Summary
Microrefugia are often located within topographically complex regions where stable environmental conditions prevail. Most of the studies concerning the distributions of climate change-sensitive species have emphasized the dominance of cold air pooling over other environmental factors, such as resource availability. There is a shortage of information on the relationships among topography-related microsite diversity, microclimate, resource availability, and species composition in microrefugia. To fill this knowledge gap, we studied the effects of microclimatic conditions and soil resources on plant species occurrence within and adjacent to 30 large topographic depressions (i.e., dolines) in two distant karst regions. Our results showed that both microclimate and soil resource availability may play a key role in maintaining climate change-sensitive species and biodiversity in dolines; therefore, they may simultaneously act as climate and resource microrefugia. Establishing climate-smart conservation priorities and strategies is required to maintain or increase the refugial capacity of such safe havens.
Subject areas: Environmental science, Nature conservation, Ecology, Plant ecology
Graphical abstract
Highlights
-
•
Topographic depressions promote cold air pooling and retain soil resources
-
•
Dolines are colder, moister and more nutrient rich than their surroundings
-
•
Complex relationships exist among microclimate, soil, and vegetation in dolines
-
•
Preserving doline microhabitats is essential to maintain biodiversity
Environmental science; Nature conservation; Ecology; Plant ecology
Introduction
Microrefugia are small areas that enable the long-term persistence of species outside their main geographic distribution.1,2,3 An increasing body of evidence suggests that these areas have played an important role in mitigating regional environmental changes over time throughout the globe,4,5 therefore they are the focus of many conservation efforts.6,7,8 Local topography—together with the effects of other abiotic and biotic factors, such as wind speed and canopy cover—may create a diverse microsite structure with the ability to decouple local climate trends from those occurring in their surroundings, which is a key factor in forming microrefugia.9,10,11 For instance, convergent environments (e.g., valleys and topographic depressions) can facilitate cold air drainage and pooling, resulting in wide ranges of temperature and humidity over short distances.12,13 This ability to provide various microclimatic conditions has contributed to the persistence of relict populations of a variety of organisms, such as vascular plants and arthropods, in “climate microrefugia”.14,15,16 In addition to thermal microsites, microsites with high resource availability (e.g., soil moisture, soil nutrients, and food sources) may also enable species to persist during regional environmental changes.2 For instance, microsites in deep sinkholes (i.e., topographic depressions) in tropical karst landscapes may provide “resource microrefugia” for frugivorous bird species after cyclically recurring costal storms, where these species can forage on a broad range of plant species.17 In landscapes where water scarcity is a critically limiting factor (e.g., in seasonally arid Mediterranean landscapes), the presence of permanently wet microsites is essential for species persistence.6 These microsites might reduce the vulnerability of wet-habitat species to drought compared to adjacent areas with seasonally dry conditions.11,18 Although many small-scale and idiosyncratic processes may provide additional water-holding capacity in such areas, their presence is difficult to predict at larger scales.19,20 Despite widespread efforts to investigate the environmental characteristics of particular landscapes and geomorphologic units, there is a shortage of information on the relationships between microclimate, soil resources and species distribution within contemporary and potential future microrefugia. To fill this knowledge gap, we studied the effects of microclimatic conditions and soil resource availability on plant species occurrence within and adjacent to topographic depressions in karst landscapes.
Karst landscapes are the foremost examples of ground-water erosion on Earth, providing topographically complex environments.21,22,23 As such landscapes cover about 15–20% of the Earth’s terrestrial surface,24 they play a crucial role in ecological speciation and maintaining global biodiversity.25,26,27 A number of studies have reported that different landforms in karst landscapes (e.g., north-facing slopes, rocky outcrops, and topographic depressions) have played an important role in buffering species populations during Quaternary climate oscillations.28 Topographic depressions—dolines, sinkholes, or tiankengs—are the most common and typical landforms in these landscapes, which can create cooler conditions than their surroundings and thus provide favourable microsites for cold-adapted species.11,29,30 In Central Europe, dolines are well known as important determinants of the presence of peripheral populations of vascular plant species as well as arthropods,15,16,31,32 highlighting their role as contemporary and potential future microrefugia.15 In the Mediterranean region of Europe and Asia Minor, dolines can serve as important habitats for endemic species with limited dispersal ability.33,34,35 Most of the studies concerning the distributions of peripheral populations in dolines have focused mainly on the microclimatic conditions of different microsites,30,36 emphasizing the prevalence of cold air pooling and temperature gradients over other environmental factors, such as edaphic heterogeneity. However, convergent environments may concentrate water and nutrient resources where wet-habitat and/or nutrient-demanding species can find suitable microsites amid unsuitable xeric and nutrient-poor conditions.6,32,37 As microclimate will likely become warmer over the next decades in dolines, they may also act as traps or transient microrefugia for at least some climate change-sensitive species (cf. McLaughlin et al.6). In addition, dolines also provide potential safe havens for species that currently occur in large areas within karst landscapes,38 if these species are able to track their preferred climates and adapt to the special soil conditions of doline microsites. To sum up, assessing plant species responses to environmental changes without considering simultaneously soil properties and climatic conditions may be misleading.39
Although spatial variation in soil resource availability may also enhance the survival of species in microrefugia,2,6,40 the relationships among topography-related microsite diversity, microclimate, soil properties, species composition, and vegetation structure have rarely been studied. In this study, we compared the patterns of environmental factors and vascular plant species among different doline microsites in Central European forested karst landscapes and analyzed species-environment relationships (Figure 1). The following questions were addressed: (1) How do microclimate and soil resource availability co-vary among the plateau and doline microsites (south-facing slopes, north-facing slopes, and bottoms) in forested karst landscapes? (2) How do microclimatic patterns and soil properties influence the species composition, vegetation pattern, and conservation value of dolines and their surroundings?
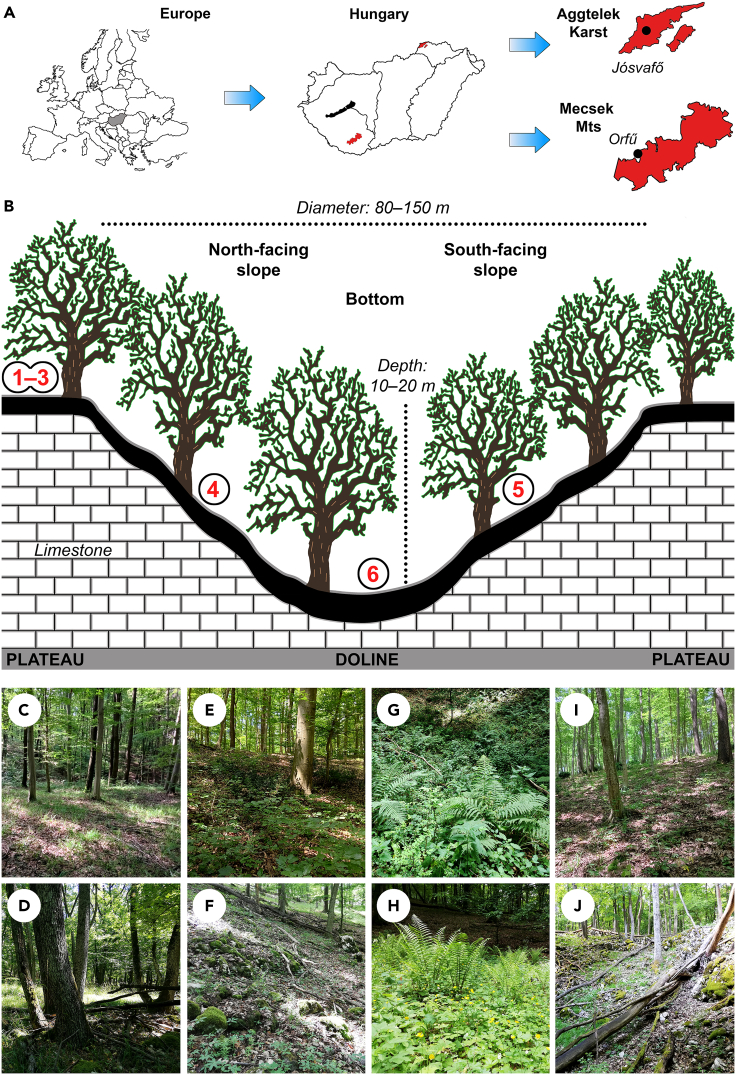
Figure 1.
Study regions and study design
(A) Location of the study areas in the Mecsek Mountains and Aggtelek Karst, Hungary, (B) Sampling locations on the plateaus (1–3) and in dolines (4–6), (C) Oak-hornbeam forest (Asperulo taurinae-Carpinetum) on the plateau in the Mecsek Mountains, (D) Rocky forest (Tilio-Fraxinetum excelsioris) on the plateau in the Aggtelek Karst, (E) Oak-hornbeam forest on the north-facing slope of a large doline in the Mecsek Mountains, (F) Scree forest (Mercuriali-Tilietum) on the north-facing slope of a large doline in the Aggtelek Karst, (G) Ravine forest (Scutellario altissimae-Aceretum) with fern species in the bottom of a large doline in the Mecsek Mountains, (H) Beech forest (Melittio-Fagetum) with fern species in the bottom of a large doline in the Aggtelek Karst, (I) Oak-hornbeam forest on the south-facing slope of a large doline in the Mecsek Mountains, and (J) Scree forest on the south-facing slope of a large doline in the Aggtelek Karst.
Results
Microclimatic patterns and soil characteristics
Air temperature and relative air humidity showed distinct spatial and daily patterns in the different doline microsites both in spring (before canopy closure) and summer (after canopy closure) (Figures 2, 3, S1, and Table S1). Microsite had a significant effect (F = 5.93–71.69, p < 0.001) on microclimatic conditions.
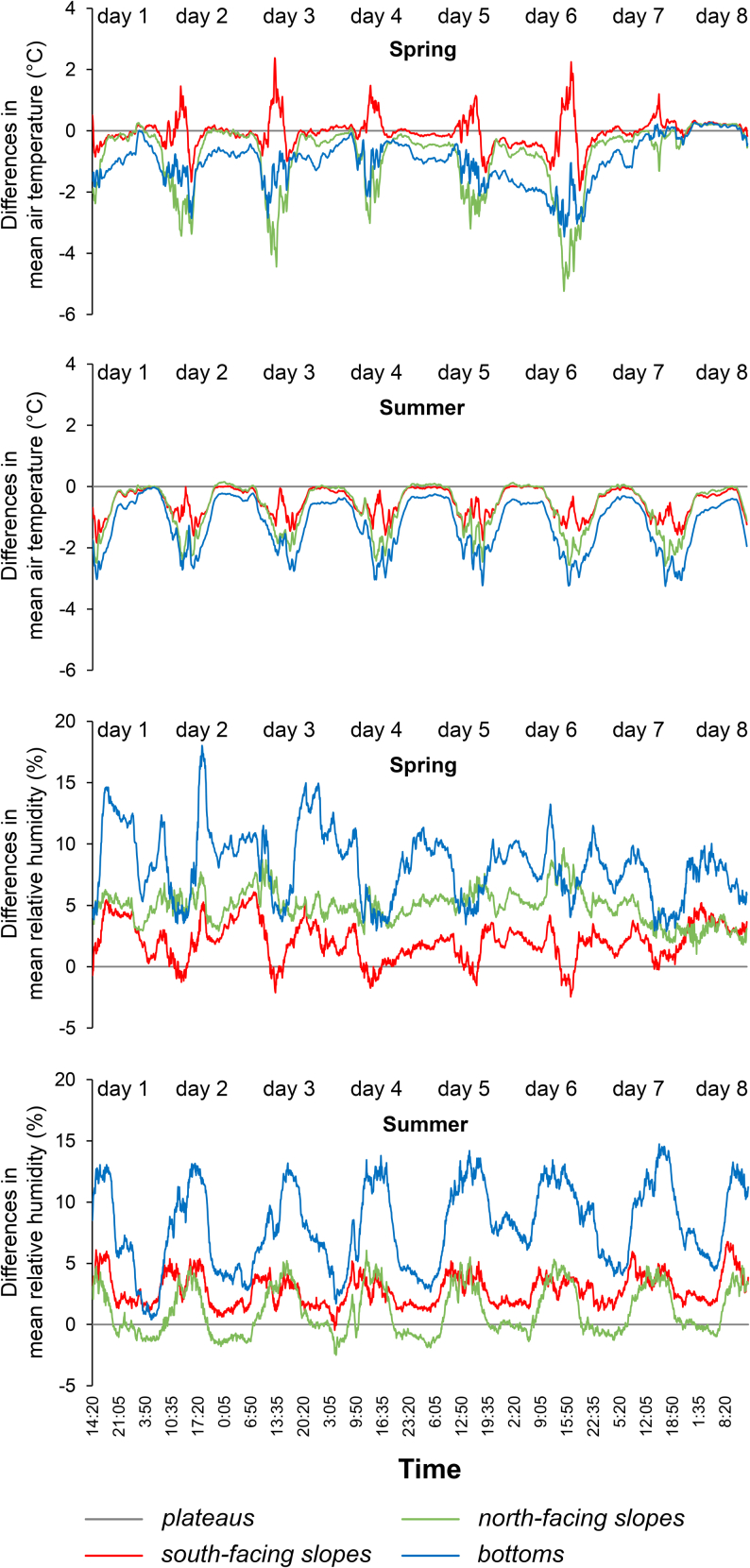
Figure 2.
General differences in mean air temperature and relative air humidity between the plateau and doline microsites in spring and summer
Air temperature and relative air humidity values were averaged across the same microsites at each measurement time point of the weeks, respectively. Negative and positive temperature and relative air humidity values indicate that doline microsites (south-facing slopes, north-facing slopes, and bottoms) had lower or higher values than the plateau (adjusted to 0) at particular times of the investigation periods, respectively. The dates of measurements in the Mecsek Mountains and Aggtelek Karst are provided in Methods and Table S1.
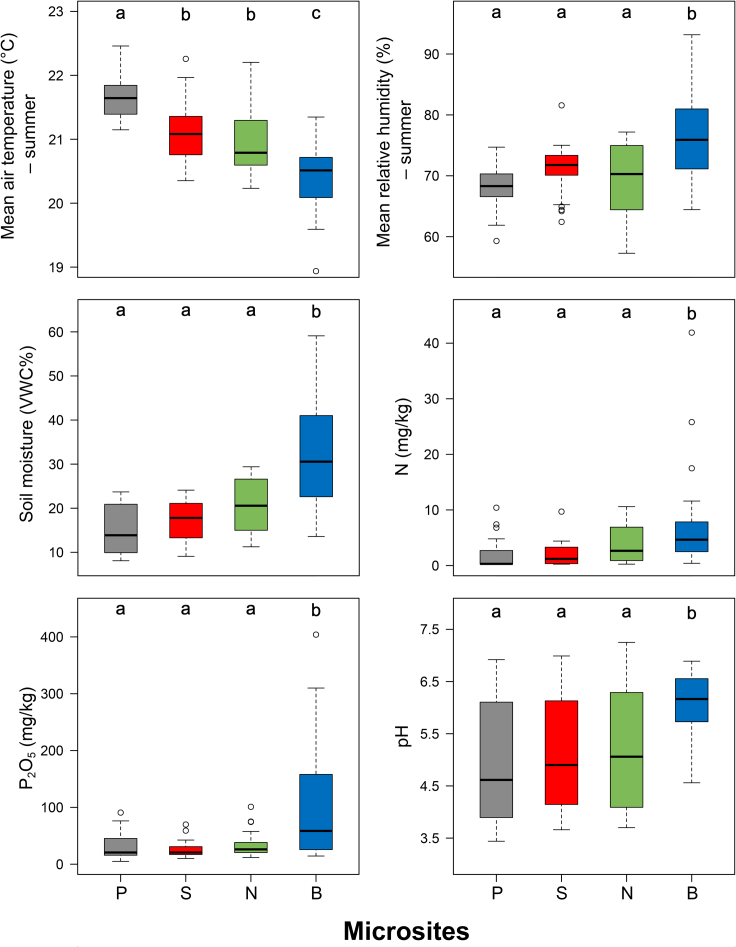
Figure 3.
Microclimatic variables and soil properties in the plateau and doline microsites
Different lowercase letters (a–c) indicate significant differences (p < 0.05) among microsites (P: plateaus, S: south-facing doline slopes, N: north-facing doline slopes, and B: doline bottoms).
In spring, mean daytime air temperature was significantly lower on north-facing slopes and bottoms than on south-facing slopes and the plateaus, while bottoms were cooler than the other microsites during the night. South-facing slopes were the warmest and north-facing slopes were the coolest around noon, while bottoms were generally cooler than the plateaus and south-facing slopes throughout the day. The largest temperature difference (6.2°C) was observed between the two slopes.
In summer, mean air temperature and mean daytime air temperature were significantly higher on the plateaus and lower in bottoms than in the other microsites. Mean night-time air temperature was lower in bottoms than in the other microsites. The largest temperature difference was 2.5°C between the two slopes. Bottoms were consistently cooler than the other microsites.
In spring, mean night-time relative humidity was significantly lower on the plateaus than on north-facing slopes and in bottoms. Bottoms showed significantly higher relative humidity values than the other microsites during the night. The largest relative humidity difference (18%) was observed between bottoms and the plateaus. Relative humidity was lowest on south-facing slopes around noon and it showed intermediate values on north-facing slopes throughout the day.
In summer, mean relative humidity was significantly higher in bottoms than in the other microsites. Mean night-time relative humidity was significantly higher in bottoms than on north-facing slopes and the plateaus. Relative humidity was lowest on north-facing slopes during night-time and it showed similar patterns on south-facing slopes and north-facing slopes during daytime. The largest relative humidity difference (14.7%) was observed between bottoms and the plateaus.
General differences in mean air temperature between microsites and open environments (i.e., climate stations) were larger in summer (plateaus – climate stations: 1.2°C, south-facing slopes – climate stations: 1.7°C, north-facing slopes – climate stations: 1.9°C, and bottoms – climate stations: 2.4°C) than in spring (plateaus – climate stations: 0.6°C, south-facing slopes – climate stations: 0.6°C, north-facing slopes – climate stations: 1.4°C, and bottoms – climate stations: 1.5°C). General differences in mean relative humidity between spring (plateaus – climate stations: 8%, south-facing slopes – climate stations: 10%, north-facing slopes – climate stations: 13%, and bottoms – climate stations: 16%) and summer (plateaus – climate stations: 7%, south-facing slopes – climate stations: 10%, north-facing slopes – climate stations: 9%, and bottoms – climate stations: 15%) were less pronounced (see also Table S1).
Microsite had a significant effect on soil moisture (F = 27.52, p < 0.001), soil N content (F = 6.69, p < 0.001), soil P2O5 content (F = 7.07, p < 0.001), and soil pH (F = 7.33, p < 0.001). Soil moisture was highest in bottoms, while it did not differ among the other microsites (Figure 3 and Table S1). This is also true for soil N and P2O5 contents, and soil pH. We did not find any difference among microsites with respect to soil K2O content (F = 0.73, p = 0.431).
Species composition
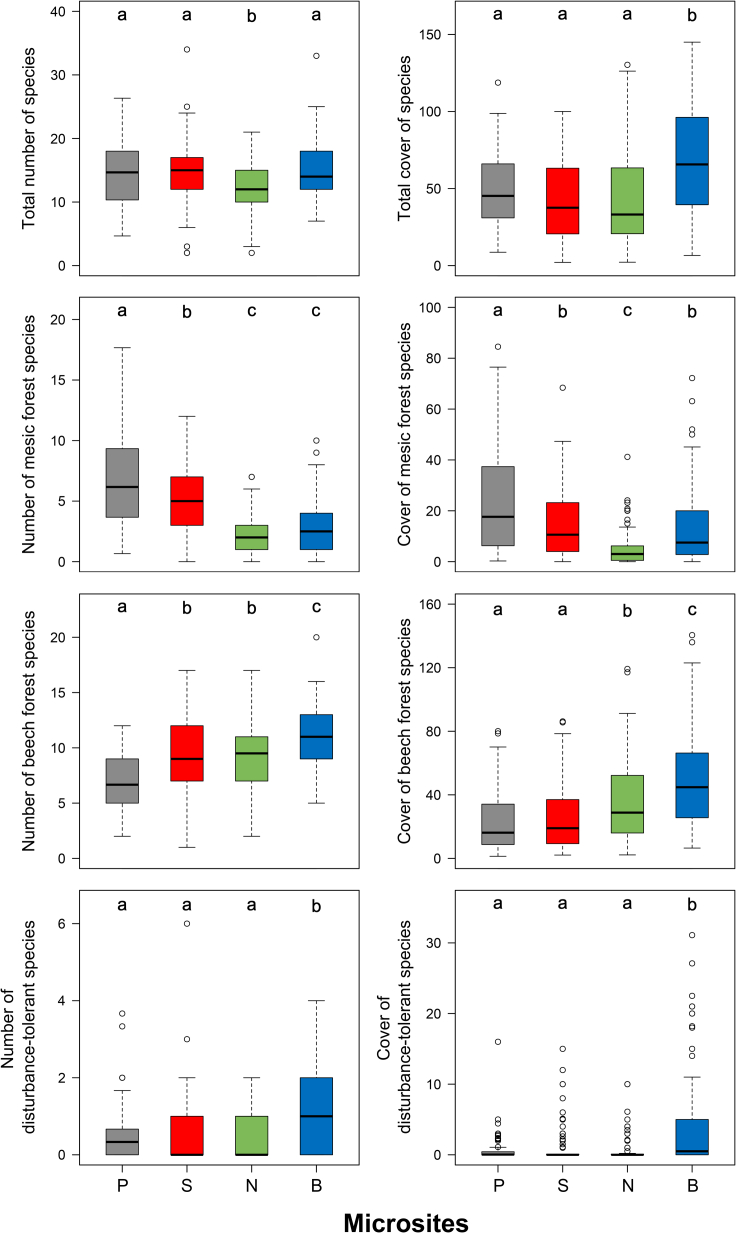
A total of 189 vascular plant species were recorded in the plots of the two study areas. Microsite had a significant effect on the total number and total cover of species (F = 13.08 and 21.92, respectively, p < 0.001), on the number and cover of mesic forest species (F = 122.27 and 35.80, respectively, p < 0.001), beech forest species (F = 48.14 and 49.04, respectively, p < 0.001), disturbance-tolerant species (F = 24.53 and 23.71, respectively, p < 0.001), and on the number of species of high conservation value (F = 6.86, p < 0.001). However, microsite did not have a significant effect on the cover of species of high conservation value (F = 0.43, p = 0.729).
The total number of species was lowest in the plots of north-facing slopes, while the plots of other microsites showed similar values (Figure 4 and Table S1). In terms of diagnostic species, the plateaus had 33 (mesic forest species: 85%, and beech forest species: 15%), south-facing slopes had 8 (mesic forest species: 50%, and beech forest species: 50%), north-facing slopes had 5 (beech forest species: 100%), and bottoms had 24 (mesic forest species: 8%, beech forest species: 71%, and disturbance-tolerant species: 21%) species (Table 1). The total cover of species was similar in the microsites, except in the bottoms, where significantly higher values were observed (Figure 4 and Table S1). The number and cover of mesic forest species were highest on the plateaus and significantly higher on south-facing slopes than on north-facing slopes. The number of beech forest species was highest in bottoms and lowest on the plateaus, while their cover was highest in bottoms and lowest on south-facing slopes and the plateaus. Bottoms contained significantly more disturbance-tolerant species than the other microsites. South-facing slopes and north-facing slopes had more species of high conservation value than the plateaus (Table S1).
Figure 4.
Number and cover of vascular plant species in the plateau and doline microsites
Different lowercase letters (a–c) indicate significant differences (p < 0.05) among microsites (P: plateaus, S: south-facing doline slopes, N: north-facing doline slopes, and B: doline bottoms).
Table 1.
Synoptic table of vascular plants associated with the plateau and doline microsites (south-facing slopes, north-facing slopes, and bottoms)
| Diagnostic species |
|---|
| Plateau |
| Dactylis glomerata subsp. lobatam (51.8), Waldsteinia geoidesm (39.9), Brachypodium sylvaticumm (34.7), Melica unifloram (31.4), Quercus petraeam (30.1), Viola hirtam (28.1), Carpinus betulusb (28.0), Rosa canina agg.m (27.7), Cruciata glabram (27.2), Carex brevicollism (26.7), Cornus masm (26.1), Aegonychon purpurocaeruleumm (23.6), Carex pilosab (23.2), Clinopodium vulgarem (23.2), Carex divulsam (22.6), Crataegus monogynam (22.3), Viola odoratam (21.6), Galium intermediumm (21.1), Stellaria holosteam (20.3), Fragaria vescam (20.1), Geum urbanumm (19.9), Glechoma hirsutab (18.2), Ruscus hypoglossumb (18.2), Euphorbia amygdaloidesb (17.9), Tanacetum corymbosumm (17.6), Ligustrum vulgarem (16.9), Campanula bononiensism (16.8), Viola mirabilism (16.8), Melica nutansm (16.7), Quercus cerrism (16.2), Potentilla micrantham (16.1), Fraxinus ornusm (15.7), Crataegus laevigatam (15.1) |
| South-facing slope |
| Veronica chamaedrysm (22.7), Galium odoratumb (21.6), Primula vulgarisb (21.6), Dioscorea communism (21.6), Helleborus odorusb (20.6), Acer campestrem (20.3), Clematis vitalbam (18.4), Viola reichenbachianab (15.9) |
| North-facing slope |
| Mercurialis perennisb (27.0), Acer pseudoplatanusb (21.7), Oxalis acetosellab (21.4), Erythronium dens-canisb (17.8), Prenanthes purpureab (17.5) |
| Bottom |
| Urtica dioicad (49.0), Athyrium filix-feminab (42.2), Dryopteris filix-masb (35.4), Chrysosplenium alternifoliumb (31.1), Geranium robertianumm (29.9), Galium aparined (29.0), Veronica montanab (29.0), Corydalis solidab (26.9), Corydalis cavab (26.8), Ulmus glabrab (26.2), Aegopodium podagrariab (25.2), Ranunculus ficariab (24.0), Stachys sylvaticab (24.0), Alliaria petiolatad (23.2), Actaea spicatab (21.6), Anemone ranunculoidesb (20.3), Circaea lutetianab (19.9), Maianthemum bifoliumb (19.7), Pulmonaria obscurab (19.5), Stellaria mediad (17.5), Moehringia trinerviam (16.2), Festuca giganteab (15.9), Lathraea squamariab (15.4), Sambucus nigrad (15.4) |
Within blocks, species are listed by decreasing values of the phi (Φ) coefficient of association between species and microsite (in parentheses, Φ × 100). Mesic forest species, beech forest species and disturbance-tolerant species are indicated with superscripts m, b and d, respectively. Non-diagnostic species were excluded with Fisher’s exact test (p < 0.05).
Species–environment relationships
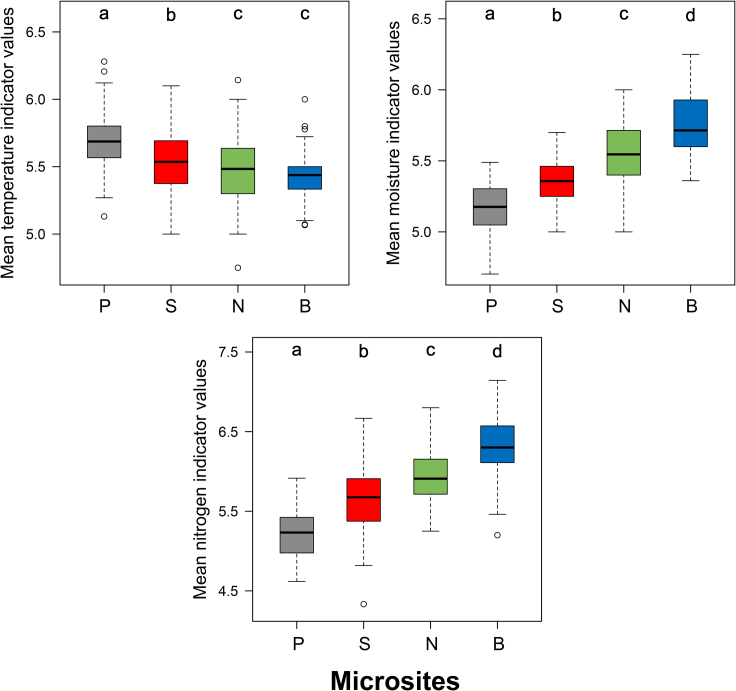
Microsite had a significant effect on mean EIVs for temperature (F = 56.40, p < 0.001), moisture (F = 351.44, p < 0.001), and soil nitrogen (F = 288.82, p < 0.001). Species on the plateaus indicated the warmest and north-facing slopes and bottoms indicated the coolest conditions (Figure 5 and Table S1). All microsites differed from each other in respect to moisture indicator values, indicating steep moisture gradients from the plateaus to bottoms. The same pattern was observed when soil nitrogen indicator values were compared among microsites. Species in bottoms indicated the highest and species on the plateaus indicated the lowest nitrogen availability (Figures 5 and 6).
Figure 5.
Mean Ellenberg indicator values in the plateau and doline microsites
Lowercase letters (a–d) indicate significant differences (p < 0.05) among microsites (P: plateaus, S: south-facing doline slopes, N: north-facing doline slopes, and B: doline bottoms).
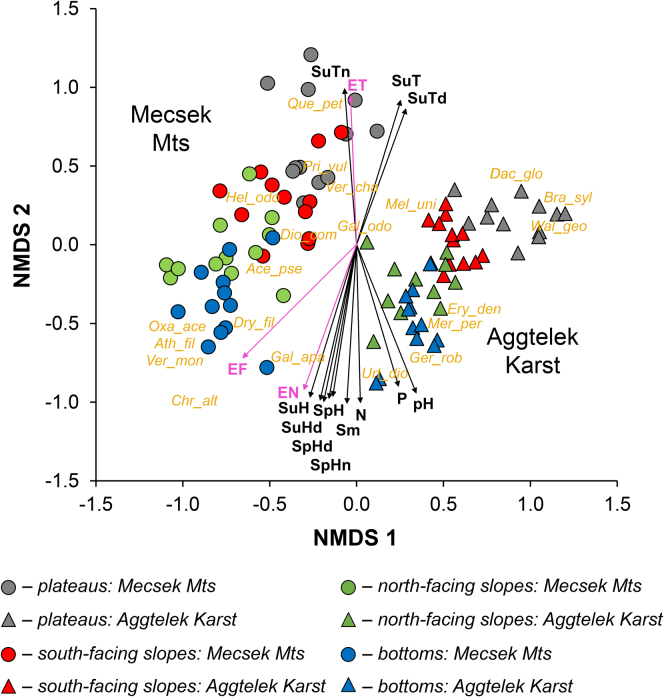
Figure 6.
Compositional patterns and vegetation–environment relationships in the plateau and doline microsites
Fitted vectors show correlations between NMDS axial scores and potential predictors (SuT: Mean air temperature (summer), SuTd: mean daytime air temperature (summer), SuTn: mean night-time air temperature (summer), SpH: mean relative humidity (spring), SuH: mean relative humidity (summer), SpHd: mean daytime relative humidity (spring), SuHd: mean daytime relative humidity (summer), SpHn: mean night-time relative humidity (spring), pH: soil pH, Sm: soil moisture, P: soil P2O5 content, N: soil (NO2– + NO3–)-N content, ET: mean Ellenberg indicator values for temperature, EW: mean Ellenberg indicator values for moisture, and EN: mean Ellenberg indicator values for soil nitrogen, p < 0.05). Arrow directions indicate the direction of the correlation, while vector length shows the strength of correlation. Abbreviated names of some diagnostic species are also plotted (see Table 1).
NMDS ordination of plots (stress factor: 0.12) showed that the two karst areas separated from each other (Figure 6). However, NMDS revealed very similar environmental gradients (from the plateaus to bottoms) for both areas in the ordination space. Differences in species assemblages among microsites were significant (permutational multivariate analysis of variance [PERMANOVA] F = 17.25, p < 0.001, Table 2). Mean air temperature (summer), mean daytime air temperature (summer), mean night-time air temperature (summer), mean relative humidity (spring and summer), mean daytime relative humidity (spring and summer), mean night-time relative humidity (spring), soil pH, soil moisture, soil P2O5 content, soil N content, and all mean EIVs were significantly related to the ordination (R2 = 0.14–0.84, p < 0.01, Table S1). Species assemblages on north-facing slopes and bottoms were associated with lower air temperature and higher relative humidity values, and higher soil moisture, soil pH, soil P2O5 and soil N contents than assemblages on south-facing slopes and the plateau.
Table 2.
Pairwise comparisons of species assemblages in the plateau (P) and doline microsites (S: south-facing slopes, N: north-facing slopes, and B: bottoms) with permutational multivariate analysis of variance (PERMANOVA)
| F | p | |
|---|---|---|
| B – N | 7.09 | <0.001 |
| B – P | 23.49 | <0.001 |
| B – S | 13.67 | <0.001 |
| N – P | 23.49 | <0.001 |
| N – S | 7.94 | <0.001 |
| P – S | 9.47 | <0.001 |
Discussion
We found that both microclimatic and resource availability variations are significantly associated with vegetation patterns in topographically complex karst landscapes and that doline microsites provide favorable conditions for a large suite of vascular plant species generally restricted to certain microsites in the studied regions. Doline bottoms in forested karst landscapes not only provide “cool islands” but also have the potential to capture and store substantially increased volumes of water and nutrients in their soil. This is the first study that has analyzed the association among topography-related microsite diversity, microclimate, resource availability, species composition and vegetation patterns inside and outside dolines using a large set of abiotic and biotic data collected from distant geographic locations. Our results are also novel with respect to the ecology of dolines. Although previous studies have demonstrated that the vegetation pattern of such depressions may markedly differ from that of the surrounding landscape, we know little about the small-scale vegetation patterns within them. Similarly, although studies conducted by early researchers found that low elevation depressions in Europe may provide important habitats for high-elevation and/or high-latitude species, the complex links between species occurrences, microclimate and soil properties have rarely been studied. In the subsections below, we further elaborate on the implications of these key findings.
Microclimate and resource availability
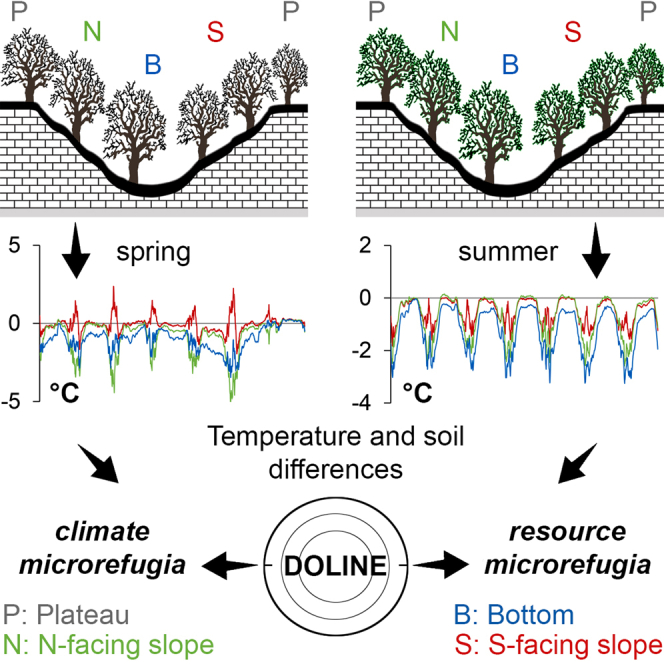
Although global atmospheric circulation patterns substantially influence macroclimatic conditions (e.g., temperature regimes), regional topography also has an impact on these global climatic patterns, and variations in local topography may lead to a broad range of microclimates.9,41 According to Keppel et al.,2 decoupling from regional climates (i.e., thermal stability), spatial variation in microclimatic factors (i.e., high diversity of microclimates) and the compounding effects of topography and climate (e.g., free-air advection and sheltered topographic depressions) are key factors in producing climate microrefugia from prevalent climate conditions. In addition, cold air pooling—a globally widespread phenomenon that sustains low temperatures in convergent environments, such as basins, topographic depressions and valleys13—results in steep temperature gradients over short distances. We found that the lowest parts of topographic depressions (i.e., doline bottoms) in the studied karst landscapes consistently promoted cold air drainage and pooling, as well as high relative humidity over the investigation periods both in spring and summer (Figures 2 and 3); however, different microclimatic patterns were observed on the slopes during the different seasons. In spring, we observed a variation of 6.2°C from north- to south-facing slopes, and dolines showed similar microclimatic patterns to those previously measured in grassland dolines, in which south-facing slopes are considerably warmer and north-facing slopes are considerably cooler around noon than the surrounding microsites.30,42 However, the maximum temperature difference found between the two slopes was only 2.5°C in summer (Figure 2). The main reason for this is the complete absence of canopy cover in spring, allowing south-facing slopes to receive high levels of direct solar radiation during daytime.43 Although summer temperature differences among microsites seem to be relatively low compared to spring differences, they may be especially important for the persistence of cold-adapted species. This microclimatic variability was also indicated by the temperature indicator values of vascular plant species (Figure 5). Previous microclimatic studies over various time periods also indicate some doline microsites (both in grassland and forested dolines) to be cooler, wetter and more humid than the surrounding plateau.11,22,32 Our results also support a growing body of evidence that topographic complexity in local depressions increases the microclimatic variability of an area over small spatial scales.11,32,44,45
Understanding limiting resources in particular landscapes can also assist the identification and conservation of species that would become extinct in future environmental changes.2 In landscapes where water becomes limiting, wet microenvironments play a crucial role in species persistence. Wet microenvironments often arise from topographic heterogeneity that enhances water inputs or reduces water losses from the soil.6,46 Although dolines get the same amount of precipitation as their surroundings, we found that doline bottoms have the capacity to retain more water than their surroundings and therefore act as “wet islands” within karst landscapes (Figure 3), potentially enhancing water availability for species. In addition to the concave topography, cool temperature, reduced wind speed, and thicker soil may enhance water retention in these sheltered topographic depressions.11,47 We also found that doline bottoms also have the potential to accumulate high amounts of nutrients (P2O5, and N) in their soil. The existence of steep moisture and nutrient gradients along doline slopes was also confirmed by the indicator values of vascular plant species (Figure 5). Our results suggest that the distribution patterns of soil moisture and nutrients may be governed by similar mechanisms in dolines. Runoff volume, litter accumulation, and soil redistribution (i.e., erosion on slopes) presumably increase from top to bottom in topographic depressions,11,37,48 resulting in wet and nutrient-rich conditions in bottoms. Soil pH was also higher in doline bottoms than in the other microsites. Cation leaching from the upper slopes and the decomposition of litter through the release of basic cations may potentially increase soil pH in this microsite. To sum up, our results suggest that topographic complexity may have significant impacts on the interactions between climate, soil and hydrologic mechanisms within dolines, which in turn may have significant consequences for biodiversity.
Biodiversity and conservation
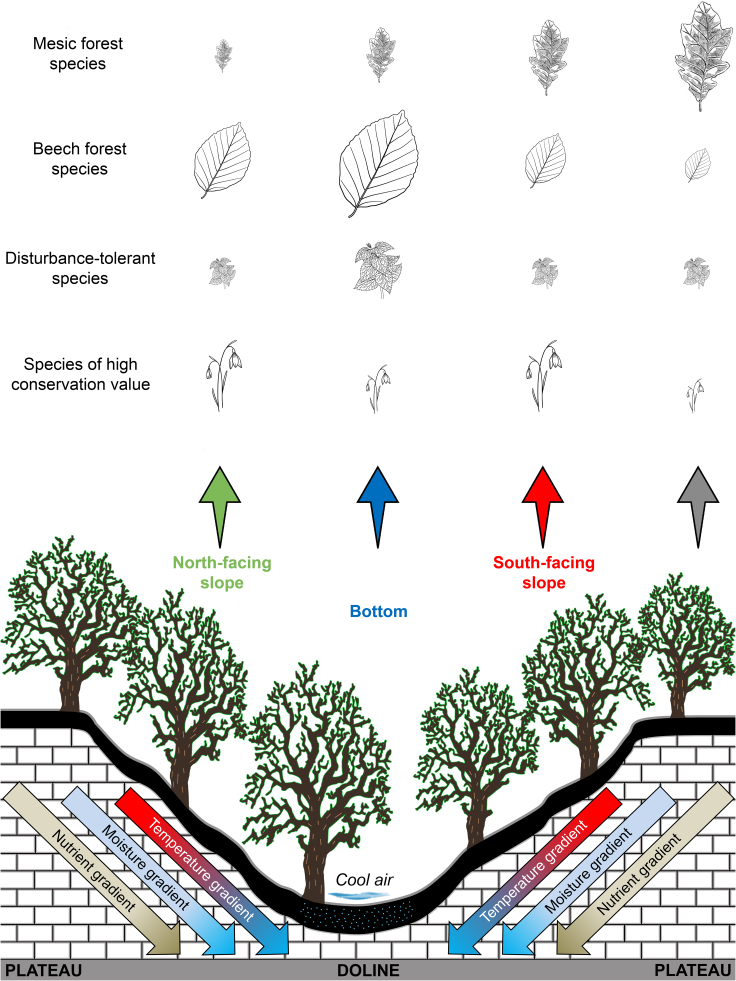
Given the high variation of microclimate and resource availability in dolines described above, doline microsites—independently of the types of regional climate15,28,29,32,37,49—allow the maintenance of various species in a given landscape (Figure 7). We found a total of 189 vascular plant species in the microsites, sampling a total area of only 2,160 m2 (our dataset contained 540 2 m × 2 m vegetation plots). All doline microsites provided safe havens for at least some species that are rare or absent in the plots of surrounding microsites (Table 1 and Figure 4), indicating the importance of microsite patterns for the survival of these species in the current climate. Within dolines, the warmer, drier and less nutrient-rich south-facing slopes provided suitable conditions for a number of mesic forest species (e.g., Acer campestre and Veronica chamaedrys), while the cooler, wetter and more nutrient-rich north-facing slopes and bottoms provided suitable conditions for a number of beech forest species (e.g., Athyrium filix-femina and Prenanthes purpurea). Presumably, natural disturbances (i.e., soil profile inversion due to uprooting trees) and high resource availability in doline bottoms may also support the maintenance of some disturbance-tolerant and/or nitrophilous species (e.g., Galium aparine and Urtica dioica).50 The dissimilarity in species assemblages was so large presumably because assemblages in dolines were not nested subsets of the assemblages on the plateau.51 Dolines may also provide the only or the main habitat for some rare and endangered species within a landscape.31,33,42 Our results confirmed and extended the results of previous findings that topographic depressions throughout the world—including dolines—may act as important biodiversity reservoirs7,28,42,52,53 and may function as “small natural features” with a disproportionately large role in maintaining local biodiversity relative to their size54 (Figure 7).
Figure 7.
Schematic illustration of the environmental conditions and species composition of the plateau and doline microsites
Different doline microsites (south- and north-facing slopes, and bottoms) and the surrounding plateau exhibit steep temperature, moisture and nutrient gradients and promote a high diversity of species groups. Illustration size for different plant species groups indicates their importance in microsites (smaller illustrations: lower importance, and larger illustrations: higher importance). Conservation of these local biodiversity hotspots is critical to maintain the resistance and resilience of karst landscapes to global warming.
We also found more species of high conservation value in some doline microsites (south- and north-facing slopes) than on the surrounding plateaus. The reason why a higher number of protected and/or red-listed species occur in these microsites is presumably due to the rare and opposing environmental conditions (warm and dry vs. cool and wet) that prevent a species from thriving everywhere in the studied karst areas (i.e., under intermediate temperature and moisture conditions). Therefore, both south- and north-facing doline slopes may provide safe havens for at least some protected and/or red-listed vascular plant species with a restricted distribution (south-facing slopes: e.g., Dioscorea communis, and north-facing slopes: e.g., Erythronium dens-canis). These results also support the view that topographically complex landscapes constitute a high conservation priority as key areas for the conservation of species diversity.7,55
Doline microrefugia in changing environment
Understanding how climate and resources influence species distributions has taken on a renewed urgency as many parts of the Earth are getting warmer and drier, influencing the persistence of biodiversity.41,56 We found that topographic depressions in karst landscapes harbor appropriate conditions for many vascular plant species adapted to cooler, moister and/or nutrient-rich conditions (Figures 4 and 6), providing climatic and resource-rich “islands” in the “ocean” of habitats that increasingly fail to provide these conditions. In addition, our results also suggest that these topographically complex landforms may reduce the distances that species must move to track suitable conditions under environmental changes.57 The presence of various species with restricted distributions (e.g., climate relicts) in dolines suggests that many of them function as contemporary microrefugia.15,31 However, microrefugia that currently buffer species from environmental extremes may not be able to do so in the future.58 Contemporary and future microrefugia must satisfy multiple conditions (i.e., climate and resource availability, disturbances, species attributes, and species interactions) that will determine the capacity of microrefugia, or their ability to support species persistence.6,59 These conditions interact to influence the distribution of species. Stable microclimatic conditions and/or persistent elevated resources are a necessary—but not sufficient—condition for a site to serve as a climatic and/or resource microrefugium. This ability also strongly depends on whether species are able to persist in such environments, while being excluded from the surrounding landscape. The functional attributes of species, such as physiological, demographic, and morphological traits, must be compatible with site conditions, and species interactions, such as competition and mutualism, must enable the target species to persist in, or disperse across such locations.6,60,61 For instance, species with higher specific leaf area (SLA) may prefer the more productive environments in dolines (cf. Dainese and Bragazza62); however, the number and strength of competitive interactions may increase as a response to drought.6 In addition, local disturbances may also influence the ability of species to access microrefugia. For instance, Bátori et al.63 found that forestry activity may influence the capacity of dolines to support species associated with wet conditions, as the dense and homogeneous canopy can significantly reduce the amount of water reaching the soil in the first decades of the forest regeneration phase. Although the results of previous studies suggest that dolines have the potential to maintain stable environmental conditions for a longer time period,11,32 further studies are required to understand fully how they may be affected by additional changes in environmental conditions, such as global warming and local disturbances.
Local anthropogenic disturbances, such as forest management, may have a detrimental impact on species persistence in a changing climate. For instance, canopy removal in dolines influences the light regime, microclimate, soil characteristics, species composition and community structure of microsites49,64 and their capacity to provide safe havens for climate change-sensitive species. As the combined effect of canopy cover and topographic concavity strongly influences the buffering and decoupling capacities of habitats,65 it would be desirable to introduce close-to-nature forestry in forested dolines and their surroundings in as many karst landscapes as possible to maintain unique environmental characteristics and species composition, and to keep pace with high rates and magnitudes of global warming (cf. Lenoir et al.12; Bátori et al.38). Establishing climate-smart conservation priorities and strategies are also required to maintain or increase resistance and resilience of karst landscapes and their contemporary and potential future microrefugia to climate change.41,66
Limitations of the study
Although our results clearly indicate that the presence of diverse microclimates and soil properties in topographic depressions can maintain various plant assemblages and species vulnerable to climate change, long-term studies would be required to reveal how regional temperature increase will influence the environmental characteristics and refugial capacity of such habitats. The results of these studies would allow the quantification of adaptive potentials and environmental niches of the investigated species.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| R package ‘lme4’ | Bates et al.67 | https://cran.r-project.org/web/packages/lme4/index.html |
| R package ‘car’ | Fox and Weisberg68 | https://cran.r-project.org/web/packages/car/index.html |
| R package ‘emmeans’ | Lenth69 | https://cran.r-project.org/web/packages/emmeans/index.html |
| R package ‘vegan’ | Oksanen et al.70 | https://cran.r-project.org/web/packages/vegan/index.html |
| JUICE | Tichý et al.71 | https://www.sci.muni.cz/botany/juice/ |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Zoltán Bátori (zbatory@gmail.com).
Materials availability
This study did not generate new unique materials.
Experimental model and subject details
No experimental model was used in this work.
Method details
Study regions
The study was conducted in a karst area of the Mecsek Mountains (near the village of Orfű, southern Hungary; 46°7'18.49"N 18°8'21.26"E; total area of the karst region: 32 km2) and in a karst area of the Aggtelek Karst (near the village of Jósvafő, northern Hungary; 48°30'37.56"N 20°33'59.27"E; total area of the karst region: 126 km2) (Figure 1A). The karst region of the Mecsek Mountains has hundreds of solution dolines (i.e. bowl- or funnel-shaped depressions formed by the dissolution of limestone at the bedrock–soil interface23), between 300–500 m asl. Most dolines are small (diameter: less than 20 m; depth: less than 5 m), while the diameter of the largest doline is about 200 m and its depth exceeds 30 m. The climate is sub-continental with sub-Mediterranean influences. The mean annual precipitation is 700–750 mm and the mean annual temperature is 9.5°C. The surface of the Aggtelek Karst is also characterized by hundreds of solution dolines, located between 300–600 m asl. Dolines are usually larger than in the Mecsek Mountains; the diameter of the largest doline is about 350 m and its depth exceeds 50 m. The climate is sub-continental with a mean annual precipitation of 640–700 mm and a mean annual temperature of 8°C.72 The dominant soil types in the study area of the Mecsek Mountains are (Leptic) Luvisols, while Rendzic Leptosols are the dominant soil types in the study area of the Aggtelek Karst.73
Both study areas are part of the Natura 2000 network (a large protected area network that was created to protect Europe's threatened species and habitats). The karst region in the Mecsek Mountains is managed by the Danube-Drava National Park Directorate, while the Aggtelek National Park Directorate is responsible for the management of the Aggtelek Karst. Oak-hornbeam forest (Asperulo taurinae-Carpinetum, Natura 2000 code: 91L0) is the dominant vegetation type on the karst plateaus in the Mecsek Mountains, while the bottoms of dolines are covered by small patches of beech forests (Helleboro odoro-Fagetum, Natura 2000 code: 91K0) or ravine forests (Scutellario altissimae-Aceretum, Natura 2000 code: 9180). Large dolines provide safe havens for rare and endangered plant species, such as Aconitum vulparia, Dryopteris dilatata and Stachys alpina.38 Oak-hornbeam forest (Carici pilosae-Carpinetum, Natura 2000 code: 91G0) and rocky forest (Tilio-Fraxinetum excelsioris, Natura 2000 code: 9180) are the dominant vegetation types on the plateaus of the Aggtelek Karst within the study area, while the slopes and bottoms of dolines are covered by patches of beech forest (Melittio-Fagetum, Natura 2000 code: 9130) or scree forest (Mercuriali-Tilietum, Natura 2000 code: 9180). These dolines also act as safe havens for rare and endangered plant species, such as Daphne mezereum, Dryopteris dilatata, and Rosa pendulina74 (Figures 1B–1J). Further information about the structure of these forests can be found in Bátori et al.38,63 and Virók et al.75
Sampling design
Fifteen large solution dolines (diameters: 80–150 m, depths: 10–20 m) were randomly selected within both study areas (Mecsek Mountains and Aggtelek Karst, 30 dolines in total). The age of forests in dolines and their surroundings was 100–120 years in the Aggtelek Karst and 70–80 years in the Mecsek Mts. Canopy cover for all investigated dolines and their surroundings was similar within a study area; it ranged between 80–95% in the Mecsek Mountains and between 75–90% in the Aggtelek Karst. Therefore, forest structure may have had very similar effects on understorey vegetation. Based on previous research,30 four microsites were investigated for each doline: the south-facing slope, north-facing slope and bottom of doline, and the surrounding plateau (Figure 1B). Six sampling locations were established in these microsites: one on the central part of the south-facing slope, one on the central part of the north-facing slope, one in the doline bottom, and three randomly selected locations on the surrounding plateau (2 study areas × 15 dolines × 6 locations = 180 locations in total). Sampling locations on the plateau were at least 25 m from each other and between 10 and 50 m from the edge of doline. Three 2 m × 2 m permanent plots were established at each location for vegetation sampling (180 locations × 3 plots = 540 vegetation plots in total). Vegetation sampling was carried out in two different seasons – in spring (before canopy closure): March–April, and in summer (after canopy closure): June–August. The percentage cover of each vascular plant species (herbs, shrubs, and tree saplings) was estimated visually in each plot. Of the spring and summer species cover values, the larger value of each species was used for subsequent data analyses (e.g., Erdős et al.76). The study was carried out in 2019 in the Mecsek Mountains, and in 2021 in the Aggtelek Karst. The nomenclature follows the World Flora Online (http://www.worldfloraonline.org/).
To reveal the microclimatic patterns of the microsites, we measured air temperature (°C) and relative air humidity (%) in 12 of the 15 dolines in both study areas in 2019 (Mecsek Mountains) and in 2021 (Aggtelek Karst). We performed the measurements using Optin Ambient Data Loggers (ADL TH3-32; accuracy: ±0.2°C for temperature, and ±1.8% for relative humidity) every 5 minutes over a one-week period in spring (before canopy closure in March–April; Mecsek Mountains: 11.03.2019–19.03.2019, Aggtelek Karst: 27.03.2021–03.04.2021) and summer (after canopy closure in July–August; Mecsek Mountains: 16.07.2019–23.07.2019, Aggtelek Karst: 07.08.2021–16.08.2021) at each location of doline microsites (south-facing slope, north-facing slope, and bottom), and at one plateau location per doline (2 study areas × 2 seasons × 12 dolines × 4 locations × 1 logger = 192 measurements in total). Loggers were encased in a radiation shield and they were suspended 10 cm above the ground (Figure S1). Measurements were carried out under similar weather conditions in both karst areas. To provide information on the thermal buffering capacity of forests in both areas, mean air temperature and mean relative air humidity data were obtained from nearby climate stations (Mecsek Mountains: Pécs, and Aggtelek Karst: Jósvafő; https://www.ogimet.com).
Soil moisture (i.e. volumetric water content, VWC%) was measured in summer after a dry period in the upper 12.2 cm of the soil using a FieldScout TDR 350 Soil Moisture Meter (rod length: 12.2 cm) at the same locations – 5 measurements per location – where microclimate was measured (2 study areas × 12 dolines × 4 locations × 5 measurements = 480 measurements in total). Location-averaged soil moisture data were used for subsequent data analyses. Soil samples were also collected from these locations – 3 samples per location – in summer from the upper 15 cm of the soil (2 study areas × 12 dolines × 4 locations × 3 samples = 288 soil samples in total). Soil samples from each location were pooled and homogenized before soil chemical analysis. The following soil properties were determined in an accredited laboratory (accreditation number: NAH-1-1437/2018): pH (H2O), CaCO3 (m/m%), humus (m/m%), P2O5 (mg/kg), K2O (mg/kg), and nitrogen forms (NO2– + NO3–)-N (mg/kg) (henceforth N). Prior to analyses, soil samples were dried in the laboratory. Soil pH was measured in aqueous solution (1:2.5 soil/distilled water ratio) using a WTW InoLab pH 720 digital pH meter. Carbonate content was determined using a Scheibler calcimeter measuring the volume of the released CO2 during hydrochloric acid (HCl) treatment. Humus content was assayed with a Helios Gamma spectrophotometer after sulphuric acid (H2SO4) digestion in the presence of potassium dichromate (K2Cr2O7). P2O5 and K2O were extracted using ammonium-lactate (AL), while nitrogen forms (NO2−+ NO3−)-N were extracted with a potassium chloride (KCl) solution. Soil N and P2O5 were then determined by a Foss FIAstar 5000 flow injection analyser, while soil K2O was determined by a Perkin Elmer Optima 7000DV ICP-OES spectrometer.77
Species classification
We classified all vascular plant species according to their habitat preferences based on the system of Soó.78 Habitat preference refers to the habitat or habitat group where the species most often occurs in the study regions. To identify protected and red-listed species (henceforth ‘species of high conservation value’), we used the Database of Hungarian Natural Values (www.termeszetvedelem.hu) and Király.79 The following four groups of plant species were analysed: (1) mesic forest species (species of Querco-Fagetea), (2) beech forest species (species of Fagetalia sylvaticae), (3) disturbance-tolerant species (adventives, cosmopolitan species and weeds – such as species of Epilobietea angustifolii), and (4) species of high conservation value. Beech forest species were considered the main target group, as the ongoing climate change threatens their populations in many parts of Europe.80,81
Quantification and statistical analysis
Air temperature and relative air humidity values were averaged across the same microsites at each measurement time point, respectively. For visualising general microclimatic differences between the plateau and doline microsites, we subtracted plateau values from the values of doline microsites (south-facing slopes, north-facing slopes, and bottoms, respectively) for every measurement time point. We also determined general differences in air temperature and relative air humidity between microsites and climate stations.
Prior to analysis, we classified each plant species according to its Ellenberg-type indicator values for temperature (ET), moisture (EF) and soil nitrogen (EN), using the system of Borhidi.82 Ellenberg indicator values express the realized optimum of the species on a nine-degree (ET and EN) or twelve-degree (EF) ordinal scale defined along environmental gradients. Unweighted mean Ellenberg indicator values (EIVs hereafter) for temperature, moisture and soil nitrogen were calculated for each plot. According to the findings of previous studies,83,84 mean EIVs provide reliable estimates of the environmental conditions of an area and provide a useful tool for investigating environmental gradients.
We used linear mixed-effects models (LMMs) to test the effects of microsites (plateau, south-facing slope, north-facing slope, and bottom) on microclimatic conditions (mean air temperature, mean daytime air temperature, mean night-time air temperature, mean relative humidity, mean daytime relative humidity, and mean night-time relative humidity in spring and summer), as well as soil properties (pH, moisture, CaCO3, humus, P2O5, K2O and N) and mean EIVs (temperature, moisture, and soil nitrogen values). Daytime was defined as the time between sunrise and sunset, while night-time was the time between sunset and sunrise. We assessed the multicollinearity among the measured environmental variables by computing the variance inflation factor (VIF). Values of VIF exceeding 5 were considered evidence of collinearity. Variables with VIF < 5 (mean daytime air temperature in spring: VIF = 2.0, mean air temperature in summer: VIF = 3.3, mean daytime air temperature in summer: VIF = 3.2, mean night-time air temperature in summer: VIF = 1.6, mean night-time relative humidity in spring: VIF = 1.7, mean relative humidity in summer: VIF = 2.1, mean night-time relative humidity in summer: VIF = 2.0, soil pH: VIF = 2.2, moisture: VIF = 4.1, P2O5: VIF = 1.6, K2O: VIF = 1.2, and N: VIF = 2.0) were retained in the modelling steps (cf. Woods et al.85). We also tested the effects of microsites on the total number and total cover of species in the vegetation plots, and on the number and cover of species in the four species groups (i.e. mesic forest species, beech forest species, disturbance-tolerant species, and species of high conservation value) in the vegetation plots. We used LMMs for the total cover of species and cover of species groups, and generalized linear mixed-effects models (GLMMs) with Poisson error distribution for the species number data. In the models, microclimatic conditions, soil properties, and the number and cover of species were used as response variables, microsite was included as a fixed factor, while doline ID (from 1 to 24 for microclimatic conditions and soil properties, and from 1 to 30 for the number and cover of species) nested within region (Mecsek Mts and Aggtelek Karst) was used as a random factor. We performed all analyses in R (v. 4.2.2),86 using the lmer and glmer functions of the ‘lme4’ package.67 We used the vif function of the ‘car’ package to check for multicollinearity in our response variables, the Anova function of the ‘car’ package to test model significance,68 and the emmeans function of the ‘emmeans’ package to calculate pairwise comparisons with adjusted p-values (Tukey method).69
The diagnostic species of each microsite (plateau, south-facing slope, north-facing slope, and bottom) were identified by calculating the fidelity – measured by using the phi (Φ) coefficient – of all species to these microsites, using the JUICE program.71 The statistical significance of fidelity was tested with Fisher's exact test (p < 0.05), and species with Φ ≥ 0.15 were considered diagnostic species.87 In the rare case when a species appeared to be diagnostic for more than one microsite, only the occurrence with the higher phi value was considered.
To test the effect of microsites (plateau, south-facing slope, north-facing slope, and bottom) on plant assemblages in dolines, we used Permutational Multivariate Analysis of Variance (PERMANOVA) based on Bray–Curtis dissimilarity and 999 permutations. We used the strata argument that constrains permutations to the region (Mecsek Mts and Aggtelek Karst) in order to account for the nonindependency of sampling. We then calculated pairwise PERMANOVAs among the microsite types (plateau, south-facing slope, north-facing slope, and bottom). We performed a non-metric multidimensional scaling (NMDS) ordination with Bray–Curtis dissimilarity to visually illustrate compositional differences among the microsites, using the Hellinger-transformed plant cover data of 24 dolines (12 from the Mecsek Mts, and 12 from the Aggtelek Karst – where we measured microclimatic conditions and collected soil samples). To assess the relationships among environmental variables (microclimatic conditions: mean air temperature, mean daytime air temperature, mean night-time air temperature, mean relative humidity, mean daytime relative humidity, and mean night-time relative humidity in spring and summer), soil properties (pH, moisture, CaCO3, humus, P2O5, K2O and N) and compositional differences, environmental vectors were fitted onto the ordination diagram and correlations were calculated between ordination values and fitted vectors. The vectors of mean EIVs (temperature, moisture, and soil nitrogen) were also added to the ordination. These analyses were run in R using the adonis2, decostand, metaMDS and envfit functions of the ‘vegan’ package (v. 4.2.2).86,70
Additional resources
No additional resources were generated from the study.
Acknowledgments
Open access funding provided by University of Szeged (grant number: 6445). This research was funded by the NKFI K 124796 and FK 142428 grants. The contribution of Z.B. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by the New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund (ÚNKP-23-5-SZTE-697). K.F. was supported by the New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund (ÚNKP-23-3-SZTE-441).
Author contributions
K.F.: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. A.V.: Conceptualization, Investigation, Writing – review & editing. T.F.: Investigation, Writing – review & editing. L.E.: Investigation, Writing – review & editing. K.B.: Investigation, Writing – review & editing. A.E-.V.: Investigation, Writing – review & editing. C.T.: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing. Z.B.: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft.
Declaration of interests
The authors declare that they have no competing interests.
Published: October 21, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108202.
Contributor Information
Kata Frei, Email: freikata98@gmail.com.
Zoltán Bátori, Email: zbatory@gmail.com.
Supplemental information
Data and code availability
-
•
All raw data are available in the supplemental information.
-
•
Any additional information required to reanalyse the data reported in this study is available from the lead contact upon request.
-
•
This paper does not report original code.
References
- 1.Rull V. J. Biogeogr. 2009;36:481–484. doi: 10.1111/j.1365-2699.2008.02023.x. [DOI] [Google Scholar]
- 2.Keppel G., Van Niel K.P., Wardell-Johnson G.W., Yates C.J., Byrne M., Mucina L., Schut A.G.T., Hopper S.D., Franklin S.E. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Global Ecol. Biogeogr. 2012;21:393–404. doi: 10.1111/j.1466-8238.2011.00686.x. [DOI] [Google Scholar]
- 3.Finocchiaro M., Médail F., Saatkamp A., Diadema K., Pavon D., Meineri E. Bridging the gap between microclimate and microrefugia: A bottom-up approach reveals strong climatic and biological offsets. Global Change Biol. 2023;29:1024–1036. doi: 10.1111/gcb.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greiser C., Ehrlén J., Meineri E., Hylander K. Hiding from the climate: Characterizing microrefugia for boreal forest understory species. Global Change Biol. 2020;26:471–483. doi: 10.1111/gcb.14874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radosavljević I., Satovic Z., di Pietro R., Jug Dujaković M., Varga F., Škrtić D., Liber Z. Phylogeographic structure of common sage (Salvia officinalis L.) reveals microrefugia throughout the Balkans and colonizations of the Apennines. Sci. Rep. 2022;12:15726. doi: 10.1038/s41598-022-20055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin B.C., Ackerly D.D., Klos P.Z., Natali J., Dawson T.E., Thompson S.E. Hydrologic refugia, plants, and climate change. Global Change Biol. 2017;23:2941–2961. doi: 10.1111/gcb.13629. [DOI] [PubMed] [Google Scholar]
- 7.Selwood K.E., Zimmer H.C. Refuges for biodiversity conservation: a review of the evidence. Biol. Conserv. 2020;245:108502. doi: 10.1016/j.biocon.2020.108502. [DOI] [Google Scholar]
- 8.Fragnière Y., Gremaud J., Pesenti E., Bétrisey S., Petitpierre B., Guisan A., Kozlowski G. Mapping habitats sensitive to overgrazing in the Swiss Northern Alps using habitat suitability modelling. Biol. Conserv. 2022;274:109742. doi: 10.1016/j.biocon.2022.109742. [DOI] [Google Scholar]
- 9.Dobrowski S.Z. A climatic basis for microrefugia: the influence of terrain on climate. Global Change Biol. 2011;17:1022–1035. doi: 10.1111/j.1365-2486.2010.02263.x. [DOI] [Google Scholar]
- 10.Deák B., Kovács B., Rádai Z., Apostolova I., Kelemen A., Kiss R., Lukács K., Palpurina S., Sopotlieva D., Báthori F., Valkó O. Linking environmental heterogeneity and plant diversity: the ecological role of small natural features in homogeneous landscapes. Sci. Total Environ. 2021;763:144199. doi: 10.1016/j.scitotenv.2020.144199. [DOI] [PubMed] [Google Scholar]
- 11.Bátori Z., Gallé R., Gallé-Szpisjak N., Császár P., Nagy D.D., Lőrinczi G., Torma A., Tölgyesi C., Maák I.E., Frei K., et al. Topographic depressions provide potential microrefugia for ground-dwelling arthropods. Elementa. 2022;10:00084. doi: 10.1525/elementa.2021.00084. [DOI] [Google Scholar]
- 12.Lenoir J., Hattab T., Pierre G. Climatic microrefugia under anthropogenic climate change: implications for species redistribution. Ecography. 2017;40:253–266. doi: 10.1111/ecog.02788. [DOI] [Google Scholar]
- 13.Pastore M.A., Classen A.T., D'Amato A.W., Foster J.R., Adair E.C. Cold-air pools as microrefugia for ecosystem functions in the face of climate change. Ecology. 2022;103:e3717. doi: 10.1002/ecy.3717. [DOI] [PubMed] [Google Scholar]
- 14.Gavin D.G., Fitzpatrick M.C., Gugger P.F., Heath K.D., Rodríguez-Sánchez F., Dobrowski S.Z., Hampe A., Hu F.S., Ashcroft M.B., Bartlein P.J., et al. Climate refugia: Joint inference from fossil records, species distribution models and phylogeography. New Phytol. 2014;204:37–54. doi: 10.1111/nph.12929. [DOI] [PubMed] [Google Scholar]
- 15.Bátori Z., Vojtkó A., Farkas T., Szabó A., Havadtői K., Vojtkó A.E., Tölgyesi C., Cseh V., Erdős L., Maák I.E., Keppel G. Large- and small-scale environmental factors drive distributions of cool-adapted plants in karstic microrefugia. Ann. Bot. 2017;119:301–309. doi: 10.1093/aob/mcw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raschmanová N., Miklisová D., Kováč Ľ. A unique small-scale microclimatic gradient in a temperate karst harbours exceptionally high diversity of soil Collembola. Int. J. Speleol. 2018;47:247–262. doi: 10.5038/1827-806X.47.2.2194. [DOI] [Google Scholar]
- 17.White T.H., Collazo J.A., Vilella F.J., Guerrero S.A. Effects of Hurricane Georges on habitat use by captive-reared Hispaniolan parrots (Amazona ventralis) released in the Dominican Republic. Ornitol. Neotrop. 2005;16:405–417. [Google Scholar]
- 18.Garfì G., Carimi F., Fazan L., Gristina A.S., Kozlowski G., Livreri Console S., Motisi A., Pasta S. From glacial refugia to hydrological microrefugia: Factors and processes driving the persistence of the climate relict tree Zelkova sicula. Ecol. Evol. 2021;11:2919–2936. doi: 10.1002/ece3.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peñuelas J., Filella I. Deuterium labelling of roots provides evidence of deep water access and hydraulic lift by Pinus nigra in a Mediterranean forest of NE Spain. Environ. Exp. Bot. 2003;49:201–208. doi: 10.1016/S0098-8472(02)00070-9. [DOI] [Google Scholar]
- 20.Jobbágy E.G., Nosetto M.D., Villagra P.E., Jackson R.B. Water subsidies from mountains to deserts: their role in sustaining groundwater-fed oases in a sandy landscape. Ecol. Appl. 2011;21:678–694. doi: 10.1890/09-1427.1. [DOI] [PubMed] [Google Scholar]
- 21.Jeanpert J., Genthon P., Maurizot P., Folio J.-L., Vendé-Leclerc M., Sérino J., Join J.-L., Iseppi M. Morphology and distribution of dolines on ultramafic rocks from airborne LiDAR data: the case of southern Grande Terre in New Caledonia (SW Pacific) Earth Surf. Process. Landforms. 2016;41:1854–1868. doi: 10.1002/esp.3952. [DOI] [Google Scholar]
- 22.Öztürk M.Z., Savran A. An oasis in the Central Anatolian steppe: The ecology of a collapse doline. Acta Biologica Turcica. 2020;33:100–113. [Google Scholar]
- 23.Čarni A., Čonč Š., Breg Valjavec M. Landform-vegetation units in karstic depressions (dolines) evaluated by indicator plant species and Ellenberg indicator values. Ecol. Indicat. 2022;135:108572. doi: 10.1016/j.ecolind.2022.108572. [DOI] [Google Scholar]
- 24.White W.B., Culver D.C., Herman J.S., Kane T.C., Mylroie J.E. Karst Lands. The dissolution of carbonate rock produces unique landscapes and poses significant hydrological and environmental concerns. Am. Sci. 1995;83:450–459. [Google Scholar]
- 25.Olesen J., Pöllabauer C., Sigvardt Z.M.S., Rogers D.C. A new species of Lynceus Müller, 1776 from New Caledonia (Crustacea. Branchiopoda: Laevicaudata) from dolines, with remarks on zoogeography. Eur J. Taxon. 2016;224:1–18. doi: 10.5852/ejt.2016.224. [DOI] [Google Scholar]
- 26.Wang K., Zhang C., Chen H., Yue Y., Zhang W., Zhang M., Qi X., Fu Z. Karst landscapes of China: patterns, ecosystem processes and services. Landsc. Ecol. 2019;34:2743–2763. doi: 10.1007/s10980-019-00912-w. [DOI] [Google Scholar]
- 27.Sutcharit C., Jeratthitikul E., Pholyotha A., Lin A., Panha S. Molecular phylogeny reveals high diversity and endemism in the limestone karst-restricted land snail genus Sophina Benson, 1859 from Myanmar (Eupulmonata: Helicarionidae), with description of four new species. J. Zool. Syst. Evol. Res. 2020;58:957–981. doi: 10.1111/jzs.12420. [DOI] [Google Scholar]
- 28.Su Y., Tang Q., Mo F., Xue Y. Karst tiankengs as refugia for indigenous tree flora amidst a degraded landscape in southwestern China. Sci. Rep. 2017;7:4249. doi: 10.1038/s41598-017-04592-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breg Valjavec M., Zorn M., Čarni A. Bioindication of human-induced soil degradation in enclosed karst depressions (dolines) using Ellenberg indicator values (Classical Karst, Slovenia) Sci. Total Environ. 2018;640–641:117–126. doi: 10.1016/j.scitotenv.2018.05.294. [DOI] [PubMed] [Google Scholar]
- 30.Bátori Z., Vojtkó A., Maák I.E., Lőrinczi G., Farkas T., Kántor N., Tanács E., Kiss P.J., Juhász O., Módra G., et al. Karst dolines provide diverse microhabitats for different functional groups in multiple phyla. Sci. Rep. 2019;9:7176. doi: 10.1038/s41598-019-43603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazarević P., Lazarević M., Krivošej Z., Stevanović V. On the distribution of Dracocephalum ruyschiana (Lamiaceae) in the Balkan Peninsula. Phytol. Balc. 2009;15:175–179. [Google Scholar]
- 32.Marcin M., Raschmanová N., Miklisová D., Kováč L.'. Microclimate and habitat heterogeneity as important drivers of soil Collembola in a karst collapse doline in the temperate zone. Invertebr. Biol. 2021;140:e12315. doi: 10.1111/ivb.12315. [DOI] [Google Scholar]
- 33.Egli B., Gerstberger P., Greuter W., Risse H. Horstrissea dolinicola, a new genus and species of umbels (Umbelliferae, Apiaceae) from Kriti (Greece) Willdenowia. 1990;19:389–399. [Google Scholar]
- 34.Özkan K., Gulsoy S., Mert A., Ozturk M., Muys B. Plant distribution-altitude and landform relationships in karstic sinkholes of Mediterranean region of Turkey. J. Environ. Biol. 2010;31:51–60. [PubMed] [Google Scholar]
- 35.Fazan L., Gwiazdowicz D.J., Fragnière Y., Fałtynowicz W., Ghosn D., Remoundou I., Rusińska A., Urbański P., Pasta S., Garfì G., Kozlowski G. Factors influencing the diversity and distribution of epiphytic lichens and bryophytes on the relict tree Zelkova abelicea (Lam.) Boiss. (Ulmaceae) Lichenologist (Lond.) 2022;54:195–212. doi: 10.1017/S0024282922000159. [DOI] [Google Scholar]
- 36.Marcin M., Raschmanová N., Miklisová D., Šupinský J., Kaňuk J., Kováč Ľ. Karst dolines support highly diversified soil Collembola communities – possible refugia in a warming climate? Diversity. 2022;14:1037. doi: 10.3390/d14121037. [DOI] [Google Scholar]
- 37.Gargano D., Vecchio G., Bernardo L. Plant-soil relationships in fragments of Mediterranean snow-beds: ecological and conservation implications. Plant Ecol. 2010;207:175–189. doi: 10.1007/s11258-009-9663-7. [DOI] [Google Scholar]
- 38.Bátori Z., Erdős L., Gajdács M., Barta K., Tobak Z., Frei K., Tölgyesi C. Managing climate change microrefugia for vascular plants in forested karst landscapes. For. Ecol. Manage. 2021;496:119446. doi: 10.1016/j.foreco.2021.119446. [DOI] [Google Scholar]
- 39.Lévesque M., Walthert L., Weber P. Soil nutrients influence growth response of temperate tree species to drought. J. Ecol. 2016;104:377–387. doi: 10.1111/1365-2745.12519. [DOI] [Google Scholar]
- 40.Hulshof C.M., Spasojevic M.J. The edaphic control of plant diversity. Global Ecol. Biogeogr. 2020;29:1634–1650. doi: 10.1111/geb.13151. [DOI] [Google Scholar]
- 41.Ackerly D.D., Kling M.M., Clark M.L., Papper P., Oldfather M.F., Flint A.L., Flint L.E. Topoclimates, refugia, and biotic responses to climate change. Front. Ecol. Environ. 2020;18:288–297. doi: 10.1002/fee.2204. [DOI] [Google Scholar]
- 42.Bátori Z., Valkó O., Vojtkó A., Tölgyesi C., Farkas T., Frei K., Hábenczyus A.A., Tóth Á., Li G., Rádai Z., et al. Environmental heterogeneity increases the conservation value of small natural features in karst landscapes. Sci. Total Environ. 2023;872:162120. doi: 10.1016/j.scitotenv.2023.162120. [DOI] [PubMed] [Google Scholar]
- 43.Erdős L., Török P., Veldman J.W., Bátori Z., Bede-Fazekas Á., Magnes M., Kröel-Dulay G., Tölgyesi C. How climate, topography, soils, herbivores, and fire control forest–grassland coexistence in the Eurasian forest-steppe. Biol. Rev. 2022;97:2195–2208. doi: 10.1111/brv.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whiteman C.D., Haiden T., Pospichal B., Eisenbach S., Steinacker R. Minimum temperatures, diurnal temperature ranges, and temperature inversion in limestone sinkholes of different sizes and shapes. J. Appl. Meteorol. 2004;43:1224–1236. doi: 10.1175/1520-0450(2004)043<1224:MTDTRA>2.0.CO;2. [DOI] [Google Scholar]
- 45.Morelli T.L., Daly C., Dobrowski S.Z., Dulen D.M., Ebersole J.L., Jackson S.T., Lundquist J.D., Millar C.I., Maher S.P., Monahan W.B., et al. Managing climate change refugia for climate adaptation. PLoS One. 2016;11:e0159909. doi: 10.1371/journal.pone.0159909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le P.V.V., Kumar P. Power law scaling of topographic depressions and their hydrologic connectivity. Geophys. Res. Lett. 2014;41:1553–1559. doi: 10.1002/2013GL059114. [DOI] [Google Scholar]
- 47.Tauc F., Houle D., Dupuch A., Doyon F., Maheu A. Microtopographic refugia against drought in temperate forests: Lower water availability but more extensive fine root system in mounds than in pits. For. Ecol. Manage. 2020;476:118439. doi: 10.1016/j.foreco.2020.118439. [DOI] [Google Scholar]
- 48.Roy S., Singh J.S. Consequences of habitat heterogeneity for availability of nutrients in a dry tropical forest. J. Ecol. 1994;82:503–509. [Google Scholar]
- 49.Bátori Z., Vojtkó A., Keppel G., Tölgyesi C., Čarni A., Zorn M., Farkas T., Erdős L., Kiss P.J., Módra G., Breg Valjavec M. Anthropogenic disturbances alter the conservation value of karst dolines. Biodivers. Conserv. 2020;29:503–525. doi: 10.1007/s10531-019-01896-4. [DOI] [Google Scholar]
- 50.Schaetzl R.J., Johnson D.L., Burns S.F., Small T.W. Tree uprooting: review of terminology, process, and environmental implications. Can. J. For. Res. 1989;19:1–11. doi: 10.1139/x89-001. [DOI] [Google Scholar]
- 51.Bátori Z., Csiky J., Farkas T., Vojtkó A., Erdős L., Kovács D., Wirth T., Körmöczi L., Vojtkó A. The conservation value of karst dolines for vascular plants in woodland habitats of Hungary: refugia and climate change. Int. J. Speleol. 2014;43:15–26. doi: 10.5038/1827-806X.43.1.2. [DOI] [Google Scholar]
- 52.Ford K.R., Ettinger A.K., Lundquist J.D., Raleigh M.S., Hille Ris Lambers J. Spatial heterogeneity in ecologically important climate variables at coarse and fine spatial scales in a high-snow mountain landscape. PLoS One. 2013;8:e65008. doi: 10.1371/journal.pone.0065008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dendoncker M., Vincke C., Bazan S., Madingou M.P.N., Taugourdeau S. The size of topographic depressions in a Sahelian savanna is a driver of woody vegetation diversity. SSRN Journal. 2023;210:104923. doi: 10.2139/ssrn.4219049. [DOI] [Google Scholar]
- 54.Hunter M.L., Acuña V., Bauer D.M., Bell K.P., Calhoun A.J., Felipe-Lucia M.R., Fitzsimons J.A., González E., Kinnison M., Lindenmayer D., et al. Conserving small natural features with large ecological roles: a synthetic overview. Biol. Conserv. 2017;211:88–95. doi: 10.1016/j.biocon.2016.12.020. [DOI] [Google Scholar]
- 55.Tang C.Q., Matsui T., Ohashi H., Nualart N., Herrando-Moraira S., Dong Y.-F., Grote P.J., Van Ngoc N., Van Sam H., Li S., et al. Identifying long-term stable refugia for dominant Castanopsis species of evergreen broad-leaved forests in East Asia: A tool for ensuring their conservation. Biol. Conserv. 2022;273:109663. doi: 10.1016/j.biocon.2022.109663. [DOI] [Google Scholar]
- 56.Ramalho Q., Vale M.M., Manes S., Diniz P., Malecha A., Prevedello J.A. Evidence of stronger range shift response to ongoing climate change by ectotherms and high-latitude species. Biol. Conserv. 2023;279:109911. doi: 10.1016/j.biocon.2023.109911. [DOI] [Google Scholar]
- 57.Ackerly D.D., Loarie S.R., Cornwell W.K., Weiss S.B., Hamilton H., Branciforte R., Kraft N.J.B. The geography of climate change: implications for conservation biogeography. Divers. Distrib. 2010;16:476–487. doi: 10.1111/j.1472-4642.2010.00654.x. [DOI] [Google Scholar]
- 58.Ulrey C., Quintana-Ascencio P.F., Kauffman G., Smith A.B., Menges E.S. Life at the top: Long-term demography, microclimatic refugia, and responses to climate change for a high-elevation southern Appalachian endemic plant. Biol. Conserv. 2016;200:80–92. doi: 10.1016/j.biocon.2016.05.028. [DOI] [Google Scholar]
- 59.Keppel G., Mokany K., Wardell-Johnson G.W., Phillips B.L., Welbergen J.A., Reside A.E. The capacity of refugia for conservation planning under climate change. Front. Ecol. Environ. 2015;13:106–112. doi: 10.1890/140055. [DOI] [Google Scholar]
- 60.Silvertown J., Araya Y., Gowing D. Hydrological niches in terrestrial plant communities: a review. J. Ecol. 2015;103:93–108. doi: 10.1111/1365-2745.12332. [DOI] [Google Scholar]
- 61.Corlett R.T., Westcott D.A. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013;28:482–488. doi: 10.1016/j.tree.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Dainese M., Bragazza L. Plant traits across different habitats of the Italian Alps: a comparative analysis between native and alien species. Alpine Bot. 2012;122:11–21. doi: 10.1007/s00035-012-0101-4. [DOI] [Google Scholar]
- 63.Bátori Z., Tölgyesi C., Li G., Erdős L., Gajdács M., Kelemen A. Forest age and topographic position jointly shape the species richness and composition of vascular plants in karstic habitats. Ann. For. Sci. 2023;80:16. doi: 10.1186/s13595-023-01183-x. [DOI] [Google Scholar]
- 64.Kermavnar J., Ferlan M., Marinšek A., Eler K., Kobler A., Kutnar L. Effects of various cutting treatments and topographic factors on microclimatic conditions in Dinaric fir-beech forests. Agric. For. Meteorol. 2020;295:e108186. doi: 10.1016/j.agrformet.2020.108186. [DOI] [Google Scholar]
- 65.De Frenne P., Lenoir J., Luoto M., Scheffers B.R., Zellweger F., Aalto J., Ashcroft M.B., Christiansen D.M., Decocq G., De Pauw K., et al. Forest microclimates and climate change: Importance, drivers and future research agenda. Global Change Biol. 2021;27:2279–2297. doi: 10.1111/gcb.15569. [DOI] [PubMed] [Google Scholar]
- 66.Hansen L., Hoffman J., Drews C., Mielbrecht E. Designing climate-smart conservation: guidance and case studies. Conserv. Biol. 2010;24:63–69. doi: 10.1111/j.1523-1739.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- 67.Bates D.W., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. BMJ Qual. Saf. 2015;24:1–3. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 68.Fox J., Weisberg S. Sage; 2019. An R Companion to Applied Regression, third ed. [Google Scholar]
- 69.Lenth R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.6.0. 2021. https://cran.r-project.org/web/packages/emmeans/index.html
- 70.Oksanen J., Blanchet F.G., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P.R. Vegan: Community ecology package. 2018. https://cran.r-project.org/web/packages/vegan/index.html
- 71.Tichý L. JUICE, software for vegetation classification. J. Veg. Sci. 2002;13:451–453. doi: 10.1111/j.1654-1103.2002.tb02069.x. [DOI] [Google Scholar]
- 72.Dövényi Z., editor. Magyarország kistájainak katasztere (Inventory of microregions in Hungary) MTA Földrajztudományi Kutatóintézet; 2010. [Google Scholar]
- 73.IUSS Working Group WRB . FAO; 2015. World Reference Base for Soil Resources 2014. Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106. [Google Scholar]
- 74.Virók V., Farkas R., Farkas T., Šuvada R., Vojtkó A. Aggteleki Nemzeti Park Igazgatóság; 2016. A Gömör-Tornai-Karszt Flórája. Enumeráció (Vascular Flora of the Gömör-Torna Karst, Enumeration) [Google Scholar]
- 75.Virók V., Farkas R., Farkas T., Szűts F., Vojtkó A. Aggteleki Nemzeti Park Igazgatóság; 2014. A Gömör-Tornai-Karszt Flórája. Általános Rész (Vascular Flora of the Gömör-Torna Karst, General Information) [Google Scholar]
- 76.Erdős L., Kröel-Dulay G., Bátori Z., Kovács B., Németh C., Kiss P.J., Tölgyesi C. Habitat heterogeneity as a key to high conservation value in forest-grassland mosaics. Biol. Conserv. 2018;226:72–80. doi: 10.1016/j.biocon.2018.07.029. [DOI] [Google Scholar]
- 77.Farsang A., Babcsányi I., Ladányi Z., Perei K., Bodor A., Csányi K.T., Barta K. Evaluating the effects of sewage sludge compost applications on the microbial activity, the nutrient and heavy metal content of a Chernozem soil in a field survey. Arabian J. Geosci. 2020;13:982. doi: 10.1007/s12517-020-06005-2. [DOI] [Google Scholar]
- 78.Soó R. Akadémiai Kiadó; 1980. A Magyar Flóra És Vegetáció Rendszertani-Növényföldrajzi Kézikönyve VI (Systematic-Geobotanical Synopsis of the Flora and Vegetation of Hungary VI) [Google Scholar]
- 79.Király G. Sopron; 2007. A Magyarországi Edényes Flóra Veszélyeztetett Fajai (Red List of the Vascular Flora of Hungary). Private Edition. [Google Scholar]
- 80.Czúcz B., Gálhidy L., Mátyás C. Present and forecasted xeric climatic limits of beech and sessile oak distribution at low altitudes in Central Europe. Ann. For. Sci. 2011;68:99–108. doi: 10.1007/s13595-011-0011-4. [DOI] [Google Scholar]
- 81.Kasper J., Weigel R., Walentowski H., Gröning A., Petritan A.M., Leuschner C. Climate warming-induced replacement of mesic beech by thermophilic oak forests will reduce the carbon storage potential in aboveground biomass and soil. Ann. For. Sci. 2021;78:89. doi: 10.1007/s13595-021-01081-0. [DOI] [Google Scholar]
- 82.Borhidi A. Social behaviour types, the naturalness and relative ecological indicator values of the higher plants in the Hungarian Flora. Acta Bot. Hung. 1995;39:97–181. [Google Scholar]
- 83.Lengyel A., Purger D., Csiky J. Classification of mesic grasslands and their transitions of South Transdanubia (Hungary) Acta Bot. Croat. 2012;71:31–50. doi: 10.2478/v10184-011-0060-7. [DOI] [Google Scholar]
- 84.Tölgyesi C., Bátori Z., Erdős L. Using statistical tests on relative ecological indicator values to compare vegetation units – Different approaches and weighting methods. Ecol. Indicat. 2014;36:441–446. doi: 10.1016/j.ecolind.2013.09.002. [DOI] [Google Scholar]
- 85.Woods C.L., Maleta K., Ortmann K. Plant–plant interactions change during succession on nurse logs in a northern temperate rainforest Ecol. Evolution. 2021;11:9631–9641. doi: 10.1002/ece3.7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.R Core Team R. Computer software R Foundation for Statistical Computing; 2022. A Language and Environment for Statistical Computing (4.2.2)https://www.R-project.org [Google Scholar]
- 87.Tichý L., Chytrý M. Statistical determination of diagnostic species for site groups of unequal size. J. Veg. Sci. 2006;17:809–818. doi: 10.1111/j.1654-1103.2006.tb02504.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All raw data are available in the supplemental information.
-
•
Any additional information required to reanalyse the data reported in this study is available from the lead contact upon request.
-
•
This paper does not report original code.