Abstract
Enzymatic activities of aminopeptidase and β-glucosidase were investigated in Antarctic Ross Sea sediments at two sites (sites B and C, 567 and 439 m deep, respectively). The sites differed in trophic conditions related to organic matter (OM) composition and bacterial distribution. Carbohydrate concentrations at site B were about double those at site C, while protein and lipid levels were 10 times higher. Proteins were mainly found in a soluble fraction (>90%). Chloropigment content was generally low and phaeopigments were almost absent, indicating the presence of reduced inputs of primary organic matter. ATP concentrations (as a measure of the living microbial biomass) were significantly higher at site B. By contrast, benthic bacterial densities at site C were about double those at site B. Bacterial parameters do not appear to be “bottom-up controlled” by the amount of available food but rather “top-down controlled” by meiofauna predatory pressure, which was significantly higher at site B. Aminopeptidase and β-glucosidase extracellular enzyme activities (EEA) in Antarctic sediments appear to be high and comparable to those reported for temperate or Arctic sediments and characterized by low aminopeptidase/β-glucosidase ratios (about 10). Activity profiles showed decreasing patterns with increasing sediment depth, indicating vertical shifts in both availability and nutritional quality of degradable OM. Vertical profiles of aminopeptidase activity were related to a decrease in protein concentration and/or to an increase in the insoluble refractory proteinaceous fraction. The highest aminopeptidase activity rates were observed at site C, characterized by much lower protein concentrations. Differences in EEA between sites do not seem to be explained by differences in the in situ temperature (−1.6 and −0.8°C at sites B and C, respectively). Aminopeptidase activity profiles are consistent with the bacterial biomass and frequency of dividing cells. Enzyme substrate affinity was generally dependent upon substrate concentrations. EEA, normalized to bacterial numbers, indicated specific activities comparable to those reported for equally deep sediments at temperate latitudes. Vertical patterns of specific enzymatic activity appeared to be controlled by chloroplastic pigment concentrations that accumulate in the deeper sediment layers. The overall conclusion from the analysis of EEA in Antarctic sediments is that enzyme-dependent transformations of OM proceed at rates similar to those measured in temperate environments. Protein carbon potentially liberated by aminopeptidase activities (12.597 to 26.190 mg of C m−2 day−1) indicates that the whole protein pool could be mobilized within 1.3 to 17 h. Carbohydrate carbon mobilization (773 to 2,552 mg of C m−2 day−1) is sufficient to turn over the carbohydrate pool within 16 to 20 h. Such rates are 6 to 45 times higher than fluxes of particulate organic proteins and carbohydrates, indicating an “uncoupled hydrolysis” by the Antarctic benthic assemblages, in which bacteria appear to be able to rapidly exploit episodic OM pulses.
In marine sediments, organic matter (OM) diagenesis is largely dependent on bacterial activity (13). Sedimentary organic material is largely composed of high-molecular-weight compounds and particles unsuitable for direct utilization by bacteria (52). Extracellular enzyme activity (EEA) is generally recognized as the key step in degradation and utilization of organic polymers by bacteria, since only compounds with molecular mass lower than 600 Da can pass through cell pores (24, 27, 41).
Microbial degradation rates are dependent upon substrate composition, as well as on molecular structure (2). The presence of highly refractory compounds might significantly slow the conversion of OM into bacterial biomass with consequences for benthic microbial loop functioning. Previous studies reported that cycling of nitrogen compounds is largely influenced by the C/N ratio of OM in sediments (33) and that bacterial carbon conversion efficiency is inversely related to detritus aging (53). Temperature has also been identified as a factor controlling EEA (38), but with few exceptions (48) the enzymes apparently do not contain particular adaptations to cold environments (54).
The latter point appears to be of particular interest for understanding OM cycling at high latitudes. In fact, compared to temperate regions, polar oceans are characterized by very low EEA in the water column (54) that, coupled with high sedimentation rates (8), result in an accumulation of organic detritus of high nutritional quality in the sediments (18, 20), potentially supporting large microbial biomasses (32). Previous studies carried out in the Antarctic revealed that benthic bacterial densities are comparable to those reported at temperate latitudes (14, 20). However, the very low bacterial production rates suggest that a large fraction of the bacterial assemblage is inactive (55). Therefore, the large accumulation of sedimentary OM could be the result of an inefficient benthic microbial loop that is not able to channel organic detritus to higher trophic levels. Determination of the enzymatic activity in the sediments represents a key parameter for understanding the actual role of bacteria in Antarctic sediments.
Despite the importance of extracellular hydrolysis to bacteria and OM cycling, factors controlling EEA in marine sediments remain poorly understood, especially in the Antarctic ecosystem (31).
This study was carried out as a part of an integrated investigation (ROSSMIZE [Ross Sea Marginal Ice Zone Ecology]) of planktonic and benthic communities aimed at investigating pelagic-benthic coupling processes in relation to melting of sea ice in the Ross Sea (Antarctica). The aims of this study were (i) to assess the abundance, biomass, and distribution of benthic bacterial assemblages in two equally deep sites of the Ross Sea characterized by different available organic matter inputs; (ii) to analyze their exoenzymatic activities in relation to distribution and composition of the sedimentary organic matter; and (iii) to compare the results with previous studies carried out at different latitudes.
MATERIALS AND METHODS
Study site.
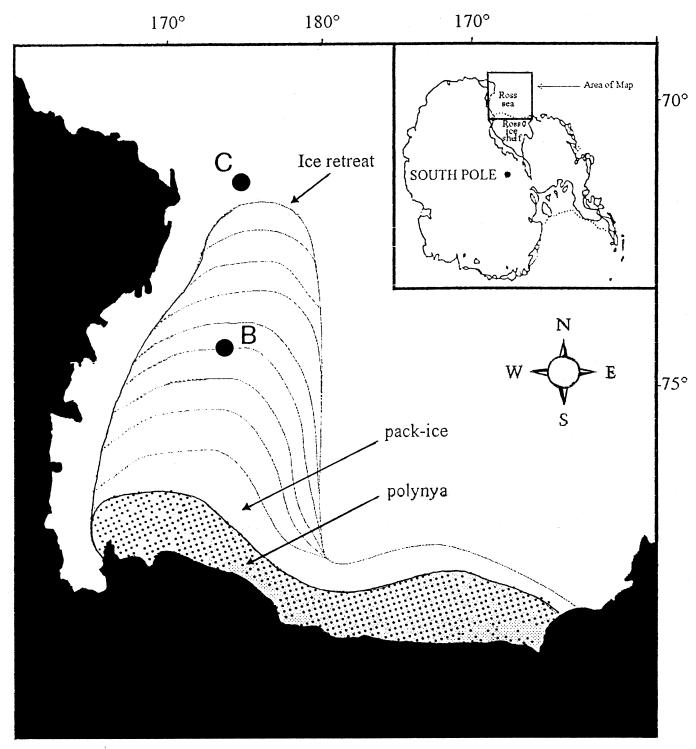
Sediment samples were collected in the Ross Sea (Antarctica) (72 to 74°S, 174 to 175°E) during the first leg of the X Italian Antarctic Expedition carried out by the R/V ITALICA between 15 November 1994 and 4 January 1995. Samples were taken from two different areas selected for mooring deployments: site B (average depth, 567 m) and site C (average depth, 439 m) (Fig. 1). Site B is located in the center of the northern part of the Joides basin and is characterized by biosiliceous olive-grey mud sediments that become anoxic at 5 to 7 cm (redox potential discontinuity layer). Site C is located in the northern flank of the Mawson Bank, very close to the shelf break. Sediments at site C are composed of sand, large amounts of gravel, pebbles, and coarse biogenic carbonate debris and are characterized by oxic conditions down to 15 cm. Site C is also characterized by high near-bottom current velocities (up to 20 cm s−1). The two sites contain different particulate organic material (19). The entire study area (Fig. 1) is characterized by a gradient of sedimentation rates: from 2.45 mg of OM m−2 day−1 in the polynyas (74°42′.411 S, 175°07′.280 E) to 1.79 mg of OM m−2 day−1 at site B (74°00′.117 S, 175°01′.696 E). OM is here considered to be the sum of protein and carbohydrate fluxes at 150 m of depth (from data collected during the survey [15a]). Since the use of multiple corers was precluded by the coarse sediment composition at site C and by the presence of dispersed stones at site B, sediments were collected with a large USNEL-type box corer (0.2 m2). At both sampling sites, three box corer samples were taken around the mooring position, at about 0.2 to 0.5 miles from each other (see Table 1). For the analysis of the biochemical composition of sedimentary OM, three replicate cores (from three different box corers) were collected at each site. Immediately after sampling, sediment cores were vertically divided into different layers: 0 to 1, 1 to 2, 2 to 5, 5 to 10, and 10 to 15 cm of depth at site B and 0 to 2, 2 to 5, and 5 to 10 cm of depth at site C. Sediments were placed in sterile petri dishes and frozen at −20°C. For microbial analyses, subsamples (1 cm3), were collected from the same cores as collected for OM analysis by using sterile syringes, with three to five replicates per sediment layer. Samples were fixed with 0.2-μm-pore-filtered seawater containing 2% buffered (sodium tetraborate, 20 g liter−1) formalin and stored at 4°C.
FIG. 1.
Sampling sites in the Ross Sea (Antarctica), with indication of the polynya position during the cruise.
TABLE 1.
Comparisons of EEA from sediments of different areas
| Area | Activity range (nM/cm3/h)a
|
Source or reference | |
|---|---|---|---|
| Aminopeptidase | β-Glucosidase | ||
| Howe Sound | 30–220 | 10–470 | 1 |
| Kiel Bay | 16,240–139,000 | ND | 47 |
| Intertidal sediments | ND | 23,200 | 31 |
| Breitenbach | ND | 35–77 | 37 |
| Damariscotta Estuary | 48–120 | ND | 38 |
| Northeastern Atlantic | 8,211–24,382 | 0.002–0.13 | 45 |
| Northeastern Atlantic | 6,000–14,700 | 9–135 | 4 |
| Celtic Sea | 25–90 | 0.05–0.7 | 46 |
| Arctic polynya | 10–10,000 | ND | 54 |
| Ross Sea | 1,312–2,728 | 81–266 | Present study |
For Howe Sound, Arctic polynya, and Ross Sea, values are given in nM/g/h. ND, not determined.
Biochemical composition of sediment organic matter.
For all biochemical analyses, 1 g of sediment was used. Lipids were extracted from dried sediment samples by direct elution with chloroform and methanol (3, 36). Lipid concentrations are reported as tripalmitine equivalents. Protein analyses were carried out after extractions with NaOH (0.5 M, 4 h) (25, 49). Soluble and total protein concentrations were determined according to Fabiano et al. (20). Concentrations are presented as albumin equivalents. Carbohydrates were analyzed according Gerchacov and Hatcher (23) and expressed as glucose equivalents. The method is based on the same principle as the widely used method of Dubois et al. (15) but is specifically adapted for carbohydrate determination in sediments. Absorbance was measured at 485 and 600 nm (for correction of the turbidity). Soluble carbohydrates were determined according to Fabiano et al. (20).
For each biochemical analysis, blanks were made with the same sediment samples as previously treated in a muffle furnace (450°C, 2 h). All analyses were carried out in four replicates per sediment layer. Protein, carbohydrate, and lipid concentrations were converted to carbon equivalents by using the following conversion factors: 0.49, 0.40, and 0.75 μg of C μg−1, respectively (16). The sum of protein, carbohydrate, and lipid carbon was referred as the biopolymeric carbon (BPC [sensu]) (21, 22).
Phytopigment analysis.
Chloroplastic pigments (chlorophyll a and phaeopigments) were extracted from about 1 g of sediment with 90% acetone. After centrifugation, the supernatant was used to determine the chlorophyll a concentration and then acidified with 0.1 N HCl to estimate phaeopigments (34). Chloroplastic pigment equivalents (CPE) were defined as the sum of chlorophyll a and phaeopigments. Chlorophyll a concentrations were converted to carbon equivalents by using the conversion factor 30 μg of C μg−1 (12).
Microbial parameters.
Each sediment replicate was added to 5 to 10 ml of 0.2-μm-pore-filtered and sterile seawater with prefiltered formaldehyde (2%). Samples were sonicated three times (Branson Sonifier 2200; 60 W for 1 min). Subsamples were diluted 100 to 500 times. Aliquots of the subsamples were stained with acridine orange (5 mg 1−1) and filtered on black Nucleopore 0.2-μm-pore-size filters. The filters were analyzed by epifluorescence microscopy (Zeiss Universal Microscope) (43). The frequency of dividing cells (FDC) was determined as the fraction of cells showing clear invagination. Bacteria were divided into different size classes, and bacterial biovolume was converted to carbon content assuming 310 fg of C per μm3 (21). Data were normalized to dry weight after desiccation (60°C, 24 h).
Immediately after sampling, triplicate subsamples (0.1 to 0.2 ml) were extracted into 5.0 ml of boiling phosphate buffer (40 mM, pH 7.70 [7]) and analyzed by the firefly (Firefly Lantern FLE 50; Sigma) bioluminescence assay with an ATP photometer (model 3000; Biospherical Instruments). Internal standards were used to correct for losses of extractable ATP. ATP concentrations were converted to carbon equivalents by using the factor 250 μg of C μg−1 (29).
Enzymatic activity.
Analyses of extracellular enzymatic activities (β-d-glucosidase, MFU–β-glucopyranoside [Glu-MFU], and aminopeptidase, l-leucine-4-methylcoumarinyl-7-amide [Leu-MCA]) were performed immediately after retrieval (39) as described previously for deep-sea sediments (42). Sediment slurries were prepared by using 1:1 dilutions (vol/vol) (44) in 0.2-μm-pore-filtered (Puradisc TM25AS), sonicated, and autoclaved bottom seawater (400 m deep). Incubations were performed at 1 atm (45) in the dark and at in situ (−0.8 to −1.5°C) temperatures (28) for 2 h. Enzymatic reactions were started by adding increasing concentrations (12.5, 25, 50, 100, and 200 μM) of Glu-MFU and Leu-MCA in replicate sediment samples. Each analysis was carried out at each sediment layer on two or three replicates for substrate concentration. The saturating concentration was 200 μM for both Glu-MFU and Leu-MCA. EEA rates increased linearly with time up to 4 h (28, 42). After incubation, the samples were centrifuged (3,000 rpm, 5 min) and the release of fluorescent dye was measured with a Perkin-Elmer spectrofluorometer (model LS50B) at 380 excitation, 440 emission (for Glu-MFU [28]) and at 365 excitation, 455 emission (for Leu-MCA [39]). Solutions of fluorescein (0.1 to 1.0 μM) were used as standards (freshly prepared with prefiltered seawater and sterilized seawater [26]). Data were normalized to dry weight (60°C, 24 h) and reported as nanomoles of fluorescein released per gram of sediment per hour.
Data analysis.
Differences in microbial parameters between sites were tested by nonparametric analysis (Kruskal-Wallis), as data did not meet the assumption for parametric analysis (analysis of variance) (51).
RESULTS
Environmental parameters.
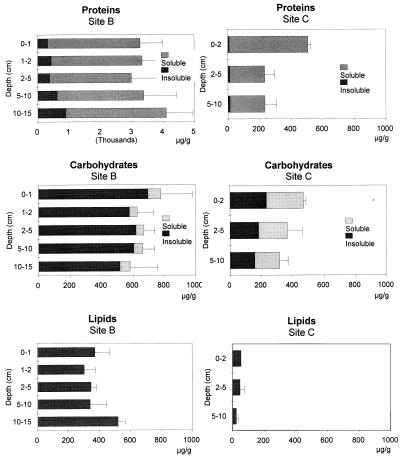
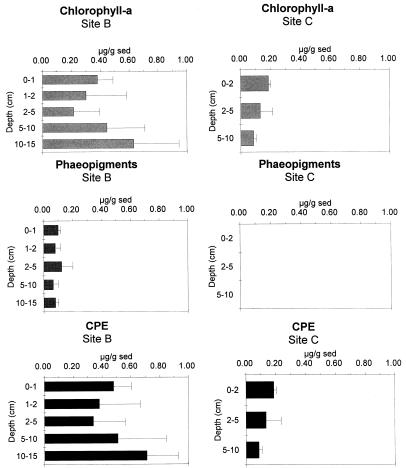
Evident differences in sedimentary structure were observed between sites. Site B was characterized by muddy sediments with interspersed stones, whereas site C showed large amounts of calcareous debris and briozoans in the top 2 cm, medium-size sands in the sediment layers down to 10 cm, and fine sand-mud in the bottom 10- to 15-cm layer (17). Such differences in grain size were reflected by qualitative and quantitative differences in OM biochemical composition. Proteins were the dominant biochemical class of organic compounds. Protein concentrations at site B were 6 to 14 times higher than at site C (Fig. 2) and ranged from 2,984 to 4,098 μg g−1 and from 507 to 232 μg g−1 (at sites B and C, respectively). Soluble proteins represented the main fraction, accounting for 78 to 89% of total protein concentrations at site B and for 95 to 98% at site C. Vertical patterns of total protein concentrations displayed a clear decrease with depth only at site C. The second main biochemical class was represented by carbohydrates (Fig. 2) that, at site B, showed concentrations about double (range, 780 to 581 μg g−1 from the 0 to 1 and 10 to 15-cm sediment layers, respectively) those at site C (range, 291 to 187 μg g−1 from the 0 to 2 and 5 to 10-cm sediment layers, respectively). Carbohydrate concentrations appear to differ significantly with depth only between the top and the lowest layers at site C. Soluble carbohydrates accounted only for a minor fraction of the total carbohydrate concentration: on average, about 10% at site B and 20% at site C. Finally, lipids (Fig. 2) displayed the same trend reported for proteins, with the highest values at site B (range, 301 to 518 μg g−1 from the 1 to 2 and 10 to 15-cm sediment layers, respectively) and the lowest concentrations at site C (on average, 7 to 14 times higher than at site B) (range, 54 to 23 μg g−1 from the 0 to 2 and 5 to 10-cm sediment layers, respectively). BPC concentrations were about 10 times higher at site B than at site C (integrated values down to the 10-cm depth, 2,285 and 231 μg of C g−1, respectively). Data on chloroplastic pigments in the sediments are reported in Fig. 3. At site B, chlorophyll a concentrations ranged from 0.6 ± 0.2 to 0.2 ± 0.2 μg g−1 (10 to 15 and 2 to 5-cm depths; on average, 0.4 ± 0.4 μg g−1). At site C, chlorophyll a concentrations ranged from 0.1 ± 0.0 to 0.2 ± 0.0 μg g−1 (5 to 10 and 0 to 2-cm depths; on average, 0.3 ± 0.2 μg g−1). No phaeopigments were found at site C, whereas at site B phaeopigment concentrations were quite constant with sediment depth (0.1 ± 0.0 μg g−1). As a result, CPE concentrations were about four times higher at site B than at site C.
FIG. 2.
Vertical distributions of the main biochemical classes of organic compounds at sites B and C. Reported are proteins, carbohydrates, and lipids. For proteins and carbohydrates, the water-soluble fraction is illustrated. Standard deviations are indicated (data are expressed in micrograms per gram [dry weight] of sediment).
FIG. 3.
Vertical distributions of phytopigment concentrations at sites B and C. Reported are chlorophyll a, phaeopigments, and CPE. Standard deviations are indicated (data are expressed in micrograms per gram [dry weight] of sediment).
Microbial parameters and enzymatic activities.
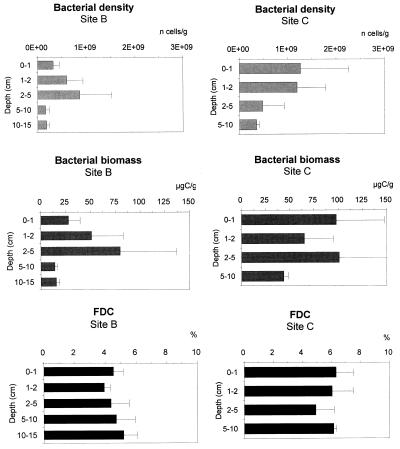
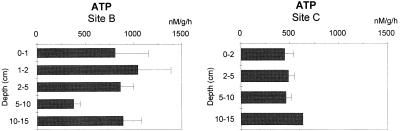
Vertical patterns of bacterial density, biomass, and frequency of dividing cells in the two sites are reported in Fig. 4. Bacterial density at site C (range, 3.6 × 108 to 12.7 × 108 cells g−1 of sediment at 5 to 10 and 0 to 2-cm depths, respectively) was about double that at site B (range, 1.7 × 108 to 8.7 × 108 cells g−1 of sediment at 10 to 15 and 2 to 5-cm depths, respectively). Bacterial density decreased regularly in the sediments at site C, while it showed a peak at 2 to 5 cm of depth at site B. Bacterial biomass followed a similar pattern at site B (range, 14.1 to 79.8 μg of C g−1 of sediment at 5 to 10 and 2 to 5-cm depths, respectively) but at site C showed a subsurface maximum at 2 to 5 cm of depth (101.0 μg of C g−1). FDC values were generally higher at site C (range, 4.9 to 6.3%) than at site B (range, 3.9 to 4.7%). By contrast, ATP concentrations were significantly higher at site B (integrated value, 718 ± 141 ng g−1) than at site C (integrated value, 523 ± 39 ng g−1) and did not display clear vertical patterns (Fig. 5). Total microbial biomass (as ATP carbon equivalents) ranged between 179.6 (integrated values at site B) and 101.6 μg of C g−1 (at site C). Bacterial contribution to the total microbial biomass was significantly different between sites, as bacterial biomass accounted for 17 and 64% at sites B and C, respectively. The ATP carbon equivalent, in turn, accounted for 8 and 41% of BPC at sites B and C, respectively.
FIG. 4.
Vertical distributions of bacterial parameters at sites B and C. Reported are bacterial‘ density (number of cells per gram [dry weight] of sediment), bacterial biomass (micrograms of carbon per gram), and FDC (percent). Standard deviations are indicated.
FIG. 5.
Vertical distributions of ATP concentrations at sites B and C. Standard deviations are indicated (data are expressed in nanomolar concentrations per gram [dry weight] of sediment per hour).
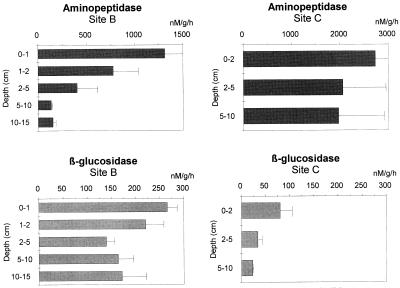
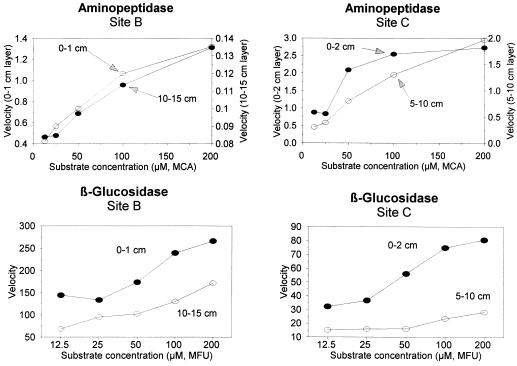
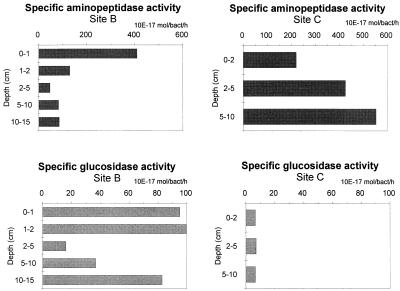
Activity profiles of the two hydrolytic enzymes in the two sites are presented in Fig. 6. Aminopeptidase activities were, on average, two to three times higher at site C than at site B, whereas β-glucosidase activities were clearly higher at site B than at site C. Aminopeptidase activities were always higher than β-glucosidase activities, with a ratio of 5:1 at site B and 34:1 at site C. Aminopeptidase activity in the top sediment layer ranged from 135.3 to 1,312.2 μM g−1 h−1 at site B and from 1,968.0 to 2,728.2 μM g−1 h−1 at site C, whereas β-glucosidase activity ranged from 139.3 to 265.8 μM g−1 h−1 at site B and from 23.4 to 80.5 at site C. The shape of the activity profile was dependent on site and enzyme. In the upper 10 cm of sediment, β-glucosidase decreased 38% at site B and 71% at site C, whereas aminopeptidase decreased 88% at site B and 28% at site C. The kinetics of enzyme activity with increasing substrate concentrations in the top 2 cm and in the deepest layers of the sediments are illustrated in Fig. 7. Substrate affinity for aminopeptidase was higher at site C than at site B (35 and 48 μM, respectively) and at both sites the highest Km values were found in subsurface sediment layers. Km values for β-glucosidase were similar (25 and 30 μM at sites B and C, respectively), but substrate affinity increased with depth in the sediments. Vertical patterns of enzymatic activity rates normalized to bacterial density are presented in Fig. 8. The highest aminopeptidase activities were observed at site C (222 × 10−17 to 409 × 10−17 mol number of bacteria−1 h−1), while the highest β-glucosidase activities were observed at site B (16.1 × 10−17 to 99.5 × 10−17 mol number of bacteria−1 h−1).
FIG. 6.
Vertical distributions of aminopeptidase and β-glucosidase at sites B and C. Standard deviations are indicated (data are expressed in nanomolar concentrations per gram [dry weight] of sediment per hour).
FIG. 7.
Substrate saturation curves for the hydrolysis of Leu-MCA and Glu-MFU in the top 1 cm of sediment. Mean values from three to five experiments are shown.
FIG. 8.
Vertical profiles of aminopeptidase and β-glucosidase normalized to bacterial numbers. Data are expressed as molar concentration times 10−17 per number of bacteria per hour.
The amounts of protein carbon potentially liberated by aminopeptidase activity in the 0- to 1-cm horizon were 12,597 and 26,190 mg of C m−2 day−1 at sites B and C, respectively, whereas the amounts of carbohydrate carbon potentially liberated by β-glucosidase activity in the 0- to 1-cm horizon were 2,552 and 773 mg of C m−2 day−1 at sites B and C, respectively. By converting total sedimentary carbohydrate and protein concentrations to carbon equivalents, it is possible to estimate the potential turnover rates of these two classes of organic compounds in the studied sediments. All carbohydrates were potentially degraded in 16.3 and 20.1 h at sites B and C, respectively, whereas all proteins were potentially degraded within 16.9 and 1.3 h at sites B and C, respectively.
DISCUSSION
In Antarctica, low temperatures and low microbial activities in the water column (9) determine low degradation rates and hence the accumulation of organic matter in the sediment (18, 20). In this study, we compared two sites characterized by quite evident trophic differences in terms of protein, carbohydrate, and lipid concentrations. A strong gradient of sedimentation rates was observed moving away from the polynyas (direction from site B to site C [Fig. 1]) so that concentrations of sedimentary organic compounds displayed significant differences between sites in agreement with the particle flux gradient. Carbohydrate concentrations were indeed about double at site B (with respect to site C), but the most evident differences were observed in terms of protein and lipid concentrations that were about 10 times higher at site B. OM concentrations (in terms of protein, carbohydrate, and lipid content of the sediments) are quite high compared with equally deep sediments in coastal areas at temperate latitudes and similar to those reported in highly productive environments (16, 38). A specific feature of OM composition in Antarctic sediments is the high concentration of proteins, mostly composed of soluble fraction (>90%). This is unusual, since in more oligotrophic environments such as the eastern Mediterranean Sea, soluble proteins generally account only for a minor fraction (10 to 20%) of the total proteinaceous material (11).
Phytopigment concentrations are assumed to represent a tracer of the primary organic input to the sediment (44). According to the patterns described for OM concentrations, chloropigment content was higher at site B. The large contribution of chlorophyll a to CPE at site B and the lack of phaeopigments at site C suggest the presence of fresh OM inputs.
It is generally recognized that bacterial distribution is largely dependent upon the amounts of utilizable OM in the sediments, which in turn is largely controlled by the sedimentation and degradation rates in the water column (10, 14). The different OM concentrations at the two sites were reflected by ATP concentrations (as an indicator of the microbial benthic biomass [29]), which displayed significant differences between sites (Kruskal-Wallis [P < 0.001]). However, benthic bacteria displayed the opposite pattern, as bacterial densities at site C were about double those at site B. These data are in contrast with most studies on bacterial distribution in marine sediments, which have found quite conservative bacterial densities in deep-sea sediments (10, 11). Data from this study indicate that bacterial abundance is not “bottom-up controlled” by the amount of potentially available compounds or by sediment structure (muddy sediments at site B versus coarse sandy sediments at site C). Therefore, other factors must be considered. The benthic microbial loop can play an important role in channelling organic detritus trough bacteria to higher trophic levels (13). A synoptic study on meiofaunal assemblages reported at site C densities six times lower than at site B (17). As the meiofauna was found to be mostly composed of selective and nonselective deposit feeders (35), it is possible that bacterial densities at site B are “top-down controlled” by meiofauna predatory pressure. If this is the case, the low predatory pressures at site C allowed a strong enhancement of the bacterial assemblages. However, other controlling factors might be hypothesized. In addition to the limitation of microbial communities by carbon, it has been shown that Antarctic bacterial populations might be limited by the availability of iron.
Enzyme activity in natural sediments might provide important indications of the OM flux crossing through the microbial loop as well as of the quantity and quality of the OM available to heterotrophs. However, despite the increasing use of fluorogenic substrates to assay enzyme activity, no standardized protocols exist. The concentrations of artificial substrate necessarily affect measured rates (39), and moreover the use of sediment slurry (i.e., sediment dilution) might underestimate the actual activity rates (45). In this study, the activity rates reported are those detected at saturating substrate concentrations (200 μM); therefore, comparisons with other studies must be made with caution, as often subsaturating concentrations were utilized (e.g., 10 μM [54]).
As previously reported for other coastal and deep-sea environments at temperate and high latitudes (9, 30, 40), aminopeptidase displayed the highest activity (4, 5, 45). However, in this study, aminopeptidase activity exceeded β-glucosidase activity by a factor of 10. This result is anomalous, as most benthic and water column studies reported aminopeptidase activity generally about 1,000 times higher than β-glucosidase (4, 9). In Celtic Sea sediments, the ratio between aminopeptidase and β-glucosidase activity rates was found to increase with increasing water depth (Leu-MCA/Glu-MFU ratio increased from 91 at 135 m to 567 at 1,680 m of depth) (46). Also, with respect to latitudinal patterns, the Leu-MCA/Glu-MFU ratio is expected to increase in Antarctica (9). As enzyme activities are stimulated by OM availability, it is possible that the relatively low aminopeptidase/β-glucosidase ratios found in our study are due to the high nutritional quality of the carbohydrate pools (soluble fraction of 10 to 20% versus about 1 to 5% in other deep-sea environments [11]).
Few data on sedimentary EEA are available in the literature for comparison. EEA rates (aminopeptidase and β-glucosidase) in Antarctic sediments appear to be comparable to those reported in temperate and Arctic sediments (Table 1). In particular, β-glucosidase activity values observed in Antarctic sediments (at 436 to 566 m of depth) appear to be in good agreement with those reported for deep-sea sediments of the northeastern Atlantic (4) although lower than those reported for coastal areas (39) and in intertidal sediments (31). Aminopeptidase activities appear to be generally lower than those reported for deep-sea or coastal sediments but within the same range as those reported for Arctic sediments (54). Values reported in this study, however, are higher than those reported in the continental slope of the Celtic Sea, where a high substrate concentration (1,000 μM [46]) was utilized. Therefore, values from the Celtic Sea are likely to be actually lower than those reported for Antarctic sediments at similar depths.
EEA activities in Antarctic sediments appeared to be highly site specific. The highest aminopeptidase activity rates were observed at site C, characterized by protein concentrations about 10 times lower than at site B. By contrast, β-glucosidase activities were significantly higher at site B in the presence of slightly higher carbohydrate concentrations. Differences in EEA activity between sites can hardly be explained by differences in the in situ temperature (−1.6 and −0.8°C at sites B and C, respectively). In this regard, Q10 values of <2 were observed in most Arctic sediment samples (54). Therefore, differences of 0.8°C between sites B and C are unlikely to determine an increase of two to three times the activity, as reported in this study. Alternatively, it is possible that lower aminopeptidase activity rates at site B are due to enzyme inhibition induced by the strong protein input. This enzymatic response was observed in experimentally manipulated deep-sea sediments (6), where a strong enhancement of β-glucosidase activity and nonresponse or inhibition of aminopeptidase activity with addition of their relative substrates were observed. However, differences in terms of aminopeptidase activity rates are consistent with the higher bacterial biomass and FDC at site C. As reported by other authors, aminopeptidase activities are the best descriptor for actual bacterial activity (6, 9).
Comparison of the kinetic parameters at the two stations revealed that substrate affinities were quite similar, although Km values for aminopeptidase were slightly higher at site B (characterized by higher protein concentrations) whereas Km values for β-glucosidase were not significantly different between the two sites (characterized by similar carbohydrate concentrations).
The overall conclusion than can be obtained from the analysis of EEA in Antarctic sediments is that enzyme-dependent transformations of OM are able to proceed at rates similar to those measured in more temperate environments.
The activity profiles showed decreasing patterns with increasing sediment depth. This might indicate vertical shifts in both the availability and nutritional quality of degradable OM (45). Vertical profiles of aminopeptidase activity at site C are consistent with a decrease in the total protein concentration. However, at site B the strong decrease in aminopeptidase activity disagrees with the increasing total protein concentration with depth into the sediment core. The reason for such different patterns of OM concentration versus EEA probably lie in the sedimentary protein pool composition. In fact, it is expected that a significant fraction of the proteinaceous material in sediment is refractory (16, 49). A detailed analysis of the protein profiles revealed that insoluble proteins (probably of more refractory composition) increased with increasing depth in the sediment. By contrast, most of the protein concentration at site C (95 to 98%) was soluble. Microbial decomposition of resistant OM is considerably stimulated by the availability of easily decomposable organic substances (50). Therefore, the observed vertical patterns suggest that enzyme activity rates depend upon OM composition (in terms of labile versus refractory compounds, e.g., insoluble proteins).
In order to test changes in specific bacterial enzyme activity rates with depth in the sediments, aminopeptidase and β-glucosidase activities were normalized to bacterial numbers (Fig. 8). Normalizing EEA is problematic, firstly because a large fraction of bacteria might be inactive and secondly because the contributions of other organisms to aminopeptidase and β-glucosidase activities are unknown. Few data are available for comparison; specific β-glucosidase activity of 17.8 × 10−17 mol cell−1 h−1 was reported previously for deep-sea sediments (4). Our results from Antarctic sediments are up to five times higher. However, much higher specific rates (up to 232 × 10−17 mol cell−1 h−1) have been reported in intertidal sediments (31). The comparison for aminopeptidase revealed that specific activities in the top sediment layer of Antarctic samples (222 × 10−17 and 409 × 10−17 mol cell−1 h−1, respectively, for sites C and B) were about 5 to 10 times lower than values reported for northeastern Atlantic deep-sea sediments (2,230 × 10−17 mol cell−1 h−1 [45] and 2,940 × 10−17 mol cell−1 h−1 [4]). Vertical patterns of enzyme specific activity did not always decrease regularly with depth in the sediment (Fig. 8). For instance, an increase in the specific β-glucosidase activity was observed at site B. Such a pattern did not reflect carbohydrate distribution but appeared to be consistent with chloroplastic pigment distribution (CPE [Fig. 3]), which accumulated in the deeper sediment layers. A similar increase of the specific aminopeptidase activity was reported at site C. In this case, again, vertical patterns of total protein concentration do not provide support for the interpretation of this result. Site C was a high-energy system. Therefore, it is possible that the strong bottom currents (up to 20 cm sec−1) inhibited enzyme production and/or removed the enzymes released from the top sediment layers.
The protein and carbohydrate carbon potentially liberated by the aminopeptidase and β-glucosidase activities was compared with total sedimentary protein and carbohydrate concentrations at the two stations and with data obtained for protein and carbohydrate fluxes (only at site B). In the top sediment horizon, 12,597 and 26,190 mg of protein C m−2 day−1 (at sites B and C, respectively) would be mobilized. These values compared to protein concentrations converted to carbon equivalents and normalized to square meters indicate that the entire protein pool could be mobilized within 17 and 1.3 h, respectively, for sites B and C. Similarly, mobilization rates of 773 and 2,552 mg of carbohydrate C m−2 day−1 (at sites C and B, respectively) are potentially able to mobilize the entire carbohydrate pool within 16 and 20 h (at sites B and C, respectively). Moreover, at site B, such rates are 6 to 45 times higher than the input of particulate organic proteins (280 mg of C m−2 day−1) and carbohydrates (433 mg of C m−2 day−1 [15a]). Similar results were reported in the northeastern Atlantic, where even higher discrepancies (a ratio of mobilized versus flux of about 200) were found (45). Such discrepancies might be partially explained with the use of potential enzyme activity rates at saturated substrate concentrations. Using subsaturating substrate concentrations (12.5 μM) instead of 200 μM, activity rates would be three to five times lower. Alternatively, EEA rates observed might be due to enzymes produced and released during periods of much higher OM inputs that remained in the system. This hypothesis has been recently proposed for deep-sea sediments at temperate latitudes (5), so differences between OM input and its biological utilization are not limited to Antarctic sediments. This fact would suggest the presence of an “uncoupled hydrolysis” strategy of the Antarctic benthic assemblages in order to be able to exploit rapidly the episodic OM pulses. In the future, the analysis of chitinase and aminoglycosidase activities should allow clarification of the extent of bacterial utilization of other carbon sources in Antarctic sediments.
ACKNOWLEDGMENTS
Roberto Danovaro is particularly indebted to F. Faranda (responsible for the project Ecology and Biogeochemistry of the Southern Ocean) and the crew of the R/V ITALICA for collaboration during sampling. Two anonymous referees greatly improved the quality of the manuscript. We also thank A. Dell’Anno and S. Vanucci for stimulating suggestions, L. Guglielmo (chief of expedition, University of Messina), M. Ravaioli (IGM, Bologna, Italy), P. Povero, and C. Misic (Genoa, Italy) for kindly providing all facilities on board.
This work has been undertaken as part of the National Programme for Antarctic Research (PNRA) funded by the Ministero dell’Università e Ricerca Scientifica e Tecnologica of Italy.
REFERENCES
- 1.Amy P S, Calwell B A, Soeldner A H, Morita R Y, Albright L J. Microbial activity and ultrastructure of mineral-based marine snow from Howe Sound, British Columbia. Can J Fish Aquat Sci. 1987;44:1135–1142. [Google Scholar]
- 2.Arnosti C, Repeta D J. Oligosaccharide degradation by anaerobic marine bacteria: characterisation of an experimental system to study polymer degradation in sediments. Limnol Oceanogr. 1994;39:1865–1877. [Google Scholar]
- 3.Bligh E G, Dyer W. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 4.Boetius A. Microbial hydrolitic enzyme activities in deep-sea sediments. Helgol Meeresunters. 1995;49:177–187. [Google Scholar]
- 5.Boetius A, Lochte K. Regulation of microbial enzymatic degradation of OM in deep-sea sediments. Mar Ecol Prog Ser. 1994;104:299–307. [Google Scholar]
- 6.Boetius A, Lochte K. Effect of organic enrichments on hydrolitic potentials and growth of bacteria in deep-sea sediments. Mar Ecol Prog Ser. 1996;140:239–250. [Google Scholar]
- 7.Bulleid N C. An improved method for extraction of adenosine triphosphate from marine sediment and seawater. Limnol Oceanogr. 1977;1:174–178. [Google Scholar]
- 8.Cattaneo-Vietti, R., and M. Fabiano. Sedimentation rates in the southern ocean: a review. Sci. Mar., in press.
- 9.Christian J R, Karl D M. Bacterial ectoenzymes in marine waters: activity ratios and temperature responses in three oceanic provinces. Limnol Oceanogr. 1995;40:1042–1049. [Google Scholar]
- 10.Danovaro R, Fabiano M, Della Croce N. Labile organic matter and microbial biomasses in deep sea sediments (Eastern Mediterranean Sea) Deep-Sea Res. 1993;40:953–965. doi: 10.1007/s002489900080. [DOI] [PubMed] [Google Scholar]
- 11.Danovaro, R., D. Marrale, A. Dell’Anno, N. Della Croce, A. Tselepides, and M. Fabiano. Bacterial response to seasonal changes in labile organic matter composition on the continental shelf and bathyal sediments of the Cretan Sea. Prog. Oceanogr., in press.
- 12.De Jonge V E. Fluctuations in the organic carbon to chlorophyll a ratios for estuarine benthic diatom populations. Mar Ecol Prog Ser. 1980;2:345–353. [Google Scholar]
- 13.Deming J W, Barross J A. The early diagenesis of organic matter: bacterial activity. In: Engel M H, Macko S A, editors. Organic geochemistry: principles and applications. New York, N.Y: Plenum Press; 1993. pp. 119–144. [Google Scholar]
- 14.Deming J W, Yager P L. Natural bacterial assemblages in deep-sea sediments: toward a global view. In: Rowe G T, Pariente V, editors. Deep-sea food chains and the global carbon cycle. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 11–28. [Google Scholar]
- 15.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 15a.Fabiano, M. Unpublished data.
- 16.Fabiano M, Danovaro R. Composition of organic matter in sediments facing a river estuary (Tyrrhenian Sea): relationship with bacteria and microphytobenthic biomass. Hydrobiologia. 1994;277:71–84. [Google Scholar]
- 17.Fabiano, M., and R. Danovaro. Meiofauna distribution and mesoscale variability in two sites of the Ross Sea (Antarctica) with contrasting food supply. Submitted for publication.
- 18.Fabiano M, Pusceddu A. Total and hydrolizable particulate organic matter (carbohydrates, proteins and lipids) at a coastal station in Terra Nova Bay (Ross Sea, Antarctica) Polar Biol. 1998;19:125–132. [Google Scholar]
- 19.Fabiano M, Povero P, Danovaro R. Distribution and composition of particulate organic matter in the Ross Sea (Antarctica) Polar Biol. 1993;13:525–533. [Google Scholar]
- 20.Fabiano, M., R. Danovaro, M. C. Chiantore, and A. Pusceddu. Bacteria, protozoa and organic matter composition in the sediment of Terra Nova Bay. In L. Guglielmo and A. Ianora (ed.), Ross Sea ecology, in press. Springer-Verlag, Berlin, Germany.
- 21.Fichez R. Composition and fate of organic matter in submarine cave sediments: implications for the biogeochemical cycle of organic carbon. Oceanol Acta. 1991;14:369–377. [Google Scholar]
- 22.Fry J C. Determination of biomass. In: Austin B, editor. Methods in aquatic bacteriology. New York, N.Y: John Wiley & Sons; 1990. pp. 27–72. [Google Scholar]
- 23.Gerchakov S M, Hatcher P G. Improved technique for analysis of carbohydrates in sediments. Limnol Oceanogr. 1972;17:938–943. [Google Scholar]
- 24.Gottschalk G. Bacterial metabolism. New York, N.Y: Springer-Verlag; 1986. [Google Scholar]
- 25.Hartree E F. Determination of proteins: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972;48:422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- 26.Hoppe H G. Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl-substrates. Mar Ecol Prog Ser. 1993;11:299–308. [Google Scholar]
- 27.Hoppe H G. Microbial extracellular enzyme activity: a new key parameter in aquatic ecology. In: Chrost R J, editor. Microbial enzymes in aquatic environments. Berlin, Germany: Springer-Verlag; 1991. pp. 60–80. [Google Scholar]
- 28.Hoppe H G. Use of fluorogenic model substrate for extracellular enzyme activity (EEA) measurement of bacteria. In: Kemp P F, editor. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 423–431. [Google Scholar]
- 29.Karl D M. Total microbial biomass estimation derived from the measurement of particulate adenosine-5′-triphosphate. In: Kemp P F, editor. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 359–368. [Google Scholar]
- 30.Kim S J. Bacterial number, heterotrophy and extracellular enzyme activity in Bransfield Strait, Antarctica. Korean J Polar Res. 1990;2:9–16. [Google Scholar]
- 31.King G M. Characterization of β-glucosidase activity in intertidal marine sediments. Appl Environ Microbiol. 1986;51:373–380. doi: 10.1128/aem.51.2.373-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knox G A. The biology of the southern ocean. Cambridge, England: Cambridge University Press; 1994. [Google Scholar]
- 33.Kuenen J G, Roberton L A. Interactions among bacteria metabolizing inorganic nitrogen compounds. In: Guerrero R, Pedròs-Aliò C, editors. Trends in microbial ecology. Barcelona, Spain: Spanish Society for Microbiology; 1993. pp. 33–36. [Google Scholar]
- 34.Lorenzen C, Jeffrey J. Determination of chlorophyll in sea water. UNESCO Tech Pap Mar Sci. 1980;35:1–20. [Google Scholar]
- 35.Manachini B. Biodiversity of Nematoda assemblages in the Antarctic Sea bed. M.S. thesis. Ghent, Belgium: University of Gent; 1997. [Google Scholar]
- 36.Marsh J B, Weinstein W J. A simple charring method for determination of lipids. J Lipid Res. 1966;7:574–576. [PubMed] [Google Scholar]
- 37.Marxsen J, Fiebig D M. Use of perfused cores for evaluating extracellular enzyme activity in stream-bed sediments. FEMS Microbiol Ecol. 1993;13:1–12. [Google Scholar]
- 38.Mayer L M. Extracellular proteolytic enzyme activity in sediments of an intertidal mudflat. Limnol Oceanogr. 1989;34:973–981. [Google Scholar]
- 39.Meyer-Reil L A. Measurement of hydrolitic activity and incorporation of dissolved organic substrate by microorganisms in marine sediments. Mar Ecol Prog Ser. 1986;31:143–149. [Google Scholar]
- 40.Meyer-Reil L A. Seasonal and spatial distribution of extracellular enzymatic activities and microbial incorporation of dissolved organic substrates in marine sediments. Appl Environ Microbiol. 1987;53:1748–1755. doi: 10.1128/aem.53.8.1748-1755.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer-Reil L A. Ecological aspects of enzymatic activity in marine sediments. In: Chrost R J, editor. Microbial enzymes in aquatic environments. Berlin, Germany: Springer-Verlag; 1991. pp. 84–95. [Google Scholar]
- 42.Meyer-Reil L A, Koster M. Microbial life in pelagic sediments: the impact of environmental parameters on enzymatic degradation of organic material. Mar Ecol Prog Ser. 1992;81:65–72. [Google Scholar]
- 43.Montagna P A. Sampling design and enumeration statistics for bacteria from marine sediments. Appl Environ Microbiol. 1982;43:1366–1372. doi: 10.1128/aem.43.6.1366-1372.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfannkuche O. Benthic response to the sedimentation of particulate organic matter at BIOTRANS station, 47°N, 20°W. Deep-Sea Res. 1993;40:135–150. [Google Scholar]
- 45.Poremba K. Hydrolytic enzymatic activity in deep-sea sediments. FEMS Microbiol Ecol. 1995;16:213–222. [Google Scholar]
- 46.Poremba K, Hoppe H G. Spatial variation of benthic microbial production and hydrolytic enzymatic activity down the continental slope of the Celtic Sea. Mar Ecol Prog Ser. 1995;118:237–245. [Google Scholar]
- 47.Reichardt W. Enzymatic potential for decomposition of detrital biopolymers in sediments from Kiel Bay. Ophelia. 1986;26:369–384. [Google Scholar]
- 48.Reichardt W. Impact of the Antarctic benthic fauna of the enrichment of biopolymer degrading psychrophilic bacteria. Microb Ecol. 1988;15:311–321. doi: 10.1007/BF02012644. [DOI] [PubMed] [Google Scholar]
- 49.Rice D L. The detritus nitrogen problem: new observations and perspectives from organic geochemistry. Mar Ecol Prog Ser. 1982;9:153–162. [Google Scholar]
- 50.Shimp R J, Pfaender F K. Influence of easily degradable naturally occurring carbon substrates on biodegradation of monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol. 1985;49:394–401. doi: 10.1128/aem.49.2.394-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sokal R R, Rohlf R J. Biometry, the principles and practice of statistics in biological research. W. H. San Francisco, Calif: Freeman; 1969. [Google Scholar]
- 52.Thurman E M. Organic geochemistry of natural waters. Dordrecht, The Netherlands: Martinus Nijhoff/Dr. W. Junk Publishers; 1985. [Google Scholar]
- 53.Turley C M, Lochte K. Microbial response to the input of fresh detritus to the deep-sea bed. Global Planet Change. 1990;89:3–23. [Google Scholar]
- 54.Vetter Y A, Deming J W. Extracellular enzyme activity in the Arctic northeast water polynya. Mar Ecol Prog Ser. 1994;114:23–34. [Google Scholar]
- 55.White D C, Smith G A, Nichols P D, Stanton G R, Palmisano A C. The lipid composition and microbial activity of selected recent Antarctic benthic marine sediments and organisms: a mechanism for monitoring and comparing microbial populations. Antarct J U S. 1985;19:130–132. [Google Scholar]