Abstract
Introduction
Severe acute respiratory virus syndrome coronavirus 2 (SARS-CoV-2) was responsible for coronavirus disease (COVID-19) pandemic. As patients recovered from COVID-19 infection, hair loss was increasingly observed as a distressing symptom.
Methods
This was a cross-sectional study of patients with post COVID-19 hair loss between July to December 2021 at a tertiary care center. Detailed history, clinical examination, trichoscopy and biochemical tests were performed and recorded. COVID-19 disease severity was assessed based on duration of COVID-19 infection and place of management.
Results
The study included 120 patients with a mean age of 39.6 years. The majority of the patients were females treated at home and had COVID-19 infection for >2 weeks. The mean visual analog scale (VAS) score for stress was 5.25. Vitamin D deficiency was present in 56.7% and low ferritin in 30% of cases. The mean time of onset of hair loss post COVID-19 was 49 days. Patients mainly presented with diffuse hair loss. Trichodynia was present in 15.8% of cases. The degree of hair loss was severe in 55.8% of the subjects. Positive hair pull test was seen in 65% of patients. Most common trichoscopic features included single hair follicles (81.7%) and vellus hair >10% (60%).
Conclusions
The mean time of onset of hair loss post COVID-19 infection was less than 2 months. Majority patients had diffuse pattern and severe degree of hair loss. Trichoscopy can aid in unmasking co-existing patterned hair loss in patients presenting clinically with diffuse hair loss.
Keywords: COVID-19, hair loss, telogen effluvium, trichoscopy, patterned hair loss
Introduction
Severe acute respiratory virus syndrome coronavirus 2 (SARS-CoV-2) caused the coronavirus disease 2019 (COVID-19) pandemic, which created public health care crisis worldwide. Besides pulmonary, it also leads to cardiovascular, gastrointestinal, neurological, hematological and dermatological manifestations [1]. Even though immediate complications of COVID-19 infection are well defined, its delayed sequalae are now being increasingly reported, and are a cause of significant concern. Post-acute COVID-19 syndrome is characterized by persistent symptoms beyond 4 weeks, which is further classified into subacute phase (symptoms present from 1–3 months after acute COVID-19 infection) and chronic or post-COVID-19 syndrome (symptoms present or persisting after 3 months of onset of acute COVID-19 infection and not attributable to alternative diagnosis) [2].
The association between telogen effluvium (TE) and COVID-19 infection is stronger than other forms of hair loss [3]. TE is categorized as either acute when it lasts for less than 6 months or chronic when it lasts for more than 6 months. It is a common cause of diffuse non-scarring hair loss that occurs 3–4 months after any acute illness like high grade fever, medical or surgical causes, medications, stress or nutritional deficiencies. However, post COVID-19 TE has been reported after 1–3 months of onset of infection, earlier than classic TE.
Androgenetic alopecia, alopecia areata, fibrosing alopecia in a patterned distribution are other types of hair loss reported post COVID-19 infection [3]. Androgenetic alopecia has been suggested to be a poor prognostic factor for severe COVID-19 infection in some studies [4,5]. However, results from recent studies refute this observation [6,7]. Alopecia areata (including totalis and universalis) may be exacerbated or induced by COVID-19 infection. Fibrosing alopecia has also been reported as isolated cases [3,8]. The mechanisms of hair loss remain unclear, although autoimmune abnormalities, immune imbalances and prolonged residual infection of hair follicle may be involved [3].
Objectives
The aim of this study was to assess the clinical, hematological, biochemical and trichoscopic features in patients presenting with post COVID-19 hair loss and to compare them with the severity of COVID-19 infection and degree of hair loss.
Methods
This cross-sectional study was conducted at a tertiary care center in North India during the post COVID-19 pandemic between July to December 2021. Consecutive patients aged 18–60 years, who presented with hair loss and had a preceding and recent COVID-19 infection, confirmed by detection of viral RNA by real time reverse transcriptase polymerase chain reaction were included. Detailed clinical history related to pre-COVID-19 hair loss, severity and duration of COVID-19 infection, associated stress, co-morbid illnesses like diabetes, hypertension and asthma and predisposing factors like pre-existing medical, surgical, metabolic illness, anemia, stress and drug intake were recorded in a pre-designed questionnaire. Severity of COVID-19 infection was arbitrarily decided by the authors based on the place of treatment (at home or hospitalized) and duration of COVID-19 infection (≤ 2 weeks or >2 weeks). Degree of hair loss was classified according to average hair shed per day as mild (<50), moderate (50–100) and severe (>100). Psychological stress due to COVID-19 infection was recorded on a visual analogue scale (VAS) of 0 to 10. Hair pull test was done by grasping around forty hair between index finger and thumb on frontal, mid scalp, vertex, temporal and occipital regions. Trichoscopy was performed using DermLite DL4 handheld dermoscope for anisotrichosis, single hair units, vellus hair percentage, interfollicular distance, yellow dots, black dots and perifollicular pigment and scale. Biochemical investigations were done to rule out concurrent thyroid disorders, iron, Vitamin D and Vitamin B12 deficiencies. The study was approved by institutional ethics committee (S.No IEC/VMMC/SJH/Project/2021-10/CC-196) and informed consent was taken from all study subjects.
Data Collection and Statistical Analysis
Statistical analysis was performed using IBM SPSS statistics version 17.0 (IBM Corp.). Baseline characteristics were analyzed using descriptive statistics. The parametric data was described as percentages and mean ± standard deviation. Comparison between groups was done using Student t test, chi-square test, Wilcoxon-Mann-Whitney U test, Kruskal Wallis test and Fishers exact test. Analysis was carried out at 5% level of significance and P-value < 0.05 was considered statistically significant.
Results
Demographic Factors and Associated Diseases
A total of 120 patients were included in the study. Mean age of the patients was 39.6±12.4 years. Age distribution was bimodal, with 54.1% in the age group of 41–60 years and 25% in the age group of 21–30 years. There were 103 (85.8%) females and 17 (14.2%) males. Of the total patients, 104 (87%) were treated for COVID-19 infection at home. The duration of illness was ≤ 2 weeks among 68 (56.7%) patients and > 2 weeks in 52 (43.3%) patients. Only 12 (10%) patients had co-morbid illnesses and 50 patients (41.7%) had underlying predisposing factors for TE. Mean VAS score for stress was found to be 5.25±2.8. Vitamin D deficiency was detected in 68 (56.7%) patients, low serum ferritin in 36 (30%), low hemoglobin in 24 (20%) and Vitamin B12 deficiency in 18 (15%) patients. Raised Thyroid Stimulating Hormone (TSH) levels were noted in 14 (11.7%) patients. Table 1 shows the demographic factors and associated diseases among study participants.
Table 1.
Demographic factors & associated diseases in patients with post COVID-19 hair loss.
| Variable | Result |
|---|---|
|
| |
| Age (in years) [Range, Mean ± SD] | [19–60, 39.6 ± 12.4 years] |
| 11–20 | 6 (5%) |
| 21–30 | 30 (25%) |
| 31–40 | 19 (15.8%) |
| 41–50 | 37 (30.8%) |
| 51–60 | 28 (23.3%) |
|
| |
| Gender (F: M ratio) | 6.05:1 |
| Females (F); Males (M) | 103 (85.8%); 17 (14.2%) |
|
| |
| Patients treated for COVID-19 at Home; Hospital | 104 (86.7%); 16 (13.3%) |
|
| |
| Duration of COVID-19 infection | 68 (56.7%) |
| ≤ 2 weeks | 52 (43.3%) |
| >2 weeks | |
|
| |
| Co-morbid illness | 12 (10%) |
| Diabetes Mellitus | 5 (4.2%) |
| Hypertension | 4 (3.3%) |
| Asthma | 1 (0.8%) |
| Diabetes and Hypertension | 2 (1.7%) |
|
| |
| Predisposing factors | 50 (41.7%) |
| Medical/Metabolic/Anemia/Surgery | 40 (33.3%) |
| Stress | 4 (3.3%) |
| Drugs | 8 (6.7%) |
| Others | 2 (1.7%) |
|
| |
| Mean VAS score | 5.25 ± 2.8 |
|
| |
| Vitamin D deficiency | 68 (56.7%) |
|
| |
| Low ferritin | 36 (30%) |
|
| |
| Low hemoglobin | 24 (20%) |
|
| |
| Vitamin B12 deficiency | 18 (15%) |
|
| |
| Raised TSH | 14 (11.7%) |
|
| |
| Family member with COVID-19 associated hair loss | 57 (47.5%) |
SD = standard deviation; TSH = Thyroid Stimulating Hormone; VAS = Visual Analog Scale.
Clinical and Trichoscopic Findings
Of 120 participants, 45 (37.5%) had pre COVID-19 hair loss. The most common pattern of hair loss among them was diffuse type in 26 patients (21.7%). Mean time of onset of hair loss post COVID-19 infection was observed to be 49 ± 30.7 days, with no significant difference between males (43.71 days) and females (50.18 days) (P-value = 0.684). Trichodynia was reported by 19 (15.8%) and scalp itch by 13 (10.8%) patients. Mild, moderate and severe hair loss (Figure 1) was seen in 6.7%, 37.5% and 55.8% of patients respectively. Hair pull test was found to be positive among 78 (65%) patients. The most common pattern of post COVID-19 hair loss was diffuse type in 80 (66.7%) patients. The most common trichoscopic finding observed was single hair follicle unit in 98 (81.7%) patients. Table 2 shows the clinical and trichoscopic features (Figures 2 and 3) in patients with COVID-19 hair loss.
Figure 1.
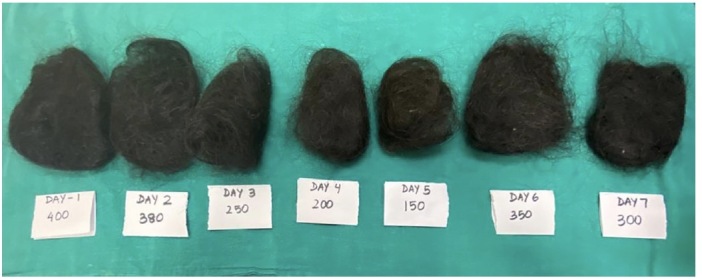
Daily hair count of seven days from a patient with severe degree of diffuse hair loss.
Table 2.
Clinical and trichoscopic findings of hair loss.
| Variable | Result |
|---|---|
|
| |
| Pattern of Pre-COVID-19 hair loss | 45 (37.5%) |
| Diffuse | 26 (21.7%) |
| FPHL | 9 (7.5%) |
| MPHL | 8 (6.7%) |
| AA | 2 (1.7%) |
|
| |
| Pattern of Post-COVID-19 hair loss | |
| Diffuse | 81 (67.5%) |
| FPHL | 24 (20%) |
| MPHL | 9 (7.5%) |
| Diffuse and FPHL | 4 (3.3%) |
| Diffuse and MPHL | 1 (0.8%) |
| Diffuse and AA | 1 (0.8%) |
|
| |
| Time of onset of hair loss post COVID-19 infection | 2–196 days, 49 ± 30.7 days |
| Range, Mean ± SD | |
|
| |
| Trichodynia | 19 (15.8%) |
|
| |
| Scalp itch | 13 (10.8%) |
|
| |
| Degree of hair loss | |
| Mild | 8 (6.7%) |
| Moderate | 45 (37.5%) |
| Severe | 67 (55.8%) |
|
| |
| Positive hair pull test | 78 (65%) |
|
| |
| Hair Density Decreased | 109 (90.8%) |
|
| |
| Scalp features (on clinical examination) | |
| Scaling | 11 (9.2%) |
| Erythema | 1 (0.8%) |
| Pustule | 1 (0.8%) |
|
| |
| Trichoscopic features | |
|
| |
| Single hair follicle | 98 (81.7%) |
| Vellus hair >10% | 72 (60%) |
| Empty hair follicle | 61 (50.8%) |
| Anisotrichosis | 52 (43.3%) |
| Increased interfollicular distance | 50 (41.2%) |
| Perifollicular scaling | 33 (27.5%) |
| Perifollicular pigment | 17 (14.2%) |
| Yellow dot | 15 (12.5%) |
AA = Alopecia areata; FPHL = Female pattern hair loss; MPHL = Male pattern hair loss; SD = standard deviation.
Figure 2.
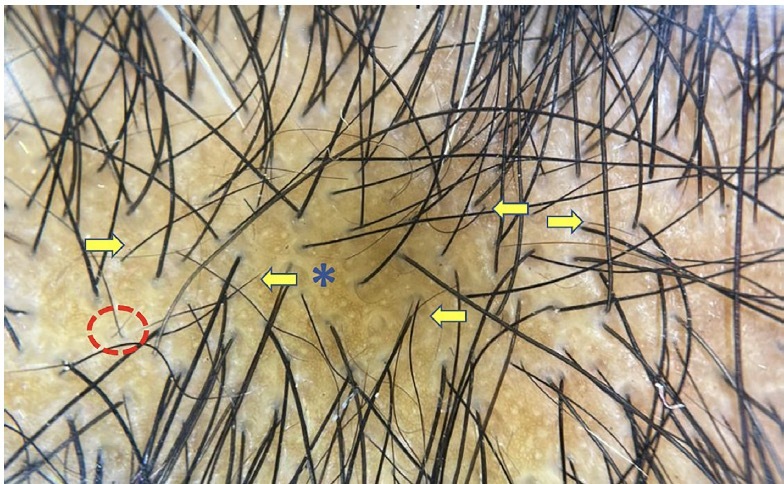
Trichoscopy showing numerous empty hair follicles (blue asterisk), increased interfollicular distance, single hair follicles, multiple short regrowing hair (yellow arrow) and few vellus hair (red circle) suggestive of telogen effluvium (DermLite DL4 ×20).
Figure 3.
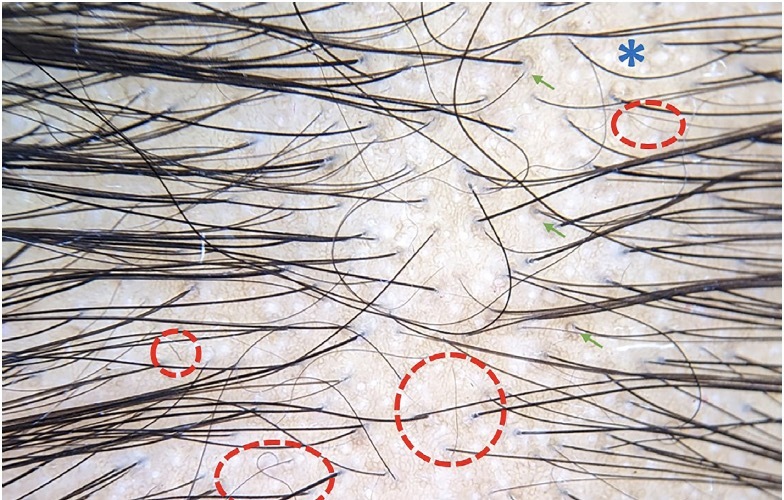
Trichoscopy showing anisotrichosis, single hair follicles, empty hair follicles (blue asterisk), increased interfollicular distance, single hair follicles, multiple vellus hair (red circle), perifollicular brown pigmentation (green arrow) suggestive of patterned hair loss (DermLite DL4 ×20).
Significant comparative findings between study parameters and degree of hair loss are enlisted in Table 3. Comparison of clinical and trichoscopic features of hair loss between patients treated for COVID-19 infection at home (Group 1) and at hospital (Group 2) are summarized in Table 4; and for patients with duration of COVID-19 infection for ≤ 2 weeks (Group 3) and for > 2 weeks (Group 4) are summarized in Table 5.
Table 3.
Significant comparative findings of study parameters based on degree of hair loss.
| Variable | Group A Mild hair loss (N = 8) |
Group B Moderate hair loss (N = 45) |
Group C Severe hair loss (N = 67) |
P-value |
|---|---|---|---|---|
|
| ||||
| Age (Years) | 39.00 ± 14.22 | 36.27 ± 11.92 | 41.94 ± 12.15 | 0.048 |
|
| ||||
| Gender | 3 (37.5%) | 10 (22.2%) | 4 (6.0%) | 0.008 |
| Male | 5 (62.5%) | 35 (77.8%) | 63 (94.0%) | |
| Female | ||||
|
| ||||
| Place of management | 6 (75.0%) | 41 (91.1%) | 57 (85.1%) | 0.395 |
| Home | 2 (25.0%) | 4 (8.9%) | 10 (14.9%) | |
| Hospitalized | ||||
|
| ||||
| Duration of COVID-19 infection | 3 (37.5%) | 33 (73.3%) | 32 (47.8%) | 0.011 |
| ≤ 2 Weeks | 5 (62.5%) | 12 (26.7%) | 35 (52.2%) | |
| > 2 Weeks | ||||
|
| ||||
| Pre-existing Hair Loss | 5 (62.5%) | 22 (48.9%) | 18 (26.9%) | 0.020 |
| Yes | 3 (37.3%) | 23 (51.1&) | 49 (73.1%) | |
| No | ||||
|
| ||||
| VAS score | 3.62 ± 2.62 | 5.00 ± 2.72 | 5.61 ± 2.83 | 0.099 |
AA = Alopecia areata; FPHL = Female pattern hair loss; MPHL = Male pattern hair loss; VAS: =Visual analogue scale.
Table 4.
Comparison of clinical and trichoscopic features of hair loss between Group 1 (treated at home) and Group 2 (hospitalized).
| Variable | Group 1 (N = 104) | Group 2 (N = 16) | P-value |
|---|---|---|---|
|
| |||
| Age (years) | 38.1± 12.2 | 49.2± 8.6 | 0.001 |
|
| |||
| Mean time of onset of hair loss post COVID-19 infection (days) | 48.9 ± 31.5 | 51.9 ± 25.9 | 0.427 |
|
| |||
| Duration | |||
| ≤ 2 weeks | 65 (62.5%) | 3 (18.8%) | 0.001 |
| > 2 weeks | 39 (37.5%) | 13 (81.2%) | |
|
| |||
| Mean VAS score | 4.8 ± 2.8 | 7.06 ± 2.38 | 0.005 |
|
| |||
| Pattern of current hair loss | |||
| Diffuse | 69 (66.3%) | 12 (75%) | 0.810 |
| MPHL | 20 (19.2%) | 4 (25%) | |
| FPHL | 9 (8.7%) | 0 (0%) | |
| Diffuse and FPHL | 4 (3.8%) | 0 (0%) | |
| Diffuse and AA | 1 (1.0%) | 0 (0%) | |
| Diffuse and MPHL | 1 (1.0%) | 0 (0%) | |
|
| |||
| Degree of hair loss | |||
| Mild | 6 (5.8%) | 2 (12.5%) | 0.395 |
| Moderate | 41 (39.4%) | 4 (25%) | |
| Severe | 57 (54.8%) | 10 (62.5%) | |
|
| |||
| Trichoscopic features | |||
|
| |||
| Single hair follicle | 83 (79.8%) | 15 (93.8%) | 0.299 |
|
| |||
| Vellus hair >10% | 58 (55.8%) | 14 (87.5%) | 0.016 |
|
| |||
| Empty hair follicles | 48 (46.2%) | 13 (81.2%) | 0.009 |
|
| |||
| Anisotrichosis | 44 (42.3%) | 8 (50.0%) | 0.563 |
|
| |||
| Increased Interfollicular distance | 40 (38.5%) | 10 (62.5%) | 0.069 |
|
| |||
| Perifollicular scaling | 30 (28.8%) | 3 (18.8%) | 0.552 |
|
| |||
| Perifollicular pigment | 14 (13.5%) | 3 (18.8%) | 0.699 |
|
| |||
| Yellow dots | 11 (10.6%) | 4 (25%) | 0.115 |
AA = Alopecia areata; FPHL = Female pattern hair loss; MPHL = Male pattern hair loss; VAS: =Visual analogue scale.
Table 5.
Comparison of clinical and trichoscopic features between Group 3 (duration of COVID-19 infection ≤ 2 weeks) and Group 4 (duration of COVID-19 infection > 2 weeks).
| Variable | Group 3 ≤ 2 weeks (N = 68) |
Group 4 >2 weeks (N = 52) |
P-value |
|---|---|---|---|
|
| |||
| Age (years) | 35.9± 11.82 | 44.48 ± 11.49 | 0.001 |
|
| |||
| Mean time of onset of hair loss post COVID-19 infection (days) | 50.3 ± 34.2 | 47.9 ± 25.6 | 0.901 |
|
| |||
| Place of management | |||
| Home | 65 (95.6%) | 39 (75.0%) | 0.001 |
| Hospitalized | 3 (4.4%) | 13 (25.0%) | |
|
| |||
| Mean VAS score | 4.60±2.80 | 6.10±2.60 | 0.004 |
|
| |||
| Pattern of current hair loss | |||
| Diffuse | 44 (64.7%) | 37 (71.2%) | 0.336 |
| MPHL | 13 (19.1%) | 11 (21.2%) | |
| FPHL | 6 (8.8%) | 3 (5.8%) | |
| Diffuse and FPHL | 4 (5.9%) | 0 (0%) | |
| Diffuse and MPHL | 1 (1.5%) | 0 (0%) | |
| Diffuse and AA | 0 (0%) | 1 (1.9%) | |
|
| |||
| Degree of hair loss | |||
| Mild | 3 (4.4%) | 5 (9.6%) | 0.011 |
| Moderate | 33 (48.5%) | 12 (23%) | |
| Severe | 32 (47.1%) | 35 (67.3%) | |
|
| |||
| Trichoscopic features | |||
|
| |||
| Single hair follicle | 56 (82.4%) | 42 (80.8%) | 0.824 |
|
| |||
| Vellus hair >10% | 38 (55.9%) | 34 (65.4%) | 0.292 |
|
| |||
| Empty hair follicles | 34 (50.0%) | 27 (51.9%) | 0.835 |
|
| |||
| Anisotrichosis | 28 (41.2%) | 24 (46.2%) | 0.586 |
|
| |||
| Increased Interfollicular distance | 27 (39.7%) | 23 (44.2%) | 0.618 |
|
| |||
| Perifollicular scaling | 15 (22.1%) | 18 (34.6%) | 0.127 |
|
| |||
| Perifollicular pigment | 10 (14.7%) | 7 (13.5%) | 0.846 |
|
| |||
| Yellow dots | 8 (11.8%) | 7 (13.5%) | 0.781 |
AA = Alopecia areata; FPHL = Female pattern hair loss; MPHL = Male pattern hair loss; VAS: =Visual analogue scale.
Conclusions
COVID-19 infection was a cause of global pandemic and there has been extensive research to guide diagnosis, management and prevention of the same. However, post recovery symptoms have been equally harrowing, with survivors showing varied symptoms ranging from fatigue, dyspnea, headache, attention deficit to hair loss. Post COVID-19 hair loss has been reported to be one of the five most common post recovery symptoms [9].
The mean age of patients reported in our study (39.6 years) was similar to that reported by Sharquie et al (41.3 years). However, both younger (mean age 31 years) and older age groups (49 years) have been affected, which could possibly be due to geographical variation in the prevalence of COVID-19 infection [10–12]. There was a female preponderance in the present study, as seen in other studies as well, possible explanations being, longer hair length and higher vulnerability of female hair follicles due to role of estrogen and progesterone hormones in the pathophysiology of hair loss [1,2,8,10,12–15]. Higher prevalence of anemia (20%) and low ferritin levels (30%) in our patients, who were predominantly females, can also be a contributing factor, as was also observed by Babaei et al who reported the presence of iron deficiency anemia in 13.8% of their patients [12].
The mean VAS score for stress in our study was 5.25, which was comparatively lower than that reported by Rivetti et al (8.2) [16]. Stress, however, has been noted to induce as well as increase the hair loss during and post COVID-19 pandemic. We also found that the mean VAS score was significantly higher in patients who were hospitalized for COVID-19 infection and who had infection for more than 2 weeks, hence, correlating with the severity of infection. However, it was not significantly associated with the degree of hair loss. The relationship between stress and hair cycle changes has given rise to the concept of “brain-hair follicle axis”, wherein the release of specific neuropeptides and hormones along this axis may promote changes in the hair growth cycle by shifting the hair from anagen to telogen, promoting hair loss [16]. An unexpected finding was that 47.5% of study population had a family member with post COVID-19 hair loss, indicating its high prevalence and illustrating the importance of taking a good family history.
Pre COVID-19 hair loss was observed more frequently in our patients (37.5%) compared to a study by Abdulwahab et al (15.5%). The latter reported a greater number of cases of TE and alopecia areata vis-à-vis the present study. We, however, observed a higher proportion of patients with patterned hair loss [2]. Interestingly, majority of our cases with pre-existing hair loss had mild to moderate degree of hair loss post COVID-19 infection.
Current hair loss in our study was predominantly diffuse type, followed by patterned type with overlap of diffuse, patterned and/or alopecia areata in a few cases. In contrast, Abdulwahab et al reported greater number of cases of TE and alopecia areata and lesser number of cases with patterned hair loss [2]. Meanwhile, Babaei et al in their study found androgenetic alopecia as the most common concomitant pattern with TE [12]. Another study on hair loss post COVID-19 infection reported majority cases of TE and only few cases of alopecia areata and fibrosing alopecia [8]. We noted a relatively higher percentage of patterned hair loss, possibly because of high prevalence of pre-existing patterned hair loss in our study population. Another possible explanation could be that COVID-19 infection exacerbated or triggered patterned hair loss [17].
The mean time of onset of hair loss post COVID-19 infection in our study was 49 days, which was comparable to that found in other studies (7–9 weeks) [8,10,12–14,18–21]. Starace et al observed earlier onset of hair loss in patients with trichodynia (3 weeks), however we observed no such difference [22]. The mean time of onset of hair loss post COVID-19 infection is earlier than the classic TE, possibly because of pathogenetic mechanisms implicated in post COVID-19 hair loss [10]. These include intense release of pro inflammatory cytokines including IL-6, IL-4, IL-10, matrix metalloproteinase (MMP) 1 and 3, which induce catagen, cause premature anagen release, have cytotoxic effects on keratinocytes, inhibit stem cells and decrease hair growth [5,10]. Moreover, anticoagulant proteins are decreased in COVID-19 infection, causing microthrombi formation which obstruct the hair follicle blood supply resulting in hair loss. Increased stress hormones, oxidative stress and hypoxia during COVID-19 infection are all potential culprits [5]. Furthermore, direct viral damage to hair follicle has also been hypothesized [10]. Co-existing anemia, deficiency of Vitamin D and Vitamin B12, increased TSH and high stress level may also contribute. A significant proportion of our study population (43.3%) had COVID-19 infection for > 2 weeks, causing more sustained inflammation, possibly resulting in earlier onset of hair loss. However, we noticed no significant difference in onset of hair loss post COVID-19 infection amongst patients with or without hospitalization or duration of COVID-19 infection (≤ or > 2 weeks). Babaei et al reported early onset of hair loss in patients with hypothyroidism, younger age group and females [12].
Trichodynia was present in 15.8% and scalp itch in 10.8% of our study subjects. On the other hand, Starace et al observed trichodynia in 58.4% and trichodynia with TE in 42.4% of patients. They also found an association of trichodynia with dysgeusia and anosmia, postulating an underlying neurogenic pathogenesis for all [22]. Trichodynia corresponds to a complex symptom comprising scalp pain, pruritus or burning sensation on touching the scalp, which may be a sign of severity or a warning symptom of imminent hair shedding [15.22].
Females showed a greater degree of hair loss compared to males (61.2% of females versus 23.5% of males), the difference being statistically significant. Older study subjects had more severe hair loss as has been previously reported [12]. Degree of hair loss also correlated with duration of COVID-19 infection in our study, as majority of patients with COVID-19 infection for >2 weeks had severe hair loss. This can be possibly explained by sustained inflammatory or viral damage. Hospitalization, however, did not have any impact on the degree of hair loss in our study. The effect of hospitalization or COVID-19 severity on hair loss has been controversial. Even though studies have found that more than a fourth of the patients with acute TE post recovery were hospitalized during COVID-19 infection, others have reported that approximately 1 in 10 patients had subclinical infection or majority had only mild infection [8,14,18]. Therefore, it is imperative that clinicians enquire about preceding COVID-19 infection in last 1–3 months in all patients with sudden hair loss in the context of the pandemic [18,21].
Trichoscopic evaluation is essential in all patients with hair loss as it helps to uncover and guide towards clinically indistinguishable causes, like, differentiating diffuse alopecia areata from TE and patterned hair loss in early stages. Even though, acute TE does not have any specific findings on trichoscopy, the most common features described are: decreased density of hair, empty hair follicles, numerous short regrowing hair of normal thickness and single hair follicles [10,15,18,19,21,23,24]. The commonest presenting complaint in our study was decreased hair density (in 90.8% of patients), which on trichoscopy was seen as increased interfollicular distance in 41.2% of study subjects. Empty hair follicle, another common trichoscopic finding in TE (which may also be seen in patterned hair loss), was observed in 50.8% of our cases. Also, single hair follicle, which has been previously reported in TE and patterned hair loss was seen in 43.3% of our patients [25]. Similar findings on trichoscopy have been described in case reports and small case series [10,13,19,24,26,27].
Anisotrichosis or hair diameter variability of 20% favors patterned hair loss over TE. Even though only 31.7% of study subjects clinically had patterned hair loss, anisotrichosis on trichoscopy was seen in 43.3% of patients, predicting that a significant proportion may eventually develop patterned hair loss in addition to diffuse hair loss. In contrast to our finding, a few case reports have reported the absence of anisotrichosis [20,26,28].
An interesting finding in our study was the presence of vellus hair in 60% of patients, even though clinically majority had diffuse hair loss suggestive of TE. Similarly, vellus hair in fronto-temporal region have been reported in a single case of TE post COVID-19 infection [24]. On the contrary, some studies observed absence of miniaturized hair in post COVID-19 TE [19,20]. As vellus hair are classically seen in patterned hair loss and alopecia areata, our patients with vellus hair could represent overlap of TE with patterned hair loss or alopecia areata [25].
Yellow dots, which are a feature of alopecia areata and patterned hair loss, was seen in only 12.5% and perifollicular pigment, which has been reported in patterned hair loss, was seen in 14.2% of study subjects [23,25]. Even though clinically, scalp seborrhea and erythema were present in only 9.2% of cases; perifollicular scaling on trichoscopy was seen in 27.5% of cases. It has been speculated previously that COVID-19 infection can exacerbate or precipitate seborrheic dermatitis [29]. Lv et al also reported scalp inflammation, capillary ectasia and seborrhea on trichoscopy [28].
Another interesting finding in the present study was that patients who were hospitalized for COVID-19 infection had significantly greater number of empty hair follicles and vellus hair. These findings are suggestive that severe COVID-19 infection led to higher release of pro-inflammatory cytokines, thereby resulting in a significant increase in diffuse and possibly patterned hair loss. Association between hospitalization for COVID-19 infection and patterned hair loss has been hypothesized in previous studies, which suggested androgen mediated SARS-CoV-2 vulnerability, and gave the eponym of Gabrin sign to visually identify people at an increased risk of hospitalization and negative outcomes. The Gabrin sign suggests that patients with higher degree of male pattern baldness are at higher risk of developing severe COVID-19 symptoms and may require hospitalization [4,5]. This has, however, been argued against, and it has been suggested by few studies that patterned baldness in both women and men is not related to COVID-19 severity [3,6,7].
Hair loss has been a significant complaint in the post-acute COVID-19 syndrome, both in the subacute and chronic phase. The underlying cause has been documented to be TE (anagen effluvium as well as chronic TE), precipitation and exacerbation of alopecia areata, or patterned hair loss [2,30]. Our findings also suggest the same, with 67% of patients presenting in the subacute phase and 13% in the chronic phase, reiterating the need of awareness in both patients and dermatologists alike.
The limitations of our study included the study population being limited to the patients specifically seeking dermatologist consultation at a tertiary care hospital and therefore, not representative of the general population. In addition, there was a lack of control group of patients with COVID-19 infection who did not suffer from hair loss. Histopathological confirmation of diagnosis was not carried out, which may have led to misdiagnosis of a particular type of hair loss.
In conclusion the present study elucidates the clinical, biochemical and trichoscopic features in patients presenting with post COVID-19 hair loss. Given the severe stress and anxiety that hair loss can have, along with potential long-term sequelae of diffuse and patterned hair loss, dermatologists need to be aware of this distressing effect of COVID-19 infection. Future areas of research include histopathological examination of hair follicles, genetic studies for susceptibility to hair loss and studies evaluating the roles of hormones (estrogen, progesterone and androgens) and inflammatory cytokines on the extent and duration of hair loss.
Acknowledgements
The authors would like to acknowledge the help rendered by Dr Prateek Sharma (Ex-senior resident Department of Dermatology and STD, Vardhman Mahavir Medical College and Safdarjung Hospital) and Dr Namita Srivastava (Statistician cum lecturer, Department of Community Medicine, Vardhman Mahavir Medical College and Safdarjung Hospital) in the preparation of the manuscript.
Footnotes
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
Funding: None.
References
- 1.Tammaro A, Adebanjo GAR, Parisella FR, Luzi F, Scarabello A. Hair and nail manifestations of COVID-19. J Cosmet Dermatol. 2022;21(4):1339–1346. doi: 10.1111/jocd.14774. [DOI] [PubMed] [Google Scholar]
- 2.Abdulwahab RA, Aldajani BM, Natto NK, et al. Prevalence of Hair Loss After COVID-19 Infection in Makkah Region, Saudi Arabia. Cureus. 2022;14(9):e29285. doi: 10.7759/cureus.29285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei KC, Yang CC. Hair loss and COVID-19. Dermatol Sin. 2021;39:167–168. doi: 10.4103/ds.ds_52_21. [DOI] [Google Scholar]
- 4.Wambier CG, Vaño-Galván S, McCoy J, et al. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The “Gabrin sign”. J Am Acad Dermatol. 2020;83(2):680–682. doi: 10.1016/j.jaad.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gentile P. Hair Loss and Telogen Effluvium Related to COVID-19: The Potential Implication of Adipose-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma as Regenerative Strategies. Int J Mol Sci. 2022;23(16):9116. doi: 10.3390/ijms23169116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torabi S, Mozdourian M, Rezazadeh R, Payandeh A, Badiee S, Darchini-Maragheh E. Androgenetic alopecia in women and men is not related to COVID-19 infection severity: a prospective cohort study of hospitalized COVID-19 patients. J Eur Acad Dermatol Venereol. 2021;35(9):e553–e556. doi: 10.1111/jdv.17353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanha HM, Medland S, Martin NG, Nyholt DR. Genetic correlation analysis does not associate male pattern baldness with COVID-19. J Am Acad Dermatol. 2021;85(4):971–973. doi: 10.1016/j.jaad.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czech T, Sugihara S, Nishimura Y. Characteristics of hair loss after COVID-19: A systematic scoping review. J Cosmet Dermatol. 2022;21(9):3655–3662. doi: 10.1111/jocd.15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv [Preprint] 20210 doi: 10.1101/2021.01.27.21250617. 2021.01.27.21250617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi A, Magri F, Sernicola A, et al. Telogen Effluvium after SARS-CoV-2 Infection: A Series of Cases and Possible Pathogenetic Mechanisms. Skin Appendage Disord. 2021;21(5):1–5. doi: 10.1159/000517223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharquie KE, Jabbar RI. COVID-19 infection is a major cause of acute telogen effluvium. Ir J Med Sci. 2022;191(4):1677–1681. doi: 10.1007/s11845-021-02754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babaei K, Kavoussi H, Rezaei M, Kavoussi R. Characteristics of telogen effluvium in COVID-19 in western Iran (2020) An Bras Dermatol. 2021;96(6):688–692. doi: 10.1016/j.abd.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roda Â, Oliveira-Soares R. Acute telogen effluvium in patients recently infected with SARS-CoV-2. J Port Soc Dermatol Venereol. 2021;79(1):21–25. doi: 10.29021/spdv.79.1.1299. [DOI] [Google Scholar]
- 14.Moreno-Arrones OM, Lobato-Berezo A, Gomez-Zubiaur A, et al. SARS-CoV-2-induced telogen effluvium: a multicentric study. J Eur Acad Dermatol Venereol. 2021;35(3):e181–e183. doi: 10.1111/jdv.17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seyfi S, Alijanpour R, Aryanian Z, Ezoji K, Mahmoudi M. Prevalence of telogen effluvium hair loss in COVID-19 patients and its relationship with disease severity. J Med Life. 2022;15(5):631–634. doi: 10.25122/jml-2021-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivetti N, Barruscotti S. Management of telogen effluvium during the COVID-19 emergency: Psychological implications. Dermatol Ther. 2020;33(4):e13648. doi: 10.1111/dth.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadzhigoroeva A, Sanchez DG, Firooz A, et al. COVID-19 Can Exacerbate Pattern Hair Loss and Trigger Telogen Effluvium - The Role of Proteoglycan Replacement Therapy with Nourkrin® in Clinical Treatment of COVID-19 Associated Hair Loss. J Dermatol Res Ther. 2021;7:103. doi: 10.23937/2469-5750/151010. [DOI] [Google Scholar]
- 18.Hussain N, Agarwala P, Iqbal K, et al. A systematic review of acute telogen effluvium, a harrowing post-COVID-19 manifestation. J Med Virol. 2022;94(4):1391–1401. doi: 10.1002/jmv.27534. [DOI] [PubMed] [Google Scholar]
- 19.Rizzetto G, Diotallevi F, Campanati A, et al. Telogen effluvium related to post severe Sars-Cov-2 infection: Clinical aspects and our management experience. Dermatol Ther. 2021;34(1):e14547. doi: 10.1111/dth.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monari P, Gualdi G, Bettoni G, et al. Post-SARS-CoV-2 Acute Telogen Effluvium: An Expected Complication. J Clin Med. 2022;11(5):1234. doi: 10.3390/jcm11051234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrantes TF, Artounian KA, Falsey R, et al. Time of onset and duration of post-COVID-19 acute telogen effluvium. J Am Acad Dermatol. 2021;85(4):975–976. doi: 10.1016/j.jaad.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: Results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11–18. doi: 10.1016/j.jdin.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ocampo-Garza J, Tosti A. Trichoscopy of Dark Scalp. Skin Appendage Disord. 2018;5(1):1–8. doi: 10.1159/000488885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mieczkowska K, Deutsch A, Borok J, et al. Telogen effluvium: a sequela of COVID-19. Int J Dermatol. 2021;60(1):122–124. doi: 10.1111/ijd.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govindarajulu SM, Srinivas RT, Kuppuswamy SK, Prem P. Trichoscopic Patterns of Nonscarring Alopecia’s. Int J Trichology. 2020;12(3):99–106. doi: 10.4103/ijt.ijt_1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domínguez-Santás M, Haya-Martínez L, Fernández-Nieto D, Jiménez-Cauhé J, Suárez-Valle A, Díaz-Guimaraens B. Acute telogen effluvium associated with SARS-CoV-2 infection. Aust J Gen Pract. 2020:49. doi: 10.31128/AJGP-COVID-32. [DOI] [PubMed] [Google Scholar]
- 27.Di Landro A, Naldi L, Glaser E, Paus R, Tosti A. Pathobiology questions raised by telogen effluvium and trichodynia in COVID-19 patients. Exp Dermatol. 2021;30(7):999–1000. doi: 10.1111/exd.14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lv S, Wang L, Zou X, Wang Z, Qu B, Lin W, Yang D. A Case of Acute Telogen Effluvium After SARS-CoV-2 Infection. Clin Cosmet Investig Dermatol. 2021;14:385–387. doi: 10.2147/CCID.S307982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aryanian Z, Balighi K, Hatami P, Afshar ZM, Mohandesi NA. The role of SARS-CoV-2 infection and its vaccines in various types of hair loss. Dermatol Ther. 2022;35(6):e15433. doi: 10.1111/dth.15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


