Key Points
Question
Do associations of sphingolipids with sudden cardiac death (SCD) vary based on the length of the acylated saturated fatty acid?
Findings
In this cohort study of 4612 participants aged 65 years or older with 215 SCD events, plasma ceramides and sphingomyelins with palmitic acid were associated with 34% and 37% higher SCD risk, respectively, per higher SD of log plasma levels.
Meaning
These findings suggest that sphingolipids with palmitic acid are associated with increased risk of SCD, whereas those containing very-long-chain saturated fatty acids are not.
This cohort study addresses whether ceramide and sphingomyelin species containing very-long-chain saturated fatty acids were associated with a reduced risk of sudden cardiac death (SCD) and whether species containing palmitic acid were associated with an increased risk of SCD among participants in the Cardiovascular Health Study.
Abstract
Importance
Sphingolipids, including ceramides and sphingomyelins, may influence the pathophysiology and risk of sudden cardiac death (SCD) through multiple biological activities. Whether the length of the fatty acid acylated to plasma sphingolipid species is associated with SCD risk is not known.
Objective
To determine whether the saturated fatty acid length of plasma ceramides and sphingomyelins influences the association with SCD risk.
Design, Setting, and Participants
In this cohort study, multivariable Cox proportional hazards regression models were used to examine the association of sphingolipid species with SCD risk. The study population included 4612 participants in the Cardiovascular Health Study followed up prospectively for a median of 10.2 (IQR, 5.5-11.6) years. Baseline data were collected from January 1992 to December 1995 during annual examinations. Data were analyzed from February 11, 2020, to September 9, 2023.
Exposures
Eight plasma sphingolipid species (4 ceramides and 4 sphingomyelins) with saturated fatty acids of 16, 20, 22, and 24 carbons.
Main Outcome and Measure
Association of plasma ceramides and sphingomyelins with saturated fatty acids of different lengths with SCD risk.
Results
Among the 4612 CHS participants included in the analysis (mean [SD] age, 77 [5] years; 2724 [59.1%] women; 6 [0.1%] American Indian; 4 [0.1%] Asian; 718 [15.6%] Black; 3869 [83.9%] White, and 15 [0.3%] Other), 215 SCD cases were identified. In adjusted Cox proportional hazards regression analyses, plasma ceramides and sphingomyelins with palmitic acid (Cer-16 and SM-16) were associated with higher SCD risk per higher SD of log sphingolipid levels (hazard ratio [HR] for Cer-16, 1.34 [95% CI, 1.12-1.59]; HR for SM-16, 1.37 [95% CI, 1.12-1.67]). Associations did not differ by baseline age, sex, race, or body mass index. No significant association of SCD with sphingolipids with very-long-chain saturated fatty acids was observed after correction for multiple testing (HR for ceramide with arachidic acid, 1.06 [95% CI, 0.90-1.24]; HR for ceramide with behenic acid, 0.92 [95% CI, 0.77-1.10]; HR for ceramide with lignoceric acid, 0.92 [95% CI, 0.77-1.09]; HR for sphingomyelin with arachidic acid, 0.83 [95% CI, 0.71-0.98]; HR for sphingomyelin with behenic acid, 0.84 [95% CI, 0.70-1.00]; HR for sphingomyelin with lignoceric acid, 0.86 [95% CI, 0.72-1.03]).
Conclusions and Relevance
The findings of this large, population-based cohort study of SCD identified that higher plasma levels of Cer-16 and SM-16 were associated with higher risk of SCD. Future studies are needed to examine the underlying mechanism of these associations.
Introduction
Sudden cardiac death (SCD) is a leading cause of mortality, accounting for 180 000 to 300 000 deaths in the US annually.1,2,3 Ventricular fibrillation is the most common rhythm at the time of SCD, and cardiovascular disease (CVD) is the most common underlying pathophysiology.2,4,5,6,7 A number of risk factors have been associated with SCD, including CVD and its risk factors as well as atrial fibrillation and electrophysiological variables. Understanding the biochemical mechanisms underlying SCD will allow for better treatment and prevention measures.
Sphingolipids have been reported to be important risk factors for CVD, including heart failure and atrial fibrillation, 2 conditions that markedly elevate cardiac arrest risk.8,9,10,11,12 Ceramides and sphingomyelins are in the family of sphingolipids. They are purported to be integral to a multitude of cardiovascular-related signaling pathways13,14 and play a role in oxidative stress, inflammation, fibrosis, and remodeling.8,15,16 Interestingly, the length of the saturated fatty acid acylated to the sphingolipid species may influence its biological activity.17 Lemaitre et al18,19 previously reported associations of sphingolipid species with heart failure and diabetes, both conditions associated with SCD, with differential associations depending on saturated fatty acid length.
Whether circulating levels of ceramides and sphingomyelins are associated with SCD and whether the associations vary with the length of the saturated fatty acid are not known. This study was hypothesis driven, based on previous work showing associations of erythrocyte membrane very-long-chain saturated fatty acids (VLSFAs) with a lower risk of SCD20; associations of ceramides and sphingomyelins with palmitic acid (Cer-16 and SM-16) with a higher risk of total mortality; and associations of VLSFA-acylated ceramides and sphingomyelins with lower total mortality.21 The goals of this analysis were to address the hypothesis that ceramide and sphingomyelin species containing a VLSFA are associated with a reduced risk of SCD and species containing palmitic acid are associated with an increased risk of SCD within the Cardiovascular Health Study (CHS).
Methods
Study Design and Setting
The CHS is a prospective cohort study of CVD risk factors among community-dwelling adults 65 years or older. The study design and procedures are described in detail elsewhere.22,23 In 1989 and 1990, 5201 participants were selected at random from lists of Medicare beneficiaries and recruited from 4 field centers located in Pittsburgh, Pennsylvania; Washington County, Maryland; Forsyth County, North Carolina; and Sacramento County, California. An additional cohort of 687 predominantly Black individuals was recruited in 1992 to 1993 with the same methods from the same counties except Washington County. The CHS was approved by the institutional review board at each field center, and all study participants gave written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Collection
Between June 10, 1989, and July 15, 1999, participants underwent annual study examinations that included personal interviews, physical examinations, laboratory assessments, and diagnostic tests. Collected data included height, weight, blood pressure, questions about tobacco and alcohol use, medical history, and an electrocardiogram. At the 1992 to 1993 examination, levels of the following analytes were measured: high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, C-reactive protein (CRP), fibrinogen, N-terminal pro–brain-type natriuretic peptide (NT-proBNP), and troponin T. Sphingolipid plasma levels were measured at the 1992-1993 or 1994-1995 study examination, and patient characteristics from that examination formed the study baseline for the analysis.
Participant race was self-reported in the CHS. Race data were collected for the present study because we wanted to determine whether the study results applied to different racial and ethnic groups.
Sphingolipid Measurement
Fasting EDTA plasma samples collected at the 1994-1995 examination (n = 4026) and at the 1992-1993 examination for participants without a 1994-1995 plasma sample (n = 586) were used for ceramide and sphingomyelin assays. Plasma samples were stored at −70 °C until they were extracted. Extracted sphingolipids were quantified using liquid chromatography–tandem mass spectrometry as described in detail previously.18 We examined 8 sphingolipid species: ceramide and sphingomyelin with acylated palmitic acid (Cer-16 and SM-16, respectively), arachidic acid (Cer-20 and SM-20, respectively), behenic acid (Cer-22 and SM-22, respectively), or lignoceric acid (Cer-24 and SM-24, respectively); Cer-24 comprised the summed concentrations of 2 ceramide species with distinct sphingoid backbones. In determining sphingolipid concentrations, a pooled EDTA plasma sample was used as a single-point calibrator and added to each batch in 5 replicates. An independent pool of EDTA plasma served to provide a quality control sample that was added to each batch in duplicate; coefficients of variation for each of the sphingolipid measurements were less than 20% over the whole period of laboratory assays, which included 52 batches.
Identification of Outcomes
Adjudication of SCD in the CHS was performed as previously published.24,25 In brief, information was gathered on cardiovascular events and deaths from hospital medical records, interviews with next of kin and primary health care clinicians, death certificates, and autopsy and coroner’s reports where available. Cause of death and nonfatal myocardial infarctions (MIs) were adjudicated by the CHS Event Committee. All adjudicated cardiac deaths were reviewed and adjudicated for SCD by a cardiologist (N.S.) using the following case definition: a pulseless condition in a previously stable individual presumed due to ventricular arrhythmia without evidence of either a noncardiac cause of death or progression of previous hypotension or heart failure. All events occurred outside the hospital or in the emergency department. By definition, cases could not have a life-threatening noncardiac comorbidity or be under hospice or nursing home care. A blinded second physician review was used, with high interreviewer agreement of 88%.24 Used for comparison analysis, nonfatal MIs were adjudicated by physician committee as previously described.24,26
Statistical Analysis
Data were analyzed from February 11, 2020, to September 9, 2023. There were no exclusions made for the SCD analyses. Associations of sphingolipid levels with SCD were assessed using Cox proportional hazards regression models, and statistical significance was determined with the Wald score. Participants began accruing time after their sphingolipid measurement and were followed up until an SCD event, death, dropout, or November 30, 2012. Sphingolipid levels were log2 transformed, and results are presented per SD difference in log concentration of each sphingolipid. We assessed 3 primary sets of models with a priori selected baseline characteristics as adjustment terms: model 1 (the minimally adjusted model) included adjustment for baseline age, sex, race, and study site; model 2 (the adjusted model) included model 1 additionally adjusted for body mass index (BMI), treated hypertension, HDL and LDL cholesterol levels, smoking, and prevalent type 2 diabetes and CHD; and model 3 (the primary model) included model 2 additionally adjusted for one of the other species: the Cer-16 and SM-16 models included adjustment for Cer-22 and SM-22, respectively; the Cer-20, Cer-22, and Cer-24 models included adjustment for Cer-16; and the SM-20, SM-22, and SM-24 models included adjustment for SM-16.
Missing values of HDL (n = 203) and LDL (n = 280) cholesterol levels were multiply imputed using information on age, sex, race, BMI, and smoking. Twenty imputed data sets were generated, and model-fitting results were pooled using standard methods.27
In sensitivity analyses, we repeated our primary analysis with additional adjustment for prevalence of heart failure, atrial fibrillation, chronic kidney disease by estimated glomerular filtration rate (eGFR), and chronic obstructive pulmonary disease (model 4). Using values measured at the 1992-1993 examination in additional models, we adjusted for heart rate, QT interval, and QRS interval (model 5); and log-transformed CRP, NT-proBNP, and troponin T levels (model 6).
For the nonfatal MI analyses, patients with a history of MI were excluded, leaving 4092 patients for the analysis. Associations of sphingolipid levels with nonfatal MI were performed using the same Cox proportional hazards regression models as with SCD. The same adjustment terms were used, and the data are presented in a comparable fashion.
We report 95% CIs for the hazard ratios (HRs). However, to correct for multiple comparisons, we assessed statistical significance at a threshold of 2-sided P < .0063 (0.05/8 sphingolipid species) by Bonferroni correction. The assumption of proportional hazards was not violated in any analysis, and there was no evidence of departure from linearity. Analyses were performed using Stata, version 16 (StataCorp LLC).
Results
The baseline characteristics of the 4612 included participants and their distributions across quartiles of each of the sphingolipids are presented in Figure 1 and eTable 1 in Supplement 1. The CHS participants examined were older adults (mean [SD] age, 77 [5] years) and predominantly female (2724 women [59.1%] vs 1888 men [40.9%]). A total of 6 participants (0.1%) were American Indian; 4 (0.1%) were Asian; 718 (15.6%) were Black, 3869 (83.9%) were White, and 15 (0.3%) were other races or ethnicities. Smoking and alcohol consumption were uncommon, and most participants were free of underlying prevalent CVD at baseline (Figure 1). Higher concentrations of LDL cholesterol were associated with higher plasma levels of all 8 sphingolipid species in univariate analysis, whereas association with age, sex, race, and other clinical factors differed by sphingolipid species type (Figure 1 and eTable 1 in Supplement 1).
Figure 1. Baseline Characteristics and Trends Across Quartiles of Sphingolipid Levels Among Cardiovascular Health Study Participants.
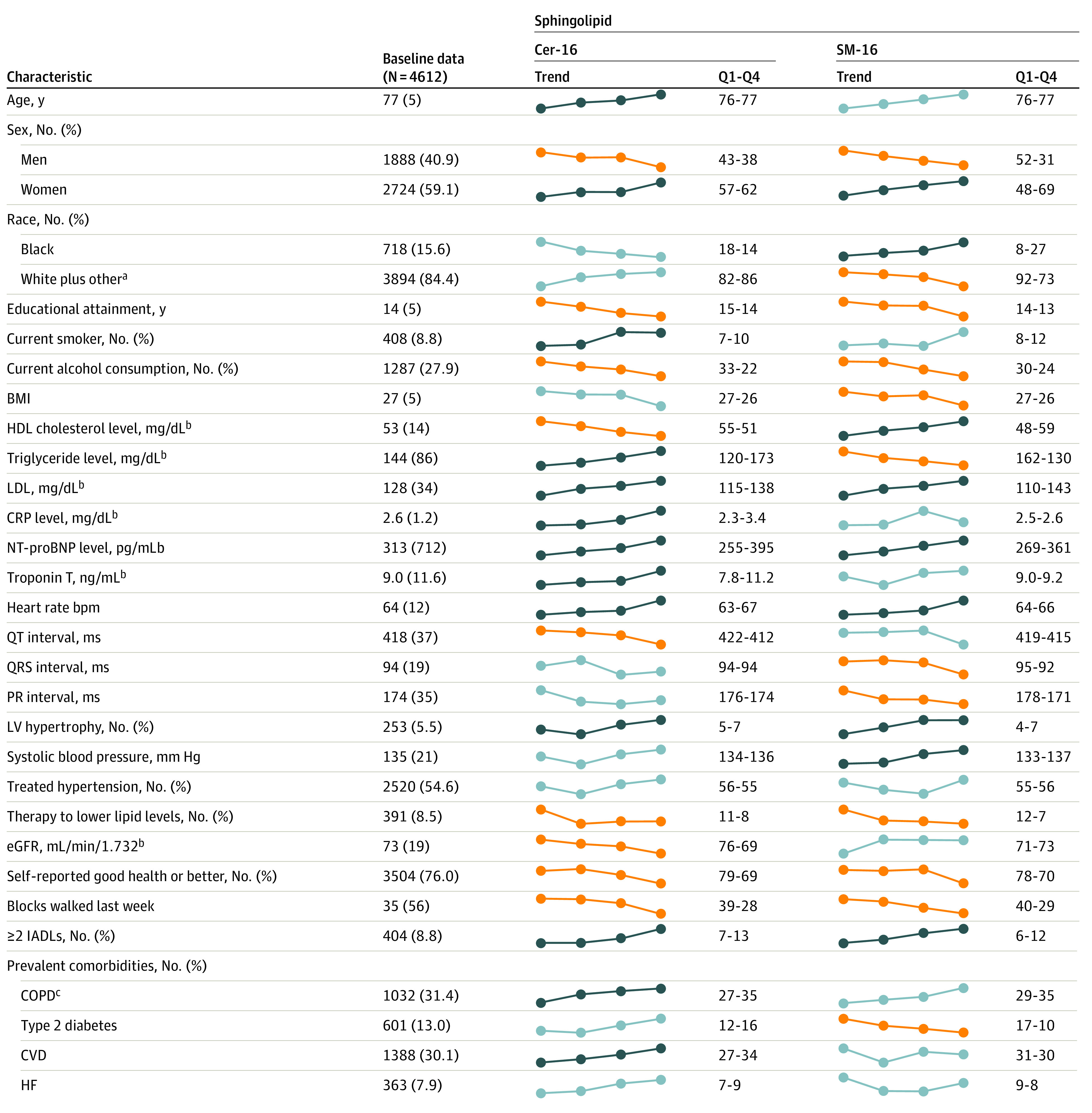
Unless otherwise indicated, data are expressed as mean (SD). Unadjusted linear and logistic regression models were used to assess significant (P < .0018; 0.05/28 characteristics) associations of log-transformed sphingolipids with each characteristic. The dotted lines represent means or percentages of each characteristic across quartiles of each of the sphingolipids; dark blue lines indicate significant positive trends, orange lines indicate statistically significant negative trends, and light blue lines indicate P > .0018. Q1 indicates the mean or percentage of each characteristic among participants with a sphingolipid level in the lowest 25% of the distribution; Q4 indicates the mean or percentage among participants with a sphingolipid level in the highest 25% of the distribution. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); Cer-16, ceramide with palmitic acid; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HF, heart failure; IADLs, instrumental activities of daily living; LDL, low-density lipoprotein; LV, left ventricle; NT-proBNP, N-terminal pro–brain-type natriuretic peptide; Q1, the lowest quartile of the distribution; Q4, highest quartile of the distribution; SM-16, sphingomyelin with palmitic acid. To convert CRP to mg/L, multiply by 10; HDL and LDL cholesterol to mmol/L, multiply by 0.0259; NT-proBNP to ng/L, multiply by 1.0; triglycerides to mmol/L, multiply by 0.0113; troponin T to μg/L, multiply by 1.0.
aOther includes American Indian or Alaska Native, Asian, and other.
bMeasured in 1992 to 1993 for all participants.
cInformation available for 3282 participants.
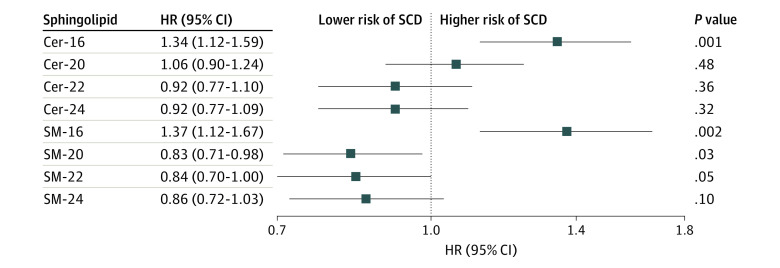
Over the median follow-up of 10.2 (IQR, 5.5-11.6) years, 215 SCD cases were identified (incidence rate, 5.5/1000 person-years). Each SD log difference in plasma Cer-16 was associated with a 34% higher risk of SCD (HR, 1.34 [95% CI, 1.12-1.59]) and in plasma SM-16, with a 37% higher risk of SCD (HR 1.37 [95% CI, 1.12-1.67]) in models adjusted for age, sex, race, study site, BMI, HDL and LDL cholesterol levels, smoking, treated hypertension, prevalent type 2 diabetes, CHD, and levels of other sphingolipids (Figure 2).
Figure 2. Risk of Sudden Cardiac Death (SCD) per Higher SD of Log Sphingolipid Levels Based on 215 Events Among 4612 Patients.
Hazard ratios (HRs) and 95% CIs were adjusted for age, sex, race, study site, body mass index, treated hypertension, high- and low-density lipoprotein cholesterol levels, smoking, and prevalent type 2 diabetes and coronary heart disease. Additionally, ceramide and sphingomyelin with palmitic acid (Cer-16 and SM-16, respectively) include adjustment for ceramide with behenic acid (Cer-22) and sphingomyelin with behenic acid (SM-22), respectively; ceramide with arachidic acid (Cer-20), behenic acid (Cer-22), and lignoceric acid (Cer-24) include adjustment for Cer-16; and sphingomyelin with arachidic acid (SM-20), behenic acid (SM-22), and lignoceric acid (SM-24) include adjustment for SM-16.
Higher levels of ceramides containing a VLSFA were not associated with a difference in SCD risk (HR for Cer-20, 1.06 [95% CI, 0.90-1.24]; HR for Cer-22, 0.92 [95% CI, 0.77-1.10]; HR for Cer-24, 0.92 [95% CI, 0.77-1.09]). Each SD log difference of sphingomyelins with a VLSFA showed a nonsignificant lower risk of SCD (HR for SM-20, 0.83 [95% CI, 0.71-0.98]; HR for SM-22, 0.84 [95% CI, 0.70-1.00]; HR for SM-24, 0.86 [95% CI, 0.72-1.03]) (Figure 2).
To further explore the mechanism of the association of sphingolipids with SCD, we performed sensitivity analyses with additional adjustment for prevalent clinical disease (chronic obstructive pulmonary disease, heart failure, atrial fibrillation, and chronic kidney disease by eGFR) or for electrocardiographic risk factors for SCD (heart rate, QT interval, and QRS interval). These only minimally attenuated the associations of Cer-16 and SM-16 with SCD (eTable 2 in Supplement 1, models 4 and 5). A model that included adjustment for CRP, troponin T, and NT-proBNP levels showed an attenuated association (eTable 2 in Supplement 1, model 6).
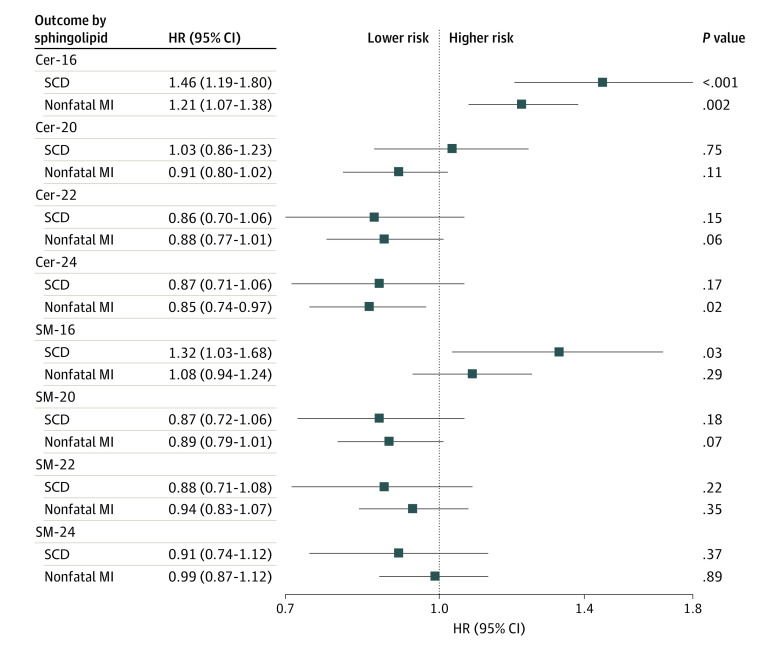
We next examined the association with nonfatal MI to determine whether the associations with SCD were specific to SCD (Figure 3). In a subset of patients without prior MI (n = 4092), each SD log difference of Cer-16 was associated with a 21% higher risk of incident MI (n = 412; HR, 1.21 [95% CI, 1.07-1.38]) and a 46% higher risk of SCD (n = 160; HR, 1.46 [95% CI, 1.19-1.80]). The SD log difference of SM-16 was not associated with nonfatal MI but was associated with a 32% higher risk of SCD (HR, 1.32 [95% CI, 1.03-1.68]).
Figure 3. Risk of Sudden Cardiac Death (SCD) and Incident Nonfatal Myocardial Infarction (MI) per Higher SD of Log Sphingolipid Levels.
Participants with a history of MI were excluded from both analyses. Of the remaining 4092 participants, there were 160 instances of SCD and 412 instances of nonfatal MI. Hazard ratios (HRs) and 95% CIs are presented, adjusted for age, sex, race, study site, body mass index, treated hypertension, high- and low-density lipoprotein cholesterol levels, smoking, and prevalent type 2 diabetes and coronary heart disease. Additionally, ceramide and sphingomyelin with palmitic acid (Cer-16 and SM-16, respectively) include adjustment for ceramide with behenic acid (Cer-22) and sphingomyelin with behenic acid (SM-22), respectively; ceramide with arachidic acid (Cer-20), behenic acid (Cer-22), and lignoceric acid (Cer-24) include adjustment for Cer-16; and sphingomyelin with arachidic acid (SM-20), behenic acid (SM-22), and lignoceric acid (SM-24) include adjustment for SM-16.
Given the moderate correlation of 0.45 between Cer-16 and SM-16 (eFigure in Supplement 1), we ran models including both sphingolipids in the same model and found attenuation of both sphingolipids’ associations with SCD (eTable 3 in Supplement 1), suggesting that both lipid species partly account for the observed SCD risk association. In interaction analysis, there was no evidence that associations of the sphingolipids with SCD were significantly modified by age, sex, race, or BMI (eTable 4 in Supplement 1).
Discussion
In this cohort study of older adults followed up prospectively for SCD, plasma levels of Cer-16 and SM-16 were associated with a higher risk of SCD. These associations were independent of other risk factors and did not differ by subgroups, including age, sex, race, and BMI.
Ceramides and sphingomyelins are membrane, intracellular, and circulating lipids in the sphingolipid family of bioactive lipids. Ceramides are composed of a fatty acid acylated to a sphingoid base, with sphingomyelins having the addition of a choline head group.28 Sphingolipids are synthesized de novo within most cells, and blood levels of sphingomyelins and ceramides may be a systemic metabolic signature.28,29,30 Ceramides as well as sphingomyelins play a role in oxidative stress, inflammation, fibrosis, and remodeling,8,15,16 all of which may influence the risk of SCD.
Sphingolipid species containing saturated fatty acids of different lengths show different biological activities in both experimental17 and patient association studies.31 Diabetes and heart failure are important risk factors for SCD, and previous investigations have found associations of ceramides and sphingomyelins with these outcomes,18,19 as well as atrial fibrillation32 and mortality,21 based on the length of the acylated fatty acid. Another group found an association with cardiovascular mortality as well.33 Previous investigations34,35,36,37 have also reported associations of plasma phospholipid VLSFAs with lower risk of these conditions. Importantly, Lemaitre et al20 have reported that higher levels of several VLSFAs (arachidic, behenic, and lignoceric acids) measured in erythrocyte phospholipids, including phosphoglycerolipid and sphingomyelin fatty acids, are associated with a lower risk of SCD.
Our hypothesis is that ceramide and sphingomyelin species with acylated palmitic acid can increase the risk of SCD in 2 compounding ways: a direct effect on cardiac electrophysiology and an indirect effect through ceramide release and a resultant accentuated inflammatory response. First, efficient electrical signaling requires concerted activation of multiple ion channels, and altered electrical coupling is a hallmark of cardiac arrhythmia. In the case of left ventricular tachyarrhythmias that are the cause of SCD, these are generally propagated via cell-to-cell electrical signaling through gap junctions, highly regulated areas of electrical signaling. Lipid rafts, which are enriched in sphingomyelins, play an important role in this process, both by their influence on ion channels38,39 and by proper assembly of these microdomains.40,41 Aberrant electrical signaling, possibly due to altered electrophysiological cellular communication, can predispose to SCD.
Second, the observed parallel associations of Cer-16 and SM-16 may exist because membrane-bound sphingomyelins can release ceramide via the enzymatic action of neutral sphingomyelinase,14 with sphingomyelins providing spatial control and sphingomyelinase providing temporal control of ceramide release. This enzyme is reported to be rapidly activated in ischemia and ischemic-reperfusion injury,42 representing a potential stress response mechanism for ceramide release in the setting of MI.43 The resulting ceramide release is associated with multiple detrimental pathways, including inflammation and apoptosis,8,16,44,45 potentially increasing both the damage caused by ischemia45,46 and the risk of SCD. Interestingly, while Cer-16 was associated with a higher risk of both nonfatal MI and SCD (though with a greater magnitude of association with SCD), SM-16 was associated only with SCD and not with nonfatal MI. Although there may be some mechanistic overlap between SCD and nonfatal MI, the potential effect of SM-16 may be more specific to SCD, considering that membrane localization of sphingomyelin may be more relevant to the aberrant electrical signaling of SCD. Interestingly, the association of Cer-16 with both MI and SCD remained after adjustment for LDL and HDL cholesterol levels, triglyceride levels, diabetes prevalence, eGFR, and race, suggesting that these associations may be mechanistically independent of these factors. Associations of ceramides and sphingomyelins with SCD were attenuated after adjustment for CRP, NT-proBNP, and troponin T levels, suggesting a possible connection to inflammatory or myocardial wall stress-related mechanisms.
Of note, the sphingolipids in the present study were associated with LDL cholesterol levels, consistent with their presence in apolipoprotein B–containing complexes.47 Future studies could help elucidate sphingolipids associated with different lipoproteins.
The primary prevention strategy for SCD is placement of implantable cardioverter defibrillators in high-risk individuals. It has been suggested that sphingolipids be included in risk assessment of coronary artery disease and cardiovascular events,48,49,50,51,52 and the present work supports their further exploration. Future studies in independent populations should evaluate whether risk prediction is improved by the inclusion of sphingolipid risk species.
Strengths and Limitations
The strengths of this study include its prospective design, the detailed and thorough assessment of ceramide and sphingomyelin species and multiple risk factors that might affect SCD risk, and the number of identified cases with SCD. The study is a first step in showing the associations of specific species of plasma sphingolipids with risk of SCD, a difficult-to-study phenotype.
This study also has some limitations. First, because it was observational in nature, we cannot infer causation; future studies, including mendelian randomization techniques and animal studies, are needed to address causality. Second, the study participants were of African and European descent, with a mean baseline age of 77 years, thus our findings may not be generalizable to individuals of other races or ethnicities or to younger populations. Third, given that the study was based on a single measurement, we were unable to distinguish confounding from mediation. Fourth, there might be variations in an individual’s sphingolipid profile over time, for example with diet,53 and circulating levels were reported to increase with age over decades.54 Any measurement error introduced by individual variation over time would likely be random and bias the study toward the null hypothesis. Finally, levels of HDL and LDL cholesterol, CRP, NT-proBNP, and troponin T were measured only at the 1992-1993 study examination, and their values may not accurately reflect their levels at baseline in participants whose sphingolipid levels were measured in the 1994-1995 examination.
Conclusions
The findings of this cohort study of participants from the CHS, suggest novel associations of ceramide and sphingomyelin species with SCD. These associations may help refine risk stratification for SCD among populations being considered for cardioverter defibrillator implantation. If the associations prove to be causal, reducing the levels of ceramide and sphingomyelin species with acylated palmitic acid may be a useful therapeutic target for the prevention of SCD. Additional studies will be needed to establish determinants of plasma levels of ceramide and sphingomyelin species and to evaluate whether ceramide and sphingomyelin levels may have clinical utility as potential components of SCD risk scores.
eTable 1. Mean Baseline Characteristics and Trends Across Quartiles of Sphingolipids Among 4612 CHS (Cardiovascular Health Study) Participants
eTable 2. Sensitivity Analyses for Risk of Sudden Cardiac Death per SD Higher Log Sphingolipid Level Based on 215 Events Among 4612 Participants
eTable 3. Mutual Adjustment of Species With Palmitic Acid, Cer-16 and SM-16, in Addition to Model 3 Adjustment
eTable 4. Interactions With Age, Sex, Race, and BMI (Body Mass Index)
eFigure. Correlation Matrix Among the Sphingolipid Species of Interest
Data Sharing Statement
References
- 1.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72(14):e91-e220. doi: 10.1016/j.jacc.2017.10.054 [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Reinier K, Teodorescu C, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51(3):213-228. doi: 10.1016/j.pcad.2008.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isbister J, Semsarian C. Sudden cardiac death: an update. Intern Med J. 2019;49(7):826-833. doi: 10.1111/imj.14359 [DOI] [PubMed] [Google Scholar]
- 4.Farb A, Tang AL, Burke AP, Sessums L, Liang Y, Virmani R. Sudden coronary death: frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation. 1995;92(7):1701-1709. doi: 10.1161/01.CIR.92.7.1701 [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Doyle JT, McNamara PM, Quickenton P, Gordon T. Precursors of sudden coronary death: factors related to the incidence of sudden death. Circulation. 1975;51(4):606-613. doi: 10.1161/01.CIR.51.4.606 [DOI] [PubMed] [Google Scholar]
- 6.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115(9):2305-2315. doi: 10.1172/JCI26381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong CX, Brown A, Lau DH, et al. Epidemiology of sudden cardiac death: global and regional perspectives. Heart Lung Circ. 2019;28(1):6-14. doi: 10.1016/j.hlc.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 8.Choi RH, Tatum SM, Symons JD, Summers SA, Holland WL. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol. 2021;18(10):701-711. doi: 10.1038/s41569-021-00536-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang XC, Goldberg IJ, Park TS. Sphingolipids and cardiovascular diseases: lipoprotein metabolism, atherosclerosis and cardiomyopathy. Adv Exp Med Biol. 2011;721:19-39. doi: 10.1007/978-1-4614-0650-1_2 [DOI] [PubMed] [Google Scholar]
- 10.Kikas P, Chalikias G, Tziakas D. Cardiovascular implications of sphingomyelin presence in biological membranes. Eur Cardiol. 2018;13(1):42-45. doi: 10.15420/ecr.2017:20:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovilakath A, Cowart LA. Sphingolipid mediators of myocardial pathology. J Lipid Atheroscler. 2020;9(1):23-49. doi: 10.12997/jla.2020.9.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovilakath A, Jamil M, Cowart LA. Sphingolipids in the heart: from cradle to grave. Front Endocrinol (Lausanne). 2020;11:652. doi: 10.3389/fendo.2020.00652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milhas D, Clarke CJ, Hannun YA. Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS Lett. 2010;584(9):1887-1894. doi: 10.1016/j.febslet.2009.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavoine C, Pecker F. Sphingomyelinases: their regulation and roles in cardiovascular pathophysiology. Cardiovasc Res. 2009;82(2):175-183. doi: 10.1093/cvr/cvp030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baranowski M, Górski J. Heart sphingolipids in health and disease. Adv Exp Med Biol. 2011;721:41-56. doi: 10.1007/978-1-4614-0650-1_3 [DOI] [PubMed] [Google Scholar]
- 16.Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R11-R26. doi: 10.1152/ajpregu.00416.2005 [DOI] [PubMed] [Google Scholar]
- 17.Grösch S, Schiffmann S, Geisslinger G. Chain length–specific properties of ceramides. Prog Lipid Res. 2012;51(1):50-62. doi: 10.1016/j.plipres.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 18.Lemaitre RN, Yu C, Hoofnagle A, et al. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: the Strong Heart Family Study. Diabetes. 2018;67(8):1663-1672. doi: 10.2337/db17-1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemaitre RN, Jensen PN, Hoofnagle A, et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ Heart Fail. 2019;12(7):e005708. doi: 10.1161/CIRCHEARTFAILURE.118.005708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemaitre RN, King IB, Rice K, et al. Erythrocyte very long-chain saturated fatty acids associated with lower risk of incident sudden cardiac arrest. Prostaglandins Leukot Essent Fatty Acids. 2014;91(4):149-153. doi: 10.1016/j.plefa.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fretts AM, Jensen PN, Hoofnagle AN, et al. Circulating ceramides and sphingomyelins and risk of mortality: the Cardiovascular Health Study. Clin Chem. 2021;67(12):1650-1659. doi: 10.1093/clinchem/hvab182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):270-277. doi: 10.1016/1047-2797(94)00092-8 [DOI] [PubMed] [Google Scholar]
- 23.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263-276. doi: 10.1016/1047-2797(91)90005-W [DOI] [PubMed] [Google Scholar]
- 24.Sotoodehnia N, Siscovick DS, Vatta M, et al. Beta2-adrenergic receptor genetic variants and risk of sudden cardiac death. Circulation. 2006;113(15):1842-1848. doi: 10.1161/CIRCULATIONAHA.105.582833 [DOI] [PubMed] [Google Scholar]
- 25.Hussein AA, Gottdiener JS, Bartz TM, et al. Cardiomyocyte injury assessed by a highly sensitive troponin assay and sudden cardiac death in the community: the Cardiovascular Health Study. J Am Coll Cardiol. 2013;62(22):2112-2120. doi: 10.1016/j.jacc.2013.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278-285. doi: 10.1016/1047-2797(94)00093-9 [DOI] [PubMed] [Google Scholar]
- 27.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammad SM. Blood sphingolipids in homeostasis and pathobiology. Adv Exp Med Biol. 2011;721:57-66. doi: 10.1007/978-1-4614-0650-1_4 [DOI] [PubMed] [Google Scholar]
- 29.Aureli M, Loberto N, Chigorno V, Prinetti A, Sonnino S. Remodeling of sphingolipids by plasma membrane associated enzymes. Neurochem Res. 2011;36(9):1636-1644. doi: 10.1007/s11064-010-0360-7 [DOI] [PubMed] [Google Scholar]
- 30.Iqbal J, Walsh MT, Hammad SM, Hussain MM. Sphingolipids and lipoproteins in health and metabolic disorders. Trends Endocrinol Metab. 2017;28(7):506-518. doi: 10.1016/j.tem.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemaitre RN, King IB. Very long-chain saturated fatty acids and diabetes and cardiovascular disease. Curr Opin Lipidol. 2022;33(1):76-82. doi: 10.1097/MOL.0000000000000806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen PN, Fretts AM, Hoofnagle AN, et al. Plasma ceramides and sphingomyelins in relation to atrial fibrillation risk: the Cardiovascular Health Study. J Am Heart Assoc. 2020;9(4):e012853. doi: 10.1161/JAHA.119.012853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigruener A, Kleber ME, Heimerl S, Liebisch G, Schmitz G, Maerz W. Glycerophospholipid and sphingolipid species and mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. PLoS One. 2014;9(1):e85724. doi: 10.1371/journal.pone.0085724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemaitre RN, McKnight B, Sotoodehnia N, et al. Circulating very long-chain saturated fatty acids and heart failure: the Cardiovascular Health Study. J Am Heart Assoc. 2018;7(21):e010019. doi: 10.1161/JAHA.118.010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemaitre RN, Fretts AM, Sitlani CM, et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2015;101(5):1047-1054. doi: 10.3945/ajcn.114.101857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi: 10.1161/JAHA.114.000889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fretts AM, Mozaffarian D, Siscovick DS, et al. Associations of plasma phospholipid SFAs with total and cause-specific mortality in older adults differ according to SFA chain length. J Nutr. 2016;146(2):298-305. doi: 10.3945/jn.115.222117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pulli I, Asghar MY, Kemppainen K, Törnquist K. Sphingolipid-mediated calcium signaling and its pathological effects. Biochim Biophys Acta Mol Cell Res. 2018;1865(11, pt B):1668-1677. doi: 10.1016/j.bbamcr.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 39.Dart C. Lipid microdomains and the regulation of ion channel function. J Physiol. 2010;588(pt 17):3169-3178. doi: 10.1113/jphysiol.2010.191585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Defamie N, Mesnil M. The modulation of gap-junctional intercellular communication by lipid rafts. Biochim Biophys Acta. 2012;1818(8):1866-1869. doi: 10.1016/j.bbamem.2011.09.023 [DOI] [PubMed] [Google Scholar]
- 41.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol. 2008;98(2-3):149-160. doi: 10.1016/j.pbiomolbio.2009.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez OM, Discher DJ, Bishopric NH, Webster KA. Rapid activation of neutral sphingomyelinase by hypoxia-reoxygenation of cardiac myocytes. Circ Res. 2000;86(2):198-204. doi: 10.1161/01.RES.86.2.198 [DOI] [PubMed] [Google Scholar]
- 43.Burrello J, Biemmi V, Dei Cas M, et al. Sphingolipid composition of circulating extracellular vesicles after myocardial ischemia. Sci Rep. 2020;10(1):16182. doi: 10.1038/s41598-020-73411-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bielawska AE, Shapiro JP, Jiang L, et al. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am J Pathol. 1997;151(5):1257-1263. [PMC free article] [PubMed] [Google Scholar]
- 45.Hadas Y, Vincek AS, Youssef E, et al. Altering sphingolipid metabolism attenuates cell death and inflammatory response after myocardial infarction. Circulation. 2020;141(11):916-930. doi: 10.1161/CIRCULATIONAHA.119.041882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hua T, Bao Q, He X, Cai W, He J. Lipidomics revealed alteration of sphingolipid metabolism during the reparative phase after myocardial infarction injury. Front Physiol. 2021;12:663480. doi: 10.3389/fphys.2021.663480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iqbal J, Walsh MT, Hammad SM, et al. Microsomal triglyceride transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycosylceramide. J Biol Chem. 2015;290(43):25863-25875. doi: 10.1074/jbc.M115.659110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laaksonen R, Ekroos K, Sysi-Aho M, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37(25):1967-1976. doi: 10.1093/eurheartj/ehw148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meeusen JW, Donato LJ, Kopecky SL, Vasile VC, Jaffe AS, Laaksonen R. Ceramides improve atherosclerotic cardiovascular disease risk assessment beyond standard risk factors. Clin Chim Acta. 2020;511:138-142. doi: 10.1016/j.cca.2020.10.005 [DOI] [PubMed] [Google Scholar]
- 50.Poss AM, Maschek JA, Cox JE, et al. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J Clin Invest. 2020;130(3):1363-1376. doi: 10.1172/JCI131838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seah JYH, Chew WS, Torta F, et al. Plasma sphingolipids and risk of cardiovascular diseases: a large-scale lipidomic analysis. Metabolomics. 2020;16(9):89. doi: 10.1007/s11306-020-01709-8 [DOI] [PubMed] [Google Scholar]
- 52.Vasile VC, Meeusen JW, Medina Inojosa JR, et al. Ceramide scores predict cardiovascular risk in the community. Arterioscler Thromb Vasc Biol. 2021;41(4):1558-1569. doi: 10.1161/ATVBAHA.120.315530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seah JYH, Chew WS, Torta F, et al. Dietary fat and protein intake in relation to plasma sphingolipids as determined by a large-scale lipidomic analysis. Metabolites. 2021;11(2):11. doi: 10.3390/metabo11020093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muilwijk M, Callender N, Goorden S, Vaz FM, van Valkengoed IGM. Sex differences in the association of sphingolipids with age in Dutch and South-Asian Surinamese living in Amsterdam, the Netherlands. Biol Sex Differ. 2021;12(1):13. doi: 10.1186/s13293-020-00353-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Mean Baseline Characteristics and Trends Across Quartiles of Sphingolipids Among 4612 CHS (Cardiovascular Health Study) Participants
eTable 2. Sensitivity Analyses for Risk of Sudden Cardiac Death per SD Higher Log Sphingolipid Level Based on 215 Events Among 4612 Participants
eTable 3. Mutual Adjustment of Species With Palmitic Acid, Cer-16 and SM-16, in Addition to Model 3 Adjustment
eTable 4. Interactions With Age, Sex, Race, and BMI (Body Mass Index)
eFigure. Correlation Matrix Among the Sphingolipid Species of Interest
Data Sharing Statement