Abstract
In the current study, we combined sociometric nominations and neuroimaging techniques to examine adolescents’ neural tracking of peers from their real-world social network that varied in social preferences and popularity. Adolescent participants from an entire school district (N = 873) completed peer sociometric nominations of their grade at school, and a subset of participants (N = 117, Mage = 13.59 years) completed a neuroimaging task in which they viewed peer faces from their social networks. We revealed two neural processes by which adolescents track social preference: (1) the fusiform face area, an important region for early visual perception and social categorization, simultaneously represented both peers high in social preference and low in social preference; (2) the dorsolateral prefrontal cortex (DLPFC), which was differentially engaged in tracking peers high and low in social preference. No regions specifically tracked peers high in popularity and only the inferior parietal lobe, temporoparietal junction, midcingulate cortex and insula were involved in tracking unpopular peers. This is the first study to examine the neural circuits that support adolescents’ perception of peer-based social networks. These findings identify the neural processes that allow youths to spontaneously keep track of peers’ social value within their social network.
Keywords: adolescent, brain development, peer status, sociometric nomination, social preference, popularity
Introduction
Most social species use knowledge about others to construct hierarchical social networks in which some individuals are highly accepted within the network (i.e. liked) and others are highly rejected (i.e. disliked; Dwortz et al., 2022). For instance, animals often judge who is more attractive, preferred as a mate or cooperative. In turn, this self-organized social structure has significant effects on animals’ social behaviors, well-being and survival by dictating who to approach and who to avoid (for review, Qu et al., 2017). Indeed, individuals who are better at understanding social hierarchies and can adapt their social behaviors accordingly are more likely to survive and thrive (Li et al., 2021). Humans, like other social species, represent others’ social value within their social hierarchies, and this ability may develop as early as infancy (Cummins, 2005; Thomsen, 2020). Nonetheless, the importance of social value may peak in adolescence, when peer-based social rank becomes especially salient and has profound impacts on adolescents’ social behaviors (McFarland et al., 2014; Prinstein, 2017). Prior research has examined the neural representation of others’ social value in adults (e.g. Chiao, 2010; Zerubavel et al., 2015; Parkinson et al., 2017; Morelli et al., 2018), but no research to date has examined how adolescents neurally represent the social value of peers in their real-world social networks. Many brain systems undergo significant reorganization during adolescence (for review, Sisk and Foster, 2004), which may subserve the emergence of a social orientation towards peers and sensitivity to peer-based social hierarchies within networks. In the present study, we examine how the brains of adolescents represent their peers who vary in peer-nominated social preference (i.e. whether peers are the most or least liked in their social network) and popularity (i.e. whether peers are rated as the most or least popular in their social network), which are two important forms of peer status (Prinstein, 2017).
Social hierarchies in adolescence
Adolescence is marked by an increased orientation towards peers (Nelson et al., 2005). This is manifested in multifarious social networks such as friendships, cliques and romantic relationships (Moody et al., 2011). By early adolescence, peer groups start to become differentiated by peer status (Coie et al., 1990; LaFontana and Cillessen, 2010). Peer status in a network refers to an individual’s socially ascribed value within a social network and can be predictive of a variety of important social attributes such as popularity and likability (Prinstein, 2017). Adolescents tend to be more concerned with their social status among peers than are children and adults (Eder, 1985; LaFontana and Cillessen, 2010). Adolescents are also more motivated than children and adults to gain social status among their peers (Prinstein, 2017) and to prioritize peers’ status when selecting friends (LaFontana and Cillessen, 2010). Adolescents’ social milieu provides a critical developmental context for adolescents to practice social behavior (e.g. cooperation and competition) and to engage in self-exploration through social comparison (Laninga-Wijnen and Veenstra, 2021). Adolescents’ representation of peers’ social status also has implications for their own health behavior: adolescents are more likely to engage in risky behaviors when endorsed by high-status peers than low-status peers (Cohen and Prinstein, 2006; Do et al., 2020). Therefore, it is essential to understand the basic processes by which adolescents represent and assess peer status in order to understand how those processes may contribute to social behavior.
Neurobiological sensitivity to social hierarchies
Despite the significance of peer status to adolescents’ social life (Prinstein and Giletta, 2016), relatively little is known about how the brains of adolescents spontaneously represent their peers’ social status in a dynamic and ecological context. It is understood that adolescents are hyper-attuned to the peer-based social networks in their environments (Jonkmann et al., 2009; McFarland et al., 2014; Lunn et al., 2021), which is reflected in greater visual attention towards high social status peers (Lansu et al., 2014). These findings suggest that the visuoperceptual system becomes particularly sensitive to peer-based social hierarchies during adolescence, but no research to date has examined the neural processes that allow youths to represent varying levels of peer statuses in social networks.
Keeping track of others’ peer status within a network requires efficiently extracting, processing and integrating social information about peers (Koski et al., 2015). Humans can make these important, yet computationally demanding, evaluations within milliseconds (Todorov et al., 2007, 2015; Koski et al., 2015). In adults, this ability is largely supported by neural systems involved in face processing, social evaluation, mentalizing and executive function (Koski et al., 2015, 2017; Zerubavel et al., 2015; Curley and Ochsner, 2017; Morelli et al., 2018). Structural neuroimaging work in primates shows that the volume of these brain regions, including the amygdala, orbitofrontal cortex, prefrontal cortex (PFC) and superior temporal sulcus, are positively associated with the sizes of an individual’s social networks (e.g. Bickart et al., 2012; Kanai et al., 2012; Powell et al., 2012). Importantly, the function of these systems undergoes significant reorganization during adolescence (for review, Sisk and Foster, 2004; Foulkes and Blakemore, 2018), which corresponds to the timeframe during which youths become highly sensitive to peer contexts (for review, Casey et al., 2008; Blakemore and Robbins, 2012; Foulkes and Blakemore, 2018). Therefore, brain development during this period may underlie the emergence of sensitivity to social hierarchies during adolescence.
Although no prior research to date has investigated the neural tracking of social status in human adolescents, neural systems involved in face processing, social evaluation, mentalizing and executive function may also represent this social attribute of peers in adolescents. The first system includes regions involved in face processing. This spatially distributed set of regions includes core visual regions such as the fusiform face area (FFA) and occipital gyrus, and extended regions such as the anterior temporal lobe (ATL; e.g. Haxby et al., 2000; Duchaine and Yovel, 2015). The FFA can be further subdivided into the FFA1 and FFA2 (e.g. Elbich and Scherf, 2017), which play a critical role in facial feature analysis and identity perception, respectively (e.g. Grill-Spector et al., 2004; Kanwisher and Yovel, 2006; Weiner and Grill-Spector, 2011). In addition, the FFA is also involved in the social categorization of emotions, gender, and race (e.g. Stolier and Freeman, 2016; Brooks and Freeman, 2018; Brooks et al., 2019). The occipital gyrus, which is critical for visual perception, elicits significantly greater activation when viewing high-status faces relative to low-status faces (e.g. Zink et al., 2008). Finally, the ATL is broadly involved in semantic storage and retrieval and has been shown to represent social category knowledge (Olson et al., 2013) and biographical information about targets (e.g. Brambati et al., 2010; Wang et al., 2014).
Second, visually tracking social status may also engage regions involved in social valuation and salience such as the ventral striatum (VS) and amygdala. Past work suggests that the VS is implicated in assessing the motivational significance of visual stimuli (e.g. Lacey et al., 2011; Lindquist et al., 2016; Graf et al., 2018) and the affective value of peer-based group members (e.g. Zerubavel et al., 2015; Morelli et al., 2018; Do et al., 2020). The amygdala is more generally involved in detecting salient stimuli in the environment such as emotionally evocative stimuli or in-group faces (e.g. Adolphs, 2002, 2009; Kubota et al., 2012; Amodio, 2014; Guassi Moreira et al., 2017). The amygdala also plays an essential role in detecting the social status of group members and representing knowledge about social hierarchies (e.g. Zink et al., 2008; Fernald and Maruska, 2012; Kumaran et al., 2012).
Finally, tracking peers’ social status might engage brain regions associated with social cognition such as the temporoparietal junction (TPJ) and PFC. The TPJ is involved in judging group members’ characteristics and inferring social members’ mental states and intentions, as well as peer status (e.g. Parkinson et al., 2017; Schmälzle et al., 2017). Moreover, the TPJ exhibits increased activity while viewing images of influential individuals compared to less essential individuals in adult networks (e.g. Morelli et al., 2018). Research has indicated that several subregions of the PFC may be involved in the recognition of group members’ social values and regulation of social behaviors towards socially valued group members (for review, Wang et al., 2014). For instance, the medial PFC (mPFC) is involved in social rank encoding (e.g. Padilla-Coreano et al., 2022) and flexibly guides behaviors on the basis of hierarchical status in rodents (e.g. Li et al., 2021). In humans, the mPFC underlies the construction and representation of others’ relative social value (Park et al., 2021), as well as reinforcement learning of human social hierarchies (Ligneul et al., 2016). In addition, the mPFC is activated while adults perceive or make judgments about high peer status members (e.g. Zink et al., 2008; Koski et al., 2015). Another important subregion of the PFC is the dorsolateral PFC (DLPFC), which underlies the perception of higher social hierarchies (Koski et al., 2015; Watanabe and Yamamoto, 2015), and supports the regulation of behaviors towards individuals with high social status (for review, Qu et al., 2017).
The present study
In the present study, we examined the neural tracking of social status in adolescents’ real-world social networks using a combination of sociometric nominations and neuroimaging techniques. One important form of peer status is Social Preference, or the extent to which an individual is on average liked vs disliked across other members of a social network (Coie et al., 1982). Although social hierarchies can take many forms in humans, social preference (i.e. likability) is one of the most widely used metrics to understand peer status in adolescents’ social world (LaFontana and Cillessen, 2010; Prinstein, 2017). A second form of peer status is Perceived Popularity, or the extent to which an individual is viewed as popular across other members of a social network, regardless of whether they are well-liked. Social preference (i.e. likeability) and perceived popularity are two different sociometric constructs and are only moderately correlated in adolescent samples (Cillessen and Mayeux, 2004; Prinstein et al., 2011b). Indeed, not all popular adolescents are liked and not all unpopular adolescents are disliked (Mayeux et al., 2008; Prinstein and Giletta, 2016).
Sociometric nominations are one of the most ecologically valid tools for investigating the structures of human social networks (Prinstein and Cillessen, 2003; Fournier, 2009). While social hierarchies can be only observed behaviorally in the context of social interaction in children (Strayer and Strayer, 1976), adolescents can perceive status hierarchies in their social ecology and characterize their social networks using multiple attributes, including which peers are most liked and least liked and most popular and least popular (Koski et al., 2015). Social Preference is computed by assessing every person in a network for their preference scores for who they like the most and like the least. Social preference scores for each individual in a network are then calculated as the standardized difference between ‘liked most’ and ‘liked least’ nomination tallies, with positive scores (i.e. above the mean) indicating high social preference and negative scores (i.e. below the mean) indicating low social preference (Coie and Dodge, 1983). Perceived Popularity is similarly computed by assessing every person in a network for who they think is the most and least popular. Importantly, adolescents exhibit an extremely high level of consistency in their assessment of peer status within a network (Anderson et al., 2006; Koski et al., 2015), suggesting that there is a fair amount of consensus about peers’ social value within social networks. We thus measured social preference and perceived popularity using sociometric peer nominations in which 6th and 7th graders in three public middle schools reported on which of their classmates they liked the most and least and which of their classmates were the most and least popular (Conway et al., 2011; Lansford et al., 2014).
Using the peer nominations, we created a novel fMRI paradigm, in which adolescents viewed their real-life peers’ images, which were selected based on having sociometric ratings that were the highest and lowest on social preference and perceived popularity. We predicted that a peer’s social status (e.g. social preference) would be spontaneously represented during the viewing of peer faces. We hypothesized two potential visuoperceptual strategies which allow adolescents to perceive their peer-based social hierarchies. First, adolescents may represent peers’ social status via a single psychological dimension, which simultaneously represents both group members high in social status and group members low in social status (e.g. most liked and least liked peers). This may be critical for individuals to quickly decide their next move when navigating their social worlds (e.g. approach or avoidance; cooperation or competition). Second, individuals may represent peers high in social status in a unique fashion in which salience detection is preferentially engaged, allowing them to detect individuals who are central in a network and most likely to influence future behavior while allocating less attention to less socially valued group members (e.g. Lansu et al., 2014; Morelli et al., 2018). To investigate these possibilities, we first examined the brain regions that similarly track peers high in social status (e.g. most liked) and low in social preference (e.g. least liked). Next, we examined the brain regions that differentially track peers high and low in social status by contrasting representations of high-status and low-status peers. We conducted these analyses for social preference and popularity separately as these two are distinct sociometric constructs.
Methods
Participants
This report consists of data collection across two waves when participants were in the 6th and 7th grade (wave 1, 2016–2017 school year) and 7th and 8th grade (wave 2, 2017–2018 school year) in three public middle schools in the rural southeast United States. Sociometric nominations were obtained in school-based testing sessions at each wave. A total of 873 consented and assented adolescents participated in school-based assessments (including sociometric nominations) at wave 1. At wave 1, 148 adolescents were enrolled in fMRI data collection (see supplementary material for recruitment details). To account for attrition, an additional 30 participants were recruited at wave 2, during which the Classmates fMRI task was collected from January to September 2018. The resulting sample included 178 participants.
Of the original 178 participants, 26 did not participate in the second wave, eight were unable to complete the scan due to braces, six were excluded due to technical errors during scanning, one quit the scanning session early, two were not from the school district and so did not have social network data and three participants were excluded from analyses for excessive head motion (> 2 mm in any direction). Of these 132 participants, 15 7th grade participants from one school were excluded due to sociometric data calculation errors, which resulted in them seeing invalid stimuli. The final sample of participants included 117 adolescents ages 12–15 years (Mage = 13.59, SD = 0.58). Sixty-one of the participants identified as female (52.6%) and participants endorsed diverse racial/ethnic identities (see Table 1 for detailed demographic information).
Table 1.
Demographic information of adolescent participants
| N = (117 in analysis) | ||
|---|---|---|
| Demographic variables | n | % |
| Biological sex | ||
| Female | 61 | 52.6% |
| Male | 56 | 47.4% |
| Age (year) | ||
| 12 ∼ 13 | 14 | 11.9% |
| 13 ∼ 14 | 74 | 63.2% |
| 14 ∼ 15 | 27 | 23.1% |
| >15 | 2 | 1.7% |
| Race/ethnicity | ||
| White | 41 | 35.0% |
| Black/African American | 28 | 23.9% |
| Asian | 2 | 1.7% |
| Native American | 6 | 5.9% |
| Multi-racial | 14 | 11.9% |
| Other | 26 | 22.2% |
| Hispanic | ||
| Yes | 40 | 34.2% |
| No | 76 | 65.7% |
| Prescription medication | ||
| Yes | 24 | 20.5% |
| No | 93 | 79.5% |
| Language | ||
| Native English speaker | 117 | 100% |
| Spanish family | 28 | 24.1% |
| SES (family total annual income) | ||
| $0—$14 999 | 11 | 9.4% |
| $15 000—$29 999 | 29 | 24.8% |
| $30 000—$44 999 | 23 | 19.7% |
| $45 000—$59 999 | 23 | 19.7% |
| $60 000—$74 999 | 10 | 8.5% |
| $75 000—$89 999 | 2 | 1.7% |
| $90 000—$99 999 | 6 | 5.1% |
| $100 000—$119 999 | 4 | 3.4% |
| $120 000—$150 000 | 4 | 3.4% |
| >$150 000 | 1 | 0.8% |
| N/A | 4 | 3.4% |
| Parent education | ||
| < 8th grade | 12 | 10.3% |
| 8th grade completed | 7 | 5.9% |
| Some high school | 8 | 6.8% |
| High school completed | 13 | 11.1% |
| Some college | 44 | 37.6% |
| Associate’s degree | 14 | 11.9% |
| Bachelor’s degree | 12 | 10.2% |
| Some graduate school | 2 | 1.7% |
| Graduate or professional degree | 4 | 3.4% |
Note: demographic information was reported by adolescent participants or parents. Medication, family total annual income and parent education were reported by adolescents’ parents or legal guardians during scanning visits. Biological sex, age and race/ethnicity were reported by adolescents during scanning visits. All adolescent participants were native English speakers; parents of 28 participants spoke Spanish. We missed some demographic data from one participant; therefore, only 116 participants’ demographic information was fully reported.
Peer sociometric nominations
Sociometric procedures were used to measure peer status during the school-based assessment (see supplementary material for school-based consent and assent procedures). During the school-based assessment, participants were given a full list of peers within their school and grade level and were asked to identify 1) whom they like the most, 2) whom they like the least, 3) who is the most popular and 4) who is the least popular. There was no limit to the number of peers they could nominate. Prior research suggests that the size of a social network may impact group members’ social cognition (for review, Smith et al., 2020); we thus provide basic demographic information about the school and network sizes to contextualize the current findings with previous findings in adult social network literature (e.g. Morelli et al., 2018). In our study, peer nominations were completed by 78.7% of students at School 1, 77.0% at School 2 and 89.2% at School 3. The numbers of students in each grade from each school who participated in this session ranged from 104 to 193 (School 1, 6th grade = 164, 7th grade = 109; School 2, 6th grade = 124, 7th grade = 104; School 3, 6th grade = 193 and 7th grade = 179).
Based on adolescents’ peer sociometric nominations, we calculated social preference and popularity scores for each individual. In line with past research (Coie et al., 1982), the social preference and popularity scores were computed for each adolescent as the standardized differences score between nomination tallies (i.e. z-score of ‘like the most’ minus the z-score of ‘like the least’ and z-score of ‘most popular’ minus the z-score of ‘least popular’). This z-score is thus based on ratings relative to all other students within their school and grade. High z-scores (e.g. > 1) represent high social status (e.g. liked) peers and low z-scores (e.g. < −1) represent low social status (e.g. disliked) peers within the same school (Coie et al., 1982). This sociometric procedure is considered the most ecologically valid and robust approach for assessing peer status among adolescents (Crick and Bigbee, 1998).
Classmates’ fMRI task
Adolescents who participated in the fMRI session completed a task-based fMRI face recognition task adapted from Parkinson et al. (2017), in which they viewed yearbook photos of their peers from their school and grade (See supplementary material for all fMRI visit procedures and scan acquisition parameters). The peer nominations collected during wave 1 were used to select the images of adolescents presented in the fMRI task, which was administered at wave 2. The associations between likability and popularity ratings across waves 1 and 2 are high (see Supplementary Figure S3), suggesting peer status is highly stable. Face stimuli were scanned and digitized (e.g. JPEG images) from school yearbooks from wave 1. Luminance and image size were standardized across images. Unlike some face recognition studies, we did not grayscale the picture, mask blemish or scars in the faces or crop out hair and clothing. Thus, images were as naturalistic as possible. Peer images were selected for the fMRI task based on their sociometric rating. To be selected as a face stimulus target for the task, the peer needed to have a sociometric z-score between 1 and 5 (representing 1–5 SD above the mean on social preference/popularity in their school and grade) or between −1 and −5 (representing 1–5 SD below the mean on social preference/popularity in their school and grade). The task was created using E-prime 2.0, and one version was made for each grade level within each school (six versions total for all subjects, see Supplementary Table S1). Figure 1 shows an example trial of the Classmates task.
Fig. 1.
Example trial of the classmates’ fMRI task during scan. Each trial consists of a jittered fixation and a picture of the peer from the same school with participants. We did not obtain permission to display adolescent peers’ faces; therefore, facial pictures from the developmental emotional face stimulus set (Meuwissen et al., 2017) are shown here.
The task had four conditions: high social preference (i.e. z-score between 1 and 5 on social preference), low social preference (i.e. z-score between −1 and −5 on social preference), high popularity (e.g. z-score between 1 and 5 on popularity) and low popularity (i.e. z-score between −1 and −5 on popularity). Within each condition, there were 10 targets (i.e. 40 targets for each participant), and we aimed for an equal number of boys and girls within each condition. Due to a data management error, z-scores for the targets in two of the six task versions were incorrect and did not fall within the criteria (i.e. z-score between ± 1 and 5). Popularity and social preference (i.e. likability) scores were recalculated for the target images in these task versions. For one group of participants (n = 20), there were a sufficient number of target images that fit the criteria for each condition (High Popularity = 9, Low Popularity = 8, High Social Preference = 8, Low Social Preference = 10), so these participants were included, resulting in five groups of participants and study stimuli. For the other groups, there were an insufficient number of target images (e.g. as little as 5) in the conditions, so these participants (n = 15) were excluded. The faces in each condition were equally divided by female and male adolescents. We also attempted to build a racially/ethnically diverse paradigm such that each condition contains some faces representing individuals from minoritized groups. Research suggests that social preference (i.e. likability) and popularity are two distinct but correlated sociometric constructs in adolescents (Cillessen and Mayeux, 2004). For our stimuli, the correlation between social preference and popularity scores ranged from 0.36 to 0.68 (Group 1: r = 0.36, P < 0.05; Group 2: r = 0.68, P < 0.001; Group 3: r = 0.56, P < 0.001; Group 4: r = 0.42, P < 0.01, Group 5: r = 0.46, P < 0.005). The correlation between social preference and popularity scores in the larger dataset from which study stimuli were drawn was 0.46, P < 0.001. Each target belonged in only one sociometric category and there was no overlap between targets in the social preference and popularity conditions. We did so to maximize the difference between neural responses to sociometric likability and popularity and increase the statistical power in our analyses. No adolescent participants in this neuroimaging study were included as face stimuli. The average z-score within each condition was approximately 2 (absolute value; see Supplementary Table S1). We ensured equal distributions of sociometric scores across schools and conditions for the stimuli.
The Classmates task consisted of 16 blocks, four blocks per condition, presented across two runs. The blocks were presented in a randomized order. Within each block, there were 10 targets chosen to comprise that condition. Peer faces within each block were shown in a fixed order using a randomization algorithm. Participants saw each face 4 times total (2 in each run), with each condition having 40 total trials each. To achieve the power for neuroimaging analysis on visual stimuli, we repeated each peer face across the conditions, in line with previous studies (e.g. Zerubavel et al., 2015; Parkinson et al., 2017). We used a 1-back task to ensure that adolescent participants were paying attention to these visual stimuli during scanning, such that each block contained one target that appeared twice in a row. In addition, implicit inferences of social attributes from faces often require face identity recognition processes (Todorov et al., 2008, 2015). A large body of work has shown that 1-back paradigms successfully elicit face identity recognition processes in which participants invoke a mental representation of face identity in the absence of percept (Kanwisher et al., 1998; Gauthier et al., 2000; Dai and Scherf, 2023). Participants were instructed to press a button with their right pointer finger when a face repeated. Adolescents were not explicitly told to track peer status, which allows us to investigate how the brain spontaneously supports adolescents’ awareness of peer-based social hierarchies in real-world networks. Each face was shown for 1750 ms, and a fixation cross was jittered around an average of 2301 ms (range: 565.8–4936.8 ms; see Figure 1). We did not include a control condition (e.g. teenagers with equal social attributes from other social networks) to account for other social attributes as it may regress out the phenomenon of our research interest, given that other social attributes (e.g. facial attractiveness, trustworthiness) are inherently correlated with social status in humans (Qu et al., 2017).
Neuroimaging analysis
In line with the analysis protocol in our previous studies (McCormick and Telzer, 2018; Kwon et al., 2022), different analysis packages were employed to take advantage of its own merits (e.g. SPM (Statistical Parametric Mapping) is proficient in parametric modulation). Preprocessing was conducted using FSL (FMRIB’s Software Library, version 6.0; www.fmrib.ox.ac.uk/fsl). See supplementary material for detailed preprocessing steps.
Individual level, fixed-effects analyses were estimated using the general linear model (GLM) convolved with a canonical hemodynamic response function in SPM12 . Parametric modulation analysis was conducted to detect the linear relationship between the BOLD response and peer status. The task was modeled as event-related with four conditions: high popularity, low popularity, high social preference and low social preference. The absolute value of the sociometric rating for each target in each condition (i.e. social preference score for the high and low social preference conditions; popularity score for the high and low popular conditions) was added as a parametric modulator (PM) at the trial level. Importantly, the sociometric ratings ranged from relatively lower to high scores within each condition, which allows us to examine whether adolescents’ brains track variation in peer status. Original low social preference (i.e. least liked) and low popularity scores were negative (i.e. −1 SD below the mean on likeability), and high social preference (i.e. most liked) and high popularity scores were positive (i.e. +1 SD above the mean). In order to compare activation across the conditions, we took the absolute values and used these absolute values as PMs at the trial level. For instance, for the low social preference peer condition, this identifies brain regions that linearly increase in BOLD as the absolute value of low-likability scores increase (i.e. more dislikable); for the high social preference peer condition, this identifies brain regions that linearly increase in BOLD as the absolute value of high-likability scores increase (i.e. more likable). Each condition was modeled at the onset of the face stimulus and the duration equal to zero. Six motion parameters were modeled as regressors of no interest. The repeated faces in this 1-back Classmates task were treated as a separate condition and modeled as a contrast of no interest. TRs with motion greater than 0.5 frame-wise displacement were modeled as a nuisance regressor. Using the parameter estimates from the GLM, linear contrast images comparing each of the face conditions of interest were calculated for each subject for the contrasts including high social preference, low social preference, high social preference vs low social preference and low social preference vs high social preference. Similar contrasts were created for the high and low popularity conditions. All conditions were performed in one GLM. Individual subject contrasts were then submitted to random effects, group-level analyses using GLMFlex (McLaren et al., 2011), which corrects for variance-covariance inequality, removes outliers and sudden activation change in the brain, partitions error terms and analyzes all voxels containing data (http://mrtools.mgh.harvard.edu/ index.php/GLM_Flex).
First, to investigate which brain areas show similar neural tracking of high social status (e.g. most liked) and low social status (e.g. least liked) peers, we conducted whole-brain analyses separately for high and low social preference conditions, with social preference scores (absolute values for high social preference and low social preference peers) as PMs for each condition. This allowed us to identify the brain regions that track (i.e. linearly increase in activation) peers of increasing likability and decreasing likability. In a subsequent analysis, we performed a conjunction analysis to formally evaluate which regions from the maps generated for the high social preference and low social preference peer conditions had similar areas (i.e. voxels) of activation using AFNI 3dcalc program (t-value as 3.14 and corrected). Regions that were identified across both conditions were then masked for overlap and plotted for descriptive purposes. This conjunction analysis allowed us to identify the brain regions that track the representation of high social preference and low social preference via a single psychological dimension. Second, to investigate which brain regions show unique neural tracking of high social preference and low social preference peers, we contrasted the high social preference condition with the low social preference condition with social preference scores as PMs (i.e. High social preference with PM > Low social preference with PM). This allowed us to identify brain regions that differentially tracked highly likable peers relative to dislikable peers (and vice versa).
Similar analyses were run for the popularity contrasts. Specifically, we conducted whole-brain analyses separately for high and low popularity conditions, with popularity scores (absolute values for high popular and low popular peers) as PMs for each condition. This allowed us to identify the brain regions that track (i.e. linearly increase in activation) peers of increasing popularity and decreasing popularity. We also included exploratory analyses in our GLM for contrasting social preference and popularity with respective sociometric scores as PMs (i.e. High social preference with PM > High popularity with PM, Low social preference with PM > Low popularity with PM, social preference with PM > popularity with PM and popularity with PM > social preference with PM). See Supplementary Table S2 for detailed results of analyses with the popularity conditions.
To correct for multiple comparisons, we employed Monte Carlo simulations using 3dClustSim in the AFNI software package (updated version Oct 2020; Ward, 2000). Specifically, we first submitted the residuals generated from the random-effects, individual-level analysis to the 3dFWHMx program to calculate the spatial group smoothness, assuming an auto-correlation function (i.e. -acf). Based on these simulations, we determined a corrected threshold of two-sided P < 0.001, with a minimum cluster extent of 84 voxels, corresponding to P < 0.05, family-wise error (FWE) corrected. Whole-brain analyses for each contrast are available on NeuroVault (Gorgolewski et al., 2015): https://identifiers.org/neurovault.collection:12781.
Results
Neural correlates of social preference
Similarity in neural tracking of peers high in social preference and low in social preference
To investigate neural tracking of social hierarchies in adolescents, we ran whole-brain analyses for the high social preference and low social preference conditions separately, each with social preference scores as a PM. Neural tracking of peers with high social preference scores (i.e. most liked peers) elicited significant activation in the occipital gyrus and bilateral FFAs (including FFA1 and FFA2). Neural tracking of peers with low social preference score (i.e. least liked peers) elicited activation in the bilateral occipital gyrus and FFA (including FFA1 and FFA2). All main effect results are reported in Table 2.
Table 2.
Brain regions that exhibited activation for tracking social preference (likability) of peers
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrasts | Regions | x | y | z | t-values | k |
| High social preference with PM | L fusiform face area* | −32 | −78 | −10 | 6.95 | 1918 |
| L middle occipital gyrus* | −32 | −96 | 10 | 6.14 | 1918 | |
| L calcarine gyrus | −8 | −104 | 4 | 4.63 | 1918 | |
| R fusiform face area* | 30 | −76 | −10 | 5.48 | 1671 | |
| R middle occipital gyrus* | 28 | −84 | 10 | 5.20 | 1671 | |
| R fusiform face area* | 28 | −56 | −10 | 5.14 | 1671 | |
| Low social preference with PM | R calcarine gyrus | 12 | −100 | 10 | 5.21 | 281 |
| L middle occipital gyrus | −12 | −102 | 6 | 5.06 | 216 | |
| L fusiform face area* | −32 | −38 | −16 | 4.97 | 203 | |
| L fusiform face area* | −32 | −78 | −10 | 4.24 | 102 | |
| R fusiform face area* | 22 | −86 | −10 | 4.51 | 478 | |
| R cerebellum | 34 | −46 | −18 | 3.98 | 537 | |
| L postcentral gyrus | −64 | −24 | 32 | −3.87 | 104 | |
| High > low social preference with PM | L middle occipital gyrus | −34 | −94 | 12 | 4.92 | 136 |
| L dorsolateral prefrontal cortex | −30 | 60 | 4 | 3.92 | 176 | |
Note: L—left, R—right, PM—PM, ACC—anterior cingulate cortex, MCC—midcingulate cortex.
represents the brain regions that were significant in conjunction analysis (multiple comparison corrected). Fusiform face areas (FFA1 and FFA2) were activated in both high social preference and low social preference conditions with PM (e.g. FFA1 [x = −32, y = −78, z = −10], FFA2 [x = −32, y = −38, z = −16]). The t-values stand for the t-score at peak activation level and k values refer to the number of activated voxels in activation clusters. Brain regions were based on whole-brain analyses using multiple comparison threshold of a minimum cluster size of 84 voxels (voxel-wise threshold of P < 0.001; 2-sided), corresponding to P < 0.05, family-wise error corrected. No activated brain regions survived this threshold in low vs high social preference with PMs.
Notably, the FFAs (FFA1 and FFA2) were both activated while adolescents tracked high social preference and low social preference peers (see Figure 2A), suggesting the FFA might be a critical region that represents both high and low peer status among adolescents. Our subsequent conjunction analysis confirmed that the regions that were simultaneously tracking high social preference and low social preference were bilateral FFA and occipital lobes (see Figure 2B and Table 2). For descriptive purposes, we extracted parameter estimates (β) from the FFA from the clusters that overlapped across both conditions and plotted the beta weights as a function of sociometric likability scores. Figure 2C shows that adolescents exhibited linear increases in FFA activity as likability scores increased in the high social preference peer condition, and linear increases in FFA activity as likability scores decreased in the low social preference peer condition, indicating that both the highest and lowest likable peers elicited the greatest FFA activation. See Supplementary Figure S1 for the distribution of β values in each FFA as a function of peer status across participants.
Fig. 2.
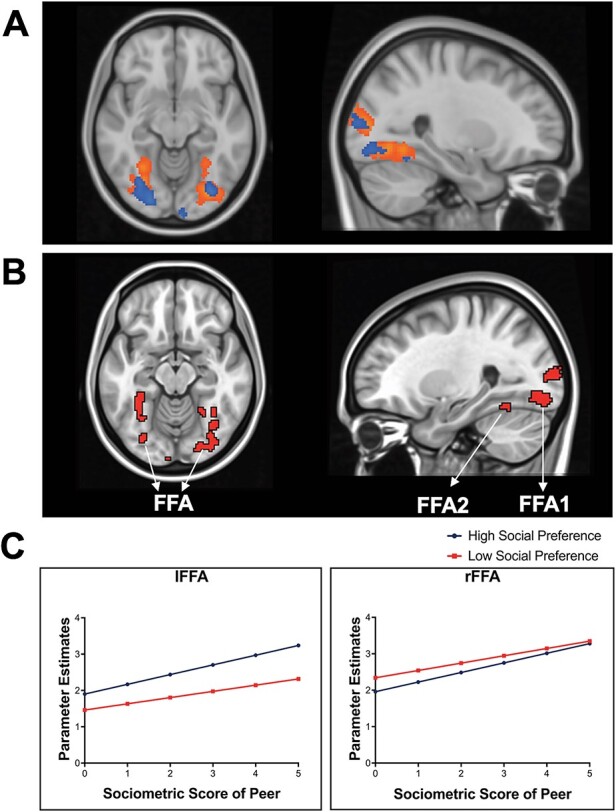
Brain activation when tracking high and low social preference peers. (A) The high and low social preference peers (i.e. most liked and least liked peers) are simultaneously represented in the fusiform face area (i.e. FFA). Data are thresholded at the voxel level P < 0.001 (2 sided) with a cluster threshold k = 84, N = 117. To visualize, group-level activation to high social preference peers (orange cluster) and low social preference peers (blue cluster) are overlaid onto an MNI anatomical image using FSL. (B) Conjunction analysis with the same multiple comparison correction criteria shows that both high and low social preference peers are represented in the FFA. (C) To visualize how the FFA tracks peer social preference, we plotted the parameter estimates (β) from the activated FFA in each condition. The x-axis represents the absolute value of social preference score, such that high z-scores represent the sociometric statuses of the most liked peers and the least liked peers, and the y-axis represents mean brain activation in the FFA. lFFA = left FFA, rFFA = right FFA. See Supplementary Figure S1 for the distribution of β values in FFA across participants.
Differential neural tracking of peers high in social preference and low in social preference
To investigate which brain regions differentially track peers high in social preference and low in social preference during adolescence, whole-brain t-tests were conducted to examine the main effects of high > low social preference peers with absolute social preference scores added as PMs. This analysis detects the brain regions that exhibit relatively greater BOLD activation as absolute values in high status peers increase relative to absolute values in low status peers (i.e. increasing dislikability—low in social preference). Analyses at the whole-brain level showed that neural tracking of peers high in social preference (i.e. most liked peers) differentially engaged the DLPFC, as well as the middle occipital lobe (see Table 2). For descriptive purposes, we extracted parameter estimates (β) from the DLPFC in the high social preference and low social preference peer conditions and plotted the beta weights as a function of sociometric likability scores. Figure 3 shows that participants exhibited linear increases in the DLPFC as likability scores increased to peers high in social preference, whereas brain activation in this region decreased with decreasing likeability for peers low in social preference. No brain regions survived the predefined threshold for the low status > high status contrast. See Supplementary Figure S2 for the distribution of β values in the DLPFC across participants.
Fig. 3.
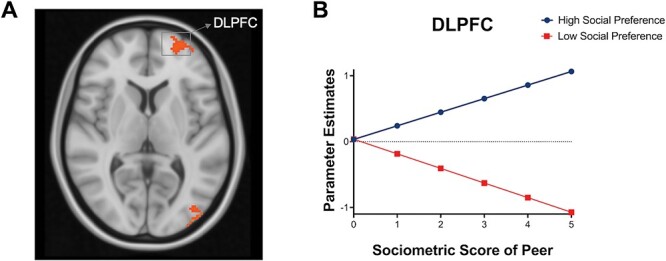
Brain activation when tracking high vs low social preference peers. (A) Whole-brain results for high > low social preference peers (with PMs). (B) For descriptive purposes to visualize how these brain regions track high social preference peers relative to low social preference peers (i.e. most liked vs least liked peers), we plotted the parameter estimates (β) from the activated regions. The x-axis represents the absolute value of social preference score, such that high z-scores represent the sociometric statuses for most liked peers and the least liked peers, and the y-axis represents mean activation in each brain region. See Supplementary Figure S2 for the distribution of β values in DLPFC across participants.
Neural correlates of popularity
Similarity in neural tracking of peers high in popularity and low in popularity
To investigate neural tracking of social hierarchies in adolescents, we ran whole-brain analyses for the high popularity and low popularity conditions separately, each with popularity scores as a PM. No brain regions survived the predefined threshold for tracking of high popularity. Neural tracking of peers low in popularity (i.e. unpopular peers) elicited increasing activation as unpopularity increased in the bilateral inferior parietal lobe (IPL; see Figure 4A, B). However, there were decreases in activation as unpopularity increased in the bilateral insula, superior temporal lobes (i.e. TPJ) and midcingulate cortex (MCC) (see Table 3 & Figure 5). Figure 4 shows that participants exhibited linear increases in the IPL as unpopularity scores increased (i.e. peers became more unpopular), whereas brain activation in this region remained consistently low as popularity scores increased to peers high in popularity, suggesting the IPL tracks peers low in popularity. Figure 5 shows that participants exhibited linear decreases in the bilateral insula, right MCC and right TPJ as popularity scores increased to peers low in popularity, whereas brain activation to peers high in popularity within this region remained consistent. Supplementary Figures S4 and S5 for the distribution of β values in the bilateral IPL, insula, right MCC, and right TPJ across participants. Conjunction analysis was not performed given there was no overlapping activation across the two conditions.
Fig. 4.
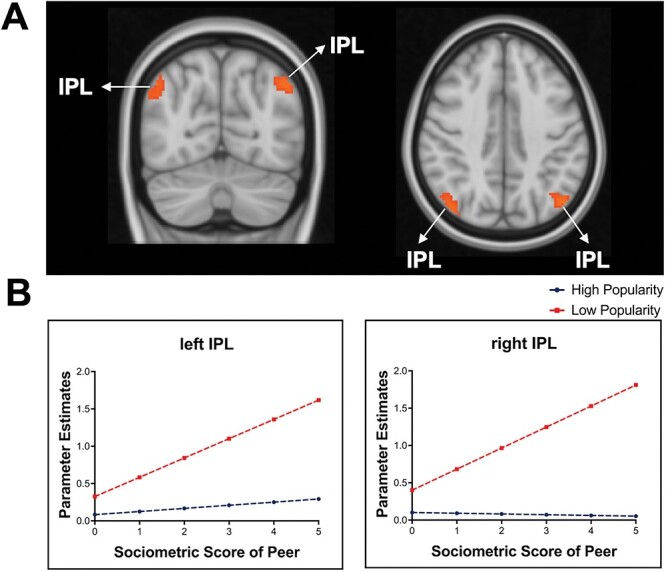
Brain activation when tracking high and low popular peers. (A) The bilateral inferior parietal lobe (IPL) significantly tracks adolescents low in perceived popularity, such that neural activation increased as unpopularity increased in low-popular peers. Data are thresholded at the voxel level P < 0.001 (2 sided) with a cluster threshold k = 84, N = 117. To visualize how the IPL tracks peer low in popularity, we plotted the parameter estimates (β) from the activated IPL in each condition (see Supplementary Figure S4. The x-axis represents the absolute value of perceived popularity score, such that high z-scores represent the sociometric statuses of the most popular peers and the most unpopular peers, and the y-axis represents mean brain activation in the IPL.
Table 3.
Brain regions that exhibited activation for tracking popularity of peers
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrasts | Regions | x | y | z | t-values | k |
| Low-popular peers with PM | L inferior parietal lobe | −46 | −70 | 42 | 4.81 | 119 |
| R inferior parietal lobe | 42 | −74 | 40 | 4.40 | 175 | |
| R insula lobe | 44 | 8 | 2 | −5.32 | 344 | |
| R MCC | 8 | 12 | 38 | −5.26 | 960 | |
| L ACC | 0 | 32 | 18 | −4.08 | 960 | |
| R posterior-medial frontal | 8 | 8 | 60 | −3.63 | 960 | |
| R temporoparietal junction | 66 | −42 | 26 | −5.04 | 883 | |
| R postcentral gyrus | 66 | −20 | 28 | −4.29 | 883 | |
| L insula lobe | −36 | 12 | 8 | −4.92 | 112 | |
| R rectal gyrus | 22 | 10 | −10 | −4.74 | 152 | |
| R linual gyrus | 24 | −60 | 2 | −4.33 | 142 | |
| R MCC | 10 | −24 | 40 | −4.31 | 123 | |
| L superior temporal gyrus | −64 | −42 | 16 | −4.26 | 574 | |
| L superior temporal gyrus | −48 | −28 | 14 | −4.00 | 574 | |
| L postcentral gyrus | −58 | −26 | 32 | −3.48 | 574 | |
| L olfactory cortex | −16 | 6 | −12 | −4.01 | 199 | |
| High- > low-popular with PM | L superior temporal gyrus | −54 | −24 | 12 | 4.55 | 327 |
| L superior temporal gyrus | −62 | −42 | 16 | 4.08 | 327 | |
| R superior temporal gyrus | 66 | −42 | 26 | 4.53 | 151 | |
| R supramarginal gyrus | 66 | −22 | 30 | 3.44 | 151 | |
| L MCC | 0 | 2 | 38 | 3.87 | 134 | |
| Low- > high-popular with PM | R inferior parietal lobe | 42 | −72 | 44 | 4.34 | 101 |
Note: L—left, R—right, PM—parametric modulator, MCC—midcingulate cortex, ACC—anterior cingulate. The t-values stand for the t-score at the peak activation level and k values refer to the number of activated voxels in activation clusters. Brain regions were based on whole-brain analyses using multiple comparison threshold of a minimum cluster size of 84 voxels (voxel-wise threshold of P < 0.001; 2-sided), corresponding to P < 0.05, family-wise error corrected.
Fig. 5.
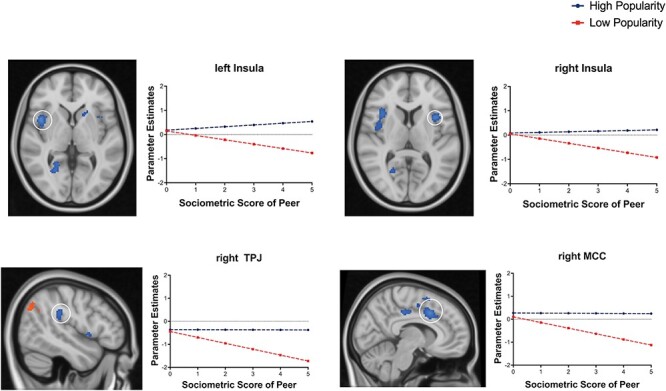
Decreasing neural tracking of peers in low popular peers. The temporoparietal junction (TPJ), insula and midcingulate cortex (MCC) significantly decreased as unpopularity increased in the low-popular peer condition. Data are thresholded at the voxel level P < 0.001 (2 sided) with a cluster threshold k = 84, N = 117. To visualize how the brain regions track peer low in popularity, we plotted the parameter estimates (β) from the activated regions in each condition (see Supplementary Figure S5 for distribution). The x-axis represents the absolute value of perceived popularity score, such that high z-scores represent the sociometric statuses of the most popular peers and the most unpopular peers, and the y-axis represents mean brain activation in the IPL.
Differential neural tracking of peers high in popularity and low in popularity
To investigate whether brain regions uniquely track peers high in popularity and low in popularity, whole-brain t-tests were conducted to examine the main effects of high-popular > low-popular peers with absolute popularity scores added as a PM. Our results showed greater activation to high-popular relative to low-popular peers in the bilateral superior temporal gyrus and left MCC (see Table 3). Given that the main effect for the low popular peers elicited decreases in activation as low popularity increased, this result suggests that low popular peers uniquely elicit decreases in activation. Moreover, we see greater activation to low-popular relative to high popular peers in the IPL.
Discussion
Adolescence is a period marked by an increased focus on the salience of peer-based social hierarchies (Cillessen et al., 2011). In this study, adolescent participants viewed images of peers from their social networks without being explicitly told to track their peers’ social statuses (i.e. social preference and popularity). Social preference (i.e. likability) and popularity scores of adolescents’ peers were used as PMs in our neuroimaging analysis to examine whether and how neural activation tracks levels of peer status. We aimed to investigate which neural regions similarly track high and low social status peers and which neural regions uniquely track high and low social status peers. For social preference, we found that the FFA subregions (i.e. FFA1 and FFA2) tracked both peers high in social preference and low in social preference. In contrast, the DLPFC differentially tracked peers high in social preference relative to peers low in social preference, such that the DLPFC exhibited increased activation to peers high in social preference as social preference scores increased (i.e. more likeable), whereas decreased activation to peers low in social preference (i.e. more dislikeable). For popularity, no regions similarly tracked peers high and low in popularity, whereas the TPJ, MCC, insula, and angular gyrus (i.e. IPL) specifically tracked the degree of unpopularity amongst lowly popular peers. Taken together, these neural findings indicate that adolescents spontaneously track social hierarchies amongst their peers and suggest that different brain regions may jointly represent and assess peer status.
Neural correlates of tracking social preference in adolescents
The FFA simultaneously tracks peers high and low in social preference
Our first goal was to examine which neural regions similarly track peers high and low in social status. We found that the FFA was activated while adolescents tracked both peers high and low in social preference. Our results, along with previous evidence, bolsters the emerging perspective that high-order social knowledge may have a downstream regulation on visuoperceptual processes by showing that both highly likable and unlikable peers are represented by the FFA. A substantial body of neuroimaging studies reveals that the FFA is highly specialized in object- and face-perception expertise (Haxby et al., 2000; Kanwisher, 2017) and in the perception of social categories, particularly in distinguishing salient faces from non-salient faces (Freeman et al., 2019). Most neuroimaging studies to date have treated the FFA as a single area; however, there is a growing recognition that the FFA contains two functionally distinct regions—FFA1 and FFA2 (Weiner and Grill-Spector, 2012; Elbich and Scherf, 2017). The FFA1, which is the posterior part of the fusiform gyrus, is linked to featural analysis of faces (e.g. Rossion et al., 2000; Yovel et al., 2008) and early perceptual learning about faces (e.g. Bi et al., 2014). In contrast, FFA2, the anterior part of the FFA, is associated with recognition accuracy of face identity and detecting the social salience of percepts (e.g. Elfgren et al., 2006; McGugin et al., 2014; Axelrod and Yovel, 2015). We found that activation in the FFA1 and FFA2 were both sensitive to social rank, which may suggest that information about social rank is relevant to both the early perception of faces as well as the social evaluation of those faces, more generally. Together, these findings may explain why inferring the social attributes of faces is an early (within hundreds of milliseconds) and primary feature of face perception (Todorov et al., 2005, 2008).
To date, most studies in the extant literature have focused on how and why individuals with more social value receive more selective attention compared to individuals with less social value (e.g. Lansu et al., 2014; Morelli et al., 2018). Relatively less attention has been paid to how individuals perceive and process low-status peers and how high- and low-status peers may be represented in the brain. Indeed, significant activation in the early visuoperceptual system in response to both high- and low-status peers might be developmentally adaptive. Our findings suggest that the visuoperceptual regions of adolescents’ brains may represent socially salient input along a single psychological dimension, which may in turn allow youths to allocate attentional resources to rapidly evaluate and identify socially desirable and undesirable members within their social networks. This information about group members, in turn, potentially guides adolescents’ subsequent social behaviors and interactions with their peers. For example, peers high in social preference may be rewarding to adolescents because these peers receive more collective attention from others; approaching and bonding with this socially valued group may provide avenues to maintain or further improve one’s status in a network (Dijkstra et al., 2013). In contrast, low social preference (i.e. being disliked) is often associated with social isolation and relational aggression (Prinstein and Cillessen, 2003; Neal, 2010; Lansu and Cillessen, 2012). Adolescents may actively seek to avoid these individuals, as bonding with this group may lower one’s own peer status or result in peer victimization. Thus, it may be optimal in adolescence to allocate attentional resources to identifying both socially rewarding and threatening members of one’s peer group. Our finding suggests that the FFA, a critical region for face perception and social categorization, plays a substantial role in representing adolescents’ peer-based social hierarchies which may subserve their navigation in their real social world.
The DLPFC differentially tracks high and low social preference peers
Our second goal was to examine neural regions which uniquely track peers high in social preference and low in social preference. We found that the DLPFC was differentially involved in tracking peers who were high and low in social preferences, such that brain activation increased for high social preference peers but decreased for low social preference peers. The DLPFC is involved in goal-directed attentional control in humans (Miller and Cohen, 2001), and a substantial body of neuroimaging work links the DLPFC to the representation of social hierarchies and social-hierarchy-dependent behaviors in non-human primates and humans. For example, in rhesus macaques, the DLPFC underlies increased attention to hierarchically superior vs inferior conspecifics (for review, Watanabe and Yamamoto, 2015). In humans, the DLPFC is broadly implicated in interpersonal judgment (Mah et al., 2004), social moral judgment (Spitzer et al., 2007) and perceiving socially valued members (Qu et al., 2017). For instance, neuroimaging studies found increased DLPFC activation during passive observation of socially valued individuals relative to socially unvalued individuals (Zink et al., 2008; Ligneul et al., 2017), suggesting its essential role in the representation of social hierarchy. Recent neuroimaging work has proposed that the DLPFC may also serve as a central mediator in behavioral regulation and social hierarchy processing (for review, Chiao, 2010; Wang et al., 2014). Indeed, empirical evidence shows that DLPFC activation is positively associated with one’s subjective levels of social norm compliance (Ruff et al., 2013), and related to one’s behaviors and attitudes associated with the affirmation of social hierarchies among group members (Ligneul et al., 2017). Our finding shows that the DLPFC differentially tracks high and low social preference peers, suggesting that the DLPFC may be specialized in tracking self-relevant social hierarchy information from visual inputs and may underlie the psychological responses to peers with higher and lower social rank. Given that social behaviors often occur in tandem with peer status inferences (Li et al., 2022) and adolescents are more susceptible to the influence of high-status peers than the influence of low-status peers (e.g. Crone and Dahl, 2012; Maheux et al., 2020), differential neural tracking in the DLPFC to high peer status and low peer status may serve as a susceptibility marker to peer influence.
Two neuropsychological processes for tracking social preference in adolescents
The current results suggest that two parallel processes may help adolescents track their peer-based social hierarchies in terms of social preference: (i) neural sensitivity that tracks both high and low social preference peers within the core face processing system (i.e. the FFA) may allow youth to represent their emerging peer-based social hierarchies; (ii) neural sensitivity that differentially tracks peers high and low in social preference within regions involved in goal-directed attention and behavioral regulation (i.e. DLPFC) may allow youth to represent high- and low-status peers differently. This differential neural tracking of peers high and low in social preference may help adolescents identify those peers who they deem behaviorally relevant. These neural processes may present important pathways via which adolescents navigate their social networks across peer contexts. First, insofar as peer-based social hierarchies provide a developmental context for adolescents to understand social relationships and practice cooperation, compromise and competition with peers who vary in peer status (Laursen and Veenstra, 2021); accurate representation of social hierarchies via the FFA may set a critical stage for adolescents’ social behaviors towards both socially valued and unvalued peers. Second, since social hierarchies have a profound impact on organizing social groups, differential neurobiological sensitivity to high and low social preference peers in the DLPFC may help explain teenagers’ social behaviors, particularly in peer influence contexts. In humans, high-status members facilitate low-status members’ social learning processes and maximize low-status members’ motivation (Henrich and McElreath, 2003; Magee and Galinsky, 2008). Moreover, studies suggest that adolescents more strongly conform to high-status peers’ than low-status peers’ behaviors, regardless of whether those behaviors are prosocial (Choukas-Bradley et al., 2015) or risky (Cohen and Prinstein, 2006; Koski et al., 2015). These behavioral changes in peer contexts often occur in tandem with peer status inference and social comparison. Our finding here may thus shed light on the neurobiological mechanisms underlying peer influence in adolescents—differential neural sensitivity in the DLPFC to high- and low-status peers. Together, we identify two potential neuropsychological processes that allow youths to represent their emerging social hierarchies and keep track of peer status, which provide insight into how teens flexibly and sufficiently navigate their real-world social networks.
Neural correlates of tracking popularity in adolescents
In the current study, we also examined how adolescents’ brains track another significant sociometric dimension—popularity. Interestingly, we did not find any overlap in regions that track both high and low popularity. Moreover, no regions significantly tracked adolescents high in perceived popularity. In contrast, we found that peers who were rated as low in popularity (i.e. unpopular) were uniquely represented at the neural level. In particular, we found that the IPL was involved in tracking more unpopular peers in adolescents’ social networks. The IPL is a key neural substrate for a variety of psychological processes, including fundamental visuospatial attention, language processing and social cognition that governs human social interactions (for review, Numssen et al., 2021). Importantly, empirical work suggests that the IPL hierarchically represents social information within a continuum. For instance, neuroimaging studies have demonstrated a graded response in IPL to emotion recognition behaviors representing intense fear vs mild fear vs neutral facial recognition behaviors (Radua et al., 2010). IPL also responds while human subjects represent the hierarchical rank of group members during passively viewing of facial or body images (Zink et al., 2008; Freeman et al., 2009; Chiao et al., 2009; Chiao, 2010; Qu et al., 2017). Along with these neuroimaging findings, our study suggests that the IPL may also hierarchically represent peer status in adolescents. Nevertheless, the IPL only appears to track the hierarchical status information amongst unpopular peers in adolescence; no significant results were found in the popular peer condition.
Interestingly, we found that the TPJ, insula and MCC were more significantly active as unpopularity decreased in the low-popular peer condition. In other words, neural responses within these regions were stronger to those mildly unpopular peers than extremely unpopular peers in adolescents. A substantial body of work links TPJ to the understanding of others’ thoughts and feelings in social contexts (Saxe and Kanwisher, 2003) and inferring social member’s status within a network (Parkinson et al., 2017), while the insula and MCC are involved in representing salient and affectively potent stimuli (Seeley, 2019) and are activated during the observation of and empathy for others’ experiences (e.g. social exclusion; Zaki and Ochsner, 2012). Indeed, recent neuroimaging work shows that the co-activation between these regions involved in mentalizing and empathy predicts adolescents’ behavioral change in peer contexts (Wasylyshyn et al., 2018), consistent with these brain regions’ vital role in navigating the social world. It is intriguing that these brain regions become less engaged the more unpopular the target is. Future studies including measures assessing additional peer characteristics and adolescents’ subjective feelings are needed to detect the psychological meanings of this neural process in adolescents.
Surprisingly, we did not find any brain region that linearly tracks increases in popularity amongst the most popular peers in adolescence. These null findings reflect two possibilities. One potential explanation is that highly popular peers may not be socially salient and valued by adolescents. However, this is unlikely to be true, as a substantial body of empirical work has suggested that popular peers play a significant role in behavioral conformity among teens (for review, Prinstein, 2017). Furthermore, adolescents are visuoperceptually sensitive to highly popular peers (Lansu et al., 2014). Alternatively, our null finding may suggest that past a certain threshold of popularity, adolescents do not distinguish neurally amongst highly popular peers within their social networks. That is, all popular peers may elicit similar activation, regardless of within-group variation in popularity levels.
Limitations and future directions
The current study uses novel methods to investigate how neural processes during adolescence allow youths to keep track of the peer status in their social networks. Using sociometric nomination techniques in which all teens in a school nominated their high and low social preference and popularity peers, paired with neuroimaging allowed us to delineate how the adolescent brain is functionally organized to map their emerging social hierarchies. Nonetheless, this study has limitations. First, we used images of peers who were most liked and least liked, as well as most popular and least popular, but did not include images of average social status peers in our study. We did so because faces of high and low social status peers are important social cues that humans preferentially pay more attention to and because they are important social agents that have profound impacts on adolescent behaviors (Prinstein et al., 2011a,b; Teunissen et al., 2012). Nonetheless, future investigations including a broader range of peer status will help us better understand how the developing brain fully represents social hierarchies during adolescence. For instance, it would be interesting to understand whether peer status is represented in a graded fashion in the adolescent brain or whether it is represented in a more categorical fashion (e.g. whereby average teens are more similar to low status teens than high status teens).
A second limitation of the present study is that sociometric nominations and yearbook images were necessarily from the prior year of data collection. We did so because of the time it took to process the massive sociometric data and the availability of photo images before the scan session. Thus, it is possible that the peer statuses of nominated teens might have changed across the year leading up to our neuroimaging session. However, both prior research and our study show that peer status is highly stable across years (i.e. correlation range from 0.7 to 0.9) during adolescence (Dijkstra et al., 2013; see Supplementary Figure S3), suggesting this likely is not a major confound. Nonetheless, it would be interesting to examine the neural representation of social hierarchy in a longitudinal fashion to examine how it might shift with shifts in social hierarchies.
Conclusions
Peers gain increased salience during adolescence and youths become hyper-attuned to their emerging social hierarchies in peer contexts during this time (Prinstein and Giletta, 2020), yet no work to date has examined how youths keep track of peer status and represent their emerging peer-based social hierarchies. This work, to our knowledge, is the first study to investigate adolescents’ neural tracking of peer status by integrating neuroimaging with sociometric nomination techniques. We revealed different neuropsychological processes which may be involved in encoding the complex social hierarchy among adolescents: (i) the early visual system for face perception and social categorization—the fusiform face area—similarly represents both high and low social preference peers (i.e. most liked and least liked peers), which may be socially and developmentally adaptive for teens’ social navigation; (ii) the DLPFC is differentially engaged in tracking high and low social preference peers in adolescents, such that adolescents exhibited increased activation to peers high in social preference while decreased activation to peers low in social preference in the DLPFC; (iii) the IPL, TPJ, MCC and insula automatically tracks the popularity of peers, but only to those lowest in popularity, with no regions tracking high popular peers. These findings make a novel and significant contribution to the study of adolescent brain development, social development, peer relationship and research on peer influences.
Supplementary Material
Acknowledgements
We would like to thank the Biomedical Research Imaging Center (BRIC) at the University of North Carolina at Chapel Hill for assistance with data collection, as well as Carina Fowler, Susannah Ivory, Amanda Benjamin, Virnaliz Jimenez, Emily Watlington, Emily Bibby, Rosario Villa, Melissa Burroughs, Kathy Do, Ethan McCormick, Paul Sharp, Lynda Lin, Christina Rogers, Jorien van Hoorn and Tae-Ho Lee for assistance with study design, data collection and analysis.
Contributor Information
Junqiang Dai, Department of Psychology and Neuroscience, The University of North Carolina at Chapel Hill, 235 E. Cameron Avenue, Chapel Hill, NC 27599-3270, USA.
Nathan A Jorgensen, Department of Psychology and Neuroscience, The University of North Carolina at Chapel Hill, 235 E. Cameron Avenue, Chapel Hill, NC 27599-3270, USA.
Natasha Duell, Department of Psychology and Neuroscience, The University of North Carolina at Chapel Hill, 235 E. Cameron Avenue, Chapel Hill, NC 27599-3270, USA.
Jimmy Capella, Department of Psychology and Neuroscience, The University of North Carolina at Chapel Hill, 235 E. Cameron Avenue, Chapel Hill, NC 27599-3270, USA.
Maria T Maza, Department of Psychology and Neuroscience, The University of North Carolina at Chapel Hill, 235 E. Cameron Avenue, Chapel Hill, NC 27599-3270, USA.
Seh-Joo Kwon, Department of Psychology and Neuroscience, The University of North Carolina at Chapel Hill, 235 E. Cameron Avenue, Chapel Hill, NC 27599-3270, USA.
Mitchell J Prinstein, Department of Psychology and Neuroscience, The University of North Carolina at Chapel Hill, 235 E. Cameron Avenue, Chapel Hill, NC 27599-3270, USA.
Kristen A Lindquist, Department of Psychology and Neuroscience, The University of North Carolina at Chapel Hill, 235 E. Cameron Avenue, Chapel Hill, NC 27599-3270, USA.
Eva H Telzer, Department of Psychology and Neuroscience, The University of North Carolina at Chapel Hill, 235 E. Cameron Avenue, Chapel Hill, NC 27599-3270, USA.
Supplementary data
Supplementary data is available at SCAN online.
Funding
This research was supported by the National Institutes of Health (R01DA039923 to E.H.T and R01DA051127 to E.H.T and K.A.L).
Conflict of interest
The authors declared that they had no conflict of interest with respect to their authorship or the publication of this article.
References
- Adolphs R. (2002). Neural systems for recognizing emotion. Current Opinion in Neurobiology, 12, 169–77. [DOI] [PubMed] [Google Scholar]
- Adolphs R. (2009). The social brain: neural basis of social knowledge. Annual Review of Psychology, 60, 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio D.M. (2014). The neuroscience of prejudice and stereotyping. Nature Reviews Neuroscience, 15(10), 670–82. [DOI] [PubMed] [Google Scholar]
- Anderson C., Srivastava S., Beer J.S., et al. (2006). Knowing your place: self-perceptions of status in face-to-face groups. Journal of Personality and Social Psychology, 91, 1094–110. [DOI] [PubMed] [Google Scholar]
- Axelrod V., Yovel G. (2015). Successful decoding of famous faces in the fusiform face area. PLoS One, 10(2), e0117126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi T., Chen J., Zhou T., et al. (2014). Function and structure of human left fusiform cortex are closely associated with perceptual learning of faces. Current Biology, 24, 222–7. [DOI] [PubMed] [Google Scholar]
- Bickart K.C., Hollenbeck M.C., Barrett L., et al. (2012). Intrinsic amygdala–cortical functional connectivity predicts social network size in humans. The Journal of Neuroscience, 32, 14729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Robbins T.W. (2012). Decision-making in the adolescent brain. Nature Neuroscience, 15, 1184–91. [DOI] [PubMed] [Google Scholar]
- Brambati S.M., Benoit S., Monetta L., et al. (2010). The role of the left anterior temporal lobe in the semantic processing of famous faces. NeuroImage, 53, 674–81. [DOI] [PubMed] [Google Scholar]
- Brooks J.A., Chikazoe J., Sadato N., et al. (2019). The neural representation of facial-emotion categories reflects conceptual structure. Proceedings of the National Academy of Sciences, 116, 15861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J.A., Freeman J.B. (2018). Conceptual knowledge predicts the representational structure of facial emotion perception. Nature Human Behaviour, 2, 581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J.B., Jones R.M., Hare T.A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao J.Y. (2010). Neural basis of social status hierarchy across species. Current Opinion in Neurobiology, 20, 803–9. [DOI] [PubMed] [Google Scholar]
- Chiao J. Y., Harada T., Oby E. R., et al. (2009). Neural representations of social status hierarchy in human inferior parietal cortex. Neuropsychologia, 47(2), 354–63. [DOI] [PubMed] [Google Scholar]
- Choukas-Bradley S., Giletta M., Cohen G.L., et al. (2015). Peer influence, peer status, and prosocial behavior: an experimental investigation of peer socialization of adolescents’ intentions to volunteer. Journal of Youth and Adolescence, 44, 2197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillessen A.H.N., Mayeux L. (2004). From censure to reinforcement: developmental changes in the association between aggression and social status. Child Development, 75(1), 147–63. [DOI] [PubMed] [Google Scholar]
- Cillessen, A.H., Schwartz, D. and Mayeux, L., editors. (2011). Popularity in the Peer System. New York, NY: Guilford Press. [Google Scholar]
- Cohen G.L., Prinstein M.J. (2006). Peer contagion of aggression and health risk behavior among adolescent males: an experimental investigation of effects on public conduct and private attitudes. Child Development, 77, 967–83. [DOI] [PubMed] [Google Scholar]
- Coie J.D., Dodge K.A., Coppotelli H. (1982). Dimensions and types of social status: a cross-age perspective. Developmental Psychology, 18, 557–70. [Google Scholar]
- Coie J.D., Dodge K.A., Kupersmidt J.B. (1990). Peer group behavior and social status. In: Asher, S.R., Coie, J.D., editors. Peer Rejection in Childhood, Cambridge, UK: Cambridge University Press, 17–59. [Google Scholar]
- Conway C.C., Rancourt D., Adelman C.B., et al. (2011). Depression socialization within friendship groups at the transition to adolescence: the roles of gender and group centrality as moderators of peer influence. Journal of Abnormal Psychology, 120, 857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick N.R., Bigbee M.A. (1998). Relational and overt forms of peer victimization: a multiinformant approach. Journal of Consulting and Clinical Psychology, 66(2), 337–47. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13, 636–50. [DOI] [PubMed] [Google Scholar]
- Cummins D.D. (2005). Dominance, status, and social hierarchies. In: Buss, D., editor. The Evolutionary Psychology Handbook, HoboKen, NJ: Wiley, 676–97. [Google Scholar]
- Curley J.P., Ochsner K.N. (2017). Neuroscience: social networks in the brain. Nature Human Behaviour, 1, 0104. [Google Scholar]
- Dai J., Scherf K.S. (2023). The privileged status of peer faces: subordinate-level neural representations of faces in emerging adults. Journal of Cognitive Neuroscience, 35(4), 715–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra J.K., Cillessen A.H.N., Borch C. (2013). Popularity and adolescent friendship networks: selection and influence dynamics. Developmental Psychology, 49, 1242–52. [DOI] [PubMed] [Google Scholar]
- Do K.T., Prinstein M.J., Telzer E.H. (2020). Neurobiological susceptibility to peer influence in adolescence. In: Kadosh, K.C., editor. The Oxford Handbook of Developmental Cognitive Neuroscience, Oxford: Oxford University Press. [Google Scholar]
- Duchaine B., Yovel G. (2015). A revised neural framework for face processing. Annual Reviews of Vision Science, 1, 393–416. [DOI] [PubMed] [Google Scholar]
- Dwortz M.F., Curley J.P., Tye K.M., et al. (2022). Neural systems that facilitate the representation of social rank. Philosophical Transactions of the Royal Society B, 377(1845), 20200444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder D. (1985). Interpersonal relations among female adolescents. Sociology of Education, 58, 154–65. [Google Scholar]
- Elbich D.B., Scherf K.S. (2017). Beyond the FFA: brain-behavior correspondences in face recognition abilities. NeuroImage, 147, 409–22. [DOI] [PubMed] [Google Scholar]
- Elfgren C., Westen D.V., Passant U., et al. (2006). fMRI activity in the medial temporal lobe during famous face processing. NeuroImage, 30, 609–16. [DOI] [PubMed] [Google Scholar]
- Fernald R.D., Maruska K.P. (2012). Social information changes the brain. Proceedings of the National Academy of Sciences, 109, 17194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes L., Blakemore S.-J. (2018). Studying individual differences in human adolescent brain development. Nature Neuroscience, 21, 315–23. [DOI] [PubMed] [Google Scholar]
- Fournier M.A. (2009). Adolescent hierarchy formation and the social competition theory of depression. Journal of Social and Clinical Psychology, 28, 1144–72. [Google Scholar]
- Freeman J.B., Rule N.O., Adams Jr, R.B., Ambady N. (2009). Culture shapes a mesolimbic response to signals of dominance and subordination that associates with behavior. Neuroimage, 47(1), 353–9. [DOI] [PubMed] [Google Scholar]
- Freeman J.B., Stolier R.M., Brooks J.A. (2019). Dynamic interactive theory as a domain-general account of social perception. Advances in Experimental Social Psychology, 61, 237–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I., Tarr M.J., Moylan J., Skudlarski P., Gore J.C., Anderson A.W. (2000). The fusiform face area is part of a network that processes faces at the individual level. Journal of Cognitive Neuroscience, 12(3), 495–504. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K.J., Varoquaux G., Rivera G., et al. (2015). NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics, 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf H., Wiegers M., Metzger C.D., et al. (2018). Differential noradrenergic modulation of monetary reward and visual erotic stimulus processing. Frontiers in Psychiatry, 9, 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K., Knouf N., Kanwisher N. (2004). The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience, 7, 555–62. [DOI] [PubMed] [Google Scholar]
- Guassi Moreira J.F., Bavel J.J., Telzer E.H. (2017). The neural development of ‘Us and Them’. Social Cognitive and Affective Neuroscience, 12, 184–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I., et al. (2000). The distributed human neural system for face perception. Trends in Cognitive Sciences, 4, 223–33. [DOI] [PubMed] [Google Scholar]
- Henrich J., McElreath R. (2003). The evolution of cultural evolution. Evolutionary Anthropology: Issues, News, and Reviews, 12, 123–35. [Google Scholar]
- Jonkmann K., Trautwein U., Lüdtke O. (2009). Social dominance in adolescence: the moderating role of the classroom context and behavioral heterogeneity. Child Development, 80, 338–55. [DOI] [PubMed] [Google Scholar]
- Kanai R., Bahrami B., Roylance R., et al. (2012). Online social network size is reflected in human brain structure. Proceedings of the Royal Society B: Biological Sciences, 279(1732), 1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N. (2017). The quest for the FFA and where it led. The Journal of Neuroscience, 37, 1056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N., Tong F., Nakayama K. (1998). The effect of face inversion on the human fusiform face area. Cognition, 68(1), B1–11. [DOI] [PubMed] [Google Scholar]
- Kanwisher N., Yovel G. (2006). The fusiform face area: a cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society B: Biological Sciences, 361, 2109–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski J.E., Collins J.A., Olson I.R. (2017). The neural representation of social status in the extended face‐processing network. European Journal of Neuroscience, 46, 2795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski J.E., Xie H., Olson I.R. (2015). Understanding social hierarchies: the neural and psychological foundations of status perception. Social Neuroscience, 10, 527–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota J.T., Banaji M.R., Phelps E.A. (2012). The neuroscience of race. Nature Neuroscience, 15, 940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D., Melo H.L., Duzel E. (2012). The emergence and representation of knowledge about social and nonsocial hierarchies. Neuron, 76, 653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S.J., Turpyn C.C., Prinstein M.J., Lindquist K.A., Telzer E.H. (2022). Self-oriented neural circuitry predicts other-oriented adaptive risks in adolescence: a longitudinal study. Social Cognitive and Affective Neuroscience, 17(2), 161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey S., Hagtvedt H., Patrick V.M., et al. (2011). Art for reward’s sake: visual art recruits the ventral striatum. NeuroImage, 55, 420–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFontana K.M., Cillessen A.H.N. (2010). Developmental changes in the priority of perceived status in childhood and adolescence. Social Development, 19, 130–47. [Google Scholar]
- Laninga-Wijnen L., Veenstra R. (2021). Types of selection and influence, and factors contributing to openness to peer influence. In: Halpern-Felsher, B., editor. The Encyclopedia of Child and Adolescent Health. Elsevier. [Google Scholar]
- Lansford J.E., Dodge K.A., Fontaine R.G., et al. (2014). Peer rejection, affiliation with deviant peers, delinquency, and risky sexual behavior. Journal of Youth and Adolescence, 43, 1742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansu T.A.M., Cillessen A.H.N. (2012). Peer status in emerging adulthood. Journal of Adolescent Research, 27, 132–50. [Google Scholar]
- Lansu T.A.M., Cillessen A.H.N., Karremans J.C. (2014). Adolescents’ selective visual attention for high-status peers: the role of perceiver status and gender. Child Development, 85, 421–8. [DOI] [PubMed] [Google Scholar]
- Laursen B., Veenstra R. (2021). Toward understanding the functions of peer influence: a summary and synthesis of recent empirical research. Journal of Research on Adolescence, 31, 889–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligneul R., Girard R., Dreher J.C. (2017). Social brains and divides: the interplay between social dominance orientation and the neural sensitivity to hierarchical ranks. Scientific Reports, 7(1), 45920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligneul R., Obeso I., Ruff C.C., Dreher J.-C. (2016). Dynamical representation of dominance relationships in the human rostromedial prefrontal cortex. Current Biology, 26(23), 3107–15. [DOI] [PubMed] [Google Scholar]
- Li S., Krueger F., Camilleri J.A., et al. (2021). The neural signatures of social hierarchy-related learning and interaction: a coordinate- and connectivity-based meta-analysis. NeuroImage, 245, 118731. [DOI] [PubMed] [Google Scholar]
- Lindquist K.A., Satpute A.B., Wager T.D., et al. (2016). The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26, 1910–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. W., Zeliger O., Strahs L. et al. (2022). Frontal neurons driving competitive behaviour and ecology of social groups. Nature, 603(7902), 661–6. [DOI] [PubMed] [Google Scholar]
- Lunn J., Wilcockson T., Donovan T., et al. (2021). The role of chronotype and reward processing in understanding social hierarchies in adolescence. Brain and Behavior, 11(5), e02090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J.C., Galinsky A.D. (2008). 8 social hierarchy: the self-reinforcing nature of power and status. Academy of Management Annals, 2(1), 351–98. [Google Scholar]
- Mah L., Arnold M.C., Grafman J. (2004). Impairment of social perception associated with lesions of the prefrontal cortex. American Journal of Psychiatry, 161, 1247–55. [DOI] [PubMed] [Google Scholar]
- Maheux A.J., Evans R., Widman L., Nesi J., Prinstein M.J., Choukas‐bradley S. (2020). Popular peer norms and adolescent sexting behavior. Journal of Adolescence, 78(1), 62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux L., Sandstrom M.J., Cillessen A.H.N. (2008). Is being popular a risky proposition? Journal of Research on Adolescence, 18(1), 49–74. [Google Scholar]
- McCormick E.M., Telzer E.H. (2018). Not doomed to repeat: Enhanced medial prefrontal cortex tracking of errors promotes adaptive behavior during adolescence. Journal of Cognitive Neuroscience, 30(3), 281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland D.A., Moody J., Diehl D., et al. (2014). Network ecology and adolescent social structure. American Sociological Review, 6, 1088–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGugin R., Newton A.T., Gore J.C., et al. (2014). Robust expertise effects in right FFA. Neuropsychologia, 63, 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren D.G., Schultz A.P., Locascio J.J., Sperling R.A., Atri A. (2011). Repeated-measures designs overestimate between-subject effects in fMRI packages using one error term. In: 17th Annual Meeting of Organization for Human Brain Mapping. Quebec City, Canada. 26-30 June 2011, 26–30.
- Meuwissen A.S., Anderson J.E., Zelazo P.D. (2017). The creation and validation of the developmental emotional faces stimulus set. Behavior Research Methods, 49, 960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. [DOI] [PubMed] [Google Scholar]
- Moody J., Brynildsen W.D., Osgood D.W., et al. (2011). Popularity trajectories and substance use in early adolescence. Social Networks, 33, 101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli S.A., Leong Y.C., Carlson R.W., et al. (2018). Neural detection of socially valued community members. Proceedings of the National Academy of Sciences, 115(32), 8149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal J.W. (2010). Social aggression and social position in middle childhood and early adolescence: burning bridges or building them? The Journal of Early Adolescence, 30, 122–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., et al. (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35, 163–74. [DOI] [PubMed] [Google Scholar]
- Numssen O., Bzdok D., Hartwigsen G. (2021). Functional specialization within the inferior parietal lobes across cognitive domains. ELife, 10, e63591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson I.R., McCoy D., Klobusicky E., et al. (2013). Social cognition and the anterior temporal lobes: a review and theoretical framework. Social Cognitive and Affective Neuroscience, 8, 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Coreano N., Batra K., Patarino M., et al. (2022). Cortical ensembles orchestrate social competition through hypothalamic outputs. Nature, 603, 667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson C., Kleinbaum A.M., Wheatley T. (2017). Spontaneous neural encoding of social network position. Nature Human Behaviour, 1(5), 0072. [Google Scholar]
- Park S.A., Miller D.S., Boorman E.D. (2021). Inferences on a multidimensional social hierarchy use a grid-like code. Nature Neuroscience, 24, 1292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J., Lewis P.A., Roberts N., Garcia-Finana M., Dunbar R.I. (2012). Orbital prefrontal cortex volume predicts social network size: an imaging study of individual differences in humans. Proceedings of the Royal Society B: Biological Sciences, 279(1736), 2157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein M.J. (2017). Popular: The Power of Likability in a Status-obsessed World. New York, NY: Viking. [Google Scholar]
- Prinstein M.J., Brechwald W.A., Cohen G.L. (2011a). Susceptibility to peer influence: using a performance-based measure to identify adolescent males at heightened risk for deviant peer socialization. Developmental Psychology, 47, 1167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein M.J., Choukas-Bradley S.C., Helms S.W., Brechwald W.A., Rancourt D. (2011b). High peer popularity longitudinally predicts adolescent health risk behavior, or does it?: an examination of linear and quadratic associations. Journal of Pediatric Psychology, 36(9), 980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein M.J., Cillessen A.H. (2003). Forms and functions of adolescent peer aggression associated with high levels of peer status. Merrill-Palmer Quarterly, 49, 310–42. [Google Scholar]
- Prinstein M.J., Giletta M. (2016). Peer relations and developmental psychopathology. Developmental psychopathology, 1–53. [Google Scholar]
- Prinstein M.J., Giletta M. (2020). Future directions in peer relations research. Journal of Clinical Child & Adolescent Psychology, 49, 1–19. [DOI] [PubMed] [Google Scholar]
- Qu C., Ligneul R., Henst J.-B.V.D., et al. (2017). An integrative interdisciplinary perspective on social dominance hierarchies. Trends in Cognitive Sciences, 21, 893–908. [DOI] [PubMed] [Google Scholar]
- Radua J., Phillips M.L., Russell T., et al. (2010). Neural response to specific components of fearful faces in healthy and schizophrenic adults. NeuroImage, 49(1), 939–46. [DOI] [PubMed] [Google Scholar]
- Rossion B., Dricot L., Devolder A., et al. (2000). Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. Journal of Cognitive Neuroscience, 12, 793–802. [DOI] [PubMed] [Google Scholar]
- Ruff C.C., Ugazio G., Fehr E. (2013). Changing social norm compliance with noninvasive brain stimulation. Science, 342(6157), 482–4. [DOI] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. (2003). People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind. NeuroImage, 19(4), 1835–42. [DOI] [PubMed] [Google Scholar]
- Schmälzle R., O’Donnell M.B., Garcia J.O., et al. (2017). Brain connectivity dynamics during social interaction reflect social network structure. Proceedings of the National Academy of Sciences, 114, 5153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W. (2019). The salience network: a neural system for perceiving and responding to homeostatic demands. The Journal of Neuroscience, 39(50), 9878–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk C.L., Foster D.L. (2004). The neural basis of puberty and adolescence. Nature Neuroscience, 7, 1040–7. [DOI] [PubMed] [Google Scholar]
- Smith E.B., Brands R.A., Brashears M.E., Kleinbaum A.M. (2020). Social networks and cognition. Annual Review of Sociology, 46, 159–74. [Google Scholar]
- Spitzer M., Fischbacher U., Herrnberger B., Grön G., Fehr E. (2007). The neural signature of social norm compliance. Neuron, 56(1), 185–96. [DOI] [PubMed] [Google Scholar]
- Stolier R.M., Freeman J.B. (2016). Neural pattern similarity reveals the inherent intersection of social categories. Nature Neuroscience, 19, 795–7. [DOI] [PubMed] [Google Scholar]
- Strayer F.F., Strayer J. (1976). An ethological analysis of social agonism and dominance relations among preschool children. Child Development, 47, 980–9. [Google Scholar]
- Teunissen H.A., Spijkerman R., Prinstein M.J., Cohen G.L., Engels R.C.M.E., Scholte R.H.J. (2012). Adolescents’ conformity to their peers’ pro-alcohol and anti-alcohol norms: the power of popularity. Alcoholism, Clinical and Experimental Research, 36, 1257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen L. (2020). The developmental origins of social hierarchy: how infants and young children mentally represent and respond to power and status. Current Opinion in Psychology, 33, 201–8. [DOI] [PubMed] [Google Scholar]
- Todorov A., Baron S.G., Oosterhof N.N. (2008). Evaluating face trustworthiness: a model based approach. Social Cognitive and Affective Neuroscience, 3, 119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A., Gobbini M.I., Evans K.K., et al. (2007). Spontaneous retrieval of affective person knowledge in face perception. Neuropsychologia, 45, 163–73. [DOI] [PubMed] [Google Scholar]
- Todorov A., Mandisodza A.N., Goren A., et al. (2005). Inferences of competence from faces predict election outcomes. Science, 308, 1623–6. [DOI] [PubMed] [Google Scholar]
- Todorov A., Olivola C.Y., Dotsch R., et al. (2015). Social attributions from faces: determinants, consequences, accuracy, and functional significance. Annual Review of Psychology, 66, 519–45. [DOI] [PubMed] [Google Scholar]
- Wang F., Kessels H.W., Hu H. (2014). The mouse that roared: neural mechanisms of social hierarchy. Trends in Neurosciences, 37, 674–82. [DOI] [PubMed] [Google Scholar]
- Ward B.D. (2000). Simultaneous inference for fMRI data. Available: http://afni.nimh.nih.gov/afni/docpdf/ [March 16, 2010].
- Wasylyshyn N., Falk B.H., Garcia J.O., et al. (2018). Global brain dynamics during social exclusion predict subsequent behavioral conformity. Social Cognitive and Affective Neuroscience, 13(2), 182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Yamamoto M. (2015). Neural mechanisms of social dominance. Frontiers in Neuroscience, 9, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner K.S., Grill-Spector K. (2011). Not one extrastriate body area: using anatomical landmarks, hMT+, and visual field maps to parcellate limb-selective activations in human lateral occipitotemporal cortex. NeuroImage, 56, 2183–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner K.S., Grill-Spector K. (2012). The improbable simplicity of the fusiform face area. Trends in Cognitive Sciences, 16, 251–4. [DOI] [PubMed] [Google Scholar]
- Yovel G., Tambini A., Brandman T. (2008). The asymmetry of the fusiform face area is a stable individual characteristic that underlies the left-visual-field superiority for faces. Neuropsychologia, 46, 3061–8. [DOI] [PubMed] [Google Scholar]
- Zaki J., Ochsner K.N. (2012). The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience, 15(5), 675–80. [DOI] [PubMed] [Google Scholar]
- Zerubavel N., Bearman P.S., Weber J., et al. (2015). Neural mechanisms tracking popularity in real-world social networks. Proceedings of the National Academy of Sciences, 112, 15072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink C.F., Tong Y., Chen Q., et al. (2008). Know your place: neural processing of social hierarchy in humans. Neuron, 58(2), 273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


