Abstract
Viruses infect all kingdoms of life; their genomes vary from DNA to RNA and in size from 2kB to 1MB or more. Viruses frequently employ disordered proteins, that is, protein products of virus genes that do not themselves fold into independent three-dimensional structures, but rather, constitute a versatile molecular toolkit to accomplish a range of functions necessary for viral infection, assembly, and proliferation. Interestingly, disordered proteins have been discovered in almost all viruses so far studied, whether the viral genome consists of DNA or RNA, and whatever the configuration of the viral capsid or other outer covering. In this review, I present a wide-ranging set of stories illustrating the range of functions of IDPs in viruses. The field is rapidly expanding, and I have not tried to include everything. What is included is meant to be a survey of the variety of tasks that viruses accomplish using disordered proteins.
Keywords: Protein disorder, protein-protein interaction, post-translational modification, virus proteins, viral oncoproteins
Graphical Abstract

I. INTRODUCTION
According to Crick’s “Central Dogma” of biology, the information stored in the DNA sequence of an organism is transcribed into the sequence of messenger RNA, and subsequently into the amino acid sequence of a protein. The protein folds to its 3-dimensional structure, which enables it to carry out its function in the organism. Intrinsically disordered proteins (IDPs) appear to contradict this model. Proteins that do not have a unique 3-dimensional structure but are nevertheless fully functional1 have begun to turn up throughout biology. The prevalence of IDPs in high-profile biological systems such as those awarded Nobel prizes has recently been pointed out.2 IDPs and intrinsically disordered regions of larger proteins (IDRs) are particularly prevalent in cancer-related proteins3 and in cell signaling,4 and are frequently found in viruses, where they assist in the hijacking of cellular function.5
Viruses consist of a nucleic acid genome and a protein coat, and have been termed “gift-wrapped nucleic acid”.6 Whether viruses are living or nonliving remains a matter of debate.6 They are clearly capable of reproduction, but only within a host cell, and require the host cell machinery for replication. On the other hand, recent analysis of the evolutionary trees of viruses and their proteins concluded that the likely origin of viruses was early in the “RNA world” and that viruses evolved from simple RNA-containing cells that adapted to an exclusively parasitic existence by losing all of the mechanics of metabolism.7 The question of whether viruses are living aside, it is clear that their genomes are exceptionally simple, frequently using multiple open reading frames to code for several proteins from the same genetic sequence. Another common characteristic of viruses is that many of these protein products are fully or partially intrinsically disordered, and that this intrinsic disorder is a vital part of their ability to “survive”.
An important characteristic of viruses (amply illustrated in the public health consequences of the SARS CoV2 pandemic) is their rapid rate of mutation. The accumulation of mutations appears especially high in disordered regions.8 This makes viruses extremely heterogeneous, and their lineages exceptionally difficult to trace and classify. Rather than classifying them in an evolutionary tree-like model, such as is used for prokaryotes, eukaryotes, or archea, viruses have been grouped into “Realms” based on the genetic material (RNA or DNA) of their genome and on the geometry of the outer covering or capsid. More recently, sequence analysis of conserved genes and proteins has been increasingly used in formal virus classification.9 The four major virus realms are the Duplodnaviria (double-stranded DNA viruses with icosahedral-shaped capsids. Examples include tailed bacteriophages and herpes viruses), the Monodnaviria (single-stranded DNA viruses, often with circular genomes. Examples infecting eukaryotes include papillomaviruses and polyomaviruses), Riboviria (RNA viruses that encode an RNA-dependent RNA polymerase or a reverse transcriptase. These are mostly eukaryotic viruses and most eukaryotic viruses are classified here. Examples include influenza, HIV, coronaviruses, ebolaviruses and rabies virus, also tobacco mosaic virus and other plant viruses), and Varidnavaria (DNA viruses with a jelly-roll fold capsid protein. Examples include adenoviruses and poxviruses). Other, less-populated realms have more unusual chemistry. These include the Adnaviria, which are archeal filamentous viruses with linear double-stranded A-form DNA genomes, and the Ribozyviria, which contain genomic and antigenomic ribozymes. This description serves to illustrate the wide varieties of nucleic acid and protein chemistries utilized by viruses. It also serves to introduce the theme of this review: viruses from all four of the major realms have been found to make extensive use of IDPs in the processes of the viral “life cycle”. The disorder prevalence in over 6000 viral proteomes was recently described.10 In some systems, detailed studies of the role of disordered and other domains in viral infection and replication have given important insights into the physiological mechanisms whereby viruses disrupt and hijack cells. Another intriguing aspect is the role of specific disordered sequences in the packaging of viruses. In particular, it appears that IDRs containing conserved arginine-rich motifs (ARMs) are important for correct packaging and self-assembly.11,12 Intriguingly, the lengths of the ARMs are correlated with the size of the viral genome,13 consistent with a role in charge neutralization and condensation of the packaged nucleic acid. Another emerging theme is the role of liquid-liquid phase separation of viral proteins and nucleic acids as an important organizing principle in viral infectivity and replication. These themes will appear frequently in the discussions below.
II. VIRUS IDPs
IDPs from the Major Eukaryotic Realm Duplodnaviria
Herpes simplex outer tegument component
Herpes simplex virus has the double-stranded DNA genome and icosahedral capsid typical of Duplodnaviria. The icosahedral capsid is enclosed by a layer termed the tegument, containing viral enzymes and other proteins associated with infection of cells and subversion of cellular processes. Surrounding the tegument is an envelope, which is derived from the host membrane and contains several glycoproteins derived from the virus. A simplified diagram of these structures is shown in Figure 1.
Figure 1.
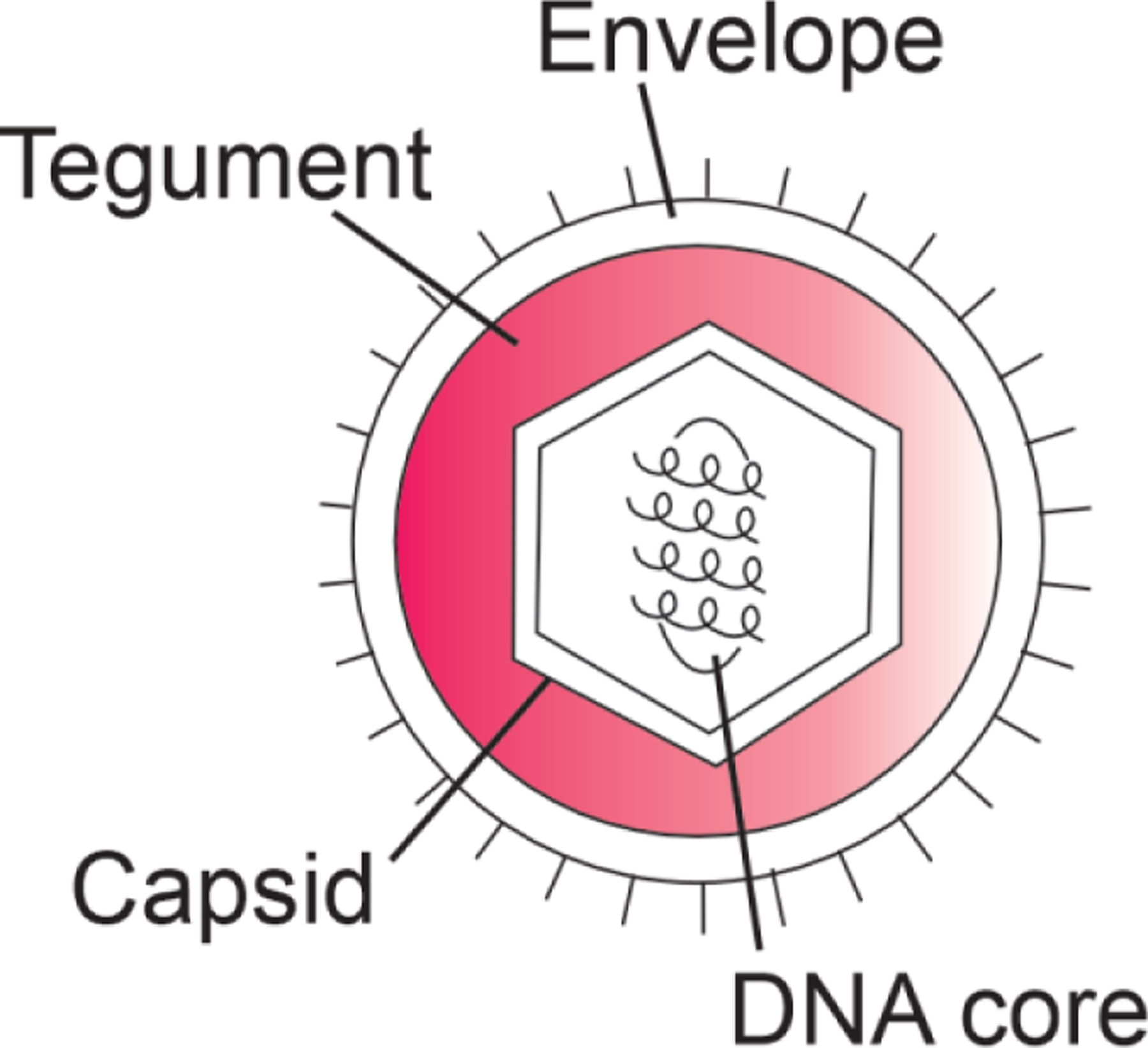
Schematic diagram of the herpes virus structure.
Herpesvirus tegument proteins frequently contain extensive disordered regions. Disorder has been characterized for one of the proteins of the tegument, termed UL11.14 This protein is of variable length in different herpes viruses, but in HSV1 and HSV2 it contains 95 amino acids, with an N-terminal domain that binds the capsid-associated protein UL16 and a C-terminal disordered domain. Extensive biophysical characterization of UL11 demonstrated that the entire sequence is likely disordered in the absence of partners, and that it undergoes temperature-dependent phase-separation. The authors suggest that “It is tempting to speculate that rather than forming an ordered structure, the outer tegument exists as a biomolecular condensate, a heterogeneous milieu composed of many partially disordered molecules that make multiple redundant nonspecific interactions.”14
Kaposi’s sarcoma herpesvirus
Herpes viruses associated with Kaposi’s sarcoma (KSHV) infect cells latently, through the formation of a chromosome-associated episome. Tethering of the episome to host mitotic chromosomes and its replication and segregation into progeny cells is the mechanism for viral latency, which is mediated by the LANA (latency-associated nuclear antigen) protein15 (Figure 2). The N-terminus of LANA binds to chromosomal H2A/H2B, while the C-terminus self-associates and interacts directly with chromatin. The central region consists of a disordered repeat region rich in acidic residues.
Figure 2.
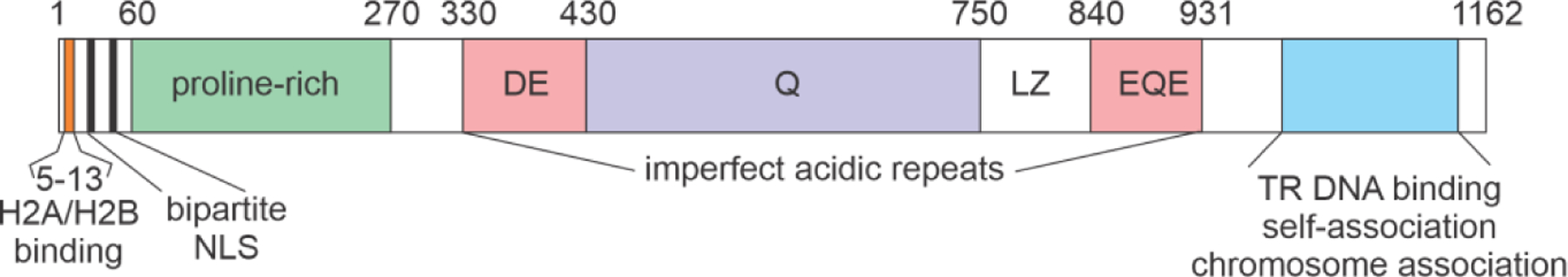
Schematic diagram showing regions of the LANA protein of KSHV (adapted from ref.15).
The presence of about 600 residues of an acid-rich region is diagnostic for protein disorder, and three regions of the sequence (residues 330–381, 372–421 and 845–895) are very similar to the composition and sequence of the acidic domain of the SET protein.16 SET proteins were recently identified as an important participants in the control of transcription of p53-responsive genes.17 The transcription factor p53 is a ubiquitous mediator of stress responses, and defects in p53 are found in many cancers, consistent with its role as a tumor suppressor. Under normal conditions, p53 levels are kept low in the cell by the interaction of the negative regulator MDM2, which promotes p53 degradation. Under stress conditions, post-translational modifications such as phosphorylation and acetylation act to stabilize p53 and promote its transcriptional activity.18 Acetylation of lysine residues in the C-terminal domain of p53 promotes interaction with DNA and recruitment of bromodomains such as that found in the transcriptional coactivator CBP and its paralog p300.19,20 Acidic SET domains specifically interact with non-acetylated lysines in a charge-dependent manner.17 Like the IDP regions of many other viral proteins, the LANA domain of KSHV interposes itself into the cellular milieu by binding to an essential protein in place of the cellular partner. In this case, the LANA protein may compete with cellular SET domains to dysregulate p53 or other lysine-containing proteins.15
Cross-reactivity of Viral IDPs with Myelin Basic Protein in Multiple Sclerosis
Another connection between viral infection and interference of viral IDPs in cellular processes is thought to occur in the case of the autoimmune disease multiple sclerosis (MS). (reviewed in 21) Myelin basic protein (MBP), which binds together with myelin to nerve fibres to form a sheath, is a 167-residue protein that is largely disordered. It contains three short amphipathic helical regions involved in membrane association, as well as binding sites for MAP kinases and calmodulin, and it is believed to function by interacting with adjacent membrane leaflets.21 The mechanism by which MBP becomes aberrantly structured, resulting in the demyelination of nerve fibres and the symptoms of MS, has been the subject of intensive research. One hypothesis for the source of the aberrant MBP structure is that viral IDPs may provide an immunological trigger for auto-antibodies by cross-reaction with MBP. In particular, the U24 protein of human herpesvirus 6 (HHV6) has a common sequence (PRTPPPS) with the central switch domain of MBP.22 Infection with Epstein-Barr virus or HHV6 in early childhood has been correlated with susceptibility to MS, but the involvement of viral triggers remains controversial. (reviewed in 23) This may be an example of viral molecular mimicry,24 a mechanism that has been widely documented, where viruses have borrowed genetic sequences from cells, precipitating autoimmunity.
IDPs from the Major Eukaryotic Realm Monodnaviria
The single-stranded DNA genome of human papillomavirus (HPV) codes for 8 proteins, early proteins E1, E2, E4, E5, E6, E7, and late proteins L1 and L2. (Figure 3). A concise description of these proteins and their functions is given in reference25, together with a summary of the structural information available for each of them.
Figure 3.
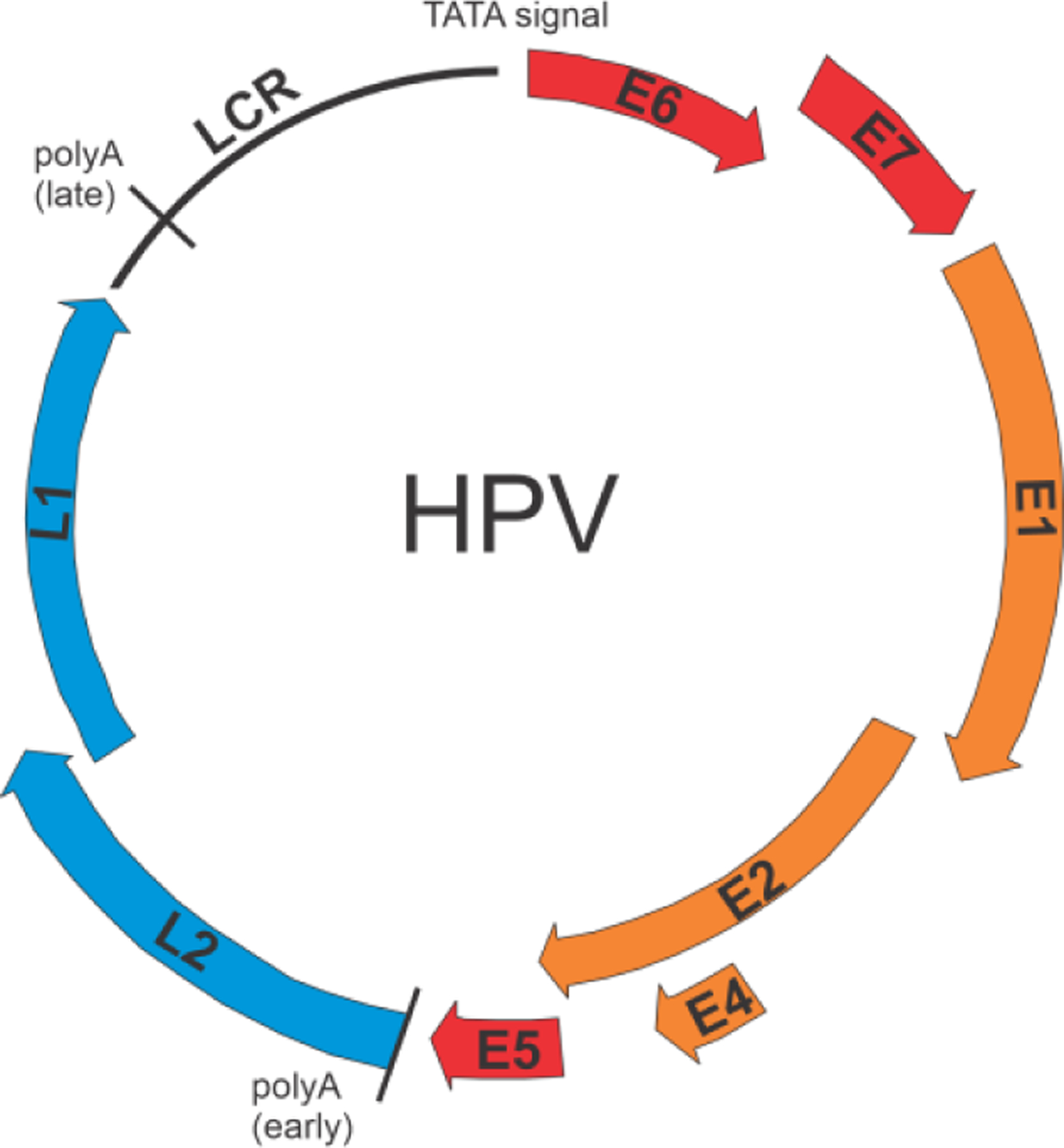
Schematic diagram of the HPV genome. E1, E2, E4 (orange) are replication and transcription proteins. E5, E6 and E7 (red) are oncoproteins. L1 and L2 (blue) are capsid proteins.
Human papillomavirus (HPV) is a ubiquitous virus that infects almost exclusively skin cells termed keratinocytes. The majority of HPVs cause benign lesions called papillomas or warts, but certain strains can induce malignant transformation leading to a number of cancers. So-called high-risk strains of HPV are responsible for a large proportion of cervical cancers throughout the world. Immunization against high-risk HPV provides one of the few avenues to prevention of cancer.26
The molecular mechanism whereby the high-risk strains of HPV cause cancer has received a lot of attention. The E7 protein has been strongly implicated in cervical cancer: whole-genome sequencing of a large collection of HPV16-infected tissues showed that the amino acid sequences of E7 proteins from cervical cancer patients were almost identical, whereas those in the non-cancer group showed much greater sequence variation.27
The E7 protein contains 98 residues, with three regions of higher relative sequence conservation, termed CR1, CR2 and CR3 (Figure 4). CR1 and CR2 are disordered domains that participate in interactions with cellular proteins; CR3 is structured, forming an E7 dimer that coordinates two zinc ions using conserved CxxC motifs.28
Figure 4.

Sequence alignment of HPV E7 sequences from several strains. The constant regions CR1, CR2, and CR3 are indicated. The LxCxE interaction motif and the two CxxC zinc-binding motifs are shown boxed.
Several research groups have targeted HPV E7 as a model system, in one case for examining evolutionary changes and hidden sequence conservation in IDPs29–32 and in another as a paradigm for dissecting the free energy landscape of a protein with distinct ordered and disordered regions.33,34
Given the sequence similarity of the E7 proteins from a number of HPV strains (Figure 4), one major question is why only some strains have a high risk for progression to cancer, while others are benign. An extensive bioinformatic survey of human papillomavirus proteins35 showed that the oncoproteins E6 and E7 likely contain high levels of disorder, and that high-risk strains appear to have a greater proportion of disordered sequences. However, a more targeted approach, examining the interactions between E7 and various cellular proteins has identified specific cellular processes that are disrupted by E7, and indicated that high-risk strains contain E7 proteins with higher affinities for these proteins than low-risk strains.
Two well-studied E7 partners are the retinoblastoma protein pRb and the transcriptional activator p300 and its paralog CREB-binding protein (CBP). pRb controls the G1 checkpoint of the cell cycle by binding to E2F and DP proteins (Figure 5). The cell cycle can proceed into the DNA synthesis phase if pRb is phosphorylated, which dissociates the complex with E2F and DP. This phosphorylation occurs as a result of a signal that dissociates the cyclin-dependent kinase inhibitor p21 from the cyclin-cyclin-dependent kinase complex. Lifting of the G1 checkpoint thus allows the cell cycle to proceed, through the second growth phase to cell division in the mitotic phase. There are many complex reasons why tumors occur, but their governing feature is a dysregulation of the cell cycle that results in uncontrolled proliferation. One example of this dysregulation, aberrant release of the E2F factors from pRb, results in premature entry into S phase; control over the cell cycle is lost, resulting in aberrant growth and tumor formation.
Figure 5.

Schematic diagram showing the cell cycle. A. Cell cycle showing phases where cell division, growth and DNA synthesis occur. B. States of pRb during mitosis and first growth phase. Dephosphorylated pRb binds the E2F and DP proteins, arresting the cell cycle at the G2 checkpoint. Cyclin dependent kinases are inhibited by the p21 cyclin dependent kinase inhibitor. C. Receipt of a signal dissociates the CDK-p21 complex, releasing the CDK to phosphorylate pRb, which frees the E2F and DP proteins to allow the cell cycle to proceed into the DNA synthesis phase. Figure adapted from reference36 with permission.
The disordered CR2 domain of HPV E7 interacts with the AB domain of the retinoblastoma protein (pRbAB), via the interaction motif LxCxE,37,38 which is also the interaction site for the TAZ2 domain of the transcriptional activator CREB-binding protein or its paralog p300.39 The E7 sequences from high-risk strains of HPV were found to have higher affinity than those from low-risk strains for both the pRbAB40 and the TAZ2 domain of CBP.39 The oncogenicity of high-risk E7 may simply be a result of a greater ability to displace the E2F proteins from pRb. However, the pRb and TAZ2 domains have similar affinity for the disordered region of E7.39 It is intriguing to consider an alternative model for the E7 interactions that might lead to cell-cycle disruption for the high-risk strains but not for the low-risk strains.36,39 This model recognizes that the E7 protein is dimerized through the CR3 region. Thus, the functional E7 protein contains two disordered CR1-CR2 regions, one from each protomer. If the LxCxE motif of one protomer interacted with pRbAB and the other with the TAZ2 domain of CBP, these two large proteins would be brought into close proximity, which would not normally occur (Figure 6). The C domain of pRb would be placed close to the acetyltransferase domain of p300/CBP, another possible source of aberrant disruption of the pRb-E2F complex.
Figure 6.
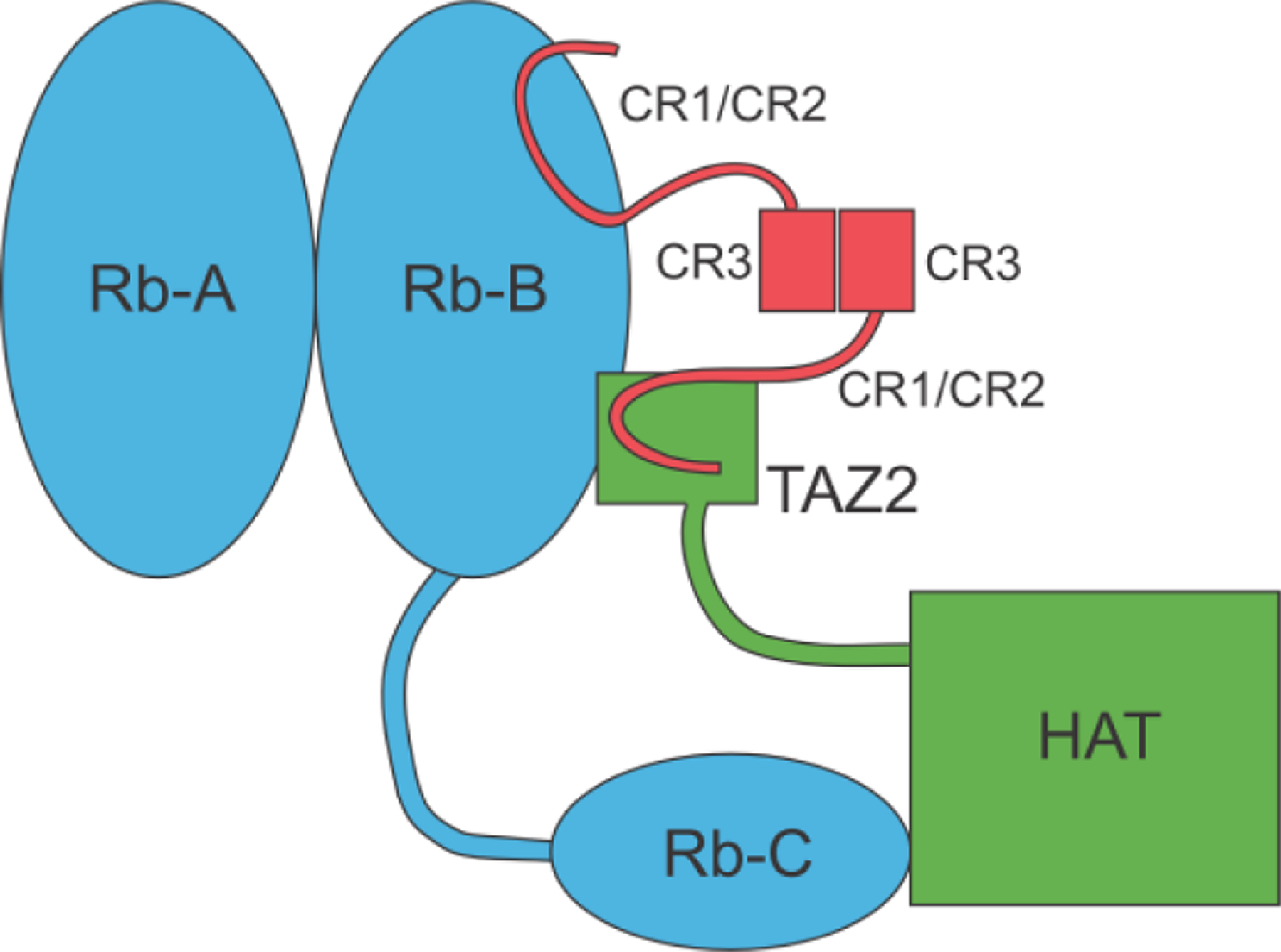
Schematic diagram of the ternary complex of HPV E7 (red) with pRb (blue) and CBP/p300 (green) (adapted from 39 with permission)
The affinity of the disordered domain of HPV E7 for TAZ2 is high (~10 nM), especially for the high-risk strain HPV16.39 Nevertheless, the structure of the complex is not well defined; the E7 N-terminus remains disordered even when bound to TAZ2,41 providing an example of a fuzzy interaction.42 Interestingly, HPV E7 has been observed to form spherical oligomers,43 perhaps anticipating the observation of punctate membraneless organelles and liquid-liquid phase separation in this system. A recent publication described the structural basis for the superior pRb affinity of the LxCxE motif from HPV E7 compared with cellular targets.44
IDPs from the Major Eukaryotic Realm Varidnaviria
Most cellular proteins interact with only a few partners, but studies of protein interactomes have shown that certain proteins, termed hub proteins, have much wider interaction networks.45,46 Hub proteins are important targets in viral infections, as takeover of one or more cellular hubs allows the virus to rapidly and comprehensively assume control of the cell. Hub proteins almost invariably contain large intrinsically disordered regions, and viral hub proteins, which are encoded by the infecting virus and which replace those of the cell, often with high efficiency, also contain large amounts of disorder. One well-studied example of a viral hub protein is the E1A protein of the small DNA tumor virus adenovirus.47,48
Like the HPV E7 protein, the adenovirus E1A protein binds to the retinoblastoma protein pRb49 and to the TAZ2 domain of CBP/p300,50 and can induce oncogenic transformation. It contains four constant regions, and the same LxCxE motif as E7 (Figure 7), but its mode of interaction is quite different.
Figure 7.
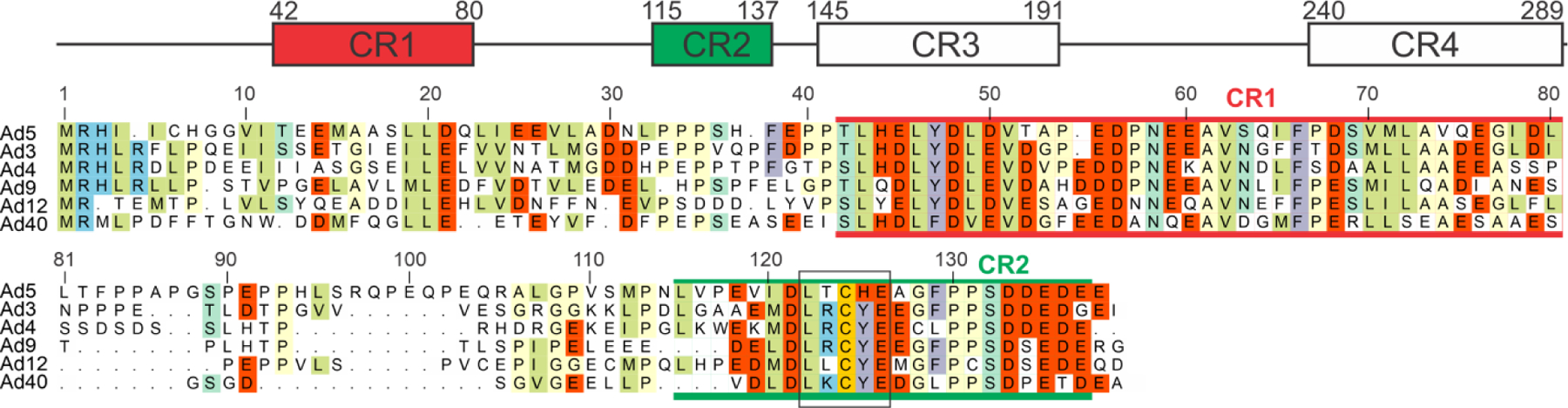
Domain schematic of E1A, showing the four conserved regions (CR1–4) and the sequence comparison for residues 1–138 for several strains of adenovirus.
E1A binds to TAZ2 primarily in CR1 (residues 53–83), giving rise to a high-affinity complex for which a solution structure was calculated (Figure 8A).51 Interestingly, the N-terminal region of E1A also interacts with TAZ2, such that the affinity of E1A(1–93) is significantly greater than that of E1A(53–93) but the interaction causes resonance broadening due to intermediate exchange,52 so this complex cannot be characterized structurally. The E1A protein interacts with pRbAB using the LxCxE site in CR2, giving rise to a ternary complex that is shown as a model in Figure 8B. The allosteric interactions involved in this complicated ternary interaction were modeled using the results of single-molecule FRET experiments.53
Figure 8.
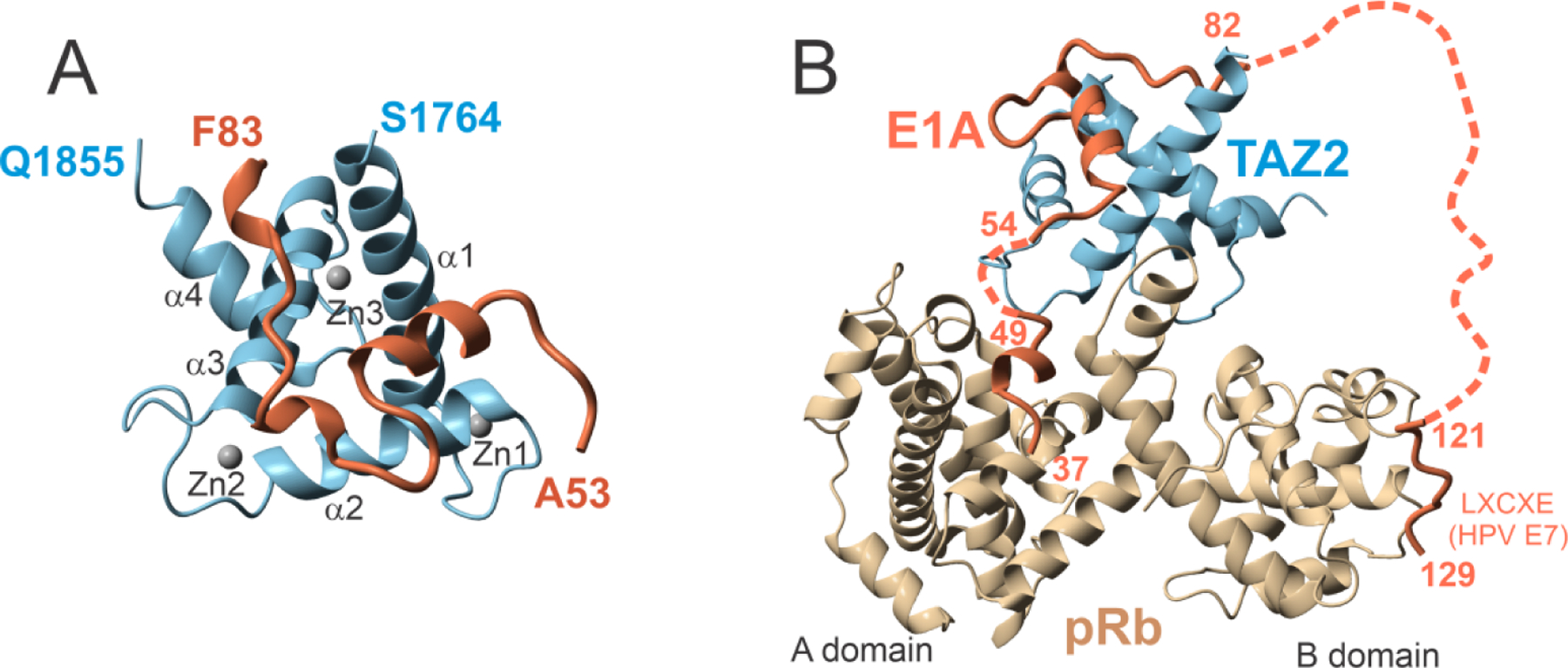
Structure of E1A with partners. A. Ribbon representation of the lowest-energy structure of TAZ2 (residues 1764–1855 of CBP) in complex with E1A (residues 53–83). Zinc atoms are shown as gray spheres. B. Structural model of the ternary complex of E1A, TAZ2 and pRb, generated using the crystal structure of pRb in complex with E1A (PDB 2R7G)54, the NMR structure of TAZ2 in complex with E1A (PDB 2KJE)51, and the crystal structure of the LxCxE region of the HPV E7 peptide (homologous to residues 122–125 of E1A) (PDB 1GUX)37. The flexible linker between CR1 and CR2 is represented by a dotted line. (Figures adapted from reference51 with permission).
Again, the disordered domains of the viral oncoproteins act by subverting normal cellular interactions (also mostly involving IDPs) and diverting normal functions towards viral processes. The versatility of the viral proteins and the role of disorder in their exceptional success is also illustrated by alternative interactions of the same E1A protein with a different domain of CBP-p300: E1A also interacts with the KIX domain55 and nuclear coactivator binding domain56 of CBP/p300. The extensive overall interactome of E1A qualifies it as a hub protein.47,57,58 A useful summary of E1A interactions is shown in Figure 1 of reference.57
IDPs from the Major Eukaryotic Realm Riboviria
Viruses with RNA genomes form the most common group that infect eukaryotes, frequently appearing as the causative agents of well-known diseases. The viral families Flaviviridae (containing Dengue, Zika, West Nile) Paramyxoviridae (containing Measles, Nipah, Hendra), Orthomyxoviridae (Influenza), Coronaviridae (SARS), Hepadnaviridae (Hepatitis), Filoviridae (Ebola), and Retroviridae (Respiratory Syncytial Virus, HIV, HTLV) constitute a catalogue of recent human plagues. The relevance of these viruses to human health has provided an incentive for study, and many of these systems have been examined – a majority if not all of them contain intrinsically disordered proteins as a vital and indispensable part of their armory.
IDPs in the Flavivirus Family
Detailed reviews of the role of IDPs in flaviviruses and their connections to disease have recently been published.59,60 Consistent with the aims of the present review, the following section contains only a selection of short stories of particular interest.
The genome of Dengue virus, and the related Zika and West Nile viruses, consists of a single positive-sense single-stranded RNA that codes for a single polyprotein, which is cleaved into 10 proteins, 3 structural (capsid C, precursor membrane prM and envelope glycoprotein E) and 7 non-structural (Figure 9).
Figure 9.

Schematic diagram showing the structural and nonstructural proteins in the Dengue polyprotein. C: capsid; prM: precursor membrane; E: envelope; NS1, 2A, 2B, 3, 4A, 4B, 5: non-structural proteins. Adapted from 61.
Dengue virus has a nucleocapsid that surrounds the viral RNA core, and an outer lipid coat that interacts with cellular membranes, especially the endoplasmic reticulum. The capsid protein is a homodimer with an N-terminal sequence that was shown to be disordered by time-resolved fluorescence anisotropy decay.62 Variability between strains occurs mostly within the disordered region, which appears to be correlated with levels of virulence.63 The disordered region is also crucial for the interaction of the virus with intracellular lipid droplets, a necessity for viral replication.64
Flavivirus IDPs Act as RNA Chaperones
The three genera of flaviviruses, Flavivirus, Pestivirus and Hepacivirus, have a common architecture, but show very little sequence similarity in their proteins. In particular the core, or capsid protein commonly contains a structured region and a disordered region, as for Dengue. The disordered region of the capsid protein has been found to act as an RNA chaperone as part of its function in packaging of the RNA genome, similar to the capsid proteins of other retroviruses such as HIV.65,66 This chaperone function has been directly tied to the intrinsic disorder of the capsid protein, through coupled folding and binding of disordered regions with kinetically trapped RNA, and has been characterized in several viruses of this type.67 The mechanism of assembly of the Hepatitis C virus was recently probed by single-molecule FRET,68 which showed that the disorder of the core protein was an important component of the complex assembly mechanism.
IDPs in the Paramyxoviruses
The Paramyxovirus family includes a large number of serious human pathogens (measles, mumps, rabies, Ebola, Hendra and Nipah). They contain negative-strand encapsidated RNA genomes that are processed by a viral RNA polymerase, through a mechanism that has received a lot of attention but is still not fully deciphered. A comprehensive review of the extensive literature on the structural biology of paramyxoviruses was recently published.69 In the following section, I will briefly review some of the features of this group that most typify the role of disorder in the “life”-cycles of these viruses.
To begin with, I show a summary in Figure 10, reproduced from reference.69 The negative-sense RNA genome (Figure 10A) codes for six proteins, the nucleoprotein N, the phosphoprotein P, matrix protein M, fusion protein F and large protein L. The genome is replicated from both the 3’ end and the 5’ end, and transcription occurs only from the 3’ end (Figure 10C). Structures of the N, P and L proteins, with disordered regions shown as dotted lines, are shown in Figure 10B.
Figure 10.
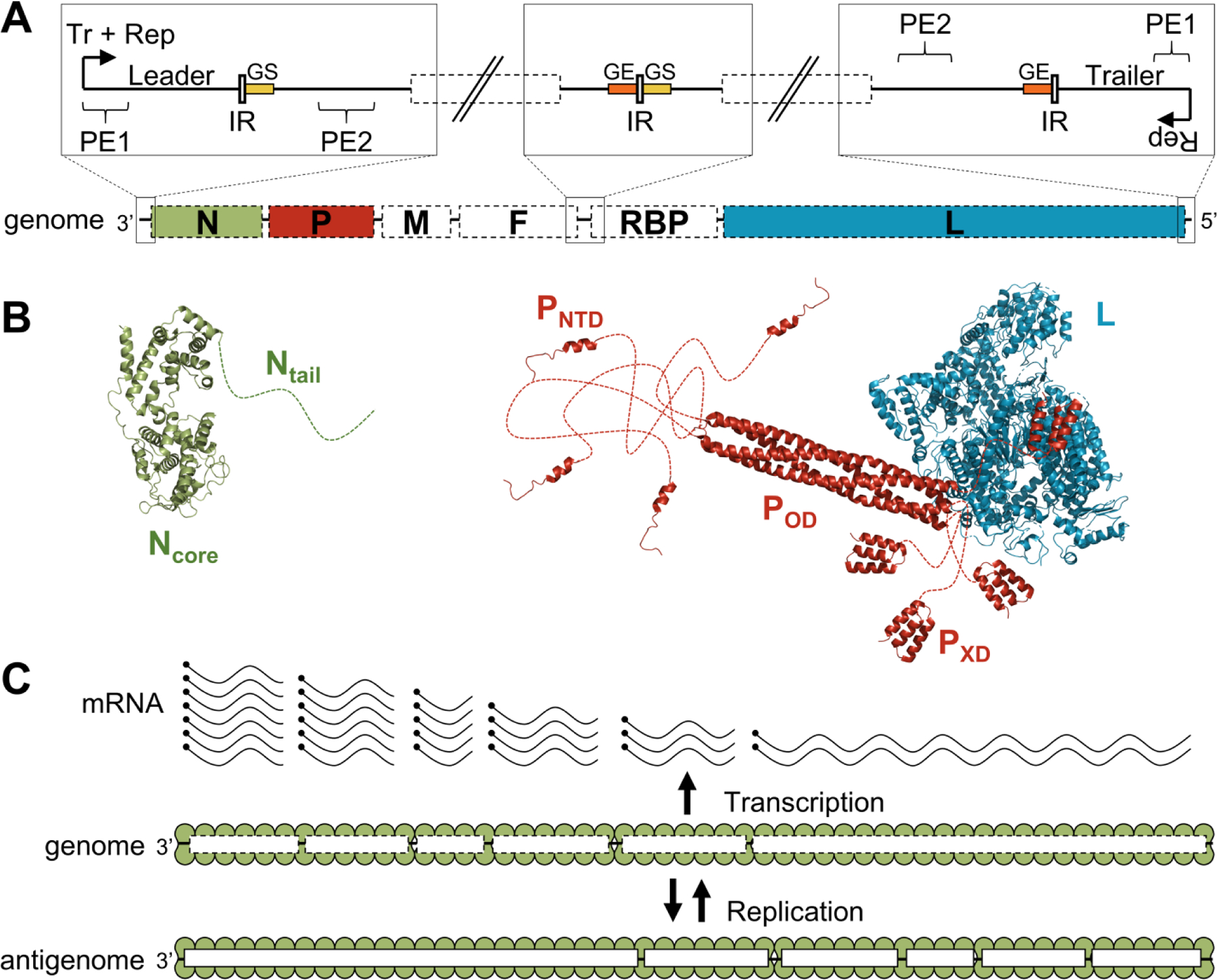
Organization and structure of the components of the RNA synthesis machinery in paramyxoviruses. (A) Schematic representation of the viral genome and the regulatory elements. The genome contains at least six conserved adjacent genes separated by intergenic regions (IR). These genes encode for the nucleoprotein (N), the phosphoprotein (P), the matrix protein (M), the fusion protein (F), the receptor-binding protein (RBP) and the large protein (L). The promoters located at the 3’ end of the genome (transcription and replication) and the antigenome (replication only) are bipartite and made of two promoter elements (PE1 and PE2). The transcription of each gene starts on a “gene start” signal (GS, in yellow) and ends on a “gene end” signal (GE, orange). (B) Cartoon representation of the structure of the protein components of the RNA synthesis machinery of parainfluenza virus 5 (PIV5) (N, PDB: 4XJN;70 PNTD, PDB: 5WKN;71 POD, PXD, and L, PDB: 6V8572). Disordered regions are represented as dotted lines. (C) Schematic representation of viral transcription and replication. Encapsidated genomes are transcribed into a gradient of mRNAs, Replication of the genomes requires the production of encapsidated genome intermediates. Figure reproduced from reference69 with permission.
Paramyxovirus Replication Proteins
The paramyxovirus nucleoprotein consists of a folded core domain NCORE and a disordered tail NTAIL.73,74 Disorder in the nucleoproteins of Nipah75 and Sendai76 viruses has been characterized by NMR, but the measles virus nucleoprotein has received the most attention. In measles virus, NCORE consists of residues 1–400, and NTAIL residues 401–525. The isolated NTAIL undergoes coupled folding upon binding to the C-terminal domain (termed XD) of its partner, the viral phosphoprotein,73 and the interaction involves two sites termed α-helical molecular recognition elements (α-MoREs).74 Further, small-angle X-ray scattering measurements on the phosphoprotein-NTAIL complex showed that the majority of the NTAIL remained disordered even in the complex,77 providing an example of a fuzzy complex.42 Nevertheless, numerous studies have implicated sequence-dependent helical structure in the NTAIL upon binding to the XD domain of the phosphoprotein.78–85 The structure and interactions of the phosphoprotein have also been described for the Sendai virus,86,87 the rabies virus,88 and the parainfluenza virus.89
Several recent studies have focused on the structure and dynamics of the phosphoprotein X domain (PXD) alone.90–92 In particular, the dynamics of this folded partner protein were recently shown to control the interactions of NTAIL.93 The complexity of the interactions between the nucleoprotein and the phosphoprotein is illustrated in Figure 11A. The paper93 provides a comprehensive experimental exploration of the structures present in the conformational ensemble of PXD, only one of which is competent for binding to NTAIL (Figure 11B).
Figure 11.
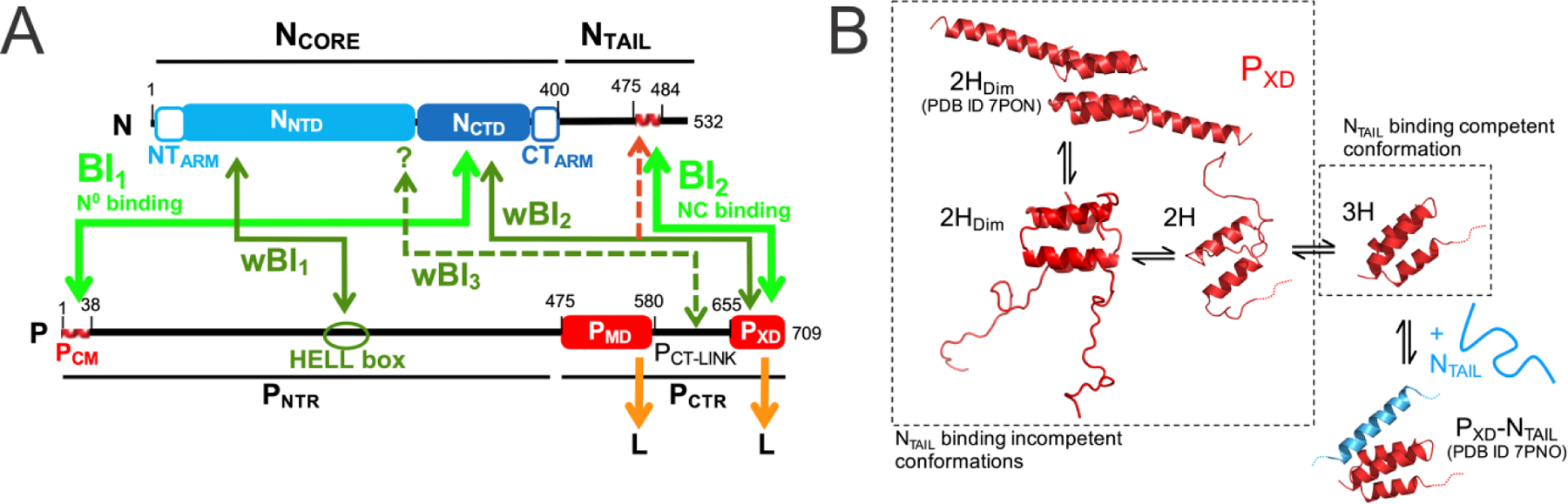
Dynamics and Interactions of the Nipah virus (NiV) phosphoprotein. A. Schematic representation of the NiV phosphoprotein (P) and nucleoprotein (N). The black lines represent disordered regions, red waved lines represent α-helical molecular recognition elements (α-MoRE) and colored boxes represent structured domains. Green arrows indicate known interactions between N and P. Dotted arrows indicate interactions with imprecisely defined binding sites. Orange arrows show the known interaction sites with L. NTARM = N-terminal arm region of N; NNTD = N-terminal domain of N; NCTD = C-terminal domain of N; CTARM = C-terminal arm region of N; PCM =N0 chaperone module of P; PMD = multimerization domain of P; PXD = X domain of P; PNTR and PCTR = N- and C-terminal regions of P. B. Schematic representation of the conformational equilibrium of PXD and of its interaction with NTAIL. Conformers labeled 2HDim, 2H and 3H co-exist in solution. Crystal structures of PXD alone (PDB 7PON)93 and in complex with NTAIL (PDB 7PNO)93 are also shown. Figure adapted from reference93 with permission.
The prevalence of structural disorder and the interplay between structured, partly-structured and disordered domains in the replication complexes of paramyxoviruses has been invoked to propose a complex mechanism for virus replication and nucleocapsid assembly,94,95 where the assembled nucleocapsid retains the NTAIL portion of the nucleocapsid as a disordered recognition sequence (Figure 12).
Figure 12.
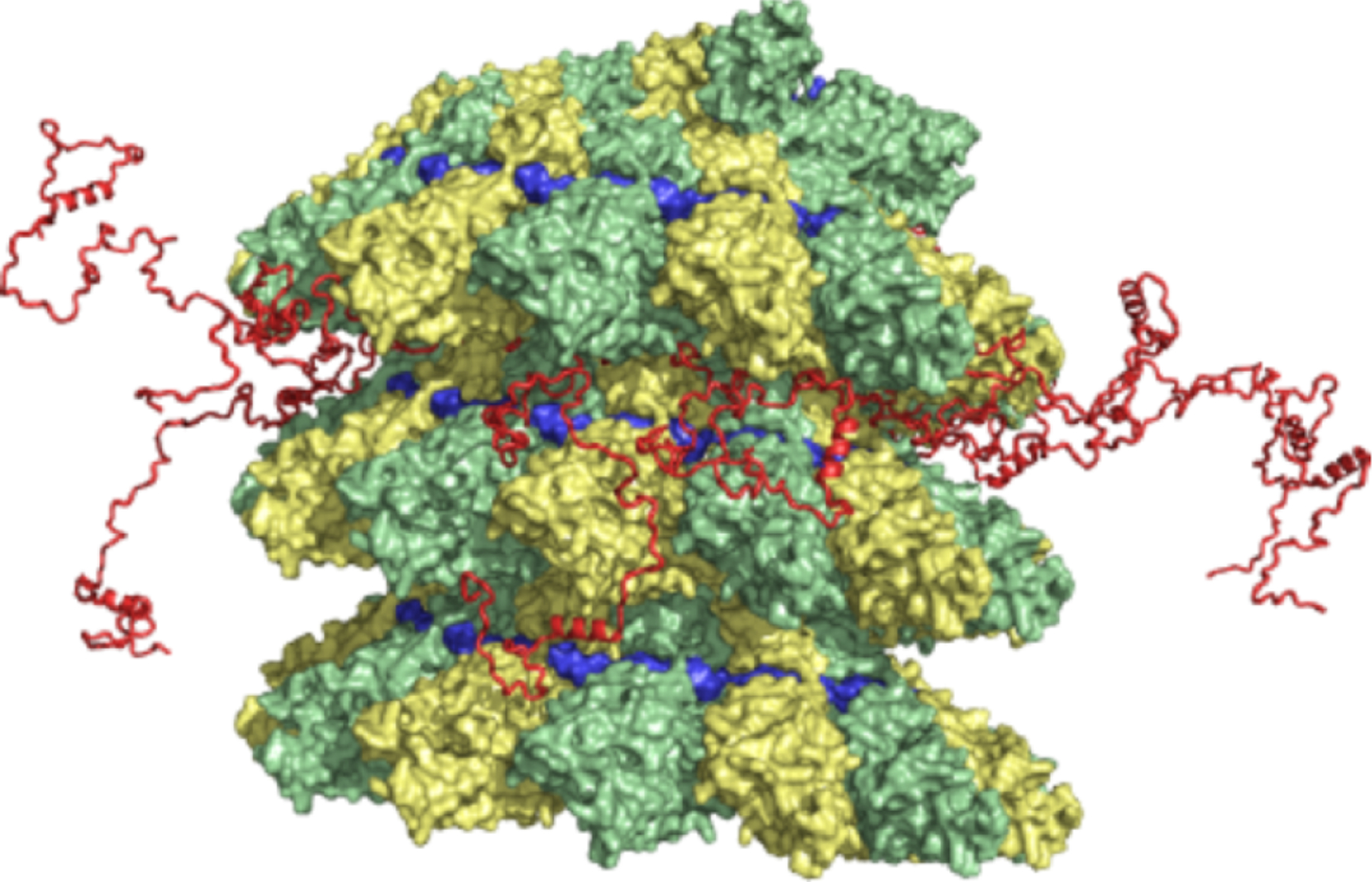
Proposed model of the location of NTAIL in intact nucleocapsids.94 The three-dimensional coordinates of the RSV N-RNA subunit docked into the EM density map of measles virus N-RNA were used.96 The conformational sampling algorithm flexible-meccano was used to build chains from the C-terminus of the folded domain of NCORE (successive NCORE monomers are colored green and yellow), allowing prediction of NMR parameters measured in solution. The modeled NTAIL is shown in red, with 13 NTAIL conformers from a single turn of capsid shown. The position of the RNA is shown in blue. Figure adapted from reference94 with permission.
IDPs in Retroviruses
Retroviruses include human immunodeficiency virus (HIV) and human T-cell lymphotrophic virus (HTLV). Several HIV proteins are intrinsically disordered and fold upon binding to their partners: the HIV transactivator of transcription (Tat) protein is extensively disordered,97,98 as is the C-terminal domain of the HIV Rev protein.99,100 The Tat protein binds to the 59-nucleotide stem-loop TAR RNA, and subsequently utilizes disorder to recruit and assemble cellular proteins to initiate viral replication.
Infection by HTLV1 causes several lymphocyte diseases, including adult T-cell leukemia.101,102 Two oncoproteins have been identified, Tax and HBZ. Tax is required for cellular transformation by the virus, activating viral gene transcription by binding to one of the interaction sites on the CBP KIX domain103 (Figure 13).
Figure 13.
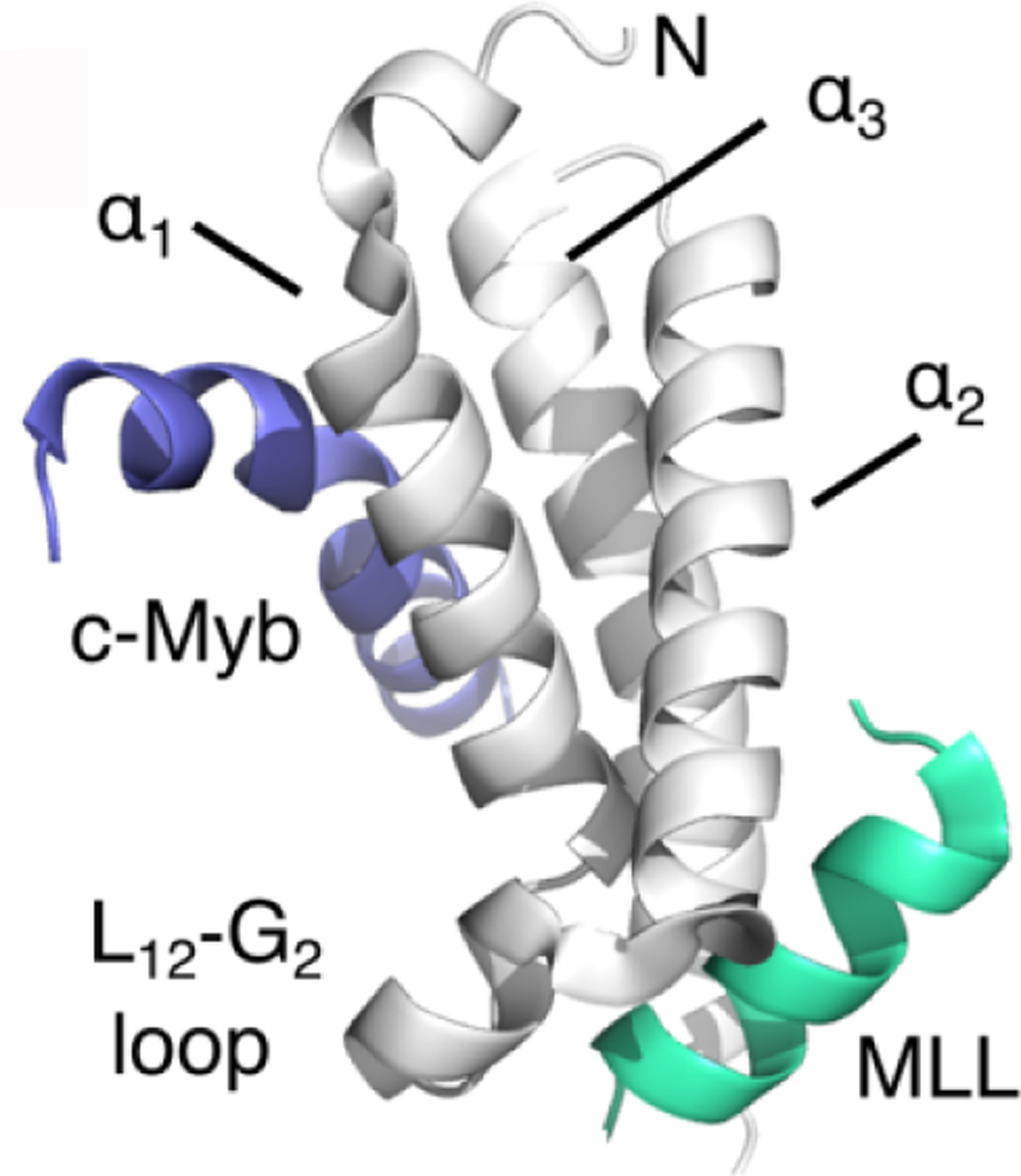
Structure of the KID:cMyb:MLL ternary complex (PDB 2AGH103) showing the binding sites on KIX (gray) occupied by the activation domains of c-Myb (blue) and MLL (green).
Simultaneous binding of Tax (in the MLL site) and pCREB (in the c-Myb site) recruits CBP/p300 to the HTLV promoter, enabling transcription of viral genes.104 The Tax protein appears to be largely disordered (there is a possible zinc finger towards the N-terminus according to UniProt), and, like many other IDPs, it has an extensive interactome.105 The Tax protein binds to the KIX domain in the MLL site and induces a strong cytotoxic T-cell response.104 The second oncoprotein, HBZ, modulates this response by competing with Tax for binding to KIX, down-regulating Tax-induced transcription, and thus contributing to the persistence of HTLV. The HBZ protein contains a disordered activation domain, several basic regions, and a C-terminal leucine zipper (Figure 14).
Figure 14.
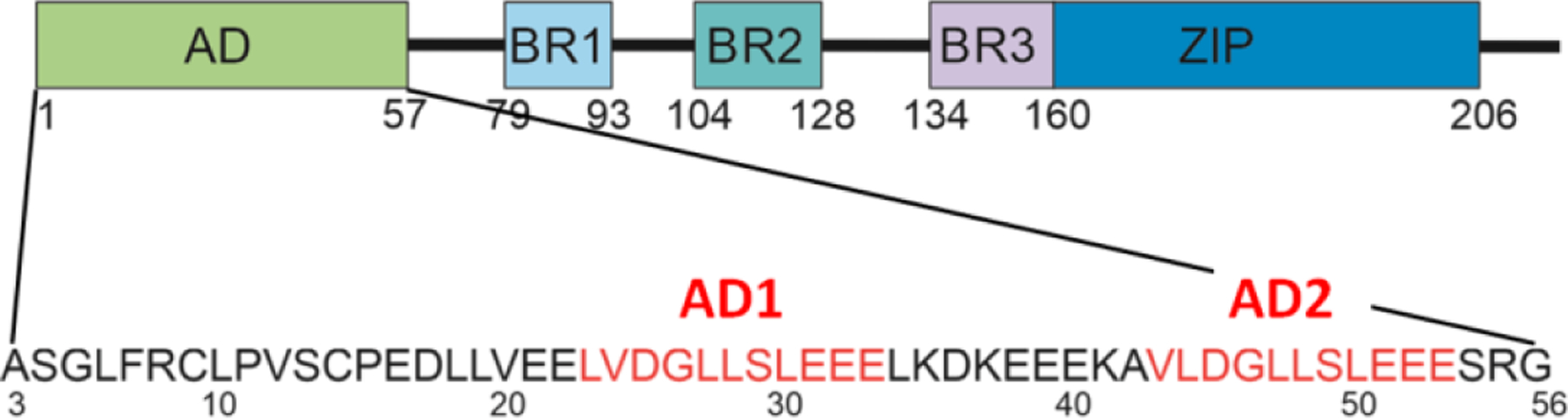
Domain structure of HBZ, showing the amino acid sequence of the disordered activation domain. AD, activation domain; BR1, 2, 3, basic regions; ZIP, leucine zipper. The two sequence motifs, AD1 and AD2, are shown in red.
The activation domain contains two motifs that interact with KIX, and participates in one of the few crystal structures obtained for disordered protein complexes106 (Figure 15).
Figure 15.
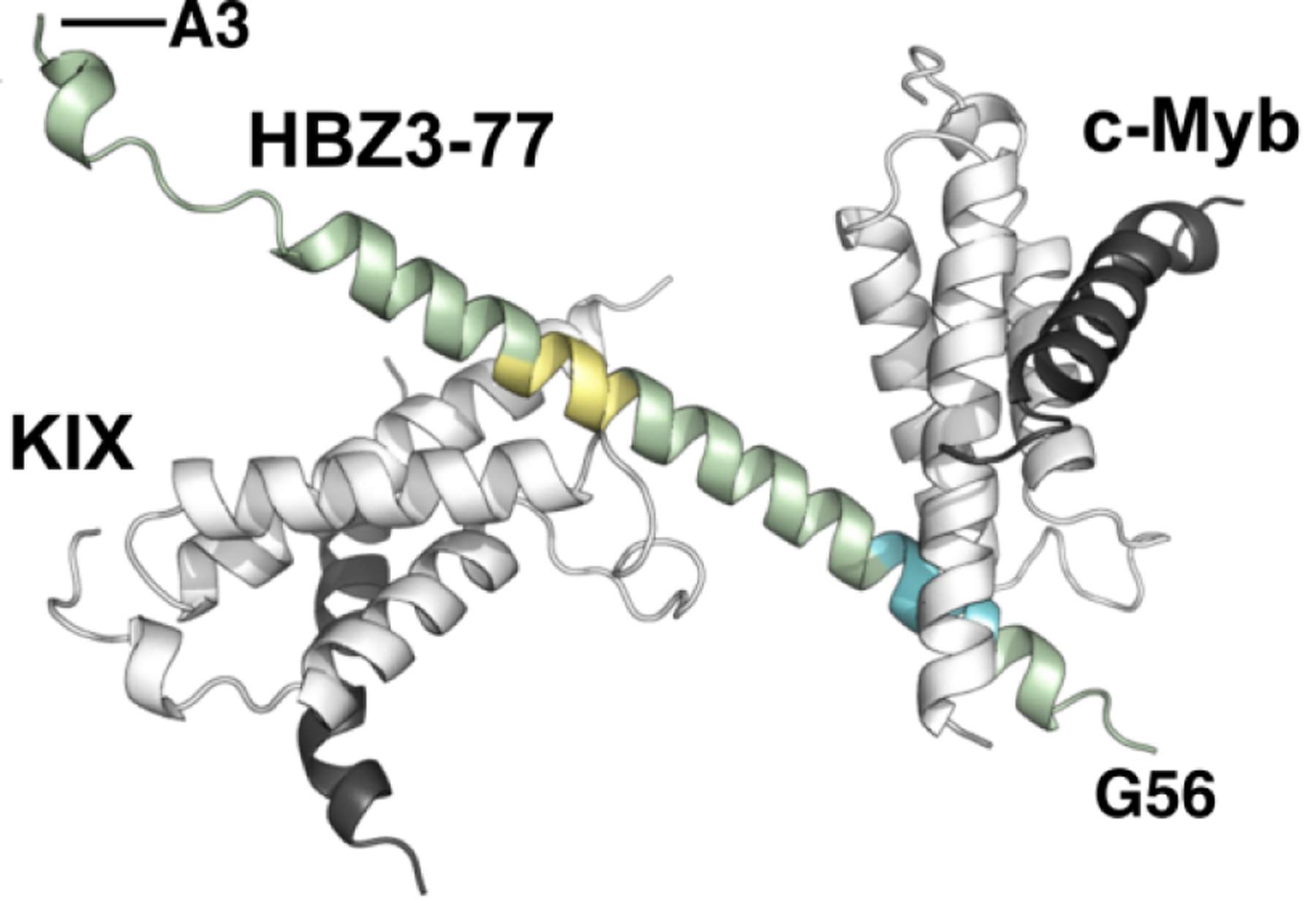
Crystal structure of the ternary complex of KIX (gray), c-Myb (black), and HBZ(3–77) (green). AD1 is shown in yellow and AD2 in blue.
Isothermal titration calorimetry (ITC) measurements show that the affinity of KIX for HBZ is greatly enhanced in a ternary KIX:HBZ:c-Myc complex, compared with that of the binary KIX:HBZ and KIX:c-Myb complexes.106 Control of hematopoiesis, the production of blood cells, is mediated by the transcription factor c-Myb. It is tempting to speculate that the enhanced affinity of c-Myb for KIX when HBZ is present provides a molecular mechanism for the loss of control of leukocyte proliferation in HTLV1 leukemia.
IDPs in Other RNA Viruses
IDPs have been detected and studied in a variety of different RNA viruses. Two virus families of particular medical interest have received a great deal of attention in the literature. Both influenza virus (Orthomyxoviridae) and the SARS viruses (Coronaviridae) employ disorder extensively, and disorder has been correlated with virulence for influenza.107 A distinctive region of the hemagglutinin protein (HA) was predicted to be disordered in virulent influenza strains, such as the 1918 H1N1 and H5N1 strains, but not in less virulent strains.107
The disordered C-terminus of the non-structural protein NS1 of the influenza virus appears to be variable between strains, and gives rise to different cellular interactions depending on the strain. The 1918 flu strain makes a high-affinity fuzzy interaction between a C-terminal disordered proline-rich domain and the cellular signaling adaptor CRKII.108,109 The same region of the same protein NS1 from the H3N2 strain was found to interact with the ATPase MORC3: its disordered C-terminal domain binds to cellular MORC3 and displaces histone H3.110,111
Coronaviruses have of course been of great interest. Both the original SARS coronavirus and the recent SARS CoV2 viruses are replete with disordered interaction domains, fuzzy interactions, and phase-separation (see following section). The SARS replicase nonstructural protein has an extensive C-terminal disordered region,112 and the disordered N-terminal region of the nucleocapsid protein is the binding site for nucleic acid.113 For SARS CoV2, the spike protein, targeted by the majority of the Covid vaccines, has been a popular subject. Mutations in variants that have arisen during the pandemic occur frequently in disordered areas of the spike protein, emphasizing the importance of disorder in immune evasion.114
Intrinsic Disorder and Biomolecular Condensates
The focus of much cellular biology has traditionally been on parts of the eukaryotic cell that are bounded by membranes: nuclei, mitochondria, endoplasmic reticulum, Golgi body. More recently, it has been recognized that a considerable amount of the chemistry of the cell takes place in non-membrane-bound organelles. Examples include (in the nucleus): nucleoli, P bodies (P granules), Cajal bodies, paraspeckles, and (in the cytoplasm) stress granules and centrosomes.(reviewed in 115). Reviews have been published on the involvement of intrinsically disordered proteins, as well as nucleic acids, in the formation of these bodies by liquid-liquid phase separation,116 and on the particular relevance of the concept of phase separation to the operation of viruses.117
Phase separation has been invoked to explain the assembly of the tegument layer of herpesviruses (Figure1)14 and also appears to be an important characteristic of the replication compartments used during lysis by herpes viruses and in the nuclear inclusions or speckles formed during viral latency.118 Phase-separated disordered proteins and nucleic acids have been described as “viral replication factories” and have been observed as the cytoplasmic Negri bodies of rabies virus,119 and for rotaviruses120,121 and measles virus.122 123 Phase separation has also been observed for the SARS CoV2 N protein124–126 and appears to be a driving force for the activation of the inflammasome in infections by RNA viruses.127
III. FUTURE PERSPECTIVES
The involvement of intrinsically disordered proteins and liquid-liquid phase separation in the interactions of viruses and their hosts is only just becoming clear. We should expect a great deal more information to be forthcoming in the next few years.
HIGHLIGHTS.
Viruses contain minimal genetic material, which they must use with the utmost efficiency
Part of this efficiency resides in the employment of intrinsically disordered proteins for interactions in the cell involved with infection, hijacking of cellular processes, viral replication, and assembly of new viruses
Disordered proteins have been found among many, if not all viruses so far studied
ACKNOWLEDGEMENTS
The analysis described in this review has been part of ongoing collaborative research between Peter Wright and Jane Dyson. By rights, Peter should have been an author on this manuscript, but since it was meant to be part of a surprise special issue of JMB, the editors and I decided that it would be written by me alone. Although Peter and I have done all of our IDP work together, I take full personal responsibility for all errors and omissions in the current manuscript.
I would like to thank Louis-Marie Bloyet, Marc Jamin, and Martin Blackledge for providing materials and helpful comments for Figures 10, 11, and 12.
The author gratefully acknowledges support from grant GM131693 from the National Institutes of Health.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
I do not know what a credit author statement is
The author declares no conflict of interest
REFERENCES
- 1.Wright PE, Dyson HJ, (1999). Intrinsically unstructured proteins: Re-assessing the protein structure-function paradigm. J. Mol. Biol, 293, 321–331. [DOI] [PubMed] [Google Scholar]
- 2.Piersimoni L, Abd el Malek M, Bhatia T, Bender J, Brankatschk C, Calvo Sánchez J, Dayhoff GW, Di Ianni A, Figueroa Parra JO, Garcia-Martinez D, Hesselbarth J, Köppen J, Lauth LM, Lippik L, Machner L, Sachan S, Schmidt L, Selle R, Skalidis I, Sorokin O, Ubbiali D, Voigt B, Wedler A, Wei AAJ, Zorn P, Dunker AK, Köhn M, Sinz A, Uversky VN, (2022). Lighting up Nobel Prize-winning studies with protein intrinsic disorder. Cell. Mol. Life Sci, 79, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iakoucheva LM, Brown CJ, Lawson JD, Obradovic Z, Dunker AK, (2002). Intrinsic disorder in cell-signaling and cancer-associated proteins. J. Mol. Biol, 323, 573–584. [DOI] [PubMed] [Google Scholar]
- 4.Wright PE, Dyson HJ, (2015). Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol, 16, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davey NE, Travé G, Gibson TJ, (2011). How viruses hijack cell regulation. Trends Biochem. Sci, 36, 159–169. [DOI] [PubMed] [Google Scholar]
- 6.Brown N, Bhella D, (2016). Are viruses alive? Microbiol. Today, 43, 58–61. [Google Scholar]
- 7.Mughal F, Nasir A, Caetano-Anollés G, (2020). The origin and evolution of viruses inferred from fold family structure. Arch. Virol, 165, 2177–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gitlin L, Hagai T, LaBarbera A, Solovey M, Andino R, (2014). Rapid Evolution of Virus Sequences in Intrinsically Disordered Protein Regions. PLoS Pathogens, 10, e1004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorbalenya AE, Krupovic M, Mushegian A, Kropinski AM, Siddell SG, Varsani A, Adams MJ, Davison AJ, Dutilh BE, Harrach B, Harrison RL, Junglen S, King AMQ, Knowles NJ, Lefkowitz EJ, Nibert ML, Rubino L, Sabanadzovic S, Sanfaçon H, Simmonds P, Walker PJ, F.M., Z., Kuhn JH, (2020). The new scope of virus taxonomy: partitioning the virosphere into 15 hierarchical ranks. Nat. Microbiol, 5, 668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar N, Kaushik R, Tennakoon C, Uversky VN, Longhi S, Zhang KYJ, Bhatia S, (2021). Comprehensive intrinsic disorder analysis of 6108 viral proteomes: from the extent of intrinsic disorder penetrance to functional annotation of disordered viral proteins. J. Proteome Res, 20, 2704–2713. [DOI] [PubMed] [Google Scholar]
- 11.McPherson A, (2005). Micelle formation and crystallization as paradigms for virus assembly. Bioessays, 27, 447–458. [DOI] [PubMed] [Google Scholar]
- 12.Rao AL, (2006). Genome packaging by spherical plant RNA viruses. Annu. Rev. Phyotopath, 44, 61–87. [DOI] [PubMed] [Google Scholar]
- 13.Cadena-Nava RD, Comas-Garcia M, Garmann RF, Rao AL, Knobler CM, Gelbart WM, (2012). Self-assembly of viral capsid protein and RNA molecules of different sizes: requirement for a specific high protein/RNA mass ratio. J. Virol, 86, 3318–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metrick CM, Koenigsberg AL, Heldwein EE, (2020). Conserved outer tegument component UL11 from herpes simplex Virus 1 Is an intrinsically disordered, RNA-binding protein. mBio, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juillard F, de Miranda MP, Li S, Franco A, Seixas AF, Liu B, Álvarez ÁL, Tan M, Szymula A, Kaye KM, Simas JP, (2020). KSHV LANA acetylation-selective acidic domain reader sequence mediates virus persistence. Proc. Natl. Acad. Sci. USA, 117, 22443–22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballestas ME, Kaye KM, (2011). The latency-associated nuclear antigen, a multifunctional protein central to Kaposi’s sarcoma-associated herpesvirus latency. Future Microbiol, 6, 1399–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Kon N, Tavana O, Gu W, (2017). The “readers” of unacetylated p53 represent a new class of acidic domain proteins. Nucleus, 8, 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks CL, Gu W, (2003). Ubiquitination, phosphorylation and acetylation: The molecular basis for p53 regulation. Curr. Opin. Cell Biol, 15, 164–171. [DOI] [PubMed] [Google Scholar]
- 19.Friedler A, Veprintsev DB, Freund SMV, von Glos KI, Fersht AR, (2005). Modulation of binding of DNA to the C-terminal domain of p53 by acetylation. Structure, 13, 629–636. [DOI] [PubMed] [Google Scholar]
- 20.Mujtaba S, He Y, Zeng L, Yan S, Plotnikova O, Sachchidanand, Sanchez R, Zeleznik L, Ronai Z, Zhou MM, (2004). Structural mechanism of the bromodomain of the coactivator CBP in p53 transcriptional activation. Mol. Cell, 13, 251–263. [DOI] [PubMed] [Google Scholar]
- 21.Vassall KA, Bamm VV, Harauz G, (2015). MyelStones: the executive roles of myelin basic protein in myelin assembly and destabilization in multiple sclerosis. Biochem. J, 472, 17–32. [DOI] [PubMed] [Google Scholar]
- 22.Tejada-Simon MV, Zang YC, Hong J, Rivera VM, Zhang JZ, (2003). Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann. Neurol, 53, 189–197. [DOI] [PubMed] [Google Scholar]
- 23.Kakalacheva K, Münz C, Lünemann JD, (2011). Viral triggers of multiple sclerosis. Biochim. Biophys. Acta, 1812, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rojas M, Restrepo-Jiménez P, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, Leung PSC, Ansari AA, Gershwin ME, Anaya JM, (2018). Molecular mimicry and autoimmunity. J Autoimmun, 95, 100–123. [DOI] [PubMed] [Google Scholar]
- 25.Xue B, Ganti K, Rabionet A, Banks L, Uversky VN, (2014). Disordered interactome of human papillomavirus. Curr. Pharm. Des, 20, 1274–1292. [DOI] [PubMed] [Google Scholar]
- 26.Monteiro J, (2017). Two basic scientists walk into a translational space. Cell, 171, 23–27. [DOI] [PubMed] [Google Scholar]
- 27.Mirabello L, Yeager M, Yu K, Clifford GM, Xiao Y, Zhu B, Cullen M, Boland JF, Wentzensen N, Nelson CW, Raine-Bennett T, Chen Z, Bass S, Song L, Yang Q, Steinberg M, Burdett L, Dean M, Roberson D, Mitchell J, Lorey T, Franceschi S, Castle PE, Walker J, Zuna R, Kreimer AR, Beachler DC, Hildesheim A, Gonzalez P, Porras C, Burk RD, Schiffman M, (2017). HPV16 E7 genetic conservation is critical to carcinogenesis. Cell, 170, 1164–1174.e1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Clements A, Zhao K, Marmorstein R, (2006). Structure of the human papillomavirus E7 oncoprotein and its mechanism for inactivation of the retinoblastoma tumor suppressor. J. Biol. Chem, 281, 578–586. [DOI] [PubMed] [Google Scholar]
- 29.Chemes LB, Glavina J, Faivovich J, De Prat-Gay G, Sánchez IE, (2012). Evolution of linear motifs within the papillomavirus E7 oncoprotein. J. Mol. Biol, 422, 336–346. [DOI] [PubMed] [Google Scholar]
- 30.Chemes LB, Glavina J, Alonso LG, Marino-Buslje C, De Prat-Gay G, Sanchez IE, (2012). Sequence evolution of the intrinsically disordered and globular domains of a model viral oncoprotein. PLoS ONE, 7, e47661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borkosky SS, Camporeale G, Chemes LB, Risso M, Noval MG, Sánchez IE, Alonso LG, de Prat Gay G, (2017). Hidden structural codes in protein intrinsic disorder. Biochemistry, 56, 5560–5569. [DOI] [PubMed] [Google Scholar]
- 32.Noval MG, Gallo M, Perrone S, Salvay AG, Chemes LB, De Prat-Gay G, (2013). Conformational dissection of a viral intrinsically disordered domain involved in cellular transformation. PLoS ONE, 8, e72760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kukic P, Lo Piccolo GM, Nogueira MO, Svergun DI, Vendruscolo M, Felli IC, Pierattelli R, (2019). The free energy landscape of the oncogene protein E7 of human papillomavirus type 16 reveals a complex interplay between ordered and disordered regions. Sci. Rep, 9, 5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calçada EO, Felli IC, Hosek T, Pierattelli R, (2013). The heterogeneous structural behavior of E7 from HPV16 revealed by NMR spectroscopy. Chembiochem, 14, 1876–1882. [DOI] [PubMed] [Google Scholar]
- 35.Uversky VN, Roman A, Oldfield CJ, Dunker AK, (2006). Protein intrinsic disorder and human papillomaviruses: Increased amount of disorder in E6 and E7 Oncoproteins from high risk HPVs. J. Proteome Res, 5, 1829–1842. [DOI] [PubMed] [Google Scholar]
- 36.Dyson HJ, Wright PE, (2018). How do intrinsically disordered viral proteins hijack the cell? Biochemistry, 57, 4045–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JO, Russo AA, Pavletich NP, (1998). Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature, 391, 859–865. [DOI] [PubMed] [Google Scholar]
- 38.Chemes LB, Sánchez IE, De Prat-Gay G, (2011). Kinetic recognition of the retinoblastoma tumor suppressor by a specific protein target. J. Mol. Biol, 412, 267–284. [DOI] [PubMed] [Google Scholar]
- 39.Jansma AL, Martinez-Yamout MA, Liao R, Sun P, Dyson HJ, Wright PE, (2014). The high-risk HPV16 E7 oncoprotein mediates interaction between the transcriptional coactivator CBP and the retinoblastoma protein pRb. J. Mol. Biol, 426, 4030–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chemes LB, Sanchez IE, Smal C, De Prat-Gay G, (2010). Targeting mechanism of the retinoblastoma tumor suppressor by a prototypical viral oncoprotein. FEBS J, 277, 973–988. [DOI] [PubMed] [Google Scholar]
- 41.Risør MW, Jansma AL, Medici N, Thomas B, Dyson HJ, Wright PE, (2021). Characterization of the high-affinity fuzzy complex between the disordered domain of the E7 oncoprotein from high-risk HPV and the TAZ2 domain of CBP. Biochemistry, 60, 3887–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tompa P, Fuxreiter M, (2008). Fuzzy complexes: polymorphism and structural disorder in protein-protein interactions. Trends Biochem. Sci, 33, 2–8. [DOI] [PubMed] [Google Scholar]
- 43.Alonso LG, Garcia-Alai MM, Smal C, Centeno JM, Iacono R, Castano E, Gualfetti P, De Prat-Gay G, (2004). The HPV 16 E7 viral oncoprotein self-assembles into defined spherical oligomers. Biochemistry, 43, 3310–3317. [DOI] [PubMed] [Google Scholar]
- 44.Putta S, Alvarez L, Lüdtke S, Sehr P, Müller GA, Fernandez SM, Tripathi S, Lewis J, Gibson TJ, Chemes LB, Rubin SM, (2022). Structural basis for tunable affinity and specificity of LxCxE-dependent protein interactions with the retinoblastoma protein family. Structure, 30, 1340–1353.e1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeong H, Mason SP, Barabási AL, Oltvai ZN, (2001). Lethality and centrality in protein networks. Nature, 411, 41–42. [DOI] [PubMed] [Google Scholar]
- 46.Han JD, Bertin N, Hao T, Goldberg DS, Berriz GF, Zhang LV, Dupuy D, Walhout AJ, Cusick ME, Roth FP, Vidal M, (2004). Evidence for dynamically organized modularity in the yeast protein-protein interaction network. Nature, 430, 88–93. [DOI] [PubMed] [Google Scholar]
- 47.Pelka P, Ablack JNG, Fonseca GJ, Yousef AF, Mymryk JS, (2008). Intrinsic structural disorder in adenovirus E1A: a viral molecular hub linking multiple diverse processes. J. Virol, 82, 7252–7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murrali MG, Felli IC, Pierattelli R, (2020). Adenoviral E1A exploits flexibility and disorder to target cellular proteins. Biomolecules, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whyte P, Buchkovich KJ, Horowitz JM, Friend SH, Raybuck M, Weinberg RA, Harlow E, (1988). Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature, 334, 124–129. [DOI] [PubMed] [Google Scholar]
- 50.Arany Z, Newsome D, Oldread E, Livingston DM, Eckner R, (1995). A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature, 374, 81–84. [DOI] [PubMed] [Google Scholar]
- 51.Ferreon JC, Martinez-Yamout MA, Dyson HJ, Wright PE, (2009). Structural basis for subversion of cellular control mechanisms by the adenoviral E1A oncoprotein. Proc. Natl. Acad. Sci. USA, 106, 13260–13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bain AD Chemical Exchange Effects in NMR☆. In: Lindon JC, Tranter GE, Koppenaal DW, editors. Encyclopedia of Spectroscopy and Spectrometry (Third Edition). Oxford: Academic Press; 2017. p. 180–187. [Google Scholar]
- 53.Ferreon AC, Ferreon JC, Wright PE, Deniz AA, (2013). Modulation of allostery by protein intrinsic disorder. Nature, 498, 390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Marmorstein R, (2007). Structure of the retinoblastoma protein bound to adenovirus E1A reveals the molecular basis for viral oncoprotein inactivation of a tumor suppressor. Genes Devel, 21, 2711–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fax P, Lipinski KS, Esche H, Brockmann D, (2000). cAMP-independent activation of the adenovirus type 12 E2 promoter correlates with the recruitment of CREB-1/ATF-1, E1A(12S), and CBP to the E2-CRE. J. Biol. Chem, 275, 8911–8920. [DOI] [PubMed] [Google Scholar]
- 56.Haberz P, Arai M, Martinez-Yamout MA, Dyson HJ, Wright PE, (2016). Mapping the interactions of adenoviral E1A proteins with the p160 nuclear receptor coactivator binding domain of CBP. Protein Sci, 25, 2256–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glavina J, Román EA, Espada R, de Prat-Gay G, Chemes LB, Sánchez IE, (2018). Interplay between sequence, structure and linear motifs in the adenovirus E1A hub protein. Virology, 525, 117–131. [DOI] [PubMed] [Google Scholar]
- 58.Hošek T, Calçada EO, Nogueira MO, Salvi M, Pagani TD, Felli IC, Pierattelli R, (2016). Structural and dynamic characterization of the molecular hub Early Region 1A (E1A) from human adenovirus. Chem. Eur. J, 22, 1–5. [DOI] [PubMed] [Google Scholar]
- 59.Kapuganti SK, Bhardwaj A, Kumar P, Bhardwaj T, Nayak N, Uversky VN, Giri R, (2022). Role of structural disorder in the multi-functionality of flavivirus proteins. Exp. Rev. Proteom, 1–14. [DOI] [PubMed] [Google Scholar]
- 60.Martins IC, Santos NC, (2020). Intrinsically disordered protein domains in flavivirus infection. Arch. Biochem. Biophys, 683, 108298. [DOI] [PubMed] [Google Scholar]
- 61.Rodenhuis-Zybert IA, Wilschut J, Smit JM, (2010). Dengue virus life cycle: viral and host factors modulating infectivity. Cell. Mol. Life Sci, 67, 2773–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faustino AF, Martins AS, Karguth N, Artilheiro V, Enguita FJ, Ricardo JC, Santos NC, Martins IC, (2019). Structural and functional properties of the capsid protein of dengue and related flavivirus. Int. J. Mol. Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goh GK-M, Dunker AK, Uversky VN, (2016). Correlating Flavivirus virulence and levels of intrinsic disorder in shell proteins: protective roles vs. immune evasion. Mol. Biosyst, 12, 1881–1891. [DOI] [PubMed] [Google Scholar]
- 64.Martins Ivo C., Gomes-Neto F, Faustino André F., Carvalho Filomena A., Carneiro Fabiana A., Bozza Patricia T., Mohana-Borges R, Castanho Miguel A.R.B., Almeida Fábio C.L., Santos Nuno C., Da Poian Andrea T., (2012). The disordered N-terminal region of dengue virus capsid protein contains a lipid-droplet-binding motif. Biochem. J, 444, 405–415. [DOI] [PubMed] [Google Scholar]
- 65.Cristofari G, Ivanyi-Nagy R, Gabus C, Boulant S, Lavergne JP, Penin F, Darlix JL, (2004). The hepatitis C virus Core protein is a potent nucleic acid chaperone that directs dimerization of the viral (+) strand RNA in vitro. Nucl. Acids Res, 32, 2623–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ivanyi-Nagy R, Kanevsky I, Gabus C, Lavergne JP, Ficheux D, Penin F, Fossé P, Darlix JL, (2006). Analysis of hepatitis C virus RNA dimerization and core-RNA interactions. Nucl. Acids Res, 34, 2618–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ivanyi-Nagy R, Lavergne JP, Gabus C, Ficheux D, Darlix JL, (2008). RNA chaperoning and intrinsic disorder in the core proteins of Flaviviridae. Nucl. Acids Res, 36, 712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holmstrom ED, Nettels D, Schuler B, (2017). Conformational plasticity of hepatitis C virus core protein enables RNA-induced formation of nucleocapsid-like particles. J. Mol. Biol, 430, 2453–2467. [DOI] [PubMed] [Google Scholar]
- 69.Bloyet LM, (2021). The nucleocapsid of paramyxoviruses: structure and function of an encapsidated template. Viruses, 13, 2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alayyoubi M, Leser GP, Kors CA, Lamb RA, (2015). Structure of the paramyxovirus parainfluenza virus 5 nucleoprotein-RNA complex. Proc. Natl. Acad. Sci. USA, 112, E1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aggarwal M, Leser GP, Kors CA, Lamb RA, (2018). Structure of the Paramyxovirus parainfluenza virus 5 nucleoprotein in complex with an amino-terminal peptide of the phosphoprotein. J. Virol, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdella R, Aggarwal M, Okura T, Lamb RA, He Y, (2020). Structure of a paramyxovirus polymerase complex reveals a unique methyltransferase-CTD conformation. Proc. Natl. Acad. Sci. USA, 117, 4931–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Longhi S, Receveur-Brechot V, Karlin D, Johansson K, Darbon H, Bhella D, Yeo R, Finet S, Canard B, (2003). The C-terminal domain of the measles virus nucleoprotein is intrinsically disordered and folds upon binding to the C-terminal moiety of the phosphoprotein. J. Biol. Chem, 278, 18638. [DOI] [PubMed] [Google Scholar]
- 74.Bourhis JM, Johansson K, Receveur-Brechot V, Oldfield CJ, Dunker KA, Canard B, Longhi S, (2004). The C-terminal domain of measles virus nucleoprotein belongs to the class of intrinsically disordered proteins that fold upon binding to their physiological partner. Virus Res, 99, 157–167. [DOI] [PubMed] [Google Scholar]
- 75.Schiavina M, Salladini E, Murrali MG, Tria G, Felli IC, Pierattelli R, Longhi S, (2020). Ensemble description of the intrinsically disordered N-terminal domain of the Nipah virus P/V protein from combined NMR and SAXS. Sci. Rep, 10, 19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jensen MR, Houben K, Lescop E, Blanchard L, Ruigrok RWH, Blackledge M, (2008). Quantitative conformational analysis of partially folded proteins from residual dipolar couplings: application to the molecular recognition element of Sendai virus nucleoprotein. J. Am. Chem. Soc, 130, 8055–8061. [DOI] [PubMed] [Google Scholar]
- 77.Bourhis JM, Receveur-Brechot V, Oglesbee M, Zhang X, Buccellato M, Darbon H, Canard B, Finet S, Longhi S, (2005). The intrinsically disordered C-terminal domain of the measles virus nucleoprotein interacts with the C-terminal domain of the phosphoprotein via two distinct sites and remains predominantly unfolded. Protein Sci, 14, 1975–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toto A, Troilo F, Visconti L, Malagrinò F, Bignon C, Longhi S, Gianni S, (2019). Binding induced folding: Lessons from the kinetics of interaction between N(TAIL) and XD. Arch. Biochem. Biophys, 671, 255–261. [DOI] [PubMed] [Google Scholar]
- 79.Bignon C, Troilo F, Gianni S, Longhi S, (2018). Modulation of measles virus NTAIL interactions through fuzziness and sequence features of disordered binding sites. Biomolecules, 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bignon C, Troilo F, Gianni S, Longhi S, (2018). Partner-mediated polymorphism of an intrinsically disordered protein. J. Mol. Biol, 430, 2493–2507. [DOI] [PubMed] [Google Scholar]
- 81.Dosnon M, Bonetti D, Morrone A, Erales J, di Silvio E, Longhi S, Gianni S, (2015). Demonstration of a folding after binding mechanism in the recognition between the Measles virus NTAIL and X domains. ACS Chem. Biol, 10, 795–802. [DOI] [PubMed] [Google Scholar]
- 82.Kavalenka A, Urbančič I, Belle V, Rouger S, Costanzo S, Kure S, Fournel A, Longhi S, Guigliarelli B, Strancar J, (2010). Conformational analysis of the partially disordered measles virus NTAIL-XD complex by SDSL EPR spectroscopy. Biophys. J, 98, 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y, Chu X, Longhi S, Roche P, Han W, Wang E, Wang J, (2013). Multiscaled exploration of coupled folding and binding of an intrinsically disordered molecular recognition element in measles virus nucleoprotein. Proc. Natl. Acad. Sci. USA, 110, E3743–E3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belle V, Rouger S, Costanzo S, Liquiere E, Strancar J, Guigliarelli B, Fournel A, Longhi S, (2008). Mapping alpha-helical induced folding within the intrinsically disordered C-terminal domain of the measles virus nucleoprotein by site-directed spin-labeling EPR spectroscopy. Proteins: Struct. Func. Bioinform, 73, 973–988. [DOI] [PubMed] [Google Scholar]
- 85.Bischak CG, Longhi S, Snead DM, Costanzo S, Terrer E, Londergan CH, (2010). Probing structural transitions in the intrinsically disordered C-terminal domain of the measles virus nucleoprotein by vibrational spectroscopy of cyanylated cysteines. Biophys. J, 99, 1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Houben K, Marion D, Tarbouriech N, Ruigrok RW, Blanchard L, (2007). Interaction of the C-terminal domains of sendai virus N and P proteins: comparison of polymerase-nucleocapsid interactions within the paramyxovirus family. J. Virol, 81, 6807–6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jensen MR, Bernadó P, Houben K, Blanchard L, Marion D, Ruigrok RW, Blackledge M, (2010). Structural disorder within Sendai virus nucleoprotein and phosphoprotein: insight into the structural basis of molecular recognition. Prot. Pept. Lett, 17, 952–960. [DOI] [PubMed] [Google Scholar]
- 88.Gerard FC, Ribeiro Ede A Jr., Leyrat C, Ivanov I, Blondel D, Longhi S, Ruigrok RW, Jamin M, (2009). Modular organization of rabies virus phosphoprotein. J. Mol. Biol, 388, 978–996. [DOI] [PubMed] [Google Scholar]
- 89.Rodriguez Galvan J, Donner B, Veseley CH, Reardon P, Forsythe HM, Howe J, Fujimura G, Barbar E, (2021). Human parainfluenza virus 3 phosphoprotein is a tetramer and shares structural and interaction features with ebola phosphoprotein VP35. Biomolecules, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gely S, Lowry DF, Bernard C, Jensen MR, Blackledge M, Costanzo S, Bourhis JM, Darbon H, Daughdrill G, Longhi S, (2010). Solution structure of the C-terminal X domain of the measles virus phosphoprotein and interaction with the intrinsically disordered C-terminal domain of the nucleoprotein. J. Mol. Recog, 23, 435–447. [DOI] [PubMed] [Google Scholar]
- 91.Baronti L, Erales J, Habchi J, Felli IC, Pierattelli R, Longhi S, (2015). Dynamics of the intrinsically disordered C-terminal domain of the Nipah virus nucleoprotein and interaction with the X domain of the phosphoprotein as unveiled by NMR spectroscopy. ChemBioChem, 16, 268–276. [DOI] [PubMed] [Google Scholar]
- 92.Jensen MR, Yabukarski F, Communie G, Condamine E, Mas C, Volchkova V, Tarbouriech N, Bourhis JM, Volchkov V, Blackledge M, Jamin M, (2020). Structural description of the Nipah virus phosphoprotein and its interaction with STAT1. Biophys. J, 118, 2470–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bourhis J-M, Yabukarski F, Communie G, Schneider R, Volchkova VA, Frénéat M, Gérard F, Ducournau C, Mas C, Tarbouriech N, Ringkjøbing Jensen M, Volchkov VE, Blackledge M, Jamin M, (2022). Structural dynamics of the C-terminal X domain of Nipah and Hendra viruses controls the attachment to the C-terminal tail of the nucleocapsid protein. J. Mol. Biol, 167551. [DOI] [PubMed] [Google Scholar]
- 94.Jensen MR, Communie G, Ribeiro EA, Martinez N, Desfosses A, Salmon L, Mollica L, Gabel F, Jamin M, Longhi S, Ruigrok RWH, Blackledge M, (2011). Intrinsic disorder in measles virus nucleocapsids. Proc. Natl. Acad. Sci. USA, 108, 9839–9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Longhi S, Bloyet LM, Gianni S, Gerlier D, (2017). How order and disorder within paramyxoviral nucleoproteins and phosphoproteins orchestrate the molecular interplay of transcription and replication. Cell. Mol. Life Sci, 74, 3091–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Desfosses A, Goret G, Farias Estrozi L, Ruigrok RW, Gutsche I, (2011). Nucleoprotein-RNA orientation in the measles virus nucleocapsid by three-dimensional electron microscopy. J. Virol, 85, 1391–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.To V, Dzananovic E, McKenna SA, O’Neil J, (2016). The dynamic landscape of the full-length HIV-1 transactivator of transcription. Biochemistry, 55, 1314–1325. [DOI] [PubMed] [Google Scholar]
- 98.Shojania S, O’Neil JD, (2006). HIV-1 Tat is a natively unfolded protein: The solution conformation and dynamics of reduced HIV-1 Tat-(1–72) by NMR spectroscopy. J. Biol. Chem, 281, 8347–8356. [DOI] [PubMed] [Google Scholar]
- 99.Faust O, Grunhaus D, Shimshon O, Yavin E, Friedler A, (2018). Protein regulation by intrinsically disordered regions: a role for subdomains in the IDR of the HIV-1 Rev protein. ChemBioChem, 19, 1618–1624. [DOI] [PubMed] [Google Scholar]
- 100.Casu F, Duggan B, Hennig M, (2013). The arginine-rich RNA-binding motif of HIV-1 Rev Is intrinsically disordered and folds upon RRE binding. Biophys. J, 105, 1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matsuoka M, Jeang KT, (2011). Human T-cell leukemia virus type 1 (HTLV-1) and leukemic transformation: viral infectivity, Tax, HBZ and therapy. Oncogene, 30, 1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsuoka M, Jeang KT, (2007). Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer, 7, 270–280. [DOI] [PubMed] [Google Scholar]
- 103.De Guzman RN, Goto NK, Dyson HJ, Wright PE, (2006). Structural basis for cooperative transcription factor binding to the CBP coactivator. J. Mol. Biol, 355, 1005–1013. [DOI] [PubMed] [Google Scholar]
- 104.Ramirez JA, Nyborg JK, (2007). Molecular characterization of HTLV-1 Tax interaction with the KIX domain of CBP/p300. J. Mol. Biol, 372, 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shallak M, Alberio T, Fasano M, Monti M, Iacobucci I, Ladet J, Mortreux F, Accolla RS, Forlani G, (2022). The endogenous HBZ interactome in ATL leukemic cells reveals an unprecedented complexity of host interacting partners involved in RNA splicing. Front. Immunol, 13, 939863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang K, Stanfield RL, Martinez-Yamout MA, Dyson HJ, Wilson IA, Wright PE, (2018). Structural basis for cooperative regulation of KIX-mediated transcription pathways by the HTLV-1 HBZ activation domain. Proc. Natl. Acad. Sci. USA, 115, 10040–10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goh GKM, Dunker AK, Uversky VN, (2009). Protein intrinsic disorder and influenza virulence: the 1918 H1N1 and H5N1 viruses. Virol. J, 6, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shen Q, Shi J, Zeng D, Zhao B, Li P, Hwang W, Cho J-H, (2018). Molecular mechanisms of tight binding through fuzzy interactions. Biophys. J, 114, 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi J, Shen Q, Cho J-H, Hwang W, (2020). Entropy hotspots for the binding of intrinsically disordered ligands to a receptor domain. Biophys. J, 118, 2502–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y, Ahn J, Green KJ, Vann KR, Black J, Brooke CB, Kutateladze TG, (2019). MORC3 Is a Target of the Influenza A Viral Protein NS1. Structure, 27, 1029–1033.e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu L, Qin J, (2019). A Viral Protein Mimics Histone to Hijack Host MORC3. Structure, 27, 883–885. [DOI] [PubMed] [Google Scholar]
- 112.Almeida MS, Johnson MA, Herrmann T, Geralt M, Wûthrich K, (2007). Novel beta-barrel fold in the nuclear magnetic resonance structure of the replicase nonstructural protein 1 from the severe acute respiratory syndrome coronavirus. J. Virol, 81, 3151–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takeda M, Chang CK, Ikeya T, Güntert P, Chang YH, Hsu Y.l., Huang TH, Kainosho M, (2008). Solution structure of the C-terminal dimerization domain of SARS coronavirus nucleocapsid protein solved by the SAIL-NMR method. J. Mol. Biol, 380, 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quaglia F, Salladini E, Carraro M, Minervini G, Tosatto SCE, Le Mercier P, (2022). SARS-CoV-2 variants preferentially emerge at intrinsically disordered protein sites helping immune evasion. FEBS J, 289, 4240–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stroberg W, Schnell S, (2017). On the origin of non-membrane-bound organelles, and their physiological function. J. Theor. Biol, 434, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Turoverov KK, Kuznetsova IM, Fonin AV, Darling AL, Zaslavsky BY, Uversky VN, (2019). Stochasticity of biological soft matter: emerging concepts in intrinsically disordered proteins and biological phase separation. Trends Biochem. Sci, 44, 716–728. [DOI] [PubMed] [Google Scholar]
- 117.Brocca S, Grandori R, Longhi S, Uversky V, (2020). Liquid–liquid phase separation by intrinsically disordered protein regions of viruses: roles in viral life cycle and control of virus–host interactions. Int. J. Mol. Sci, 21, 9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caragliano E, Brune W, Bosse JB, (2022). Herpesvirus replication compartments: dynamic biomolecular condensates? Viruses, 14, 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nikolic J, Le Bars R, Lama Z, Scrima N, Lagaudrière-Gesbert C, Gaudin Y, Blondel D, (2017). Negri bodies are viral factories with properties of liquid organelles. Nat. Commun, 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Papa G, Borodavka A, Desselberger U, (2021). Viroplasms: assembly and functions of rotavirus replication factories. Viruses, 13, 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Geiger F, Acker J, Papa G, Wang X, Arter WE, Saar KL, Erkamp NA, Qi R, Bravo JP, Strauss S, Krainer G, Burrone OR, Jungmann R, Knowles TP, Engelke H, Borodavka A, (2021). Liquid–liquid phase separation underpins the formation of replication factories in rotaviruses. EMBO J, 40, e107711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guseva S, Milles S, Jensen MR, Salvi N, Kleman J-P, Maurin D, Ruigrok RWH, Blackledge M, (2020). Measles virus nucleo- and phosphoproteins form liquid-like phase-separated compartments that promote nucleocapsid assembly. Sci. Adv, 6, eaaz7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guseva S, Milles S, Jensen MR, Schoehn G, Ruigrok RWH, Blackledge M, (2020). Structure, dynamics and phase separation of measles virus RNA replication machinery. Current Opinion in Virology, 41, 59–67. [DOI] [PubMed] [Google Scholar]
- 124.Carlson CR, Asfaha JB, Ghent CM, Howard CJ, Hartooni N, Safari M, Frankel AD, Morgan DO, (2020). Phosphoregulation of phase separation by the SARS-CoV-2 N protein suggests a biophysical basis for its dual functions. Mol. Cell, 80, 1094–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iserman C, Roden CA, Boerneke MA, Sealfon RSG, McLaughlin GA, Jungreis I, Fritch EJ, Hou YJ, Ekena J, Weidmann CA, Theesfeld CL, Kellis M, Troyanskaya OG, Baric RS, Sheahan TP, Weeks KM, Gladfelter AS, (2020). Genomic RNA elements drive phase separation of the SARS-CoV-2 nucleocapsid. Mol. Cell, 80, 1078–1091.e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cubuk J, Alston JJ, Incicco JJ, Singh S, Stuchell-Brereton MD, Ward MD, Zimmerman MI, Vithani N, Griffith D, Wagoner JA, Bowman GR, Hall KB, Soranno A, Holehouse AS, (2021). The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat. Commun, 12, 1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shen C, Li R, Negro R, Cheng J, Vora SM, Fu T-M, Wang A, He K, Andreeva L, Gao P, Tian Z, Flavell RA, Zhu S, Wu H, (2021). Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell, 184, 5759–5774.e5720. [DOI] [PMC free article] [PubMed] [Google Scholar]


