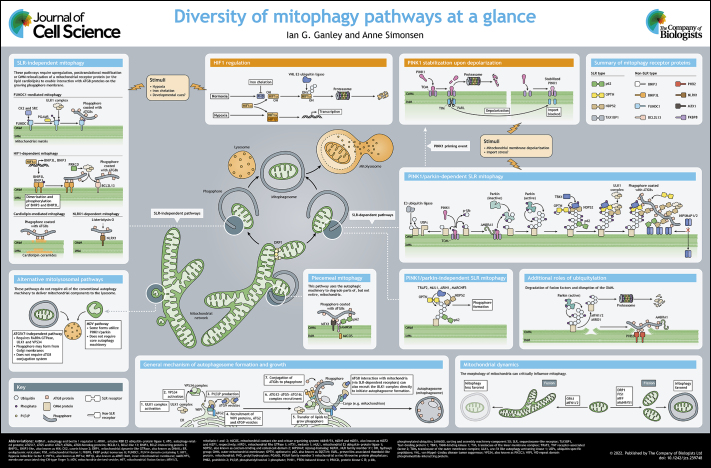
ABSTRACT
Mitochondria are crucial organelles that play a central role in various cell signaling and metabolic pathways. A healthy mitochondrial population is maintained through a series of quality control pathways and requires a fine-tuned balance between mitochondrial biogenesis and degradation. Defective targeting of dysfunctional mitochondria to lysosomes through mitophagy has been linked to several diseases, but the underlying mechanisms and the relative importance of distinct mitophagy pathways in vivo are largely unknown. In this Cell Science at a Glance and the accompanying poster, we describe our current understanding of how parts of, or whole, mitochondria are recognized by the autophagic machinery and targeted to lysosomes for degradation. We also discuss how this might be regulated under different physiological conditions to maintain mitochondrial and cellular health.
Keywords: Mitochondria, Mitophagy, SLR, PINK1, Parkin, HIF1, BNIP3, NIX, Selective autophagy
Summary: This Cell Science at a Glance provides insights into how mitochondria are recognized and targeted to lysosomes for degradation under different physiological conditions to maintain cellular homeostasis.
Introduction
Mitophagy is a type of selective autophagy that involves sequestration of mitochondria by a double-membrane phagophore that closes to form a mitophagosome, which upon fusion with a lysosome leads to degradation of the sequestered mitochondrial material. Mitophagosome biogenesis can be initiated by various cellular and environmental stressors, such as starvation, hypoxia and mitochondrial damage, but mitophagy is also important for the regulation of mitochondrial abundance in response to oocyte fertilization, erythroid cell maturation, stem cell pluripotency and neuronal differentiation (Montava-Garriga and Ganley, 2020; Ng et al., 2021; Onishi et al., 2021). Importantly, mitochondrial stress is a hallmark of several diseases, such as neurodegeneration and cancer (Bernardini et al., 2017; Moehlman and Youle, 2020), but further elucidation of different mitolysosomal pathways is needed to specifically address whether lysosomal degradation of mitochondria prevents or rather promotes disease development.
Several mitochondrial proteins and lipids have been implicated in the recruitment and activation of the core autophagy machinery (see Box 1) to initiate mitophagosome biogenesis. Mitophagy generally involves the binding of mitophagy receptor proteins to ATG8-homolog proteins (hereafter referred to as ATG8 proteins or ATG8s) in the mitophagosome membrane. Such mitophagy receptors can either be integral mitochondrial proteins localized to the outer mitochondrial membrane (OMM) or soluble autophagy receptor proteins of the sequestosome-like receptor (SLR) family that bind to ubiquitylated OMM proteins. The complexities of the molecular machineries involved in different mitophagy pathways, which are induced in response to various cellular and environmental stressors, are just starting to be unveiled, and little is known about how these machineries are regulated in time and space in vivo. Here, we provide a general overview of our current knowledge about the signals and mechanisms involved in different mitophagy pathways, which are summarized in the accompanying poster.
Box 1. Core autophagy machinery.
Macroautophagy, and hence mitophagy, requires proteins encoded by the core autophagy-related genes (ATGs) that are essential for autophagosome formation (for an in-depth review see Bento et al., 2016; Chang et al., 2021). These ATG proteins operate in complexes that can be divided into four main functional systems. (1) The ULK1 kinase complex, consisting of the ULK1 serine/threonine kinase, ATG13, ATG101 and FIP200, is thought to be the most upstream complex and acts as a node to convert stress signals into autophagosome formation. Major autophagy-regulating signaling pathways, such as the mechanistic target of rapamycin (MTOR) or AMP-activated protein kinase (AMPK) pathways, all converge at this kinase complex. (2) A major downstream target of the ULK1 complex is the next system, the class III phosphatidylinositol 3-kinase complex I, which consists of the VPS34 (PIK3C3) catalytic subunit, VPS15, Beclin1 and ATG14. This complex phosphorylates phosphatidylinositol at position 3 of its inositol ring to produce phosphatidylinositol 3-phosphate [PI(3)P]. This occurs on a specialized endoplasmic reticulum subdomain, termed the omegasome, which is thought to act as a cradle or platform to help form the autophagosome. Here, PI(3)P recruits downstream PI(3)P-binding proteins, including the WIPI family of proteins, that aid in the targeting of the next autophagy core system: the ubiquitin-like conjugation system. (3) The ubiquitin-like conjugation system conjugates the ubiquitin-like ATG8 family of proteins (see Box 2) to phosphatidylethanolamine (PE) in the growing autophagosomal membrane. This is achieved by two series of ubiquitin-like conjugation reactions. In the first, the ubiquitin-like protein ATG12 is conjugated to ATG5 via ATG7 (the E1 enzyme) and ATG10 (an E2 enzyme). The ATG12–ATG5 conjugate then binds to ATG16L1 to form the ATG12–ATG5–ATG16L1 complex, which acts as a novel E3 ligase enzyme in the conjugation of activated ATG8 proteins to PE. Prior to their lipidation, ATG8s are cleaved by ATG4 and conjugated to ATG7 and ATG3 (an E2 enzyme). The correct membrane targeting of ATG8 conjugation is achieved by the ATG12–ATG5–ATG16L1 complex through its direct membrane binding and interaction with the PI(3)P-binding protein WIPI2. As discussed in Box 2, the ATG8 proteins play key roles in controlling autophagosome size, cargo selection, fusion and lysosomal degradation. (4) The final system involved in autophagosome formation is the ATG9 system. ATG9A and ATG9B, the only transmembrane ATG proteins, are lipid scramblases that together with the lipid transfer proteins ATG2A and ATG2B play a key role in transferring lipid to the growing autophagosome.
Key mitophagy stimuli
For mitophagy receptors to trigger mitophagy, they must first be recruited to and/or activated at the target mitochondrion. While this is likely to occur under many diverse pathophysiological scenarios, our understanding of the nature of stimuli that lead to this is limited. However, mitochondrial depolarization and hypoxia-inducible factor 1 (HIF1)-dependent signaling play important roles in many of the mitophagy pathways studied to date.
Mitochondrial depolarization
Normal mitochondrial function requires polarization across the inner mitochondrial membrane (IMM). This proton electrochemical gradient, which is generated by the electron transport chain, is essential for ATP production and other mitochondrial transport functions. It is a key indicator of mitochondrial health, and depolarization frequently occurs as a result of mitochondrial damage or dysfunction. Depolarization can also act as a critical early step in the triggering of mitophagy, to maintain mitochondrial homeostasis (Ng et al., 2021). A major initial consequence of mitochondrial depolarization is an impairment or block of mitochondrial protein import (Zorova et al., 2018). This leads to stabilization of PTEN-induced kinase 1 (PINK1) in the translocase of the outer membrane complex (TOM complex) (Lazarou et al., 2012; Okatsu et al., 2013) and, as detailed below, mitophagy initiation. Indeed, inhibition of mitochondrial import itself is believed to be a critical mitophagy signaling event, and blockage of this by import stress, for example, is sufficient to trigger mitophagy (Burbulla et al., 2014; Jin and Youle, 2013). Depolarization also leads to relocalization, or accessibility to the mitophagy machinery, of other key mitochondrial components, such as the nipsnap homologs NIPSNAP1 and NIPSNAP2 (Princely Abudu et al., 2019), prohibitin 2 (PHB2) (Wei et al., 2017) and the mitochondrial lipid cardiolipin (Chu et al., 2013), all of which help to establish a combinatorial set of interactions that help to mount a robust mitophagy response to mitochondrial damage. Experimentally, depolarization-induced mitophagy is often stimulated using small-molecule mitochondrial uncouplers, such as carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (Narendra et al., 2008), or a combination of oligomycin A and antimycin A1 (Allen et al., 2013).
HIF1α stabilization
Hypoxia
Mitochondria are the major oxygen-consuming organelles of the cell; hence, they must be able to adapt to changes in oxygen tension and demand. A failure to do this under low (or high) oxygen tension can lead to increased reactive oxygen species (ROS) production, mitochondrial damage and cell death. Under these conditions, in particular in hypoxia, mitophagy can be activated. An important response to low oxygen is stabilization of HIF1α (also known as HIF1A), which drives a transcriptional program to optimize cell survival under low oxygen, including the upregulation of SLR-independent mitophagy via transcription of the OMM mitophagy receptors BCL2-interacting protein 3 (BNIP3) and BNIP3-like (BNIP3L, also known as NIX) (Bellot et al., 2009; Zhao et al., 2020) (see poster). However, hypoxia can also induce mitophagy independently of HIF1; for example, the mitophagy receptor FUN14 domain-containing 1 (FUNDC1) can activate mitophagy under hypoxia in platelets, which lack a nucleus and, hence, transcription (Zhang et al., 2016).
Iron depletion
In addition to low oxygen, HIF1α can be stabilized by other factors, such as intracellular iron depletion or a reduction in the levels of the tricarboxylic acid cycle metabolite α-ketoglutarate. Both iron and α-ketoglutarate are essential co-factors for the family of prolyl hydroxylases that are responsible for the targeted degradation of HIF1α; hence, conditions that drastically reduce these components can lead to HIF1-dependent mitophagy under normoxic conditions (Pugh, 2016). While this can occur physiologically through changes in metabolism, it can also be induced pharmacologically with the use of iron chelators, such as deferiprone (DFP) or desferrioxamine (DFO), and synthetic α-ketoglutarate analogs including dimethyloxalylglycine (DMOG) (Allen et al., 2013).
Differentiation and metabolism
HIF1α stabilization also results in cellular metabolic remodeling through an upregulation of genes encoding glycolytic enzymes (Kierans and Taylor, 2021). Enhancement of glycolysis affords conditions permissive for mitophagy: it helps to maintain energy balance by providing increased non-mitochondrial ATP production. However, it also appears that mitophagy can facilitate this glycolytic shift, as BNIP3L-mediated mitophagy is critical for the glycolytic change that drives differentiation during neuronal development and macrophage activation (Esteban-Martinez et al., 2017).
Different mitophagy pathways
Our mechanistic knowledge of mitophagy in response to different mitochondrial stressors is still limited, making it difficult to segregate distinct pathways. With our current understanding, two main types of mitophagy can be distinguished based on the requirement for soluble mitophagy receptor proteins of the SLR family versus mitophagy receptors being localized to the OMM (see poster). The former is generally dependent on ubiquitylation of OMM proteins by the RING-between-RING (RBR) ubiquitin–protein ligase parkin (encoded by PRKN) and PINK1, both of which are mutated in some forms of hereditary Parkinson's disease (McWilliams and Muqit, 2017). Given this, this type of mitophagy is often referred to as PINK1/parkin-dependent mitophagy. However, there are now known examples of SLR-dependent pathways that appear to be independent of PINK1 and parkin, and in the following sections, we therefore discuss these two pathways separately and in contrast to the SLR-independent mitophagy pathways that rely on mitochondrial receptors.
SLR-dependent mitophagy – a soluble receptor pathway
PINK1/parkin-dependent SLR mitophagy
This ubiquitin-dependent mitophagy pathway is typically stimulated by mitochondrial membrane depolarization, leading to stabilization of the serine/threonine kinase PINK1 on the OMM (see poster). PINK1 is normally imported into healthy mitochondria, leading to its cleavage by mitochondrial proteases (Greene et al., 2012; Jin et al., 2010) and subsequent proteasomal degradation (Yamano and Youle, 2013). However, when at the OMM, PINK1 becomes activated, leading to phosphorylation of ubiquitin (at Ser65) conjugated to OMM proteins, and mitochondrial recruitment and activation of the E3 ubiquitin ligase parkin (Kane et al., 2014; Kazlauskaite et al., 2014; Okatsu et al., 2015, 2013; Wauer et al., 2015). Parkin further ubiquitylates OMM proteins to generate polyubiquitin chains (Narendra et al., 2008). Other E3 ubiquitin ligases have been found to cooperate with parkin in polyubiquitylation of OMM proteins and mitophagy, including mitochondrial E3 ubiquitin–protein ligase 1 (MUL1) (Rojansky et al., 2016; Yun et al., 2014), ariadne RBR E3 ubiquitin–protein ligase 1 (ARIH1) (Villa et al., 2017) and membrane-associated ring-CH-type finger 5 (MARCHF5, also known as MITOL) (Koyano et al., 2019).
Ubiquitylated OMM proteins are then recognized by autophagy receptors of the SLR family, which includes sequestosome 1 (SQSTM1, hereafter referred to as p62), NBR1, optineurin (OPTN), Tax1-binding protein 1 (TAX1BP1) and calcium-binding and coiled-coil domain 2 (CALCOCO2, hereafter referred to as NDP52). The SLRs all have specific ubiquitin-binding domains, as well as an LC3-interacting region (LIR) that facilitates their interaction with ATG8 proteins in the autophagosome membrane (Dikic and Elazar, 2018; Johansen and Lamark, 2020) (see Box 2). NDP52 and OPTN have been found to be absolutely required for PINK1/parkin-dependent mitophagy in HeLa cells (Lazarou et al., 2015), but other SLRs have also been implicated in mitophagy – for instance, p62 in macrophages and leukemia cells (Nguyen et al., 2019; Zhong et al., 2016). The interaction of SLRs with an ATG8 protein can be modulated by phosphorylation of residues within and surrounding the core LIR motif (Box 2). As an example, the function of OPTN in parkin-dependent mitophagy is regulated by phosphorylation mediated by both TANK-binding kinase 1 (TBK1) and unc-51 like autophagy activating kinase 1 (ULK1) (Harding et al., 2021). The pro-autophagic autophagy and beclin 1 regulator 1 (AMBRA1) also has an LIR and becomes recruited to the OMM of depolarized mitochondria, where it promotes PINK1 stability and interacts with ATG8s to induce mitophagy (Di Rienzo et al., 2022; Strappazzon et al., 2015). The interaction between AMBRA1 and ATG8 proteins is positively regulated by both ubiquitylation and phosphorylation (Di Rita et al., 2018).
Box 2. ATG8 proteins and LIR motifs.
Mammalian ATG8 proteins include seven paralogs of the single yeast Atg8 protein and can be grouped in two subfamilies: microtubule-associated protein light chain 3 proteins (referred to collectively as LC3 and comprising LC3A, encoded by MAP1LC3A; LC3B, encoded by MAP1LC3B; LC3B2, encoded by MAP1LC3B2; and LC3C, encoded by MAP1LC3C) and γ-aminobutyric acid receptor-associated proteins (GABARAP, GABARAPL1, GABARAPL2). ATG8s are ubiquitin-like proteins that become covalently linked to PE on both sides of the forming autophagosome double membrane (see Box 1). The exact functions of the various ATG8s are still poorly understood, but they do have in common the ability to bind to proteins containing specific LIR motifs (for an in-depth review please see Johansen and Lamark, 2020; Nguyen and Lazarou, 2022; Slobodkin and Elazar, 2013; Stolz et al., 2014). The core LIR motif consists of an aromatic amino acid and a hydrophobic amino acid that are separated by any two amino acids – [W/F/Y]-X1-X2-[I/L/V] – and is often surrounded by negatively charged residues or phosphorylatable serine/threonine residues that can regulate interactions between the LIR and ATG8 proteins. The side chains of the core aromatic and hydrophobic LIR residues interact with two hydrophobic pockets on the surface of ATG8s. While some LIR-containing proteins appear to interact with all ATG8s, others might have a preference for LC3 or GABARAP family members. Based on a comparison of LIR sequences and mutation analysis, a specific GABARAP-interacting motif (GIM) has been defined: [W/F]-[V/I]-X2-V. The main function of ATG8s appears to be to recruit LIR-containing proteins that act as autophagy receptors or autophagy adaptors. Autophagy receptors link the cargo to be degraded to ATG8s on the inner autophagic membrane, leading to lysosomal delivery and degradation of both cargo and receptor proteins. In contrast, autophagy adaptor proteins interact with ATG8s on the outer autophagic membrane to facilitate autophagosome formation, autophagosome transport or autophagosome–lysosome fusion, without being themselves degraded by autophagy. It is, however, important to note that ATG8s are not absolutely required for autophagosome biogenesis, but rather that they appear to regulate autophagosome size and kinetics. Moreover, conjugation of ATG8s to single membranes (referred to as CASM) can take place in processes other than autophagy, such as secretion, phagocytosis and endosomal repair.
In addition to their role in bridging polyubiquitylated mitochondria to the phagophore membrane via binding to ubiquitin and ATG8s, it has recently been found that SLRs promote de novo phagophore membrane biogenesis by recruiting the ULK1 kinase complex (also known as the ULK1/ATG1 kinase complex; Box 1) (Lazarou et al., 2015; Ravenhill et al., 2019; Turco et al., 2019; Vargas et al., 2019). For PINK1/parkin-dependent mitophagy, NDP52 has been found to promote mitochondrial recruitment of FIP200 (also known as RB1-inducible coiled-coil 1, RB1CC1) (Vargas et al., 2019), likely by binding of its LIR domain to the claw domain of FIP200 (Fu et al., 2021), further triggering membrane binding of the ULK1 complex (Shi et al., 2020). Furthermore, binding of the SKICH domain of NDP52 to FIP200 is essential for xenophagy (Ravenhill et al., 2019), suggesting that this is also important for mitophagosome biogenesis.
Binding of autophagy receptors to ubiquitylated OMM proteins can be further modulated by their interaction with internal mitochondrial proteins and lipids that function as specific ‘eat me’ signals on the surface of damaged mitochondria. Mitochondrial recruitment of NDP52, as well as other SLRs, has been found to depend on binding to the mitochondrial matrix proteins NIPSNAP1 and NIPSNAP2, which become stabilized on the surface of depolarized mitochondria (Princely Abudu et al., 2019) (see poster). Moreover, the IMM protein PHB2 interacts with LC3 upon proteasomal-dependent OMM rupture (Wei et al., 2017) and has been implicated in stabilization of PINK1 on the OMM following membrane depolarization (Yan et al., 2020).
It is interesting to note that ATG8s are important, but not essential, for cargo selection during PINK1/parkin-dependent mitophagy (Nguyen et al., 2016; Vaites et al., 2018). In line with this, LIR-independent recruitment of SLRs to mitochondrial phagophores appears to amplify the mitophagy signal, as the SLRs promote further recruitment of the cargo receptors and autophagy machineries (Padman et al., 2019). Thus, complex multivalent interactions of SLRs and ATG8s with mitochondrial proteins and lipids underlie the coordination of events required for mitophagosome membrane biogenesis around damaged or dysfunctional mitochondria. Little is known about how SLR-dependent mitophagy is turned off, but several deubiquitylating enzymes (DUBs) have been shown to antagonize parkin-mediated ubiquitylation and mitophagy, including the ubiquitin-specific peptidases USP15, USP30, USP33, USP35 and USP36 (Bingol et al., 2014; Cornelissen et al., 2014; Geisler et al., 2019; Gersch et al., 2017; Niu et al., 2020; Wang et al., 2015). Furthermore, protein phosphatase with EF-hand domain 2 (PPEF2), which dephosphorylates ubiquitin at Ser65, has been shown to inhibit PINK1-dependent mitophagy (Wall et al., 2019).
PINK1/parkin-independent SLR mitophagy
Several E3 ubiquitin ligases appear to have a redundant function with parkin to promote SLR-dependent mitophagy, including TNF receptor-associated factor 2 (TRAF2) (Yang et al., 2015), CIAP (also known as BIRC2) (Zachari et al., 2019) and the α isoform of tripartite motif-containing protein 5 (TRIM5α) (Saha et al., 2022). Induction of mitophagy by the lactone ivermectin, causing a rapid fragmentation of mitochondria, results in mitochondrial recruitment of OPTN. Mitochondrial OPTN recruitment requires TBK1-mediated activation of FIP200 and ATG13, but not the activity of the kinases ULK1 and ULK2 (Zachari et al., 2019). At mitochondria, OPTN further promotes de novo synthesis of mitophagosomes by recruiting vesicles containing ATG9A (Yamano et al., 2020), a lipid scramblase required for phagophore biogenesis (Matoba et al., 2020).
SLR-independent mitophagy – a mitochondrial receptor pathway
Induction of mitophagy in response to other cellular or environmental stressors, such as iron depletion and hypoxia, does not appear to depend on ubiquitylation of mitochondrial OMM proteins and SLRs. However, there seems to be a general requirement for integral mitochondrial proteins that have a LIR domain that can interact directly with ATG8 proteins in the phagophore membrane. Such mitophagy receptors are generally mitochondrial proteins anchored on the cytosolic face of the OMM and include BNIP3 (Quinsay et al., 2010), BNIP3L (Novak et al., 2010; Sandoval et al., 2008; Schwarten et al., 2009), FUNDC1 (Chen et al., 2016; Liu et al., 2012), BCL2-like 13 (BCL2L13) (Murakawa et al., 2015), FKBP prolyl isomerase 8 (FKBP8) (Bhujabal et al., 2017) and NLR family member X1 (NLRX1) (Zhang et al., 2019) in humans (see poster). In yeast, the OMM protein Atg32 interacts with Atg8 and Atg11 to mediate mitophagy (Kanki et al., 2009; Okamoto et al., 2009).
Although our knowledge about how the mitophagy functions of these receptors are regulated is still sparse, it is becoming evident that their activity is tightly regulated at several levels. BNIP3L and BNIP3 levels are transcriptionally upregulated by HIF1α during hypoxia (Bellot et al., 2009) and iron depletion (Allen et al., 2013; Zhao et al., 2020). At basal levels, expression of both BNIP3L and FUNDC1 are regulated by miR137, a microRNA that is downregulated in response to hypoxia, thereby preventing mitophagy under normoxia conditions (Li et al., 2014). As is the case for SLRs, the affinity of mitochondrial mitophagy receptors towards ATG8 proteins is closely controlled by phosphorylation of their LIR motifs (Poole et al., 2021; Rogov et al., 2017; Zhu et al., 2013). As an example, the LIR motif of BNIP3 is enclosed by two serine residues (Ser17 and Ser24), where phosphorylation of Ser17 is essential for BNIP3 binding to LC3B, whereas phosphorylation of both Ser17 and Ser24 facilitates its binding to GABARAPL2, further promoting mitophagy (see Box 2) (Zhu et al., 2013). Intriguingly, the interaction of FUNDC1 with ATG8 proteins is negatively regulated under normoxic conditions by phosphorylation of the LIR-surrounding residues Ser13 and Tyr18 [by casein kinase 2 (CK2) and SRC kinase, respectively]. These residues become dephosphorylated upon hypoxia, when ULK1-mediated phosphorylation of Ser17 promotes the interaction of FUNDC1 with LC3 and induction of mitophagy (Chen et al., 2014; Liu et al., 2012; Wu et al., 2014) (see poster). It is interesting to note that phosphorylation of FUNDC1 on Tyr18 decreases its interaction with NIPSNAP1 and NIPSNAP2 on the OMM of damaged mitochondria upon ischemic reperfusion injury, resulting in an accumulation of damaged mitochondria (Li et al., 2021). Thus, the phosphorylation status of FUNDC1 appears to regulate mitophagy in both a positive and negative manner.
Although mitochondrial mitophagy receptors can be phosphorylated by the ULK1 kinase, it remains unknown whether they interact directly with FIP200, as is seen for SLRs (Ravenhill et al., 2019; Turco et al., 2019; Vargas et al., 2019), to facilitate mitochondrial recruitment of the ULK1 complex and de novo mitophagosome formation. The mitochondria-localized protein kinase C δ (PRKCD) has recently been shown to stimulate mitochondrial recruitment of ULK1 and ATG13 upon hypoxia-induced mitophagy, but its specific substrates remain unknown (Munson et al., 2021).
Dimerization of the BNIP3L receptor further promotes recruitment of the autophagy machinery and mitophagy progression (Marinkovic et al., 2021), suggesting that receptor oligomerization might increase the ATG8 binding avidity. In support of this, the intracellular bacterial pathogen Listeria monocytogenes has been found to induce mitophagy in macrophages through the virulence factor listeriolysin O (LLO), which promotes oligomerization of NLRX1 and binding of its LIR motif to LC3, thereby aiding bacterial survival by induction of mitophagy and reducing ROS levels (Zhang et al., 2019).
The phosphorylation status of mitophagy receptors can also modulate their interaction with proteins that regulate mitochondrial morphology. For instance, phosphorylation of FUNDC1 on Ser13 promotes its interaction with the mitochondrial dynamin-like GTPase OPA1 and decreases its interaction with the mitochondrial dynamin-like GTPase DRP1 (also known as DNM1L), thus inhibiting mitochondrial fission (Chen et al., 2016). In contrast, the mitochondrial serine/threonine protein phosphatase PGAM family member 5 (PGAM5) dephosphorylates FUNDC1 Ser13 and DRP1, thereby stimulating mitochondrial fission (Chen et al., 2014; Sugo et al., 2018). The correlation between mitochondrial morphology and mitophagy is, however, not so clear. Several studies have found that mitochondrial fission is important for mitophagy (Abeliovich et al., 2013; Ikeda et al., 2015; Kageyama et al., 2014; Mao et al., 2013; Rambold et al., 2011; Tanaka et al., 2010; Twig et al., 2008), but other studies have reported that mitophagosome formation occurs independently of mitochondrial fission (Burman et al., 2017; Yamashita et al., 2016). This is likely dependent on the type of mitophagy-inducing signal, as well as cell or tissue type. Moreover, in addition to the mitochondrial fission and fusion machinery, it is possible that the endosomal sorting complexes required for transport (ESCRT) machinery, which is involved in mitophagosome closure (Zhen et al., 2020), can facilitate ‘pinching off’ of small fragments of mitochondria. Additionally, sequestration of small pieces of mitochondria through ‘piecemeal mitophagy’ (see below) or budding of mitochondria-derived vesicles (MDVs) contribute to the turnover of specific mitochondrial fragments that can be targeted for lysosomal degradation.
In addition to protein-based mitophagy receptors, lipids might also play a similar critical role. For example, the IMM lipid cardiolipin translocates to the OMM following mitochondrial injury, and once on the surface it can interact with LC3 to facilitate mitophagy (Chu et al., 2013).
Piecemeal mitophagy
While macromitophagy is believed to involve sequestration and lysosomal targeting of whole mitochondria, piecemeal mitophagy involves the sequestration and delivery of selected mitochondrial cargo to lysosomes, seemingly in a DRP1-independent manner (Abudu et al., 2021; Le Guerroué et al., 2017). Mechanistically, little is known about this type of mitophagy, but it appears to rely on SLRs and ATG8 proteins (see poster). The OMM protein metaxin 1 (MTX1) has been found to directly interact with LC3C and p62 to promote phagophore sequestration of mitochondrial fragments (Le Guerroué et al., 2017). Moreover, the sorting and assembly machinery (SAM) component SAMM50 has been found to function as a receptor for basal mitophagy of components of the SAM and mitochondrial contact site and cristae organizing system (MICOS) complexes through its interaction with p62 and ATG8 proteins (Abudu et al., 2021; Le Guerroué et al., 2017).
Alternative mitolysosomal pathways
ATG8 conjugation-independent mitophagy
The autophagy ubiquitin-like conjugation system is essential for conventional macroautophagy (see Box 1). However, instances of mitophagy have been observed in cells where this system has been inhibited through loss of ATG5 or ATG7. Certain cytotoxic stressors, such as etoposide, in ATG5-null mouse embryonic fibroblasts have been found to induce a form of macroautophagy that appears to involve autophagosomes derived from Golgi membranes and requires the small GTPase RAB9A (Nishida et al., 2009). Although much less is known about the mechanism, this process does require some of the conventional autophagy machinery, including the ULK1 complex and class III phosphatidylinositol 3-kinase complex I (also known as the VPS34 complex or PIK3C3–C1 complex; Box 1). Of note, this pathway appears to be relevant for certain physiological instances of mitophagy and can occur during reticulocyte development (Honda et al., 2014; Nishida et al., 2009) and ischemic stress in the heart (Saito et al., 2019).
Mitochondria-derived vesicles
Mitochondrial components can also be delivered to lysosomes via a conventional membrane trafficking pathway, where MDVs bud from mitochondria and fuse with the endolysosomal system (Soubannier et al., 2012; Sugiura et al., 2014) (see poster). Although this is a separate pathway and is independent of the core autophagy machinery, it has a close relationship to mitophagy. It can be stimulated under conditions that also promote mitophagy, such as mitochondrial depolarization and oxidative stress (Soubannier et al., 2012), and may even require some of the same machinery, such as PINK1 and parkin (McLelland et al., 2014). The MDV pathway can also be upregulated under conditions where conventional mitophagy is blocked, where it is thought to act in a compensatory manner (Towers et al., 2021).
Concluding remarks and future perspectives
Mammalian mitophagy is clearly a physiologically important process with an in vivo prevalence that has been highlighted by novel reporter models allowing high-resolution visualization of mitophagy within specialized cells of distinct tissues (McWilliams et al., 2016; Sun et al., 2015). However, despite uncovering these physiological instances, less is known about which mitophagy pathway is responsible. Likewise, although we have advanced our understanding of the mechanisms involved in lysosomal degradation of mitochondrial components, there are still many open questions. Is the mitochondrial cargo the same for different types of mitophagy? How can we distinguish different mitophagy pathways mechanistically, especially if distinct pathways can occur at the same time? It is important to keep in mind that key players of mitophagy might be regulated oppositely under different metabolic conditions – for example, phosphorylation of FUNDC1 under normoxic versus hypoxic conditions – thus making it difficult to draw conclusions from protein-depletion experiments. Moreover, as mitochondrial turnover is often studied in the context of severe damage to mitochondria in cell culture, it will be important to characterize the different pathways under more physiologically relevant conditions, as well as how they are regulated in time (during aging or disease onset) and space (in different cells and tissues). Such knowledge is essential to eventually be able to target mitophagy therapeutically and to address whether lysosomal degradation of surplus and/or dysfunctional mitochondria indeed protects against disease development.
Panel 1. Diversity of mitophagy pathways
Panel 2. HIF1 regulation
Panel 3. General mechanism of autophagosome formation and growth
Panel 4. Summary of mitophagy receptor proteins
Panel 5. SLR-independent mitophagy
Panel 6. PINK1/parkin-dependent SLR mitophagy
Panel 7. PINK1 stabilization upon depolarization
Panel 8. PINK1/parkin-independent SLR mitophagy
Panel 9. Piecemeal mitophagy
Panel 10. Alternative mitolysosomal pathways
Panel 11. Mitochondrial dynamics
Panel 12. Additional roles of ubiquitylation
Footnotes
Funding
I.G.G. is funded by a grant from the Medical Research Council, UK (MC_UU_00018/2). A.S. is funded by Norges Forskningsråd through the Centres of Excellence funding scheme (project 262652) and FRIPRO grants (project 314684), Kreftforeningen (project 223278) and Helse Sør-Øst RHF (project 2020032). Open access funding provided by University of Dundee. Deposited in PMC for immediate release.
Cell science at a glance
Individual poster panels are available for downloading at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.259748#supplementary-data
Contributor Information
Ian G. Ganley, Email: i.ganley@dundee.ac.uk.
Anne Simonsen, Email: anne.simonsen@medisin.uio.no.
References
- Abeliovich, H., Zarei, M., Rigbolt, K. T. G., Youle, R. J. and Dengjel, J. (2013). Involvement of mitochondrial dynamics in the segregation of mitochondrial matrix proteins during stationary phase mitophagy. Nat. Commun. 4, 2789. 10.1038/ncomms3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abudu, Y. P., Shrestha, B. K., Zhang, W., Palara, A., Brenne, H. B., Larsen, K. B., Wolfson, D. L., Dumitriu, G., Oie, C. I., Ahluwalia, B. S.et al. (2021). SAMM50 acts with p62 in piecemeal basal- and OXPHOS-induced mitophagy of SAM and MICOS components. J. Cell Biol. 220, e202009092. 10.1083/jcb.202009092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, G. F. G., Toth, R., James, J. and Ganley, I. G. (2013). Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. 14, 1127-1135. 10.1038/embor.2013.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellot, G., Garcia-Medina, R., Gounon, P., Chiche, J., Roux, D., Pouyssegur, J. and Mazure, N. M. (2009). Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol. 29, 2570-2581. 10.1128/MCB.00166-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento, C. F., Renna, M., Ghislat, G., Puri, C., Ashkenazi, A., Vicinanza, M., Menzies, F. M. and Rubinsztein, D. C. (2016). Mammalian autophagy: how does it work? Annu. Rev. Biochem. 85, 685-713. 10.1146/annurev-biochem-060815-014556 [DOI] [PubMed] [Google Scholar]
- Bernardini, J. P., Lazarou, M. and Dewson, G. (2017). Parkin and mitophagy in cancer. Oncogene 36, 1315-1327. 10.1038/onc.2016.302 [DOI] [PubMed] [Google Scholar]
- Bhujabal, Z., Birgisdottir, Å. B., Sjøttem, E., Brenne, H. B., Øvervatn, A., Habisov, S., Kirkin, V., Lamark, T. and Johansen, T. (2017). FKBP8 recruits LC3A to mediate Parkin-independent mitophagy. EMBO Rep. 18, 947-961. 10.15252/embr.201643147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingol, B., Tea, J. S., Phu, L., Reichelt, M., Bakalarski, C. E., Song, Q., Foreman, O., Kirkpatrick, D. S. and Sheng, M. (2014). The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 510, 370-375. 10.1038/nature13418 [DOI] [PubMed] [Google Scholar]
- Burbulla, L. F., Fitzgerald, J. C., Stegen, K., Westermeier, J., Thost, A. K., Kato, H., Mokranjac, D., Sauerwald, J., Martins, L. M., Woitalla, D.et al. (2014). Mitochondrial proteolytic stress induced by loss of mortalin function is rescued by Parkin and PINK1. Cell Death Dis. 5, e1180. 10.1038/cddis.2014.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman, J. L., Pickles, S., Wang, C., Sekine, S., Vargas, J. N. S., Zhang, Z., Youle, A. M., Nezich, C. L., Wu, X., Hammer, J. A.et al. (2017). Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J. Cell Biol. 216, 3231-3247. 10.1083/jcb.201612106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C., Jensen, L. E. and Hurley, J. H. (2021). Autophagosome biogenesis comes out of the black box. Nat. Cell Biol. 23, 450-456. 10.1038/s41556-021-00669-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G., Han, Z., Feng, D., Chen, Y., Chen, L., Wu, H., Huang, L., Zhou, C., Cai, X., Fu, C.et al. (2014). A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol. Cell 54, 362-377. 10.1016/j.molcel.2014.02.034 [DOI] [PubMed] [Google Scholar]
- Chen, M., Chen, Z., Wang, Y., Tan, Z., Zhu, C., Li, Y., Han, Z., Chen, L., Gao, R., Liu, L.et al. (2016). Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy 12, 689-702. 10.1080/15548627.2016.1151580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, C. T., Ji, J., Dagda, R. K., Jiang, J. F., Tyurina, Y. Y., Kapralov, A. A., Tyurin, V. A., Yanamala, N., Shrivastava, I. H., Mohammadyani, D.et al. (2013). Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 15, 1197-1205. 10.1038/ncb2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen, T., Haddad, D., Wauters, F., Van Humbeeck, C., Mandemakers, W., Koentjoro, B., Sue, C., Gevaert, K., De Strooper, B., Verstreken, P.et al. (2014). The deubiquitinase USP15 antagonizes Parkin-mediated mitochondrial ubiquitination and mitophagy. Hum. Mol. Genet. 23, 5227-5242. 10.1093/hmg/ddu244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rienzo, M., Romagnoli, A., Ciccosanti, F., Refolo, G., Consalvi, V., Arena, G., Valente, E. M., Piacentini, M. and Fimia, G. M. (2022). AMBRA1 regulates mitophagy by interacting with ATAD3A and promoting PINK1 stability. Autophagy 18, 1752-1762. 10.1080/15548627.2021.1997052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rita, A., Peschiaroli, A., D Acunzo, P., Strobbe, D., Hu, Z., Gruber, J., Nygaard, M., Lambrughi, M., Melino, G., Papaleo, E.et al. (2018). HUWE1 E3 ligase promotes PINK1/PARKIN-independent mitophagy by regulating AMBRA1 activation via IKKalpha. Nat. Commun. 9, 3755. 10.1038/s41467-018-05722-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic, I. and Elazar, Z. (2018). Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349-364. 10.1038/s41580-018-0003-4 [DOI] [PubMed] [Google Scholar]
- Esteban-Martinez, L., Sierra-Filardi, E., Mcgreal, R. S., Salazar-Roa, M., Marino, G., Seco, E., Durand, S., Enot, D., Grana, O., Malumbres, M.et al. (2017). Programmed mitophagy is essential for the glycolytic switch during cell differentiation. EMBO J. 36, 1688-1706. 10.15252/embj.201695916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, T., Zhang, M., Zhou, Z., Wu, P., Peng, C., Wang, Y., Gong, X., Li, Y., Wang, Y., Xu, X.et al. (2021). Structural and biochemical advances on the recruitment of the autophagy-initiating ULK and TBK1 complexes by autophagy receptor NDP52. Sci. Adv. 7, eabi6582. 10.1126/sciadv.abi6582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler, S., Jager, L., Golombek, S., Nakanishi, E., Hans, F., Casadei, N., Terradas, A. L., Linnemann, C. and Kahle, P. J. (2019). Ubiquitin-specific protease USP36 knockdown impairs Parkin-dependent mitophagy via downregulation of Beclin-1-associated autophagy-related ATG14L. Exp. Cell Res. 384, 111641. 10.1016/j.yexcr.2019.111641 [DOI] [PubMed] [Google Scholar]
- Gersch, M., Gladkova, C., Schubert, A. F., Michel, M. A., Maslen, S. and Komander, D. (2017). Mechanism and regulation of the Lys6-selective deubiquitinase USP30. Nat. Struct. Mol. Biol. 24, 920-930. 10.1038/nsmb.3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene, A. W., Grenier, K., Aguileta, M. A., Muise, S., Farazifard, R., Haque, M. E., Mcbride, H. M., Park, D. S. and Fon, E. A. (2012). Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 13, 378-385. 10.1038/embor.2012.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, O., Evans, C. S., Ye, J., Cheung, J., Maniatis, T. and Holzbaur, E. L. F. (2021). ALS- and FTD-associated missense mutations in TBK1 differentially disrupt mitophagy. Proc. Natl. Acad. Sci. USA 118, e2025053118. 10.1073/pnas.2025053118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, S., Arakawa, S., Nishida, Y., Yamaguchi, H., Ishii, E. and Shimizu, S. (2014). Ulk1-mediated Atg5-independent macroautophagy mediates elimination of mitochondria from embryonic reticulocytes. Nat. Commun. 5, 4004. 10.1038/ncomms5004 [DOI] [PubMed] [Google Scholar]
- Ikeda, Y., Shirakabe, A., Maejima, Y., Zhai, P., Sciarretta, S., Toli, J., Nomura, M., Mihara, K., Egashira, K., Ohishi, M.et al. (2015). Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 116, 264-278. 10.1161/CIRCRESAHA.116.303356 [DOI] [PubMed] [Google Scholar]
- Jin, S. M. and Youle, R. J. (2013). The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy 9, 1750-1757. 10.4161/auto.26122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, S. M., Lazarou, M., Wang, C., Kane, L. A., Narendra, D. P. and Youle, R. J. (2010). Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 191, 933-942. 10.1083/jcb.201008084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen, T. and Lamark, T. (2020). Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J. Mol. Biol. 432, 80-103. 10.1016/j.jmb.2019.07.016 [DOI] [PubMed] [Google Scholar]
- Kageyama, Y., Hoshijima, M., Seo, K., Bedja, D., Sysa-Shah, P., Andrabi, S. A., Chen, W., Höke, A., Dawson, V. L., Dawson, T. M.et al. (2014). Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 33, 2798-2813. 10.15252/embj.201488658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, L. A., Lazarou, M., Fogel, A. I., Li, Y., Yamano, K., Sarraf, S. A., Banerjee, S. and Youle, R. J. (2014). PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 205, 143-153. 10.1083/jcb.201402104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki, T., Wang, K., Cao, Y., Baba, M. and Klionsky, D. J. (2009). Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev. Cell 17, 98-109. 10.1016/j.devcel.2009.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskaite, A., Kondapalli, C., Gourlay, R., Campbell, D. G., Ritorto, M. S., Hofmann, K., Alessi, D. R., Knebel, A., Trost, M. and Muqit, M. M. (2014). Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem. J. 460, 127-141. 10.1042/BJ20140334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierans, S. J. and Taylor, C. T. (2021). Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J. Physiol. 599, 23-37. 10.1113/JP280572 [DOI] [PubMed] [Google Scholar]
- Koyano, F., Yamano, K., Kosako, H., Tanaka, K. and Matsuda, N. (2019). Parkin recruitment to impaired mitochondria for nonselective ubiquitylation is facilitated by MITOL. J. Biol. Chem. 294, 10300-10314. 10.1074/jbc.RA118.006302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou, M., Jin, S. M., Kane, L. A. and Youle, R. J. (2012). Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev. Cell 22, 320-333. 10.1016/j.devcel.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou, M., Sliter, D. A., Kane, L. A., Sarraf, S. A., Wang, C., Burman, J. L., Sideris, D. P., Fogel, A. I. and Youle, R. J. (2015). The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309-314. 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guerroué, F., Eck, F., Jung, J., Starzetz, T., Mittelbronn, M., Kaulich, M. and Behrends, C. (2017). Autophagosomal content profiling reveals an LC3C-dependent piecemeal mitophagy pathway. Mol. Cell 68, 786-796.e6. 10.1016/j.molcel.2017.10.029 [DOI] [PubMed] [Google Scholar]
- Li, W., Zhang, X., Zhuang, H., Chen, H. G., Chen, Y., Tian, W., Wu, W., Li, Y., Wang, S., Zhang, L.et al. (2014). MicroRNA-137 is a novel hypoxia-responsive microRNA that inhibits mitophagy via regulation of two mitophagy receptors FUNDC1 and NIX. J. Biol. Chem. 289, 10691-10701. 10.1074/jbc.M113.537050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., Zhou, Y., Gu, X., Zhang, X. and Jia, Z. (2021). NLRX1/FUNDC1/NIPSNAP1-2 axis regulates mitophagy and alleviates intestinal ischaemia/reperfusion injury. Cell Prolif. 54, e12986. 10.1111/cpr.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L., Feng, D., Chen, G., Chen, M., Zheng, Q., Song, P., Ma, Q., Zhu, C., Wang, R., Qi, W.et al. (2012). Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177-185. 10.1038/ncb2422 [DOI] [PubMed] [Google Scholar]
- Mao, K., Wang, K., Liu, X. and Klionsky, D. J. (2013). The scaffold protein Atg11 recruits fission machinery to drive selective mitochondria degradation by autophagy. Dev. Cell 26, 9-18. 10.1016/j.devcel.2013.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic, M., Šprung, M. and Novak, I. (2021). Dimerization of mitophagy receptor BNIP3L/NIX is essential for recruitment of autophagic machinery. Autophagy 17, 1232-1243. 10.1080/15548627.2020.1755120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba, K., Kotani, T., Tsutsumi, A., Tsuji, T., Mori, T., Noshiro, D., Sugita, Y., Nomura, N., Iwata, S., Ohsumi, Y.et al. (2020). Atg9 is a lipid scramblase that mediates autophagosomal membrane expansion. Nat. Struct. Mol. Biol. 27, 1185-1193. 10.1038/s41594-020-00518-w [DOI] [PubMed] [Google Scholar]
- Mclelland, G. L., Soubannier, V., Chen, C. X., Mcbride, H. M. and Fon, E. A. (2014). Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 33, 282-295. 10.1002/embj.201385902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcwilliams, T. G. and Muqit, M. M. (2017). PINK1 and Parkin: emerging themes in mitochondrial homeostasis. Curr. Opin. Cell Biol. 45, 83-91. 10.1016/j.ceb.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Mcwilliams, T. G., Prescott, A. R., Allen, G. F., Tamjar, J., Munson, M. J., Thomson, C., Muqit, M. M. and Ganley, I. G. (2016). mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J. Cell Biol. 214, 333-345. 10.1083/jcb.201603039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehlman, A. T. and Youle, R. J. (2020). Mitochondrial quality control and restraining innate immunity. Annu. Rev. Cell Dev. Biol. 36, 265-289. 10.1146/annurev-cellbio-021820-101354 [DOI] [PubMed] [Google Scholar]
- Montava-Garriga, L. and Ganley, I. G. (2020). Outstanding questions in mitophagy: what we do and do not know. J. Mol. Biol. 432, 206-230. 10.1016/j.jmb.2019.06.032 [DOI] [PubMed] [Google Scholar]
- Munson, M. J., Mathai, B. J., Ng, M. Y. W., Trachsel-Moncho, L., De La Ballina, L. R., Schultz, S. W., Aman, Y., Lystad, A. H., Singh, S., Singh, S.et al. (2021). GAK and PRKCD are positive regulators of PRKN-independent mitophagy. Nat. Commun. 12, 6101. 10.1038/s41467-021-26331-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakawa, T., Yamaguchi, O., Hashimoto, A., Hikoso, S., Takeda, T., Oka, T., Yasui, H., Ueda, H., Akazawa, Y., Nakayama, H.et al. (2015). Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat. Commun. 6, 7527. 10.1038/ncomms8527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra, D., Tanaka, A., Suen, D.-F. and Youle, R. J. (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795-803. 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, M. Y. W., Wai, T. and Simonsen, A. (2021). Quality control of the mitochondrion. Dev. Cell 56, 881-905. 10.1016/j.devcel.2021.02.009 [DOI] [PubMed] [Google Scholar]
- Nguyen, T. N. and Lazarou, M. (2022). A unifying model for the role of the ATG8 system in autophagy. J. Cell Sci. 135, jcs258997. 10.1242/jcs.258997 [DOI] [PubMed] [Google Scholar]
- Nguyen, T. N., Padman, B. S., Usher, J., Oorschot, V., Ramm, G. and Lazarou, M. (2016). Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J. Cell Biol. 215, 857-874. 10.1083/jcb.201607039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. D., Shaid, S., Vakhrusheva, O., Koschade, S. E., Klann, K., Thölken, M., Baker, F., Zhang, J., Oellerich, T., Sürün, D.et al. (2019). Loss of the selective autophagy receptor p62 impairs murine myeloid leukemia progression and mitophagy. Blood 133, 168-179. 10.1182/blood-2018-02-833475 [DOI] [PubMed] [Google Scholar]
- Nishida, Y., Arakawa, S., Fujitani, K., Yamaguchi, H., Mizuta, T., Kanaseki, T., Komatsu, M., Otsu, K., Tsujimoto, Y. and Shimizu, S. (2009). Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461, 654-658. 10.1038/nature08455 [DOI] [PubMed] [Google Scholar]
- Niu, K., Fang, H., Chen, Z., Zhu, Y., Tan, Q., Wei, D., Li, Y., Balajee, A. S. and Zhao, Y. (2020). USP33 deubiquitinates PRKN/parkin and antagonizes its role in mitophagy. Autophagy 16, 724-734. 10.1080/15548627.2019.1656957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak, I., Kirkin, V., Mcewan, D. G., Zhang, J., Wild, P., Rozenknop, A., Rogov, V., Lohr, F., Popovic, D., Occhipinti, A.et al. (2010). Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45-51. 10.1038/embor.2009.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, K., Kondo-Okamoto, N. and Ohsumi, Y. (2009). Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev. Cell 17, 87-97. 10.1016/j.devcel.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Okatsu, K., Uno, M., Koyano, F., Go, E., Kimura, M., Oka, T., Tanaka, K. and Matsuda, N. (2013). A dimeric PINK1-containing complex on depolarized mitochondria stimulates Parkin recruitment. J. Biol. Chem. 288, 36372-36384. 10.1074/jbc.M113.509653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okatsu, K., Koyano, F., Kimura, M., Kosako, H., Saeki, Y., Tanaka, K. and Matsuda, N. (2015). Phosphorylated ubiquitin chain is the genuine Parkin receptor. J. Cell Biol. 209, 111-128. 10.1083/jcb.201410050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi, M., Yamano, K., Sato, M., Matsuda, N. and Okamoto, K. (2021). Molecular mechanisms and physiological functions of mitophagy. EMBO J. 40, e104705. 10.15252/embj.2020104705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padman, B. S., Nguyen, T. N., Uoselis, L., Skulsuppaisarn, M., Nguyen, L. K. and Lazarou, M. (2019). LC3/GABARAPs drive ubiquitin-independent recruitment of Optineurin and NDP52 to amplify mitophagy. Nat. Commun. 10, 408. 10.1038/s41467-019-08335-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole, L. P., Bock-Hughes, A., Berardi, D. E. and Macleod, K. F. (2021). ULK1 promotes mitophagy via phosphorylation and stabilization of BNIP3. Sci. Rep. 11, 20526. 10.1038/s41598-021-00170-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princely Abudu, Y., Pankiv, S., Mathai, B. J., Håkon Lystad, A., Bindesbøll, C., Brenne, H. B., Yoke Wui Ng, M., Thiede, B., Yamamoto, A., Mutugi Nthiga, T.et al. (2019). NIPSNAP1 and NIPSNAP2 Act as “eat me” signals for mitophagy. Dev. Cell 49, 509-525.e12. 10.1016/j.devcel.2019.03.013 [DOI] [PubMed] [Google Scholar]
- Pugh, C. W. (2016). Modulation of the hypoxic response. Adv. Exp. Med. Biol. 903, 259-271. 10.1007/978-1-4899-7678-9_18 [DOI] [PubMed] [Google Scholar]
- Quinsay, M. N., Thomas, R. L., Lee, Y. and Gustafsson, Å. B. (2010). Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophagy 6, 855-862. 10.4161/auto.6.7.13005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold, A. S., Kostelecky, B., Elia, N. and Lippincott-Schwartz, J. (2011). Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl. Acad. Sci. USA 108, 10190-10195. 10.1073/pnas.1107402108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenhill, B. J., Boyle, K. B., Von Muhlinen, N., Ellison, C. J., Masson, G. R., Otten, E. G., Foeglein, A., Williams, R. and Randow, F. (2019). The cargo receptor NDP52 initiates selective autophagy by recruiting the ULK complex to cytosol-invading bacteria. Mol. Cell 74, 320-329.e6. 10.1016/j.molcel.2019.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogov, V. V., Suzuki, H., Marinkovic, M., Lang, V., Kato, R., Kawasaki, M., Buljubasic, M., Sprung, M., Rogova, N., Wakatsuki, S.et al. (2017). Phosphorylation of the mitochondrial autophagy receptor Nix enhances its interaction with LC3 proteins. Sci. Rep. 7, 1131. 10.1038/s41598-017-01258-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojansky, R., Cha, M. Y. and Chan, D. C. (2016). Elimination of paternal mitochondria in mouse embryos occurs through autophagic degradation dependent on PARKIN and MUL1. Elife 5, e17896. 10.7554/eLife.17896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, B., Salemi, M., Williams, G. L., Oh, S., Paffett, M. L., Phinney, B. and Mandell, M. A. (2022). Interactomic analysis reveals a homeostatic role for the HIV restriction factor TRIM5alpha in mitophagy. Cell Rep. 39, 110797. 10.1016/j.celrep.2022.110797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, T., Nah, J., Oka, S. I., Mukai, R., Monden, Y., Maejima, Y., Ikeda, Y., Sciarretta, S., Liu, T., Li, H.et al. (2019). An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. J. Clin. Invest. 129, 802-819. 10.1172/JCI122035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval, H., Thiagarajan, P., Dasgupta, S. K., Schumacher, A., Prchal, J. T., Chen, M. and Wang, J. (2008). Essential role for Nix in autophagic maturation of erythroid cells. Nature 454, 232-235. 10.1038/nature07006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarten, M., Mohrlüder, J., Ma, P., Stoldt, M., Thielmann, Y., Stangler, T., Hersch, N., Hoffmann, B., Merkel, R. and Willbold, D. (2009). Nix directly binds to GABARAP: a possible crosstalk between apoptosis and autophagy. Autophagy 5, 690-698. 10.4161/auto.5.5.8494 [DOI] [PubMed] [Google Scholar]
- Shi, X., Chang, C., Yokom, A. L., Jensen, L. E. and Hurley, J. H. (2020). The autophagy adaptor NDP52 and the FIP200 coiled-coil allosterically activate ULK1 complex membrane recruitment. Elife 9, e59099. 10.7554/eLife.59099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodkin, M. R. and Elazar, Z. (2013). The Atg8 family: multifunctional ubiquitin-like key regulators of autophagy. Essays Biochem. 55, 51-64. 10.1042/bse0550051 [DOI] [PubMed] [Google Scholar]
- Soubannier, V., Mclelland, G. L., Zunino, R., Braschi, E., Rippstein, P., Fon, E. A. and Mcbride, H. M. (2012). A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr. Biol. 22, 135-141. 10.1016/j.cub.2011.11.057 [DOI] [PubMed] [Google Scholar]
- Stolz, A., Ernst, A. and Dikic, I. (2014). Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 16, 495-501. 10.1038/ncb2979 [DOI] [PubMed] [Google Scholar]
- Strappazzon, F., Nazio, F., Corrado, M., Cianfanelli, V., Romagnoli, A., Fimia, G. M., Campello, S., Nardacci, R., Piacentini, M., Campanella, M.et al. (2015). AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ. 22, 517. 10.1038/cdd.2014.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura, A., Mclelland, G. L., Fon, E. A. and Mcbride, H. M. (2014). A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 33, 2142-2156. 10.15252/embj.201488104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugo, M., Kimura, H., Arasaki, K., Amemiya, T., Hirota, N., Dohmae, N., Imai, Y., Inoshita, T., Shiba-Fukushima, K., Hattori, N.et al. (2018). Syntaxin 17 regulates the localization and function of PGAM5 in mitochondrial division and mitophagy. EMBO J. 37, e98899. 10.15252/embj.201798899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, N., Yun, J., Liu, J., Malide, D., Liu, C., Rovira, I. I., Holmstrom, K. M., Fergusson, M. M., Yoo, Y. H., Combs, C. A.et al. (2015). Measuring in vivo mitophagy. Mol. Cell 60, 685-696. 10.1016/j.molcel.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, A., Cleland, M. M., Xu, S., Narendra, D. P., Suen, D.-F., Karbowski, M. and Youle, R. J. (2010). Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 191, 1367-1380. 10.1083/jcb.201007013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers, C. G., Wodetzki, D. K., Thorburn, J., Smith, K. R., Caino, M. C. and Thorburn, A. (2021). Mitochondrial-derived vesicles compensate for loss of LC3-mediated mitophagy. Dev. Cell 56, 2029-2042.e5. 10.1016/j.devcel.2021.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco, E., Witt, M., Abert, C., Bock-Bierbaum, T., Su, M. Y., Trapannone, R., Sztacho, M., Danieli, A., Shi, X., Zaffagnini, G.et al. (2019). FIP200 claw domain binding to p62 promotes autophagosome formation at ubiquitin condensates. Mol. Cell 74, 330-346.e11. 10.1016/j.molcel.2019.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig, G., Elorza, A., Molina, A. J. A., Mohamed, H., Wikstrom, J. D., Walzer, G., Stiles, L., Haigh, S. E., Katz, S., Las, G.et al. (2008). Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27, 433-446. 10.1038/sj.emboj.7601963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaites, L. P., Paulo, J. A., Huttlin, E. L. and Harper, J. W. (2018). Systematic analysis of human cells lacking ATG8 proteins uncovers roles for GABARAPs and the CCZ1/MON1 regulator C18orf8/RMC1 in macroautophagic and selective autophagic flux. Mol. Cell. Biol. 38, e00392-17. 10.1128/MCB.00392-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas, J. N. S., Wang, C., Bunker, E., Hao, L., Maric, D., Schiavo, G., Randow, F. and Youle, R. J. (2019). Spatiotemporal control of ULK1 activation by NDP52 and TBK1 during selective autophagy. Mol. Cell 74, 347-362.e6. 10.1016/j.molcel.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa, E., Proics, E., Rubio-Patino, C., Obba, S., Zunino, B., Bossowski, J. P., Rozier, R. M., Chiche, J., Mondragon, L., Riley, J. S.et al. (2017). Parkin-independent mitophagy controls chemotherapeutic response in cancer cells. Cell Rep. 20, 2846-2859. 10.1016/j.celrep.2017.08.087 [DOI] [PubMed] [Google Scholar]
- Wall, C. E., Rose, C. M., Adrian, M., Zeng, Y. J., Kirkpatrick, D. S. and Bingol, B. (2019). PPEF2 opposes PINK1-mediated mitochondrial quality control by dephosphorylating ubiquitin. Cell Rep. 29, 3280-3292.e7. 10.1016/j.celrep.2019.10.130 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Serricchio, M., Jauregui, M., Shanbhag, R., Stoltz, T., Di Paolo, C. T., Kim, P. K. and Mcquibban, G. A. (2015). Deubiquitinating enzymes regulate PARK2-mediated mitophagy. Autophagy 11, 595-606. 10.1080/15548627.2015.1034408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauer, T., Simicek, M., Schubert, A., Jr. and Komander, D. (2015). Mechanism of phospho-ubiquitin-induced PARKIN activation. Nature 524, 370-374. 10.1038/nature14879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Y., Chiang, W. C., Sumpter, R., Mishra, P. and Levine, B. (2017). Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell 168, 224-238.e10. 10.1016/j.cell.2016.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, W., Tian, W., Hu, Z., Chen, G., Huang, L., Li, W., Zhang, X., Xue, P., Zhou, C., Liu, L.et al. (2014). ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO Rep. 15, 566-575. 10.1002/embr.201438501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano, K. and Youle, R. J. (2013). PINK1 is degraded through the N-end rule pathway. Autophagy 9, 1758-1769. 10.4161/auto.24633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano, K., Kikuchi, R., Kojima, W., Hayashida, R., Koyano, F., Kawawaki, J., Shoda, T., Demizu, Y., Naito, M., Tanaka, K.et al. (2020). Critical role of mitochondrial ubiquitination and the OPTN–ATG9A axis in mitophagy. J. Cell Biol. 219, e201912144. 10.1083/jcb.201912144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, S.-., Jin, X., Furukawa, K., Hamasaki, M., Nezu, A., Otera, H., Saigusa, T., Yoshimori, T., Sakai, Y., Mihara, K.et al. (2016). Mitochondrial division occurs concurrently with autophagosome formation but independently of Drp1 during mitophagy. J. Cell Biol. 215, 649-665. 10.1083/jcb.201605093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C., Gong, L., Chen, L., Xu, M., Abou-Hamdan, H., Tang, M., Desaubry, L. and Song, Z. (2020). PHB2 (prohibitin 2) promotes PINK1-PRKN/Parkin-dependent mitophagy by the PARL-PGAM5-PINK1 axis. Autophagy 16, 419-434. 10.1080/15548627.2019.1628520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K. C., Ma, X., Liu, H., Murphy, J., Barger, P. M., Mann, D. L. and Diwan, A. (2015). Tumor necrosis factor receptor-associated factor 2 mediates mitochondrial autophagy. Circ. Heart Fail 8, 175-187. 10.1161/CIRCHEARTFAILURE.114.001635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, J., Puri, R., Yang, H., Lizzio, M. A., Wu, C., Sheng, Z. H. and Guo, M. (2014). MUL1 acts in parallel to the PINK1/parkin pathway in regulating mitofusin and compensates for loss of PINK1/parkin. Elife 3, e01958. 10.7554/eLife.01958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachari, M., Gudmundsson, S. R., Li, Z., Manifava, M., Shah, R., Smith, M., Stronge, J., Karanasios, E., Piunti, C., Kishi-Itakura, C.et al. (2019). Selective autophagy of mitochondria on a ubiquitin-endoplasmic-reticulum platform. Dev. Cell 50, 627-643.e625. 10.1016/j.devcel.2019.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., Ren, H., Xu, C., Zhu, C., Wu, H., Liu, D., Wang, J., Liu, L., Li, W., Ma, Q.et al. (2016). Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. Elife 5, e21407. 10.7554/eLife.21407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Yao, Y., Qiu, X., Wang, G., Hu, Z., Chen, S., Wu, Z., Yuan, N., Gao, H., Wang, J.et al. (2019). Listeria hijacks host mitophagy through a novel mitophagy receptor to evade killing. Nat. Immunol. 20, 433-446. 10.1038/s41590-019-0324-2 [DOI] [PubMed] [Google Scholar]
- Zhao, J. F., Rodger, C. E., Allen, G. F. G., Weidlich, S. and Ganley, I. G. (2020). HIF1alpha-dependent mitophagy facilitates cardiomyoblast differentiation. Cell Stress 4, 99-113. 10.15698/cst2020.05.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen, Y., Spangenberg, H., Munson, M. J., Brech, A., Schink, K. O., Tan, K.-W., Sørensen, V., Wenzel, E. M., Radulovic, M., Engedal, N.et al. (2020). ESCRT-mediated phagophore sealing during mitophagy. Autophagy 16, 826-841. 10.1080/15548627.2019.1639301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, Z., Umemura, A., Sanchez-Lopez, E., Liang, S., Shalapour, S., Wong, J., He, F., Boassa, D., Perkins, G., Ali, S. R.et al. (2016). NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164, 896-910. 10.1016/j.cell.2015.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., Massen, S., Terenzio, M., Lang, V., Chen-Lindner, S., Eils, R., Novak, I., Dikic, I., Hamacher-Brady, A. and Brady, N. R. (2013). Modulation of serines 17 and 24 in the LC3-interacting region of Bnip3 determines pro-survival mitophagy versus apoptosis. J. Biol. Chem. 288, 1099-1113. 10.1074/jbc.M112.399345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorova, L. D., Popkov, V. A., Plotnikov, E. Y., Silachev, D. N., Pevzner, I. B., Jankauskas, S. S., Babenko, V. A., Zorov, S. D., Balakireva, A. V., Juhaszova, M.et al. (2018). Mitochondrial membrane potential. Anal. Biochem. 552, 50-59. 10.1016/j.ab.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.