Abstract
Purpose
CPAP is the “gold standard” treatment for obstructive sleep apnea (OSA). Current CPAP models have developed additional functions including automatic CPAP and pressure relief. However, CPAP adherence has not improved over the last three decades. Many patients in low-income countries cannot afford these CPAP devices. A novel simple CPAP device with a fixed pressure without pressure controller was developed.
Methods
Manual CPAP pressure titration was performed in 127 patients with OSA. Six patients with a titration pressure higher than 11 cmH2O and 14 patients who could not tolerate CPAP were excluded, leaving 107 participating in the following 2 studies. In study one, 54 of 107 patients were treated by both conventional fixed CPAP and simple CPAP in random order. In the second study, another 53 patients were treated by both autoCPAP in automatic function and simple CPAP in random order. Simple CPAP was fixed at 10 cmH2O, 8 cmH2O, and 6 cmH2O for patients whose titration pressure was between 9–10, 7–8, and ≤ 6 cmH2O, respectively. Conventional fixed CPAP device was set exactly the same as manual titration pressure.
Results
All patients whose manual titration pressure ≤ 10 cmH2O were effectively treated by simple CPAP (AHI 40.7 ± 2.3 events/h before vs 2.5 ± 0.3 events/h after, p < 0.001). Patients expressed similar preferences for simple CPAP, autoCPAP, and conventional fixed CPAP (p > 0.05).
Conclusions
We conclude that a novel simple CPAP is an alternative treatment for most patients with OSA, which may widen access to CPAP therapy in the developing countries because of its low cost.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11325-023-02823-2.
Keywords: OSA, Pressure titration, Automatic CPAP, Fixed CPAP
Introduction
Obstructive sleep apnea (OSA) is a common disease. Moderate to severe OSA defined as an apnea–hypopnea index (AHI) of more than 15 events/h affects between 6 and 17% of the general population [1]. The prevalence of OSA has continuously increased, mainly because of the increasing prevalence of obesity [2], although increased life expectancy [3] may also be pertinent because age is a risk factor for OSA [4, 5].
The major physiological abnormalities in OSA are repetitive upper airway occlusions that result in intermittent hypoxia, increased inspiratory pressure swings, and frequent arousals from sleep. Approximately half of individuals affected experience daytime sleepiness that contributes to an increased risk of road traffic accidents, cardiovascular events, depression, and diabetes [6].
Effective intervention, using nasal continuous positive airway pressure (CPAP), can significantly improve OSA-related outcomes including daytime sleepiness, quality of life, and insulin resistance, although outcomes for cardiovascular disease may not necessarily be improved [7]. One of the challenges of OSA treatment is that many patients cannot afford CPAP if the costs need to be covered by themselves [8, 9]. Unsurprisingly, this problem is most acute in low-income countries [8, 10]. In these countries, more than 50% of patients with severe OSA may not be able to afford to buy a CPAP device. This impediment to OSA therapy may be not confined to low-income countries. For example, financial issue related to CPAP therapy has also been reported among low-income residents of high-income countries [9]. Developing an effective simple CPAP device is essential to enhance uptake of this therapy globally [11].
One reason that current CPAP devices may be costly is that current models offer multiple additional functions such as remote telemonitoring interactions, leak measurement, bi-level and variable level pressure, pressure relief during expiration, and ramping of pressure, as well as significant data storage and processing functions. Interestingly, despite these additional functions being added to CPAP devices, acceptance and adherence to CPAP or the efficacy of treatment has remained largely unchanged since its invention in the 1980s [12, 13]. This suggests that the additional functions added to CPAP devices do not materially improve patient comfort or treatment efficacy.
The main principle of CPAP therapy in patients with OSA is to maintain upper airway patency with positive airway pressure while asleep. Standard CPAP devices can be adjusted to any pressure level between 4 and 20 cmH2O, but the controller hardware and associated software needed to make this adjustment significantly contribute to the cost of modern CPAP devices. However, based on manual titration studies done by us [14] and others [15, 16], the CPAP pressure required for the effective treatment in the majority of patients is less than or equal to 10 cmH2O.
We therefore developed a simple CPAP with the aim of reducing the cost of CPAP. Our simple CPAP device has no pressure controller components and no additional functions such as ramp, pressure relief, or automatic change in pressure, and it only requires a power switch for full operation. The pressures delivered by the simple CPAP device were pre-set in the factory to a default at 10, 8, or 6 cmH2O. In the current study, we compared this device with conventional fixed pressure CPAP and automatic CPAP (autoCPAP) devices to determine whether or not a simple CPAP could effectively treat patients with OSA.
Methods
The study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University, China, and was registered on ClinicalTrials.Gov (registration no. NCT03782844). A written informed consent was obtained from all the participants.
Patient selection
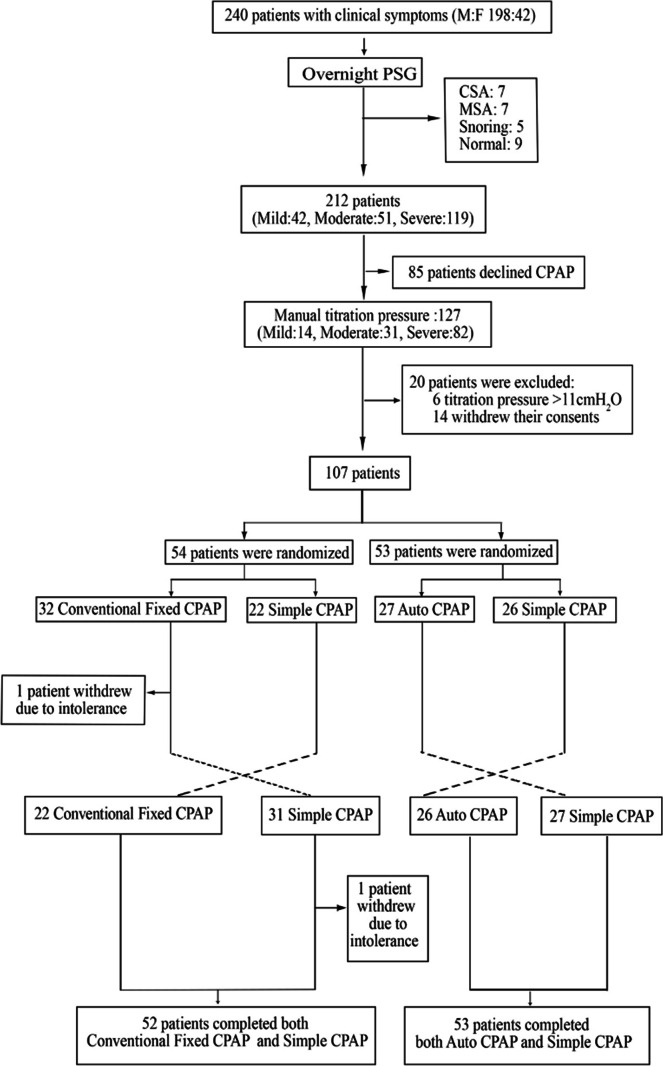
Patient recruitment flow is shown in Fig. 1. Briefly, 240 patients (aged 48 ± 0.9 year) with daytime sleepiness or snoring visiting outpatient clinic between June 2017 and January 2019 were referred for overnight full polysomnography at the Sleep Center of the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China, for clinical reasons. Patients with obstructive pulmonary disease, severe neuromuscular disease, and respiratory failure were excluded.
Fig. 1.
Flow of patient recruitment
Of these, 212 patients were diagnosed with OSA based on polysomnography using a criterion of AHI ≥ 5 events/h and deemed to require CPAP therapy. These patients were invited to participate in the study. Eighty-five of 212 patients declined to accept CPAP therapy, leaving 127 patients with OSA who proceeded to have a second night sleep study in the sleep laboratory during which manual titration was undertaken to identify the optimal CPAP pressure.
Of the 127, fourteen patients could not tolerate CPAP and withdrew their consent from the study during pressure titration, and 6 further patients whose titration pressure was higher than 11 cmH2O were excluded for further comparison between simple CPAP and conventional CPAP or autoCPAP.
Consequently, 107 patients were randomized to compare simple CPAP with conventional fixed pressure CPAP (study 1, n = 54) or autoCPAP (study 2, n = 53). To avoid bias during the study, all devices for comparison (simple CPAP, conventional fixed CPAP, and autoCPAP) were sealed inside an unlabeled box. Patients did not know the device with which they were treated. The mask and tubing used for the study were exactly the same for each patient. At the end of the study, patients were asked for their preference as to which device they preferred for the future treatment.
Polysomnography
Full-attended polysomnography (Alice 5, Philips Respironics, USA) was performed in all cases. Two channels of electroencephalogram (C4A1, C3A2), left and right electrooculograms (EOGs), submental EMG (EMGchin), airflow from both thermistor and nasal pressure, chest and abdominal movements, as measured by uncalibrated respiratory inductance plethysmography (RIP), arterial oxygen saturation (SaO2), and body position were recorded. In the first (diagnostic) night of polysomnography, we also recorded the Epworth sleepiness scale (ESS) score and demographic data including height, weight, and neck and waist circumference.
In the second and therapeutic night, CPAP was manually titrated with a device (REMstar Auto, CFlex, Philips Respironics, PA, USA) used in manual mode. Patients were again studied in the sleep laboratory monitored with full PSG (Alice 5, Philips Respironics, PA, USA) based on our standard procedures, as previously described [14]. In brief, CPAP was started at 4.0 cmH2O and gradually increased in steps of 1 cmH2O every 10 min until apneas and hypopneas were controlled. When no further respiratory events were observed for half an hour, CPAP was decreased again by 1 cmH2O every 10 min until apnea–hypopnea events recurred. This cycle was repeated until the optimal therapeutic pressure was obtained, defined as the lowest effective pressure for eliminating apnea and hypopnea events in all body positions and sleep stages including rapid eye movement (REM) sleep during supine position. The minimum acceptable time for this titration process was 3 h.
Study 1: comparison between simple CPAP and conventional fixed CPAP
The first 54 of the 107 newly diagnosed patients who had completed manual titration were treated with both conventional fixed CPAP (REMstar Auto A-Flex (557P), Philips Respironics, USA) and simple CPAP (Respiratory Medical Science Ltd. Co, Guangzhou, China) on two separate nights in random order, and the effects were monitored by full polysomnography. The simple CPAP device commercially named as Sui-Kang (SK) had a default pressure at low level (6 cmH2O), at middle level (8 cmH20), or at high level (10 cmH2O). For the conventional fixed CPAP treatment, pressure was set at the level the same as that derived from the manual titration. However, selection of the type of simple CPAP was based on the following criteria: if manual titration pressure was 6 cmH2O or less, then 6 cmH2O was selected; if the manual titration pressure was 7–8 cmH2O, 8 cmH2O was selected; and if the CPAP pressure derived from manual titration was 9–10 cmH2O, 10 cmH2O was selected. Two of the 54 patients dropped out from the study before completion of the comparison. Consequently, comparison between conventional fixed CPAP and simple CPAP was possible in 52 patients. All the patients used the conventional fixed CPAP treatment without the ramp, pressure release, and humidity.
Study 2: comparison between simple CPAP and autoCPAP
The second group consisted of the subsequent 53 of the 107 patients with OSA after titration. They were treated both by autoCPAP (REMstar Auto, A-Flex, 557P, Philips Respironics, PA, USA) in automatic function and simple CPAP in random order on two different nights within a week. The pressure for the simple CPAP device was selected based on the criteria, as described above (see study 1). The autoCPAP was set in automatic mode with the permit pressures between 4 and 20 cmH2O. Additional functions such as pressure release (A-flex level 3), humidity, and pressure ramp were on.
Data analysis
Data obtained from overnight full polysomnography were analyzed based on American Academy of Sleep Medicine (AASM) criteria 2012 [17] for scoring respiratory events by a qualified technician with more than 10 years of experience in PSG. The technician who analyzed the data was blinded to the device allocation. AHI, 3% oxygen desaturation index (ODI), arousal events/hour (arousal index), sleep efficacy, and duration of CPAP treatment were analyzed and reported. Data analyses were performed using SPSS for Windows, version 20.0 (SPSS, Inc., Chicago, IL, USA). A paired t-test was used to assess the difference between different CPAP devices. Patients’ preference to CPAP model was assessed by chi-square test. All tests were considered statistically significant if p < 0.05.
Results
Baseline PSG data including AHI, ODI, arousal index, sleep efficacy and participant characteristics in 127 patients are reported in Table 1 and online supplementary table E-1 and table E-2. In six patients, the effective titration pressure was above 11cmH20 (three patients at 11 cmH2O and three others at 12 cmH2O). However, no patients had a manual titration pressure higher than 12 cmH20 in the study. For most patients (95.3%), the effective manually titrated pressure was below 10cmH2O (Table 2): 48.0% with ≤ 6 cmH2O, 32.3% with 7–8 cmH2O, and 15% with 9–10 cmH2O.
Table 1.
Participant characteristics (mean ± SE; n = 127)
| Variables | Results |
|---|---|
| Age (years) | 51.0 ± 1.3 |
| Sex (male/female) | 104/23 |
| Body mass index (kg/m2) | 27.2 ± 0.3 |
| Neck circumference (cm) | 38.7 ± 0.3 |
| Waist circumference (cm) | 96.5 ± 1.0 |
| Hip circumference (cm) | 101.3 ± 0.7 |
| Epworth sleepiness scale score | 7.2 ± 0.5 |
| Systolic BP (mmHg) | 129.5 ± 1.4 |
| Diastolic BP (mmHg) | 77.2 ± 1.1 |
| Hypertension (n, %) | 45 (35.4) |
| Coronary heart disease (n, %) | 16 (12.6) |
| Stroke (n, %) | 11 (8.7) |
| Diabetes (n, %) | 13 (10.2) |
| Asthma (n, %) | 1 (0.8) |
| COPD (n, %) | 3 (2.4) |
| Current cigarette smoking (n, %) | 59 (46.5) |
| Alcohol use (n, %) | 11 (8.7) |
| Polysomnography | |
| Total record time (min) | 497.5 ± 4.3 |
| Total sleep time (min) | 396.7 ± 5.9 |
| Sleep onset latency (min) | 19.7 ± 2.2 |
| Sleep efficiency (%) | 80.2 ± 1.2 |
| Arousal index (events/h) | 33.2 ± 1.8 |
| REM sleep (%) | 16.8 ± 0.6 |
| Stage 1 (%) | 24.8 ± 1.3 |
| Stage 2 (%) | 52.1 ± 1.1 |
| Stage 3 (%) | 6.2 ± 0.6 |
| AHI (events/h) | 42.5 ± 2.2 |
| OAI (events/h) | 20.5 ± 1.7 |
| CAI (events/h) | 2.3 ± 0.3 |
| MAI (events/h) | 4.8 ± 0.7 |
| HI (events/h) | 14.8 ± 1.0 |
| Sleep time mean SaO2 (%) | 94.0 ± 0.3 |
| Lowest SaO2 (%) | 75.9 ± 1.1 |
| 3% ODI (events/h) | 30.6 ± 2.1 |
Table 2.
Respiratory events, sleep structure during overnight titration, and the subject number at different titrated pressure (mean ± SE)
| Parameter | Baseline | Manual titration | p value |
|---|---|---|---|
| Total record time (min) | 497.5 ± 4.3 | 502.4 ± 4.6 | 0.373 |
| Total sleep time (min) | 396.7 ± 5.9 | 415.1 ± 6.1 | 0.006 |
| Sleep onset latency (min) | 19.7 ± 2.2 | 22.3 ± 3.1 | 0.491 |
| Sleep Efficiency (%) | 80.2 ± 1.2 | 82.4 ± 1.3 | 0.120 |
| Arousal index (events/h) | 33.2 ± 1.8 | 10.8 ± 0.4 | 0.000 |
| REM sleep (%) | 16.8 ± 0.6 | 23.2 ± 0.7 | 0.000 |
| Stage 1 (%) | 24.8 ± 1.3 | 15.4 ± 0.9 | 0.000 |
| Stage 2 (%) | 52.1 ± 1.1 | 53.0 ± 1.1 | 0.482 |
| Stage 3 (%) | 6.2 ± 0.6 | 8.4 ± 0.8 | 0.006 |
| AHI (events/h) | 42.5 ± 2.2 | 4.7 ± 0.4 | 0.000 |
| OAI (events/h) | 20.5 ± 1.7 | 0.9 ± 0.1 | 0.000 |
| CAI (events/h) | 2.3 ± 0.3 | 0.9 ± 0.1 | 0.000 |
| MAI (events/h) | 4.8 ± 0.7 | 0.1 ± 0.0 | 0.000 |
| HI (events/h) | 14.8 ± 1.0 | 2.8 ± 0.2 | 0.000 |
| Sleep time mean SaO2 (%) | 94.0 ± 0.3 | 96.2 ± 0.1 | 0.000 |
| Lowest SaO2 (%) | 75.9 ± 1.1 | 88.0 ± 0.9 | 0.000 |
| ODI (events/h) | 30.6 ± 2.1 | 3.6 ± 0.3 | 0.000 |
| Pressure ≤ 6 cmH2O (n, %) | 61 (48.0%) | ||
| Pressure 7–8 cmH2O (n, %) | 41(32.3%) | ||
| Pressure 9–10 cmH2O (n, %) | 19 (15.0%) | ||
| Pressure ≥ 11 cmH2O | 6 (4.7%) |
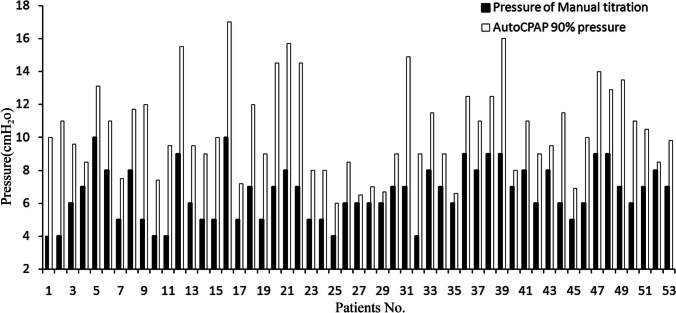
Manual titration vs automatic titration
Both manual titration and automatic titration of CPAP pressure were performed in 53 patients with OSA (study 2). The mean CPAP pressure derived from manual titration (6.6 ± 1.7 cmH2O) was significantly lower than that derived from automatic titration (10.5 ± 2.8 cmH20, p < 0.001) (Fig. 2), as determined by the 90% of the pressure time.
Fig. 2.
The pressure from manual titration and automatic titration
The effect of simple CPAP on patients with OSA
In total, simple CPAP was used in 105 patients and showed that it could satisfactorily improve sleep structure. The mean AHI and ODI normalized on treatment with simple CPAP; the AHI and ODI were 40.7 ± 2.3 and 28.4 ± 2.1events/h at baseline, respectively, and were 2.5 ± 0.3 and 1.7 ± 0.2 events/h while on simple CPAP, respectively (all p < 0.001). The arousal index decreased from 31.8 ± 2.0 to 11.8 ± 0.5 events/h on treatment (p < 0.001; Table 3 and online supplementary table E-3).
Table 3.
Sleep structure and respiratory events before and after treatment with the simple CPAP device in 105 patients (mean ± SE)
| Parameter | Baseline | Simple CPAP | p value |
|---|---|---|---|
| Total record time (min) | 495.9 ± 4.8 | 468.6 ± 5.3 | 0.000 |
| Total sleep time (min) | 392.2 ± 6.4 | 382.5 ± 6.7 | 0.232 |
| Sleep onset latency (min) | 20.5 ± 2.3 | 22.4 ± 2.3 | 0.524 |
| Sleep efficiency (%) | 79.6 ± 1.3 | 81.7 ± 1.2 | 0.113 |
| Arousal index (events/h) | 31.8 ± 2.0 | 11.8 ± 0.5 | 0.000 |
| REM sleep (%) | 17.2 ± 0.7 | 21.8 ± 0.6 | 0.000 |
| Stage 1 (%) | 24.5 ± 1.4 | 13.7 ± 0.8 | 0.000 |
| Stage 2 (%) | 51.6 ± 1.3 | 51.5 ± 1.0 | 0.907 |
| Stage 3 (%) | 6.6 ± 0.7 | 13.0 ± 0.9 | 0.000 |
| AHI (events/h) | 40.7 ± 2.3 | 2.5 ± 0.3 | 0.000 |
| OAI (events/h) | 18.4 ± 1.7 | 0.6 ± 0.1 | 0.000 |
| CAI (events/h) | 2.4 ± 0.3 | 0.5 ± 0.1 | 0.000 |
| MAI (events/h) | 4.7 ± 0.7 | 0.1 ± 0.0 | 0.000 |
| HI (events/h) | 15.1 ± 0.9 | 1.4 ± 0.2 | 0.000 |
| Sleep time mean SaO2 (%) | 94.3 ± 0.3 | 96.3 ± 0.1 | 0.000 |
| Lowest SaO2 (%) | 76.9 ± 1.2 | 90.8 ± 0.4 | 0.000 |
| ODI (events/h) | 28.4 ± 2.1 | 1.7 ± 0.2 | 0.000 |
Comparison between simple CPAP and conventional fixed CPAP
Fifty-two patients completed treatment both with conventional fixed CPAP and simple CPAP devices. The AHI, ODI, and arousal index improved significantly both with conventional fixed CPAP and simple CPAP. There were no significant differences between the treatment effects using simple CPAP and conventional fixed CPAP (AHI, 3.1 ± 0.6 events/h vs 3.3 ± 0.6 events/h; ODI 2.1 ± 0.4 events/h vs 2.3 ± 0.4 events/h; arousal index 12.2 ± 0.7 events/h vs 11.0 ± 0.6 events/h; lowest SaO2 90.7% ± 0.7% vs 90.3% ± 0.6% respectively, all p > 0.05). Moreover, sleep structure including REM sleep and non-REM sleep stage 3 was also similar (Table 4 and online supplementary table E-4). Blinded to device allocation, 26.9% patients preferred the treatment with simple CPAP, and 36.5% patients preferred treatment with fixed pressure CPAP, whereas 36.6% of patients reported no preference (p = 0.218).
Table 4.
Effects of simple CPAP and fixed CPAP in 52 patients (mean ± SE)
| Parameter | Baseline | Simple CPAP | Conventional CPAP | p value† |
|---|---|---|---|---|
| Total record time (min) | 490.2 ± 7.2 | 474.0 ± 4.9a | 482.3 ± 7.0 | 0.300 |
| Total sleep time (min) | 400.2 ± 8.8 | 391.9 ± 8.2 | 398.1 ± 9.2 | 0.514 |
| Sleep onset latency (min) | 14.6 ± 1.8 | 22.5 ± 3.4a | 20.8 ± 3.9 | 0.643 |
| Sleep efficiency (%) | 82.0 ± 1.6 | 82.7 ± 1.5 | 82.8 ± 1.8 | 0.944 |
| Arousal index (events/h) | 33.3 ± 2.8 | 12.2 ± 0.7a | 11.0 ± 0.6a | 0.080 |
| REM sleep (%) | 16.6 ± 0.9 | 21.1 ± 0.6a | 21.7 ± 0.7a | 0.479 |
| Stage 1 (%) | 28.7 ± 2.4 | 16.3 ± 1.3a | 16.1 ± 1.1a | 0.798 |
| Stage 2 (%) | 48.6 ± 2.0 | 51.2 ± 1.5 | 50.9 ± 1.5 | 0.755 |
| Stage 3 (%) | 6.0 ± 0.9 | 11.4 ± 1.2a | 11.3 ± 1.2a | 0.843 |
| AHI (events/h) | 41.6 ± 3.2 | 3.1 ± 0.6a | 3.3 ± 0.6a | 0.686 |
| OAI (events/h) | 19.2 ± 2.6 | 0.7 ± 0.2a | 1.0 ± 0.4a | 0.243 |
| CAI (events/h) | 2.6 ± 0.5 | 0.6 ± 0.9a | 0.5 ± 0.1a | 0.791 |
| MAI (events/h) | 3.4 ± 0.8 | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.119 |
| HI (events/h) | 16.3 ± 1.4 | 1.8 ± 0.4a | 1.6 ± 0.3a | 0.504 |
| Sleep time mean SaO2 (%) | 94.2 ± 0.4 | 96.3 ± 0.2a | 96.5 ± 0.2a | 0.184 |
| Lowest SaO2 (%) | 77.2 ± 1.7 | 90.7 ± 0.7a | 90.3 ± 0.6a | 0.512 |
| ODI (events/h) | 29.1 ± 3.0 | 2.1 ± 0.4a | 2.3 ± 0.4a | 0.521 |
†p value indicates the difference between simple CPAP and conventional fixed CPAP; ap < 0.05 compared with baseline
Comparison between simple CPAP and autoCPAP
Fifty-three patients with OSA were treated using both simple CPAP and autoCPAP devices. The AHI, ODI, and arousal index improved significantly both with simple CPAP and autoCPAP. There was no difference between treatment with simple CPAP or autoCPAP in AHI (1.9 ± 0.2 events/h vs 1.9 ± 0.2 events/h) and ODI (1.3 ± 0.2 vs 1.3 ± 0.2 times/h, p > 0.05). The arousal index and lowest SaO2 during treatment with the two devices were also similar (11.0 ± 0.7 events/h vs 9.9 ± 0.7 events/h for arousal index and 90.9% ± 0.5% vs 91.2% ± 0.5% for lowest SaO2, p > 0.05; Table 5 and online supplementary table E-5). The proportion of patients who preferred treatment with the simple CPAP (38%) was similar to that who preferred treatment with autoCPAP (34%), and 28% of the patients reported no preference (p = 0.646).
Table 5.
Effects of simple CPAP and autoCPAP in 53 patients (mean ± SE)
| Parameter | Baseline | Simple CPAP | autoCPAP | p value† |
|---|---|---|---|---|
| Total record time (min) | 501.6 ± 6.4 | 463.3 ± 9.3a | 482.0 ± 7.6a | 0.088 |
| Total sleep time (min) | 384.3 ± 9.2 | 373.3 ± 10.5 | 390.8 ± 7.8 | 0.181 |
| Sleep onset latency (min) | 26.3 ± 4.1 | 22.3 ± 3.1 | 22.9 ± 5.1 | 0.929 |
| Sleep efficiency (%) | 77.2 ± 2.0 | 80.8 ± 1.8 | 81.6 ± 1.5a | 0.696 |
| Arousal index (events/h) | 30.2 ± 2.7 | 11.0 ± 0.7a | 9.9 ± 0.7a | 0.054 |
| REM sleep (%) | 17.8 ± 1.0 | 22.6 ± 0.9a | 24.5 ± 1.0a | 0.059 |
| Stage 1 (%) | 20.5 ± 1.3 | 11.2 ± 0.8a | 12.0 ± 0.9a | 0.312 |
| Stage 2 (%) | 54.6 ± 1.6 | 51.7 ± 1.3a | 50.3 ± 1.3a | 0.252 |
| Stage 3 (%) | 7.2 ± 1.0 | 14.6 ± 1.2a | 12.8 ± 1.4a | 0.061 |
| AHI (events/h) | 39.8 ± 3.4 | 1.9 ± 0.2a | 1.9 ± 0.2a | 0.915 |
| OAI (events/h) | 17.6 ± 2.3 | 0.5 ± 0.1a | 0.3 ± 0.1a | 0.19 |
| CAI (events/h) | 2.2 ± 0.4 | 0.4 ± 0.1a | 0.4 ± 0.1a | 0.585 |
| MAI (events/h) | 6.0 ± 1.2 | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.87 |
| HI (events/h) | 13.9 ± 1.2 | 1.0 ± 0.1a | 1.1 ± 0.1a | 0.438 |
| Sleep time mean SaO2 (%) | 94.4 ± 0.3 | 96.2 ± 0.2a | 96.3 ± 0.2a | 0.403 |
| Lowest SaO2 (%) | 76.5 ± 1.7 | 90.9 ± 0.5a | 91.2 ± 0.5a | 0.561 |
| ODI (events/h) | 27.7 ± 3.0 | 1.3 ± 0.2a | 1.3 ± 0.2a | 0.953 |
†p value indicates the difference between simple CPAP and autoCPAP; ap < 0.05 compared with baseline
Discussion
Our results show that the therapeutic effect using a simple CPAP device is non-inferior to either conventional fixed pressure CPAP or autoCPAP for the treatment of patients with OSA, judged by the sleep architecture, conventional indices of sleep-disordered breathing, and sleep fragmentation. Blinded to the device allocation, patients expressed no specific preferences for any of the devices used in the study.
Clinical significance of the findings
Given that our study and patient preference failed to show an advantage for state-of-the-art CPAP devices, it is worthwhile to consider the differences in the functional components of these devices. One function is pressure release which provides a temporary drop in airway pressure during early expiration. Because normal expiration is passive and relied on elastic recoil of the lung which is largest at early expiration and becomes smaller over the expiration, an ideal pressure release should be arranged at the later rather than the early part of expiration for facilitating expiration during CPAP. However, pressure release is usually developed at the early expiration for the modern CPAP. It is not surprising that CPAP with pressure release function may not improve expiration during CPAP. Indeed, we and others have previously shown that the efficacy of CPAP treatment and preferences for CPAP devices with or without pressure release is similar [18, 19].
Similarly, since airway closure is more likely when supine or in REM sleep, it could be hypothesized that at these times, therapeutic pressures need to be higher to maintain airway patency. This was the origin of the concept of autoCPAP which is able to track the resistance and adjust the pressures required to maintain airway patency and, thus, reduce mean pressures for parts of the night during CPAP treatment [20]. However, pressure delivered by autoCPAP depends on sensitivity and accuracy in detecting changes in airway resistance, which may be influenced by many factors including flow-derived algorithm used for automatic titration and frequency of arousals [21]. Indeed, it is shown that pressure titrated by autoCPAP is usually 2–3 cmH2O higher rather than lower than that obtained by manual titration, although the treatment efficacy and preference for the pressure derived from both titrations are similar [14]. Other study also showed that pressure derived from 95th percentile of treatment pressure during APAP therapy was usually 2 cmH2O higher than that from manual titration [22]. Consequently, it is not surprising that many studies show that the percentage of patients who prefer to be treated by fixed CPAP is actually more than or similar to those treated by autoCPAP [23–25].
The third function present in most current CPAP devices, which was deliberately omitted from our simple device to reduce cost, was a pressure ramp. Theoretically, if a CPAP device could respond automatically to sleep onset and increase the airway pressure to the required level to maintain upper airway patency, the ramp function might be useful to improve comfort prior to sleep onset. However, this is hypothetical as current commercially available CPAP devices do not have the ability to detect sleep onset, and the times set for pressure ramps are necessarily arbitrary, and this may be why we found no difference in sleep latency and overall quality or patient preference between the devices with and without a pressure ramp. Consistent with our observation, there are few data to support that pressure ramps improve patients’ long-term compliance to CPAP [26].
The humidification function for CPAP devices may be useful for some patients, particularly those who are under treatment with full-face mask rather than nasal mask and breathe through their mouth at night. However, CPAP increases lung volume, but it does not actually increase ventilation through the nose and it does not necessarily generate airway dryness when breathing room air. Indeed, many studies showed that humidification did not significantly improve long-term compliance [27, 28].
CPAP devices with fixed pressures may not be adjusted to increase pressure in the future, perhaps in response to weight gain. However, with good routine multidisciplinary clinical advice and management, this may not be a significant problem. Effective treatment of OSA should go beyond CPAP to encompass dietetic advice and weight loss in patients who are overweight [29]. It has been reported that weight in patients with OSA changes little during CPAP treatment [30, 31], and in long-term clinical trials with careful follow-up, CPAP pressures require little change following initiation of the treatment [7].
Although we did not record long-term compliance in a large population, it has previously been shown that initial tolerance to CPAP treatment predicts long-term compliance of CPAP treatment [32, 33]. In the current study, patients had similar preferences for autoCPAP, fixed pressure CPAP, and simple CPAP suggesting that long-term compliance to simple CPAP could be similar to autoCPAP or fixed pressure CPAP, although a further study is required to determine the long-term compliance of the simple CPAP.
Conclusion
In summary, we found that the use of a simple CPAP device was not different to “state-of-the-art” CPAP devices for treating indices of sleep architecture in patients with OSA who required pressures up to 10cmH2O.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank the participants for contributing to the current study.
Author contribution
Qiu and Liang contributed equally to the work. Study design: YML, ZHQ, and SFL. Data acquisition: ZHQ, XBD, QSW, LW, SFL, YXW, and YML. Data analysis and interpretation: ZHQ, JS, RDM, and YML. Manuscript preparation: ZHQ, YML, JS, RDM, and YML.
Funding
This study was supported by grants from Guangdong Major Project (Grant No. 2016B010108011) and Zhong Nanshan Medical Foundation of Guangdong Province (ZNSA 2020013).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The study was approved by the First Affiliated Hospital of Guangzhou Medical University (2011 No. 5; Registry: Clinical trials.GOV; Name: Efficacy of Simple Continuous Positive Airway Pressure on Patients with Obstructive Sleep Apnea; URL: https://clinicaltrials.gov/ct2/show/NCT03782844; Identifier: NCT03782844).
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Inoue Y, Qin B, Poti J, et al. Epidemiology of obesity in adults: latest trends. Curr Obes Rep. 2018;7(4):276–288. doi: 10.1007/s13679-018-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontis V, Bennett JE, Mathers CD, et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389(10076):1323–1335. doi: 10.1016/S0140-6736(16)32381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Garcia MA, Duran-Cantolla J, Montserrat JM. Sleep apnea-hypopnea syndrome in the elderly. Arch Bronconeumol. 2010;46(9):479–488. doi: 10.1016/j.arbres.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152(5):1070–1086. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mcevoy RD, Antic NA, Heeley E, et al. Cpap for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 8.Goyal A, Agarwal N, Pakhare A. Barriers to cpap use in India: an exploratory study. J Clin Sleep Med. 2017;13(12):1385–1394. doi: 10.5664/jcsm.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarasiuk A, Reznor G, Greenberg-Dotan S, et al. Financial incentive increases cpap acceptance in patients from low socioeconomic background. Plos One. 2012;7(3):e33178. doi: 10.1371/journal.pone.0033178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezaie L, Phillips D, Khazaie H. Barriers to acceptance and adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea: a report from kermanshah province, western iran. Patient Prefer Adherence. 2018;12:1299–1304. doi: 10.2147/PPA.S165905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan CE, Issa FG, Berthon-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 13.Rotenberg BW, Murariu D, Pang KP. Trends in cpap adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45(1):43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo J, Xiao S, Qiu Z, et al. Comparison of manual versus automatic continuous positive airway pressure titration and the development of a predictive equation for therapeutic continuous positive airway pressure in chinese patients with obstructive sleep apnoea. Respirology. 2013;18(3):528–533. doi: 10.1111/resp.12014. [DOI] [PubMed] [Google Scholar]
- 15.Stradling JR, Hardinge M, Paxton J, et al. Relative accuracy of algorithm-based prescription of nasal cpap in osa. Respir Med. 2004;98(2):152–154. doi: 10.1016/j.rmed.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Teschler H, Berthon-Jones M, Thompson AB, et al. Automated continuous positive airway pressure titration for obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;154(3 Pt 1):734–740. doi: 10.1164/ajrccm.154.3.8810613. [DOI] [PubMed] [Google Scholar]
- 17.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 aasm manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo YM, Qiu ZH, Wu HD, et al. Neural drive during continuous positive airway pressure (cpap) and pressure relief cpap. Sleep Med. 2009;10(7):731–738. doi: 10.1016/j.sleep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Nilius G, Happel A, Domanski U, et al. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest. 2006;130(4):1018–1024. doi: 10.1378/chest.130.4.1018. [DOI] [PubMed] [Google Scholar]
- 20.Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep. 2004;27(2):249–253. doi: 10.1093/sleep/27.2.249. [DOI] [PubMed] [Google Scholar]
- 21.Lloberes P, Ballester E, Montserrat JM, et al. Comparison of manual and automatic cpap titration in patients with sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1755–1758. doi: 10.1164/ajrccm.154.6.8970366. [DOI] [PubMed] [Google Scholar]
- 22.Galetke W, Anduleit N, Richter K, et al. Comparison of automatic and continuous positive airway pressure in a night-by-night analysis: a randomized, crossover study. Respiration. 2008;75(2):163–169. doi: 10.1159/000097767. [DOI] [PubMed] [Google Scholar]
- 23.Nolan GM, Doherty LS, Mc NW. Auto-adjusting versus fixed positive pressure therapy in mild to moderate obstructive sleep apnoea. Sleep. 2007;30(2):189–194. doi: 10.1093/sleep/30.2.189. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy B, Lasserson TJ, Wozniak DR, et al. Pressure modification or humidification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2019;12:D3531. doi: 10.1002/14651858.CD003531.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alves A, Gigante AR, Machado D, et al. Transition from apap to cpap may be a cost-effective health intervention in osa patients. J Bras Pneumol. 2021;47(6):e20210286. doi: 10.36416/1806-3756/e20210286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gay P, Weaver T, Loube D, et al. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29(3):381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 27.Boyer L, Philippe C, Covali-Noroc A, et al. Osa treatment with cpap: randomized crossover study comparing tolerance and efficacy with and without humidification by thermosmart. Clin Respir J. 2019;13(6):384–390. doi: 10.1111/crj.13022. [DOI] [PubMed] [Google Scholar]
- 28.Zhu D, Wu M, Cao Y, et al. Heated humidification did not improve compliance of positive airway pressure and subjective daytime sleepiness in obstructive sleep apnea syndrome: a meta-analysis. Plos One. 2018;13(12):e207994. doi: 10.1371/journal.pone.0207994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owens RL, Shafazand S. Effects of cpap and weight loss on osa outcomes. J Clin Sleep Med. 2014;10(12):1365–1367. doi: 10.5664/jcsm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou Q, Chen B, Loffler KA, et al. The effects of long-term cpap on weight change in patients with comorbid osa and cardiovascular disease: data from the save trial. Chest. 2019;155(4):720–729. doi: 10.1016/j.chest.2018.08.1082. [DOI] [PubMed] [Google Scholar]
- 31.Redenius R, Murphy C, O'Neill E, et al. Does cpap lead to change in bmi? J Clin Sleep Med. 2008;4(3):205–209. doi: 10.5664/jcsm.27181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pengo MF, Czaban M, Berry MP, et al. The effect of positive and negative message framing on short term continuous positive airway pressure compliance in patients with obstructive sleep apnea. J Thorac Dis. 2018;10(Suppl 1):S160–S169. doi: 10.21037/jtd.2017.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Ryswyk E, Anderson CS, Antic NA, et al. Predictors of long-term adherence to continuous positive airway pressure in patients with obstructive sleep apnea and cardiovascular disease. Sleep. 2019;42(10):1–9. doi: 10.1093/sleep/zsz152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.