Abstract
Vascular dysfunction resulting from endothelial hyperpermeability is a common and important feature of critical illness due to sepsis, trauma, and other conditions associated with acute systemic inflammation. Clarkson disease [monoclonal gammopathy-associated idiopathic systemic capillary leak syndrome (ISCLS)] is a rare, orphan disorder marked by spontaneous and recurrent episodes of hypotensive shock and peripheral edema due to widespread vascular leakage in peripheral tissues. Mortality from acute flares approaches 30% due to lack of effective therapies. We evaluated a monoclonal antibody (4E2) specific for the endothelial receptor tyrosine kinase Tie2 in ISCLS models. 4E2 activated Tie2 in ISCLS patient-derived endothelial cells and reduced baseline and proinflammatory mediator-induced barrier dysfunction. 4E2 also reduced mortality and/or vascular leakage associated with systemic histamine challenge or influenza infection in the SJL/J mouse model of ISCLS. These findings support a critical role for Tie2 dysregulation in ISCLS and highlight a viable therapeutic approach to this catastrophic disorder.
ATie2 specific antibody improves endothelial function in Clarkson disease.
INTRODUCTION
Vascular leakage during acute ISCLS episodes leads to vasopressor-resistant shock due to the loss of up to 70% of plasma volume, massive edema in the trunk and limbs after fluid resuscitation, and hypoalbuminemia (1). Clinical complications of ISCLS-associated endotheliopathy include multi-organ dysfunction syndrome (MODS) resulting from hypoperfusion, rhabdomyolysis, thrombosis, and compartment syndrome in the extremities, which necessitates fasciotomies and limb amputation (2). Although triggers for ISCLS crises are not always apparent, nearly half are preceded by a flu-like illness, suggesting that even mild inflammation induces outsized vascular permeability responses in these patients (3). Inexplicably, vascular leakage ceases spontaneously after several days, when fluids trapped in tissues return rapidly to the intravascular space. Patients may develop pulmonary edema at this stage due to transient cardiac dysfunction.
Mortality from acute ISCLS flares results primarily from MODS, prothrombotic complications, the sequelae of iatrogenic administration of excessive fluids, and the absence of effective interventions other than supportive measures (1, 3). Because ISCLS-specific humoral leakage factors have not yet been identified, generic anti-inflammatory agents (e.g., corticosteroids) or more narrowly targeted drugs counteracting established mediators of endothelial permeability [e.g., vascular endothelial (VE) growth factor (VEGF), histamine, bradykinin, and leukotrienes] have no impact on clinical outcomes (1, 4). Although published studies of more highly prevalent conditions like sepsis or COVID-19 provide rationale for targeting endothelial dysregulation (5), the utility of this approach in acute ISCLS is unclear. Methylxanthines and/or β-adrenergic agonists, which broadly antagonize endothelial permeability-related signaling pathways, were used historically as prophylaxis and in acute ISCLS flares (6). The use of these drugs led to intolerable side effects and did not reduce episode frequency, lessen the duration or severity of acute episodes, or prevent complications (7). By contrast, indefinite monthly prophylaxis with high-dose intravenous immunoglobulins (IVIGs) is disease sparing and improves survival in patients with an established diagnosis (7). However, the mechanism of action of IVIG and its utility in acute ISCLS episodes is unclear (1, 8).
ISCLS typically occurs in middle-aged adults with no family history of the disease (2). Consequently, identification of genetic abnormalities contributing to disease pathogenesis and/or specific therapeutic targets has proven difficult. The angiopoietin (Angpt)–Tie2 pathway has emerged as a promising candidate for treatment of vascular dysfunction in diverse conditions including sepsis (9), pneumonia (10), and age-related macular degeneration (11). Tie2 binds two ligands (Angpt-1 and Angpt-2) competitively (12). Angpt-1 ligation activates Tie2 by promoting heterotypic receptor phosphorylation (13). The downstream signaling pathway activated by phosphorylated Tie2, in turn, promotes endothelial quiescence and barrier function and is required for angiogenesis and lymphatic development (14). Increased serum Angpt-2 levels are detected during inflammatory stress because of critical illness and correlate with poor clinical outcomes (15). Angpt-2 antagonizes tonic Angpt-1–mediated Tie2 signaling and induces vessel leakiness (12).
Transient but sustained increases in serum Angpt-2 are also detected during acute ISCLS crises (16, 17). Pretreatment of normal dermal microvascular endothelial cells with an Angpt-2–neutralizing Tie2-Fc fusion protein reduces permeability elicited by acute ISCLS sera in vitro (16). On the basis of these preliminary findings, we studied the effects of the recently developed Tie2-activating antibody 4E2 in patient-derived primary endothelial cells and mouse models of ISCLS. Our results strongly support a pathogenic role for Angpt-Tie2 dysregulation in ISCLS episodes and point to the utility of Tie2-targeted therapeutics for acute flares.
RESULTS
A Tie2-activating antibody (4E2) fortifies barrier function in quiescent ISCLS-derived endothelial cells
4E2 is a chimeric mouse immunoglobulin G2a (IgG2a) antibody with a human variable region derived from a clone with high Tie2 binding activity that was isolated by biopanning a HuPhage single-chain variable fragment (ScFv) library. 4E2 binds human and mouse Tie2 with nanomolar affinity in a ligand-independent manner, partially inhibits binding of Angpt-1 to Tie2, and completely blocks Angpt-2 binding in cell-free assays, suggesting shared binding epitopes (18). Application of 4E2 to human umbilical vein endothelial cells (HUVECs) elicits Tie2 phosphorylation and activation of downstream signaling pathways (Akt-mediated FOXO1 phosphorylation) (18). To evaluate 4E2 as a candidate for the treatment of ISCLS, we first studied its effects on endothelial cells expanded from whole blood obtained from patients with ISCLS during convalescent periods or healthy controls by venipuncture [blood-outgrowth endothelial cells (BOECs)] (19). To precisely quantify the impact of 4E2 on barrier function, we used electrical cell impedance sensing (ECIS), which reflects transendothelial resistance (TER) (20). Application of 4E2 to quiescent ISCLS-derived or healthy control BOECs significantly increased baseline TER compared to untreated or isotype control IgG2a-treated cells like that induced by the addition of exogenous Angpt-1 (Fig. 1A). Notably, the 4E2-induced increase in TER in ISCLS-derived BOECs was nearly double that observed in control BOECs, whereas the response to Angpt-1 was equivalent in the two groups (Fig. 1B). Neither surface Tie2 expression determined by flow cytometry (Fig. 1C and fig. S1) nor total Tie2 expression in cell lysates (fig. S2, A and B) differed between BOECs from patients with ISCLS and those from healthy controls. Compared to no or control IgG2a-treatment, 4E2 induced a rapid and sustained (2 hours) increase in Tie2 phosphorylation in control or ISCLS-derived BOECs (Fig. 1, D and E, and fig. S3, A and B).
Fig. 1. 4E2 activates Tie2 in human primary endothelial cells and fortifies barrier function.
(A) TER over time in control or ISCLS BOECs treated as indicated. Curves are representative of n = 3 cell lines per group; arrow denotes time of antibody addition. (B) Composite percent increase in TER at t = 1 hour after treatment. Means ± SEM of n = 3 cell lines per group analyzed in more than four independent experiments; *P = 0.03, two-way analysis of variance (ANOVA) and Benjamini-Hochberg–corrected multiple comparisons. (C) Tie2 expression [net geometric mean fluorescence intensity (ΔGMFI)] in BOECs; means ± SEM of n = 4 per group. (D and E) Representative immunoblot (D) and quantification (pTie2/Tie2) (E) in BOECs treated with 4E2. Means ± SEM of n = 3 to 6 experiments.
4E2 inhibits stress-induced endothelial barrier dysfunction
Genetic and pharmacological studies point to the sensitizing effect of reduced circulating Angpt-1/Angpt-2 ratios on endothelial permeability induced by cytokines (e.g., VEGF and bradykinin) and other organic humoral mediators (e.g., histamine) (21). To evaluate the impact of 4E2 on the stress-induced endothelial phenotype in ISCLS, we first measured TER after histamine treatment. Histamine elicited a progressive decrease in TER over time in ISCLS BOECs in a dose-dependent fashion (fig. S4, A and B). To facilitate comparison of antibody efficacy across cell lines from individual subjects, we derived median effective concentration (EC50) values for various mediators in each cell line (fig. S4C and table S1) and determined the impact of 4E2 pretreatment on TER in cells treated with submaximal agonist concentrations (at or near EC50). Compared to control IgG2a-treated cells, pretreatment with 4E2 significantly prevented the histamine-evoked decrease in TER in BOECs from both healthy donors and patients with ISCLS, like that induced by preincubation with Angpt1 (Fig. 2, A and B). Likewise, 4E2 effectively dampened barrier disruption elicited by the ISCLS-related mediator VEGF (Fig. 2, C and D) (16).
Fig. 2. 4E2 reduces responsiveness of patient-derived endothelial cells to canonical permeability mediators.
(A and C) TER over time in BOECs pretreated as indicated and stimulated with submaximal concentrations of histamine (A) or VEGF (C). Curves representative of n = 3 cell lines per group. The arrow denotes time of antibody addition; the arrowhead denotes time of stimulus addition, and the vertical line indicates approximate time of maximal decrease in TER. (B and D) Maximal decrease in TER as a percentage of the response of mock-pretreated cells. Means ± SEM of n = 3 cell lines per group analyzed in more than four independent experiments; *P = 0.03, **P < 0.009, and ***P = 0.0001, two-way ANOVA and Tukey multiple comparisons. (E) Morphology of BOECs left untreated (mock) or pretreated with either control IgG2a or 4E2 and then not treated (NT) or stimulated with either histamine or VEGF and fixed. Cells were stained with VE-cadherin antibody (green), phalloidin (red), and 4′,6-diamidino-2-phenylindole (DAPI; blue). Arrowheads denote areas of membrane disruption. Images represent three different cell lines evaluated in three independent experiments. Scale bar, 10 μm. (F) Representative blot of Tie2 knockdown in BOECs transfected with CTRL or Tie2 siRNA. (G) TER (maximal decrease as a percentage of mock treated cells) in untreated cells or cells pretreated with 4E2 and stimulated with VEGF. Means ± SEM from n = 2 independent experiments (six biological replicates per condition). *P = 0.03 and **P = 0.008, one-way ANOVA and Tukey multiple comparisons. ns, not significant.
To clarify the mechanism(s) underlying the effect of 4E2 on stress-induced endothelial barrier dysfunction, we examined dynamic changes in morphology and molecular structure of BOECs by immunofluorescence. Analogous to the effect of inflammatory mediators, treatment of normal dermal microvascular endothelial cell monolayers with acute ISCLS sera promotes formation of gaps between adjacent endothelial cells due to dissolution of intercellular adhesion junctions and remodeling of the actin cytoskeleton (16, 22, 23). As expected, treatment of either control or ISCLS-derived BOEC monolayers with histamine or VEGF reduced expression and localization of VE-cadherin at cell-cell junctions and cortical actin at the periphery of individual cells (Fig. 2E). Compared to IgG2a-pretreated cells, however, preincubation with 4E2 before agonist stimulation largely preserved membrane VE-cadherin localization and cortical actin in histamine- or VEGF-treated cells. Last, we evaluated the effect of 4E2 treatment on TER in BOECs transfected with Tie2-specific small interfering RNA (siRNA). Compared to mock or control siRNA-treated cells, Tie2 siRNA substantially reduced Tie2 expression (Fig. 2F). Moreover, the protective effect of 4E2 on the VEGF-induced decrease in TER was significantly reduced in Tie2-depleted cells compared to cells treated with control siRNA (Fig. 2G). These results strongly suggest that the impact of 4E2 on barrier function of BOECs depends on Tie2 activation.
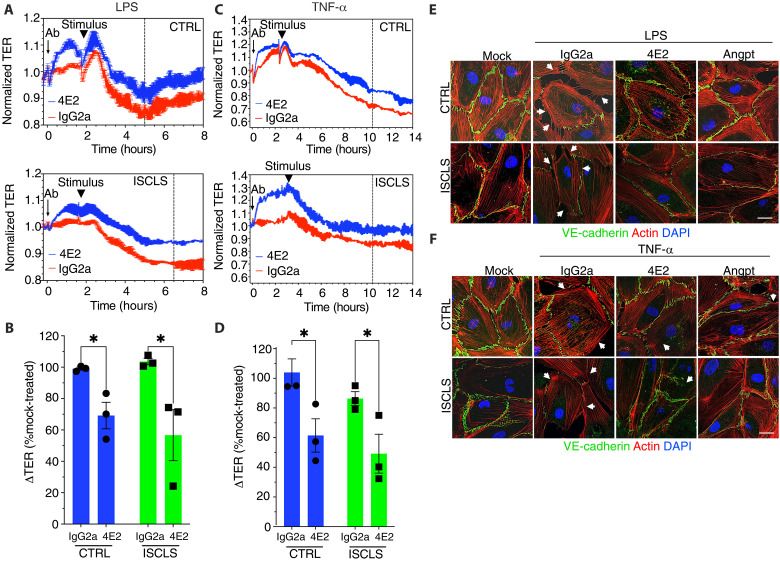
Acute ISCLS crises are frequently preceded by acute bacterial or viral infections (3). Accordingly, acute ISCLS sera contain increased levels of proinflammatory cytokines including CXCL10, CCL2, VEGF, interleukin-6, and tumor necrosis factor–α (TNF-α) (24, 25). We therefore tested the effect of 4E2 on inflammation-related endothelial barrier function by stimulating cells with either lipopolysaccharide (LPS) or TNF-α. 4E2 pretreatment significantly attenuated the maximal decrease in TER elicited by LPS (Fig. 3, A and B) or TNF-α (Fig. 3, C and D). Moreover, 4E2 substantially reduced the disruptions in junctional VE-cadherin and loss of cortical actin induced by either mediator (Fig. 3, E and F). These studies indicated that 4E2 dampens endothelial barrier dysfunction induced by a diverse group of inflammatory mediators in vitro.
Fig. 3. 4E2 attenuates inflammation-related endothelial barrier disruption.
(A and C) TER over time in BOECs pretreated as indicated and stimulated with submaximal concentrations of LPS (A) or TNF-α (C). Curves are representative of n = 3 cell lines per group. The arrow denotes time of antibody addition; arrowheads denote time of stimulus addition, and the vertical line indicates approximate time of maximal decrease in TER. (B and D) Maximal decrease in TER as a percentage the response of mock-pretreated cells. Means ± SEM of n = 3 cell lines per group analyzed in more than four independent experiments; *P = 0.01, two-way ANOVA and Tukey multiple comparisons. (E and F) Morphology of BOECs left untreated (mock) or pretreated with either control IgG2a, 4E2, or Angpt-1 and then stimulated with either LPS or TNF-α and fixed. Cells were stained with VE-cadherin antibody (green), phalloidin (red), and DAPI (blue). Arrowheads denote areas of membrane disruption. Images represent three different cell lines evaluated in two independent experiments. Scale bars, 10 μm.
4E2 attenuates leakage of dermal vasculature to histamine and VEGF in mice
The results of our in vitro studies revealed that Tie2 activation may control vascular leakage in ISCLS crises precipitated by systemic inflammation. To determine the significance of these findings for vascular leakage in vivo, we first examined the impact of 4E2 on the dermal microvasculature of mice challenged with classical permeability provocateurs. Pharmacokinetic analysis supported the suitability of 4E2 for these experiments. A single dose of 4E2 administered intravenously to mice (10 to 30 mg/kg) was well tolerated and had a prolonged half-life of ~180 hours (Supplementary text and tables S2 to S4). For analysis of vascular leakage, we studied aged (>6 months of age) SJL/J mice, which we have previously used to model acute ISCLS (26, 27). Similar to adult patients with ISCLS, aged SJL/J mice have no overt vascular phenotype at homeostasis but are unusually susceptible to vascular leakage, particularly in peripheral tissues such as skin and skeletal muscle, after systemic challenge with histamine or antecedent viral (influenza) infection. Unlike most other inbred strains of mice, a high proportion of SJL/J mice die within 30 min of systemic challenge with very low doses of histamine (26, 27). The autosomal recessive trait underlying this phenotype (termed “Histh”) maps to a ~83 Mb quantitative trait locus (QTL) on chromosome 6 (Chr6), a region most closely aligned with an interval on human Chr3 (325.3p) that was previously linked to ISCLS in a genome-wide association study (27, 28).
Compared to treatment with a control IgG2a, systemic injection of 4E2 into SJL/J mice significantly attenuated vascular leakage in skin [extravasation of Evans Blue (EB) dye] induced by intradermal injection of histamine (Fig. 4, A and B). Because the role of histamine in acute ISCLS is uncertain (29), we also examined 4E2’s impact on vascular sensitivity to an ISCLS-related humoral factor (VEGF). Plasma VEGF levels tend to be elevated at the onset of ISCLS episodes and wane over the course of the illness coincident with clinical improvement (16, 24). Intradermal injection of VEGF promoted EB extravasation into skin in a dose-dependent manner, and 4E2 pretreatment nearly abolished this response (Fig. 4, C and D).
Fig. 4. 4E2 dampens cutaneous vascular leakage to canonical permeability provocateurs in mice.
(A) Representative images of EB accumulation in skin of aged SJL/J mice pretreated as indicated and injected intradermally with histamine. (B) Quantification of EB content in skin. Means ± SEM of n = 7 to 9 mice per group. *P = 0.01 and ***P = 0.0002, two-way ANOVA and Sidak multiple comparisons. (C) Representative images of EB accumulation in skin of SJL/J mice pretreated as indicated and injected intradermally with VEGF. (D) Quantification of EB content in skin. Means ± SEM of n = 5 to 6 mice per group. *P = 0.04 and **P = 0.004, two-way ANOVA and Sidak multiple comparisons. Scale bars, 5 mm.
4E2 reduces mortality in the systemic mouse model of ISCLS
In our previous study, we mapped the Histh QTL mouse model of ISCLS narrowly on the basis of mortality of aged (>6 months of age) SJL/J mice within 30 min of challenge with histamine and enhanced vascular leakage, predominantly within skin and skeletal muscle, compared to histamine-resistant strains (26, 27). SJL/J mice have higher baseline circulating Angpt-2 levels than histamine-resistant strains like B10.S (26), suggesting the potential contribution of Tie2 dysregulation to the Histh phenotype in mice. To examine the viability of 4E2 as a therapeutic candidate for acute ISCLS episodes, we first assessed mortality of aged SJL/J mice after systemic administration of an otherwise lethal dose of histamine (10 mg/kg; fig. S5). While a majority (80%) of the mice pretreated with control IgG2a before histamine administration had died by the 30-min cutoff point that was originally used to define the Histh trait, nearly 65% of the mice pretreated with 4E2 remained alive at this time point (Fig. 5A). Moreover, while all the mice pretreated with control IgG2a subsequently died over a 2-hour observation period, 36% of 4E2-pretreated mice survived (Fig. 5B). Thus, 4E2 pretreatment significantly reduces overall mortality in the Histh ISCLS model.
Fig. 5. 4E2 reduces mortality and vascular leakage in an ISCLS mouse model.
(A) Mortality in SJL/J mice pretreated with either IgG2a or 4E2 at 30 min after systemic challenge with histamine (10 mg/kg body weight); n = 5 to 6 mice per group; *P = 0.01, t test. (B) Overall mortality in histamine-challenged mice; P = 0.01, log rank (Mantel-Cox) test. (C and D) Representative EB in leg muscle (C) and quantified in tissues (D) of SJL/J mice after systemic challenge with either PBS or histamine (2.5 mg/kg body weight). Means ± SEM of n = 6 to 11 mice per group. ****P < 0.0001, two-way ANOVA and Sidak multiple comparisons. (E and F) Representative EB in leg muscle (E) and quantified in tissues (F) of SJL/J mice pretreated with either IgG2a or 4E2 and challenged systemically with histamine. Means ± SEM of n = 9 to 11 mice per group; *P < 0.03, t test with Holm-Sidak multiple comparisons corrections. (G) Skeletal muscle sections from histamine-challenged mice pretreated as indicated and immunostained with phospho-Tie2 antibody. Arrows denote positive staining in microvasculature. Images representative on n = 6 mice per group. Scale bar, 100 μm.
Similar to ISCLS patients in crisis, SJL/J mice challenged with histamine die from cardiovascular collapse induced by widespread fluid extravasation into peripheral tissues, particularly in skin and skeletal muscle (26). To specifically evaluate the effect of 4E2 treatment on systemic vascular leakage, we administered submaximal, nonlethal doses of histamine (2.5 mg/kg) to aged SJL/J mice and measured EB extravasation into tissues. Consistent with our previous findings and the pattern of vascular leakage in ISCLS patients, histamine challenge increased EB extravasation primarily in skeletal muscle and, to a lesser extent, skin, but not in visceral organs, compared to challenge with phosphate-buffered saline (PBS) alone (Fig. 5, C and D). 4E2 pretreatment significantly reduced histamine-induced EB extravasation in skin and skeletal muscle compared to that observed in mice treated with control IgG2a (Fig. 5, E and F). To confirm that 4E2 elicited Tie activation in vivo, we examined phosphorylated Tie2 in skeletal muscle tissue sections from histamine-challenged mice using immunohistochemistry. Consistent with prior findings (18), we observed substantially more immunoreactive pTie2 in the muscle-associated microvasculature of 4E2-treated mice compared to IgG2a-treated controls (Fig. 5G).
4E2 dampens vascular leakage in an infection-related model of ISCLS
ISCLS crises are frequently associated with respiratory viral infections including influenza and COVID-19 (8, 30). Likewise, Histh is also unmasked in otherwise resistant younger (<6 months of age) SJL/J mice when they have been primed with an inflammatory stimulus (complete Freund’s adjuvant) before histamine challenge (26). To determine the impact of 4E2 on infection-associated vascular leakage, we inoculated young (~16 weeks of age) SJL/J mice intranasally with a sublethal dose of influenza virus A (H3N2). We then treated mice with 4E2 or control IgG2a at the onset of clinical symptoms (weight loss) (Fig. 6A). Both groups experienced a similar degree of weight loss (~25% of initial body weight) that began days 3 to 4 after inoculation (Fig. 6B). We then euthanized mice at the peak of clinical symptoms (day 7) and quantified EB extravasation in various tissues. Compared to mice treated with control IgG, 4E2-treated mice had significantly less postinfection vascular leakage as evidenced by reduced EB content in all organs examined including lung, liver, intestine, skin, and muscle (Fig. 6C).
Fig. 6. 4E2 ameliorates influenza-associated vascular leakage in the ISCLS mouse model.
(A) Experimental schematic. Mice were inoculated with H3N2 (~45,000 virions) intranasally on day 0 and treated with IgG2a or 4E2 (30 mg/kg) on days 4 and 6 after infection. (B) Weight loss in mice inoculated with H3N2 over time. Means ± SEM of n = 8 mice per group. (C) EB content in various tissues in H3N2-infected mice treated with either IgG2a or 4E2 and euthanized on day 7. Means ± SEM of n = 8 mice per group; **P = 0.006, two-way ANOVA and Tukey multiple comparisons.
DISCUSSION
Effective therapy for acute ISCLS episodes remains elusive. No diagnostic criteria exist to predict the frequency or severity of episodes in each patient. In nearly half of ISCLS crises, there is no obvious infectious trigger (3). Although numerous proinflammatory cytokines are detected in acute ISCLS sera, typically, their levels have already ebbed by the time the patient presents in extremis (16). Rather, compelling evidence is emerging to suggest that ISCLS flares result from an exaggerated endothelial response to otherwise mundane inflammatory stressors. Patients have increased skin edema after intradermal challenge with unrelated leak provocateurs (histamine or morphine) (26). Preliminary studies suggest that patient-derived endothelial cells exhibit outsized permeability responses to proinflammatory cytokines in culture over multiple passages, suggesting a genetically or epigenetically determined defect underlying the functional hyperresponsiveness. These findings provide strong rationale for targeting the host vascular response to inflammation in ISCLS rather than specific humoral mediators.
In this proof-of-principle study, we demonstrated that the Tie2 agonist antibody 4E2 fortifies the barrier function of ISCLS-derived endothelial cells in vitro at homeostasis and under stress and dampens vascular leakage in the SJL/J mouse model of ISCLS induced by histamine or VEGF challenge. These findings demonstrate the promise of 4E2 prophylaxis to reduce the frequency and/or severity of ISCLS flares. 4E2 treatment of SJL/J mice post-influenza exposure also effectively reduced vascular leakage, suggesting the applicability of 4E2 as rescue therapy in patients who present with signs and symptoms of acute ISCLS (hypotension and hemoconcentration) after several days of flu-like symptoms.
Tie2 may be a uniquely attractive target for the prevention and/or treatment of acute ISCLS flares. Serum Angpt-2 levels are already elevated at baseline in ISCLS sera, rise further during episodes as clinical symptoms progress, and wane with resolution of hypotension and edema (16, 17), suggesting a role for this cytokine in the induction and/or maintenance of vascular leakage. Genetic and pharmacological studies of related conditions featuring prominent endothelial barrier dysregulation strongly support the vascular protective role of Tie2. Mice with Tie2 haploinsufficiency and patients with predicted loss of function haplotypes in Tie2 are more susceptible to sepsis-induced vascular leakage and mortality (31). A synthetic Angpt-1 mimic peptide (vasculotide) (32) or an Angpt-2 binding, Tie2-activating antibody (ABTAA) reduces vessel leakiness and mortality in mouse models of sepsis (9).
While these and several other Angpt-Tie2–directed therapeutics developed over the past decade have demonstrated considerable efficacy in preclinical models (12), none has yet proven to be efficacious in treating systemic conditions in human clinical trials. An engineered version of Angpt-1, cartilage oligomeric matrix protein (COMP)–Angpt-1, was generated to improve upon the poor solubility of the native protein and prolong bioavailability. COMP–Angpt-1 nonetheless has a relatively short half-life in plasma (~30 min) and is poorly distributed in peripheral microvasculature after systemic injection (9). Neither COMP–Angpt-1 or Angpt-2–blocking antibodies reduces mortality in mouse sepsis models (9).
4E2 may thus represent an advance over these and other agents because of its specificity and ability to activate Tie2 in a ligand-independent manner. Razuprotafib (AKB-9778), a small molecule inhibitor of VE protein tyrosine phosphatase, enhances Tie2 activation and improves mortality in mouse sepsis (33), but its applicability to ISCLS may be limited by the occurrence of drug-associated hypotension. Likewise, the vascular protective effects of ABTAA are inherently dependent upon circulating Angpt-2 levels. More recently, another ligand-independent Tie2-activating antibody has been generated, but its effects on endothelial permeability have not yet been tested (13).
Our study has several limitations and raises further questions. 4E2 activity depends on Tie2 expression. Endotoxemia is associated with reduced Tie2 protein abundance (34), and inflammatory mediators may induce Tie2 ectodomain cleavage and shedding into circulation (15), potentially reducing 4E2’s efficacy. However, we found that Tie2 expression in ISCLS-derived BOECs was like that in BOECs from healthy controls and have not detected increased soluble Tie2 levels in acute ISCLS sera (16, 25). Second, while Tie2-targeted therapies like vasculotide or ABTAA reduce parameters of inflammation (serum cytokine storm and glycocalyx degradation) in mouse models of infection, we did not address 4E2’s potential anti-inflammatory effects. However, these studies may be less important for ISCLS, as there is typically no correlation between the severity of infection and the degree of vascular leakage and shock due to ISCLS. As but one prominent example, severe acute respiratory syndrome coronavirus 2–positive ISCLS patients experiencing little to no respiratory signs and symptoms have nevertheless presented with severe acute flares characterized by rapidly progressive and ultimately fatal hypotensive shock resistant to resuscitation (4, 30).
Potential impediments to implementation of 4E2 therapy in ISCLS include the difficulty of randomized controlled trials due to disease rarity and frequently missed or delayed diagnosis. The rapid rate of progression of acute ISCLS episodes may also limit its therapeutic window of efficacy. Last, we did not address in this study the potential effects of chronic Tie2 activation by 4E2. Forced expression of COMP–Angpt-1 in hematopoietic stem cells in mice leads to aberrant angiogenesis and impaired hematopoiesis (35). Chronic Angpt-2 blockade may lead to disruption of lymphangiogenesis, implying a risk for lymphedema with chronic use (14). Further studies will be needed to determine the efficacy of 4E2 as IVIG-sparing prophylactic therapy in ISCLS.
MATERIALS AND METHODS
Experimental design
The main objectives of the present study were to clarify the role of the endothelial receptor Tie2 in Clarkson disease and to evaluate the efficacy of the Tie2-activating monoclonal antibody 4E2 on vascular integrity in disease models. We first studied the effects of 4E2 on Tie2 activation and permeability of patient-derived endothelial cells at quiescence and after exposure to several different ISCLS-related permeability provocateurs. We then assessed the impact of 4E2 on SJL/J mice in models of ISCLS induced by three different inflammatory stressors. Sample size justification was derived from previous animal studies that were sufficiently powered to detect differences in vascular permeability (26). Animals were randomly assigned to treatment with 4E2 or IgG2a before commencement of each study.
Patients
Patients were enrolled in a study protocol approved by the National Institutes of Health (NIH) Institutional Review Board (09-I-0184) after providing informed consent. BOECs were isolated and expanded as described previously (19, 36). BOECs from anonymized healthy donors were used as controls. BOECs were cultured in collagen type I–coated flasks containing EGM2 media (Lonza) supplemented with 10% fetal bovine serum and VEGF-A (3 ng/ml), in an incubator containing 5% CO2.
Mice
SJL/J (SJL) and Balb/cJ mice were obtained from the Jackson Laboratory. Animals were housed, and experiments were conducted under a protocol approved by the National Institute of Allergy and Infectious Diseases (NIAID) Institutional Animal Care and Use Committee (protocol #LAD3E).
Pharmacokinetic analysis
CT26 colon cancer syngeneic BALB/c mice were treated with 4E2 mouse IgG2a (10 or 30 mg/kg; table S2). In each group, the treatment (n = 3/time point, n = 33 in total) was administered through a single or repeated intravenous bolus injection in the tail vein. Blood was collected on day 1 before dose; after dosing at 0.083, 0.5, 3, 6, and 24 hours, and on postdose days 2, 3, 4, 7, and 14. At each sampling time, three mice were euthanized; blood was collected via cardiac puncture, and serum was harvested by centrifugation. Serum 4E2 concentrations were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well microtiter plate wells were coated with human recombinant Tie2 (1.5 μg/ml; Sino Biological) in PBS at 4°C overnight. Wells were washed three times with a PBS/0.05% Tween 20 and incubated with blocking buffer [PBS containing 0.5% bovine serum albumin (BSA) and 0.05% Tween 20/0.05% Proclin300] for 2 hours. After washing, assay calibrators and quality control solutions representing the lower limit of quantification, LQC, middle quality control, high quality control, and upper limit of quantification were prepared by diluting known amounts of 4E2 in blank serum (BioIVT). Serum samples were serially diluted from 1- to 1000-fold in blank serum, and calibrators, quality controls, serum blanks, and samples were diluted with predetermined factor, i.e., 100 of the minimum required dilution in blocking buffer. Each component was added into the plate well in duplicate and incubated at 25°C for 1 hour. After washing, the plate was incubated with detection antibody [horseradish peroxidase (HRP)–conjugated goat anti-mouse IgG (Fcγ) antibody (Jackson ImmunoResearch), 1:2000 in a PBS/0.05% Tween 20] at 25°C for 30 min. After wash with a PBS/0.05% Tween 20 solution, plates were incubated with 3,30,5,50-tetramethylbenzidine chromogenic solution (TMB; BD Biosciences) at 25°C for 20 min. Reactions were quenched by adding 1 M sulfuric acid stop solution. Optical density was measured at 450 nm with the correction at 650 nm and analyzed using Softmax Pro 5 software (Molecular Devices). Serum 4E2 concentrations were interpolated from a four-parameter logistic fit of the standard curve on the same plate. Assay sensitivity ranged from 0.156 to 10 μg/ml, and the minimum required dilution for the assay was 1:100 for mouse serum. Pharmacokinetic analysis was conducted using Phoenix WinNonlin (version 8.3; Certara Inc.) following noncompartmental analysis based on the serum concentration curve (tables S2 and S3). Terminal elimination half-life (t1/2) was calculated using the terminal elimination rate constant using [t1/2 = 0.693/Kel (terminal elimination rate constant)]. The maximum observed peak serum concentration (Cmax) and the time to reach Cmax (Tmax) were determined on the basis of the observed data. The AUClast was calculated using the area under the serum concentration–time curve from 0 hours to the last quantifiable time point with linear trapezoidal method with linear interpolation, and the AUCinf was calculated using the area under the serum concentration–time curve from 0 hours to infinity, with linear trapezoidal rule and the addition of Clast/Kel. Total body clearance (CL) was calculated using (CL = dose/AUCinf). Volume of distribution at steady state (Vss) was calculated using [Vss = (AUMCinf × dose)/(AUCinf)2], where AUMCinf is the area under the first moment curve from time zero to infinity.
Electrical cell impedance sensing
TER was assessed in BOECs using the ECIS Z-Theta system (Applied Biophysics) as previously described (16, 37). Briefly, cells were plated in electrode-containing wells coated with collagen I (50 μg/ml) and equilibrated overnight. Cells were serum-starved in EBM medium (Lonza) containing 0.2% BSA for 4 hours; isotype control IgG2a or 4E2 (30 μg/ml) was added 2 hours before stimulation. Normalized TER values were normalized by dividing raw readings by TER at t = 0.
RNA interference
On-TARGETplus Smartpool TEK siRNA (catalog no. L-003178-00-0005) or nontargeting control siRNA (5 μM; catalog no. D-001810-10-20; Horizon Discovery) was transfected into BOECs using DharmaFECT 4 transfection reagent according to the manufacturer’s instructions. BOECs were analyzed 48 hours after transfection.
Immunoblotting
BOECs were grown to confluence on collagen type I–coated dishes and were serum-starved for four hours in EBM containing 0.2% BSA. Cells were pretreated with IgG2a or 4E2 (30 μg/ml) for 2 hours before stimulation. Treated cells were washed twice with ice-cold PBS supplemented phenylmethylsulfonyl fluoride (1 mM). Lysates were prepared using 1× radioimmunoprecipitation buffer (EMD Millipore) supplemented with 1× PhosSTOP and 1× cOmplete Protease inhibitor cocktails (Roche). Proteins were electrophoresed using NuPAGE Bis-Tris gels (Thermo Fisher Scientific) and transferred to nitrocellulose membranes. Blots were incubated overnight with primary antibodies (data file S1) detected with near-infrared–conjugated secondary antibodies using the LI-COR Odyssey 3000 imager (LI-COR Biosciences).
Immunofluorescence and immunohistochemistry
BOECs (25,000 cells per well) were plated in collagen type I–coated ibiTreat eight-well slides and grown to confluence. Cells were serum-starved for 4 hours in total and treated with IgG2a or 4E2 (30 μg/ml) for 2 hours before agonist treatment. Cells were fixed with 4% paraformaldehyde in PBS and then permeabilized using PBS containing 0.2% Triton X-100 and 2% BSA. Cells were stained overnight at 4°C with primary antibodies and then fluorophore-conjugated secondary antibodies for 1 hour at room temperature (RT). Images were acquired at ×63 magnification using a Leica Sp8 DMI8000 confocal microscope. For immunohistochemistry, formalin-fixed samples were embedded into paraffin blocks and sectioned. Sections (5 μm) were generated using a microtome, and the slides were deparaffinized through graded alcohols and brought to water. Slides were then retrieved using heat retrieval and blocked with hydrogen peroxidase. Next, slides were incubated with pTie2 antibody and detected with a cross-adsorbed goat-anti-rabbit-HRP secondary antibody and visualized with DAB. Slides were counterstained with hematoxylin, dehydrated in graded alcohols, cleared in xylene, and mounted with a permanent mounting medium.
Flow cytometry
BOECs were fixed in PBS containing 4% paraformaldehyde for 15 min at RT. Fixed cells were washed twice with PBS containing 2% BSA and blocked with PBS containing 1% BSA and 0.05% Triton X100 and 0.05% NaN3 for 30 min at RT. Cells were stained with primary antibodies for 2.5 hours on ice and washed twice with PBS containing 1% BSA. Fluorescence signals were acquired using a LSRFortessa flow cytometer (BD Biosciences) and analyzed using FlowJo software.
Miles assay
EB extravasation was assessed as previously described (26). Briefly, the dorsal back skin of mice was depilated at least 2 days before experiments. Animals were first injected with or IgG2a or 4E2 intravenously (30 mg/kg body weight) 2 hours before administration of PBS containing 2% Evans Blue (Sigma-Aldrich) via the retro-orbital route (100 μl). PBS, histamine, or VEGF-A (Sigma-Aldrich) were then injected intradermally 30 min later (volume, 50 μl). Mice were euthanized, and the dorsal skin was harvested using a 12-mm biopsy punch. Samples were dried at 95°C overnight. EB was extracted from dried samples with formamide (Sigma-Aldrich; 65°C overnight). EB was quantified by measuring absorbance at 620 nm and interpolating amounts from a standard curve. For experiments using VEGF, pyrilamine maleate (4 mg/kg body weight; Sigma-Aldrich) was injected intraperitoneally 30 min before intradermal challenge to reduce background permeability.
Systemic histamine challenge
Mice were given control IgG2a or 4E2 (30 mg/kg body weight) intravenously 2 hours before challenge. Mortality was recorded over a period of 2 hours. For assessment of vascular leakage, mice were pretreated as above and injected with PBS containing 2% EB via the retro-orbital route (volume, 100 μl). Mice were then immediately challenged with histamine via intraperitoneal injection. Fifteen minutes after challenge, mice were anesthetized by isoflurane inhalation and perfused with 20 ml of heparinized PBS through the left ventricle. Tissues were harvested, and EB content was measured as above.
Influenza virus infection
Mice were anesthetized with isoflurane and inoculated intranasally with influenza A/HK/1/68 (H3N2) virus (~45,000 virions). Weight loss was monitored, and mice were given IgG2a or 4E2 (30 mg/kg) intraperitoneally on day 4 and again on day 6 after infection. Mice were euthanized on day 7 after inoculation for analysis of vascular leakage.
Statistical analysis
Unless otherwise noted, data are presented as means ± SEM. Statistical analyses were done using GraphPad Prism. Significance was assessed using two-tailed unpaired t tests for two groups or analysis of variance (ANOVA) for comparison of multiple groups. Log-rank analysis was used to compare survival distributions. P < 0.05 was considered statistically significant.
Acknowledgments
Funding: This work was supported by the Division of Intramural Research, NIAID/NIH (grant AI001083-14 to K.M.D.) and by a Collaborative Research and Development Agreement (CRADA) between NIAID/NIH and PharmAbcine Inc. (M-CRADA 2020-0274-1). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Author contributions: Conceptualization: J.-S.Y., C.H.P., E.-A.L., B.Y.K., W.S.L., H.P., and K.M.D. Methodology: A.J.A., A.D., J.-S.Y., C.H.P., E.-A.L., B.Y.K., W.S.L., H.P., Y.A.L., S.R.S., and K.M.D. Investigation: A.J.A. and A.D. Visualization: A.J.A., A.D., J.-S.Y., C.H.P., E.-A.L., B.Y.K., W.S.L., H.P., and K.M.D. Funding acquisition: K.M.D. Project administration: K.M.D. Supervision: K.M.D. Writing (original draft): A.J.A. and K.M.D. Writing (review and editing): A.J.A, A.D., J.-S.Y., C.H.P., E.-A.L., B.Y.K., W.S.L., H.P., and K.M.D.
Competing interests: J.-S.Y., C.H.P., E.-A.L., B.Y.K., W.S.L., and H.P. are coinventors of 4E2 and stockholders of PharmAbcine Inc. The authors declare that they have no other competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Materials used in the analysis are available upon request to PharmAbcine (jinsan.yoo@pharmabcine.com) after execution of a material transfer agreement.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S4
Tables S1 to S4
Legend for data file S1
Other Supplementary Material for this manuscript includes the following:
Data file S1
REFERENCES AND NOTES
- 1.M. P. de Chambrun, C.-E. Luyt, F. Beloncle, M. Gousseff, W. Mauhin, L. Argaud, S. Ledochowski, A.-S. Moreau, R. Sonneville, B. Verdière, S. Merceron, N. Zappella, M. Landais, D. Contou, A. Demoule, S. Paulus, B. Souweine, B. Lecomte, A. Vieillard-Baron, N. Terzi, E. Azoulay, R. Friolet, M. Puidupin, J. Devaquet, J.-M. Mazou, Y. Fedun, J.-P. Mira, J.-H. Raphalen, A. Combes, Z. Amoura, The clinical picture of severe systemic capillary-leak syndrome episodes requiring ICU admission. Crit. Care Med. 45, 1216–1223 (2017). [DOI] [PubMed] [Google Scholar]
- 2.K. M. Druey, S. M. Parikh, Idiopathic systemic capillary leak syndrome (Clarkson disease). J. Allergy Clin. Immunol. 140, 663–670 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.T. S. Eo, K. J. Chun, S. J. Hong, J. Y. Kim, I. R. Lee, K. H. Lee, M. Eisenhut, A. Kronbichler, J. I. Shin, Clinical presentation, management, and prognostic factors of idiopathic systemic capillary leak syndrome: A systematic review. J. Allergy Clin. Immunol. Pract. 6, 609–618 (2018). [DOI] [PubMed] [Google Scholar]
- 4.P. C. Cheung, R. Eisch, N. Maleque, D. M. Polly, S. C. Auld, K. M. Druey, Fatal exacerbations of systemic capillary leak syndrome complicating coronavirus disease. Emer. Infect. Dis. 27, 2529–2534 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.N. P. Juffermans, C. E. van den Brom, D. J. B. Kleinveld, Targeting endothelial dysfunction in acute critical illness to reduce organ failure. Anesth. Analg. 131, 1708–1720 (2020). [DOI] [PubMed] [Google Scholar]
- 6.V. Dhir, V. Arya, I. C. Malav, B. S. Suryanarayanan, R. Gupta, A. B. Dey, Idiopathic systemic capillary leak syndrome (SCLS): Case report and systematic review of cases reported in the last 16 years. Intern. Med. 46, 899–904 (2007). [DOI] [PubMed] [Google Scholar]
- 7.M. P. de Chambrun, M. Gousseff, W. Mauhin, J.-C. Lega, M. Lambert, S. Rivière, A. Dossier, M. Ruivard, F. Lhote, G. Blaison, L. Alric, C. Agard, D. Saadoun, J. Graveleau, M. Soubrier, M.-J. Lucchini-Lecomte, C. Christides, A. Bosseray, H. Levesque, J.-F. Viallard, N. Tieulie, P.-Y. Lovey, S. L. Moal, B. Bibes, G. Malizia, P. Abgueguen, F. Lifermann, J. Ninet, P.-Y. Hatron, Z. Amoura; EurêClark Study Group , Intravenous immunoglobulins improve survival in monoclonal gammopathy-associated systemic capillary-leak syndrome. Am. J. Med. 130, 1219.e1219–1219.e1227 (2017). [DOI] [PubMed] [Google Scholar]
- 8.M. S. Pecker, M. Hammudi, R. Melchio, A. R. Eisch, F. Verlicchi, K. M. Druey, Management of acute episodes of clarkson disease (monoclonal gammopathy-associated systemic capillary leak syndrome) with intravenous immunoglobulins. Ann Intern Med Clin Cases 1, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.S. Han, S.-J. Lee, K. E. Kim, H. S. Lee, N. Oh, I. Park, E. Ko, S. J. Oh, Y.-S. Lee, D. Kim, S. Lee, D. H. Lee, K.-H. Lee, S. Y. Chae, J.-H. Lee, S.-J. Kim, H.-C. Kim, S. Kim, S. H. Kim, C. Kim, Y. Nakaoka, Y. He, H. G. Augustin, J. Hu, P. H. Song, Y.-I. Kim, P. Kim, I. Kim, G. Y. Koh, Amelioration of sepsis by TIE2 activation-induced vascular protection. Sci. Transl. Med. 8, 335ra355 (2016). [DOI] [PubMed] [Google Scholar]
- 10.A. Lask, B. Gutbier, O. Kershaw, G. Nouailles, A. D. Gruber, H. C. Müller-Redetzky, S. Chackowicz, D. A. Hamilton, P. van Slyke, M. Witzenrath, Adjunctive therapy with the Tie2 agonist vasculotide reduces pulmonary permeability in Streptococcus pneumoniae infected and mechanically ventilated mice. Sci. Rep. 12, 15531 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.J. Kim, J. R. Park, J. Choi, I. Park, Y. Hwang, H. Bae, Y. Kim, W. J. Choi, J. M. Yang, S. Han, T. Y. Chung, P. Kim, Y. Kubota, H. G. Augustin, W. Y. Oh, G. Y. Koh, Tie2 activation promotes choriocapillary regeneration for alleviating neovascular age-related macular degeneration. Sci. Adv. 5, eaau6732 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.P. Saharinen, L. Eklund, K. Alitalo, Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 16, 635–661 (2017). [DOI] [PubMed] [Google Scholar]
- 13.G. Jo, J. Bae, H. J. Hong, A. R. Han, D. K. Kim, S. P. Hong, J. A. Kim, S. Lee, G. Y. Koh, H. M. Kim, Structural insights into the clustering and activation of Tie2 receptor mediated by Tie2 agonistic antibody. Nat. Commun. 12, 6287 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.E. A. Korhonen, A. Murtomäki, S. K. Jha, A. Anisimov, A. Pink, Y. Zhang, S. Stritt, I. Liaqat, L. Stanczuk, L. Alderfer, Z. Sun, E. Kapiainen, A. Singh, I. Sultan, A. Lantta, V. M. Leppänen, L. Eklund, Y. He, H. G. Augustin, K. Vaahtomeri, P. Saharinen, T. Mäkinen, K. Alitalo, Lymphangiogenesis requires Ang2/Tie/PI3K signaling for VEGFR3 cell-surface expression. J. Clin. Invest. 132, e155478 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.K. D. Sack, J. A. Kellum, S. M. Parikh, The angiopoietin-Tie2 pathway in critical illness. Crit. Care Clin. 36, 201–216 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Z. Xie, C. C. Ghosh, R. Patel, S. Iwaki, D. Gaskins, C. Nelson, N. Jones, P. R. Greipp, S. M. Parikh, K. M. Druey, Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome). Blood 119, 4321–4332 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.S. Yamanouchi, M. Yamagishi, S. Kaneko, M. Shimizu, K. Kaneko, Dysregulation of angiopoietin-1 and angiopoietin-2 in an infant with fatal Clarkson disease. Pediatr. Int. 62, 1400–1401 (2020). [DOI] [PubMed] [Google Scholar]
- 18.E. Lee, E. A. Lee, E. Kong, H. Chon, M. Llaiqui-Condori, C. H. Park, B. Y. Park, N. R. Kang, J. S. Yoo, H. S. Lee, H. S. Kim, S. H. Park, S. W. Choi, D. Vestweber, J. H. Lee, P. Kim, W. S. Lee, I. Kim, An agonistic anti-Tie2 antibody suppresses the normal-to-tumor vascular transition in the glioblastoma invasion zone. Exp. Mol. Med. 55, 470–484 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.A. C. Sek, Z. Xie, K. Terai, L. M. Long, C. Nelson, A. Z. Dudek, K. M. Druey, Endothelial expression of endothelin receptor a in the systemic capillary leak syndrome. PLOS ONE 10, e0133266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.I. Bischoff, M. C. Hornburger, B. A. Mayer, A. Beyerle, J. Wegener, R. Fürst, Pitfalls in assessing microvascular endothelial barrier function: Impedance-based devices versus the classic macromolecular tracer assay. Sci. Rep. 6, 23671 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A. V. Benest, K. Kruse, S. Savant, M. Thomas, A. M. Laib, E. K. Loos, U. Fiedler, H. G. Augustin, Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLOS ONE 8, e70459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Z. Xie, C. C. Ghosh, S. M. Parikh, K. M. Druey, Mechanistic classification of the systemic capillary leak syndrome: Clarkson disease. Am. J. Respir. Crit. Care Med. 189, 1145–1147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.L. Claesson-Welsh, E. Dejana, D. M. McDonald, Permeability of the endothelial barrier: Identifying and reconciling controversies. Trends Mol. Med. 27, 314–331 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Z. Xie, E. Chan, Y. Yin, C. C. Ghosh, L. Wisch, C. Nelson, M. Young, S. M. Parikh, K. M. Druey, Inflammatory markers of the systemic capillary leak syndrome (Clarkson disease). J. Clin. Cell. Immunol. 5, 1000213 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Z. Xie, D. B. Kuhns, X. Gu, H. H. Otu, T. A. Libermann, J. I. Gallin, S. M. Parikh, K. M. Druey, Neutrophil activation in systemic capillary leak syndrome (Clarkson disease). J. Cell. Mol. Med. 23, 5119–5127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A. Raza, Z. Xie, E. C. Chan, W. S. Chen, L. M. Scott, A. Robin Eisch, D. N. Krementsov, H. F. Rosenberg, S. M. Parikh, E. P. Blankenhorn, C. Teuscher, K. M. Druey, A natural mouse model reveals genetic determinants of systemic capillary leak syndrome (Clarkson disease). Commun Biol 2, 398 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A. L. Tyler, A. Raza, D. N. Krementsov, L. K. Case, R. Huang, R. Z. Ma, E. P. Blankenhorn, C. Teuscher, J. M. Mahoney, Network-based functional prediction augments genetic association to predict candidate genes for histamine hypersensitivity in mice. G3 (Bethesda) 9, 4223–4233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Z. Xie, V. Nagarajan, D. E. Sturdevant, S. Iwaki, E. Chan, L. Wisch, M. Young, C. M. Nelson, S. F. Porcella, K. M. Druey, Genome-wide SNP analysis of the systemic capillary leak syndrome (Clarkson disease). Rare Dis 1, e27445 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.J. P. Atkinson, T. A. Waldmann, S. F. Stein, J. A. Gelfand, W. J. Macdonald, L. W. Heck, E. L. Cohen, A. P. Kaplan, M. M. Frank, Systemic capillary leak syndrome and monoclonal IgG gammopathy; studies in a sixth patient and a review of the literature. Medicine (Baltimore) 56, 225–239 (1977). [DOI] [PubMed] [Google Scholar]
- 30.M. P. de Chambrun, Q. Moyon, S. Faguer, G. Urbanski, A. Mathian, N. Zucman, M. Werner, C.-E. Luyt, F. Verlicchi, Z. Amoura; EurêClark Study Group , The consequences of COVID-19 pandemic on patients with monoclonal gammopathy-associated systemic capillary leak syndrome (Clarkson disease). J. Allergy Clin. Immunol. Pract. 10, 626–629 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.C. C. Ghosh, S. David, R. Zhang, A. Berghelli, K. Milam, S. J. Higgins, J. Hunter, A. Mukherjee, Y. Wei, M. Tran, F. Suber, L. Kobzik, K. C. Kain, S. Lu, A. Santel, K. Yano, P. Guha, D. J. Dumont, D. C. Christiani, S. M. Parikh, Gene control of tyrosine kinase TIE2 and vascular manifestations of infections. Proc. Natl. Acad. Sci. U.S.A. 113, 2472–2477 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.P. Kümpers, F. Gueler, S. David, P. Van Slyke, D. J. Dumont, J.-K. Park, C. L. Bockmeyer, S. M. Parikh, H. Pavenstädt, H. Haller, N. Shushakova, The synthetic tie2 agonist peptide vasculotide protects against vascular leakage and reduces mortality in murine abdominal sepsis. Crit. Care 15, R261 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.M. Frye, M. Dierkes, V. Küppers, M. Vockel, J. Tomm, D. Zeuschner, J. Rossaint, A. Zarbock, G. Y. Koh, K. Peters, A. F. Nottebaum, D. Vestweber, Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J. Exp. Med. 212, 2267–2287 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.M. van Meurs, N. F. Kurniati, F. M. Wulfert, S. A. Asgeirsdottir, I. A. de Graaf, S. C. Satchell, P. W. Mathieson, R. M. Jongman, P. Kümpers, J. G. Zijlstra, P. Heeringa, G. Molema, Shock-induced stress induces loss of microvascular endothelial Tie2 in the kidney which is not associated with reduced glomerular barrier function. Am. J. Physiol. Renal Physiol. 297, F272–F281 (2009). [DOI] [PubMed] [Google Scholar]
- 35.R. G. Wallace, K. D. Rochfort, P. Barabas, T. M. Curtis, H. Uehara, B. K. Ambati, P. M. Cummins, COMP-Ang1: Therapeutic potential of an engineered Angiopoietin-1 variant. Vascul. Pharmacol. 141, 106919 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Z. Xie, M. Børset, K. Svéen, O. W. Bøe, E. C. Chan, J. B. Lack, K. M. Hornick, F. Verlicchi, A. R. Eisch, R. Melchio, A. Z. Dudek, K. M. Druey, Markers of endothelial glycocalyx dysfunction in Clarkson disease. J. Transl. Med. 20, 380 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Z. Xie, W. S. Chen, Y. Yin, E. C. Chan, K. Terai, L. M. Long, T. G. Myers, A. Z. Dudek, K. M. Druey, Adrenomedullin surges are linked to acute episodes of the systemic capillary leak syndrome (Clarkson disease). J. Leukoc. Biol. 103, 749–759 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S4
Tables S1 to S4
Legend for data file S1
Data file S1