Abstract
Face memory deficits may be a bipolar disorder (BD) endophenotype. BD (n = 27) and unaffected youth at risk (n = 13) exhibited middle frontal gyrus hypoactivation during successful vs. unsuccessful encoding. Parahippocampal gyrus dysfunction was found in BD and at-risk youth (vs. low-risk, n = 37). Middle occipital gyrus hypoactivation was only present in BD.
Keywords: fMRI, Pediatric bipolar disorder, At risk, Face memory, Endophenotype
Deficits in face memory, or the capacity to encode and recall faces, may be a bipolar disorder (BD) endophenotype [7]. These deficits have been observed in both adults [1,8] and children [1,4,12] with BD, regardless of mood state [8], and in unaffected individuals at familiar risk [7]. Medial temporal [1,8,4] and frontal cortex [1,8] dysfunction have been implicated in face memory deficits in BD. For example, BD youth, relative to typically-developing youth, display increased activation in the middle temporal gyrus, caudate, and parahippocampal gyrus [4] and decreased activation in the middle frontal gyrus [1] during successful vs. unsuccessful face encoding. However, researchers have not examined the neural correlates mediating face memory deficits in youth at familial risk for BD.
We compared neural activation during face encoding in pediatric BD, youth at risk for BD, and healthy comparisons (HC). We expected that BD and at-risk youth would demonstrate behavioral deficits in face memory [7] and attention (i.e., increased intrasubject variability in response time [ISV-RT]) during encoding [3]. We also hypothesized that, during successful vs. unsuccessful face encoding, BD and at-risk youth would show neural dysfunction in the frontal cortex and medial temporal regions previously implicated in face memory processing [1,8,4].
1. Methods
1.1. Participants
Twenty-seven BD, 13 at-risk, and 37 HC youth (9–19 years) participated in an Institutional Review Board-approved study at NIMH. Parental/child informed consent/assent was obtained. BD and HC data have been published previously [1,4]. Data from at-risk youth (n = 13), the focus of this study, have not been published.
BD met narrow phenotype criteria [10]. At-risk youth had a first-degree BD relative (8 parent; 5 sibling). At-risk youth with attention-deficit/hyperactivity disorder (ADHD) or anxiety disorders were included; a history of depression or mood disorder was exclusionary.
Exclusion criteria for all participants were: IQ < 70, head trauma, neurological disorder, pervasive developmental disorder, chronic medical illness, or substance abuse. BD and at-risk youth completed the following clinical ratings within 48 h of the scan: Young Mania Rating Scale [17], Children’s Depression Rating Scale [13], Pediatric Anxiety Rating Scale [14], and Children’s Global Assessment of Severity [15] (Table 1).
Table 1.
Sample characteristics.
| Characteristic | BD (n = 27) | At-risk (n = 13) | HC (n = 37) | F, t, or Chi2 | P value |
|---|---|---|---|---|---|
|
|
|||||
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| Age (years) | 14.44 ± 2.82 | 13.71 ± 2.28 | 14.69 ± 2.29 | 0.73 | .48 |
| Full-scale IQ | 109.70 ± 12.99 | 112.87 ± 12.74 | 108.92 ± 11.71 | 0.47 | .63 |
| YMRS | 9.56 ± 6.19 | 4.08 ± 3.45 | – | 2.96 | .005 |
| CDRS | 27.22 ± 9.21 | 19.38 ± 3.36 | – | 2.96 | .005 |
| PARS | 13.61 ± 4.59 | 4.00 ± 4.04 | – | 6.29 | <.001 |
| CGAS | 51.44 ± 12.00 | 75.46 ± 10.89 | – | −6.10 | <.001 |
| n (%) | n (%) | n (%) | |||
|
| |||||
| Male | 15 (55.6) | 8 (61.5) | 16 (43.2) | 1.69 | .43 |
| Bipolar type | |||||
| Bipolar I | 23 (85.2) | 0 | – | – | – |
| Bipolar II | 4 (14.8) | 0 | – | – | – |
| Any anxiety disordera | 16 (59.3) | 1 (7.7) | – | – | – |
| ADHD | 11 (40.7) | 2 (15.4) | – | – | – |
| ODD or CD | 8 (29.6) | 0 | – | – | – |
| Mood stateb | |||||
| Euthymic | 18 (66.7) | 13 (100) | – | – | – |
| Depressed | 2 (7.4) | 0 | – | – | – |
| Hypo/manic | 5 (18.5) | 0 | – | – | – |
| Mixed | 2 (7.4) | 0 | – | – | – |
| Medication at scan | |||||
| Unmedicated | 3 (11.1) | 13 (100) | 37 (100) | – | – |
| Atypical antipsychotic | 13 (48.1) | 0 | 0 | – | – |
| Lithium | 12 (44.4) | 0 | 0 | – | – |
| Antiepileptic | 16 (59.3) | 0 | 0 | – | – |
| Antidepressant | 7 (25.9) | 0 | 0 | – | – |
| Stimulants | 8 (29.6) | 0 | 0 | – | – |
BD: bipolar disorder; HC: healthy comparisons; YMRS: Young Mania Rating Scale; CDRS: Children’s Depression Rating Scale; PARS: Pediatric Anxiety Rating Scale; CGAS: Clinical Global Assessment Scale; ADHD: attention-deficit hyperactivity disorder; ODD: oppositional defiant disorder; CD: conduct disorder; SD: standard deviation.
Includes generalized anxiety disorder, separation anxiety disorder, social phobia, panic disorder, posttraumatic stress disorder, and obsessive compulsive disorder.
Euthymic state was defined as a CDRS score ≤ 40 and a YMRS score ≤ 12; depressed state was defined as a CDRS score > 40 and a YMRS score ≤ 12; hypomanic or manic state was defined as a CDRS score ≤ 40 and a YMRS score > 12.
1.2. Paradigm and fMRI data acquisition
Details on the paradigms, scan parameters, and image processing are described in the supplement and elsewhere [1,4]. In brief, participants viewed gray-scale faces of 32 actors displaying different emotions in an event-related design, while providing ratings or viewing faces passively during scanning. After scanning, participants completed a surprise memory task with neutral faces of 24 novel and 24 previously viewed actors, and indicated whether they had seen each face.
1.3. Data analyses
1.3.1. Clinical data and demographics
Analyses of variance (ANOVA) examined between-group differences in age and IQ; Chi2 examined sex distribution. T-tests compared clinical ratings between BD and at-risk youth.
1.3.2. Behavior
A d′ score indexed post-scan memory performance: d′ = Zhits – Zfalse alarms.“Hits” were defined as faces recalled correctly; “false alarms” were faces misidentified as previously seen. Higher d′ indicates better memory performance. ANOVA examined group differences in d′, mean reaction time (RT), and ISV-RT during encoding. Post-hoc comparisons used Fisher’s Least Significant Difference.
1.3.3. fMRI
Image preprocessing, individual-level, and whole-brain analyses were conducted in Statistical Parametric Mapping 8 (SPM 8, Wellcome Trust Centre for Neuroimaging, London). After slice timing and motion correction, data were spatially normalized to standard Montreal Neurological Institute (MNI) brain space, and voxel size was resampled to 2 × 2 × 2 mm. We employed an 8-mm Gaussian smoothing kernel followed by intensity normalization to ensure activation was measured in units of local percent signal change. To map brain regions engaged during successful vs. unsuccessful encoding, imaging data were “binned” according to whether participants correctly or incorrectly recognized previously viewed faces (“Hits” vs. “Misses” contrast). This approach, rather than Hits vs. fixation or Misses vs. fixation, was used because prior studies indicated that fixation trials provide an ambiguous baseline in memory paradigms [9]. Whole-brain ANOVA focused on the “Hits” vs. “Misses” contrast, with group as a between-subject variable. We collapsed across face emotion types (angry, fearful, happy) to increase total trials per condition to approximately 45–50 Hits and Misses trials each (instead of ~ 15 trials each for Hits and Misses per emotion) to maximize statistical power. Clusters surpassing a threshold of P < .001 and k ≥ 10 voxels [11] were considered significant. Blood-oxygen-level-dependent signal changes at significant clusters were extracted with an 8 mm sphere around the peak. These extracted values were then used in the SPSS analyses. ANOVAs were first performed. To decompose significant group effects, we then conducted post-hoct-tests for pairwise comparisons using Fisher’s Least Significant Difference. The significance threshold for these analyses was set at P < .05.
We also conducted post-hoc analyses, using the extracted blood-oxygen-level-dependent signal changes to examine the correlations between significant activation and memory performance and the effects of potential confounding variables (demographics, d′, mood state, medications, comorbidities) in BD. In at-risk youth, we tested the effect of proband status (sibling vs. parent) and the presence of ADHD or anxiety disorders on the results. The significance threshold for these analyses was also set at P < .05.
2. Results
2.1. Clinical data and demographics
Groups did not differ in age, IQ, or sex distribution (Table 1). BD youth had higher scores on the mania, depression, and anxiety rating scales and were more impaired (i.e., lower scores on the Children’s Global Assessment of Severity) than at-risk youth (Table 1).
2.2. Behavioral data
Groups tended to differ in memory performance (d′) (F[2,74] = 2.46, P = .09). Post-hoc comparisons indicated that BD performed more poorly than HC (P = .05, d = .51); at-risk youth differed from HC, approaching trend, with a medium effect size (P = .14, d = .50). BD and at-risk youth did not differ (P = .93).
Groups did not differ in mean RT (F[2,74] = 0.38, P = .69), but differed in ISV-RT (F[2,70] = 11.08, P < .001). BD (P < .001, d = 1.01) and at-risk youth (P < .001, d = 1.31) had higher ISV-RT than HC. BD and at-risk youth did not differ (P = .55).
2.3. fMRI data
Whole-brain ANOVA revealed group effects in several regions (Table 2).
Table 2.
Between-group whole-brain analyses during encoding of successfully vs. unsuccessfully recalled faces.
| Area of activation | Brodmann area | Side | Cluster size | MNI coordinates | F (2, 74) | P uncorrected | Between-group differences | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| X | y | z | |||||||
| Middle frontal gyrus | BA 6/8 | L | 35 | −42 | 12 | 48 | 8.21 | .001 | BD < HC*** AR < HC* |
| Parahippocampal gyrus, uncus | BA 34 | R | 127 | 12 | −10 | −22 | 9.05 | < .001 | BD < HC* HC < AR*** BD < AR*** |
| Middle occipital gyrus | BA 19 | R | 18 | 42 | −80 | 2 | 7.51 | .001 | BD < HC*** BD < AR* |
MNI: Montreal Neurological Institute; BD: bipolar disorder; AR: at-risk; HC: healthy comparisons; BA: Brodmann area; L: left; R: right.
P ≤ .05;
P ≤ .01;
P ≤ .001.
2.3.1. Left middle frontal gyrus
BD and at-risk youth, relative to HC, showed hypoactivation during Hits vs. Misses (ps < .05; Fig. 1a and b).
Fig. 1.
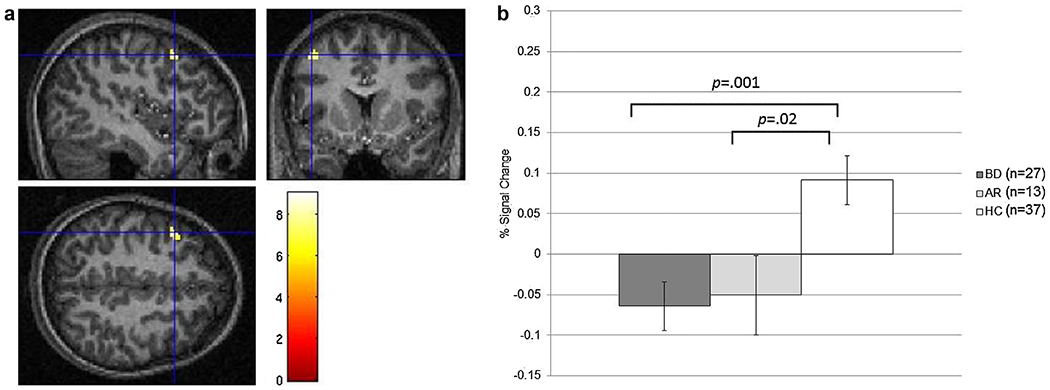
a and b: left middle frontal gyrus activation during successful vs. unsuccessful face encoding.
2.3.2. Right parahippocampal gyrus/uncus
BD exhibited hypoactivation vs. at-risk youth and HC (ps ≤ .05). At-risk youth showed hyperactivation vs. HC (ps < .001; Fig. 2a and b).
Fig. 2.
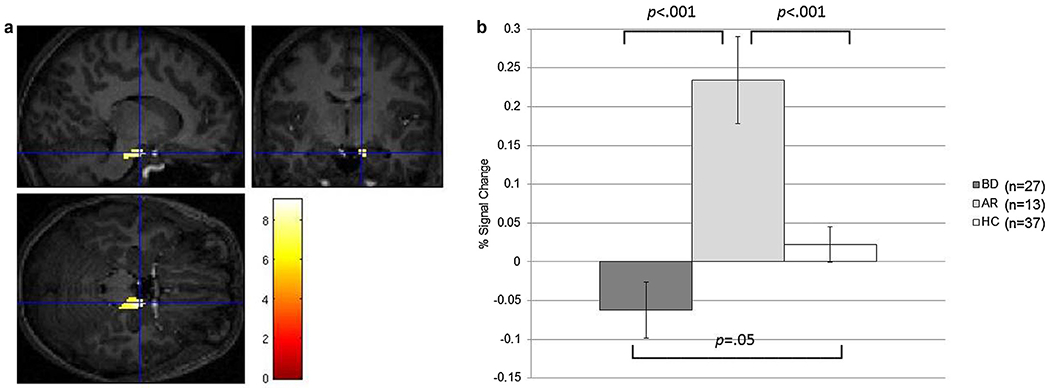
a and b: right parahippocampal gyrus, uncus activation during successful vs. unsuccessful face encoding.
2.3.3. Right middle occipital gyrus
BD showed hypoactivation vs. HC and at-risk youth (ps < .05).
2.3.4. Post-hoc analyses
We calculated Pearson correlations between activation and memory performance to explore possible associations between neural activation and behavior. There was a positive correlation between left middle frontal gyrus activation and d′ in HC (r = .33, P = .04), but not BD (r = −.15, P = .44) and at-risk youth (r = .46, P = .12). Correlations were not significant in other regions in any group.
We also conducted Analyses of Covariance (ANCOVA), with d′, age, IQ, and gender as a covariate, to evaluate the effect of these potential confounding variables. Group effects on the middle frontal gyrus (ps = .006), parahippocampal gyrus (ps < .001), and middle occipital gyrus (ps < .001) remained significant. Analyses including only euthymic BD vs. HC did not change the main findings, i.e., euthymic BD showed hypoactivation in the middle frontal gyrus (P = .005), parahippocampal gyrus (P = .05), and middle occipital gyrus (P = .02), relative to HC. In BD, mania and depression scores, number of medications, and comorbidities did not correlate with neural activation in any of the regions (ps > .09), although anxiety scores correlated negatively with parahippocampal gyrus activation (r = −.46, P = .03). At-risk youth with an affected parent did not differ from those with an affected sibling (P ≥ .68). All findings remained when at-risk youth with ADHD or anxiety were excluded (ps < .05).
3. Discussion
Face memory deficits are a potential BD endophenotype [7]. This is the first study to examine the neural correlates of this deficit in at-risk youth, who showed behavioral memory deficits at a trend level, although effect sizes were medium. Despite the small sample of at-risk youth, fMRI revealed hypoactivation in the middle frontal gyrus during successful vs. unsuccessful face encoding in both BD and at-risk youth. Parahippocampal gyrus activation also may be a neural marker for BD or BD risk status. Middle occipital gyrus dysfunction was only present following illness onset.
Extending prior research on BD [1], both BD and at-risk youth displayed hypoactivation in the middle frontal gyrus, suggesting that this may be a neural endophenotype for BD. Furthermore, middle frontal gyrus activation correlated positively with memory performance in HC. The middle frontal gyrus is involved in executive functions that require demands in working memory [6]. Our findings suggest that increased activation in this area may be necessary for successful face encoding. Additionally, both BD and at-risk youth showed parahippocampal gyrus dysfunction [8,4]; this region plays a central role in familiarity-based memory encoding and retrieval [5]. Whereas BD patients showed hypoactivation, at-risk youth demonstrated hyperactivation, suggesting that parahippocampal gyrus dysfunction may serve as a compensatory neural process in unaffected youth at risk for BD. In the middle occipital gyrus, an area involved in early visual processing [16], hypoactivation was present only in BD. Dysfunction in this region may only manifest following presence of the illness.
Unlike previous research in youth with BD that demonstrates neural dysfunction in the traditional face processing areas, e.g., fusiform gyrus, amygdala, and superior temporal sulcus, we did not find between-group differences in these regions. This may be explained by the discrepancy in the neural contrasts across studies (e.g., Hits vs. Misses in this study and Face Viewing vs. Fixation in previous research). Additionally, while our sample sizes compare favorably to others in the literature, the at-risk sample is relatively small. Thus, our null findings in the traditional face processing regions may be attributable to type II error. Future studies with larger samples, particularly the at-risk youth, are needed.
Limitations of the study include heterogeneity of the at-risk group, i.e., some had an Axis I diagnosis, and BD proband differed (sibling vs. parent). However, post-hoc analyses showed that these variables did not impact the findings. Furthermore, most BD youth had comorbid illnesses (88.9%), were medicated (88.9%), and were in a variety of mood states at the time of scanning, which may have influenced neural activation. However, post-hoc analyses suggested that comorbidities, medications, and mood state did not contribute to our findings. Finally, although the three groups did not differ in important demographics including age, gender, and IQ, it is unclear how well they match to each other on SES. Future research is needed to rule out the contribution of SES variables on our findings.
This study suggests that dysfunction in the middle frontal gyrus and parahippocampal gyrus may be neural markers for BD or BD risk. Neural dysfunction in these regions may contribute to the social cognition and face emotion labeling deficits seen in both BD and at-risk youth [2]. Future studies with larger samples and a longitudinal design would help determine the extent to which neural dysfunction associated with face memory deficits can predict the onset of BD in youth at risk for the illness.
Acknowledgements
This research was supported (in part) by the Intramural Research Program of the NIMH. Dr. Olsavsky’s research was made possible through the Clinical Research Training Program, a public–private partnership supported jointly by the NIH and Pfizer Inc. (via a grant to the Foundation for NIH from Pfizer Inc.).
Footnotes
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
References
- [1].Adleman NE, Kayser RR, Olsavsky AK, Bones BL, Muhrer EJ, Fromm SJ, et al. Abnormal fusiform activation during emotional face encoding assessed with functional magnetic resonance imaging. Psychiatry Res 2013;212:161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brotman MA, Guyer AE, Lawson ES, Horsey SE, Rich BA, Dickstein DP, et al. Facial emotion labeling deficits in children and adolescents at-risk for bipolar disorder. Am J Psychiatry 2008;165:385–9. [DOI] [PubMed] [Google Scholar]
- [3].Brotman MA, Rooney MH, Skup M, Pine DS, Leibenluft E. Increased intrasubject variability in response time in youths with bipolar disorder and at-risk family members. J Am Acad Child Adolesc Psychiatry 2009;48:628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, et al. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disord 2007;9:679–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci 2007;30:123–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Garavan H, Ross TJ, Li SJ, Stein EA. A parametric manipulation of central executive functioning. Cereb Cortex 2000;10:585–92. [DOI] [PubMed] [Google Scholar]
- [7].Glahn DC, Almasy L, Barguil M, Hare E, Peralta JM, Kent JW Jr, et al. Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Arch Gen Psychiatry 2010;67:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Glahn DC, Robinson JL, Tordesillas-Gutierrez D, Monkul ES, Holmes MK, Green MJ, et al. Fronto-temporal dysregulation in asymptomatic bipolar I patients: a paired associate functional MRI study. Hum Brain Mapp 2010;31:1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2001;2:685–94. [DOI] [PubMed] [Google Scholar]
- [10].Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry 2003;160:430–7. [DOI] [PubMed] [Google Scholar]
- [11].Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci 2009;4:423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McClure EB, Treland JE, Snow J, Dickstein DP, Towbin KE, Charney DS, et al. Memory and learning in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44:461–9. [DOI] [PubMed] [Google Scholar]
- [13].Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Psychiatry 1984;23:191–7. [DOI] [PubMed] [Google Scholar]
- [14].Research on Pediatric Psychopharmacology Anxiety Study Group. The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Am Acad Child Adolesc Psychiatry; 2002;41:1061–9. [DOI] [PubMed] [Google Scholar]
- [15].Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry 1983;40:1228–31. [DOI] [PubMed] [Google Scholar]
- [16].Thomas LA, Brotman MA, Bones BL, Chen G, Rosen BH, Pine DS, et al. Neural circuitry of masked emotional face processing in youth with bipolar disorder, severe mood dysregulation, and healthy volunteers. Dev Cogn Neurosci 2014;8:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978;133:429–35. [DOI] [PubMed] [Google Scholar]


