Abstract
Postmenopause, the secretion of female hormones changes, causing excessive fat accumulation in the body and leading to chronic inflammation, which increases the incidence of cardiovascular diseases (CVD). Walking is an easily accessible daily exercise and effective non-pharmacological treatment for reducing obesity and the incidence of CVD. The aim of this study was to investigate the effect of moderate intensity walking exercises on body composition, vascular inflammatory factors, and vascular endothelial growth factor (VEGF) in postmenopausal women with obesity. Twenty-six older postmenopausal women with obesity (ages 68–72) were randomly assigned to control (n = 12, BMI 26.06 ± 1.37) or exercise (n = 14, BMI 26.04 ± 1.94) groups. Following a 12-week moderate intensity walking exercise program, we measured the participants’ body composition with an InBody S10 analyzer and assessed blood sera using enzyme-linked immunosorbent assays. There was a significant clustering by weight (p < 0.01), body mass index (p < 0.01), percentage body fat (p < 0.001), high-sensitivity C-reactive protein (p < 0.05), interleukin-6, and tumor necrosis factor-α (p < 0.05) being significantly decreased in the exercise group. Although VEGF levels did not change significantly, a tendency to increase was observed in participants that exercised. Our results indicate that walking exercise may help prevent CVD in postmenopausal women with obesity by reducing obesity and vascular inflammatory factors.
Subject terms: Biochemistry, Immunology, Physiology, Cardiology, Endocrinology, Health care
Introduction
Menopause is a naturally occurring aging process in women, and cardiovascular diseases (CVD) notably increase after menopause1. In addition, postmenopausal hormonal changes, such as a deficiency in the female hormone estrogen, accelerate fat accumulation in the abdomen, possibly leading to obesity2. A previous comparative study reported that the body fat mass is higher after than before menopause3. Longitudinal Study reported that changes in body weight were associated with an increased risk of cardiovascular diseases4. Cross-sectional studies reported associations between obesity and inflammatory factors5. The Korea National Health and Nutrition Examination Survey between 2016 and 2017 reported that obesity is a risk factor for the elevation of inflammatory markers in postmenopausal women6. When excess fat accumulates in the body, the inflammatory response intensifies, leading to chronic inflammation. Chronic inflammatory conditions in women after menopause in turn, increase the incidence of CVD7,8.
High-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are crucial vascular inflammatory factors9. They have at high levels of hs-CRP10, IL-611, and TNF-α12 in obese individuals. Inflammation factors known to play a key role in the pathogenesis of arteriosclerosis and CVD13. Hs-CRP is produced in the liver upon stimulation by TNF-α and IL-69, and its levels are used to measure various inflammatory responses and diseases14. In particular, hs-CRP serves as an index indicating the degree of the inflammatory response of atherosclerotic vascular15. Hs-CRP inhibits the stimulation of angiogenesis by vascular endothelial growth factor (VEGF), with the latter playing a vital role in forming new capillaries by promoting endothelial cell proliferation16,17. In addition, blood VEGF levels were reported to be low in obese people in studies on the relationship between body mass index and VEGF18. Obesity worsens the condition of vascular, such as reducing the density of blood vessels and capillary activity. As such, an increase in inflammatory factors caused by obesity increases the risk of CVD in women who have undergone menopause.
Regular exercise is recommended as a non-pharmacological method to reduce this risk of cardiovascular disease. A review and meta-analysis study by Zheng et al.19 reported that regular aerobic exercise effectively reduces inflammatory cytokine levels, thereby improving resistance to cardiovascular disease20. Among various aerobic exercises, walking is a physical activity with low impact compared to running. With less impact on the musculoskeletal system and joints, it is highly recommended for women who are obese, older or possess weak physical strength21. Walking is also an accessible form of exercise that increases physical activity in daily life, and the American Heart Association recommends walking over 7000 steps daily. In previous cohort studies reported that people who walked a minimum of 7000 steps per day had a 50–70% lower risk of mortality compared to those who walked fewer than 7000 steps per day22. In addition, as the number of steps increases by 1000, the levels of IL-6 and CRP decrease by 13%23. In a cohort study demonstrated with every increase of 1000 steps, there was a reduction in the levels of inflammation within the body in elderly individuals24.
The objective of the present study has been refined as follows: This research endeavored to assiduously explore the consequences of engaging in walking exercise of moderate intensity quantitatively defined as a regimen involving 7000–9999 steps per day at a pace of 100 steps per minute22 on several health parameters, including body mass index, hs-CRP, IL-6, TNF-α, and VEGF levels, among postmenopausal women navigating through obesity. A comparative analysis was implemented against a cohort of demographically analogous individuals who refrained from regular exercise. The guiding hypothesis of this research speculated that a walking exercise protocol, characterized by its moderate intensity, would elicit tangible enhancements in vascular inflammation and VEGF levels, as evidenced by a juxtaposition with the outcomes derived from the non-exercising cohort.
Methods
Study design
A randomized controlled trial was conducted to confirm the effect of moderate intensity walking for 12 weeks. The block randomization method in SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA) was used. The 26 participants were divided into either a control (n = 12, age 69.91 ± 1.14, BMI 26.06 ± 1.37) or an exercise (n = 14, age 70.17 ± 1.21, BMI 26.04 ± 1.94) group. Anthropometrics and Blood collection were performed on each participant at the same time in the morning (08:00 AM, ± 1 h) pre and post the 12-week intervention. Individuals in the control group had not exercised regularly (Table 1). Participants were restricted from taking any medications and engaging in exercises that could influence the trial results during the study period. Subsequent to these restrictions, safety matters were additionally verified through medical examinations.
Table 1.
The number of steps taken by the participants.
| Variables | Exercise (n = 14) | Control (n = 12) |
|---|---|---|
| Walking step | 9106.90 ± 1191.44*** | 1960.91 ± 90.60 |
| Sedentary time (min) | 831.60 ± 96.33** | 1202.91 ± 125.17 |
EX exercise group, CON control group.
Values are presented as mean ± standard deviation.
**p < 0.01, ***p < 0.001 EX vs. CON.
Participants
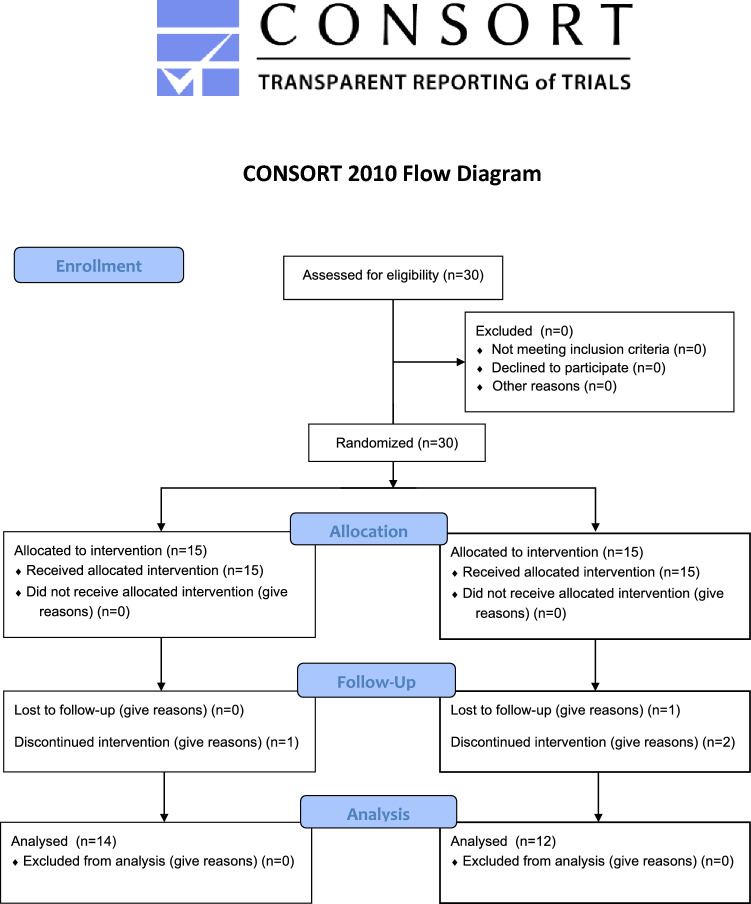
Participants were recruited with flyers in a metropolitan city in South Korea at a senior community center and a singing class from November 1, 2021, to December 1, 2022. The inclusion and exclusion criteria were set to ensure that the participants had not received drug treatments in the past six months, did not exercise regularly, and did not possess known musculoskeletal disorders. We used G-power version 3.1 software (Kiel University, Kiel, Germany) with an effect size of 0.25 (default), a significance level of 0.05, and a power of 0.70 as parameters to determine the effective sample size required, which was calculated as 26 participants. Therefore, a total of 30 people were recruited for this study, considering potential dropouts. Our participants were postmenopausal women with obesity (68–72 years old) who were fully aware of the purpose and contents of the study and submitted informed consent for participation. Among them, three people from the control group and one from the exercise group discontinued due to personal circumstances, rendering a final sample population of 26 individuals (Fig. 1). Table 2 summarizes the characteristics of all participants. Written signed consent was collected from each participant before the start of the study. This study received approval from the Institutional Review Board designated by the Ministry of Health and Welfare of Korea (Approval Number: 2-1040709-AB-N-01-202109-HR-066-0 4) and was conducted in strict adherence to the principles of the Helsinki Declaration and ethical guidelines for research. Furthermore, this trial was retrospectively registered with the Clinical Research Information Service (CRIS), Republic of Korea (Registration Number: KCT0008621, Date of Registration: 17/07/2023, Website: https://cris.nih.go.kr).
Figure 1.
CONSORT flow diagram for the individual randomized controlled trial.
Table 2.
Characteristics of the participants at baseline.
| Variables | EX (n = 14) | CON (n = 12) | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Age (years) | 70.2 ± 1.21 | 69.9 ± 1.14 | ||
| Height (cm) | 154.69 ± 4.15 | 155.82 ± 2.52 | ||
| Weight (kg) | 62.23 ± 4.19 | 61.26 ± 3.80 | 63.22 ± 2.49 | 63.44 ± 2.17 |
| BMI (kg/m2) | 26.04 ± 1.94 | 25.63 ± 1.71 | 26.06 ± 1.37 | 26.15 ± 1.26 |
| Body fat (%) | 37.33 ± 3.37 | 36.12 ± 3.21 | 37.0 ± 1.57 | 37.11 ± 1.77 |
| Skeletal muscle mass (kg) | 20.18 ± 1.50 | 20.41 ± 1.58 | 20.98 ± 1.57 | 20.85 ± 1.57 |
Values are presented as mean ± standard deviation.
EX exercise group, CON control group, BMI body mass index.
Exercise program
The walking route was pre-determined and close to the participants' residential areas. Each exercise session started with a 20-min warm-up. Participants performed a walking exercise at an intensity between 64–76% of their maximum heart rate (HRmax) measured using a Polar RS400sd monitor (APAC model 90026360; Polar Electro, Bethpage, NY, USA). The baseline cadence was 100 steps/min25,26, adjusted according to the walking conditions in question. For their safety, the wearable heart rate monitor sounds an alarm when your heart rate exceeds 64–76% of your HRmax. They activated heart rate monitor alarms to control heart rate at 64–76% HRmax (i.e., 110–130 b/min) during walking sessions27. Haptic vibratory feedback was sent to participants to help maintain walking intensity. The control group maintained their conventional physical activity and diet. We monitored compliance once a week via a Fitbit Charge 4 activity tracker (Fitbit, San Francisco, CA, USA), which participants had to wear throughout the day except for sleeping and bathing.
Anthropometrics
The participants wore simple clothes that contained no metal to ensure accurate measurements and minimum of 8 h of fasting to retain an empty stomach until measurements were taken. Height was measured using a portable extensometer InlabS50 (Biospace Corp., Seoul, Korea). Body composition was determined using Inbody S10 (Biospace Corp., Seoul, Korea) before and after 12 weeks, evaluating weight (kg), percentage of body fat (PBF; %), and skeletal muscle mass (SMM; kg). Body mass index (BMI) was calculated by taking the body mass divided by height squared (kg/m2).
Blood sampling and analysis
All participants were instructed to fast for ≥ 8 h before sample collection. In the morning, 8 − 10 a.m., 10 mL of blood was collected from the antebrachial vein by a clinical pathologist. The blood was centrifuged at 3000 rpm for 10 min in Combi-514R (Hanil, Seoul, Korea) for further analysis. An enzyme-linked immunosorbent assay (ELISA) was used to measure circulating plasma levels of TNF-α and IL-6 (Quantikine HS Human TNF-α and Quantikine HS Human IL-6 kits, respectively; R&D Systems, Minneapolis, USA). For data acquisition and analysis of the ELISA assays, we used a VersaMax absorbance microplate reader (Molecular Devices, Sunnyvale, CA, USA). Blood CRP was analyzed by turbidimetric immunoassay using a CRP4 kit (Roche, Basel, Switzerland) Cobas 8000 equipment (Roche). Serum VEGF was analyzed using a Human VEGF Quantikine ELISA kit (R&D Systems) and the VersaMax absorbance microplate (Molecular Devices).
Data analysis
The data were processed and analyzed using SPSS 27.0 (IBM, New York, NY, USA). The means and standard deviations of data were calculated. First, by conducting a normality test on our research results, we confirmed that they were normally distributed. To investigate the effect of 12 weeks of moderate intensity walking exercises on changes in vascular inflammatory factors and VEGF, we set the treatment groups (control or exercise) and time (pre- and post-treatment) as independent variables and performed two-way repeated measures ANOVA. Bonferroni post hoc analysis was used. We set statistical significance at p < 0.05.
Ethics approval and consent to participate
The study protocol was approved by the Ministry of Health and Welfare of Korea (2-1040709-AB-N-01-202109-HR-066-04) and was performed in compliance with the Helsinki Declaration and ethical research principles. Written signed consent was collected from each participant before the start of the study. This trial was retrospectively registered in the Clinical Research Information Service (CRIS) (Republic of Korea, KCT0008621, 17/07/2023, https://cris.nih.go.kr).
Results
Effect of moderate intensity walking exercises on changes in body composition
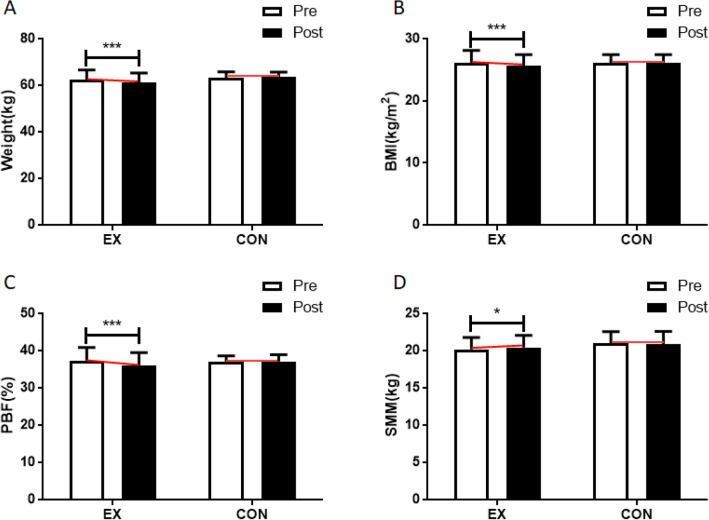
Walking exercises affected changes in body weight (interaction effect: F = 7.446, p = 0.013; time effect: F = 2.971, p = 0.099; group effect: F = 1.259, p = 0.274). The post hoc analysis of body weight by pre- and post-treatment period revealed a significant decrease in body weight in the walking group (F = 10.363, p = 0.004). Body mass index (BMI) was also affected by walking exercises (interaction effect: F = 9.203, p = 0.006; time effect: F = 3.894, p = 0.062; group effect: F = 0.152, p = 0.701), with a significant decrease in BMI in participants of the exercise group (F = 13.105, p = 0.002). Body fat percentage was equally changed through the exercises (interaction effect: F = 11.181, p = 0.003; time effect: F = 7.804, p = 0.011; group effect: F = 0.085, p = 0.773), with participants who did walking exercises showing a significant decrease in body fat percentage at the conclusion of the study (F = 19.689, p = 0.000). Lastly, skeletal muscle mass was affected by walking exercises (interaction effect: F = 5.656, p = 0.027; time effect: F = 0.435, p = 0.517; group effect: F = 0.852, p = 0.367), as evident in its significant increase in the walking group (F = 4.824, p = 0.039). The above results are summarized in Fig. 2 and Table S1.
Figure 2.
Effect of 12 weeks of moderate intensity walking exercise on body composition in postmenopausal women with obesity. After the intervention, compared with the baseline values, (A) Weight levels were decreased in the moderate intensity walking exercise group and (B) BMI levels were decreased in the moderate intensity walking exercise group, (C) PBF levels were decreased in the moderate intensity walking exercise group, (D) SMM levels were increased in the moderate intensity walking exercise group. Data are presented as the mean ± standard deviation; *p < 0.05, ***p < 0.001 before vs. after intervention. BMI body mass index, PBF percentage of body fat, SMM skeletal muscle mass.
Effect of moderate intensity walking exercises on vascular inflammation factors
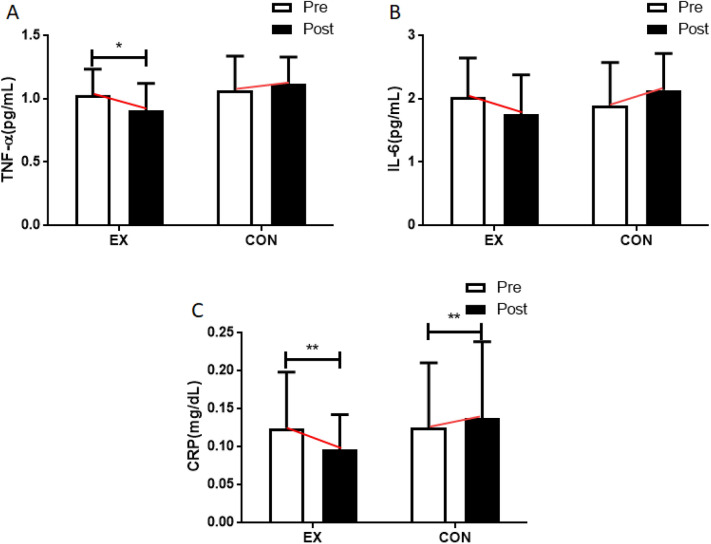
TNF-α was affected by walking exercises (interaction effect: F = 6.895, p = 0.016; time effect: F = 1.111, p = 0.304; group effect: F = 1.887, p = 0.184). Post hoc analysis of TNF-α levels before and after the experiment showed a significant decrease of this inflammation factor in the walking group (F = 7.078, p = 0.015). However, IL-6 was not affected by the exercises (interaction effect: F = 5.792, p = 0.025; time effect: F = 0.007, p = 0.933; group effect: F = 0.286, p = 0.598), at the conclusion of the experiment, a significant difference was observed in the interaction effect, but no significant differences were detected at the main effect levels.
The walking program did affect hs-CRP levels in the blood (interaction effect: F = 7.493, p = 0.012; time effect: F = 1.268, p = 0.273; group effect: F = 0.450, p = 0.510), which were significantly decreased post-treatment in participants in the walking group (F = 7.802, p = 0.011). Figure 3 and Table S1 illustrates the above findings.
Figure 3.
Effect of 12 weeks of moderate intensity walking exercise on vascular inflammation factors in postmenopausal women with obesity. After the intervention, compared with the baseline values, (A) TNF-α levels were decreased in the moderate intensity walking exercise group and (B) IL-6 levels were not changed in all groups, (C) CRP levels were decreased in the moderate intensity walking exercise group and increased in the control group. Data are presented as the mean ± standard deviation; *p < 0.05, ** p < 0.01 before vs after intervention. TNF-α tumor necrosis factor-alpha, IL-6 interleukin-6, CRP C-reactive protein.
Effect of moderate intensity walking exercise on vascular endothelial growth factor
VEGF showed no significant changes due to walking exercises (interaction effect: F = 0.326, p = 0.574; time effect: F = 0.020, p = 0.889; group effect: F = 0.037, p = 0.849. This is depicted in Fig. 4 and Table S1.
Figure 4.
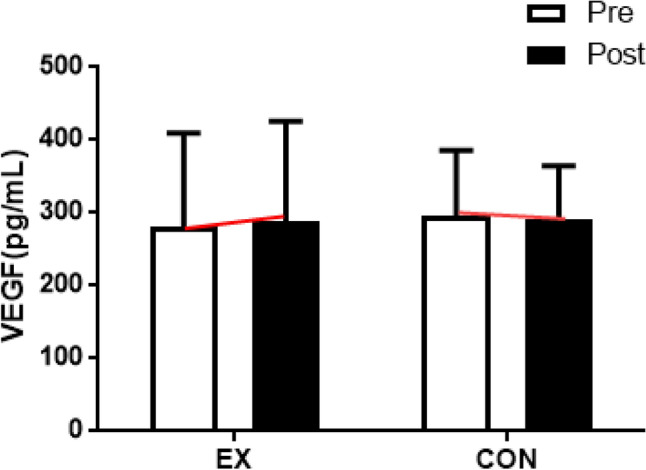
Effect of 12 weeks of moderate intensity walking exercise on VEGF in postmenopausal women with obesity. After the intervention, VEGF levels were not changed compared with the baseline values in all groups. Data are presented as the mean ± standard deviation. VEGF Vascular endothelial growth factor.
Discussion
This study was conducted to investigate the effect that twelve weeks of moderate intensity walking exercise has on the body composition, vascular inflammatory factors, and VEGF levels of women to assess whether such exercise can assist with potential postmenopausal obesity. We hypothesized that vascular inflammatory and endothelial growth factor levels would improve after a moderate intensity walking program. Our data confirmed that moderate intensity walking improved body composition and reduced vascular inflammatory factors, but we observed no changes in VEGF levels.
Postmenopausal, rapid physiological and hormonal changes, such as decreased estrogen levels, reduce muscle mass and accelerate fat accumulation1,28. As a result, obesity may be induced while the basal metabolic rate decreases29. Poehlman30 reported that the body composition of women remains constant before menopause, but after menopause, the lean body mass decreases by ~ 3 kg, and body fat increases by ~ 2.5 kg. This increase in body fat is highly correlated with metabolic syndrome and is a risk factor for CVD31.
Regular exercise can help improve lean body mass and reduce body fat32. A walking program has previously been demonstrated to reduce the body fat percentage in postmenopausal women33. This can be ascribed to the use of triacylglycerol in adipose tissue as an energy source during and after exercise, inducing changes in body fat mass34 and increasing the energy utilization capacity of fatty tissues35. Our study similarly revealed a statistically significant decrease in body fat percentage due to moderate intensity walking exercises. These results suggest that regular moderate intensity walks can help postmenopausal women with obesity prevent and reduce obesity and improve body composition.
When triglycerides and adipose tissue increase in musculature, there is a corresponding increase in inflammatory factors TNF-α and IL-6 in vascular8,36. CVD is caused by such vascular inflammation and the formation of blood clots, which can result in arteriosclerosis37. TNF-α is a major inflammatory response factor secreted mainly from macrophages and vascular endothelial cells and is primarily involved in the initial stage of inflammation38. Arteriosclerosis occurs when excessive TNF-α secretion causes cholesterol accumulation along blood vessel walls39. Plasma TNF-α levels can also predict the risk of myocardial infarction40, and high serum levels thereof have been reported in obese people41. TNF-α serum levels are correlated with those of IL-642. When IL-6 increases, lipoprotein lipase expression is suppressed, inducing hyperlipidemia43. Obesity, in particular, is a major factor that increases plasma IL-6 levels, and Vozarava et al.44 demonstrated that obesity and IL-6 are positively correlated. Conversely, TNF-α45 and IL-646 levels decrease after an obese person has lost weight, indicating the effect of regular physical activity47.
A previous study showed that TNF-α48 and IL-649 decrease with walking exercise. It is known that body fat reduction through exercise induces positive changes in TNF-α and IL-650. In this study, both TNF-α alevels were significantly decreased in participants from the moderate intensity walking exercise group. This confirms that regular moderate intensity walking exercises can reduce body weight and fat51 and decrease inflammatory factors in blood vessels and, consequently, may reduce the risk of cardiovascular disease.
Hs-CRP is an inflammatory factor produced in the liver by stimulating inflammatory substances such as TNF-α and IL-652. Therefore, it is a valuable indicator of various inflammatory conditions and the risk of cardiovascular diseases. Obesity increases the serum level hs-CRP11, which can result in the formation of thrombi, dysfunction of vascular endothelial cells53, and induction of CVD such as atherosclerosis54,55. In the Japan Collaborative Cohort study reported that hs-CRP levels were positively associated with CVD56. Cohort study of data from 3,119 participants reported that High hs-CRP serum levels strongly associated with the incidence and mortality of cardiovascular disease57, whereas decreased hs-CRP levels have been shown to reduce the risk of CVD58. Such decreases in hs-CRP levels occur when the body fat and BMI are reduced59. Exercise, in particular, has an anti-inflammatory effect that lowers the levels of hs-CRP60.
Taghian et al.61 reported that walking exercises reduce CRP in older women, and this can be due to both a decrease in inflammation and an increase in anti-inflammation through exercise62. Furthermore, decreases in hs-CRP have been correlated with decreases in IL-6, which stimulates the secretion of hs-CRP63,64. In this study, we observed a significant drop in hs-CRP levels following regular moderate intensity walking exercise. This suggests that moderate intensity walking exercises can help prevent cardiovascular diseases by reducing inflammatory factors.
Obesity disrupts vascular endothelial cell function via heightened inflammation and oxidative stress65, and vascular endothelial dysfunction is recognized as a prognostic symptom leading to atherosclerosis64. Indeed, increases in adipose tissue have been demonstrated to reduce the density and activity of capillaries in skeletal muscle66. VEGF controls the permeability of blood vessels67 and plays a vital role in the formation of new capillaries by promoting the proliferation of endothelial cells68. Exercise has been shown to increase VEGF expression and, as a result, increase capillary number and density as well as blood flow, thereby promoting angiogenesis and improving the structure and function of blood vessels69.
In a previous study, patients with PAD (peripheral arterial disease) experienced an increase in VEGF as a result of treadmill walking70. This was ascribed to increases in shear stress and nitric oxide through exercise71. On the contrary, Izzicupo et al.72 observed no change in VEGF levels in postmenopausal women after participating in 13 weeks of walking exercises. The authors suggested that VEGF fluctuation may be associated with the intensity and nature of exercises73. In this study, we similarly encountered no significant difference in VEGF levels between postmenopausal women with obesity who did and did not follow a moderate intensity walking exercise program. However, VEGF showed a tendency to increase slightly in those that exercised. This suggests that continued participation in moderate intensity walking exercises may have a positive effect on VEGF levels.
A potential limitation of this study is that we did not measure direct markers of vascular changes in response to moderate intensity walking. Therefore, further studies need to investigate the direct indices of walking exercise and the relationship between vascular inflammatory factors and VEGF. In addition, there are some limitations in generalizing our findings. First, as the focus of the study was postmenopausal women with obesity, it is difficult to extrapolate the results to men or women of other ages. Second, due to the small sample size, the effect size in this study was confined to a statistical power of 70%, posing a limitation that could potentially influence the outcomes. Therefore, subsequent studies involving a larger number of participants should be conducted to reinforce our findings. Lastly, our study did not implement dietary control to assess the effects of walking exercise while maintaining participants' daily routines. This omission represents a limitation of our research. Therefore, for future studies, incorporating dietary control is important to provide a more specific understanding of the observed changes.
Conclusion
In summary, moderate intensity walking is an effective therapeutic expected improving percent body fat, TNF- and hs CRP in postmenopausal women with obesity. In addition, since VEGF showed a tendency to increase in participants that exercised, we anticipate that additional research may provide evidence for a similar improvement in VEGF levels as a result of moderate intensity walking exercises. These results support that moderate intensity walking exercise can be expected to prevent CVD by reducing obesity and vascular inflammatory factors in postmenopausal women with obesity.
Supplementary Information
Acknowledgements
We would like to express our gratitude to all participants who helped with our research. We would also like to express our gratitude to the managers and facility workers from various welfare organizations who supported the recruitment of study participants and the research.
Author contributions
Conceptualization, W.H.S. and M.-S.H.; methodology, M.-S.H.; software, M.-S.H.; validation, W.H.S.; formal analysis, M.-S.H.; investigation, W.H.S.; resources, W.H.S.; data curation, M.-S.H.; writing—original draft preparation, W.H.S. and M.-S.H.; writing—review and editing, B.H.J., H.-T.P. and M.-S.H.; visualization, M.-S.H.; supervision, W.H.S. and M.-S.H.; project administration, W.H.S. and B.H.J.; funding acquisition, W.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2021S1A5B5A16078209).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hyun-Tae Park and Min-Seong Ha.
Contributor Information
Hyun-Tae Park, Email: htpark@dau.ac.kr.
Min-Seong Ha, Email: haminseong@uos.ac.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-47403-2.
References
- 1.El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA, American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing Menopause transition and cardiovascular disease risk: Implications for timing of early prevention: A scientific statement from the American Heart Association. Circulation. 2020;142:e506–e532. doi: 10.1161/CIR.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 2.Karvonen-Gutierrez C, Kim C. Association of mid-life changes in body size, body composition and obesity status with the menopausal transition. Healthcare (Basel) 2016 doi: 10.3390/healthcare4030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winneker RC, Harris HA. Progress and prospects in treating postmenopausal vaginal atrophy. Clin. Pharmacol. Ther. 2011;89:129–132. doi: 10.1038/clpt.2010.161. [DOI] [PubMed] [Google Scholar]
- 4.Wu W, Zheng X. Weight change over 4 years and risk of cardiovascular diseases in China: The China health and retirement longitudinal study. Obes. Facts. 2022;15:694–702. doi: 10.1159/000526419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Festa A, D'Agostino R, Jr, Williams K, Karter A, Mayer-Davis E, Tracy R, Haffner S. The relation of body fat mass and distribution to markers of chronic inflammation. Int. J. Obes. 2001;25:1407–1415. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 6.Kim JK, Chung YT, Kim HY, Han JA, Kim JW. The factors associated with high-sensitivity C-reactive protein in postmenopausal women: Based on Korea National health and nutrition examination survey 2016–2017. Korean J. Family Pract. 2020;10:96–102. doi: 10.21215/kjfp.2020.10.2.96. [DOI] [Google Scholar]
- 7.Khanna D, Welch BS, Rehman A (2022) Pathophysiology of Obesity. In: StatPearls. Treasure Island (FL) [PubMed]
- 8.Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glynn RJ, Cook NR. C-reactive protein and coronary heart disease. N. Engl. J. Med. 2004;351:295–298. doi: 10.1056/NEJM200407153510318. [DOI] [PubMed] [Google Scholar]
- 10.Lee H. Association between obesity and high-sensitivity C-reactive protein in Korean adults without cardiovascular disease. J. Korean Biol. Nurs. Sci. 2023;25:32–42. doi: 10.7586/jkbns.23.343. [DOI] [Google Scholar]
- 11.Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi: 10.1016/j.cytogfr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001;280:E745–751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 13.Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: Effects of dietary weight loss and exercise training. CMAJ. 2005;172:1199–1209. doi: 10.1503/cmaj.1040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am. J. Cardiol. 2001;88:1264–1269. doi: 10.1016/s0002-9149(01)02088-4. [DOI] [PubMed] [Google Scholar]
- 15.Moriya J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019;73:22–27. doi: 10.1016/j.jjcc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA, Stewart DJ. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 17.Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv. Wound Care (New Rochelle) 2014;3:647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int. J. Obes. (Lond.) 2005;29:1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 19.Zheng G, Qiu P, Xia R, Lin H, Ye B, Tao J, Chen L. Effect of aerobic exercise on inflammatory markers in healthy middle-aged and older adults: A systematic review and meta-analysis of randomized controlled trials. Front. Aging Neurosci. 2019;11:98. doi: 10.3389/fnagi.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abd El-Kader SM, Al-Shreef FM, Al-Jiffri OH. Impact of aerobic exercise versus resisted exercise on endothelial activation markers and inflammatory cytokines among elderly. Afr. Health Sci. 2019;19:2874–2880. doi: 10.4314/ahs.v19i4.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nabkasorn C, Miyai N, Sootmongkol A, Junprasert S, Yamamoto H, Arita M, Miyashita K. Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur. J. Public Health. 2006;16:179–184. doi: 10.1093/eurpub/cki159. [DOI] [PubMed] [Google Scholar]
- 22.Paluch AE, Gabriel KP, Fulton JE, Lewis CE, Schreiner PJ, Sternfeld B, Sidney S, Siddique J, Whitaker KM, Carnethon MR. Steps per day and all-cause mortality in middle-aged adults in the coronary artery risk development in young adults study. JAMA Netw. Open. 2021;4:e2124516. doi: 10.1001/jamanetworkopen.2021.24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb MA, Mani H, Robertson SJ, Waller HL, Webb DR, Edwardson CL, Bodicoat DH, Yates T, Khunti K, Davies MJ. Moderate increases in daily step count are associated with reduced IL6 and CRP in women with PCOS. Endocr. Connect. 2018;7:1442–1447. doi: 10.1530/EC-18-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moy ML, Teylan M, Weston NA, Gagnon DR, Danilack VA, Garshick E. Daily step count is associated with plasma C-reactive protein and IL-6 in a US cohort with COPD. Chest. 2014;145:542–550. doi: 10.1378/chest.13-1052. [DOI] [PubMed] [Google Scholar]
- 25.MacDougall HG. Moore ST (2005) Marching to the beat of the same drummer: The spontaneous tempo of human locomotion. J. Appl. Physiol. 1985;99:1164–1173. doi: 10.1152/japplphysiol.00138.2005. [DOI] [PubMed] [Google Scholar]
- 26.Styns F, van Noorden L, Moelants D, Leman M. Walking on music. Hum. Mov. Sci. 2007;26:769–785. doi: 10.1016/j.humov.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Guo X, Liu L, Xie M, Lam WK. Effects of Tai-Chi and running exercises on cardiorespiratory fitness and biomarkers in sedentary middle-aged males: A 24-week supervised training study. Biology (Basel) 2022 doi: 10.3390/biology11030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, Villaseca P, Writing Group of the International Menopause Society for World Menopause D Understanding weight gain at menopause. Climacteric. 2012;15:419–429. doi: 10.3109/13697137.2012.707385. [DOI] [PubMed] [Google Scholar]
- 29.Gold EB, Crawford SL, Shelton JF, Tepper PG, Crandall CJ, Greendale GA, Matthews KA, Thurston RC, Avis NE. Longitudinal analysis of changes in weight and waist circumference in relation to incident vasomotor symptoms: The Study of Women's Health Across the Nation (SWAN) Menopause. 2017;24:9–26. doi: 10.1097/GME.0000000000000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poehlman ET. Menopause, energy expenditure, and body composition. Acta Obstet. Gynecol. Scand. 2002;81:603–611. doi: 10.1034/j.1600-0412.2002.810705.x. [DOI] [PubMed] [Google Scholar]
- 31.De Lucia RE, Ong KK, Sleigh A, Dunger DB, Norris SA. Abdominal fat depots associated with insulin resistance and metabolic syndrome risk factors in black African young adults. BMC Public Health. 2015;15:1013. doi: 10.1186/s12889-015-2147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aragao FR, Abrantes CG, Gabriel RE, Sousa MF, Castelo-Branco C, Moreira MH. Effects of a 12-month multi-component exercise program on the body composition of postmenopausal women. Climacteric. 2014;17:155–163. doi: 10.3109/13697137.2013.819328. [DOI] [PubMed] [Google Scholar]
- 33.Roussel M, Garnier S, Lemoine S, Gaubert I, Charbonnier L, Auneau G, Mauriege P. Influence of a walking program on the metabolic risk profile of obese postmenopausal women. Menopause. 2009;16:566–575. doi: 10.1097/gme.0b013e31818d4137. [DOI] [PubMed] [Google Scholar]
- 34.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993;265:E380–391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 35.Kolnes KJ, Petersen MH, Lien-Iversen T, Hojlund K, Jensen J. Effect of exercise training on fat loss-energetic perspectives and the role of improved adipose tissue function and body fat distribution. Front. Physiol. 2021;12:737709. doi: 10.3389/fphys.2021.737709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stolarczyk E. Adipose tissue inflammation in obesity: A metabolic or immune response? Curr. Opin. Pharmacol. 2017;37:35–40. doi: 10.1016/j.coph.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Bartekova M, Radosinska J, Jelemensky M, Dhalla NS. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 2018;23:733–758. doi: 10.1007/s10741-018-9716-x. [DOI] [PubMed] [Google Scholar]
- 38.Olszewski MB, Groot AJ, Dastych J, Knol EF. TNF trafficking to human mast cell granules: Mature chain-dependent endocytosis. J. Immunol. 2007;178:5701–5709. doi: 10.4049/jimmunol.178.9.5701. [DOI] [PubMed] [Google Scholar]
- 39.Oberoi R, Vlacil AK, Schuett J, Schosser F, Schuett H, Tietge UJF, Schieffer B, Grote K. Anti-tumor necrosis factor-alpha therapy increases plaque burden in a mouse model of experimental atherosclerosis. Atherosclerosis. 2018;277:80–89. doi: 10.1016/j.atherosclerosis.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 40.Tian M, Yuan YC, Li JY, Gionfriddo MR, Huang RC. Tumor necrosis factor-alpha and its role as a mediator in myocardial infarction: A brief review. Chronic Dis. Transl. Med. 2015;1:18–26. doi: 10.1016/j.cdtm.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan W, Xu Y, Liu Y, Zhang Z, Lu L, Ding Z. Obesity or overweight, a chronic inflammatory status in male reproductive system, leads to mice and human subfertility. Front. Physiol. 2017;8:1117. doi: 10.3389/fphys.2017.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popko K, Gorska E, Stelmaszczyk-Emmel A, Plywaczewski R, Stoklosa A, Gorecka D, Pyrzak B, Demkow U. Proinflammatory cytokines Il-6 and TNF-alpha and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010;15(Suppl 2):120–122. doi: 10.1186/2047-783x-15-s2-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams L, Bagley J, Iacomini J. The role of IL-6 in hyperlipidemia-induced accelerated rejection. Am. J. Transplant. 2022;22:427–437. doi: 10.1111/ajt.16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes. Res. 2001;9:414–417. doi: 10.1038/oby.2001.54. [DOI] [PubMed] [Google Scholar]
- 45.Beltowski J. Adiponectin and resistin-new hormones of white adipose tissue. Med. Sci. Monit. 2003;9:RA55–61. [PubMed] [Google Scholar]
- 46.Chae JS, Paik JK, Kang R, Kim M, Choi Y, Lee SH, Lee JH. Mild weight loss reduces inflammatory cytokines, leukocyte count, and oxidative stress in overweight and moderately obese participants treated for 3 years with dietary modification. Nutr. Res. 2013;33:195–203. doi: 10.1016/j.nutres.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Izzicupo P, D'Amico MA, Bascelli A, Di Fonso A, D'Angelo E, Di Blasio A, Bucci I, Napolitano G, Gallina S, Di Baldassarre A. Walking training affects dehydroepiandrosterone sulfate and inflammation independent of changes in spontaneous physical activity. Menopause. 2013;20:455–463. doi: 10.1097/gme.0b013e31827425c9. [DOI] [PubMed] [Google Scholar]
- 48.Jennersjo P, Ludvigsson J, Lanne T, Nystrom FH, Ernerudh J, Ostgren CJ. Pedometer-determined physical activity is linked to low systemic inflammation and low arterial stiffness in Type 2 diabetes. Diabet. Med. 2012;29:1119–1125. doi: 10.1111/j.1464-5491.2012.03621.x. [DOI] [PubMed] [Google Scholar]
- 49.Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA. Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity (Silver Spring) 2011;19:1131–1136. doi: 10.1038/oby.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J. Physiol. Pharmacol. 2006;57(Suppl 10):43–51. [PubMed] [Google Scholar]
- 51.Monteiro MRP, Aragao-Santos JC, Vasconcelos ABS, Resende-Neto AG, Chaves L, Cardoso AP, Nogueira AC, Carnero-Diaz A, Marcos-Pardo PJ, Correa CB, Moura TR, Da Silva-Grigoletto ME. Bodyweight and combined training reduce chronic low-grade inflammation and improve functional fitness of postmenopausal women. Sports (Basel) 2022 doi: 10.3390/sports10100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shrivastava AK, Singh HV, Raizada A, Singh SK. C-reactive protein, inflammation and coronary heart disease. Egypt. Heart J. 2015;67:89–97. doi: 10.1016/j.ehj.2014.11.005. [DOI] [Google Scholar]
- 53.Jialal I, Kaur H, Devaraj S, Smith G. Human C-reactive protein induces endothelial dysfunction in biobreeding diabetic rats. Diab. Vasc. Dis. Res. 2013;10:550–553. doi: 10.1177/1479164113503971. [DOI] [PubMed] [Google Scholar]
- 54.Halcox JP, Roy C, Tubach F, Banegas JR, Dallongeville J, De Backer G, Guallar E, Sazova O, Medina J, Perk J, Steg PG, Rodriguez-Artalejo F, Borghi C. C-reactive protein levels in patients at cardiovascular risk: EURIKA study. BMC Cardiovasc. Disord. 2014;14:25. doi: 10.1186/1471-2261-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laaksonen DE, Niskanen L, Nyyssonen K, Punnonen K, Tuomainen TP, Salonen JT. C-reactive protein in the prediction of cardiovascular and overall mortality in middle-aged men: A population-based cohort study. Eur. Heart J. 2005;26:1783–1789. doi: 10.1093/eurheartj/ehi237. [DOI] [PubMed] [Google Scholar]
- 56.Iso H, Cui R, Date C, Kikuchi S, Tamakoshi A, Group JS. C-reactive protein levels and risk of mortality from cardiovascular disease in Japanese: The JACC study. Atherosclerosis. 2009;207:291–297. doi: 10.1016/j.atherosclerosis.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 57.Bernabe-Ortiz A, Carrillo-Larco RM, Gilman RH, Smeeth L, Checkley W, Miranda JJ. High-sensitivity C-reactive protein and all-cause mortality in four diverse populations: The CRONICAS cohort study. Ann. Epidemiol. 2022;67:13–18. doi: 10.1016/j.annepidem.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopes WA, Leite N, da Silva LR, Brunelli DT, Gaspari AF, Radominski RB, Chacon-Mikahil MP, Cavaglieri CR. Effects of 12 weeks of combined training without caloric restriction on inflammatory markers in overweight girls. J. Sports Sci. 2016;34:1902–1912. doi: 10.1080/02640414.2016.1142107. [DOI] [PubMed] [Google Scholar]
- 59.Fedewa MV, Hathaway ED, Ward-Ritacco CL. Effect of exercise training on C reactive protein: A systematic review and meta-analysis of randomised and non-randomised controlled trials. Br. J. Sports Med. 2017;51:670–676. doi: 10.1136/bjsports-2016-095999. [DOI] [PubMed] [Google Scholar]
- 60.Mohammadi HR, Khoshnam MS, Khoshnam E. Effects of different modes of exercise training on body composition and risk factors for cardiovascular disease in middle-aged men. Int. J. Prev. Med. 2018;9:9. doi: 10.4103/ijpvm.IJPVM_209_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taghian F, Rahnama N, Esfarjani F, Sharifi G. Does aerobic exercise effect on the levels of interlukin-6, TNF-α and plasma CRP in the elderly women. Gazz. Med. Ital. Arch. Sci. Med. 2012;171:767–773. [Google Scholar]
- 62.Pedersen BK. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Invest. 2017;47:600–611. doi: 10.1111/eci.12781. [DOI] [PubMed] [Google Scholar]
- 63.Sena CM, Leandro A, Azul L, Seica R, Perry G. Vascular oxidative stress: Impact and therapeutic approaches. Front. Physiol. 2018;9:1668. doi: 10.3389/fphys.2018.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwaifa IK, Bahari H, Yong YK, Noor SM. Endothelial dysfunction in obesity-induced inflammation: Molecular mechanisms and clinical implications. Biomolecules. 2020 doi: 10.3390/biom10020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mendez-Barbero N, Gutierrez-Munoz C, Blanco-Colio LM. Cellular crosstalk between endothelial and smooth muscle cells in vascular wall remodeling. Int. J. Mol. Sci. 2021 doi: 10.3390/ijms22147284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J. Am. Coll. Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 67.Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Moscow) 2008;73:751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- 68.Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: Similarities and differences. J. Cell. Mol. Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zbinden R, Zbinden S, Meier P, Hutter D, Billinger M, Wahl A, Schmid JP, Windecker S, Meier B, Seiler C. Coronary collateral flow in response to endurance exercise training. Eur. J. Cardiovasc. Prev. Rehabil. 2007;14:250–257. doi: 10.1097/HJR.0b013e3280565dee. [DOI] [PubMed] [Google Scholar]
- 70.da Silva ND, Jr, Andrade-Lima A, Chehuen MR, Leicht AS, Brum PC, Oliveira EM, Wolosker N, Pelozin BRA, Fernandes T, Forjaz CLM. Walking training increases microRNA-126 expression and muscle capillarization in patients with peripheral artery disease. Genes (Basel) 2022 doi: 10.3390/genes14010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez I, Gonzalez M. Physiological mechanisms of vascular response induced by shear stress and effect of exercise in systemic and placental circulation. Front. Pharmacol. 2014;5:209. doi: 10.3389/fphar.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Izzicupo P, D'Amico MA, Di Blasio A, Napolitano G, Nakamura FY, Di Baldassarre A, Ghinassi B. Aerobic training improves angiogenic potential independently of vascular endothelial growth factor modifications in postmenopausal women. Front. Endocrinol. (Lausanne) 2017;8:363. doi: 10.3389/fendo.2017.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wahl P, Zinner C, Achtzehn S, Behringer M, Bloch W, Mester J. Effects of acid-base balance and high or low intensity exercise on VEGF and bFGF. Eur. J. Appl. Physiol. 2011;111:1405–1413. doi: 10.1007/s00421-010-1767-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.