Abstract
Background & Aims:
Acetaminophen (APAP)-induced acute liver failure (ALF) is a rare disease associated with high mortality. This study aimed to evaluate changes in interventions, psychosocial profile and clinical outcomes over a 21-year period using data from the ALF Study Group registry.
Methods:
A retrospective review of this prospective, multicenter cohort study of all APAP-ALF patients enrolled during the study period (1998–2018) was completed. Primary outcomes evaluated were 21-day transplant-free survival (TFS) and neurological complications. Covariates evaluated included enrollment cohort (early: 1998–2007; recent: 2008–2018), intentionality, psychiatric comorbidity, and use of organ support including continuous renal replacement therapy (CRRT).
Results:
Of 1190 APAP-ALF patients, recent cohort patients (n=608) had significantly improved TFS (recent: 69.8% vs. early: 61.7%; p=0.005). Recent cohort patients were more likely to receive CRRT (22.2% vs. 7.6%; p<0.001), and less likely to develop intracranial hypertension (ICH; 29.9% vs. 51.5%; p<0.001) or die by day 21 due to cerebral edema (CE; 4.5% vs. 11.6%; p<0.001). Grouped by TFS status (non-TFS: n=365 vs. TFS: n=704), there were no differences in psychiatric comorbidity (51.5% vs. 55.0%; p=0.28) or intentionality (intentional: 39.7% vs. 41.6%; p=0.58). On multivariable logistic regression adjusting for vasopressor support, development of grade 3/4 hepatic encephalopathy, King’s College Criteria, and MELD score, the use of CRRT (OR 1.62; p=0.023) was associated with significantly increased TFS (c-statistic 0.86). In a second model adjusting for the same covariates, recent enrollment was significantly associated with TFS (OR 1.42; p=0.034; c-statistic 0.86).
Conclusions:
TFS in APAP-ALF has improved in recent years and rates of ICH/CE have declined, possibly related to increased CRRT use.
Keywords: cerebral edema, continuous renal replacement therapy, intracranial hypertension, transplantation, transplant-free survival
INTRODUCTION
Acetaminophen (APAP) is the most common cause of acute liver failure (ALF) in Europe and North America.1–3 Injury and recovery follow a hyper-acute pattern, in which maximum hepatocyte destruction is complete by 72 hours following ingestion, often necessitating intensive care unit (ICU) admission.4, 5 Resulting intracranial hypertension (ICH) and multisystem organ failure are associated with substantial morbidity and mortality, with cerebral edema (CE) responsible for up to 25% of ALF deaths.6, 7 Management is largely supportive and aims to control or prevent CE, correct metabolic derangements, and maintain hemodynamic stability.3, 8 For those failing maximal medical therapy, liver transplantation (LT) may be required; however, severity of critical illness and presence of concomitant psychosocial factors may complicate listing decisions for LT.9–11
Outcomes over time have improved overall for ALF patients;1, 9 however, contributing factors for this warrant further exploration. N-acetylcysteine (NAC) administration is accepted to minimize APAP-related hepatotoxicity and may also improve outcomes in non-APAP ALF.12–14 More recently, continuous renal replacement therapy (CRRT) has been demonstrated to improve 21-day transplant-free survival (TFS) in all-etiology ALF.15 What is not clear is whether changes in psychological profile (intentional overdose vs. therapeutic misadventure, psychiatric comorbidity) have changed with time or impacted APAP-ALF outcomes.1, 16–20 Recent, multicenter epidemiologic studies evaluating changes in interventions, psychosocial profile, and clinical outcomes in the context of APAP-ALF are lacking.1–3
Analyzing prospectively collected APAP-ALF patient data from the multicenter United States Acute Liver Failure Study Group (US ALFSG) registry between 1998 and 2018, we evaluated clinical parameters, intensive care interventions, rates of LT, and TFS stratifying the patient cohort in two eras; (1998–2007 ~ early; 2008–2018 ~ recent). Our primary objectives were to test the following hypotheses in APAP-ALF:
TFS is significantly higher in the recent cohort compared to the early cohort.
ICH development and CE-related deaths are significantly lower in the recent cohort.
The impact of psychosocial profile on important clinical outcomes has not changed with time and may not factor into clinical decision-making or outcomes.
PATIENTS AND METHODS
Study Design
We performed a retrospective cohort study of all APAP-ALF patients prospectively enrolled in the US ALFSG registry between January 1998 and December 2018 (n=1190). The study’s protocol was approved by all respective institutional review boards/health research ethics boards at participating sites (tertiary liver transplantation referral centers) within the US ALFSG. Written informed consent was obtained from each participant/next of kin (in cases of hepatic encephalopathy (HE) at time of enrollment). All research procedures were conducted according to the principles of the 1975 Declaration of Helsinki. Therapeutic interventions and monitoring were implemented according to participating institutional standards of care. Criteria for listing and performing liver transplantation were those utilized at participating centers.
Participants
Inclusion criteria were as follows: (1) evidence of ALF according to the enrollment criteria of the US ALFSG (see operational definitions), (2) participant age ≥18 years, and (3) primary diagnosis of APAP-ALF as determined by the site investigator and further adjudicated by an external review committee. Exclusion criteria were as follows: (1) evidence of cirrhosis/acute-on-chronic liver failure and (2) non-APAP ALF etiology. No patients with severe acute liver injury were enrolled in this cohort study.21
Operational Definitions
For the purpose of this study, ALF was defined using the following criteria: (1) international normalized ratio (INR) ≥1.5, (2) HE of any grade (West Haven Criteria), (3) illness onset less than 26 weeks from hepatic insult, and (4) absence of existing cirrhosis. The King’s College Criteria (KCC) qualify poor prognostic signs in ALF. In APAP-ALF, KCC is defined as either (1) arterial pH <7.3, or (2) all three of i) INR >6.5, ii) creatinine >300 μmol/L (3.4 mg/dL), and iii) the presence of grade 3/4 HE. The Acute Liver Failure Study Group Prognostic Index (ALFSG-PI) is an internally-validated mathematical model that predicts 21-day TFS of patients with ALF using hospital admission data and has been previously described.22 The model for end stage liver disease (MELD) is calculated as follows: [3.78*ln(bilirubin in mg/dL) + 11.2*ln(INR) + 9.57*ln(creatinine in mg/dL) + 6.43]; a serum creatinine value of 354 μmol/L (4 mg/dL) is substituted for dialyzed patients. RRT included both intermittent hemodialysis (IHD) and continuous hemofiltration (CRRT). Patients receiving CRRT and IHD during days 1–7 were coded accordingly. The use of RRT within the US ALFSG is not standardized; thus, modality, replacement fluid, anticoagulation, dose, and indications for initiation and cessation of therapy were based on intensivist judgement at the enrolling center. Development of ICH was defined as any recorded intracranial pressure (ICP) measurement ≥25 mmHg, computed tomography/magnetic resonance imaging findings consistent with CE, and/or neurologic cause of death within 21 days of enrollment. Overdose intent was classified based on patient self-reporting and chart review: intentional overdose was considered an excessive APAP ingestion in a patient indicating suicidal intent (either single timepoint or multi-timepoint); unintentional overdose was considered an ingestion of excessive APAP quantities to relieve somatic symptoms with an absence of suicidal intent. Intentionality classified as “unknown” was excluded from analysis1, 19.
Clinical Variables and Endpoints
The US ALFSG registry (data coordinating center at Medical University of South Carolina, Department of Public Health Sciences, Charleston, South Carolina) contains prospectively collected demographic, clinical (days 1–7), biochemical (days 1–7), and outcome data. Data assessed in this study included baseline patient characteristics (age, sex, overdose intent, psychiatric comorbidities), requirement of organ support (mechanical ventilation, vasopressors, RRT), early and late biochemistry profile (complete blood count, INR, transaminases, bilirubin, pH, ammonia, creatinine, lactate, phosphate), HE grade, NAC use, and clinical outcomes (LT listing, receipt of LT, 21-day TFS, and overall 21-day survival). The primary endpoint for this study was 21-day TFS. Participants were stratified into two enrollment time cohorts as follows: 1998–2007 (“early” cohort) and 2008–2018 (“recent” cohort).
Statistical Analysis
Categorical variables were presented as proportions and compared using the Chi-squared test. Continuous variables were presented as medians with interquartile range (IQR) following assessment for normality using skewness (±0.5) and kurtosis (±2) and subsequently compared using the Mann-Whitney U test (all continuous variables were non-normally distributed). The study of associations with 21-day TFS was completed using logistic regression. Clinically relevant covariates or those yielding p<0.10 on univariate analysis were initially chosen for multivariable analysis including sex, age, HE grade, use of vasopressors, use of RRT (CRRT versus IHD/no RRT), KCC classification, MELD, overdose intent, psychiatric history, and enrollment time cohort. Final models were derived using a backward elimination process with a p-value threshold of 0.05. Multicollinearity was assessed using variance inflation factors. A variance inflation factor value greater than 5 was considered high multicollinearity and avoided, where appropriate. Model performance was assessed using area under the receiver operating curve (AUROC). All analyses were two‐tailed. We used a threshold for statistical significance of 0.05. Statistical analysis was performed using Stata (version 15.1; StataCorp, College Station, Texas), SAS (version 9.4; SAS Institute, Cary, North Carolina), and R (version 0.99.879; RStudio, Boston, Massachusetts).
RESULTS
Baseline APAP-ALF Cohort Parameters
A total of 1190 patients with ALF secondary to APAP toxicity were identified within the US ALFSG data registry between January 1998 and December 2018. Median (IQR) age was 37 (28–47) years and 895 (75.2%) patients were female. During the first seven days of inpatient study, 733 (63.3%) patients developed grade 3/4 HE, and 216 (18.2%) patients met APAP-specific KCC for consideration of LT listing. Mechanical ventilation, vasopressor therapy, and CRRT were required in 735 (61.8%), 394 (33.1%), and 179 (15.0%) patients, respectively. When overdose intention was known (n=1062), 445 patients (41.9%) presented with intentional overdose. Pre-existing psychiatric diagnoses were present in 641 patients (53.9%). Median (IQR) admission ALFSG-PI predicted probability of TFS was 74.1% (49.5%−86.8%). Demographic and clinical outcomes of the APAP-ALF cohort are described in Table 1.
Table 1.
Demographic, clinical and outcome parameters in the APAP-ALF patient cohort.
| Parameter | Overall (N = 1190) | |
|---|---|---|
| N | ||
| Age (years) | 1190 | 37 (28–47) |
| Sex (male) | 1190 | 295 (24.8%) |
| King’s College Criteria met (days 1–7) | 1190 | 216 (18.2%) |
| ALFSG Prognostic Index (admission)* | ||
| Predicted Probability (%) | 1104 | 74.1 (49.5-86.8) |
| Predicted Probability ≥ 80% | 1104 | 430 (38.9%) |
| Highest MELD (median; days 1–7) | 1176 | 27.5 (17.9-34.0) |
| Coma Grade 3/4 (days 1–7) | 1158 | 733 (63.3%) |
| Organ Support (days 1–7) | ||
| Mechanical Ventilation | 1190 | 735 (61.8%) |
| Vasopressors | 1190 | 394 (33.1%) |
| Continuous Renal Replacement Therapy | 1190 | 179 (15.0%) |
| ICP Directed Therapies (days 1–7) | ||
| ICP monitor | 1190 | 144 (12.1%) |
| Mannitol | 1190 | 230 (19.3%) |
| Barbiturate | 1190 | 81 (6.8%) |
| Hypothermia | 1190 | 73 (6.1%) |
| Sedatives | 1190 | 740 (62.2%) |
| Blood Products (days 1–7) | ||
| Red Blood Cells | 1190 | 354 (29.7%) |
| Fresh Frozen Plasma | 1190 | 539 (45.3%) |
| Recombinant Factor VIIA | 1190 | 21 (1.8%) |
| Platelets | 1190 | 224 (18.8%) |
| ICU Complications (days 1–7) | ||
| Seizures | 1190 | 73 (6.1%) |
| Arrhythmia | 1190 | 247 (20.8%) |
| Gastrointestinal Bleeding | 1190 | 101 (8.5%) |
| N-acetylcysteine a | ||
| Intravenous | 1190 | 984 (82.7%) |
| Oral | 1190 | 754 (63.4%) |
| Psychological Comorbidities | 1190 | 641 (53.9%) |
| Depression | 983 | 434 (44.2%) |
| Schizophrenia | 565 | 16 (2.8%) |
| Chronic Pain | 553 | 4 (0.7%) |
| Bipolar Disorder | 664 | 115 (17.3%) |
| Anxiety | 679 | 130 (19.1%) |
| Overdose Intent b | ||
| Intentional | 1062 | 445 (41.9%) |
| Unintentional | 1062 | 617 (58.1%) |
| Intravenous Drug Use | 1178 | 95 (8.1%) |
| Intracranial Hypertension (days 1–21) | 577 | 208 (36.0%) |
| Death (days 1–21) | 1048 | 273 (26.0%) |
| Cerebral Edema Death | 1046 | 83 (7.9%) |
| Listed for Liver Transplantation | 1189 | 273 (23.0%) |
| Received Liver Transplant (days 1–21) | 1186 | 100 (8.4%) |
| Transplant-free Survival (day 21) | 1069 | 704 (65.9%) |
Some subjects received both intravenous and oral N-acetylcysteine
Overdose intent could not be determined (i.e., unknown) in 128 subjects Abbreviations: ALFSG, Acute Liver Failure Study Group; ICP, intracranial pressure;
ICU, intensive care unit; MELD, model for end stage liver disease.
Univariate Analysis of APAP-ALF Patients: Enrollment Time Cohort
Comparisons of enrollment time cohort (recent: 2008–2018 vs. early: 1998–2007) demographic and clinical outcome parameters are shown in Table 2. During the first seven days of inpatient study, there were no significant differences comparing recent and early cohorts in terms of meeting KCC (17.4% vs. 18.9%; p=0.51), having grade 3/4 HE (62.0% vs. 64.5%; p=0.38), and requiring mechanical ventilation (59.9% vs. 63.7%; p=0.17) or vasopressors (30.8% vs. 35.6%; p=0.08). Comparing the admission ALFSG-PI for the recent vs. early cohorts, there were no significant differences in median predicted probability of TFS (72.7% vs. 74.8%; p=0.83) or proportion reaching the optimal survival probability prediction threshold of 80% (40.1% vs. 37.8%; p=0.43). Recent time cohort patients were more likely to receive CRRT (22.2% vs. 7.6%; p<0.001) and were less likely to receive IHD (14.4% vs. 31.0%, p<0.001). Recent time cohort patients demonstrated significantly higher 21-day TFS (69.8% vs. 61.7%; p=0.005), and lower rates of ICH (29.9% vs. 51.5%; p<0.001) and 21-day CE-related death (4.5% vs. 11.6%; p<0.001).
Table 2.
Patient parameters stratified by time cohort (1998–2007 vs. 2008–2018).
| “Early” (1998–2007) (N = 582) | “Recent” (2008–2018) (N = 608) | P-value | |||
|---|---|---|---|---|---|
| N | N | ||||
| Age (years) | 582 | 36 (28–45) | 608 | 37 (28–49) | 0.026 |
| Sex (male) | 582 | 147 (25.3%) | 608 | 148 (24.3%) | 0.72 |
| King’s College Criteria met (days 1–7) | 582 | 110 (18.9%) | 608 | 106 (17.4%) | 0.51 |
| ALFSG Prognostic Index (admission) | |||||
| Survival Predicted Probability ≥ 80% | 566 | 214 (37.8%) | 538 | 216 (40.1%) | 0.43 |
| Highest MELD (days 1–7) | 576 | 29.0 (18.7–35.7) |
600 | 25.8 (16.3–32.5) |
<0.001 |
| Coma Grade 3/4 (days 1–7) | 581 | 375 (64.5%) | 577 | 358 (62.0%) | 0.38 |
| Organ Support (days 1–7) | |||||
| Mechanical Ventilation | 582 | 371 (63.7%) | 608 | 364 (59.9%) | 0.17 |
| Vasopressors | 582 | 207 (35.6%) | 608 | 187 (30.8%) | 0.08 |
| Continuous Renal Replacement Therapy | 582 | 44 (7.6%) | 608 | 135 (22.2%) | <0.001 |
| Intermittent Hemodialysis | 577 | 179 (31.0%) | 604 | 87 (14.4%) | <0.001 |
| ICP Directed Therapies (days 1–7) | |||||
| ICP monitor | 582 | 95 (16.3%) | 608 | 49 (8.1%) | <0.001 |
| Mannitol | 582 | 125 (21.5%) | 608 | 105 (17.3%) | 0.07 |
| Barbiturate | 582 | 59 (10.1%) | 608 | 22 (3.6%) | <0.001 |
| Hypothermia | 582 | 18 (3.1%) | 608 | 55 (9.0%) | <0.001 |
| Sedatives | 582 | 398 (68.4%) | 608 | 342 (56.2%) | <0.001 |
| Blood Products (days 1–7) | |||||
| Red Blood Cells | 582 | 225 (38.7%) | 608 | 129 (21.2%) | <0.001 |
| Fresh Frozen Plasma | 582 | 341 (58.6%) | 608 | 198 (32.6%) | <0.001 |
| Recombinant Factor VIIA | 582 | 0 (0.0%) | 608 | 21 (3.5%) | <0.001 |
| Platelets | 582 | 123 (21.1%) | 608 | 101 (16.6%) | 0.046 |
| ICU Complications (days 1–7) | |||||
| Seizures | 582 | 46 (7.9%) | 608 | 27 (4.4%) | 0.013 |
| Arrhythmia | 582 | 159 (27.3%) | 608 | 88 (14.5%) | <0.001 |
| Gastrointestinal Bleeding | 582 | 68 (11.7%) | 608 | 33 (5.4%) | <0.001 |
| N-acetylcysteine | |||||
| Intravenous | 582 | 504 (86.6%) | 608 | 480 (78.9%) | <0.001 |
| Oral | 582 | 516 (88.7%) | 608 | 238 (39.1%) | <0.001 |
| Psychological Comorbidities | 582 | 286 (49.1%) | 608 | 355 (58.4%) | 0.001 |
| Depression | 497 | 201 (40.4%) | 486 | 233 (47.9%) | 0.018 |
| Schizophrenia | 300 | 4 (1.3%) | 265 | 12 (4.5%) | 0.022 |
| Chronic Pain | 299 | 3 (1.0%) | 254 | 1 (0.4%) | 0.40 |
| Bipolar Disorder | 333 | 37 (11.1%) | 331 | 78 (23.6%) | <0.001 |
| Anxiety | 332 | 36 (10.8%) | 347 | 94 (27.1%) | <0.001 |
| Overdose Intent | 0.12 | ||||
| Intentional | 526 | 233 (44.3%) | 536 | 212 (39.6%) | |
| Unintentional | 526 | 293 (55.7%) | 536 | 324 (60.4%) | |
| Intravenous Drug Use | 572 | 31 (5.4%) | 606 | 64 (10.6%) | 0.001 |
| Intracranial Hypertension (days 1–21) | 165 | 85 (51.5%) | 412 | 123 (29.9%) | <0.001 |
| Death (days 1–21) | 509 | 156 (30.6%) | 539 | 117 (21.7%) | 0.001 |
| Cerebral Edema Death | 509 | 59 (11.6%) | 537 | 24 (4.5%) | <0.001 |
| Listed for Liver Transplantation | 582 | 152 (26.1%) | 607 | 121 (19.9%) | 0.011 |
| Received Liver Transplant (days 1–21) | 581 | 50 (8.6%) | 605 | 50 (8.3%) | 0.83 |
| Transplant-free Survival (day 21) | 519 | 320 (61.7%) | 550 | 384 (69.8%) | 0.005 |
Abbreviations: ALFSG, Acute Liver Failure Study Group; ICP, intracranial pressure; ICU, intensive care unit; MELD, model for end stage liver disease.
Univariate Analysis of Admission (Day 1) Parameters: Enrollment Time Cohort
Comparisons of biochemical and clinical admission parameters by enrollment time cohort are shown in Table 3. Comparing recent vs. early cohorts on admission, there were no significant differences in patients meeting KCC (11.7% vs. 13.2%; p=0.42), having high grade (3/4) HE (52.5% vs. 51.5%; p=0.71), and requiring mechanical ventilation (52.8% vs. 53.3%; p=0.87), or vasopressor support (23.7% vs. 22.3%; p=0.58). Significantly more patients were treated with CRRT on admission in the recent cohort over early cohort (15.8% vs. 4.1%; p<0.001).
Table 3.
Biochemical and organ support parameters at admission, stratified by time cohort (1998–2007 vs. 2008–2018).
| “Early” (1998–2007) (N = 582) | “Recent” (2008–2018) (N = 608) | P-value | ||||
| Biochemistry | ||||||
| Hemoglobin (g/L) | 578 | 111 (96–128) | 579 | 106 (93–121) | <0.001 | |
| White Blood Cells (109/L) | 579 | 9.4 (6.3–14.1) | 599 | 9.3 (6.3–13.5) | 0.41 | |
| Platelets (109/L) | 579 | 126 (84–179) | 592 | 125.5 (81.5–181) | 0.71 | |
| INR | 570 | 2.8 (2.0–4.6) | 590 | 3.05 (2.1–4.4) | 0.37 | |
| AST (IU/L) | 579 | 4110 (1543–8160) | 594 | 3093 (1374–6981) | 0.004 | |
| ALT (IU/L) | 578 | 4024 (2121–6702) | 595 | 3543 (1916–5733) | 0.005 | |
| Bilirubin | (μmol/L) | 579 | 78.7 (49.6–112.9) | 589 | 71.8 (42.8–107.7) | 0.037 |
| (mg/dL) | 4.6 (2.9–6.6) | 4.2 (2.5–6.3) | ||||
| pH | 527 | 7.42 (7.36–7.48) | 468 | 7.41 (7.34–7.46) | 0.002 | |
| Ammonia (venous) (μmol/L) | 170 | 110.5 (70–159) | 288 | 97 (68–168) | 0.94 | |
| Creatinine | (μmol/L) | 581 | 168.0 (88.4–309.4) | 602 | 141.4 (76.0–260.8) | <0.001 |
| (mg/dL) | 1.9 (1.0–3.5) | 1.6 (0.86–2.95) | ||||
| Lactate (mmol/L) | 345 | 4.9 (2.7–9.3) | 410 | 3.3 (2.1–6.78) | <0.001 | |
| Phosphate (mmol/L) | 513 | 0.81 (0.52–1.26) | 520 | 0.81 (0.58–1.15) | 0.47 | |
| King’s College Criteria met | 582 | 77 (13.2%) | 608 | 71 (11.7%) | 0.42 | |
| ALFSG Prognostic Index | ||||||
| Survival Predicted Probability ≥ 80% | 566 | 214 (37.8%) | 538 | 216 (40.1%) | 0.43 | |
| MELD | 566 | 31.4 (22.8–38.7) | 574 | 29.6 (21.0–36.7) | 0.005 | |
| Coma Grade 3/4 | 581 | 299 (51.5%) | 569 | 299 (52.5%) | 0.71 | |
| Organ support | ||||||
| Mechanical Ventilation | 582 | 310 (53.3%) | 608 | 321 (52.8%) | 0.87 | |
| Vasopressors | 582 | 130 (22.3%) | 608 | 144 (23.7%) | 0.58 | |
| Continuous Renal Replacement Therapy | 581 | 24 (4.1%) | 608 | 96 (15.8%) | <0.001 | |
Abbreviations: ALFSG, Acute Liver Failure Study Group; ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio, MELD, model for end stage liver disease.
Univariate analysis of APAP-ALF patients: 21-day TFS
In comparing subjects who were alive at day 21 without LT (TFS) with those that either were transplanted or died (non-TFS), there were no significant differences in pre-existing psychiatric comorbidity (51.5% vs. 55.0%; p=0.28) and intentional overdose (39.7% vs. 41.6%; p=0.58). On admission, non-TFS patients had worse biochemical profiles and required greater organ support. By day 21, non-TFS patients displayed greater incidence of ICH and, among 273 deceased patients, 83 of 271 known causes of death (30.6%) were secondary to CE. Comparisons of non-TFS and TFS patients on admission and at day 21 are shown in Supplementary Table 1 and Supplementary Table 2.
Multivariable Analysis: Associations with TFS
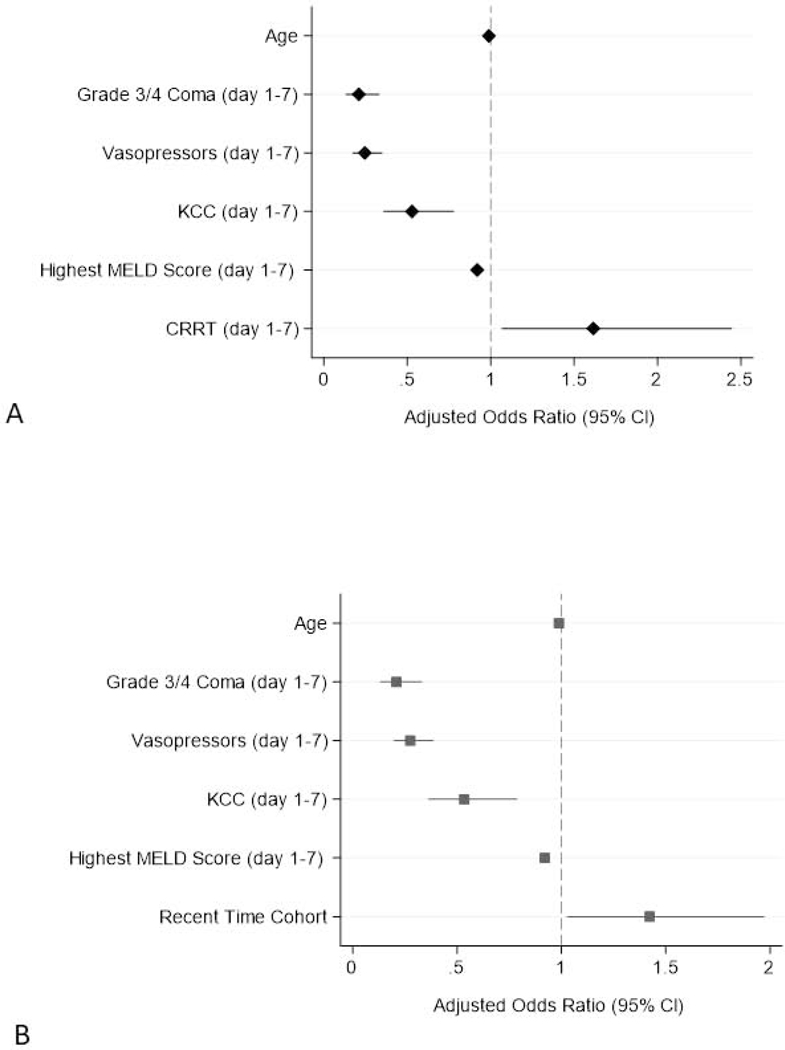
Multivariable logistic regression was performed to determine associations with 21-day TFS (Table 4 and Figure 1). Two models utilizing the same covariates were developed based on univariate logistic regression and previous publications.15, 23 Sex, overdose intent, and presence of pre-existing psychiatric comorbidity were not significantly associated with 21-day TFS on univariate analysis. To analyze CRRT and enrollment cohort separately (collinearity), we developed two models. Model 1 included use of CRRT, while Model 2 included enrollment time cohort. Adjustment for participant age was retained in both models due to clinical significance.
Table 4.
Predictors of 21-day transplant-free survival in APAP-ALF patients.
| Variable | Univariate | |||
|---|---|---|---|---|
| N | OR | 95% OR CI | P-value | |
| Sex a | 1069 | 0.92 | (0.69, 1.24) | 0.60 |
| Age | 1069 | 0.99 | (0.98, 1.00) | 0.046 |
| Vasopressors (days 1–7) | 1069 | 0.13 | (0.09, 0.17) | <0.001 |
| CRRTb (days 1–7) | 1069 | 0.58 | (0.42, 0.81) | 0.001 |
| Grade 3/4 Coma (days 1–7) | 1038 | 0.10 | (0.07, 0.15) | <0.001 |
| King’s College Criteria (days 1–7) | 1069 | 0.23 | (0.17, 0.32) | <0.001 |
| Highest MELD Score (days 1–7) | 1058 | 0.91 | (0.89, 0.92) | <0.001 |
| Overdose Intent c | 956 | 1.08 | (0.82, 1.42) | 0.58 |
| Psych Comorbidity | 1069 | 1.15 | (0.89, 1.48) | 0.28 |
| Time Cohort d | 1069 | 1.44 | (1.12, 1.85) | 0.005 |
| Variable | Multivariate Model 1 N = 1028 AUROC = 0.86 | |||
| Included in Model | aOR | 95% aOR CI | P-value | |
| Sex a | No | -- | -- | -- |
| Age | Yes | 0.99 | (0.98,1.00) | 0.10 |
| Vasopressors (days 1–7) | Yes | 0.25 | (0.17, 0.35) | <0.001 |
| CRRTb (days 1–7) | Yes | 1.62 | (1.07, 2.44) | 0.023 |
| Grade 3/4 Coma (days 1–7) | Yes | 0.21 | (0.13, 0.33) | <0.001 |
| King’s College Criteria (days 1–7) | Yes | 0.53 | (0.36, 0.78) | 0.001 |
| Highest MELD Score (days 1–7) | Yes | 0.92 | (0.90, 0.94) | <0.001 |
| Overdose Intent c | No | -- | -- | -- |
| Psych Comorbidity | No | -- | -- | -- |
| Time Cohort d | Noe | -- | -- | -- |
| Variable | Multivariate Model 2 N = 1028 AUROC = 0.86 | |||
| Included in Model | aOR | 95% aOR CI | P-value | |
| Sex a | No | -- | -- | -- |
| Age | Yes | 0.99 | (0.98, 1.00) | 0.06 |
| Vasopressors (days 1–7) | Yes | 0.28 | (0.20, 0.39) | <0.001 |
| CRRTb (days 1–7) | Noe | -- | -- | -- |
| Grade 3/4 Coma (days 1–7) | Yes | 0.21 | (0.13, 0.33) | <0.001 |
| King’s College Criteria (days 1–7) | Yes | 0.53 | (0.36, 0.79) | 0.002 |
| Highest MELD Score (days 1–7) | Yes | 0.92 | (0.90, 0.94) | <0.001 |
| Overdose Intent c | No | -- | -- | -- |
| Psych Comorbidity | No | -- | -- | -- |
| Time Cohort d | Yes | 1.42 | (1.03, 1.97) | 0.034 |
Reference group: male sex
Reference group: receipt of intermittent hemodialysis only or no renal replacement therapy
Reference group: unintentional overdose
Reference group: 1998–2007 enrollment cohort
Use of CRRT (Model 1) and enrollment time cohort (Model 2) were evaluated in separate models
Abbreviations: (a)OR, (adjusted) odds ratio; AUROC, area under receiver operator curve; CI, confidence interval; CRRT, continuous renal replacement therapy; MELD, model for end stage liver disease.
Figure 1.
Adjusted associations with 21-day transplant-free survival in 1190 APAP-ALF patients. (A) Model 1 and (B) Model 2.
Abbreviations: CRRT, continuous renal replacement therapy; CI, confidence interval; KCC, King’s College Criteria; MELD, model for end stage liver disease.
In Model 1, the following covariates (over days 1–7) were significantly associated with 21-day TFS; vasopressor support (OR: 0.25; 95% CI: 0.17–0.35; p<0.001), development of grade 3/4 HE (OR 0.21; 95% CI: 0.13–0.33; p<0.001), fulfillment of KCC (OR 0.53; 95% CI: 0.36–0.78; p=0.001), MELD (per unit increase: OR 0.92; 95% CI: 0.90–0.94; p<0.001) and the use of CRRT (OR 1.62; 95% CI: 1.07–2.44; p=0.023), but not age (per unit increase: OR 0.99; 95% CI: 0.98–1.00; p=0.10). This model had AUROC of 0.86.
In Model 2, the following covariates were significantly associated with 21-day TFS; vasopressor support (OR 0.28; 95% CI: 0.20–0.39; p<0.001), grade 3/4 HE (OR 0.21; 95% CI: 0.13–0.33; p<0.001), KCC (OR 0.53; 95% CI: 0.36–0.79; p=0.021), MELD (per unit increase: OR 0.92; 95% CI: 0.90–0.94; p<0.001), and enrollment time cohort (for 2008–2018: OR 1.42; 95% CI: 1.03–1.97; p=0.034), but not age (per unit increase: OR 0.99; 95% CI: 0.98–1.00; p=0.06). This model also had AUROC of 0.86.
DISCUSSION
Key Results
Outcomes in APAP-ALF within the US ALFSG have significantly improved over the last 21 years, with 21-day TFS significantly increasing more than 8 percent, from 61.7% during 1998–2007 to 69.8% during 2008–2018. Incidence of ICH and 21-day mortality secondary to CE have significantly decreased, from 51.5% to 29.9% and from 11.6% to 4.5%, respectively, between the same time periods. After adjusting for covariates reflecting severity of illness (vasopressor use, high coma grade, KCC, and MELD), both the use of CRRT and recent enrollment cohort were significantly associated with improved 21-day TFS. Between 1998–2007 and 2008–2018, use of CRRT significantly increased (7.6% to 22.2% during first 7 days). Overdose intent, and presence of pre-existing psychiatric comorbidity were not associated with 21-day TFS.
Comparison with the Literature
In this study, 21-day TFS significantly improved over time without a change in the rate of LT. Admission ALFSG-PI that predicted the probability of TFS did not differ across enrollment time cohorts, suggesting the protective role of one or more post-admission factors associated with recent enrollment. Bernal et al., in a large single center cohort (Kings College Hospital’s) 33-year experience with 3300 all-etiology ALF patients, noted a progressive rise in all-etiology TFS from 17% in 1973–1978 to 48% in 2004–2008, with 25.4% of the APAP-ALF cohort undergoing emergent LT.3 This may reflect improved care in a highly specialized liver critical care/transplant center with evolving intensive care strategies.2, 3
Cerebral edema/herniation is a well described complication of APAP-ALF.24 Both 21-day ICH development and CE-related death significantly decreased between the 1998–2007 and 2008–2018 enrollment cohorts from 51.5% to 29.9% and 11.6% to 4.5%, respectively. These APAP-ALF-specific findings echo those of serial all-etiology ALF Japanese studies where development of CE declined from 35.3% in 1998–2003 to 24.1% in 2004–2009.25, 26 Similarly, Bernal et al also demonstrated a significant decline in ICH incidence from 76% in 1984–1988 to 19.8% in 2004–2008 in all-etiology ALF, with ICH-associated mortality significantly decreasing from 95% in 1973–1978 to 55% in 2003–2008.3
Explaining the observed reductions in incidence of ICH/CE-death is speculative. In this study, serum ammonia levels on admission were not statistically different, and similar proportions developed high grade HE, and required mechanical ventilation or vasopressor support across enrollment cohorts. Equivalent/reduced use of ICP monitoring, ICP-lowering therapies (apart from increased use of hypothermia), and NAC administration were observed during the recent time period since NAC use depends on early recognition of APAP injury, but is often applied too late in those with severe liver injury upon arrival. Notably, recent time cohort patients were significantly more likely to receive CRRT on admission (15.8% vs. 4.1%) and over days 1–7 (22.2% vs. 7.6%), while early enrollment cohort patients were significantly more likely to receive IHD over days 1–7 (31.0% vs. 14.4%).
High serum ammonia levels are believed to play a role in the pathogenesis of CE and are associated with worsening HE and ICH.27–29 In 2014, Slack and colleagues first described the use of CRRT with hemofiltration to achieve a statistically significant reduction of ammonia clearance in ALF and in acute-on-chronic liver failure patients that correlated with the dose of ultrafiltration employed.30 In evaluating the role of RRT in all-etiology ALF, Cardoso et al reported statistically significant ammonia clearance with CRRT, but not with IHD. An improvement in 21-day TFS was associated with CRRT. Conversely, IHD was associated with a decrease in 21-day TFS.15 Most recently, Warrillow et al demonstrated in 54 ALF patients in Australia who underwent CRRT (continuous venovenous hemofiltration, median time to initiation ~ 4 hours) that CRRT was associated with significant reduced ammonia concentrations in ALF patients with its effect proportionate to cumulative dose.31
Unlike CRRT, IHD has previously been shown to be associated with significant increases in ICP, and significant decreases in mean arterial pressure and cardiac index.32 High blood flow rates, swings in hemodynamic stability, and rapid osmotic shifts associated with IHD reduce cerebral perfusion pressure and may induce or exacerbate CE.15, 32 After adjusting for significant covariates reflecting likelihood of TFS, we have shown that CRRT is associated with improved 21-day TFS in APAP-ALF. In ALF patients at high risk of ICH and CE (i.e. ventilated, encephalopathic patients with hemodynamic instability, acute renal injury etc.), CRRT is seen as a safer modality, as it minimizes sudden shifts in serum osmolality and cerebral perfusion pressure, and offers additional neuroprotective cooling, while normalizing metabolic parameters and hyperammonemia.3, 33–35
Finally, we did not find an association between psychosocial profile and 21-day TFS in APAP-ALF. No differences in overdose intentionality and presence of pre-existing psychiatric diagnosis were observed between 21-day TFS and non-TFS patients. Furthermore, there were no differences in rates of intentional overdose vs. therapeutic misadventure between the two time cohorts. Listing for LT among APAP patients does not appear to be impacted by psychiatric history.20 Recurrent suicidality and poorer compliance with pharmacotherapy and follow-up have been highlighted as potential problems in post-LT APAP-ALF patients;36 however, APAP-ALF patients have been reported to display similar long-term outcomes post-LT to those of non-APAP ALF patients.10, 37
Strengths and Limitations
This study should be interpreted in light of its strengths and limitations. The strengths consist of inclusion of APAP-ALF patients from multiple intensive care units across several geographic regions in North America. Patients in this study were largely young, female, and had similar demographics to those reported in other ALF studies from both Europe and North America.38 Therefore, the results of this study appear to have reasonable generalizability. Regarding its limitations, this retrospective analysis of prospectively collected observational data may comment only on association; we are unable to conclusively exclude sources of selection bias.39 Given that the ALFSG registry does not have complete clinical information prior to day 1 of study enrollment, we cannot exclude a possible referral bias of patients from referring hospital to ALFSG enrolling sites. Diagnosis of ICH was established retrospectively and dependent on the availability of ICP measurements, imaging features, and/or recorded cause of death. Data confirming the presence or absence of ICH was available in 577 of 1190 patients (48.5%), with greater availability in recent period over early period patients (66.8% vs. 28.4%). Clinically, while patients without any of the aforementioned data sources were plausibly less likely to have had ICH, the impact of missing data should be considered. Despite these limitations, this study represents the most recent and largest cohort of consecutive APAP-ALF patients evaluating clinical and neurological outcome trends over the last 21 years across multiple tertiary care centers leading to broad generalizability of the results.
Conclusions
In patients with APAP-ALF, TFS has significantly improved with time, along with a significant decline in the incidence of ICH and CE-related death. These findings have occurred in association with increased early CRRT (and decreased IHD) use within the intensive care setting possibly reflecting improvements in ICU management. Psychiatric comorbidities and overdose intent do not appear to be significantly associated with likelihood of TFS in APAP ALF.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background
Using the United State Acute Liver Failure (ALF) Study Group registry, we evaluated changes in medical interventions, psychosocial profile, and clinical outcomes over 21years in all (entire cohort) acetaminophen (APAP)-induced ALF patients.
Findings
Transplant-free survival has increased over time, while intracranial hypertension and cerebral edema-related mortality have decreased. These findings have occurred in the setting of increased use of continuous renal replacement therapy.
Implications for patient care
Recent improvements in critical care management strategies may improve outcome in APAP-ALF and warrant further study. Psychosocial profile and overdose intentionality are associated with transplant-free survival in APAP-ALF.
ACKNOWLEDGEMENTS
Members and institutions participating in the Acute Liver Failure Study Group 19982018 are as follows: W.M. Lee, M.D. (Principal Investigator); Anne M. Larson, M.D., Iris Liou, M.D., University of Washington, Seattle, WA; Oren Fix, M.D., Swedish Medical Center, Seattle, WA; Michael Schilsky, M.D., Yale University, New Haven, CT; Timothy McCashland, M.D., University of Nebraska, Omaha, NE; J. Eileen Hay, M.B.B.S., Mayo Clinic, Rochester, MN; Natalie Murray, M.D., Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, M.D., University of Pittsburgh, Pittsburgh, PA; Andres Blei, M.D., Northwestern University, Chicago, IL (deceased), Daniel Ganger, M.D., Northwestern University, Chicago, IL; Atif Zaman, M.D., University of Oregon, Portland, OR; Steven H.B. Han, M.D., University of California, Los Angeles, CA; Robert Fontana, M.D., University of Michigan, Ann Arbor, MI; Brendan McGuire, M.D., University of Alabama, Birmingham, AL; Raymond T. Chung, M.D., Massachusetts General Hospital, Boston, MA; Alastair Smith, M.B., Ch.B., Duke University Medical Center, Durham, NC; Robert Brown, M.D., Cornell/Columbia University, New York, NY; Jeffrey Crippin, M.D., Washington University, St Louis, MO; Edwin Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, M.B.B.S., Medical University of South Carolina, Charleston, SC; Santiago Munoz, M.D., Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, M.D., University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, M.D., Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, M.D., University of California Davis, Sacramento, CA; Raj Satyanarayana, M.D., Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, M.D., University of California, San Diego, CA; Constantine J. Karvellas MD, University of Alberta, Edmonton, AB; Jodi Olson MD, University of Kansas, Kansas City, KA; Ram Subramanian MD, Emory, Atlanta, GA; James Hanje MD, Ohio State University, Columbus, OH; Bilal Hameed MD, University of California San Francisco, CA. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia, and Corron Sanders, Ph.D., Nahid Attar, Linda S. Hynan, Ph.D., and the Medical University of South Carolina Data Coordination Unit included Valerie Durkalski, Ph.D., Wenle Zhao, Ph.D., Jaime Speiser, Catherine Dillon, Holly Battenhouse and Michelle Gottfried.
Disclosures:
AJM, JLS, DRG, KNM, BJO, AML, and CJK have no personal or funding conflicts of interest. WML receives research support from Merck, Conatus, Intercept, Bristol-Myers Squibb, Novo Nordisk, Synlogic, Eiger, Cumberland, Exalenz, Instrumentation Laboratory and Ocera Therapeutics, now Mallinkrodt Pharmaceuticals, and has received personal fees for consulting from Forma, Sanofi, Seattle Genetics, Affibody, Karuna, and Genentech.
Grant Support:
The study was sponsored by NIH grant U-01 58369 (from NIDDK).
Abbreviations:
- (a)OR
(adjusted) odds ratio
- ALF
acute liver failure
- ALFSG-PI
Acute Liver Failure Study Group Prognostic Index
- APAP
acetaminophen
- AUROC
area under receiver operator curve
- CE
cerebral edema
- CI
confidence interval
- (C)RRT
(continuous) renal replacement therapy
- HE
hepatic encephalopathy
- ICH
intracranial hypertension
- ICP
intracranial pressure
- ICU
intensive care unit
- IHD
intermittent hemodialysis
- INR
international normalized ratio
- IQR
interquartile range
- KCC
King’s College Criteria
- LT
liver transplantation
- MELD
model for end stage liver disease
- NAC
N-acetylcysteine
- TFS
transplant-free survival
- US ALFSG
United States Acute Liver Failure Study Group
STROBE Statement—checklist of items that should be included in reports of observational studies
| Item No | Recommendation | Page No | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1, 3 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 3–4 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 5 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 6 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 7 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 7 |
| Participants | 6 | (a) Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Case-control study—Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants |
7–9 |
| (b) Cohort study—For matched studies, give matching criteria and number of exposed and unexposed Case-control study—For matched studies, give matching criteria and the number of controls per case |
N/A | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 7–9 |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 7–9 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 7–8 |
| Study size | 10 | Explain how the study size was arrived at | N/A |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 9–10 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 9–10 |
| (b) Describe any methods used to examine subgroups and interactions | 9–10 | ||
| (c) Explain how missing data were addressed | 8–9 | ||
| (d) Cohort study—If applicable, explain how loss to follow-up was addressed Case-control study—If applicable, explain how matching of cases and controls was addressed Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy |
N/A | ||
| (e) Describe any sensitivity analyses | N/A | ||
| Results | |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 11 |
| (b) Give reasons for non-participation at each stage | N/A | ||
| (c) Consider use of a flow diagram | N/A | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 11 |
| (b) Indicate number of participants with missing data for each variable of interest | 25–30 | ||
| (c) Cohort study—Summarise follow-up time (eg, average and total amount) | N/A | ||
| Outcome data | 15* | Cohort study—Report numbers of outcome events or summary measures over time | 25–30 |
| Case-control study—Report numbers in each exposure category, or summary measures of exposure | N/A | ||
| Cross-sectional study—Report numbers of outcome events or summary measures | N/A | ||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 1314, 31–32 |
| (b) Report category boundaries when continuous variables were categorized | N/A | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | N/A | ||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | 11–12 |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 15 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 18–19 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 18–19 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 19 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 1 |
Give information separately for cases and controls in case-control studies and, if applicable, for exposed and unexposed groups in cohort and cross-sectional studies.
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at www.strobe-statement.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–72. Epub 2005/12/01. [DOI] [PubMed] [Google Scholar]
- 2.Reuben A, Tillman H, Fontana RJ, et al. Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann Intern Med. 2016;164(11):724–32. Epub 2016/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernal W, Hyyrylainen A, Gera A, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59(1):74–80. Epub 2013/02/27. [DOI] [PubMed] [Google Scholar]
- 4.O’Grady J, Williams R. Classification of acute liver failure. The Lancet. 1993;342(8873):743. [PubMed] [Google Scholar]
- 5.Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369(26):2525–34. Epub 2013/12/27. [DOI] [PubMed] [Google Scholar]
- 6.Ware AJ, D’Agostino AN, Combes B. Cerebral edema: a major complication of massive hepatic necrosis. Gastroenterology. 1971;61(6):877–84. Epub 1971/12/01. [PubMed] [Google Scholar]
- 7.Bernal W, Wendon J. Acute liver failure; clinical features and management. Eur J Gastroenterol Hepatol. 1999;11(9):977–84. Epub 1999/09/30. [DOI] [PubMed] [Google Scholar]
- 8.Stravitz RT, Kramer AH, Davern T, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35(11):2498–508. Epub 2007/09/29. [DOI] [PubMed] [Google Scholar]
- 9.Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947–54. Epub 2002/12/18. [DOI] [PubMed] [Google Scholar]
- 10.Karvellas CJ, Safinia N, Auzinger G, et al. Medical and psychiatric outcomes for patients transplanted for acetaminophen-induced acute liver failure: a case-control study. Liver Int. 2010;30(6):826–33. Epub 2010/04/23. [DOI] [PubMed] [Google Scholar]
- 11.Pezzia C, Sanders C, Welch S, et al. Psychosocial and behavioral factors in acetaminophen-related acute liver failure and liver injury. J Psychosom Res. 2017;101:51–7. Epub 2017/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prescott LF, Critchley JA. The treatment of acetaminophen poisoning. Annu Rev Pharmacol Toxicol. 1983;23(1):87–101. Epub 1983/01/01. [DOI] [PubMed] [Google Scholar]
- 13.Hamlyn AN, Douglas AP, James O. The spectrum of paracetamol (acetaminophen) overdose: clinical and epidemiological studies. Postgrad Med J. 1978;54(632):400–4. Epub 1978/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856–64, 64 e1. Epub 2009/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso FS, Gottfried M, Tujios S, et al. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology. 2018;67(2):711–20. Epub 2017/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerman HJ, Maddrey WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology. 1995;22(3):767–73. Epub 1995/09/01. [PubMed] [Google Scholar]
- 17.Maddrey WC. Hepatic effects of acetaminophen. Enhanced toxicity in alcoholics. J Clin Gastroenterol. 1987;9(2):180–5. Epub 1987/04/01. [DOI] [PubMed] [Google Scholar]
- 18.Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA. 1994;272(23):1845–50. Epub 1994/12/21. [DOI] [PubMed] [Google Scholar]
- 19.Schiodt FV, Rochling FA, Casey DL, et al. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337(16):1112–7. Epub 1997/10/27. [DOI] [PubMed] [Google Scholar]
- 20.Simmons OL, Meinzer C, Rule J, et al. Liver transplantation for acetaminophen-induced acute liver failure: role of psychiatric comorbidity in listing decisions and outcomes. Dig Dis Sci. 2019. Epub 2019/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch DG, Speiser JL, Durkalski V, et al. The natural history of severe acute liver injury. Am J Gastroenterol. 2017;112(9):1389–96. Epub 2017/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch DG, Tillman H, Durkalski V, et al. Development of a model to predict transplant-free survival of patients with acute liver failure. Clin Gastroenterol Hepatol. 2016;14(8):1199–206 e2. Epub 2016/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karvellas CJ, Speiser JL, Tremblay M, et al. Elevated FABP1 serum levels are associated with poorer survival in acetaminophen-induced acute liver failure. Hepatology. 2017;65(3):938–49. Epub 2016/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blei AT. Medical therapy of brain edema in fulminant hepatic failure. Hepatology. 2000;32(3):666–9. Epub 2000/08/29. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara K, Mochida S, Matsui A, et al. Fulminant hepatitis and late onset hepatic failure in Japan. Hepatol Res. 2008;38(7):646–57. Epub 2008/03/11. [DOI] [PubMed] [Google Scholar]
- 26.Oketani M, Ido A, Nakayama N, et al. Etiology and prognosis of fulminant hepatitis and late-onset hepatic failure in Japan: Summary of the annual nationwide survey between 2004 and 2009. Hepatol Res. 2013;43(2):97–105. Epub 2013/02/16. [DOI] [PubMed] [Google Scholar]
- 27.Bernal W, Hall C, Karvellas CJ, et al. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46(6):1844–52. Epub 2007/08/10. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia V, Singh R, Acharya SK. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut. 2006;55(1):98–104. Epub 2005/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clemmesen JO, Larsen FS, Kondrup J, et al. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology. 1999;29(3):648–53. Epub 1999/03/03. [DOI] [PubMed] [Google Scholar]
- 30.Slack AJ, Auzinger G, Willars C, et al. Ammonia clearance with haemofiltration in adults with liver disease. Liver Int. 2014;34(1):42–8. Epub 2013/06/22. [DOI] [PubMed] [Google Scholar]
- 31.Warrillow S, Fisher C, Bellomo R. Correction and Control of Hyperammonemia in Acute Liver Failure: The Impact of Continuous Renal Replacement Timing, Intensity, and Duration. Crit Care Med. 2020;48(2):218–24. Epub 2020/01/16. [DOI] [PubMed] [Google Scholar]
- 32.Davenport A, Will EJ, Davidson AM. Improved cardiovascular stability during continuous modes of renal replacement therapy in critically ill patients with acute hepatic and renal failure. Crit Care Med. 1993;21(3):328–38. Epub 1993/03/01. [DOI] [PubMed] [Google Scholar]
- 33.Davenport A Continuous renal replacement therapies in patients with acute neurological injury. Semin Dial. 2009;22(2):165–8. Epub 2009/05/12. [DOI] [PubMed] [Google Scholar]
- 34.Davenport A Is there a role for continuous renal replacement therapies in patients with liver and renal failure? Kidney International. 1999;56:S62–S6. [PubMed] [Google Scholar]
- 35.Wendon J, Cordoba J, Dhawan A, et al. EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66(5):1047–81. Epub 2017/04/19. [DOI] [PubMed] [Google Scholar]
- 36.Germani G, Theocharidou E, Adam R, et al. Liver transplantation for acute liver failure in Europe: outcomes over 20 years from the ELTR database. J Hepatol. 2012;57(2):288–96. Epub 2012/04/24. [DOI] [PubMed] [Google Scholar]
- 37.Fontana RJ, Ellerbe C, Durkalski VE, et al. Two-year outcomes in initial survivors with acute liver failure: results from a prospective, multicentre study. Liver Int. 2015;35(2):370–80. Epub 2014/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karvellas CJ, Todd Stravitz R, Battenhouse H, et al. Therapeutic hypothermia in acute liver failure: a multicenter retrospective cohort analysis. Liver Transpl. 2015;21(1):4–12. Epub 2014/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connors AF Jr., Pitfalls in estimating the effect of interventions in the critically ill using observational study designs. Crit Care Med. 2001;29(6):1283–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.