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Imbria Pharmaceuticals, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Pfizer, Sanofi, scPharmaceuticals, Structure Therapeutics, Vifor Pharma, and Youngene Therapeutics; and has stock options from Artera Health and Saghmos Therapeutics. Dr Sauer is a consultant for Bayer, Abbott, Acorai, Biotronik, Boston Scientific, Edwards Life Sciences, Impulse Dynamics, Medtronics, Story Health, and Vifor Pharma and owns stock in ISHI. The other authors report no disclosures.
Supplemental Material
Table S1
Figure S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 6MWT
- 6-minute walking test
- AF
- atrial fibrillation
- BMI
- body mass index
- BNP
- B-type natriuretic peptide
- eGFR
- estimated glomerular filtration rate
- EMPERIAL-Preserved
- Effect of Empagliflozin on Exercise ability and HF symptoms in Patients with Chronic Heart Failure
- HF
- heart failure
- HFpEF
- heart failure with preserved ejection fraction
- HFrEF
- HF with reduced ejection fraction
- KCCQ
- Kansas City Cardiomyopathy Questionnaire
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- NYHA
- New York Heart Association
- OR
- odds ratio
- PRESERVED-HF
- Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients With Preserved Ejection Fraction Heart Failure
- SGLT2
- sodium-glucose cotransporter-2
M. Kosiborod and A. Sauer are co-senior authors and contributed equally.
For Sources of Funding and Disclosures, see page 951.
This article was sent to Dr Ryan J. Tedford, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.123.010633.
Contributor Information
Gregory D. Lewis, Email: glewis@partners.org.
Kensey Gosch, Email: kgosch@saint-lukes.org.
Laura P. Cohen, Email: LCOHEN0@partners.org.
Michael E. Nassif, Email: mnassif@saint-lukes.org.
Sheryl L. Windsor, Email: swindsor@saint-lukes.org.
Barry A. Borlaug, Email: borlaug.barry@mayo.edu.
Dalane W. Kitzman, Email: dkitzman@wakehealth.edu.
Sanjiv J. Shah, Email: Sanjiv.shah@northwestern.edu.
Taiyeb Khumri, Email: tkhumri@saint-lukes.org.
Guillermo Umpierrez, Email: geumpie@emory.edu.
Sumant Lamba, Email: slamba@firstcoastcardio.com.
Kavita Sharma, Email: ksharma8@jhmi.edu.
Sadiya S. Khan, Email: s-khan-1@northwestern.edu.
Andrew J. Sauer, Email: asauer@saint-lukes.org.
REFERENCES
- 1.Gevaert AB, Kataria R, Zannad F, Sauer AJ, Damman K, Sharma K, Shah SJ, Van Spall HGC. Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management. Heart. 2022;108:1342–1350. doi: 10.1136/heartjnl-2021-319605 [DOI] [PubMed] [Google Scholar]
- 2.Nayor M, Houstis NE, Namasivayam M, Rouvina J, Hardin C, Shah RV, Ho JE, Malhotra R, Lewis GD. Impaired exercise tolerance in heart failure with preserved ejection fraction: quantification of multiorgan system reserve capacity. JACC Heart Fail. 2020;8:605–617. doi: 10.1016/j.jchf.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verbrugge FH, Omote K, Reddy YNV, Sorimachi H, Obokata M, Borlaug BA. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur Heart J. 2022;43:1941–1951. doi: 10.1093/eurheartj/ehab911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–1960. doi: 10.1038/s41591-021-01536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabler NB, French B, Strom BL, Palevsky HI, Taichman DB, Kawut SM, Halpern SD. Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation. 2012;126:349–356. doi: 10.1161/CIRCULATIONAHA.112.105890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wessler BS, Kramer DG, Kelly JL, Trikalinos TA, Kent DM, Konstam MA, Udelson JE. Drug and device effects on peak oxygen consumption, 6-minute walk distance, and natriuretic peptides as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction. Circ Heart Fail. 2011;4:578–588. doi: 10.1161/CIRCHEARTFAILURE.111.961573 [DOI] [PubMed] [Google Scholar]
- 7.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, et al. ; RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Udelson JE, Lewis GD, Shah SJ, Zile MR, Redfield MM, Burnett J, Jr, Parker J, Seferovic JP, Wilson P, Mittleman RS, et al. Effect of praliciguat on peak rate of oxygen consumption in patients with heart failure with preserved ejection fraction: the CAPACITY HFpEF randomized clinical trial. JAMA. 2020;324:1522–1531. doi: 10.1001/jama.2020.16641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah SJ, Voors AA, McMurray JJV, Kitzman DW, Viethen T, Bomfim Wirtz A, Huang E, Pap AF, Solomon SD. Effect of neladenoson bialanate on exercise capacity among patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2019;321:2101–2112. doi: 10.1001/jama.2019.6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey A, Shah SJ, Butler J, Kellogg DL, Jr, Lewis GD, Forman DE, Mentz RJ, Borlaug BA, Simon MA, Chirinos JA, et al. Exercise intolerance in older adults with heart failure with preserved ejection fraction: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:1166–1187. doi: 10.1016/j.jacc.2021.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. ; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 12.Swank AM, Horton J, Fleg JL, Fonarow GC, Keteyian S, Goldberg L, Wolfel G, Handberg EM, Bensimhon D, Illiou MC, et al. ; HF-ACTION Investigators. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail. 2012;5:579–585. doi: 10.1161/CIRCHEARTFAILURE.111.965186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saberi S, Wheeler M, Bragg-Gresham J, Hornsby W, Agarwal PP, Attili A, Concannon M, Dries AM, Shmargad Y, Salisbury H, et al. Effect of moderate-intensity exercise training on peak oxygen consumption in patients with hypertrophic cardiomyopathy: a randomized clinical trial. JAMA. 2017;317:1349–1357. doi: 10.1001/jama.2017.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman DE, Arena R, Boxer R, Dolansky MA, Eng JJ, Fleg JL, Haykowsky M, Jahangir A, Kaminsky LA, Kitzman DW, et al. ; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135:e894–e918. doi: 10.1161/CIR.0000000000000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, et al. ; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy YNV, Rikhi A, Obokata M, Shah SJ, Lewis GD, AbouEzzedine OF, Dunlay S, McNulty S, Chakraborty H, Stevenson LW, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. 2020;22:1009–1018. doi: 10.1002/ejhf.1788 [DOI] [PubMed] [Google Scholar]
- 18.Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, Filippatos G, Gniot J, Fu M, Gullestad L, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42:700–710. doi: 10.1093/eurheartj/ehaa943 [DOI] [PubMed] [Google Scholar]
- 19.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel-Perez F, et al. ; EMPA-TROPISM (ATRU-4) Investigators. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77:243–255. doi: 10.1016/j.jacc.2020.11.008 [DOI] [PubMed] [Google Scholar]
- 20.Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. 2021;143:516–525. doi: 10.1161/CIRCULATIONAHA.120.052186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannitsi S, Bougiakli M, Bechlioulis A, Kotsia A, Michalis LK, Naka KK. 6-minute walking test: a useful tool in the management of heart failure patients. Ther Adv Cardiovasc Dis. 2019;13:1753944719870084. doi: 10.1177/1753944719870084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sami F, Acharya P, Noonan G, Maurides S, Al-Masry AA, Bajwa S, Parimi N, Boda I, Tran C, Goyal A, et al. Palliative inotropes in advanced heart failure: comparing outcomes between milrinone and dobutamine. J Card Fail. 2022;28:1683–1691. doi: 10.1016/j.cardfail.2022.08.007 [DOI] [PubMed] [Google Scholar]
- 23.Lewis GD, Docherty KF, Voors AA, Cohen-Solal A, Metra M, Whellan DJ, Ezekowitz JA, Ponikowski P, Böhm M, Teerlink JR, et al. Developments in exercise capacity assessment in heart failure clinical trials and the rationale for the design of METEORIC-HF. Circ Heart Fail. 2022;15:e008970. doi: 10.1161/CIRCHEARTFAILURE.121.008970 [DOI] [PubMed] [Google Scholar]
- 24.Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD, Lewis GD. Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized O(2) pathway analysis. Circulation. 2018;137:148–161. doi: 10.1161/CIRCULATIONAHA.117.029058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masson W, Lavalle-Cobo A, Nogueira JP. Effect of SGLT2-inhibitors on epicardial adipose tissue: a meta-analysis. Cells. 2021;10:2150–2160. doi: 10.3390/cells10082150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salah HM, Verma S, Santos-Gallego CG, Bhatt AS, Vaduganathan M, Khan MS, Lopes RD, Al’Aref SJ, McGuire DK, Fudim M. Sodium-glucose cotransporter 2 inhibitors and cardiac remodeling. J Cardiovasc Transl Res. 2022;15:944–956. doi: 10.1007/s12265-022-10220-5 [DOI] [PubMed] [Google Scholar]
- 27.Selvaraj S, Fu Z, Jones P, Kwee LC, Windsor SL, Ilkayeva O, Newgard CB, Margulies KB, Husain M, Inzucchi SE, et al. ; DEFINE-HF Investigators. Metabolomic profiling of the effects of dapagliflozin in heart failure with reduced ejection fraction: DEFINE-HF. Circulation. 2022;146:808–818. doi: 10.1161/CIRCULATIONAHA.122.060402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nassif ME, Spertus JA, Tang F, Windsor SL, Jones P, Thomas M, Khariton Y, Brush J, Gordon RA, Jermyn R, et al. Association between change in ambulatory hemodynamic pressures and symptoms of heart failure. Circ Heart Fail. 2021;14:e008446. doi: 10.1161/CIRCHEARTFAILURE.121.008446 [DOI] [PubMed] [Google Scholar]
- 29.Packer M, Kitzman DW. Obesity-related heart failure with a preserved ejection fraction: the mechanistic rationale for combining inhibitors of aldosterone, neprilysin, and sodium-glucose cotransporter-2. JACC Heart Fail. 2018;6:633–639. doi: 10.1016/j.jchf.2018.01.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.