Summary
Renewable energy-driven bipolar membrane water electrolyzers (BPMWEs) are a promising technology for sustainable production of hydrogen from seawater and other impure water sources. Here, we present a protocol for assembling BPMWEs and operating them in a range of water feedstocks, including ultra-pure deionized water and seawater. We describe steps for membrane electrode assembly preparation, electrolyzer assembly, and electrochemical evaluation.
For complete details on the use and execution of this protocol, please refer to Marin et al. (2023).1
Subject areas: Energy, Chemistry, Material Sciences
Graphical abstract

Highlights
-
•
Fabrication of bipolar membrane electrode assembly components
-
•
Detailed walkthrough of assembly, operation, and electrochemical testing
-
•
Operation of bipolar membrane water electrolyzers with pure water and seawater
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Renewable energy-driven bipolar membrane water electrolyzers (BPMWEs) are a promising technology for sustainable production of hydrogen from seawater and other impure water sources. Here, we present a protocol for assembling BPMWEs and operating them in a range of water feedstocks including ultra-pure deionized water and seawater. We describe steps for membrane electrode assembly preparation, electrolyzer assembly, and electrochemical evaluation.
Before you begin
Background
Bipolar membranes (BPMs) are attracting significant attention toward addressing a diverse range of sustainability challenges. BPMs play a critical role in desalination and electrodialysis, and have recently been considered for novel uses in electrochemical reduction of CO2, fuel cells, flow batteries, water electrolyzers, and other next-generation sustainable devices.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19 Bipolar membrane water electrolyzers (BPMWEs) have been proposed and studied as an enabling technology for H2 production from both pure water and impure water sources — this protocol outlines the fabrication of BPMWEs for H2 production based on a recently published report.1 H2 is a promising vector that can address the challenge of long-term energy storage, and it already serves as an important industrial feedstock in the production of fertilizer, steel, and fuels. Surging demand for clean H2 worldwide has spurred a growing interest in its sustainable production.20,21 H2 generation from commercial proton exchange membrane water electrolyzers (PEMWEs) requires ultra-pure water feeds,22 which may impose equity, economic, and scalability issues.
Seawater is an abundant resource, and using it to produce H2 could have inherent advantages, such as enabling broader access to water feedstocks while reducing capital costs by mitigating the need for on-site water purification. However, generating H2 and O2 from seawater poses challenges that differ from those encountered during the electrolysis of ultra-pure water.23 Due to the complex composition of seawater, numerous ions (Na+, Mg2+, Ca2+, Cl-, SO42-, HCO3-, CO32-, etc.) can interfere with H2 evolution and O2 evolution reaction (OER) and can negatively affect the stability of the BPMWE device. One critical challenge arises from the presence of Cl- — the Cl- oxidation reaction (Cl-OR) competes with OER to generate corrosive free chlorine species (Cl2, HOCl, OCl-) at the anode. Cl-OR is a 2e- reaction and is therefore often kinetically favored in comparison to OER which requires a 4e- transfer.23
BPMWEs offer a promising solution to address challenges associated with using impure water sources during electrolysis.1 Herein, we use a ‘zero-gap’ BPM membrane electrode assembly (MEA) architecture. The BPM is composed of a cation exchange layer (CEL) combined with an anion exchange layer (AEL). The BPM is inserted between catalyst-coated porous transport layers (PTLs) serving as the cathode and anode. This BPM architecture suppresses Cl-OR by regulating ion flow and providing an alkaline environment at the anode/membrane interface. The locally alkaline environment at the anode thermodynamically disfavors Cl-OR with respect to OER.24 The following report contains a step-by-step protocol for BPMWE device assembly and testing developed in our laboratories. We view this protocol as a detailed guide for the fabrication utilized in our previous work,1 and as a report on which the community can build on to optimize the design and construction of next-generation BPM-based devices. We provide a detailed, step-by-step description of the fabrication method of each component needed, as well as the assembly and testing of the electrolyzer. We outline the fabrication of BPM MEA components, including catalyst ink preparation (Steps 1–3) and the subsequent spray coating of both the electrodes and membrane (Steps 4–7). We further provide a detailed BPMWE assembly guide (Step 8–11), followed by a comprehensive description of overarching operational steps and electrochemical testing (Steps 12–13), offering valuable insights into the performance evaluation of this BPMWE system.
Electrolyzer test station assembly set up
Timing: 1 h
In this section you will assemble the electrolysis station used for all BPMWEs tests.
-
1.Water Circulation Assembly (Figure 1)
-
a.Place two hot plates side by side with two support stands behind them in a large secondary container.
-
b.Place one 1 L glass container for the water on each hot plate.
-
c.Position a temperature probe on each stand so that each can reach inside their respective glass container.
-
a.
-
2.Electrolyzer Hardware Support
-
a.Place a large non-metallic secondary container (e.g., plastic) next to the water circulation assembly.
-
b.Position the electrolyzer hardware (Fuel Cell Technologies, Inc. ©) on a stand inside the container so any leaks will be contained and prevent the device from being in contact with excess water (Figure 1).
-
c.Using polyethylene tubing (0.250 in outer diameter, 0.170 in inner diameter) and stainless-steel compression fittings, connect the electrolyzer intake ports to the water reservoirs.
-
d.Use in-line diaphragm pumps (KNF Neuberger Inc.) on the electrolyzer intake side to pump the water into the electrolyzer, drawing from the reservoirs positioned on the hot plates.
-
e.Run similar polyethylene lines to connect the electrolyzer exit ports back to the reservoirs. Polypropylene push-to-connect on/off valves can be used on the intake and/or exit lines to stop water flow during device set up and take down.
-
f.Secure the pump inlet lines and the electrolyzer output lines in place using clamps or lab-grade label tape.
-
a.
Note: Diaphragm pumps are driven by a power supply (we used a Kungber 30 V, 10 A, DC variable supply) with an adjustable power controller (MRC model 1300) to allow for control overflow rate which can be calibrated using a flowmeter or a graduated cylinder and a stopwatch. We used a flow rate of 60 mL min-1.
CRITICAL: Elevate the pumps over a plastic secondary container to contain any water leakage. Raise the pump wires to avoid contact with water. Vibration from the diaphragm pumps has the potential to dislodge the lines from the reservoir over time which can result in water loss and subsequent dry electrolyzer operation.
Note: Electrolyzer station should be equipped with a ventilation system (e.g. exhaust snorkel) to control potential emission of Cl2 and/or H2 generated during electrolysis.
Figure 1.
Electrolyzer test station and components
(A) An electrolyzer test station with labeled components. The labeled components include the stand, electrolyzer, secondary container, thermocouple, glass container, temperature probes, power supply, and on/off valves. The image highlights the arrangement and positioning of these components.
(B) Labeled electrolyzer hardware. The labeled components include the flow fields, current collectors, gaskets, and electrolyzer body.
Experiment preparation
In this section, you will assemble a subset of the supplies and materials that should be prepared prior to the experiment — these are the supplies/materials that can be stocked for multiple experiments or are reusable. We describe membrane hydration, gaskets & spacer construction, and water feedstock collection.
Membrane preparation
Timing: 2.5 h
In this section you will convert the membranes from an as-received dry form to the required hydrated form and store them for later use.
-
3.Nafion Preparation
-
a.Cut a 1.5 cm × 10 cm wide Nafion 212 (Fuel Cell Store, 50 μm) strip using clean scissors.
-
b.Peel off any protective coatings to expose the membrane using clean stainless steel or plastic tweezers.
-
c.Add membrane to a container containing >25 mL UPDI water. We used a 125 mL polypropylene container.
-
d.Soak for >2 h.
-
e.Drain off UPDI water and exchange water with fresh UPDI water.
-
f.Store in the container for up to 2 weeks.
-
g.Retrieve with tweezers when needed. Do not leave Nafion strip outside of UPDI water long enough for it to become dehydrated.
-
a.
-
4.PiperION preparation
-
a.Cut a 1.5 cm × 10 cm PiperION (Fuel Cell Store, TP-85, 40 μm) strip using clean scissors.
-
b.Peel off any protective coating to expose membrane using clean tweezers.
-
c.Add membrane to an alkaline-stable plastic container (we used a 125 mL polypropylene jar) with ∼100 mL of 0.5 M KOH (Sigma-Aldrich, trace metal grade).
-
d.Soak for >2 h.
-
e.Pour off old KOH and replace with fresh 0.5 M KOH (Sigma-Aldrich, trace metal grade).
-
f.Store in the container for up to 2 weeks.
-
g.Retrieve as needed with chemically resistant tweezers. Do not leave PiperION strip outside of 0.5 M KOH long enough for it to become dehydrated.
-
a.
Note: If membranes show wrinkles, look discolored, or show poor performance, the storage container may be contaminated. Refresh the storage solution several times and let the membrane soak for >2 h.
Cutting electrolyzer gaskets & titanium spacers
Timing: 30 min
In this section you will cut gaskets to be used to maintain appropriate compression on the MEA (related to steps 8–11, electrolyzer assembly).
-
5.
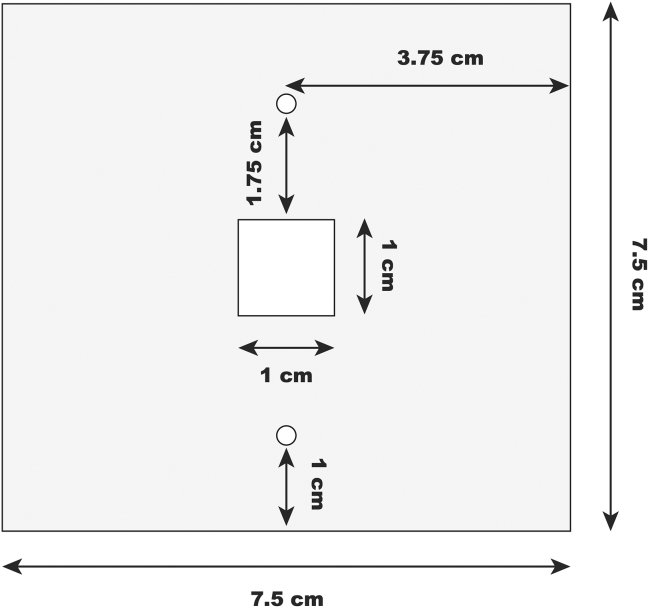
Cut several 0.010, 0.005, and 0.002 in gaskets from polyester sheets (McMaster-Carr) so they have a 1 cm × 1 cm hole for the electrodes, and two alignment holes according to Figure 2. We used a laser cutter (Universal, PLS 6.120).
-
6.
Cut 1 cm × 1 cm titanium spacers from a 400 μm thick sintered titanium plate (Baoji Yingao Metal Materials Inc.). We used a laser cutter (Coherent ExactCut 430, fiber laser, argon assist gas, cut speed = 300, piercing = 0.15, peak power = 150, pulse width = 0.15 ms, burst frequency = 2000 Hz).
Figure 2.
Gasket design and dimensions
Gasket design to be cut out of polyester sheets with varying thicknesses. The design includes circular cutouts with a diameter of 0.3 cm, along with other dimensions labeled in the diagram. Scale bars defined in image.
Water feedstock preparation
Timing: 1 day
In this section, you will prepare the water feedstocks for electrolysis during seawater testing.
-
7.Seawater Collection
-
a.Go to a local source of seawater and collect a sample using a plastic collection bucket.
-
b.Slowly retrieve the seawater sample, keeping it close to the sea surface to minimize the amount of sediment collected. The United States Geological Survey (USGS) provides guidelines for water collection methods.25
-
c.Store the sample in a durable plastic container (we used a polyester container).
-
a.
-
8.Seawater Filtration
-
a.Remove residual sediment by vacuum filtration through 70 mm Cytiva Whatman™ Qualitative Filter Paper.
-
b.Filter seawater through a Stericup® Quick Release Filter Millipore Express® PLUS 0.22 μm polyethersulfone (PES) Membrane to remove biotic organisms.
-
c.Store filtered water in inert plastic containers (we used polyethylene terephthalate).
-
a.
Note: When possible, collect water from a deep enough region to prevent sand from entering vessel, as this will reduce filtration time and improve water quality.
CRITICAL: Collect enough seawater to conduct all planned experiments. Seawater composition can vary based on time of day and weather conditions.26,27,28
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Core/shell Ir/IrOx, conductive catalyst | Fuel Cell Store | Product code: 3151663 |
| Pt catalyst | Fuel Cell Store | Product code: 590078 |
| TiO2 catalyst (P25) | Nippon Aerosil Co., Ltd | |
| Nafion solution (D-520 dispersion, 5% w/w in water and 1-propanol) | Sigma-Aldrich or Ion Power | |
| PiperION solution | Fuel Cell Store | 74820000 |
| Nafion membrane (212, 50 μm) | Fuel Cell Store or Ion Power | |
| PiperION membrane (Versogen, TP-85, 40 μm) | Fuel Cell Store | Product code: 73800014 |
| Potassium hydroxide (semiconductor grade) | Fisher | 15663080 |
| Isopropyl alcohol (IPA, ACS Reagent Grade, >99.5%) | Fisher | 18-603-551 |
| Ethanol (ACS Reagent Grade, >99.5%) | Fisher | AC615095000 |
| Ultra-pure deionized (UPDI) water (18.2 MΩ cm) | Milli-Q | |
| Software and algorithms | ||
| EC-Lab (Version 11.4) | BioLogic | www.BioLogic.net |
| Other | ||
| Potentiostat | BioLogic | |
| Electrolyzer hardware (custom electrolyzer designed based on fuel cell, 5 cm2, product code: 5SCH) | ||
| Polyester (PETE) sheets for gaskets (0.002, 0.005, and 0.01 in thick) | McMaster-Carr | 8567K22, 8567K52, & 8567K92 |
| Sintered titanium plate for porous titanium spacer (400 μm thick) | Baoji Yinggao Metal Materials Inc | |
| Toray Carbon paper (120, wet proofed) | Fuel Cell Store | Product code: 591637 |
| Steel Felt (25 AL3) | Bekaert Bekipor | |
| Polyethylene tubing for water lines (0.250 in outer diameter, 0.170 in inner diameter) | Dixon | 0817CR |
| Water pumps | KNF | UNF 1.25 RTDCB-4B |
| Airbrush | Testors | A220 |
| Bath sonicator | Branson | 1510 |
| Horn sonicator | Qsonica | Model: CL-18 |
| Polystyrene Petri dish | Thermo Fisher Scientific | 263991 |
| Lab-grade label tape (lavender) | Fisher | 159015L |
| Stirring hot plate | Corning | PC-420D |
| Polypropylene ball valve | Parker | PP4VUC6-MG |
| Parafilm | Millipore Sigma | P7668-1EA |
| Stainless steel compression fitting | Swagelok | SS-400-1-4 |
Note: This protocol most closely details the procedures followed in Marin et al.1 We encourage the community to optimize the design, refine materials, and construction of next-generation BPM-based devices.
Step-by-step method details
Catalyst ink preparation
Timing: 1.5 h
In this set of steps, prepare the inks that will be used on the anode, cathode, and Nafion membrane that will be integrated into the MEA.
-
1.Anode ink preparation – Catalyst: IrOx
-
a.Weigh 0.1 g of IrOx (Fuel Cell Store) in 20 mL glass scintillation vial.
 CRITICAL: Always add water to the catalyst first before any alcohols to reduce the risk of a potential fire.
CRITICAL: Always add water to the catalyst first before any alcohols to reduce the risk of a potential fire. -
b.Add 0.4 g UPDI water. By volume, this is ∼0.4 mL.
-
c.Add 1.7 g IPA (Fisher, ACS reagent grade). By volume, this is ∼2.2 mL.
-
d.Add 0.1 g PiperION 5% (w/w) solution (Fuel Cell Store). By volume, this is ∼0.1 mL.
-
e.Close cap and seal with parafilm.
-
f.Bath sonicate ink for at least 10–20 min or until dispersed.
Note: The total mass of ink is 2.2 g, and total volume is ∼2.7 mL in one vial. Two vials of ink will be necessary for step 5. -
a.
-
2.Cathode ink preparation – Catalyst: Pt
-
a.Weigh 0.1 g of Pt (Fuel Cell Store) in 20 mL glass scintillation vial.
 CRITICAL: Always add water to the catalyst first before any alcohols to reduce the risk of a potential fire.
CRITICAL: Always add water to the catalyst first before any alcohols to reduce the risk of a potential fire. -
b.Add 1.5 g UPDI water. By volume, this is ∼1.5 mL.
-
c.Add 1.7 g IPA (Fisher, ACS reagent grade). By volume, this is ∼2.2 mL.
-
d.Add 0.1 g Nafion 5% (w/w) solution (Sigma Aldrich). By volume, this is ∼0.1 mL.
-
e.Close cap and seal with parafilm.
-
f.Bath sonicate ink for >1 h and then horn sonicate for ∼1 min or until dispersed.Note: w/w refers to the ratio of the mass of the component to total mass.Note: Bath sonication refers to sonication using the Branson sonicator, while horn-sonication refers to sonication using the Qsonica sonicator. Bath sonication is often enough to disperse the inks, but if aggregates remain, horn sonicate for ∼1 min or until dispersed. All sonication was done at ∼20°C.Note: We used a mass balance to determine the amount of materials required to increase precision and ease of measure when possible.Note: The total mass of ink is 3.3 g, and total volume is ∼3.8 mL in one vial. Two vials of ink will be necessary for step 6.
-
a.
-
3.Water dissociation layer ink preparation – Catalyst: TiO2
-
a.Preparation of TiO2 stock solution
-
i.Add 0.1 g TiO2 (Nippon Aerosil Co., Ltd, P25) to a 20 mL glass scintillation vial.
-
ii.Add 4.9 g of UPDI water. By volume, this is ∼4.9 mL.
-
iii.Cover with vial cap and wrap parafilm around the edge of the cap.
-
iv.Bath sonicate for >5 min.
-
v.Store for up to 2 months, sonicate immediately before use for >5 min.Note: TiO2 stock solution can be stored for 2 months.
-
i.
-
b.Add 30 mg of the TiO2 stock solution to a 20 mL glass scintillation vial using a micro pipette.
-
c.Add 0.47 g of UPDI water. By volume, this is ∼0.5 mL.
-
d.Add 1.7 g IPA (Fisher, ACS reagent grade). By volume, this is ∼2.2 mL.
-
e.Bath sonicate for >5 min before spraying.
 CRITICAL: Uniform and well dispersed inks (Figure 3) are essential for preparing reproducible and high-performance electrodes and membranes. We recommend (re-)sonicating the inks immediately prior to spraying.
CRITICAL: Uniform and well dispersed inks (Figure 3) are essential for preparing reproducible and high-performance electrodes and membranes. We recommend (re-)sonicating the inks immediately prior to spraying.
-
a.
Figure 3.
Schematic of catalyst ink preparation
Schematic representation of catalyst ink preparation. The diagram highlights the need for sonication to achieve a homogeneous catalyst ink with minimal nanoparticle agglomerates.
Spray coating MEA components
Timing: 2 h
In this set of steps, coat the anode porous transport layer (PTL) (Methods video S1), cathode PTL, and Nafion cation exchange membrane (Methods video S2) with catalyst layers (Figure 4).
-
4.Prepare airbrush (Methods video S1).
-
a.Connect a straw to the airbrush (Testors, A220).
-
b.Connect airbrush to an inert gas supply controlled with a regulator, adjust until pressure is ∼10 psi. We used house nitrogen (>99.99% purity).
-
a.
Note: We cut a disposable transfer pipette (Fisher, polyethylene, CAT NO. 13-711-9AM) at both ends to act as straw. Cut once directly below the bulb, and once directly below the tip. It should fit snugly into the airbrush.
Note: We use designated airbrush tips to prevent cross contamination.
Note: Airbrush handling is critical for this process. Hold the airbrush with both hands, dominant hand on the lever that will spray the ink and other hand in contact with both the vial and the tip (Figure 5).
-
5.Prepare anode (Methods video S1)
-
a.Substrate preparation
-
i.Cut a 5 cm × 5 cm square of stainless steel felt PTL (25AL3, Bekaert Bekipor®).Note: The number of electrodes prepared can be scaled up or down by cutting out larger or smaller amounts of PTL. When preparing large amounts of electrodes, it can be helpful to use an automated spray coater if available (e.g., SonoTek ExactaCoat).
-
ii.Using a mechanical pencil, mark 1 cm × 1 cm squares on the back (‘shiny side’) to facilitate cutting electrodes for later use (Figure 6).
-
iii.Weigh and record the initial sample mass.
-
iv.Use lab-grade label tape to tape down the four corners of the PTL onto a tinfoil-covered hot plate.Note: Minimize the amount of PTL covered by tape to avoid losing catalyst coverage surface area (Figure 7). Alternatively, a larger PTL than required can be cut out and the 4 edges taped down.
-
v.Heat the hot plate surface to 100°C.
-
i.
-
b.Ink deposition
-
i.Spray ink in short 1 s puffs from a 5–8 cm distance, moving in a serpentine pattern along the felt square (Figure 7). Spray with a 45° angle to the PTL surface. Verify that you see a uniform coating of the catalyst ink on the PTL.Note: Let the solvent evaporate between puffs — do not let the liquid pool on the surface. Pooling of ink will cause non-uniform coating and can lead to poor contact between the catalyst and the membrane.
-
ii.After one full cycle, rotate hot plate 90°.
-
iii.Continue this process until desired loading is reached as calculated via mass difference from the initial mass of PTL substrate. Ink should have even coverage over the entire PTL surface.Note: Remove sample from hot place and record mass periodically to prevent overloading. We normally require ∼2 vials (∼7.6 mL) of ink to reach 2.5 mg cm-2.Note: Visually inspect the deposition periodically to assure the ink has been sprayed evenly on the substrate (Figure 8).
-
iv.Clean airbrush by spraying ethanol (ACS reagent grade, >99.5%) through nozzle into a waste container for ∼10 s.
-
i.
-
c.Spray ionomer overlayer
-
i.Make a 2% (w/w) PiperION solution by diluting 4 g of 5% (w/w) PiperION solution with 6 g of ethanol (ACS reagent grade, >99.5%)
-
ii.Spray this diluted PiperION ionomer solution using same technique as described above.
-
iii.Total ionomer loading should be between 10 – 20% of the total catalyst loading (0.25–0.5 mg cm-2; 6.25–12.5 mg total).
-
iv.Wash airbrush with IPA into a waste container for ∼10 s.
 CRITICAL: The airbrush tips can clog if not cleaned within ∼1 min after use.
CRITICAL: The airbrush tips can clog if not cleaned within ∼1 min after use.
-
i.
-
d.Store anodes in a sealed container. We used a closed polystyrene petri dish (ThermoFisherScientific).
-
a.
-
6.Prepare cathodeNote: Cathode and anode preparation techniques are very similar in technique but use different materials. Methods video S1 provides relevant preparation/techniques for cathode preparation.
-
a.Substrate preparation
-
i.Cut a 5 cm × 5 cm square of Toray carbon paper PTL (Fuel Cell Store).
-
ii.Weigh and record initial sample mass.
-
iii.Use lab-grade label tape to tape down the four corners of the PTL onto a tinfoil-covered hot plate.Note: Tape the smallest amount of PTL possible to avoid losing catalyst coverage surface area. Alternatively, a larger PTL than required can be cut out and the four edges taped down.
-
iv.Heat hot plate surface to 100°C.
-
i.
-
b.Spray ink
-
i.Spray ink in short 1 s puffs, moving in a serpentine pattern along the carbon paper PTL (Figure 7). Spray at a 45° angle to the PTL surface.Note: Let the solvent evaporate between puffs, do not let the liquid pool on the surface. Pooling of ink will cause non-uniform coating.
 CRITICAL: The airbrush tips can clog if not cleaned within ∼1 min after use.
CRITICAL: The airbrush tips can clog if not cleaned within ∼1 min after use. -
ii.After one full cycle, rotate hot plate 90°
-
iii.Continue until desired loading is reached as calculated via mass difference from the initial carbon paper.Note: Remove sample from hot place and record mass periodically to prevent overloading. We normally require ∼1.5 vial (∼5.4 mL) to reach 2.0 mg cm-2.
-
iv.Wash airbrush by spraying IPA into a waste container for ∼10 s.
-
i.
-
c.Spray ionomer overlayer
-
i.Spray 5% (w/w) Nafion ionomer solution using same technique as described above (step 6b).
-
ii.Total loading should be between 10 – 20% of the total catalyst loading (0.2–0.4 mg cm-2; 5–10 mg total).
-
iii.Wash airbrush with IPA into a waste container for ∼10 s.
-
i.
-
a.
-
7.Water dissociation catalyst-coated membrane preparation (Methods video S2)
-
a.Substrate preparation
-
i.Cut a 1.5 cm × 1.5 cm square of hydrated Nafion.
-
ii.Lay down onto glass petri dish assuring no air bubbles get trapped between the membrane and petri dish. Fold a Kimwipe into a square and press lightly against the Nafion to dry it onto the glass (Figure 9).
-
iii.Lightly tape down the sides with lab grade label tape leaving a 1.2 cm × 1.2 cm area exposed.
-
iv.Squeeze out all air bubbles that could have formed between the petri dish and the tape by pressing down outwards on the taped region and make a strong seal.
 CRITICAL: If air bubbles are present, they can lead to air movement that will deform the membrane during spray coating. We have observed that careful preparation of the Nafion/glass/tape interface is strongly correlated with assembly of acceptably performing bipolar membrane electrolyzers.
CRITICAL: If air bubbles are present, they can lead to air movement that will deform the membrane during spray coating. We have observed that careful preparation of the Nafion/glass/tape interface is strongly correlated with assembly of acceptably performing bipolar membrane electrolyzers. -
v.Place petri dish on a 90°C hot plate.
-
i.
-
b.Spray coating
-
i.Spray TiO2 ink using 8–10 psi in ∼1 s puffs at a 90° angle to the surface from a 5–6 in distance.
-
ii.After 10 puffs, rotate the hot plate 90°.
-
iii.Continue spraying and rotating until ink from the prepared vial (step 3) is completely spent.Note: It is important to not let the membrane get too wet. Excess water can lead to bubble formation.Note: Label the back of the glass petri dish with a 1.2 cm × 1.2 cm square to properly align the tape on the Nafion membrane.
-
i.
-
c.Remove petri dish from hot plate.
-
d.Carefully remove two pieces of tape that are parallel to each other.
-
e.Mark the front side of membrane with a number so proper orientation of active side can be determined later. Do not write on the TiO2 covered portion (to avoid contamination).
-
f.Remove the other two pieces of tape from the sides.
-
g.Store sprayed membrane in a plastic container containing UPDI water until ready to use. We used a 125 mL polypropylene jar.
-
a.
Figure 4.
Membrane spray coating process
The image highlights the key components involved in the membrane spraycoating process, including the catalyst ink, airbrush, membrane or PTL, hot plate, and nitrogen regulator. The airbrush, controlled by the nitrogen regulator, then sprays a fine mist of catalyst ink onto the PTL/membrane surface.
Figure 5.
Airbrush handling
Photograph of proper airbrush handling for catalyst spraying coating. The figure emphasizes the grip, hand position, and location of airbrush straw.
Figure 6.
Stainless steel PTL preparation
Back (left) and front (right) image of stainless steel PTL (25 cm2) before catalyst ink spraying. The left photograph showcases pencil markings on the backside indicating twenty-five 1 cm2 squares. These markings serve as a reference for the subsequent catalyst ink spraying process, ensuring accurate placement of the catalyst material on the front surface of the PTL. Scale bars defined in image.
Figure 7.
Electrode spray coating setup
(A) Photograph illustrating the PTL securely taped onto a foil-covered hot plate prepared for the spraying procedure.
(B) Schematic representation of the serpentine spray coating pattern applied to the PTL surface, followed by a 90° rotation of the hot plate. The diagram demonstrates the sequential actions involved in achieving an even and uniform distribution of the catalyst ink across the PTL surface (Methods video S2). Scale bars defined in image, 5 cm.
Figure 8.
Spray coated PTL
Image of sprayed PTL coated with anode catalyst, IrOx.
(A) Low quality PTL, showcasing an irregular and poorly adhered coating of IrOx. (B) High quality PTL, exhibiting uniform coating. The image highlights a successful deposition of the catalyst material onto the PTL surface. Color enhanced images of (A) and (B) are included in panels (C) and (D), respectively, to highlight the differences in deposition quality. Scale bars defined in image, 2.5 cm.
Figure 9.
Spray coated membranes
Photographs of prepared membranes for comparison.
(A) Successfully prepared membrane, exhibiting a uniform and smooth surface.
(B) Unusable membrane with commonly observed defect from presence of air bubbles (marked with a black arrow). Scale bars defined in image, 1 cm.
Electrolyzer assembly
Timing: 1 h
In this set of steps, assemble the electrolyzer hardware and MEA (Figure 10, Methods video S3).
-
8.Water circulation system preparation
-
a.Add 1 L of UPDI water to the anode and cathode reservoirs.
-
b.Place reservoirs on hot plates.
-
c.Add PTFE-coated magnetic stir bar and thermocouple each to anode and cathode reservoirs.
-
d.Turn on hot plates to ∼60°C with ∼180 rpm stirring.
-
a.
Note: To achieve the desired operation temperature of 50°C, we found it was necessary to heat the water to approximately 63°C. The exact value will depend on the extent of thermal losses along the water supply lines. We measured the electrolyzer temperature using a thin metal K-type thermocouple inserted into a hole on the cathode side of the electrolyzer, and the water temperature using a thermocouple inserted directly into the water reservoirs on the hot plates (see Figure 1). Water heating can take >30 minutes, during which time assembly of the other components can be done in parallel.
-
9.Cathode/Anode Preparation
-
a.Anode
-
i.Cut a 1 cm × 1 cm square from the previously sprayed anodes using clean scissors (see step 5).
-
ii.Gently flatten the 1 cm × 1 cm electrode using two flat surfaces.Note: Flattening prevents any edges which may have curled up during cutting from piercing the membranes and causing leaks and possibly an electrical short during operation. We used two heavy steel blocks covered with clean Kimwipes.
-
iii.Gently rinse anode with UPDI water.
-
i.
-
b.Cathode (Figure 11)
-
i.Cut a 1 cm × 1 cm square from the previously sprayed cathodes (step 6). Carbon-based PTLs do not need to be flattened.
-
ii.Gently rinse cathode with UPDI water.
-
i.
-
a.
-
10.Electrolyzer assembly (Figure 12)
-
a.Place two Teflon straws into the holes in the electrolyzer chassis base.
-
b.Put O-rings over the straws.
-
c.Place gold current collector onto electrolyzer base, the side coated with insulator should face down and O-ring should fit snugly into the holes cut into the current collector.
 CRITICAL: If the O-rings are not seated snugly, when you tighten the electrolyzer you will not get a good seal and water will leak out.Note: The Teflon straws and rubber O-rings we used were supplied by Fuel Cell Technologies, Inc. © with the electrolyzer hardware. The exact straws and O-rings required will be dependent on the electrolyzer hardware acquired, the most important considerations are that the materials used do not react with the feedstocks. In our design, the straws were approximately 0.5 in long with 0.04 in inner, and 0.1 in outer diameter. The O-rings were approximately 0.1 in thick with 0.1 in inner, and 0.2 in outer diameter.
CRITICAL: If the O-rings are not seated snugly, when you tighten the electrolyzer you will not get a good seal and water will leak out.Note: The Teflon straws and rubber O-rings we used were supplied by Fuel Cell Technologies, Inc. © with the electrolyzer hardware. The exact straws and O-rings required will be dependent on the electrolyzer hardware acquired, the most important considerations are that the materials used do not react with the feedstocks. In our design, the straws were approximately 0.5 in long with 0.04 in inner, and 0.1 in outer diameter. The O-rings were approximately 0.1 in thick with 0.1 in inner, and 0.2 in outer diameter. -
d.Place titanium anode flow field onto the current collector such that Teflon straws fit into the two holes on the backside of the flow field.Note: The as-supplied straws are sometimes too long and may need to be cut down in length slightly. The flow field should sit flat on the current collector to ensure a good seal.
-
e.Place two alignment pegs into the holes machined into top of anode flow field.
-
f.Stack polyester gaskets (from 0.010, 0.005, and 0.002 in thick sheets, see before you begin section) until the thickness is 0.047 in.
-
g.Put a 1 cm × 1 cm porous titanium spacer (400 μm thick) into the hole cut into the gaskets.
-
h.Place the anode, catalyst side up, on top of the titanium spacer.
-
i.Cut a 1.5 cm × 1.5 cm piece of pre-treated PiperION (see membrane preparation in before you begin section).
-
j.Wash PiperION in UPDI water for ∼10 s.
-
k.Place onto a clean area of gasket material such that the membrane lays completely flat.
-
l.Retrieve a TiO2-coated Nafion membrane.
-
m.Place TiO2-coated Nafion on top of the PiperION, TiO2 side down, ensuring quality lamination without air bubbles between the two membranes.
-
n.Gently slide the membranes over the anode electrode ensuring the TiO2 area fully overlaps the electrode.Note: If Nafion has a pale-yellow coloration, it may be a sign of dehydration. Let membrane sit in UPDI overnight or replace.
-
o.Stack additional polyester gaskets (from 0.010, 0.005, and 0.002 in thick sheets) until the added thickness is 0.037 in (total polyester gasket stack thickness of 0.084 in).Note: Variations in the MEA component thickness may require adjustments in the gasket thickness to maintain appropriate compression.
-
p.Place cathode catalyst side down into hole left by gaskets cut in.
-
q.Place 1 cm × 1 cm porous titanium spacer (400 μm thick) on top of the cathode.
-
r.Place graphite cathode flow field on top of gasket, allowing the alignment pegs to fit in the two holes machined into top and bottom of the flow field.
-
s.Place Teflon straws into holes on top of graphite flow field.
-
t.Place O-rings on straws.
-
u.Place current collector (insulating side up) onto flow field, making sure O-rings fit snugly in holes.
-
v.Put the top plate of the electrolyzer chassis onto current collector.
-
w.Tighten bolts to 30 lbs·in (∼3.4 Nm) in a star pattern using a torque wrench. The star pattern tightening helps evenly distribute the force on the MEA.
-
x.Tighten bolts to a final torque of 50 lbs·in (∼5.7 Nm).
-
a.
-
11.Begin water circulation and electrolyzer heatingNote: We run >1 L of ∼20°C UPDI water through the BPMWE to minimize any contamination from previous experiments. This water is discarded prior to circulating heated water.
-
a.If tubing between water reservoirs and electrolyzer body is not attached, connect it now as described in electrolyzer test station assembly set up (before you begin section) .
-
b.Attach thermocouple to monitor temperature. We used a thin metal thermocouple placed into a hole on the cathode side of the electrolyzer.
-
c.Open on/off valves to enable water flow.
-
d.Turn on diaphragm pumps, by turning on the power controller and power source.
-
e.Adjust pump power to achieve a calibrated 60 mL min-1 flow rate.
-
f.Wait for electrolyzer to heat to a stable 50 +/- 1°C. Adjust hot plate heating as necessary (see: electrolyzer assembly, step 8).Note: Failure to open butterfly valves will result in the pump line failure and water leakage.Note: The flow rate can be calibrated using a stopwatch and graduated cylinder. Adjust the power supplied to achieve the desired flow rate of 60 mL min-1.
-
a.
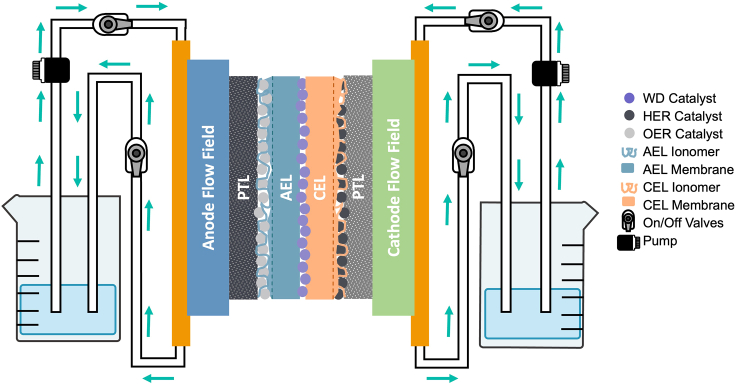
Figure 10.
Diagram of BPMWE with additional hardware components
Cross-sectional schematic of the catalyst/ionomer-coated porous transport layers (PTL) with their corresponding ion-exchange membranes and the water circulation scheme. The cation exchange membrane (CEL) refers to the Nafion membrane and ionomer overlayer, while the anion exchange layer (AEL) refers to the PiperION membrane and ionomer overlayer.
Figure 11.

Prepared cathode
Photograph of a 1 cm2 cathode, precisely cut to the required size, and prepared for insertion into an electrolyzer. Scale bar defined in image, 1 cm.
Figure 12.
Schematic BPMWE architecture
Depiction of the architecture of an assembled BPMWE, with a zoomed-in view of a cross-sectional graphic illustrating a zero-gap BPMWE configuration. The diagram showcases the overall architecture of the MEA setup, highlighting key components such as the anode, cathode, and membranes.
Electrolyzer testing
Timing: >1 h
In this set of steps (steps 12–13), prepare for the electrochemical measurements, execute electrolyzer break-in, and introduce impure water feeds such as 0.5 M NaCl or seawater.
CRITICAL: Chlorine gas is severely toxic, oxidizing, and corrosive. Seek immediate medical attention if exposure occurs. A more detailed discussion of the safety concerns is given in the publicly available safety data sheet (SDS).29 While production during a typical experiment using 0.5 M NaCl or seawater should be minimal, appropriate safety precautions must be taken to minimize the risk of exposure. We bubble the electrolyzer exhaust through water, so any potential chlorine is converted to hypochlorous acid and hypochlorite which are soluble. We also have an exhaust snorkel directly above the water containers, so any potential fumes are not released into the lab. Never mix chlorine containing chemical waste with acids (See File S1 - Example of Environmental Health and Safety Standard Operating Procedure).
-
12.Connect potentiostat and energize electrolyzer (Figure 13)
-
a.Connect potentiostat leads to the electrolyzer.Note: For our experiments, we connected the anode to the working electrode voltage sensing and current leads, and the cathode to the counter electrode voltage sensing and current leads. The reference voltage sensing lead was connected to the cathode. We used EC-Lab (BioLogic) and have included the basic BioLogic setting files that are common to many of our experiments (See File S2- BioLogic ECLab Setup File).
-
b.Execute electrochemical experiments. The steps are summarized below.
-
i.CP1: Chronopotentiometry for 1 min at 10 mA cm-2, ramp from 50 – 450 mA cm-2 in 50 mA cm-2 increments holding each current for 1 min, hold at 500 mA cm-2 for 10 min. This serves as an electrolyzer ‘break-in’/conditioning process.Note: We use the performance at 500 mA cm-2 in CP1 to assess the quality of the electrolyzer. If a BPMWE requires >2.5 V at 500 mA cm-2 (for a 1 cm2 electrode) we take the device apart and reassemble with new MEA components to achieve higher performance.
-
ii.CP2: Chronopotentiometry ramp from 500 – 50 mA cm-2 in 50 mA cm-2 increments, holding each current for 1 min, followed by applying 10 mA cm-2 for 1 min at the end. This gently returns the electrolyzer to a low applied current state from which a polarization curve can be collected in CP3.
-
iii.CP3: Chronopotentiometry starting with a current hold at 10 mA cm-2 for 10 s, then ramping from 50 – 500 mA cm-2 in 50 mA cm-2 increments and holding each current for 10 s. This serves to collect the data necessary to construct a polarization curve.
-
iv.GEIS 4–8: Galvanostatic electrochemical impedance spectroscopy with a 5–10% current modulation at 4 current densities (500, 250, 100, and 50 mA cm-2) collected in decreasing order. The fifth current density should be the current density at which the long-term CP experiment will be held. This serves to support analysis of electrochemical performance and performance limiters.
-
v.CP9: Chronopotentiometry that is generally performed for extended periods of time. This serves to test the long-term performance of the electrolyzer. This is often where impure water sources are introduced to the electrolyzer system.
 CRITICAL: To switch to 0.5 M NaCl, add solid NaCl to the cathode and/or anode and allow to dissolve. To switch to seawater, preheat filtered seawater (see before you begin section) to the same temperature as the UPDI water and switch out the UPDI water containers for those containing the hot seawater, ensuring fast transfer of the pump lines to the seawater. When testing impure water feeds, we often switched out UPDI water for 0.5 M NaCl or seawater during CP9.Note: Samples can be collected from the anode or cathode by removing a small 5–10 mL of liquid via volumetric pipet for further analysis.Note: Additional GEIS can be collected by starting and stopping the constant current experiment (CP9) to monitor changes to the device over time. We avoided allowing the electrolyzer to return to open circuit voltage in between experiments to avoid degradation of the catalyst/ionomer due to changing current/voltage conditions.
CRITICAL: To switch to 0.5 M NaCl, add solid NaCl to the cathode and/or anode and allow to dissolve. To switch to seawater, preheat filtered seawater (see before you begin section) to the same temperature as the UPDI water and switch out the UPDI water containers for those containing the hot seawater, ensuring fast transfer of the pump lines to the seawater. When testing impure water feeds, we often switched out UPDI water for 0.5 M NaCl or seawater during CP9.Note: Samples can be collected from the anode or cathode by removing a small 5–10 mL of liquid via volumetric pipet for further analysis.Note: Additional GEIS can be collected by starting and stopping the constant current experiment (CP9) to monitor changes to the device over time. We avoided allowing the electrolyzer to return to open circuit voltage in between experiments to avoid degradation of the catalyst/ionomer due to changing current/voltage conditions.
-
i.
-
a.
-
13.Finish experiment
-
a.Turn off pumps.
-
b.Turn off stirring and heating.
-
c.Close electrolyzer water line valves to prevent water leakage.
-
d.Loosen electrolyzer bolts.
-
e.Disassemble electrolyzer.
-
f.Clean components
-
i.For experiments in UPDI water, flushing with UPDI water can be sufficient.
-
ii.For experiments using salt or seawater, components should be flushed with >1 L UPDI water. Any components which have visible precipitation or salt build up should be bath sonicated in 5% HCl or 5% citric acid until precipitate has been fully removed, then bath sonicated again in UPDI water.
-
i.
-
g.Safely dispose of any waste generated.
-
a.
Figure 13.
Schematic representation of the BPMWE setup coupled with a potentiostat
The diagram illustrates the assembled electrolyzer horizontally oriented with the anode on the lower portion and the cathode on top, both connected to the potentiostat via two electrical leads. The diagram illustrated on the computer captures the first step in the break-in process (CP1). Note that the electrical connection to the electrolyzer should be made carefully—4 point connections to the anode and cathode are recommended to minimize undesired resistive losses. Stable, low resistance connections are also important to minimize resistive losses under high-current operation.
Expected outcomes
The BPMWE should ideally exhibit consistent performance at the end of the break-in period (j = 500 mA cm-2 at Vcell< 2.5 V). It is important to note that poor electrochemical performance during/after break-in can be due to a variety of errors in the methodology — we have provided a flowchart summarizing assembly and testing with key checkpoints to promote successful electrolyzer operation (Figure 14). An example of non-ideal electrolyzer behavior can be seen in Figure 15 — we attribute this I-V behavior to an improperly cleaned electrolyzer with residual salts in the system.
Figure 14.
Flowchart for BPMWE assembly and testing
The chart illustrates the decision points and key actions required at each stage. The chart outlines the process, starting from the electrolyzer assembly and preparation to electrochemical testing, with key troubleshooting points highlighted.
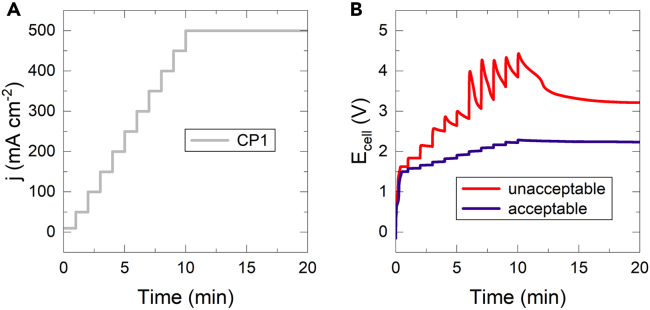
Figure 15.
Comparison of voltage profiles during break-in of a BPMWE
(A) Current density and (B) voltage of an acceptable and unacceptable BPMWE break-in. We used Ecell < 2.5 V at 500 mA as an additional performance metric to indicate successful BPMWE fabrication.
Limitations
This protocol outlines two-electrode measurements wherein voltages are read as total cell voltage difference between the cathode and anode. This configuration limits the information obtained — three- and four-electrode experiments using independent reference electrodes can be performed to understand the nature of microenvironments and reactions occurring at individual interfaces.30
Practical limitations, such as membrane batch availability and fluctuating seawater composition, can also affect the outcomes of experiments that follow this protocol. We have found anion exchange membrane properties can differ from batch to batch. This source of error can have severe effects on the resistance at electrode and membrane interfaces, leading to anomalously high voltages. Additionally, surface water composition can widely differ depending on geographic location, recent precipitation, season, among other factors. To reduce this source of error it is important to capture enough seawater at once for all planned experiments. Measuring pH and conductivity can help characterize these differences.26,27,28
Troubleshooting
Problem 1
Nafion membrane exhibits bubbles while or after spray deposition of water dissociation catalyst (Step 7b; Figure 9).
Potential solution
Tape the membrane so it is airtight against the Petri dish before placing it on a hot plate and initializing the catalyst spraying. After taping, turn the Petri dish upside down and inspect for air bubbles. If there are any bubbles in the membrane, retape the membrane. If there are any bubbles in the tape, squeeze them out towards the side with your finger or a flat edge (Methods video S2).
Problem 2
The electrode pierces the membrane, creating a hole where the water from one reservoir escape to the other. An indicator of this issue is the volume of the electrolyte reservoirs fluctuating or an observed short circuit during electrochemical testing (Steps 12–13).
Potential solution
Stop the experiment, open the electrolyzer, and exchange the punctured membrane for a new membrane. The most probable source of piercing will be the anode. Before assembling a new electrolyzer, flatten the anode between two flat surfaces as described in step 9.
Problem 3
Membranes of BPM are not adhering correctly to each other; improper adhesion can result in a discontinuous interface. When placing the Nafion membrane on the PiperION membrane, a small bubble may form between them, interfering with electrolysis (Steps 8–11).
Potential solution
Place the Nafion membrane from one edge and slowly work your way to the other side. On the edge of the gasket, drop a small amount of UPDI water, use this water to wet the Nafion membrane, and then guide it with tweezers on top of the PiperION membrane. With a Kimwipe apply pressure from the top down to remove any excess water. Re-start as many times as necessary.
Resource availability
Lead contact
Adam C. Nielander (anieland@slac.stanford.edu).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
Primary funding was provided by the US Office of Naval Research under grant N00014-20-1-2517. Partial support for long-term seawater durability measurements was provided by the Stanford Doerr School of Sustainability Accelerator. Partial support for developing the protocols for fabrication was provided by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, Catalysis Science Program through the SUNCAT Center for Interface Science and Catalysis. Partial support for G.A.L. was provided by the US Department of Energy, Office of Energy Efficiency and Renewable Energy (EERE), Fuel Cell Technologies Office (FCTO) award DE-EE0008841. D.H.M. was supported by a National GEM Consortium GEM fellowship and a TomKat Center for Sustainable Energy fellowship for Translational Research.
Author contributions
Conceptualization, I.R.A., R.T.H., D.H.M., T.F.J., S.W.B., A.C.N., L.C., G.A.L., C.R., M.A.H., J.T.P.; Methodology, I.R.A., R.T.H., D.H.M., L.C., J.T.P., M.A.H., A.C.N.; Investigation, D.H.M., J.T.P; Writing, Original Draft, I.R.A., R.T.H.; Writing, Review & Editing, I.R.A., R.T.H., D.H.M., T.F.J., S.W.B., A.C.N., L.C., G.A.L., C.R., M.A.H., J.T.P; Visualization, I.R.A., R.T.H., D.H.M.; Supervision, T.F.J., S.W.B., A.C.N., M.B.S.; Project Administration, T.F.J., S.W.B., A.C.N.; Funding Acquisition, T.F.J., S.W.B., A.C.N.
Declaration of interests
The authors have patents submitted and issued (US patent # 11,268,200) related to the content of this manuscript.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2023.102606.
Contributor Information
Daniela H. Marin, Email: dhm2121@stanford.edu.
Shannon W. Boettcher, Email: swb@uoregon.edu.
Adam C. Nielander, Email: anieland@slac.stanford.edu.
Thomas F. Jaramillo, Email: jaramillo@stanford.edu.
Supplemental information
Data and code availability
The data generated during this study are available upon request to anieland@slac.stanford.edu.
References
- 1.Marin D.H., Perryman J.T., Hubert M.A., Lindquist G.A., Chen L., Aleman A.M., Kamat G.A., Niemann V.A., Stevens M.B., Regmi Y.N., et al. Hydrogen production with seawater-resilient bipolar membrane electrolyzers. Joule. 2023;7:765–781. doi: 10.1016/j.joule.2023.03.005. [DOI] [Google Scholar]
- 2.Salvatore D.A., Gabardo C.M., Reyes A., O’Brien C.P., Holdcroft S., Pintauro P., Bahar B., Hickner M., Bae C., Sinton D., et al. Designing anion exchange membranes for CO2 electrolysers. Nat. Energy. 2021;6:339–348. doi: 10.1038/s41560-020-00761-x. [DOI] [Google Scholar]
- 3.Pärnamäe R., Mareev S., Nikonenko V., Melnikov S., Sheldeshov N., Zabolotskii V., Hamelers H.V.M., Tedesco M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021;617 doi: 10.1016/j.memsci.2020.118538. [DOI] [Google Scholar]
- 4.Yan Z., Hitt J.L., Zeng Z., Hickner M.A., Mallouk T.E. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer. Nat. Chem. 2021;13:33–40. doi: 10.1038/s41557-020-00602-0. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Rossi R., Yan Z., Yang W., Hickner M.A., Mallouk T.E., Logan B.E. Balancing Water Dissociation and Current Densities To Enable Sustainable Hydrogen Production with Bipolar Membranes in Microbial Electrolysis Cells. Environ. Sci. Technol. 2019;53:14761–14768. doi: 10.1021/acs.est.9b05024. [DOI] [PubMed] [Google Scholar]
- 6.Vermaas D.A., Smith W.A. Synergistic Electrochemical CO2 Reduction and Water Oxidation with a Bipolar Membrane. ACS Energy Lett. 2016;1:1143–1148. doi: 10.1021/acsenergylett.6b00557. [DOI] [Google Scholar]
- 7.Lindquist G.A., Xu Q., Oener S.Z., Boettcher S.W. Membrane Electrolyzers for Impure-Water Splitting. Joule. 2020;4:2549–2561. doi: 10.1016/j.joule.2020.09.020. [DOI] [Google Scholar]
- 8.Bui J.C., Digdaya I., Xiang C., Bell A.T., Weber A.Z. Understanding Multi-Ion Transport Mechanisms in Bipolar Membranes. ACS Appl. Mater. Interfaces. 2020;12:52509–52526. doi: 10.1021/acsami.0c12686. [DOI] [PubMed] [Google Scholar]
- 9.White W., Sanborn C.D., Fabian D.M., Ardo S. Conversion of Visible Light into Ionic Power Using Photoacid-Dye-Sensitized Bipolar Ion-Exchange Membranes. Joule. 2018;2:94–109. doi: 10.1016/j.joule.2017.10.015. [DOI] [Google Scholar]
- 10.Blommaert M.A., Aili D., Tufa R.A., Li Q., Smith W.A., Vermaas D.A. Insights and Challenges for Applying Bipolar Membranes in Advanced Electrochemical Energy Systems. ACS Energy Lett. 2021;6:2539–2548. doi: 10.1021/acsenergylett.1c00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wrubel J.A., Chen Y., Ma Z., Deutsch T.G. Modeling Water Electrolysis in Bipolar Membranes. J. Electrochem. Soc. 2020;167 doi: 10.1149/1945-7111/ab9ccb. [DOI] [Google Scholar]
- 12.Giesbrecht P.K., Freund M.S. Recent Advances in Bipolar Membrane Design and Applications. Chem. Mater. 2020;32:8060–8090. doi: 10.1021/acs.chemmater.0c02829. [DOI] [Google Scholar]
- 13.Weekes D.M., Salvatore D.A., Reyes A., Huang A., Berlinguette C.P. Electrolytic CO2 Reduction in a Flow Cell. Acc. Chem. Res. 2018;51:910–918. doi: 10.1021/acs.accounts.8b00010. [DOI] [PubMed] [Google Scholar]
- 14.Xie K., Miao R.K., Ozden A., Liu S., Chen Z., Dinh C.-T., Huang J.E., Xu Q., Gabardo C.M., Lee G., et al. Bipolar membrane electrolyzers enable high single-pass CO2 electroreduction to multicarbon products. Nat. Commun. 2022;13:3609. doi: 10.1038/s41467-022-31295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X., Liu R., Sun K., Chen Y., Verlage E., Francis S.A., Lewis N.S., Xiang C. Solar-Driven Reduction of 1 atm of CO2 to Formate at 10% Energy-Conversion Efficiency by Use of a TiO2 -Protected III–V Tandem Photoanode in Conjunction with a Bipolar Membrane and a Pd/C Cathode. ACS Energy Lett. 2016;1:764–770. doi: 10.1021/acsenergylett.6b00317. [DOI] [Google Scholar]
- 16.Lucas É., Bui J., Hwang M., Wang K., Bell A., Weber A., Ardo S., Atwater H., Xiang C. 2023. Asymmetric Bipolar Membrane for High Current Density Electrodialysis Operation with Exceptional Stability. [DOI] [Google Scholar]
- 17.Ahlfield J.M., Liu L., Kohl P.A. PEM/AEM Junction Design for Bipolar Membrane Fuel Cells. J. Electrochem. Soc. 2017;164:F1165–F1171. doi: 10.1149/2.1041712jes. [DOI] [Google Scholar]
- 18.Tongwen X. Electrodialysis processes with bipolar membranes (EDBM) in environmental protection—a review. Resour. Conserv. Recycl. 2002;37:1–22. doi: 10.1016/S0921-3449(02)00032-0. [DOI] [Google Scholar]
- 19.Peng S., Xu X., Lu S., Sui P.-C., Djilali N., Xiang Y. A self-humidifying acidic–alkaline bipolar membrane fuel cell. J. Power Sources. 2015;299:273–279. doi: 10.1016/j.jpowsour.2015.08.104. [DOI] [Google Scholar]
- 20.Farias C.B.B., Barreiros R.C.S., da Silva M.F., Casazza A.A., Converti A., Sarubbo L.A. Use of Hydrogen as Fuel: A Trend of the 21st Century. Energies. 2022;15:311. doi: 10.3390/en15010311. [DOI] [Google Scholar]
- 21.Tiwari A. Hydrogen Leading the Green Energy Future. Adv. Mater. Lett. 2022;13 doi: 10.5185/amlett.2022.021690. 2202-1690. [DOI] [Google Scholar]
- 22.Wang X.X., Li J., Wang T.X., Yang Y.N., Zhang H.K., Zhou M., Kang L., Wei L.Y. PEM water electrolysis for hydrogen production: fundamentals, advances, and prospects. Insect Sci. 2022;29:21–32. doi: 10.1007/s43979-022-00022-8. [DOI] [PubMed] [Google Scholar]
- 23.Mohammed-Ibrahim J., Moussab H. Recent advances on hydrogen production through seawater electrolysis. Materials Science for Energy Technologies. 2020;3:780–807. doi: 10.1016/j.mset.2020.09.005. [DOI] [Google Scholar]
- 24.Dresp S., Dionigi F., Klingenhof M., Strasser P. Direct Electrolytic Splitting of Seawater: Opportunities and Challenges. ACS Energy Lett. 2019;4:933–942. doi: 10.1021/acsenergylett.9b00220. [DOI] [Google Scholar]
- 25.Wilde F.D. Water-quality sampling by the U.S. Geological Survey-Standard protocols and procedures. U.S. Geological Survey. 2010 doi: 10.3133/fs20103121. 2010-3121, 1-2. [DOI] [Google Scholar]
- 26.Laxague N.J.M., Zappa C.J. The Impact of Rain on Ocean Surface Waves and Currents. Geophys. Res. Lett. 2020;47 doi: 10.1029/2020GL087287. [DOI] [Google Scholar]
- 27.Ludwig H. In: Reverse Osmosis Seawater Desalination Volume 1: Planning, Process Design and Engineering – A Manual for Study and Practice. Ludwig H., editor. Springer International Publishing; 2022. Seawater: Composition and Properties; pp. 73–203. [DOI] [Google Scholar]
- 28.Jiang L.-Q., Carter B.R., Feely R.A., Lauvset S.K., Olsen A. Surface ocean pH and buffer capacity: past, present and future. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-55039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chlorine; SDS No. 001015 [Online]; Airgas: Radnor, PA, Feb. 11, 2021. https://www.airgas.com/msds/001015.pdf
- 30.Chen L., Xu Q., Boettcher S. Kinetics and Mechanism of Heterogeneous Voltage-Driven Water-Dissociation Catalysis. Joule. 2023;7:1867–1886. doi: 10.1016/j.joule.2023.06.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated during this study are available upon request to anieland@slac.stanford.edu.