Highlights
-
•
The incidence of hospital-diagnosed acute pyelonephritis doubled from 2000-2018.
-
•
The increase was largely driven by a prominent rise among young children.
-
•
The prevalence of comorbidity did not increase over time among the young children.
Keywords: Epidemiology, Cohort studies, Hospitalization, Incidence, Pyelonephritis, Urinary tract infections
Abstract
Objectives
To examine temporal changes in the incidence of hospital-diagnosed acute pyelonephritis (APN) and characterize associated demographics.
Methods
Cohort study including Danish patients with hospital-diagnosed APN during 2000-2018, identified by International Classification of Diseases, 10th Revision codes. Annual sex- and age-standardized incidence rates per 10,000 person years with 95% confidence intervals (CIs) were stratified by sex, age group, diagnosis code, and region of residence. Incidence rates for selected urinary tract infections and sepsis diagnoses were also computed.
Results
We included 66,937 hospital-diagnosed APN episodes in 57,162 patients. From 2000 to 2018, the incidence increased from 6.8 (95% CI: 6.8-6.8) to 15.4 (95% CI: 15.4-15.4) in women and from 2.7 (95% CI: 2.7-2.7) to 4.5 (95% CI: 4.5-4.5) in men. Among infants, the rate rose from 7.4 (95% CI: 7.4-7.4) to 64.8 (95% CI: 64.7-64.9) in girls and from 17.1 (95% CI: 17.1-17.2) to 52.5 (95% CI: 52.4-52.6) in boys. Concomitant declines were observed in incidences of hospital-diagnosed unspecified urinary tract infections and sepsis.
Conclusion
The APN incidence roughly doubled during 2000-2018. The increase was largely driven by a prominently increasing incidence among young children which was not explained by the enlarging prevalence of congenital anomalies of the kidney and urinary tract.
Introduction
Acute pyelonephritis (APN) is a severe infection of the upper urinary tract and kidney parenchyma, which may lead to sepsis, acute kidney injury, kidney scarring, chronic kidney disease, and death [1], [2], [3], [4], [5], [6]. Few population-based studies have described APN incidence over time across all age groups [7], [8], [9]. Thus, most prior studies have been confined to subgroups, small samples, single-center settings, or a cross-sectional design. Consequently, APN incidence estimates diverge greatly in the existing literature [7], [8], [9], [10], [11].
While the majority of patients with APN are treated in primary care or in an outpatient setting, the most severe cases, comprising an estimated 20%, require hospitalization [5,7,8]. Modern treatment regimens favor discharge-based strategies including home treatment of selected patients after initial care in the emergency department (ED), thereby avoiding regular inpatient admission [5,12]. Yet, numerous other time-varying factors can lead to increasing admission rates. Firstly, changes in demography, lifestyle, medical and surgical treatments may contribute by enlarging the proportion of the population living with risk factors for APN such as chronic diseases and foreign bodies in the urinary tract [5,7]. Secondly, the last decade's rising prevalence of antimicrobial resistance in uropathogens carries the risk of complicating urinary tract infections (UTIs) [13], [14], [15], [16], [17]. Thirdly, advances in diagnostic technologies, e.g., ultrasound-guided bladder puncture for retrieving urine samples, may promote diagnostic accuracy [18].
Thus, up-to-date epidemiological data describing the APN incidence in all age groups are warranted. We therefore examined temporal changes in the APN incidence rate in Denmark and characterized the associated demographics.
Methods
Design and setting
We conducted this descriptive, population-based cohort study in Denmark from 2000-2018. The Danish healthcare system provides tax-supported health care for all residents [19].
Variables and data sources
We used the Danish National Patient Registry (DNPR) to identify patients with hospital-diagnosed APN. This register contains prospectively collected data, including admission date and type, on all somatic hospital inpatient admissions from 1977 (according to the International Classification of Diseases, 8th Revision [ICD-8] until 1994 and 10th Revision [ICD-10] hereafter) and all acute and planned outpatient visits and ED visits from 1995. The quality of the administrative data in the DNPR is generally high [20]. Further, the estimated positive predictive value was 76% for pyelonephritis recorded from 1990-2009 [21] and 80% (95% confidence interval [CI]: 70%-88%) for acute pyelonephritis in children younger than 2 years between 2002 and 2004 [22]. We defined APN by ICD-10 diagnosis codes including both primary and secondary discharge diagnoses recorded during a hospital inpatient admission or acute outpatient/ED visit (Figure S1) [20]. APN diagnoses with admission dates registered less than 30 days apart were considered a single episode, with the index date defined by the first recorded admission date. While we included recurrent hospital-diagnosed APN episodes, we excluded patients with a diagnosis indicating chronic pyelonephritis from the year 1994 until the APN diagnosis (Table S1).
Descriptive variables were obtained from the DNPR from 1977 until index date and included urolithiasis, congenital anomalies of the kidney and urinary tract (CAKUT), and for patients 15 years or older Charlson Comorbidity Index (CCI) score [23], diabetes mellitus, and kidney disease (not including acute nor chronic pyelonephritis). Furthermore, we recorded selected UTI and sepsis diagnoses in the same source population (i.e. the Danish population without chronic pyelonephritis) and calendar period.
Information on sex, age, and region of residence (the five Danish Regions form the main administrative unit of the Danish healthcare system and are defined geographically) [19] was collected from the Danish Civil Registration System, which also ensures virtually complete follow-up and enables data linkage [24].
Finally, we obtained data on the Danish population numbers according to calendar year, sex, age, and region of residence from Statistics Denmark [25].
Statistical analysis
The study population was characterized according to sex, age group (0-2 years, 3-14 years, 15-29 years, 30-49 years, 50-74 years, and ≥75 years), calendar period (2000-2004, 2005-2009, 2010-2014, and 2015-2018), CCI score (0 vs ≥1), diabetes mellitus, kidney disease, urolithiasis, CAKUT, and region of residence at the time of diagnosis. Descriptive statistics included frequency (n) with proportion (%) and median with interquartile range (IQR).
In the primary analysis, we estimated annual APN incidence rates per 10,000 person years (PY) with 95% CIs, using the Danish population on 1 January of each respective year as the denominator. We computed crude rates and rates standardized to the sex and age (in years) distribution of the year-2000 population. Finally, rates were stratified by sex and age groups and presented graphically.
We performed several subgroup and sensitivity analyses. First, we stratified the standardized incidence rate in 0-2-year-olds by age (i.e. 1-year age groups) to elucidate changes in this heterogenic age group. Second, we stratified by specific diagnosis code to examine changes in coding practice. Third, we stratified by region of residence to identify potential regional differences. Lastly, we restricted the analysis to inpatient admissions to examine the robustness of the included diagnoses.
Additionally, we similarly estimated standardized incidence rates of selected UTI and sepsis diagnoses per 10,000 PY with 95% CIs to identify systematic changes in coding practice that could potentially affect the use of APN diagnoses.
Analyses were performed in R version 3.6.1 (R Foundation for Statistical Computing).
Results
We identified 67,954 episodes of hospital-diagnosed APN in 57,543 patients during 2000-2018 and excluded 1017 episodes in 579 patients with prior chronic pyelonephritis. This yielded inclusion of 66,937 hospital-diagnosed APN episodes in 57,162 patients (Figure S1).
Patient characteristics
Over time, we observed an increasing number of hospital-diagnosed APN episodes, from 13,458 in 2000-2004 to 19,744 in 2015-2018 (Table 1). The majority of episodes occurred in female patients (73.6%) who generally were younger than male patients (median age 28 years [IQR: 11-54] vs 57 years [IQR: 8-73]). The median age fell from 37 years (IQR: 18-67) in 2000-2004 to 30 years (IQR: 9-61) in 2015-2018, largely attributable to decreasing median ages in the Capital Region of Denmark, Central Denmark Region, and Region of Southern Denmark (Table S2).
Table 1.
Baseline characteristics of patients at the time of hospital-diagnosed acute pyelonephritis, overall and stratified by sex and calendar period of diagnosis.
| Total, n | Overall | Sex |
Calendar period of diagnosis |
||||
|---|---|---|---|---|---|---|---|
| Female | Male | 2000-2004 | 2005-2009 | 2010-2014 | 2015-2018 | ||
| 66,937 | 49,269 | 17,668 | 13,458 | 15,858 | 17,877 | 19,744 | |
| Sex, n (%) | |||||||
| Female | 49,269 (73.6) | 49,269 (100.0) | - | 9560 (71.0) | 11,460 (72.3) | 13,348 (74.7) | 14,901 (75.5) |
| Age in years, n (%) | |||||||
| 0-2 | 9124 (13.6) | 5540 (11.2) | 3584 (20.3) | 1396 (10.4) | 1978 (12.5) | 2677 (15.0) | 3073 (15.6) |
| 3-14 | 8848 (13.2) | 7554 (15.3) | 1294 (7.3) | 1550 (11.5) | 2181 (13.8) | 2611 (14.6) | 2506 (12.7) |
| 15-29 | 13,299 (19.9) | 12,659 (25.7) | 640 (3.6) | 2633 (19.6) | 3008 (19.0) | 3538 (19.8) | 4120 (20.9) |
| 30-49 | 11,263 (16.8) | 9344 (19.0) | 1919 (10.9) | 2429 (18.0) | 2,750 (17.3) | 3002 (16.8) | 3082 (15.6) |
| 50-74 | 15,184 (22.7) | 8996 (18.3) | 6188 (35.0) | 3060 (22.7) | 3641 (23.0) | 3860 (21.6) | 4623 (23.4) |
| ≥75 | 9219 (13.8) | 5176 (10.5) | 4043 (22.9) | 2390 (17.8) | 2300 (14.5) | 2189 (12.2) | 2340 (11.9) |
| Median (interquartile range) | 33 (10, 63) | 28 (11, 54) | 57 (8, 73) | 37 (18, 67) | 34 (11, 64) | 30 (8, 61) | 30 (9, 61) |
| CCI scorea,b = 0, n (%) | |||||||
| Yes | 28,957 (59.1) | 23,743 (65.6) | 5214 (40.8) | 6063 (57.7) | 6876 (58.8) | 7571 (60.1) | 8447 (59.6) |
| Diabetes mellitusb, n (%) | |||||||
| Yes | 4437 (9.1) | 2626 (7.3) | 1811 (14.2) | 877 (8.3) | 1032 (8.8) | 1165 (9.3) | 1363 (9.6) |
| Kidney diseaseb, n (%) | |||||||
| Any | 4343 (8.9) | 2661 (7.4) | 1682 (13.2) | 957 (9.1) | 1057 (9.0) | 1113 (8.8) | 1216 (8.6) |
| Urolithiasis, n (%) | |||||||
| Yes | 3724 (5.6) | 2111 (4.3) | 1613 (9.1) | 777 (5.8) | 860 (5.4) | 922 (5.2) | 1165 (5.9) |
| CAKUT, n (%) | |||||||
| Any | 2719 (4.1) | 1657 (3.4) | 1062 (6.0) | 500 (3.7) | 622 (3.9) | 803 (4.5) | 794 (4.0) |
| Region of residence, n (%) | |||||||
| Capital Region of Denmark | 25,863 (38.6) | 19,389 (39.4) | 6474 (36.6) | 5378 (40.0) | 5969 (37.6) | 6320 (35.4) | 8196 (41.5) |
| Central Denmark Region | 14,100 (21.1) | 10,321 (20.9) | 3779 (21.4) | 2657 (19.7) | 3232 (20.4) | 4148 (23.2) | 4063 (20.6) |
| North Denmark Region | 5741 (8.6) | 4194 (8.5) | 1547 (8.8) | 1180 (8.8) | 1518 (9.6) | 1447 (8.1) | 1596 (8.1) |
| Region of Southern Denmark | 12,174 (18.2) | 8659 (17.6) | 3515 (19.9) | 2211 (16.4) | 3044 (19.2) | 3512 (19.6) | 3407 (17.3) |
| Region Zealand | 9059 (13.5) | 6706 (13.6) | 2353 (13.3) | 2032 (15.1) | 2095 (13.2) | 2450 (13.7) | 2482 (12.6) |
CCI score, excluding non-melanoma skin cancer (International Classification of Diseases, 8th Revision: 173 and International Classification of Diseases, 10th Revision: C44).
Ascertained only in patients ≥15 years of age.
CAKUT: congenital anomalies of the kidney and urinary tract; CCI: Charlson Comorbidity Index.
A larger proportion of female than male patients had no pre-existing comorbidities at the time of diagnosis (65.6% vs 40.8%) (Table 1), and these proportions remained largely stable over time (Table 2). The prevalence of diabetes mellitus, kidney disease, and urolithiasis were lower in female (7.3%, 7.4%, and 4.3%) than in male patients (14.2%, 13.2%, and 9.1%), and the prevalence of diabetes mellitus and urolithiasis rose over time in patients older than 50 years. Conversely, the prevalence of CAKUT decreased with time from 7.7% to 4.9% in female patients and from 13.3% to 10.6% in male patients aged 0-2 years. The prevalence of diabetes, kidney disease, urolithiasis, and CAKUT was similar across regions with relatively low prevalence observed in the Capital Region of Denmark (Table S2).
Table 2.
Baseline characteristics of female and male patients at the time of hospital-diagnosed acute pyelonephritis, stratified by calendar period and age group.
| Age group, years | Female population |
Male population |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Calendar period of diagnosis |
Calendar period of diagnosis |
||||||||
| 2000-2004 | 2005-2009 | 2010-2014 | 2015-2018 | 2000-2004 | 2005-2009 | 2010-2014 | 2015-2018 | ||
| CCI scorea,b = 0, n (%) | |||||||||
| ≥15 | Yes | 4863 (64.4) | 5,567 (65.6) | 6,294 (66.8) | 7,019 (65.5) | 1200 (40.6) | 1309 (40.7) | 1277 (40.3) | 1428 (41.4) |
| 15-29 | Yes | 2243 (90.2) | 2,531 (88.8) | 2,976 (87.5) | 3,411 (87.0) | 106 (72.6) | 121 (76.6) | 105 (76.1) | 141 (71.2) |
| 30-49 | Yes | 1378 (70.6) | 1,712 (74.8) | 1,909 (75.7) | 1,942 (75.3) | 332 (69.7) | 324 (70.1) | 331 (69.1) | 368 (73.3) |
| 50-74 | Yes | 813 (46.1) | 966 (46.4) | 1,058 (46.2) | 1,260 (44.0) | 527 (40.7) | 645 (41.3) | 618 (39.3) | 721 (41.0) |
| ≥75 | Yes | 429 (31.8) | 358 (28.3) | 351 (29.0) | 406 (30.0) | 235 (22.6) | 219 (21.1) | 223 (22.8) | 198 (20.0) |
| Diabetesb, n (%) | |||||||||
| ≥15 | Yes | 521 (6.9) | ≤ 608 | ≤ 666 | 834 (7.8) | 356 (12.0) | ≤ 427 | ≤ 504 | 529 (15.3) |
| 15-29 | Yes | 45 (1.8) | ≤ 55 | ≤ 60 | 61 (1.6) | 0 (0.0) | ≤ 5 | ≤ 5 | 9 (4.5) |
| 30-49 | Yes | 101 (5.2) | 131 (5.7) | 142 (5.6) | 169 (6.6) | 29 (6.1) | 29 (6.3) | 30 (6.3) | 24 (4.8) |
| 50-74 | Yes | 220 (12.5) | 246 (11.8) | 285 (12.5) | 403 (14.1) | 190 (14.7) | 221 (14.2) | 297 (18.9) | 312 (17.7) |
| ≥75 | Yes | 155 (11.5) | 176 (13.9) | 179 (14.8) | 201 (14.9) | 137 (13.2) | 172 (16.6) | 172 (17.6) | 184 (18.6) |
| Kidney diseaseb, n (%) | |||||||||
| ≥15 | Any | 565 (7.5) | 603 (7.1) | 710 (7.5) | 783 (7.3) | 392 (13.3) | 454 (14.1) | 403 (12.7) | 433 (12.6) |
| 15-29 | Any | 48 (1.9) | 65 (2.3) | 88 (2.6) | 125 (3.2) | 20 (13.7) | 17 (10.8) | 10 (7.2) | 21 (10.6) |
| 30-49 | Any | 203 (10.4) | 189 (8.3) | 165 (6.5) | 148 (5.7) | 71 (14.9) | 55 (11.9) | 35 (7.3) | 44 (8.8) |
| 50-74 | Any | 191 (10.8) | 229 (11.0) | 315 (13.8) | 393 (13.7) | 165 (12.7) | 220 (14.1) | 214 (13.6) | 217 (12.3) |
| ≥75 | Any | 123 (9.1) | 120 (9.5) | 142 (11.7) | 117 (8.7) | 136 (13.1) | 162 (15.6) | 144 (14.7) | 151 (15.3) |
| Urolithiasis, n (%) | |||||||||
| All | Any | ≤ 433 | ≤ 474 | ≤ 529 | ≤ 687 | ≤ 354 | ≤ 393 | ≤ 395 | ≤ 485 |
| 0-2 | Any | ≤ 5 | ≤ 5 | 0 (0.0) | ≤ 5 | ≤ 5 | ≤ 5 | 0 (0.0) | ≤ 5 |
| 3-14 | Any | 7 (0.6) | 7 (0.4) | ≤ 5 | 12 (0.5) | ≤ 5 | 9 (2.7) | 8 (2.1) | ≤ 5 |
| 15-29 | Any | 57 (2.3) | 58 (2.0) | 79 (2.3) | 80 (2.0) | 12 (8.2) | 12 (7.6) | ≤ 5 | 14 (7.1) |
| 30-49 | Any | 127 (6.5) | 133 (5.8) | 162 (6.4) | 188 (7.3) | 65 (13.7) | 53 (11.5) | 58 (12.1) | 54 (10.8) |
| 50-74 | Any | 162 (9.2) | 194 (9.3) | 208 (9.1) | 281 (9.8) | 156 (12.0) | 194 (12.4) | 198 (12.6) | 244 (13.9) |
| ≥75 | Any | 75 (5.6) | 77 (6.1) | 75 (6.2) | 121 (8.9) | 111 (10.7) | 120 (11.6) | 126 (12.9) | 163 (16.5) |
| CAKUT, n (%) | |||||||||
| All | Any | ≤ 285 | ≤ 353 | 514 (3.9) | 509 (3.4) | ≤ 219 | ≤ 273 | 289 (6.4) | 285 (5.9) |
| 0-2 | Any | 57 (7.7) | 54 (4.8) | 110 (6.5) | 97 (4.9) | 88 (13.3) | 133 (15.7) | 118 (12.0) | 116 (10.6) |
| 3-14 | Any | 59 (4.6) | 126 (6.8) | 164 (7.3) | 139 (6.3) | 52 (18.5) | 60 (18.1) | 91 (24.0) | 65 (21.5) |
| 15-29 | Any | 51 (2.1) | 61 (2.1) | 104 (3.1) | 102 (2.6) | 25 (17.1) | 19 (12.0) | 13 (9.4) | 35 (17.7) |
| 30-49 | Any | 60 (3.1) | 54 (2.4) | 51 (2.0) | 73 (2.8) | 14 (2.9) | 18 (3.9) | 14 (2.9) | 21 (4.2) |
| 50-74 | Any | 53 (3.0) | 43 (2.1) | 68 (3.0) | 82 (2.9) | 30 (2.3) | 33 (2.1) | 45 (2.9) | 37 (2.1) |
| ≥75 | Any | ≤ 5 | ≤ 15 | 17 (1.4) | 16 (1.2) | ≤ 10 | ≤ 10 | 8 (0.8) | 11 (1.1) |
CCI score, excluding non-melanoma skin cancer (International Classification of Diseases, 8th Revision: 173 and International Classification of Diseases, 10th Revision: C44).
Ascertained only in patients ≥15 years of age.
CAKUT: congenital anomalies of the kidney and urinary tract; CCI: Charlson Comorbidity Index.
Incidence rate
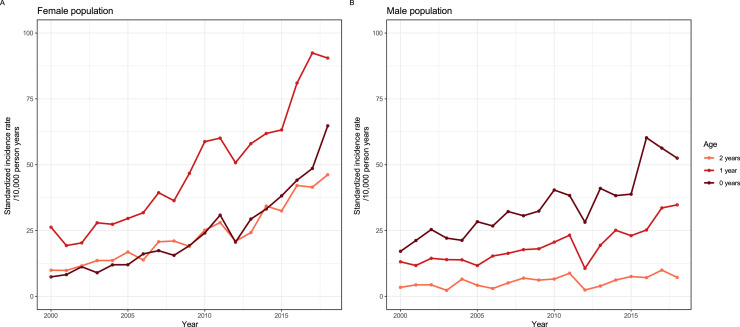
During the 19-year period, the annual sex- and age-standardized APN incidence rate increased from 6.8 (95% CI: 6.8-6.8) to 15.4 (95% CI: 15.4-15.4) per 10,000 PY in the female population and from 2.7 (95% CI: 2.7-2.7) to 4.5 (95% CI: 4.5-4.5) per 10,000 PY in the male population (Figure 1a and Table S3). The rate rose steadily over time aside from a decline around the year 2012, coincident with an ICD-10 code update. The standardized rates did not differ substantially from the crude rates (Table S3).
Figure 1.
Sex- and age-standardized incidence rates of hospital-diagnosed acute pyelonephritis in the Danish female (a and b) and male (a and c) population, 2000-2018, stratified by sex (a) and age group (b and c).
The standardized rate increased in all age groups (Figure 1b-c) with the highest rates observed in the age groups 0-2 years and ≥75 years, and in 3-14 and 15-29-year-old female population (Figure 1b-c).
The largest absolute increase occurred among girls aged 1 year (from 26.3 [95% CI: 26.2-26.3] to 90.5 [95% CI: 90.4-90.6] per 10,000 PY), and boys younger than 1 year (from 17.1 [95% CI: 17.1-17.2] to 52.5 [95% CI: 52.4-52.6] per 10,000 PY) (Figure 2a-b), with the largest relative increase observed in girls and boys younger than 1 year (girls: from 7.4 [95% CI: 7.4-7.4] to 64.8 [95% CI: 64.7-64.9] per 10,000 PY).
Figure 2.
Sex- and age-standardized incidence rate of hospital-diagnosed acute pyelonephritis in the Danish female (a) and male (b) population aged 0-2 years, 2000-2018, stratified by age.
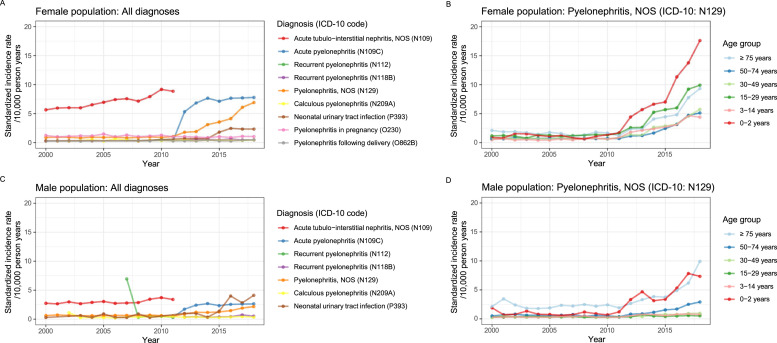
The temporal increase in APN incidence among 0-2-year-old girls varied between regions, increasing by 1.5-fold in the North Denmark Region and by 4-4.5-fold in the Capital Region of Denmark, Region of Southern Denmark, and Region Zealand (Figure S2). Moreover, a greater use, especially in the 0-2-year-olds, of the unspecified pyelonephritis diagnosis was observed from the year 2012 (Figure 3a-d).
Figure 3.
Sex- and age-standardized incidence rate of hospital-diagnosed acute pyelonephritis in the Danish female (a and b) and male (c and d) population, 2000-2018, stratified by diagnosis code (a and c) and age group (b and d).
ICD-10: International Classification of Diseases, 10th Revision; NOS: not otherwise specified.
When restricting to APN diagnoses from inpatient admissions, the total number of APN episodes decreased to 62,405 in 52,849 patients. Yet, the resulting decrease in incidence rates was modest, and the temporal changes remained. Hence, when restricting to inpatient admission, the rates in the female population changed from 6.8 (95% CI: 6.8-6.8) to 6.4 (95% CI: 6.1-6.7) per 10,000 PY in the year 2000 and from 15.4 (95% CI: 15.4-15.4) to 12.9 (95% CI: 12.5-13.3) per 10,000 PY in 2018.
During the study period, we observed an overall increasing use of the selected UTI and sepsis diagnoses; although the incidence of unspecified bacterial sepsis of newborns fluctuated greatly, and the rates for diagnoses such as unspecified sepsis and unspecified UTI decreased slightly from the year 2015 (Figure S3a and S3c).
Discussion
In this nationwide study, we found the incidence rate of hospital-diagnosed APN to have roughly doubled in the Danish female and male populations from 2000-2018. Interestingly, it was largely driven by prominent increases among the youngest children which was not accompanied by an enlarging prevalence of CAKUT. Furthermore, the temporal changes in APN incidence were associated with notable regional differences and concomitant declines in incidences of hospital-diagnosed unspecified UTI and sepsis.
Our observations extend previous findings of a rising incidence of infections caused by uropathogens in the Danish population over the past decade [14,16]. The temporally increasing APN incidence is also supported by international findings, including observations from South Korea during 1997-1999 and 2010-2014 [8,11]. However, compared with our estimates, the incidences reported in the adult female population of South Korea were much higher (inpatient rate: 18.9 per 10,000 persons; and outpatient rate: 54.4 per 10,000 persons) [11]. This discrepancy may be explained by differences in settings, coding practices, and methodologies; notably, the APN definition used in the South Korean studies likely yielded higher estimates as it was based on insurance claims data with a 14-day period defining an APN episode. Also, the overall APN incidence rates reported in a study from the United States during 1997-2001, including all ages but not recurrent episodes, were approximately two-fold greater (inpatient and outpatient female population: 15-17 per 10,000 persons; and male population: 3-5 per 10,000 persons) than our year-2000 estimates [7]. In contrast, the inpatient-only hospitalization rates were lower in the United States (female population: 3-4 per 10,000 persons; and male population: 1-2 per 10,000 persons) than in Denmark. The finding of incidences being highest in infants, young women, and the elderly was similar between studies. Moreover, consistent with our findings, other studies have reported the APN hospitalization rate to have increased over time in infants [26,27]. Thus, a nearly nine-fold increased incidence was reported among infants in California during 1985-2006 [26]. The trend is further supported by another American study covering the period 2006-2011, showing an increasing incidence of female ED visits for UTIs, including pyelonephritis, with the highest incidences in infants and adolescents [27].
The strengths of our study include its population-based design and considerable size and time span. The use of prospectively collected registry data reduced the risk of recall bias. Furthermore, retrieving APN diagnoses from the DNPR allowed us to consider acute hospital contacts in all age groups, including children. In addition, we expect that essentially all APN diagnoses in children are captured in the DNPR as Danish guidelines recommend hospital referral of children with suspected pyelonephritis and that all UTIs in children under the age of 1-2 years are considered pyelonephritis [28,29].
The limitations of our study include the lack of information on clinical symptoms to validate the diagnoses. While we excluded patients with a diagnosis indicating chronic pyelonephritis, we cannot entirely rule out some degree of misclassification of diagnoses (e.g. noninfectious nephritis etc.). Yet, an evaluation of microbiological test results from 4773 patients performed at the Department of Clinical Microbiology, Aalborg University Hospital found that an overall 52% (children aged 0-2 years: 81%) of hospital-based APN diagnoses were confirmed by urine culture within day −7 to +2 from admission date (Svingel et al., 2022, unpublished results). Moreover, we sought to unveil potential shifts in the coding of infectious diseases originating from the urinary system by examining incidence rates for selected UTI and sepsis diagnoses. Confounding by demographic changes was controlled by sex- and age-standardization. Still, it is important to bear in mind that by focusing on hospital-diagnosed APN, the results inherently underestimate the total all-setting APN incidence by a varying degree between sexes and age groups; expectedly to the largest extent in young women who are often treated outside hospital [5,10].
Plausible explanations for the rising APN incidence include a growing comorbidity burden [26,27]. In the present study, we did demonstrate increasing prevalence of diabetes mellitus and urolithiasis in patients older than 50 years over time. Yet, the prominent increase among the 0-2-year-olds could not be explained by an increasing prevalence of CAKUT in this age group over time.
Although, the past decades have seen growing implementation of outpatient management of APN [5,10,12], the increase observed in the present study cannot be explained solely by a surge in hospital outpatient management as it was consistent over time whether considering all acute hospital contacts or only inpatient admissions. However, the observations may still to some extent be explained by changes in clinical and coding practices. Firstly, the coincidental changes observed in the incidence rate for unspecified UTIs may reflect a growing inclination for doctors to provide an APN diagnosis to patients with upper UTIs assessed as suited for outpatient management. Secondly, across age groups, the increasing incidence rates during 2013-2018 were possibly incited by the implementation (2010-2013) of a national program urging timely diagnostic examinations, including microbiological tests, and treatment of patients with sepsis [30]. This likely heightened disease recognition and diagnostic specificity in infected patients. This is supported by our evaluation of microbiological tests showing that hospital-diagnosed APN was increasingly accompanied by urine culture analysis (from 75% in 2000-2006 to 93% in 2013-2018) (Svingel et al., 2022, unpublished results). Thirdly, advances in diagnostic technologies, such as ultrasound-guided bladder puncture [18], may also herald a shift towards more specific diagnostic coding. This suggestion is in accordance with the recent decline in the use of the unspecified UTI and sepsis diagnoses. In concord, data from the Department of Clinical Microbiology, Aarhus University Hospital showed that the proportion of urine samples retrieved by bladder puncture grew from 12.5% in 2010 to 33.7% in 2018 among infants residing in the uptake area (Breinbjerg [August 16, 2021], personal communication). Hence, our findings may to some degree unveil an under-coding of APN in the earlier years of our study period.
Additional knowledge on potential risk factors such as invasive procedures and immunomodulatory therapies and on the impact of antimicrobial resistance on APN incidence, disease severity, and clinical course is needed to continuously optimize preventive and treatment strategies. Furthermore, updated knowledge on the long-term prognosis following APN is crucial to mitigate morbidity and mortality.
In conclusion, the incidence rate of hospital-diagnosed APN in Denmark roughly doubled from 2000 to 2018. The increase was largely driven by a prominent increase among 0-2-year-old children which was not explained by the enlarging prevalence of CAKUT. Our findings warrant continued efforts targeted towards preventing this serious infection and its potential consequences.
CRediT authorship contribution statement
Lise Skovgaard Svingel: Conceptualization, Formal analysis, Methodology, Project administration, Software, Visualization, Writing – original draft. Christian Fynbo Christiansen: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – review & editing. Henrik Birn: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. Kirstine Kobberøe Søgaard: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. Mette Nørgaard: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – review & editing.
Declarations of competing interest
L.S.S., C.F.C., K.K.S., and M.N. are employees at Department of Clinical Epidemiology, Department of Clinical Medicine, Aarhus University Hospital and Aarhus University, Denmark. The Department of Clinical Epidemiology is involved in studies with funding from various companies as research grants to and administered by Aarhus University. None of these studies are related to the current study. L.S.S., C.F.C., K.K.S., and M.N. declare no personal conflicts of interest. H.B. has received research funding and honoraria from Astra Zeneca, Alexion, GlaxoSmithKline, Novo Nordisk, Netdoktor.dk, and Vifor Pharma; has been a consultant to Astra Zeneca; and has been on the advisory board of Astra Zeneca, Boehringer Ingelheim, Galapagos IDMC, and Vifor Pharma.
Acknowledgments
Funding
This work was supported by the Karen Elise Jensen Foundation, Denmark. The sponsor did not have any involvement in study design; collection, analysis, and interpretation of data; writing of the article; nor decision to submit the article for publication.
Ethical approval
The study was approved by the Danish Data Protection Agency (record number 2015–57-0002, Aarhus University record number 2016–051-000001/812). According to Danish legislation, registry-based studies do not require approval from an ethics committee nor informed consent.
Acknowledgments
Thanks to Anders Breinbjerg, MD and PhD student at the Department of Pediatrics, Aarhus University Hospital, for providing data on urine cultures.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2023.10.003.
Appendix. Supplementary materials
References
- 1.Buonaiuto VA, Marquez I, De Toro I, Joya C, Ruiz-Mesa JD, Seara R, et al. Clinical and epidemiological features and prognosis of complicated pyelonephritis: a prospective observational single hospital-based study. BMC Infect Dis. 2014;14:639. doi: 10.1186/s12879-014-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebäck C, Hansson S, Martinell J, Sandberg T, Sixt R, Jodal U. Renal function in adult women with urinary tract infection in childhood. Pediatr Nephrol. 2015;30:1493–1499. doi: 10.1007/s00467-015-3084-8. [DOI] [PubMed] [Google Scholar]
- 3.Graversen HV, Nørgaard M, Nitsch D, Christiansen CF. Preadmission kidney function and risk of acute kidney injury in patients hospitalized with acute pyelonephritis: a Danish population-based cohort study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson SH, Eklöf O, Eriksson CG, Lins LE, Tidgren B, Winberg J. Development of hypertension and uraemia after pyelonephritis in childhood: 27 year follow up. BMJ. 1989;299:703–706. doi: 10.1136/bmj.299.6701.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JR, Russo TA. Acute pyelonephritis in adults. N Engl J Med. 2018;378:48–59. doi: 10.1056/nejmcp1702758. [DOI] [PubMed] [Google Scholar]
- 6.Orellana P, Baquedano P, Rangarajan V, Zhao JH, Eng ND, Fettich J, et al. Relationship between acute pyelonephritis, renal scarring, and vesicoureteral reflux. Results of a coordinated research project. Pediatr Nephrol. 2004;19:1122–1126. doi: 10.1007/s00467-004-1501-5. [DOI] [PubMed] [Google Scholar]
- 7.Czaja CA, Scholes D, Hooton TM, Stamm WE. Population-based epidemiologic analysis of acute pyelonephritis. Clin Infect Dis. 2007;45:273–280. doi: 10.1086/519268. [DOI] [PubMed] [Google Scholar]
- 8.Ki M, Park T, Choi B, Foxman B. The epidemiology of acute pyelonephritis in South Korea, 1997–1999. Am J Epidemiol. 2004;160:985–993. doi: 10.1093/aje/kwh308. [DOI] [PubMed] [Google Scholar]
- 9.Nicolle LE, Friesen D, Harding GK, Roos LL. Hospitalization for acute pyelonephritis in Manitoba, Canada, during the period from 1989 to 1992; impact of diabetes, pregnancy, and aboriginal origin. Clin Infect Dis. 1996;22:1051–1056. doi: 10.1093/clinids/22.6.1051. [DOI] [PubMed] [Google Scholar]
- 10.Brown P, Ki M, Foxman B. Acute pyelonephritis among adults: cost of illness and considerations for the economic evaluation of therapy. Pharmacoeconomics. 2005;23:1123–1142. doi: 10.2165/00019053-200523110-00005. [DOI] [PubMed] [Google Scholar]
- 11.Kim B, Myung R, Kim J, Lee MJ, Pai H. Descriptive epidemiology of acute pyelonephritis in Korea, 2010–2014: population-based study. J Korean Med Sci. 2018;33:e310. doi: 10.3346/jkms.2018.33.e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sehested LT, Kamperis K, Winding L, Bjerre CK, Neland M, Hagstrøm S, et al. Children with acute pyelonephritis need medical re-evaluation when home-treated with oral antibiotics. Acta Paediatr. 2021;110:2627–2634. doi: 10.1111/apa.15958. [DOI] [PubMed] [Google Scholar]
- 13.Córdoba G, Holm A, Hansen F, Hammerum AM, Bjerrum L. Prevalence of antimicrobial resistant Escherichia coli from patients with suspected urinary tract infection in primary care. Denmark. BMC Infect Dis. 2017;17:670. doi: 10.1186/s12879-017-2785-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DANMAP. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark, https://www.danmap.org/Reports/2019; 2019 [accessed DD Month YYYY].
- 15.European Center for Disease Control and Prevention. Antimicrobial resistance in the EU/EEA (EARS-Net)—annual Epidemiological Report 2019, https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2019.pdf; 2020 [accessed 07 June 2023].
- 16.Richelsen R, Smit J, Anru PL, Schønheyder HC, Nielsen H. Incidence of community-onset extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae infections: an 11-year population-based study in Denmark. Infect Dis (Lond) 2020;52:547–556. doi: 10.1080/23744235.2020.1763452. [DOI] [PubMed] [Google Scholar]
- 17.Walker E, Lyman A, Gupta K, Mahoney MV, Snyder GM, Hirsch EB. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960–965. doi: 10.1093/cid/ciw396. [DOI] [PubMed] [Google Scholar]
- 18.Diviney J, Jaswon MS. Urine collection methods and dipstick testing in non-toilet-trained children. Pediatr Nephrol. 2021;36:1697–1708. doi: 10.1007/s00467-020-04742-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt M, Schmidt SAJ, Adelborg K, Sundbøll J, Laugesen K, Ehrenstein V, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graversen ME, Dalgaard LS, Jensen-Fangel S, Jespersen B, Østergaard L, Søgaard OS. Risk and outcome of pyelonephritis among renal transplant recipients. BMC Infect Dis. 2016;16:264. doi: 10.1186/s12879-016-1608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roed C, Friis-Møller A, Høgh B. [A study of the validity of urinary tract infection diagnosis in children younger than two years of age at Hvidovre Hospital] Ugeskr Laeger. 2008;170:2432–2434. [PubMed] [Google Scholar]
- 23.Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 25.Statistics Denmark . 2020. Population and elections.https://www.statbank.dk/statbank5a/default.asp?w=1440 [accessed 05 May 2021] [Google Scholar]
- 26.Copp HL, Halpern MS, Maldonado Y, Shortliffe LD. Trends in hospitalization for pediatric pyelonephritis: a population based study of California from 1985 to 2006. J Urol. 2011;186:1028–1034. doi: 10.1016/j.juro.2011.04.101. [DOI] [PubMed] [Google Scholar]
- 27.Sood A, Penna FJ, Eleswarapu S, Pucheril D, Weaver J, Abd-El-Barr AE, et al. Incidence, admission rates, and economic burden of pediatric emergency department visits for urinary tract infection: data from the nationwide emergency department sample, 2006 to 2011. J Pediatr Urol. 2015;11 doi: 10.1016/j.jpurol.2014.10.005. 246.e1–8. [DOI] [PubMed] [Google Scholar]
- 28.Hansen A, Andersen KV, Cortes D, Nathan E, Nielsen OH. A reference program for children with urinary tract infection. A proposal for diagnosis and treatment of children with urinary tract infection. Ugeskr Laeger. 1999;161:5775–5777. [PubMed] [Google Scholar]
- 29.Sehested LT, Nørgaard H, Hagstrøm S, Winding L, Cortes D, Færch M. Pyelonefritis hos børn og unge, http://paediatri.dk/images/dokumenter/Retningslinjer_2020/DPS-Pyelonefrit_2020.pdf; 2020 [accessed DD Month YYYY].
- 30.2014. Evaluering COWI af Patientsikkert Sygehus.https://patientsikkerhed.dk/content/uploads/2015/11/pss_evaluering_cowi_april2014.pdf [accessed 27 January 2022] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.