The Phenomenon of Overdiagnosis Is Not Specific to Melanoma
Overdiagnosis is a relevant health challenge worldwide, not just in cancer but across several medical conditions. Overdiagnosis is the diagnosis of a condition/disease that will never cause symptoms or death during a person’s expected lifetime [1]. In the case of cancer, it either represents a tumor that will not progress (or potentially regress), or a slowly progressing tumor where a patient will die of other causes before becoming symptomatic [1]. Melanoma overdiagnosis is an increasing topical issue today particularly as the number of in situ melanomas is skyrocketing. In this opinion piece, we focus on the phenomenon which has been referred to as the ‘epidemic of overdiagnosis’ in melanoma.
Overdiagnosis Versus True Increase
The most credible evidence for the concept of overdiagnosis is the significant rise in melanoma incidence with no corresponding increase in mortality. This may also be explained by a true increase due to improvements in detection given the advances in diagnostic imaging, and the lower threshold of histopathologic diagnosis known as ‘diagnostic drift’ [2]. Harms et al. discusses other factors including the introduction of screening and patient awareness campaigns, increased biopsy rates, and improved medical record keeping [3]. The increasing incidence, therefore, must be analyzed in the context of changing public awareness, dermatologic care and sun exposure [4]. Thus, Kurtansky et al. analyzed these trends adjusting for age and sex, and accounting for period and cohort effects, and identified evidence of both a true increase – most apparent in older males – and overdiagnosis – most apparent in middle aged and younger females [4].
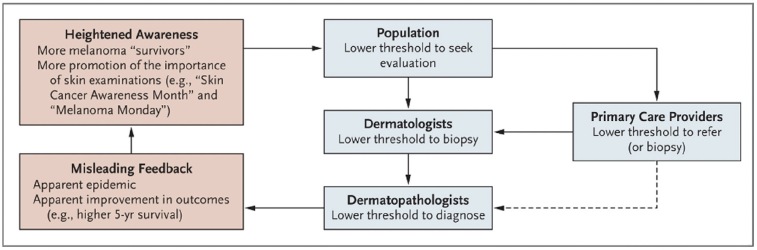
Drivers of melanoma overdiagnosis have formed a somewhat self-perpetual cycle where heighted awareness leads to more screening, leading to more biopsies and thus more melanoma diagnoses, leading again to higher awareness (Figure 1) [5]. In hope of identifying areas to address overdiagnosis, Pathirana et al. mapped potential drivers to five interrelated domains, namely, i) culture – eg beliefs that more is better, ii) financial incentives at the health system level, iii) technological change, iv) professional angst of missing disease and v) public expectations in clinicians to do something [6].
Figure 1.
Self-perpetuating cycle of melanoma over-diagnosis [5].
Is the Melanoma Epidemic Mostly an In Situ Melanoma Epidemic?
While both invasive and in situ melanoma rates are rising, the increase of in situ melanoma is alarming [4,5]. The rise of in situ melanoma has been well documented, however there is sparse evidence on its natural history. What evidence exists suggests a low probability of progression to invasive melanoma [2]. Further, Olsen et al. postulate that if in situ melanoma is indeed an obligate precursor lesion, excising them should correspond to fewer invasive melanomas, and thus then the mean age of those diagnosed with in situ melanoma should be younger than those diagnosed with invasive melanoma [7]. However, they found similar or higher mean ages of those with in situ melanoma. Coupled with the increasing ratio of in situ to invasive melanomas this finding is suggestive of the detection of more indolent lesions. Additionally, screening has been shown to be associated with a higher risk of in situ, but not invasive melanoma [8]. Also adding to this epidemic is the diagnostic shift from dysplastic nevus to in situ melanoma [2], which is further complicated by low concordance between histopathologists [9].
3D Total Body Photography, Sequential Dermoscopy Imaging and Artificial Intelligence (AI)
Improvements can be introduced to screening practices that will allow for those at high risk to still benefit from early detection, while minimizing potential overdiagnosis. Combining total body photography and sequential dermoscopy imaging has been shown to improve diagnostic accuracy in high risk individuals, as has the use of AI, particularly in less experienced clinicians [10,11]. 3D total body photography now allows unprecedented monitoring of the total skin surface and the longitudinal collection of such images. This has the potential to reduce excisions and correspondingly reduce the potential for overdiagnosis. For example, if a clinician or patient is concerned about a lesion, the clinician can now show it has not changed over time. This allows the clinician to adopt ‘watchful waiting’, a strategy we are currently evaluating in a high-risk melanoma cohort in Brisbane, Australia [12]. While AI on digital images has the potential to reduce excisions and thus overdiagnosis, this has yet to be been shown in the real world clinical setting. Algorithms must be developed with a clear clinical application in mind, on diverse datasets and reported using standardized criteria, ensuring potential bias due to training datasets clearly described [13]. Also of concern is the lack of gold standard in the algorithm training sets, due to the aforementioned discordance in pathologist diagnosis [9]. AI applied to whole slide pathology images could therefore be a promising approach, however to date models have been limited by small, largely homogenous datasets, with few models externally validated or compared head-to-head with pathologists [14]. We hypothesize combining clinical information, total body photography, sequential dermoscopy images and whole slide pathology images associated by AI will provide a holistic, more robust diagnostic approach.
Conclusions
In sum, while the term overdiagnosis lacks precision it is a very relevant and complex problem that needs to be tackled. A large driver of overdiagnosis is the increasing incidence of in situ melanomas and in this context we refer to the recent research letter by Semsarian et al. asking the question: “Do we need to rethink the diagnoses melanoma in situ and severely dysplastic naevus?” [2]. A robust discussion as initiated by this Kramer vs Kramer series in the clinical, pathology and AI community is recommended and should be followed by an international summit on the topic. We foresee that combining 3D Total Body Photography, sequential dermoscopy images and whole slide pathology images supported by AI will lead to a diagnostic solution in the best interest of our patients.
Footnotes
Competing Interests: HPS is a shareholder of MoleMap NZ Limited and e-derm consult GmbH and undertakes regular teledermatological reporting for both companies. HPS is a Medical Consultant for Canfield Scientific Inc, Blaze Bioscience Inc, and a Medical Advisor for First Derm.
Authorship: All authors have contributed significantly to this publication.
Funding: None.
References
- 1.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 2.Semsarian CR, Ma T, Nickel B, et al. Do we need to rethink the diagnoses melanoma in situ and severely dysplastic naevus? Br J Dermatol. 2022;186(6):1030–1032. doi: 10.1111/bjd.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harms PW, Chan MP, Bresler SC, et al. Comment on “An Epidemiologic Analysis of Melanoma Overdiagnosis in the United States, 1975–2017”. J Invest Dermatol. 2022;142(11):3120–3122. doi: 10.1016/j.jid.2022.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Kurtansky NR, Dusza SW, Halpern AC, et al. An Epidemiologic Analysis of Melanoma Overdiagnosis in the United States, 1975–2017. J Invest Dermatol. 2022;142(7):1804–1811.e6. doi: 10.1016/j.jid.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch HG, Mazer BL, Adamson AS. The Rapid Rise in Cutaneous Melanoma Diagnoses. N Engl J Med. 2021;384(1):72–79. doi: 10.1056/NEJMsb2019760. [DOI] [PubMed] [Google Scholar]
- 6.Pathirana T, Clark J, Moynihan R. Mapping the drivers of overdiagnosis to potential solutions. BMJ. 2017;358:j3879. doi: 10.1136/bmj.j3879. [DOI] [PubMed] [Google Scholar]
- 7.Olsen CM, Pandeya N, Rosenberg PS, Whiteman DC. Incidence of in Situ vs Invasive Melanoma: Testing the “Obligate Precursor” Hypothesis. J Natl Cancer Inst. 2022;114(10):1364–1370. doi: 10.1093/jnci/djac138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteman DC, Olsen CM, MacGregor S, et al. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187(4):515–522. doi: 10.1111/bjd.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piepkorn MW, Eguchi MM, Barnhill RL, et al. Reproducibility of the histopathologic diagnosis of melanoma and related melanocytic lesions: Results from a testing study and a reference guide for providers. JAAD Int. 2022;9:7–10. doi: 10.1016/j.jdin.2022.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moloney FJ, Guitera P, Coates E, et al. Detection of primary melanoma in individuals at extreme high risk: a prospective 5-year follow-up study. JAMA Dermatol. 2014;150(8):819–827. doi: 10.1001/jamadermatol.2014.514. [DOI] [PubMed] [Google Scholar]
- 11.Tschandl P, Rinner C, Apalla Z, et al. Human-computer collaboration for skin cancer recognition. Nat Med. 2020;26(8):1229–1234. doi: 10.1038/s41591-020-0942-0. [DOI] [PubMed] [Google Scholar]
- 12.Primiero CA, McInerney-Leo AM, Betz-Stablein B, et al. Evaluation of the efficacy of 3D total-body photography with sequential digital dermoscopy in a high-risk melanoma cohort: protocol for a randomised controlled trial. BMJ Open. 2019;9(11):e032969. doi: 10.1136/bmjopen-2019-032969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daneshjou R, Barata C, Betz-Stablein B, et al. Checklist for Evaluation of Image-Based Artificial Intelligence Reports in Dermatology: CLEAR Derm Consensus Guidelines From the International Skin Imaging Collaboration Artificial Intelligence Working Group. JAMA Dermatol. 2022;158(1):90–96. doi: 10.1001/jamadermatol.2021.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant SR, Andrew TW, Alvarez EV, Huss WJ, Paragh G. Diagnostic and Prognostic Deep Learning Applications for Histological Assessment of Cutaneous Melanoma. Cancers (Basel) 2022;14(24):6231. doi: 10.3390/cancers14246231. [DOI] [PMC free article] [PubMed] [Google Scholar]