Abstract
Background:
National guidelines recommend systolic blood pressure (SBP) in patients with heart failure with reduced ejection fraction (HFrEF) and hypertension be maintained below 130 mmHg.
Objectives:
To determine associations of SBP <130 mmHg with outcomes in patients with HFrEF.
Methods:
Of the 25,345 patients in the Medicare-linked OPTIMIZE-HF, 10,356 had EF ≤40%. Of these, 5615 had stable SBP (≤20 mmHg admission to discharge variation) and 3805 (68%) had a discharge SBP <130 mmHg. Propensity scores for SBP <130 mmHg, estimated for each of the 5615 patients, were used to assemble a matched cohort of 1189 pairs of patients with SBP <130 vs. ≥130 mmHg, balanced on 58 baseline characteristics (mean age, 76 years; mean EF, 28%, 45% women, 13% African American). We repeated the above process in 3946 patients, after excluding 1669 (30% of 5615) patients with a discharge SBP <110 mmHg and assembled a second matched balanced cohort of 1099 pairs of patients with SBP 110–129 vs. ≥130 mmHg.
Results:
30-day all-cause mortality occurred in 7% and 4% of matched patients with SBP <130 vs. ≥130 mmHg, respectively (hazard ratio {HR}, 1.76; 95% CI, 1.24–2.48; p=0.001). HRs (95% CIs) for all-cause mortality, all-cause readmission, and HF readmission at 1 year, associated with SBP <130 mmHg, were 1.32 (1.15–1.53; p<0.001), 1.11 (1.01–1.23; p=0.030) and 1.24 (1.09–1.42; p=0.001), respectively. HRs (95% CIs) for 30-day and 1-year all-cause mortality associated with SBP 110–129 (vs. ≥130) mmHg were 1.50 (1.03–2.19; p=0.035) and 1.19 (1.02–1.39; p=0.029), respectively.
Conclusions:
Among hospitalized older patients with HFrEF, SBP <130 mmHg is associated with poor outcomes. This association persisted when the analyses were repeated after excluding patients with SBP <110 mmHg. There is an urgent need for randomized controlled trials to evaluate optimal SBP reduction goals in patients with HFrEF.
Keywords: systolic blood pressure, heart failure, outcomes
Condensed Abstract:
National guidelines recommend that in patients with heart failure with reduced ejection fraction (HFrEF) and hypertension, systolic blood pressure (SBP) should be maintained below 130 mmHg. Findings from our propensity score-matched cohorts of hospitalized older patients with HFrEF demonstrate that a discharge SBP <130 mmHg is associated with a higher risk of mortality and readmission, and that this risk persists when patients with SBP <110 mmHg are excluded. These findings suggest that there is an urgent need for randomized controlled trials to evaluate optimal SBP reduction goals in patients with HFrEF.
Introduction
The American College of Cardiology/American Heart Association/Heart Failure Society (ACC/AHA/HFSA) guideline for heart failure (HF) recommends that in patients with HF with reduced ejection fraction (HFrEF) and hypertension, systolic blood pressure (SBP) should be treated to below 130 mmHg (1). A low SBP has been shown to be associated with poor outcomes in patients with HFrEF (2–4). However, the association of SBP <130 with outcomes has not been specifically examined in patients with HFrEF. In the current study, we examined the association of SBP <130 mmHg and outcomes in a propensity score-matched cohort of older patients with HFrEF
Methods
Data Source and Study Population
A deidentified copy of the Medicare-linked Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry dataset was used, the details of which have been presented before (5–7). Briefly, 48,612 medical records of hospitalizations with ICD-9 code for HF as primary discharge diagnosis between March 1, 2003 and December 31, 2004 from 259 hospitals in 48 states were abstracted using a Web-based information system. Detailed data on demographics, medical history, symptoms, signs, admission and discharge medications, inpatient procedures, and short-term outcomes were collected (5,8). In OPTIMIZE-HF, data on outcomes were limited to outcomes at 60 to 90 days on about a 10% select subset of patients (5,8). Long-term outcomes data in the current analysis were obtained by probabilistic linking of OPTIMIZE-HF with Medicare data up to December 31, 2008, which identified 26,376 unique patients, of which 25,354 were discharged alive (7,8). Of these, 10,625 had HFrEF defined as EF ≤40% (9).
Assembly of a Cohort with Stable SBP
Data on blood pressure were collected with patients in the supine position from times closest to admission and discharge, and automated electronic data checks were used to prevent outlying SBP values such as those >300 mmHg (10,11). After excluding 90 patients with discharge SBP >300 mmHg or <60 mm Hg, our sample size consisted of 10,535 patients. SBP variability has been shown to be associated with poor outcomes (12,13). To minimize bias due to measurement errors and acute inpatient events affecting SBP, we excluded patients whose admission to discharge SBP varied >20 mm Hg. This also allowed us to avoid potential exposure misclassification due to substantial admission to discharge change in SBP (11). Of the remaining 5615 patients with stable inpatient SBP (admission to discharge SBP variation of ≤20 mm Hg), 3805 (68%) had a discharge SBP of <130 mmHg (Online Figure 1).
Assembly of a Balanced Cohort
We used propensity score matching to assemble a cohort in which patients with SBP <130 vs. ≥130 mmHg would be expected to be balanced on all measured baseline characteristics (6,11,14–17). We began by estimating propensity scores for discharge SBP <130 mmHg for each of the 5615 patients using a non-parsimonious multivariable logistic regression model. In the model, SBP <130 mmHg was used as the dependent variable and the 58 baseline variables displayed in Online Figure 2 were used as covariates. The propensity score for SBP <130 mmHg for a patient is that patient’s data-driven (based on 58 measured baseline characteristics) probability of having a SBP <130 mm Hg. The model discriminated well (c statistic, 0.844). However, because propensity score models are sample-specific adjusters and are not used for out-of-sample prediction or estimation of coefficients, the measure of discrimination is less relevant to the assessment of the model’s quality (18,19). Instead, the reduction in baseline covariate imbalance is a better marker of the efficacy of a propensity score model, which is best assessed by estimating absolute standardized differences (20,21). We used a greedy matching algorithm, described elsewhere (6,11) to match 1189 (66% of 1810) patients with an SBP ≥130 mmHg with 1189 patients with SBP <130 mmHg by propensity scores (Online Figure 1). Between-group balance in baseline characteristics were compared in the 5615 pre-match and the 2378 post-match cohorts by estimating absolute standardized differences for each of the 58 baseline characteristics (6,11). An absolute standardized difference of <10% indicates inconsequential residual bias and a value of 0% would indicate no residual bias.
Finally, we repeated the above process in a subset of 3946 patients after excluding 1669 (30% of 5615) patients with a discharge SBP <110 mmHg. This was done to compare SBP 110–129 mmHg with SBP >130 mmHg patients to evaluate whether adverse outcomes associated with SBP <130 mmHg were accounted for by the subset of patients with SBP <110 mmHg in which the low SBP may indicate greater impairment in myocardial function. Of the 3946 patients, 2136 had discharge SBP 110-129 mmHg and 1810 had SBP ≥130 mmHg. The ensuing propensity score-matched cohort had 2198 patients, 1099 in each group.
Assembly of Sensitivity Cohorts
We repeated the above process three times to assemble three separate sensitivity cohorts to examine if the association of SBP and outcomes in our primary cohort would vary by a change in the measurement or methodological approaches. The first sensitivity cohort is based on 4172 patients without stable SBP (admission to discharge SBP drop of >20 mm Hg), of whom 3209 had an SBP <130 mm Hg, from which we assembled a propensity score-matched cohort of 1424 patients (712 pairs; Online Figure 1). We excluded patients whose admission to discharge SBP rose >20 mmHg as these patients would be expected to be characteristically and prognostically different from those with a drop in SBP. Our second and third sensitivity cohorts are based on all 10,535 patients regardless of admission to discharge SBP variations, but the exposure variable was defined as discharge SBP <130 mmHg (2247 matched pairs) and admission SBP <130 mmHg (3348 matched pairs; Online Figure 1).
Outcomes Data
Outcomes included all-cause mortality, all-cause readmission and HF readmission at 30 days, 1 year and during overall follow-up of 6 (median, 2.3) years up to December 31, 2008. Secondary outcomes included combined end points of all-cause readmission or all-cause mortality and HF readmission or all-cause mortality. All outcomes data were obtained by linking OPTIMIZE-HF to Medicare data (7).
Statistical Analyses
Descriptive analyses included between-group comparison of baseline characteristics using Pearson’s Chi-square and Wilcoxon rank-sum tests, as appropriate. Outcome analyses included Kaplan-Meier survival analysis comparing time to death by discharge SBP <130 mmHg in matched data. Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for all outcomes associated with SBP <130 mm Hg. For the mortality model, patients who did not die were censored at study end; for the readmission model, patients who did not have a readmission were censored at time to death or study end, whichever occurred first. To assess for nonlinearity in the relationship between SBP as a continuous variable and all-cause mortality during the overall follow-up of 6 years, we fitted restricted cubic spline models with 6 knots at SBPs 110, 120, 130 (reference), 140, 150, and 160 mm Hg, using matched data, repeating the process in the pre-match data, adjusting for age, sex, and race as well as adjusting for propensity scores. We then examined the associations between discharge SBP <130 mmHg and various outcomes at different timepoints in subgroups of matched patients with and without hypertension. We also conducted another subgroup analysis to assess the homogeneity of the association between SBP <130 mmHg and 6-year all-cause mortality in other relevant subgroups of patients.
To determine if a significant relationship between SBP <130 mmHg and all-cause mortality in our matched data could be explained away by an unmeasured baseline confounder, we conducted formal sensitivity analyses using Rosenbaum’s approach (22). From the 1189 pairs of propensity score-matched patients with SBP <130 vs. ≥130 mmHg, we identified the pairs in which time-to-death data were available for both members of the pair. This is necessary to be able to determine which patient in a pair clearly had a longer survival. If one member of the pair is censored before death, then it is not possible to determine which patient within the pair had a longer survival. It is also not possible to determine a clear winner when both members of the pair have the same time to death. Of the 1189 matched pairs, in 11% (131/1189) and 92% (1095/1189) of the pairs, we were able to determine which patient within a pair had a longer 30-day and 6-year survival, respectively. We then tested whether, in the absence of a hidden bias, patients with SBP <130 mmHg had shorter survival times than their matched counterparts with SBP ≥130 mmHg. We used sign-score test to calculate “sensitivity bounds” for a hypothetical unmeasured confounder to determine how much it would need to increase the odds of having SBP <130 mmHg to explain away any significant association between SBP <130 mmHg and mortality. A significant sign-score test provides strong evidence of a relationship between SBP <130 mmHg and time to death. Our sensitivity analysis assumes that the potential unmeasured confounder is a binary baseline characteristic that is a near perfect predictor of the death, which is also not strongly correlated with any of 58 baseline characteristics used in our propensity score model. However, sensitivity analysis cannot determine if such an unmeasured confounder exists. All statistical analyses were conducted using IBM SPSS Statistics for Windows software, version 24 (IBM Corp., Armonk, NY, USA), and SAS software for Windows, version 9.4 (SAS Institute, Cary, North Carolina).
Results
The 2378 matched patients had a mean (±SD) age of 76 (±10) years, a mean (±SD) EF of 28 (±8) percent, 45% were women, and 13% were African American. These patients had a mean discharge SBP of 128 mmHg (median, 130, minimum 82 and maximum 198 mmHg), 305 (26%) had SBP <110, 12 had SBP <90, and none had <80 mm Hg. Before matching, patients with SBP <130 mmHg had a lower prevalence of hypertension, a higher prevalence of prior myocardial infarction and atrial fibrillation, and lower mean EF (Table 1). All 58 baseline characteristics were balanced after matching, with absolute standardized differences <10% (Table 1, Online Figure 2).
Table 1.
Baseline Patient Characteristics by Discharge Systolic Blood Pressure (SBP) in Heart Failure Patients with Left Ventricular Ejection Fraction ≤ 40%
| Before propensity score matching (n=5615) | After propensity score matching (n=2378) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| n (%) or mean (±SD) | Discharge SBP | Discharge SBP | ||||
|
|
||||||
| <130 mm Hg (n=3805) | ≥130 mm Hg (n=1810) | P value | <130 mm Hg (n=1189) | ≥130 mm Hg (n=1189) | P value | |
| Age (years) | 75 (11) | 76 (11) | 0.042 | 76 (10) | 77 (10) | 0.848 |
| Female | 1316 (35%) | 838 (46%) | <0.001 | 534 (45%) | 527 (44%) | 0.773 |
| African American | 396 (10%) | 320 (18%) | <0.001 | 167 (14%) | 153 (13%) | 0.400 |
| Smoking history | 488 (13%) | 230 (13%) | 0.901 | 147 (12%) | 146 (12%) | 0.950 |
|
| ||||||
| Past medical history | ||||||
| Prior heart failure | 3502 (92%) | 1640 (91%) | 0.072 | 1067 (90%) | 1075 (90%) | 0.583 |
| Hypertension | 2170 (57%) | 1358 (75%) | <0.001 | 834 (70%) | 832 (70%) | 0.929 |
| Acute myocardial infarction | 1243 (33%) | 514 (28%) | 0.001 | 342 (29%) | 357 (30%) | 0.500 |
| Coronary revascularization | 1520 (40%) | 650 (36%) | 0.004 | 441 (37%) | 440 (37%) | 0.966 |
| Diabetes mellitus | 1429 (38%) | 829 (46%) | <0.001 | 519 (44%) | 530 (45%) | 0.650 |
| Cerebrovascular disease | 563 (15%) | 317 (18%) | 0.009 | 220 (19%) | 210 (18%) | 0.594 |
| Peripheral vascular disease | 564 (15%) | 313 (17%) | 0.017 | 195 (16%) | 198 (17%) | 0.868 |
| Atrial fibrillation | 1397 (37%) | 584 (32%) | 0.001 | 414 (35%) | 400 (34%) | 0.545 |
| Ventricular arrhythmia | 436 (12%) | 123 (7%) | <0.001 | 93 (8%) | 89 (8%) | 0.758 |
| Implantable cardioverter defibrillator | 451 (12%) | 108 (6%) | <0.001 | 80 (7%) | 86 (7%) | 0.629 |
| Biventricular pacemaker | 298 (8%) | 68 (4%) | <0.001 | 52 (4%) | 59 (5%) | 0.496 |
| Chronic obstructive pulmonary disease | 1015 (27%) | 440 (24%) | 0.059 | 292 (25%) | 298 (25%) | 0.776 |
| Depression | 367 (10%) | 174 (10%) | 0.970 | 116 (10%) | 110 (9%) | 0.675 |
|
| ||||||
| Admission clinical characteristics | ||||||
| Dyspnea on exertion | 2415 (64%) | 1116 (62%) | 0.189 | 738 (62%) | 744 (63%) | 0.800 |
| Orthopnea | 1049 (28%) | 482 (27%) | 0.460 | 311 (26%) | 326 (27%) | 0.487 |
| Paroxysmal nocturnal dyspnea | 629 (17%) | 286 (16%) | 0.489 | 183 (15%) | 183 (15%) | 1.000 |
| Dyspnea at rest | 1593 (42%) | 777 (43%) | 0.451 | 499 (42%) | 512 (43%) | 0.590 |
| Chest pain | 772 (20%) | 395 (22%) | 0.185 | 252 (21%) | 260 (22%) | 0.690 |
| Jugular venous pressure elevation | 1284 (34%) | 532 (29%) | 0.001 | 339 (29%) | 343 (29%) | 0.856 |
| Pulmonary rales | 2272 (60%) | 1150 (64%) | 0.006 | 759 (64%) | 754 (63%) | 0.831 |
| Peripheral edema | 2347 (62%) | 1117 (62%) | 0.982 | 722 (61%) | 734 (62%) | 0.614 |
| Pulse (beats/minute) | 77 (±13) | 75 (±13) | <0.001 | 76 (±13) | 76 (±13) | 0.904 |
| Discharge SBP (mmHg)* | 110 (±11) | 144 (±13) | N/A | 115 (±10) | 142 (±11) | N/A |
| Discharge SBP <90 mmHg* | 153 (4%) | 0 (0%) | N/A | 12 (0.5%) | 0 (0%) | N/A |
| Diastolic BP (mmHg) | 63 (±10) | 74 (±11) | <0.001 | 70 (±9) | 70 (±10) | 0.856 |
| Serum creatinine (mEq/L) | 1.7 (±0.9) | 1.8 (±1.4) | <0.001 | 1.7 (±1.1) | 1.7 (±1.0) | 0.352 |
| Left ventricular ejection fraction (%) | 25 (±8) | 29 (±8) | <0.001 | 28 (±8) | 28 (±8) | 0.996 |
|
| ||||||
| Discharge medications | ||||||
| ACE inhibitors or ARBs | 2515 (66%) | 1251 (69%) | 0.024 | 815 (69%) | 807 (68%) | 0.725 |
| Beta blockers | 2673 (70%) | 1303 (72%) | 0.180 | 845 (71%) | 842 (71%) | 0.892 |
| Aldosterone antagonists | 711 (19%) | 256 (14%) | <0.001 | 182 (15%) | 171 (14%) | 0.526 |
| Digoxin | 1624 (43%) | 605 (33%) | <0.001 | 422 (36%) | 432 (36%) | 0.669 |
| Loop diuretics | 3210 (84%) | 1455 (80%) | <0.001 | 996 (84%) | 978 (82%) | 0.326 |
| Amlodipine | 96 (3%) | 164 (9%) | <0.001 | 71 (6%) | 59 (5%) | 0.279 |
| Other calcium channel blockers | 162 (4%) | 166 (9%) | <0.001 | 82 (7%) | 90 (8%) | 0.527 |
| Hydralazine | 130 (3%) | 111 (6%) | <0.001 | 56 (5%) | 56 (5%) | 1.000 |
| Nitrates | 914 (24%) | 557 (31%) | <0.001 | 325 (27%) | 336 (28%) | 0.615 |
|
| ||||||
| Hospital characteristics | ||||||
| Academic | 1933 (51%) | 885 (49%) | 0.182 | 602 (51%) | 587 (49%) | 0.538 |
| Transplant | 774 (20%) | 282 (16%) | <0.001 | 208 (18%) | 189 (16%) | 0.296 |
| Interventional | 3108 (82%) | 1438 (79%) | 0.046 | 967 (81%) | 964 (81%) | 0.875 |
| Bed (number) † | 375 (251) | 375 (280) | 0.729 | 375 (241) | 375 (230) | 0.870 |
|
| ||||||
| Length of hospital stay (days) † | 4 (4) | 5 (5) | <0.001 | 4 (4) | 4 (4) | 0.999 |
SBP is the exposure variable and would not be expected to be balanced in the matched cohort; presented for descriptive purposes only.
Median (interquartile range), p values based on non-parametric independent sample median test.
ACE = angiotensin converting enzyme; ARBs = angiotensin receptor blockers
Discharge SBP <130 mmHg and All-Cause Mortality
Among the matched cohort (n = 2,378), 30-day all-cause mortality occurred in 7% and 4% of patients with a discharge SBP <130 vs. ≥130 mmHg, respectively (HR, 1.76; 95%CI, 1.24–2.48; p=0.001; Table 2). Findings from our sensitivity analyses demonstrate that the significant associations between SBP <130 mmHg and 30-day all-cause mortality was insensitive to an unmeasured confounder. Of the 131 matched pairs in which both members of the pair died within 30 days, in 63% (83/131) of those pairs, those with a shorter time to death belonged to the SBP <130 mmHg group (sign-score test p=0.002). An unmeasured baseline characteristic would need to increase the odds of having SBP <130 mmHg by 21% to be a confounder and explain away this association.
Table 2.
Outcomes by Discharge Systolic Blood Pressure (SBP) in 2378 Propensity Score-Matched Patients with Heart Failure with Ejection Fraction ≤ 40%
| Events (%), by discharge SBP |
Hazard ratio associated with SBP <130 mm Hg (95% confidence intervals) | ||
|---|---|---|---|
| Duration of follow-up | <130 mm Hg (n=1189) | ≥130 mm Hg (n=1189) | |
| 30 days | |||
| All-cause mortality | 7% (88) | 4% (51) | 1.76 (1.24–2.48); p=0.001 |
| All-cause readmission | 25% (296) | 22% (263) | 1.16 (0.99–1.37); p=0.075 |
| Heart failure readmission | 11% (135) | 9% (109) | 1.27 (0.99–1.64); p=0.060 |
| All-cause readmission or all-cause mortality | 29% (348) | 25% (300) | 1.20 (1.03–1.40); p=0.022 |
| Heart failure readmission or all-cause mortality | 18% (208) | 13% (154) | 1.39 (1.13-1.71); p=0.002 |
|
| |||
| 12 months | |||
| All-cause mortality | 35% (410) | 28% (331) | 1.32 (1.15–1.53); p<0.001 |
| All-cause readmission | 69% (821) | 68% (804) | 1.11 (1.01–1.23); p=0.030 |
| Heart failure readmission | 40% (475) | 35% (421) | 1.24 (1.09–1.42); p=0.001 |
| All-cause readmission or all-cause mortality | 78% (927) | 74% (880) | 1.15 (1.05–1.26); p=0.004 |
| Heart failure readmission or all-cause mortality | 59% (705) | 52% (616) | 1.26 (1.13-1.40); p<0.001 |
|
| |||
| 6 (median, 2.3) years | |||
| All-cause mortality | 73% (870) | 71% (843) | 1.15 (1.04-1.26); p=0.005 |
| All-cause readmission | 87% (1032) | 88% (1042) | 1.15 (1.05-1.25); p=0.002 |
| Heart failure readmission | 55% (658) | 54% (640) | 1.17 (1.05-1.30); p=0.005 |
| All-cause readmission or all-cause mortality | 98% (1160) | 96% (1140) | 1.17 (1.08-1.27); p<0.001 |
| Heart failure readmission or all-cause mortality | 87% (1032) | 85% (1011) | 1.17 (1.07-1.27); p=0.001 |
The association between SBP <130 mmHg and mortality persisted during overall follow-up of 6 years (HR, 1.15; 95% CI, 1.04–1.26; p=0.005; Table 2, Central Illustration). This association too was insensitive to an unmeasured confounder. Of the 1095 matched pairs in which both members of the pair died during 6 years of follow-up, 55% (603/1095) had a shorter time to death, which occurred in the SBP <130 mmHg group (sign-score test p<0.001). An unmeasured baseline characteristic would need to increase the odds of having SBP <130 mmHg by 9% to be a confounder and explain away this association. Findings from our restricted cubic spline analysis demonstrate that there was evidence of a non-linear relationship between SBP and all-cause mortality (p for non-linearity <0.001; Figure 1). The association between SBP <130 mmHg and 6-year mortality was rather homogeneous in various clinically relevant subgroups of matched patients (Online Figure 3).
Central Illustration: Kaplan Meier Plots by Systolic Blood Pressure <130 mmHg.
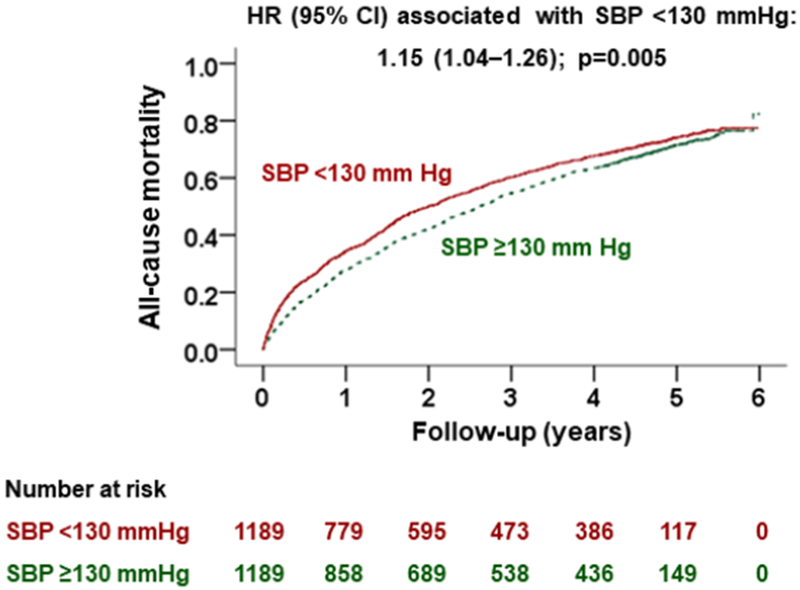
This study assessed the association of discharge systolic blood pressure (SBP) with outcomes in patients with heart failure with reduced ejection fraction (HFrEF) in a propensity score-matched cohort of 1,189 pairs of patients with an SBP of <130 versus ≥130 mmHg. During over 5 years of follow-up, SBP of <130 mmHg was associated with a significantly higher risk of death and readmission, compared with SBP of ≥130 mmHg.
Figure 1: All-Cause Mortality by Systolic Blood Pressure: Restricted Cubic Spline Plots.
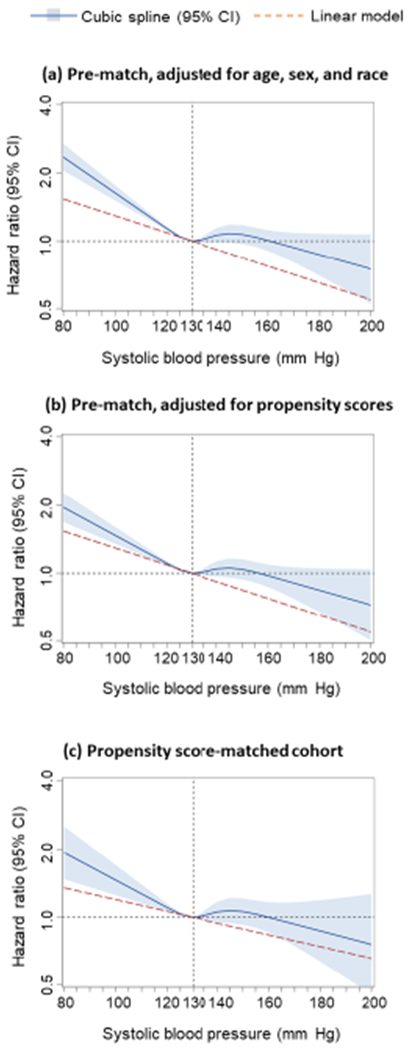
In cubic spline analysis, nonlinear relationship between discahrge systolic blood pressure and all-cause mortality was found in (a) 5615 pre-match patients, adjusted for demographics (age, sex, and race), (b) 5615 pre-match patients, adjusted for propensity scores, and (c) 2378 propensity score-matched patients balanced on 58 baseline characteristics (non-linearity p <0.001 for all three analyses). Spline curves excluded patients with systolic blood pressure values <80 mmHg (n=14) and >200 mmHg (n=2; none of the matched patients had systolic blood pressure values outside that range). Solid dark blue lines represent hazard ratios, and light blue shaded areas represent 95% CIs. CI = confidence interval.
Discharge SBP <130 mmHg and Other Outcomes
SBP <130 mmHg had no significant association with 30-day all-cause or HF readmission but was associated with a higher risk of both outcomes at 12 months and 6 years (Table 2).
SBP 110-129 vs. ≥130 mmHg and Outcomes
In the matched cohort (n=2198) that excluded patients with a discharge SBP <110 mmHg, 30-day all-cause mortality occurred in 6% and 4% of patients with a discharge SBP 110–129 mmHg compared to those with SBP ≥130 mmHg, respectively (HR, 1.50; 95% CI, 1.03–2.19; p=0.035). The association persisted during 12 months of follow-up (HR, 1.19; 95% CI, 1.02–1.39; p=0.029). HRs (95% CIs) for all-cause mortality at 2, 4 and 6 years associated with SBP 110–129 mmHg were 1.13 (0.99–1.28; p=0.062), 1.10 (0.99–1.22; p=0.086), and 1.09 (0.99–1.21; p=0.078), respectively. SBP 110–129 mmHg had no significant association with all-cause or HF readmissions.
Subgroup Analyses by Hypertension
Of the 2378 matched patients, 1666 had a history of hypertension. HRs (95% CIs) for 6-year all-cause mortality associated with SBP <130 mmHg were 1.10 (0.98–1.23; p=0.111) and 1.28 (1.08–1.52; p=0.005) in the subgroups with and without hypertension (p for interaction, 0.148; Figure 2). Respective HRs (95% CIs) for 6-year all-cause readmission were 1.11 (1.00–1.23; p=0.044 and 1.23 (1.05–1.44; p=0.011; p for interaction, 0.343). There was also no evidence of heterogeneity in the association between SBP <130 mmHg and 6-year HF readmission. HRs (95% CIs) for the associations of SBP <130 mmHg with these outcomes at 30 days and 1 year after hospital discharge among patients with and without hypertension are displayed in Figure 2.
Figure 2: Forest Plots for Subgroup Analyses of Mortality by Hypertension.

In subgroups with (n=1666) and without (n=712) hypertension, patients with systolic blood pressure <130 mmHg had higher risk of mortality and readmission compared to patients with systolic blood pressure ≥130 mmHg. CI = confidence interval.
Discharge SBP <130 mmHg and Outcomes in Sensitivity Cohorts
Among the 1424 matched patients with variable SBP (admission to discharge drop of >20 mm Hg), SBP <130 (vs. ≥130) mm Hg had no significant association with any outcomes at any of the three timepoints (Online Table). Among matched patients regardless of SBP variability, both admission and discharge SBP <130 mmHg were associated with a higher risk of all-cause mortality at all three time points. Associations with other outcomes are displayed in Online Table 1.
Discussion
Findings from the current study demonstrate that among hospitalized patients with HFrEF whose SBP was stable during hospitalization, a discharge SBP of <130 mmHg was associated with a significantly higher risk of 30-day all-cause mortality that remained significant during longer follow-up. The associations with all-cause and HF readmissions, on the other hand, were weak and non-significant at 30 days, but became significant at 12-month and during the overall follow-up of 6 years. We also observed that when patients with SBP <110 mmHg were excluded, SBP 110–129 mmHg was associated with a significantly higher risk of 30-day and 12-month all-cause mortality. These findings suggest that in patients with HFrEF, SBP <130 mmHg, even SBP values between 110 and 129 mmHg, are associated with poor outcomes.
The mean SBP of matched patients in the discharge SBP <130 mmHg group was 27 mmHg lower than that in the SBP ≥130 mmHg group. Patients with HFrEF who develop low SBP may have more progressive systolic dysfunction and the resultant neurohormonal activation may lead to hospitalization and death due to worsening symptoms as well as sudden cardiac death (23). Before matching, a higher proportion of patients in the group with SBP <130 mmHg had prior myocardial infarction and arrhythmias, and had lower mean EF, all of which are associated with poor outcomes. They were also more likely to be on diuretics and digoxin and less likely to be on inhibitors of the renin-angiotensin system. We were able to reduce these and other pre-match imbalances to an inconsequential level; however, it may not balance the underlying reasons that led to their pre-match imbalances.
Another important pre-match imbalance was in the prevalence of hypertension. Hypertension is a major risk factor for HF (24), but has been shown to be paradoxically associated with better outcomes in those with HF (25–27). The pre-match prevalence of hypertension was significantly higher in the group with a discharge SBP ≥130 mmHg (75% vs. 57% in SBP <130 mmHg group) suggesting that patients with HFrEF and hypertension are less likely to develop a low SBP. Findings from our subgroup analysis suggest that there is no evidence of a heterogeneous association of SBP <130 mmHg and poor outcomes between subgroups with vs. without hypertension, although during longer follow-up, the association appeared to be weaker in the subgroup with hypertension. The association of a low SBP with mortality was also more pronounced in the subgroup without hypertension among older patients with heart failure with preserved ejection fraction (HFpEF) (11).
According to the 2016 AHA scientific statement on management of hypertension in chronic HF, there are no compelling data to justify a single blood pressure target in treating hypertension in patients with established HF (28). However, the 2017 ACC/AHA/HFSA update of the HF guideline recommends that SBP in patients with HFrEF and hypertension should be maintained at <130 mmHg (1). This Class I recommendation is based on a Level C evidence derived from expert opinion and adapted from the Systolic Blood Pressure Intervention Trial (SPRINT) that excluded patients with HF (1,29). The guideline notes that the goal of BP reduction in patients with HFrEF and hypertension has not been specifically tested in randomized control trials (RCTs). Of note, although low SBP was associated with worse outcomes in RCTs involving patients with HFrEF, those randomized to receive HF medications that further lowered SBP, also reduced mortality (30). Thus, a low SBP should not preclude the use or up-titration of evidence-based HF medications that lower SBP.
It is currently unknown if in patients with HFrEF who have SBP ≥130 mmHg who are already on guideline-directed medical therapy (GDMT) at optimal doses, if lowering SBP to <130 mmHg with anti-hypertensive medications that are not HF guideline-recommended neurohormonal antagonists, would further improve or worsen outcomes. It is also unknown if patients with HFrEF on optimal dose GDMT who have SBP <130 mmHg and are also receiving other anti-hypertensive medications, raising SBP to ≥130 mmHg with discontinuation or down-titration of those anti-hypertensive medications, would further improve or worsen outcomes (31–33). Findings from our study that even a relatively normal SBP (110–129 mmHg) may be associated with a higher risk of death in patients with HFrEF are of potential concern, and suggest that there is an urgent need for RCTs to evaluate optimal SBP reduction goals in these patients.
Several prior studies have examined the association of a low SBP with outcomes in patients with HF (2–4,10,34). Findings from the Acute Decompensated Heart Failure National Registry (ADHERE) and OPTIMIZE-HF registries have demonstrated that a low admission SBP is associated with a higher risk of in-hospital mortality (10,34). In OPTIMIZE-HF, a subset of 2720 patients were followed for 60-90 days and patients with a low admission SBP had a significantly higher risk of mortality (10). We have recently reported a higher risk of death associated with SBP <130 mmHg in older patients with HFpEF (11). However, to the best of our knowledge, the current study is the first to examine the association of SBP <130 mmHg with outcomes in older patients with HFrEF. Our study is also distinguished from prior studies by the use of propensity score matching to assemble balanced cohorts, the use of sensitivity cohorts and extensive subgroup analyses, and the conduction of formal sensitivity analyses.
Limitations
Despite the use of propensity score-matched balanced cohorts and sensitivity analyses, bias due to residual and unmeasured confounding cannot be ruled out. Findings from our study need to be interpreted with caution, as a low SBP may be marker, mediator or both of poor outcomes. Findings of our study may not be extrapolated to patients with HFrEF without hypertension as the guideline recommendation to lower SBP to <130 mmHg is currently restricted to those with hypertension. We had no data on dosages of the HF drugs and had limited data on non-HF drugs used to treat hypertension. We also had no data on upright-seated SBP used in hypertension trials (29), although little is known about the difference between supine and seated SBP in HF. The medical, device and surgical management of HFrEF have evolved since OPTIMIZE-HF and these findings need to be replicated in more contemporary HF patients. We had no data on SBP during follow-up and any potential crossover of SBP during follow-up may have attenuated between-group differences in outcomes (35). Findings of our study, based on older fee-for-service Medicare beneficiaries hospitalized with decompensated HFrEF, may not be generalized to other HF populations, especially younger ambulatory patients with chronic HFrEF. However, the vast majority of HF patients are older adults (36,37), for whom HF is a leading cause for mortality and hospitalization (38)
Conclusions
In hospitalized older patients with HFrEF, compared with SBP ≥130 mmHg, SBP <130 mmHg, even when patients with SBP <110 mmHg are excluded, is associated with poor outcomes. Clinicians need to exercise caution in lowering SBP with agents other than guideline-recommended neurohormonal antagonists in patients with HFrEF who have SBP ≥130 mmHg and are already receiving GDMT in optimal doses. There is an urgent need for RCTs to evaluate optimal SBP reduction goals in patients with HFrEF and SBP ≥130 mmHg despite receiving guideline-directed medical therapy.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
Among patients hospitalized with HFrEF receiving guideline-directed medical therapy, systolic blood pressure (SBP) <130 mmHg is associated with higher mortality whether or not those with SBP <110 mmHg are included in the analysis.
Translational Outlook:
Prospective studies are needed to define optimum SBP for patients with HFrEF.
Acknowledgments
Funding: Dr. Ali Ahmed was in part supported by the National Institutes of Health through grants (R01-HL085561, R01-HL085561-S and R01-HL097047) from the National Heart, Lung, and Blood Institute. OPTIMIZE-HF was sponsored by GlaxoSmithKline.
Abbreviations
- HFrEF:
Heart failure reduced ejection fraction
- HFpEF:
Heart failure preserved ejection fraction
- OPTIMIZE-HF:
Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure
- HF:
Heart Failure
- SBP:
Systolic Blood Pressure
- ICD-9:
International Classification of Diseases, Ninth Revision
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Fonarow reports consulting with Abbott, Amgen, Bayer, Janssen, Novartis, and Medtronic and was the Principle Investigator of OPTIMIZE-HF. None of the other authors report any conflicts of interest related to this manuscript.
References
- 1.Yancy CW, Jessup M, Bozkurt B et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 2.Desai RV, Banach M, Ahmed MI et al. Impact of baseline systolic blood pressure on long-term outcomes in patients with advanced chronic systolic heart failure (insights from the BEST trial). Am J Cardiol 2010;106:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meredith PA, Ostergren J, Anand I et al. Clinical outcomes according to baseline blood pressure in patients with a low ejection fraction in the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity) Program. J Am Coll Cardiol 2008;52:2000–7. [DOI] [PubMed] [Google Scholar]
- 4.Banach M, Bhatia V, Feller MA et al. Relation of baseline systolic blood pressure and long-term outcomes in ambulatory patients with chronic mild to moderate heart failure. Am J Cardiol 2011;107:1208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonarow GC, Abraham WT, Albert NM et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J 2004;148:43–51. [DOI] [PubMed] [Google Scholar]
- 6.Lam PH, Dooley DJ, Deedwania P et al. Heart rate and outcomes in hospitalized patients with heart failure with preserved ejection fraction. J Am Coll Cardiol 2017;70:1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Kilgore ML, Arora T et al. Design and rationale of studies of neurohormonal blockade and outcomes in diastolic heart failure using OPTIMIZE-HF registry linked to Medicare data. Int J Cardiol 2013;166:230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonarow GC, Stough WG, Abraham WT et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007;50:768–77. [DOI] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Abraham WT, Albert NM et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006;296:2217–26. [DOI] [PubMed] [Google Scholar]
- 11.Tsimploulis A, Lam PH, Arundel C et al. Systolic Blood Pressure and Outcomes in Patients With Heart Failure With Preserved Ejection Fraction. JAMA Cardiol 2018;3:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossignol P, Girerd N, Gregory D, Massaro J, Konstam MA, Zannad F. Increased visitto-visit blood pressure variability is associated with worse cardiovascular outcomes in low ejection fraction heart failure patients: Insights from the HEAAL study. Int J Cardiol 2015;187:183–9. [DOI] [PubMed] [Google Scholar]
- 13.Schmid FA, Schlager O, Keller P et al. Prognostic value of long-term blood pressure changes in patients with chronic heart failure. Eur J Heart Fail 2017;19:837–842. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed A, Husain A, Love TE et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J 2006;27:1431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC. Primer on statistical interpretation or methods report card on propensity-score matching in the cardiology literature from 2004 to 2006: a systematic review. Circ Cardiovasc Qual Outcomes 2008;1:62–7. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum PR RD. The central role of propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 17.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services Outcomes Research Methodol 2001;2:169–188. [Google Scholar]
- 18.Westreich D, Cole SR, Funk MJ, Brookhart MA, Sturmer T. The role of the c-statistic in variable selection for propensity score models. Pharmacoepidemiol Drug Saf 2011;20:317–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan CJ. Reducing bias using propensity score matching. J Nucl Cardiol 2018;25:404–406. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor Observational Studies. New York: Springer-Verlag, 2002:105–170. [Google Scholar]
- 23.Cohn JN. Blood pressure and the therapy of advanced heart failure. J Am Coll Cardiol 2004;43:1430–1. [DOI] [PubMed] [Google Scholar]
- 24.Ekundayo OJ, Allman RM, Sanders PW et al. Isolated systolic hypertension and incident heart failure in older adults: a propensity-matched study. Hypertension 2009;53:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raphael CE, Whinnett ZI, Davies JE et al. Quantifying the paradoxical effect of higher systolic blood pressure on mortality in chronic heart failure. Heart 2009;95:56–62. [DOI] [PubMed] [Google Scholar]
- 26.Ventura HO, Messerli FH, Lavie CJ. Observations on the blood pressure paradox in heart failure. Eur J Heart Fail 2017;19:843–845. [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol 2004;43:1439–44. [DOI] [PubMed] [Google Scholar]
- 28.Bozkurt B, Aguilar D, Deswal A et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016;134:e535–e578. [DOI] [PubMed] [Google Scholar]
- 29.Wright JT Jr., Williamson JD, Whelton PK et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouleau JL, Roecker EB, Tendera M et al. Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure: the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. J Am Coll Cardiol 2004;43:1423–9. [DOI] [PubMed] [Google Scholar]
- 31.Ruskin JN. The cardiac arrhythmia suppression trial (CAST). N Engl J Med 1989;321:386–8. [DOI] [PubMed] [Google Scholar]
- 32.Cardiac Arrhythmia Suppression Trial I. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989;321:406–12. [DOI] [PubMed] [Google Scholar]
- 33.Study of the Effectiveness of Additional Reductions in C, Homocysteine Collaborative G, Armitage JM et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA 2010;303:2486–94. [DOI] [PubMed] [Google Scholar]
- 34.Yancy CW, Lopatin M, Stevenson LW et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 35.MacMahon S, Peto R, Cutler J et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990;335:765–74. [DOI] [PubMed] [Google Scholar]
- 36.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol 2013;61:1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamin EJ, Muntner P, Alonso A et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56–e66. [DOI] [PubMed] [Google Scholar]
- 38.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009;360:1418–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


