Abstract
Purpose
To identify patient characteristics associated with successful isolated immature oocyte retrieval (IsO) during ovarian tissue cryopreservation (OTC) and to determine whether they are predictive of the collection of larger numbers of oocytes.
Methods
We retrospectively analyzed all patients undergoing OTC with IsO for fertility preservation over three years of activity at a university hospital. Univariate and multivariate analyses were used to identify the patients with the highest and lowest chances of oocyte recovery, and those with the largest numbers of oocytes. We also analyzed the correlation of IsO with the number of ovarian fragments collected and histological parameters.
Results
We analyzed 257 consecutive patients undergoing these procedures, at a median age of 17.1 years [0.3–38.3 years]. Isolated oocytes were obtained from 47.1% of patients, and IsO was more likely in patients with ovulatory cycles (63.0% vs. 38.6%; P≤ .001), without chemotherapy before OTC (61.4% vs. 33.1; P< .001) and with non-malignant diseases other than Turner syndrome (77.5%). Oocyte collection failure rates were highest in patients with Turner syndrome (OR 25.0, 95% CI 3.99–157.0; P< .001) or undergoing chemotherapy with alkylating agents before OTC (OR 37.6, 95% CI 8.36–168.8; P< .001). Prepubescent status (P= .043) and large numbers of ovarian fragments (P< .001) were associated with the retrieval of larger numbers of oocytes. Oocyte recovery was correlated with the presence of follicles in the medulla, but not with follicular density.
Conclusion
The chances of IsO differ between patients. Identifying patients with the highest chances of success facilitates appropriate resource allocation.
Keywords: Fertility preservation, Ovarian tissue cryopreservation, Isolated oocytes, Ex vivo oocyte retrieval
Introduction
Ovarian tissue cryopreservation (OTC) is a fertility preservation technique offered to women exposed to highly gonadotoxic treatments or suffering from diseases associated with a risk of premature ovarian failure. This fertility preservation technique is also the only option available for young prepubescent girls [1] and for women who have already received urgent chemotherapy [2]. It is now considered to be a validated technique for fertility preservation [3].
The first birth of a child following the autologous transplantation of cryopreserved ovarian tissue was reported in 2004 by Jacques Donnez [4]. More than 200 children have now been born following the autologous transplantation of ovarian tissue [5].
In 2003, Revel et al. provided the first description of the recovery of oocytes at the germinal vesicle stage by the aspiration of antral follicles visible at the surface of the ovary and in the preparation medium during OTC [6].
The recovery and preservation of isolated oocytes during OTC can now also be used as an additional technique for fertility preservation [7]. Oocytes have been recovered in this way from prepubescent girls, some of whom were very young, the youngest being only 3.5 months old [8]. They also have been collected from patients undergoing chemotherapy initiated before OTC [9], regardless of the phase of the cycle [10, 11], and from patients with solid tumors [6, 12], hematological malignancies [10], or non-malignant diseases [6, 10]. With in vitro maturation (IVM) culture practices, many mature oocytes can often be obtained in culture medium supplemented with gonadotropin, without the need for ovarian stimulation [13]. This technique has led to the birth of some children [13–18]. The cryopreservation of isolated oocytes also has the advantage of providing patients at risk of the reintroduction of a disease following the transplantation of ovarian tissue with an opportunity to benefit from an alternative fertility preservation technique.
However, the collection of isolated oocytes during ovary cryopreservation is time-consuming and costly [18]. It is still rarely used in current practice. Given the limited success of this technique in terms of the number of births reported to date, is it pertinent to research systematically for isolated immature oocytes during OTC?
The primary aim of our study was to identify women for whom the chances of obtaining isolated oocytes were highest and lowest by determining a priori parameters associated with the retrieval of isolated oocytes during OTC, as a function of oocyte origin (follicular fluid or preparation medium). The secondary aim was, among women who had isolated oocytes collected, to determine the parameters associated with number of isolated oocytes collected. We also analyzed the correlation of isolated oocyte retrieval with a posteriori parameters as the number of ovarian fragments collected and histological parameters.
Patients and methods
Patients
We performed a retrospective study of data prospectively recorded of consecutive patients undergoing OTC at the Reproductive Biology Laboratory of Pitié-Salpêtrière Hospital, Paris, over a period of three years. We excluded patients who had undergone ovarian stimulation in the month preceding OTC. Clinical data and information about OTC were extracted from the patients’ medical records. Data on isolated were extracted from the retrospective biology laboratory database. Histological data were extracted from pathology laboratory database. There is no missing data, except in the case of very young girls where no medulla was available for histological analysis. This study was approved by the local ethics committee for clinical research of Avicenne Hospital, Bobigny, France. (no. CLEA-2021-210)
Preparation of the ovarian tissue and collection of isolated oocytes
On arrival at the laboratory, the recovered ovarian tissue was prepared in a sterile single-use culture plate, as previously described [19]. However, before ovarian tissue preparation, any oocytes from antral follicles visible at the surface of the ovary were collected by puncture, with aspiration of the follicular fluid with a 30G needle mounted on a sterile insulin syringe (BD Micro-Fine 0.5 mL, Becton Dickinson, Franklin Lakes, USA). The follicular fluid was examined under a stereomicroscope to collect for cumulus-oocyte complexes. The ovarian cortex was then isolated from the medulla. The ovarian cortex was cut into fragments 4 to 10 mm long and 1 to 2 mm thick, placed in the freezing solution and frozen according to a slow-freezing protocol. The ovarian tissue preparation medium was also examined under a stereomicroscope to collect for cumulus-oocyte complexes. In each case, the cumulus-oocyte complexes recovered were placed in culture medium (Universal IVF medium, Medicult, Limonest, France) and incubated at 37°C, under an atmosphere containing 5% CO2, until freezing.
Histological examination
For each patient, a sample of ovarian cortex, selected at random from the fragments prepared for freezing before cryopreservation, and the removed medulla were fixed in formaldehyde and embedded in paraffin for histological examination. Sections (5 μm thick) were cut and stained with hematoxylin–eosin–saffron. All the follicles present in the ovarian cortex sample and the medulla were systematically counted once. Each follicle was classified according to the modified Oktay classification [20]. Follicular density was defined as the total number of primordial, early primary, and primary follicles per mm2.
Variables of interest
The outcome variables of interest were (1) the presence or absence of isolated collected oocytes, (2) the total number of oocytes collected, (3) the number of oocytes collected from follicles visible at the ovary surface, (4) the number of oocytes collected from the medium after ovarian tissue preparation. We analyzed the correlation of patient and disease variables with oocyte collection, by defining subgroups of patients with or without successful oocyte retrieval (total, from follicles, and from medium).
The covariates considered were: patient age at the time of OTC, ovarian status at the time of OTC (prepubescent or not; for postpubescent patients, the presence of ovulatory cycles), disease (classified into four groups: hematological malignancies, solid tumors, non-malignant diseases other than Turner syndrome, and Turner syndrome), chemotherapy before OTC, and, for patients already exposed to chemotherapy, the use of regimens with or without alkylating agents, the side of the body from which the ovarian tissues were retrieved, the embryologist responsible for ovary preparation and oocyte identification, and the number of frozen ovarian fragments. In addition to the parameters known before OTC, we felt that it was important to study the correlations between the number of fragments obtained, histological data (follicular density, the presence of tertiary follicles, and the presence of follicles in the medulla), and isolated oocyte retrieval during OTC.
Statistical analysis
Comparisons between the subgroups with and without oocyte collection were performed with Mann–Whitney tests for continuous variables and Fisher’s exact tests for categorical variables. The factors associated with the number of oocytes collected were investigated by zero-inflated negative binomial (ZINB) regression. Given the excess number of zeros in the distribution (absence of oocyte collection), ZINB regression was used to investigate the variables associated with an absence of oocyte retrieval (inflated component of the model). The same covariates were included in the negative binomial regression and zero-inflated components of the analysis: ovarian status at OTC (pubescent with ovulation cycles, pubescent without ovulation cycles, prepubescent), prior exposure to chemotherapy (no prior chemotherapy, prior chemotherapy without alkylating agent, prior chemotherapy with alkylating agent), type of disease (non-malignant other than Turner syndrome, Turner syndrome, hematological malignancies, solid tumors). Age at OTC was not considered in the model because of its strong correlation with ovarian status. A P value < 0.05 was considered statistically significant. Statistical analyses were performed with Stata 16.1 (StataCorp LLC, TX, USA).
Results
Characteristics of the population (Table 1)
Table 1.
Characteristics of the patients according to the success or failure of isolated oocyte retrieval and the source of the oocytes (antral follicles or preparation medium) retrieved during ovarian tissue cryopreservation
| Study parameters | General population | Oocytes collected | No oocytes collected | Oocytes collected from follicles | No oocytes collected from follicles | Oocytes collected from medium | No oocytes collected from medium |
|---|---|---|---|---|---|---|---|
| Patients n (%) | 257 (100) | 121 (47.1) | 136 (52.9) | 45 (17.5) | 212 (82.5) | 115 (44.7) | 142 (55.3) |
| Median age at OTC [range] | 17.1 [0.3–38.3] | 19.8a [0.3–38.3] | 15.6a [0.6–36.6] | 22.8b [4.0–34.8] | 15.6b [0.3–38.3] | 19.0 [0.3–38.3] | 15.9 [0.6–36.6] |
| Ovarian status at OTC n (%) | |||||||
| Prepubescent | 96(37.4) | 41 (42.7)b | 55 (57.3) b | 9 (20.0)b | 87 (41.0)b | 41 (35.6) | 55 (38.7) |
| Post-pubescent | 161 (62.6) | 80 (49.7) | 81 (50.3) | 36 (22.4)b | 125 (77.6)b | 74 (46.0) | 87 (54.0) |
| Post-pubescent without cycle | 88 (34.2) | 34 (38.6)b | 54 (61.4)b | 12 (26.7)b | 76 (35.9)b | 30 (26.1)b | 58 (40.9)b |
| Post-pubescent with cycles | 73 (28.4) | 46 (63.0)b | 27 (37.0)b | 24 (53.3)b | 49 (23.1)b | 44 (38.3)b | 29 (20.4)b |
| Prior chemotherapy n (%) | |||||||
| No | 127 (49.4) | 78 (61.4)c | 49 (38.6)c | 30 (23.6)a | 97 (76.4)a | 75 (59.1)c | 52 (40.9)c |
| Yes | 130 (50.6) | 43 (33.1)c | 87 (66.9)c | 15 (11.5)a | 115 (88.5)a | 40 (30.8)c | 90 ( 69.2)c |
| Yes, without alkylating agents | 68 (26.5) | 33 (48.5) | 35 (51.5) | 11 (16.2) | 57 (83.8) | 31 (45.6)c | 37 (54.4)c |
| Yes, with alkylating agents | 62 (24.1) | 10 (16.1)c | 52 (83.9)c | 4 (6.4) | 58 (93.6) | 9 (14.5)c | 53 (85.5)c |
| Diseases n (%) | |||||||
| Non-malignant diseases** | 40 (15.6) | 31 (77.5)c | 9 (22.5) c | 10 (25.0) | 30 (75.0) | 30 (75.0) c | 10 (25.0)c |
| Hematological malignancies | 89 (34.6) | 43 (48.3) | 46 (51.7) | 17 (19.1) | 72 (80.9) | 39 (43.8) | 50 (56.2) |
| Solid tumors | 100 (38.9) | 40 (40.0) | 60 (60.0) | 16 (16.0) | 84 (84.0) | 39 (39.0) | 61 (61.0) |
| Turner syndrome | 28 (10.9) | 7 (25.0)a | 21 (75.0)a | 2 (7.1) | 26 (92.9) | 7 (25.0)a | 21 (75.0)a |
Statistical comparisons (oocytes vs. no oocytes collected, oocytes vs. no oocytes collected from follicles, oocytes vs. no oocytes collected from medium): a: P <.05 and ≥.01, b: P <.01 and ≥.001, c: P<.001
**Non-malignant diseases other than Turner syndrome
During the study period, 259 patients underwent OTC. Two of these patients were excluded because they had undergone ovulation stimulation in the days immediately preceding OTC. In total, 257 patients were, therefore, included in the study. The median age of the patients at the time of OTC was 17.1 years [0.3 – 38.3 years]. Ninety-six patients (37.4%) were prepubescent at the time of OTC, and 161 patients were postpubescent (62.6%). These postpubescent patients included 73 (28.4%) with ovulatory cycles at the time of OTC, and 88 patients (34.2%) without ovulatory cycles for various reasons, such as oral contraception, recent chemotherapy or recent abortion because they were pregnant at the time of disease diagnosis.
The 257 patients included 38.9% with solid tumors (n=100), 34.6% with hematological malignancies (n=89), 10.9% with Turner syndrome (n=28), and 15.6% with other non-malignant diseases (n=40), 75% of whom had sickle cell disease (n=30). Chemotherapy had already been administered before OTC in 130 patients (50.6%); the remaining 127 patients (49.4%) had never undergone chemotherapy. Alkylating agents were included in the chemotherapy regimens of 62 of the 130 patients who had already undergone chemotherapy before OTC (48.5%). Fragments from a single ovary were cryopreserved for 249 patients, whereas fragments from both ovaries were cryopreserved for the remaining eight patients.
Collection of isolated oocytes
Oocytes were found in 47.1% of the patients (n=121). We retrieved 841 oocytes, 755 of which (89.8%) were collected from the ovarian preparation medium, and 86 (10.2%) of which were obtained by puncture of the antral follicles present at the surface of the ovary. Oocytes were collected solely from the preparation medium for 62.0% of the patients (n=75), solely by the aspiration of visible follicles for 5.0% of patients (n=6) and from both the preparation medium and follicles for 33.1% of patients (n=40).
Women with the highest chances of success of isolated oocytes retrieval
In univariate analysis (Table 1), the patients for whom oocytes were collected were significantly older (19.8 years versus 15.6 years, P = .02) than those for whom no isolated oocytes were obtained. The median age of the patients for whom oocytes were collected from the follicles visible at the surface of the ovary was 22.8 years, and that of the patients for whom oocytes were not collected from the follicles was 15.6 years (P= .001). Median age did not differ significantly between the groups of patients for whom oocytes were and were not recovered from the preparation medium (P = .09).
For the patients from whom isolated oocytes were collected, the chances of oocyte retrieval were found to be higher for (Table 1):
-
(i)
Postpubescent patients with menstrual cycles (63.0%) than for postpubescent women without ovulatory cycles (38.6%) (n=34/88; P = .003) and prepubescent patients (42.7%) (n=41/96; P = .01), regardless of oocyte origin (follicle or medium)
-
(ii)
Patients without prior chemotherapy (61.4%) than for those who had already undergone chemotherapy, regardless of its type (33.1%) (P< .001). This significant difference was observed both for oocytes obtained by follicular aspiration (P=.01) and for oocytes obtained from the preparation medium (P< .001). The impact of prior treatment on the chances of obtaining oocytes was greatest for regimens containing an alkylating agent. Oocytes were obtained from only 16.1% (10/62) of the patients treated with chemotherapy regimens containing alkylating agents, versus 48.5% (33/68) of those who had already been treated but without alkylating agents (P< .001). This significant difference was observed for oocytes recovered from the preparation medium (P< .001), whereas no significant difference was found for oocytes recovered from the follicles.
-
(iii)
Patients with non-malignant diseases other than Turner syndrome (77.5%).
Women with the lowest chances of success of isolated oocytes retrieval
By contrast, the patients with the lowest chances of oocyte retrieval were postpubescent patients without menstrual cycles (no oocytes retrieved in 61.4%), patients previously exposed to alkylating agents (83.9%), and patients with Turner syndrome (75.0%) (Table 1).
In multivariate analysis, only prior chemotherapy, whether without alkylating agents (OR 5.75, 95% CI 1.61–20.4, P= .007) or with alkylating agents (OR 37.6, 95% CI 8.36–168.8, P< .001), was associated with the failure of oocyte retrieval. Patients with hematological malignancies (OR 3.7, 95% CI 1.26–10.8, P = .017) or Turner syndrome (OR 25.0, 95% CI 3.99–157.0, P = .001) had higher rates of oocyte collection failure than patients with non-malignant diseases other than Turner syndrome. We also found that the collection of a smaller number of fragments was associated with a higher risk of oocyte collection failure (Table 2).
Table 2.
Multivariate binary logistic regression for the success of oocyte retrieval and the number of oocytes collected
| Variables | Zero-inflated | Negative binomial regression | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | IRR | 95%CI | P | |
| Age at OTC* | 0.97 | [0.90;1.04] | 0.35 | 1.03 | [0.99;1.07] | 0.12 |
| Ovarian status at OTC | ||||||
| Pubescent with ovulatory cycles (baseline) | 1 | - | 1 | - | ||
| Pubescent without ovulatory cycles | 2.49 | [0.75;8.27] | 0.14 | 0.97 | [0.60;1.57] | 0.904 |
| Prepubescent | 0.76 | [0.16;3.50] | 0.73 | 2.02 | [1.02;3.98] | 0.043 |
| Prior chemotherapy | ||||||
| No prior chemotherapy (baseline) | 1 | - | 1 | - | ||
| Prior chemotherapy, without alkylating agent | 5.75 | [1.61;20.4] | 0.007 | 0.77 | [0.46–1.29] | 0.32 |
| Prior chemotherapy, with alkylating agent | 37.6 | [8.36–168.8] | <0.001 | 1.3 | [0.62–2.74] | 0.49 |
| Diseases | ||||||
| Non-malignant diseases** (baseline) | 1 | - | 1 | - | ||
| Hematological malignancies | 3.70 | [1.26–10.8] | 0.017 | 0.87 | [0.50–1.52] | 0.62 |
| Solid tumors | 2.10 | [0.33–13.3] | 0.429 | 1.52 | [0.73;3.13] | 0.26 |
| Turner syndrome | 25.0 | [3.99–157.0] | 0.001 | 0.98 | [0.39;2.45] | 0.96 |
| Number of fragments* | 0.93 | [0.89;0.97] | 0.001 | 1.05 | [1.03–1.07] | <0.001 |
Abbreviations: OTC ovarian tissue cryopreservation, OR odds ratio, IRR incidence rate ratio, 95%CI 95% confidence interval
*Continuous variables
**Non-malignant diseases other than Turner syndrome
The other parameters analyzed, such as the side from which the ovary was collected, the embryologist responsible for ovary preparation and oocyte identification, and the regularity of ovulatory cycles, were not associated with the successful retrieval of oocytes.
Factors associated with the number of oocytes collected (Table 2)
Multivariate analysis of the association between the number of oocytes collected and these covariates was performed with the ZINB model. The ZINB model combines logistic regression analysis to predict the risk of oocyte collection failure (excess of 0 values) with a negative binomial regression analysis to predict the number of oocytes collected.
The negative binomial regression part of the model indicated that both prepubescent status (IRR 2.02, 95% CI 1.02–3.98, P = .043) and a large number of fragments (IRR 1.05, 95% CI 1.03–1.07, P< .001) were associated with the retrieval of larger numbers of oocytes (Table 2). The increase in the number of isolated oocytes retrieved with the number of fragments was particularly marked in prepubescent patients (Fig. 1A), patients with no prior chemotherapy (Fig. 1B), and patients with non-malignant diseases (Fig. 1C).
Fig. 1.
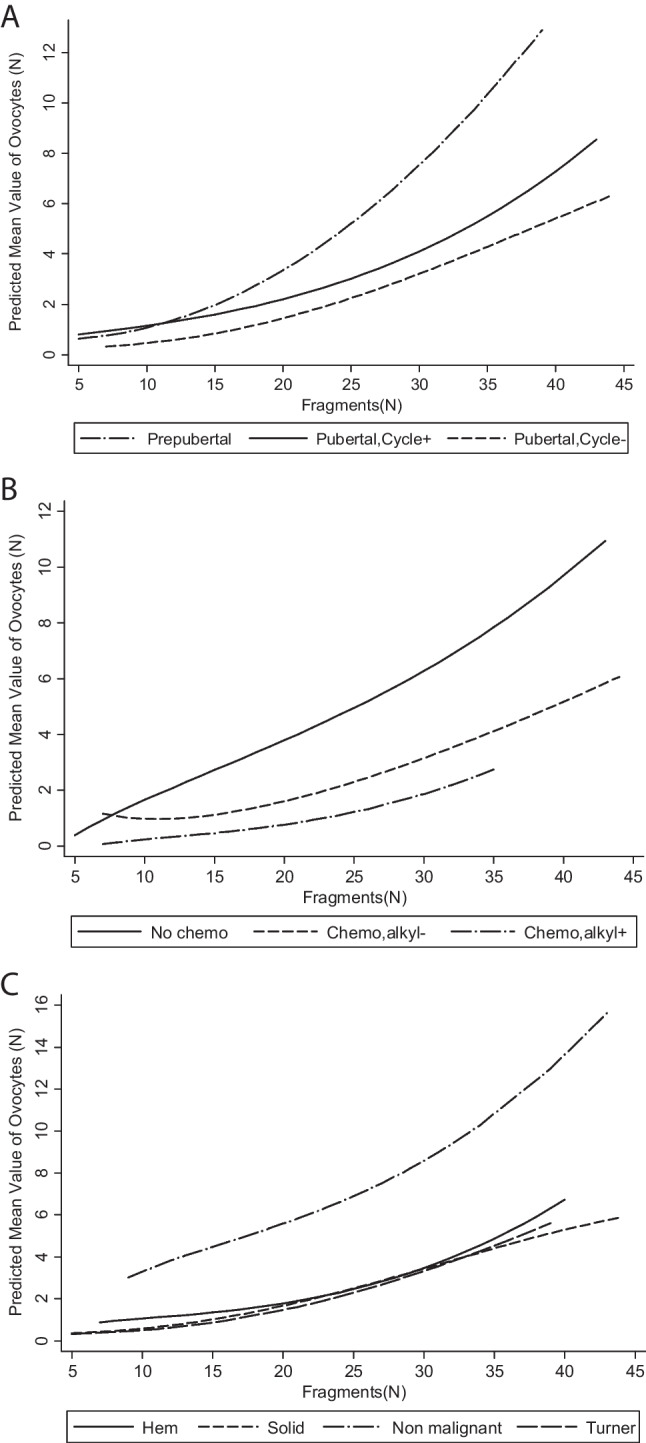
Predicted mean number of isolated oocytes obtained according to the number of fragments and the ovarian status of the patient (A), chemotherapy before OTC (B), and disease (C)
Histological parameters (Table 3)
Table 3.
Histological parameters, according to the success or failure of oocyte retrieval and the source of the oocytes (antral follicles or preparation medium) retrieved during ovarian tissue cryopreservation
| General population | Oocytes collected | No oocytes collected | P | Oocytes collected | No oocytes collected | P | Oocytes collected | No oocytes collected | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| From follicles | From medium | |||||||||
| Follicular density* | ||||||||||
| Median [range] | 1.75 [0–111.8] | 2 [0–107.6] | 1.63 [0–111.8] | 0.41 | 1.38 [0–49.8] | 2.17 [0–111.8] | 0.37 | 2.17 [0–107.6] | 1.59 [0–111.8] | 0.31 |
| Tertiary follicle(s) | ||||||||||
| Yes (%) | 21 | 14(11.6) | 7(5.2) | 0.07 | 6(13.3) | 15(7.8) | 0.26 | 13(11.3) | 8(5.6) | 0.08 |
| No (%) | 236 | 107(88.4) | 129(94.8) | 39(86.7) | 197(92.9) | 102(88.7) | 134(94.7) | |||
| Follicles in the medulla | ||||||||||
| (n=245) | ||||||||||
| Yes (%) | 198 | 102(89.5) | 96(73.3) | 0.002 | 41(93.2) | 157(78.1) | 0.01 | 97(89.8) | 101(73.7) | 0.001 |
| No (%) | 47 | 12(10.5) | 35(26.7) | 3(6.8) | 44(21.9) | 11(10.2) | 36(26.3) | |||
OTC ovarian tissue cryopreservation
*Continuous variables
Oocyte retrieval, whether from the medium or from follicles, was not correlated with either follicular density or with the presence of tertiary follicles within the tissue. However, oocyte retrieval from the medium and from follicles was positively correlated with the presence of ovarian follicles within the medulla (P = .001 and P = .01, respectively).
Discussion
We found that the likelihood of retrieving isolated oocytes was higher in older patients with ovulatory cycles at the time of OTC, and in patients who did not undergo chemotherapy before OTC. The risk of oocyte collection failure was highest if the ovarian tissue had been exposed to chemotherapy containing alkylating agents and in patients with Turner syndrome. In patients from whom isolated oocytes were retrieved, both prepubescent status and a large number of ovarian fragments were associated with the retrieval of a larger number of oocytes.
We found a significant difference in the chances of obtaining oocytes during OTC between postpubescent patients with ovulatory cycles, postpubescent patients without ovulatory cycles, and prepubescent patients. The chances of oocyte retrieval were higher in postpubescent women with ovulatory cycles than in the other groups. Another study found no difference according to the period in the ovulatory cycle [10].
In the study by Fasano cited above on a cohort of 57 patients, only four of whom were prepubescent, the number of oocytes was inversely correlated with patient age. However, as in our study, the mean number of oocytes retrieved was higher for prepubescent than for postpubertal patients. These results were confirmed in a larger study that nevertheless included only a small number of prepubescent individuals, at only six of the 136 patients included [21]. Our results conflict with those of a recent study of a smaller cohort containing a much larger proportion of prepubescent patients (43 prepubescent and 38 pubescent individuals) in which the mean number of oocytes retrieved per patient with positive retrieval results was significantly higher in postpubertal women than in prepubescent girls, and the mean number of oocytes retrieved per oophorectomy was greater in postpubertal than in prepubescent patients [22]. Karavani’s evaluation of in vitro maturation rates for young pre-menarche patients revealed no significant difference in the number of oocytes retrieved between the post- and pre-menarche groups, whereas, in comparisons of children aged 5–10 years and children aged 0–4 years, a significant positive correlation was found between age and the number of oocytes retrieved [23]. Another study of 39 postpubescent women found no link between the number of oocytes retrieved and patient age, but the 95% confidence interval for age was narrow (29.7 to 33.3 years) [11].
It is difficult to compare our results with published findings as a function of age for various reasons. Few studies have considered the rates of success for oocyte retrieval before focusing on the number of oocytes obtained. The cohorts studied are often small. Trends are sometimes revealed, but statistical analyses generally find no significant differences, and the populations studied have different characteristics (distribution of diseases, exclusion of patients on chemotherapy, etc.).
Our study is the first to show that disease can have repercussions for the retrieval and number of oocytes. Indeed, the risk of failure for oocyte retrieval was higher for Turner syndrome and malignant hematological diseases than for other diseases. This observation contrasts with the lack of impact of disease on the number of oocytes retrieved reported by Escriba [11]. However, the population studied by Escriba was highly homogeneous, with 85% of the patients undergoing OTC in the context of breast cancer, which was not the case for our population.
Few studies have considered the influence of prior chemotherapy on the chances of obtaining isolated oocytes from the follicles or preparation medium during OTC. Indeed, certain authors consider prior chemotherapy to be a contraindication for the retrieval of isolated oocytes [21, 23, 24]. Some authors have reported no significant effect of chemotherapy on the number of oocytes retrieved [9]. By contrast, we observed a clearly deleterious effect of alkylating agents on the chances of obtaining isolated oocytes.
The oocytes collected from the medium were of various sizes and constituted a highly heterogeneous population, whereas those from follicles had characteristics similar to those of oocytes retrieved by IVM. However, very few studies have considered this distinction. The distinction between oocytes from follicles and those from the medium appears to be important, because oocytes from these two sources probably differ in terms of competence [16] and future use. Oocytes harvested from ovarian follicles can be used in conventional oocyte in vitro maturation. Oocytes collected in the ovarian preparation medium could be more likely to be used, for example, in an artificial ovary program.
By distinguishing between oocytes from these two sources, we show here that age at OTC affects the likelihood of obtaining oocytes from follicles by puncture, but not the chances of obtaining isolated oocytes from the preparation medium used for the ovary tissue. Conversely, treatment with alkylating agents before OTC decreased the chances of obtaining oocytes from the medium, whereas it had no significant effect on the likelihood of obtaining oocytes from follicles. The disease also influenced the chances of obtaining oocytes from the medium, but not from follicles. Given the time-consuming nature of searches of the medium for oocytes, and the low likelihood of success in this context, such searches do not appear to be pertinent for patients treated with alkylating agents before OTC.
Several studies have reported a link between the amount of tissue collected and the number of oocytes obtained [10, 25], but our study is the first to demonstrate a significant positive correlation between the number of fragments obtained and the retrieval of isolated oocytes, regardless of the origin of the oocytes (follicle or preparation medium).
To our knowledge, this is also the first study to try to establish a correlation between the retrieval of isolated oocytes during OTC and histological data for the tissue samples collected, revealing an absence of relationship between the retrieval of isolated oocytes during OTC and follicular density.
Surprisingly, there was also no association between the presence of tertiary follicles and the number of oocytes retrieved by puncture from the follicles. This may be due to the rarity of tertiary follicles on histological examinations. By contrast, a significant positive correlation was found between the presence of follicles in the medulla and the presence of isolated oocytes. The cleavage plane between the cortex and the medulla of the ovary is not clearcut. If the cut is made too close to the cortex, it could lead to a release of isolated oocytes.
In conclusion, although our results have not been tested prospectively, this study reveals the existence of a population of patients for whom the chances of finding isolated oocytes during OTC procedures are higher (postpubescent patients with ovulatory cycles, a non-malignant disease other than Turner syndrome, and no chemotherapy before OTC), but also of a population for which the chances of finding isolated oocytes are very low (patients with Turner syndrome and patients undergoing chemotherapy with alkylating agents before OTC). These results should help the teams involved in OTC to mobilize resources more efficiently for this time-consuming technique and to provide women with additional chances of parenthood.
Acknowledgements
The authors thank Corinne Journo and Lilia Grira for technical assistance.
Author contribution
MP, FM, NB, and CP designed the study and contributed to the literature search, data collection, data interpretation, writing, and revision of the manuscript. NB performed statistical analysis and created the figure used in the manuscript. MP, FM, BS, LS, CJ, LG undertook the preparation, freezing and cryopreservation of the ovarian tissue, and the retrieval of isolated oocytes. They contributed to data collection and interpretation. AF was responsible for the ovarian tissue pick-up program. CG performed all the histological studies.
All the authors contributed to the report through review and discussion, and all agree to publication of the manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nicolas Boissel, Catherine Poirot, Marie Prades and Flora Marzouk contributed equally to this work.
References
- 1.Poirot CJ, Martelli H, Genestie C, Golmard JL, Valteau-Couanet D, Helardot P, et al. Feasibility of ovarian tissue cryopreservation for prepubertal females with cancer. Pediatr Blood Cancer. 2007;49:74–78. doi: 10.1002/pbc.21027. [DOI] [PubMed] [Google Scholar]
- 2.Poirot C, Fortin A, Dhédin N, Brice P, Socié G, Lacorte JM, et al. Post-transplant outcome of ovarian tissue cryopreserved after chemotherapy in hematologic malignancies. Haematologica. 2019;104:360–363. doi: 10.3324/haematol.2018.211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Practice Committee of the American Society for Reproductive Medicine Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022–1033. doi: 10.1016/j.fertnstert.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 5.Donnez J, Dolmans MM. Fertility preservation in women for medical and social reasons: oocytes vs. ovarian tissue. Best Pract Res Clin Obstet Gynaecol. 2021;70:63–80. doi: 10.1016/j.bpobgyn.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Revel A, Koler M, Simon A, Lewin A, Laufer N, Safran A. Oocyte collection during cryopreservation of the ovarian cortex. Fertil Steril. 2003;79:1237–1239. doi: 10.1016/S0015-0282(02)04963-4. [DOI] [PubMed] [Google Scholar]
- 7.Isachenko E, Rahimi G, Isachenko V, Nawroth F. In-vitro maturation of germinal-vesicle oocytes and cryopreservation in metaphase I/II: a possible additional option to preserve fertility during ovarian tissue cryopreservation. Reprod Biomed Online. 2004;8:553–557. doi: 10.1016/S1472-6483(10)61102-9. [DOI] [PubMed] [Google Scholar]
- 8.Poirot C, Brugieres L, Yakouben K, Prades-Borio M, Marzouk F, de Lambert G, et al. Ovarian tissue cryopreservation for fertility preservation in 418 girls and adolescents up to 15 years of age facing highly gonadotoxic treatment. Twenty years of experience at a single center. Acta Obstet Gynecol Scand. 2019;98:630–637. doi: 10.1111/aogs.13616. [DOI] [PubMed] [Google Scholar]
- 9.Abir R, Ben-Aharon I, Garor R, Yaniv I, Ash S, Stemmer SM, et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum Reprod. 2016;31:750–762. doi: 10.1093/humrep/dew007. [DOI] [PubMed] [Google Scholar]
- 10.Fasano G, Moffa F, Dechène J, Englert Y, Demeestere I. Vitrification of in vitro matured oocytes collected from antral follicles at the time of ovarian tissue cryopreservation. Reprod Biol Endocrinol. 2011;9:150–155. doi: 10.1186/1477-7827-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escribá MJ, Grau N, Escrich L, Novella-Maestre E, Sánchez-Serrano M. Spontaneous in vitro maturation of oocytes prior to ovarian tissue cryopreservation in natural cycles of oncologic patients. J Assist Reprod Genet. 2012;29:1261–1265. doi: 10.1007/s10815-012-9860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park CW, Lee SH, Yang KM, Lee IH, Lim KT, Lee KH, et al. Cryopreservation of in vitro matured oocytes after ex vivo oocyte retrieval from gynecologic cancer patients undergoing radical surgery. Clin Exp Reprod Med. 2016;43:119–125. doi: 10.5653/cerm.2016.43.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silber SJ, Goldsmith S, Castleman L, Hurlbut K, Fan Y, Melnick J, et al. In-vitro maturation and transplantation of cryopreserved ovary tissue: understanding ovarian longevity. Reprod Biomed Online. 2022;44:504–514. doi: 10.1016/j.rbmo.2021.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Prasath EB, Chan ML, Wong WH, Lim CJ, Tharmalingam MD, Hendricks M, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29:276–278. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- 15.Uzelac PS, Delaney AA, Christensen GL, Bohler HC, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104:1258–1260. doi: 10.1016/j.fertnstert.2015.07.1148. [DOI] [PubMed] [Google Scholar]
- 16.Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32:1221–1231. doi: 10.1007/s10815-015-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segers I, Bardhi E, Mateizel I, Van Moer E, Schots R, Verheyen G, et al. Live births following fertility preservation using in-vitro maturation of ovarian tissue oocytes. Hum Reprod. 2020;35:2026–2036. doi: 10.1093/humrep/deaa175. [DOI] [PubMed] [Google Scholar]
- 18.Kedem A, Yerushalmi GM, Brengauz M, Raanani H, Orvieto R, Hourvitz A, et al. Outcome of immature oocytes collection of 119 cancer patients during ovarian tissue harvesting for fertility preservation. J Assist Reprod Genet. 2018;35:851–856. doi: 10.1007/s10815-018-1153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirot C, Vacher-Lavenu MC, Helardot P, Guibert J, Brugières L, Jouannet P. Human ovarian tissue cryopreservation: indications and feasibility. Hum Reprod. 2002;17:1447–1452. doi: 10.1093/humrep/17.6.1447. [DOI] [PubMed] [Google Scholar]
- 20.Oktay K, Schenken RS, Nelson JF. Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol Reprod. 1995;53:295–301. doi: 10.1095/biolreprod53.2.295. [DOI] [PubMed] [Google Scholar]
- 21.Fasano G, Dechène J, Antonacci R, Biramane J, Vannin AS, Van Langendonckt A, et al. Outcomes of immature oocytes collected from ovarian tissue for cryopreservation in adult and prepubertal patients. Reprod Biomed Online. 2017;34:575–582. doi: 10.1016/j.rbmo.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Brouillet S, Ferrieres-Hoa A, Fournier A, Martinez G, Bessonnat J, Gueniffey A, et al. Cryopreservation of oocytes retrieved from ovarian tissue to optimize fertility preservation in prepubertal girls and women. J Vis Exp. 2020;23:164–171. doi: 10.3791/61777. [DOI] [PubMed] [Google Scholar]
- 23.Karavani G, Schachter-Safrai N, Revel A, Mordechai-Daniel T, Bauman D, Imbar T. In vitro maturation rates in young premenarche patients. Fertil Steril. 2019;112:315–322. doi: 10.1016/j.fertnstert.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Hourvitz A, Yerushalmi GM, Maman E, Raanani H, Elizur S, Brengauz M, et al. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod Biomed Online. 2015;31:497–505. doi: 10.1016/j.rbmo.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Huang JY, Tulandi T, Holzer H, Tan SL, Chian RC. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril. 2008;89:567–572. doi: 10.1016/j.fertnstert.2007.03.090. [DOI] [PubMed] [Google Scholar]


