Highlights
-
•
We prospectively enrolled 35 patients with Parkinson's disease in this study.
-
•
Concomitant use of magnesium oxide decreased levodopa Cmax by 24 % and AUC3h by 23 %.
-
•
Concomitant use of magnesium oxide decreased carbidopa Cmax by 39 % and AUC3h by 41 %.
-
•
This effect on pharmacokinetics was confirmed even when analyzed by sex and age.
-
•
In vitro study showed that benserazide may be more susceptible to magnesium oxide.
Keywords: Parkinson's disease, Levodopa, Magnesium oxide, Pharmacokinetics, Carbidopa
Abstract
Introduction
Constipation is one of the most frequent non-motor symptoms of Parkinson's disease (PD), and magnesium oxide (MgO) is a frequently used laxative. This study aimed to investigate the effect of concomitant use of MgO on the pharmacokinetics of levodopa preparations in patients with PD.
Methods
We prospectively enrolled 35 patients with PD and compared the pharmacokinetics of levodopa and carbidopa and motor symptoms with and without MgO. The impact of alterations in pH and the addition of MgO on the solubility of levodopa formulations were also evaluated under in vitro conditions.
Results
Concomitant use of MgO significantly reduced the maximum plasma concentration of levodopa (Cmax) (from 7.66 ± 3.74 μmol/L to 5.82 ± 3.69 μmol/L; p = 0.006) and area under the plasma concentration–time curve 3 h after drug administration (AUC3h, from 9.64 ± 3.23 μmol·h/L to 7.39 ± 3.15 μmol·h/L; p < 0.001), and further decreased carbidopa Cmax (from 64.02 ± 27.02 ng/mL to 38.83 ± 21.94 μmol/L; p < 0.001) and AUC3h (from 130.58 ± 65.64 ng/mL to 76.48 ± 52.24 ng·h/mL; p < 0.001). The Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale part III score also deteriorated significantly (from 30.71 ± 11.34 to 32.06 ± 11.22; p = 0.007). MgO significantly affected the pharmacokinetics of levodopa and carbidopa. This also applied when the findings were analyzed by sex and age. In vitro dissolution experiments revealed a decrease in the relative concentrations of levodopa, carbidopa, and benserazide as the pH increased and in the presence of MgO suspension, with the most prominent impact on benserazide.
Conclusions
Concomitant use of MgO and levodopa should be discouraged to improve levodopa absorption.
1. Introduction
Among many anti-parkinsonian agents available, levodopa remains the most effective symptomatic treatment for Parkinson's disease (PD). Levodopa has a short blood half-life of less than 90 min and is largely metabolized peripherally, reaching the brain only to a limited extent [1]. Metabolism of levodopa to dopamine by l-3,4-dihydroxyphenylalanine decarboxylase (DDC) is the main metabolic pathway, and DDC inhibitors such as carbidopa are commonly administered concurrently in combination. As the disease progresses, the striatal dopamine concentrations become closely correlated with the plasma concentrations of levodopa. Impaired oral levodopa absorption can result in a delayed, or even a lack of, response to levodopa therapy [2]. Thus, efficient absorption of levodopa is one of the key challenges in the treatment of PD.
Absorption of levodopa is primarily limited to the proximal small intestine and is affected by various factors such as meal timing, dietary protein, dysphagia, delayed gastric emptying, upper gastrointestinal structure, Helicobacter pylori infection, and gut microbiota [3], [4], [5], [6], [7], [8]. We recently reported that magnesium oxide (MgO) induces the degradation of carbidopa, leading to reduced bioavailability of levodopa, which is alleviated by ascorbic acid [9]. MgO is an osmotic laxative frequently used as a treatment for chronic constipation, which is one of the most frequent non-motor symptoms of PD. A previous study in a small number of healthy participants showed that MgO can cause a decrease in plasma concentrations of levodopa and carbidopa [10]. However, our retrospective study found no significant association between oral administration of antacids, including MgO, and levodopa pharmacokinetics in patients with PD [11]. The interaction between MgO and levodopa preparations has not been prospectively investigated in patients with PD, and the effect of MgO co-administration on plasma concentrations of levodopa and carbidopa remains unclear.
In this study, we evaluated the effect of concomitant use of MgO on the pharmacokinetics of levodopa and carbidopa in patients with PD by comparing their plasma concentrations with and without MgO. We also assessed whether these results differed by sex and age, because these factors affect levodopa pharmacokinetics [12]. In addition, the impact of the altered pH and addition of MgO on the solubility of levodopa formulations were assessed under in vitro conditions.
2. Methods
2.1. Study design and participants
Patients with PD were prospectively enrolled in this study at Saiseikai Matsuyama Hospital from November 2019 to April 2021. All patients were diagnosed with PD according to the UK Brain Bank Clinical Diagnostic Criteria [13]. The exclusion criteria were as follows: (i) current use of MgO; (ii) concomitant use of proton pump inhibitor, potassium-competitive acid blockers, or histamine H2 receptor antagonists within the past month; (iii) concomitant use of gastrointestinal prokinetic agents (e.g., domperidone); or (iv) antecedent history of gastrointestinal surgery.
Plasma concentrations of levodopa and carbidopa were assessed after an overnight fast and a 12-h washout period after administration of levodopa formulations. Blood samples were collected via an indwelling catheter before and 15, 30, 45, 60, 90, 120, and 180 min after oral administration of a single tablet containing 100 mg of levodopa and 10 mg of carbidopa. Concentration measurements were conducted on two different days: one day without the concomitant use of MgO and the other day with the prior evening intake of 1000 mg of MgO and simultaneous intake of 1000 mg of MgO with a levodopa formulation on the morning of the study day. These two evaluations were conducted within a period of 2 weeks, during which time the anti-parkinsonian medication was not changed. Age, sex, disease duration, body weight, body mass index, and Hoehn and Yahr stage were recorded for all participants. Motor symptoms were evaluated using the Movement Disorder Society-sponsored revision of the Unified Parkinson Disease Rating Scale (MDS-UPDRS) [14] part III score 60 to 90 min after oral administration on each study day.
This study was approved by the Institutional Review Board for Clinical Research Ethics at Saiseikai Matsuyama Hospital and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants prior to enrollment.
2.2. Determination of plasma concentrations of levodopa and carbidopa
The blood samples were centrifuged at 1,000 g and 4 °C for 10 min to separate plasma, which was stored at –80 °C until analysis. Levodopa and carbidopa were quantified using high-performance liquid chromatography (HPLC) with electrochemical (EC) detection. Plasma samples of 100 µL were mixed with 500 µL (for measurement of levodopa) or 200 µL (for measurement of carbidopa) of ice-cold 0.2 mol/L perchloric acid containing 0.1 mmol/L ethylenediaminetetraacetic acid and 0.15 ng/µL 3,4-dihydroxybenzamine. The samples were then vortexed, placed on ice for 30 min, and centrifuged at 20,000 g for 15 min at 4 °C. The supernatant was filtered through a 0.45-µm membrane filter (Chromatodisc 4A, GL Science Inc., Tokyo, Japan) and 10 μL of this solution were injected into the HPLC with EC detection (HTEC-500; Eicom, Kyoto, Japan). The analytical separation was performed at 30 °C using a reversed-phase column (C18 phase; 150 mm × 2.1 mm; EICOMPAK SC-5ODS, Eicom). The mobile phase consisted of 12 % (v/v) methanol containing 0.1 mol/L phosphate buffer (pH 2.7) and 1 mmol/L sodium octyl sulfate, and the flow rate was maintained at 0.25 mL/min.
2.3. Statistical analysis
The maximum plasma concentration (Cmax), time to maximum concentration (Tmax), and area under the plasma concentration–time curve 3 h after oral administration of a single tablet containing 100 mg of levodopa and 10 mg of carbidopa (AUC3h) were estimated according to the linear trapezoidal rule. Parameters before and after concomitant use of MgO were evaluated for significant differences using a paired t-test. We used a student's t-test for continuous data and a chi-square test for categorical data to compare independent groups. Statistical significance was set at p < 0.05. All analyses were conducted using R statistical software (version 4.2.2, The R Project for Statistical Computing, Vienna, Austria).
2.4. In vitro study
To investigate the effect of pH variation, KH2PO4 solution (136 g/L: pH 4.2) and Na2HPO4 solution (142 g/L: pH 9.5) were mixed to prepare 900 mL solutions with different pH levels (pH 4.5, pH 5.8, pH 7.4). In addition, to investigate the effect of MgO, 250 mg of MgO was dissolved in 900 mL of purified water or a strongly acidic solution with HCl (pH 1.2). Each solution was placed in an 11-cm diameter beaker with a magnetic stirrer, and a basket made of stainless-steel wire with 5 mm × 3 mm gaps was fixed in the center of the beaker to prevent movement (Supplementary Fig. 1). A single tablet containing 100 mg of levodopa and 10 mg of carbidopa (NEODOPASTON Combination Tablets L100, Daiichi Sankyo Co., Ltd., Tokyo, Japan) was placed in the center of the basket and stirred at 36 °C and 50 rpm in each solution. Similar tests were performed with a single tablet containing 100 mg of levodopa and 25 mg of beserazide (EC-DOPARL Tablets. Kyowa Kirin Co., Ltd., Tokyo, Japan) or a single tablet containing 100 mg of levodopa, 10 mg of carbidopa, and 100 mg of entacapone (STALEVO Combination Tablets L100, Novartis Pharma K.K., Tokyo, Japan) at a rotation speed of 100 rpm.
The alterations in the concentrations of levodopa, carbidopa, and benserazide in the solutions were measured as follows. Samples (20 µL) collected at each time point were diluted with purified water (200 µL) and filtered through a 0.45-µm pore filter. Aliquots (50 μL) were injected into the liquid chromatography-mass spectrometry/mass spectrometry system to quantify levodopa, carbidopa, and benserazide concentrations. Chromatographic separations were executed on an Agilent 1260 Infinity chromatographic system (Agilent Technologies, Santa Clara, CA, USA), utilizing a ZORBAX Eclipse Plus C18 (2.1 mm × 50 mm, 1.8 µm) to separate analytes. The mobile phase comprised a combination of 85 % acetonitrile and 0.5 % formic acid (95:5, v/v) and flowed at a rate of 0.2 mL/min. Mass spectrometry was performed using an amaZon speed ion trap mass spectrometer (Bruker Daltonics, Bremen, Germany) in the positive electrospray ionization mode. Levodopa was quantified at m/z 197.9–181.0, carbidopa at m/z 226.8–181.0, and benserazide at m/z 258.0–120.0 using multiple reaction monitoring transitions.
The concentrations of levodopa, carbidopa, and benserazide were expressed as a proportion of their maximum value, which was 100. All experiments were replicated thrice, and the data are presented as the mean outcome.
3. Results
3.1. Effects of concomitant use of MgO on the pharmacokinetics of levodopa and carbidopa in patients with PD
In this study, 35 patients with PD were enrolled, comprising 19 male and 16 female participants. The average age was 72.57 ± 7.75 years (mean ± standard deviation), disease duration was 7.54 ± 6.70 years, body weight was 53.15 ± 10.71 kg, and Hohen and Yahr stage was 2.97 ± 0.82. The detailed clinical profiles of the patients are presented in Table 1. Comparisons of clinical profiles by sex showed that body weight was significantly lower in female (46.61 ± 8.00 kg) than in male patients (58.66 ± 9.65 kg; p < 0.001), but no significant differences were found in other clinical profiles (Supplementary Table 1).
Table 1.
Clinical characteristics of the patients.
| PD (n = 35) | |
|---|---|
| Age, years; range | 72.57 (7.75); 53–87 |
| Male/Female | 19/16 |
| Disease duration, years | 7.54 (6.70) |
| Hohen and Yahr stage | 2.97 (0.82) |
| Body weight, kg | 53.15 (10.71) |
| Body mass index, kg/m2 | 21.07 (3.51) |
| Levodopa-induced dyskinesia (%) | 7 (20.0) |
| Wearing-off phenomenon (%) | 15 (42.9) |
| Levodopa daily dose, mg/day | 403.57 (156.26) |
| Levodopa equivalent daily dose, mg/day | 570.85 (217.10) |
| Anti-parkinsonian medication, n (%) | |
| Levodopa | 35 (100.0) |
| Pramipexole | 9 (25.7) |
| Ropinirole | 7 (20.0) |
| Selegiline | 7 (20.0) |
| Rasagiline | 5 (14.3) |
| Safinamide | 2 (5.7) |
| Entacapone | 10 (28.6) |
| Amantadine | 8 (22.9) |
| Zonisamide | 10 (28.6) |
| Istradefylline | 2 (5.7) |
Values are shown as mean (standard deviation) or n (%).
PD, Parkinson’s disease.
The pharmacokinetic parameters and motor symptoms before and after concomitant use of MgO are summarized in Supplementary Table 2. Concomitant use of MgO significantly reduced the Cmax of levodopa by 24 % (from 7.66 ± 3.74 μmol/L to 5.82 ± 3.69 μmol/L; p = 0.006) and the AUC3h of levodopa by 23 % (from 9.64 ± 3.23 μmol·h/L to 7.39 ± 3.15 μmol·h/L; p < 0.001) (Fig. 1A). Furthermore, the Cmax of carbidopa decreased by 39 % (from 64.02 ± 27.02 ng/mL to 38.83 ± 21.94 μmol/L; p < 0.001) and the AUC3h of carbidopa decreased by 41 % (from 130.58 ± 65.64 ng/mL to 76.48 ± 52.24 ng·h/mL; p < 0.001) with concomitant use of MgO (Fig. 1B). The MDS-UPDRS part III score also increased significantly by 1.35 (from 30.71 ± 11.34 to 32.06 ± 11.22; p = 0.007).
Fig. 1.
Plasma concentrations of levodopa (A) and carbidopa (B) following oral administration of a single tablet containing 100 mg of levodopa and 10 mg of carbidopa with (dotted line) or without (solid line) concomitant use of magnesium oxide in all participating patients (n = 35). The vertical bars indicate the 95 % confidence interval.
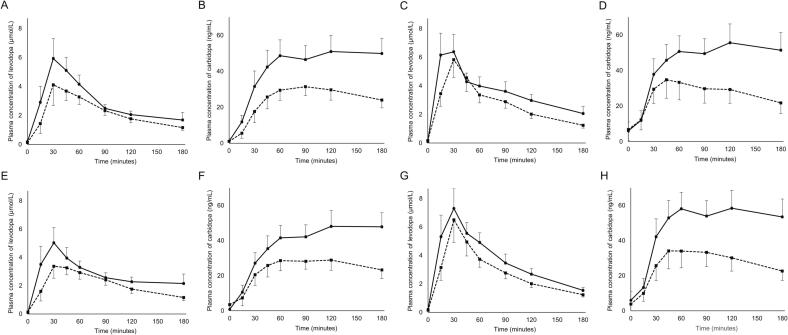
We investigated disparities in the impact of MgO on the pharmacokinetic parameters in relation to sex. MgO exerted a significant effect on the pharmacokinetics of levodopa and carbidopa in both male and female patients (Fig. 2A–D and Supplementary Table 3): the Cmax of levodopa decreased from 7.14 ± 3.65 μmol/L to 5.15 ± 3.90 μmol/L (p = 0.018), AUC3h of levodopa from 8.69 ± 2.83 μmol·h/L to 6.61 ± 2.79 μmol·h/L (p = 0.001), Cmax of carbidopa from 60.07 ± 25.70 ng/mL to 36.87 ± 16.65 ng/mL (p < 0.001), and AUC3h of carbidopa from 126.21 ± 65.00 ng·h/mL to 73.36 ± 39.60 ng·h/mL (p < 0.001) in male patients (n = 19); the Cmax of levodopa decreased from 8.27 ± 3.87 μmol/L to 6.60 ± 3.37 μmol/L (p = 0.130), AUC3h of levodopa from 10.77 ± 3.40 μmol·h/L to 8.32 ± 3.38 μmol·h/L (p = 0.004), Cmax of carbidopa from 68.71 ± 28.61 ng/mL to 41.15 ± 27.34 ng/mL (p = 0.002), and AUC3h of carbidopa from 135.77 ± 68.14 ng·h/mL to 80.19 ± 65.40 ng·h/mL (p = 0.001) in female patients (n = 16).
Fig. 2.
Comparison of pharmacokinetics categorized by sex (A–D) and age (E–H). Plasma concentrations of levodopa (A, C, E, G) and carbidopa (B, D, F, H) following oral administration of a single tablet containing 100 mg of levodopa and 10 mg of carbidopa with (dotted line) or without (solid line) concomitant use of magnesium oxide in male (n = 19) (A, B) and female patients (n = 16) (C, D) or in patients younger (n = 18) (E, F) and older than 73 years of age (n = 17) (G, H). The vertical bars indicate the 95 % confidence interval.
To assess whether age influences the effect of MgO on pharmacokinetic parameters, and because the median age was 73 years, patients were divided into two groups: those younger than 73 years and those older than 73 years. The results showed that MgO significantly affected the pharmacokinetics of levodopa and carbidopa regardless of patients’ age (Fig. 2E–H and Supplementary Table 4): the Cmax of levodopa decreased from 6.83 ± 3.18 μmol/L to 4.73 ± 2.22 μmol/L (p = 0.011), AUC3h of levodopa from 8.43 ± 2.32 μmol·h/L to 6.28 ± 2.16 μmol·h/L (p = 0.003), Cmax of carbidopa from 60.06 ± 23.70 ng/mL to 36.99 ± 16.16 ng/mL (p = 0.003), and AUC3h of carbidopa from 115.00 ± 53.60 ng·h/mL to 71.97 ± 41.02 ng·h/mL (p = 0.005) in patients under 73 years of age (n = 18); the Cmax of levodopa decreased from 8.53 ± 4.17 μmol/L to 6.97 ± 4.57 μmol/L (p = 0.150), AUC3h of levodopa from 10.93 ± 3.62 μmol·h/L to 8.58 ± 3.64 μmol·h/L (p = 0.003), Cmax of carbidopa from 68.21 ± 30.30 ng/mL to 40.78 ± 27.15 ng/mL (p < 0.001), and AUC3h of carbidopa from 147.08 ± 74.43 ng·h/mL to 81.26 ± 62.97 ng·h/mL (p < 0.001) in patients over 73 years of age (n = 17).
3.2. Effects of pH variation and MgO on the dissolution of levodopa formulations in vitro
When examined in a tablet containing 100 mg of levodopa and 10 mg of carbidopa, the relative concentrations of both levodopa and carbidopa were lower in the pH 7.4 solution than in the pH 4.5 and 5.8 solutions (Fig. 3A and B). The addition of MgO suspension to purified water decreased the relative concentration of levodopa and markedly decreased that of carbidopa (Fig. 3C and D). The pH at the final time point was 6.1 and 9.7 for solutions without and with MgO, respectively. The relative concentrations of levodopa and carbidopa decreased as MgO was suspended in a strongly acidic solution of HCl (pH 1.2), even though the pH of the solution did not change (Fig. 3E and F).
Fig. 3.
Time-course change of levodopa (A, C, E) and carbidopa (B, D, F) concentrations. A single tablet containing 100 mg of levodopa and 10 mg of carbidopa is dissolved under different pH conditions (A, B), in purified water (C, D) or in strongly acidic solution (pH 1.2) (E, F) with (dotted line) or without (solid line) magnesium oxide suspension.
The results for other levodopa formulations were generally similar, although a tablet containing 100 mg of levodopa and 25 mg of benserazide showed a pronounced decrease in relative concentration of benserazide with increasing pH (Supplementary Fig. 2), and a tablet containing 100 mg of levodopa, 10 mg of carbidopa, and 100 mg of entacapone was relatively difficult to dissolve (Supplementary Fig. 3).
4. Discussion
This study showed that plasma concentrations of both levodopa and carbidopa were significantly reduced by concomitant use of MgO in patients with PD who took a single tablet containing 100 mg of levodopa and 10 mg of carbidopa orally. Furthermore, carbidopa was more strongly affected with concomitant MgO than levodopa.
Several studies have shown that female patients have higher AUC and Cmax of levodopa than male patients, even after accounting for differences in body weight [12], [15]. Comparison of the effect of MgO by sex in this study confirmed that MgO significantly affected the pharmacokinetics of levodopa and carbidopa in both male and female patients. In addition, the effect of MgO may vary with age, because age affects the bioavailability of levodopa in patients with PD [12], [16], and gastric pH can increase because of decreased gastric acid secretion in elderly participants [17]. This study demonstrated that MgO significantly affects the pharmacokinetics of levodopa and carbidopa in both patients younger than 73 years and older than 73 years.
Research has shown that constipation appears 20 years before the onset of motor symptoms in patients with PD [18], and the severity of constipation is associated with the risk of developing PD in the future [19]. Constipation may also exacerbate the absorption of levodopa and contribute to fluctuations in motor symptoms [20]. Many patients with PD take oral laxatives [21], and appropriate management of bowel movements is important to improve the absorption of levodopa. However, our results indicate that concomitant use of MgO as a laxative may decrease plasma concentrations of levodopa and carbidopa and worsen symptoms. We suggest prescribing laxatives other than MgO as a strategy for treating constipation in patients with PD taking oral levodopa preparations.
We further conducted in vitro dissolution experiments to elucidate the mechanism of the effect of MgO on levodopa and carbidopa. When the pH of the solution was varied, the relative concentrations of both levodopa and carbidopa decreased as the pH increased from acidic to more neutral. Levodopa and carbidopa dissolve more readily in acidic conditions, and elevated gastric pH can contribute to poor absorption of levodopa [22], [23]. However, suspension of MgO in a strongly acidic solution decreased the relative concentrations of levodopa and carbidopa without affecting the pH in the solution. These results suggest that the solubility of levodopa and carbidopa may be affected by their interaction with MgO in addition to the acidity of the solutions. In addition, when MgO was suspended in purified water, the decrease in relative concentration of carbidopa was more pronounced than that of levodopa. Our previous study showed that the concentration of carbidopa decreased over time in solutions mixed with MgO, whereas no change was observed for levodopa, suggesting that MgO is involved in the degradation of carbidopa [9]. We believe that the neutralization of gastric acid and the interaction with MgO reduced the dissolution of levodopa and carbidopa, and the degradation of carbidopa was accelerated by MgO, resulting in a significant decrease in the plasma concentration of carbidopa compared to that of levodopa.
Both the levodopa/benserazide and levodopa/carbidopa/entacapone formulations, as well as the levodopa/carbidopa formulation, demonstrated a decrease in the relative concentrations of levodopa, carbidopa, and benserazide as the pH increased and in the presence of MgO, with the most prominent impact on benserazide. Both a crossover study in healthy participants and a retrospective study in patients with PD confirmed higher plasma concentrations of levodopa in combination with benserazide compared to carbidopa [12], [24]. Differences in DDC inhibitor content between the two preparations can be associated with differences in plasma levodopa concentrations, as in some countries, including Japan, carbidopa is formulated at 10 mg per 100 mg of levodopa while benserazide is formulated at 25 mg. However, another recent retrospective study reported higher plasma concentrations of levodopa in the benserazide formulation than in the carbidopa formulation, even when the combination ratio with levodopa was the same [15]. Given that benserazide is more susceptible to MgO, the decrease in plasma levodopa concentrations associated with concomitant use of MgO may be more pronounced in patients receiving oral levodopa/benserazide formulations.
This study had several limitations. We evaluated patients who received a total of 2000 mg of MgO orally over a two-day period. As patients ingest variable amounts of MgO in the real-world scenario, it is necessary to examine whether even small amounts of MgO can affect the pharmacokinetics of levodopa and carbidopa. Our retrospective study demonstrated no significant impact of MgO on levodopa pharmacokinetics [11]. This discrepancy may be because of the difference between taking MgO and levodopa on an empty stomach and after meals in addition to MgO dose differences. Additionally, the MDS-UPDRS part III score, measured at the time when plasma concentrations of levodopa are considered near peak, was significantly elevated with oral administration of MgO, but did not reach the score reported as clinically minimal pertinent deterioration [25]. However, we believe that concomitant use of MgO has a significant impact on clinical symptoms in patients with PD because it may reduce the AUC3h of levodopa by 23 % resulting in an increase in off time, especially in the advanced stages of the disease.
In conclusion, this study showed that concomitant use of MgO significantly reduced plasma concentrations of levodopa and carbidopa in patients with PD, regardless of patients' sex or age, leading to worsening of clinical symptoms. Our results also indicated that patients treated with levodopa/benserazide formulations may be more susceptible to the effects of MgO. Although patients with PD may often require prescription of laxatives, we believe that the concomitant use of MgO and levodopa should be avoided to improve levodopa absorption.
5. Authors’ contributions
NM conceived and designed the study. NM and YH acquired and analyzed the data. NM drafted the manuscript, and YH and MN critically revised the manuscript. All authors gave final approval for publication of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Madoka Kubo for technical assistance with the measurement of plasma levodopa and carbidopa concentrations.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prdoa.2023.100227.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hauser R.A. Levodopa: past, present, and future. Eur. Neurol. 2009;62:1–8. doi: 10.1159/000215875. [DOI] [PubMed] [Google Scholar]
- 2.Wright B.A., Waters C.H. Continuous dopaminergic delivery to minimize motor complications in Parkinson's disease. Expert Rev. Neurother. 2013;13:719–729. doi: 10.1586/ern.13.47. [DOI] [PubMed] [Google Scholar]
- 3.Baruzzi A., Contin M., Riva R., Procaccianti G., Albani F., Tonello C., Zoni E., Martinelli P. Influence of meal ingestion time on pharmacokinetics of orally administered levodopa in parkinsonian patients. Clin. Neuropharmacol. 1987;10:527–537. doi: 10.1097/00002826-198712000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Wang L., Xiong N., Huang J., Guo S., Liu L., Han C., Zhang G., Jiang H., Ma K., Xia Y., Xu X., Li J., Liu J.Y., Wang T. Protein-restricted diets for ameliorating motor fluctuations in Parkinson's disease. Front. Aging Neurosci. 2017;9:206. doi: 10.3389/fnagi.2017.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukae J., Fujioka S., Umemoto G., Arahata H., Yanamoto S., Mishima T., Tsuboi Y. Impact of residual drug in the pharynx on the delayed-on phenomenon in Parkinson's disease patients. Mov Disord Clin Pract. 2020;7:273–278. doi: 10.1002/mdc3.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyaue N., Yabe H., Nagai M., Nomoto M. Abnormal upper gastrointestinal structures underlying levodopa malabsorption. J. Neurol. Sci. 2020;414 doi: 10.1016/j.jns.2020.116855. [DOI] [PubMed] [Google Scholar]
- 7.Pierantozzi M., Pietroiusti A., Brusa L., Galati S., Stefani A., Lunardi G., Fedele E., Sancesario G., Bernardi G., Bergamaschi A., Magrini A., Stanzione P., Galante A. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology. 2006;66:1824–1829. doi: 10.1212/01.wnl.0000221672.01272.ba. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., He X., Mo C., Liu X., Li J., Yan Z., Qian Y., Lai Y., Xu S., Yang X., Xiao Q. Association between microbial tyrosine decarboxylase gene and levodopa responsiveness in patients with Parkinson disease. Neurology. 2022;99:e2443–e2453. doi: 10.1212/WNL.0000000000201204. [DOI] [PubMed] [Google Scholar]
- 9.Miyaue N., Kubo M., Nagai M. Ascorbic acid can alleviate the degradation of levodopa and carbidopa induced by magnesium oxide. Brain Behav. 2022;12:e2672. doi: 10.1002/brb3.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashihara Y., Terao Y., Yoda K., Hirota T., Kubota T., Kimura M., Matsuki S., Hirakawa M., Irie S., Ieiri I. Effects of magnesium oxide on pharmacokinetics of L-dopa/carbidopa and assessment of pharmacodynamic changes by a model-based simulation. Eur. J. Clin. Pharmacol. 2019;75:351–361. doi: 10.1007/s00228-018-2568-4. [DOI] [PubMed] [Google Scholar]
- 11.Nagai M., Tada S., Yamanishi Y., Miyaue N., Ando R. Differing Patterns of Diurnal Variation and Impact of Antacids on Levodopa Pharmacokinetics in Patients with Parkinson's Disease. Jpn. J. Clin. Pharmacol. Ther. 2023;54:3–8. doi: 10.3999/jscpt.54.1_3. [DOI] [Google Scholar]
- 12.Nishikawa N., Iwaki H., Shiraishi T., Mukai Y., Takahashi Y., Murata M. Female, aging, difference formulations of DCI, or lower body weight increases AUC(4hr) of levodopa in patients with Parkinson's disease. Parkinsonism Relat. Disord. 2020;76:16–20. doi: 10.1016/j.parkreldis.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A.E., Lees A., Leurgans S., LeWitt P.A., Nyenhuis D., Olanow C.W., Rascol O., Schrag A., Teresi J.A., van Hilten J.J., LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 15.Contin M., Lopane G., Belotti L.M.B., Galletti M., Cortelli P., Calandra-Buonaura G. Sex Is the main determinant of levodopa clinical pharmacokinetics: evidence from a large series of levodopa therapeutic monitoring. J. Parkinsons Dis. 2022;12:2519–2530. doi: 10.3233/JPD-223374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagayama H., Ueda M., Kumagai T., Tsukamoto K., Nishiyama Y., Nishimura S., Hamamoto M., Katayama Y. Influence of ageing on the pharmacokinetics of levodopa in elderly patients with Parkinson's disease. Parkinsonism Relat. Disord. 2011;17:150–152. doi: 10.1016/j.parkreldis.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Iijima K., Ohara S., Koike T., Sekine H., Shimosegawa T. Gastric acid secretion of normal Japanese subjects in relation to Helicobacter pylori infection, aging, and gender. Scand. J. Gastroenterol. 2004;39:709–716. doi: 10.1080/00365520410005911. [DOI] [PubMed] [Google Scholar]
- 18.Kalia L.V., Lang A.E. Parkinson's disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 19.Lin C.H., Lin J.W., Liu Y.C., Chang C.H., Wu R.M. Risk of Parkinson's disease following severe constipation: a nationwide population-based cohort study. Parkinsonism Relat. Disord. 2014;20:1371–1375. doi: 10.1016/j.parkreldis.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Fasano A., Visanji N.P., Liu L.W., Lang A.E., Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol. 2015;14:625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- 21.Kaye J., Gage H., Kimber A., Storey L., Trend P. Excess burden of constipation in Parkinson's disease: a pilot study. Mov. Disord. 2006;21:1270–1273. doi: 10.1002/mds.20942. [DOI] [PubMed] [Google Scholar]
- 22.Krisai K., Charnvanich D., Chongcharoen W. Increasing the solubility of levodopa and carbidopa using ionization approach. Thai J. Pharmaceut. Sci. 2020;44 [Google Scholar]
- 23.Yazawa I., Terao Y., Sai I., Hashimoto K., Sakuta M. Gastric acid secretion and absorption of levodopa in patients with Parkinson's disease–the effect of supplement therapy to gastric acid. Rinsho Shinkeigaku. 1994;34:264–266. [PubMed] [Google Scholar]
- 24.Iwaki H., Nishikawa N., Nagai M., Tsujii T., Yabe H., Kubo M., Ieiri I., Nomoto M. Pharmacokinetics of levodopa/benserazide versus levodopa/carbidopa in healthy subjects and patients with Parkinson's disease, Neurol. Clin. Neurosci. 2015;3:68–73. doi: 10.1111/ncn3.152. [DOI] [Google Scholar]
- 25.Horváth K., Aschermann Z., Ács P., Deli G., Janszky J., Komoly S., Balázs É., Takács K., Karádi K., Kovács N. Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Parkinsonism Relat. Disord. 2015;21:1421–1426. doi: 10.1016/j.parkreldis.2015.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.