Abstract
Background
Digital health interventions (DHIs) are defined as digital technologies such as digital health applications and information and communications technology systems (including SMS text messages) implemented to meet health objectives. DHIs implemented using various technologies, ranging from electronic medical records to videoconferencing systems and mobile apps, have experienced substantial growth and uptake in recent years. Although the clinical effectiveness of DHIs for children and adolescents has been relatively well studied, much less is known about the cost-effectiveness of these interventions.
Objective
This study aimed to systematically review economic evaluations of DHIs for pediatric and adolescent populations. This study also reviewed methodological issues specific to economic evaluations of DHIs to inform future research priorities.
Methods
We conducted a database search in PubMed from 2011 to 2021 using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist. In total, 2 authors independently screened the titles and abstracts of the search results to identify studies eligible for full-text review. We generated a data abstraction procedure based on recommendations from the Panel on Cost-Effectiveness in Health and Medicine. The types of economic evaluations included in this review were cost-effectiveness analyses (costs per clinical effect), cost-benefit analyses (costs and effects expressed in monetary terms as net benefit), and cost-utility analyses (cost per quality-adjusted life year or disability-adjusted life year). Narrative analysis was used to synthesize the quantitative data because of heterogeneity across the studies. We extracted methodological issues related to study design, analysis framework, cost and outcome measurement, and methodological assumptions regarding the health economic evaluation.
Results
We included 22 articles assessing the cost-effectiveness of DHI interventions for children and adolescents. Most articles (14/22, 64%) evaluated interventions delivered through web-based portals or SMS text messaging, most frequently within the health care specialties of mental health and maternal, newborn, and child health. In 82% (18/22) of the studies, DHIs were found to be cost-effective or cost saving compared with the nondigital standard of care. The key drivers of cost-effectiveness included population coverage, cost components, intervention effect size and scale-up, and study perspective. The most frequently identified methodological challenges were related to study design (17/22, 77%), costing (11/22, 50%), and economic modeling (9/22, 41%).
Conclusions
This is the first systematic review of economic evaluations of DHIs targeting pediatric and adolescent populations. We found that most DHIs (18/22, 82%) for children and adolescents were cost-effective or cost saving compared with the nondigital standard of care. In addition, this review identified key methodological challenges directly related to the conduct of economic evaluations of DHIs and highlighted areas where further methodological research is required to address these challenges. These included the need for measurement of user involvement and indirect effects of DHIs and the development of children-specific, generic quality-of-life outcomes.
Keywords: digital health, cost-effectiveness, economic evaluation, children, adolescents
Introduction
Background
Digital health interventions (DHIs) administered through various technologies have experienced substantial growth and uptake in recent years [1,2]. DHIs are defined as digital technologies such as digital health applications and information and communications technology systems (including SMS text messages) implemented to meet health objectives [3]. DHIs can range from electronic medical records used by providers to mobile apps tailored to patients for remote monitoring, videoconferencing systems for treatment administration training, and SMS text message reminders to promote treatment adherence [4,5]. The diverse roles that DHIs can play in the health system include replacement of face-to-face meetings with health care professionals; provision of patient education and counseling services; data collection and access; health information sharing; promotion of healthy behaviors and prevention; and facilitation of patient monitoring and support through clinical examination, diagnosis, and treatment [2,6]. DHIs compete for scarce National Health Service resources (fixed budget) with other digital and nondigital technologies in the health care system. In this context of scarcity, health economic evaluations provide an assessment of the relative benefits and costs of DHIs and competing options, which is crucial evidence for informing resource allocation decisions [2]. In the context of pediatric and adolescent care settings, the most common uses of DHIs are in mental health, particularly web-based cognitive behavioral therapies (CBTs) that include both children and their families, and in weight management programs involving patient- and caregiver-reported outcomes through mobile apps [7-9].
There is an abundance of literature evaluating the clinical effectiveness of DHIs in both children and adolescents [7-9]. Several studies have found a small but substantial effect of DHIs on health outcomes in these patient groups, primarily for interventions for depression, anxiety, and weight management [7-9]. In contrast, clinical evidence on the effectiveness of DHIs and the ability to compare effectiveness across studies is limited for other diseases such as asthma, attention-deficit/hyperactivity disorder, and eating disorders [8]. This is mainly because of limitations and inconsistencies in clinical trial design, such as small sample size, variable uptake and user engagement with DHIs, lack of blinded outcome assessment, short-term follow-up, and poor specification of the extent of human support (ie, DHIs that are fully self-administered by a patient without any elements of intervention delivery or monitoring by a clinician) [8,9]. As clinical effectiveness data are essential for parameterizing cost-effectiveness models, these inconsistencies in clinical evidence can pose limitations in conducting economic evaluations of DHIs.
The current understanding of the value for money of DHIs for children and adolescents is considerably more limited. Adoption of and engagement with DHIs are likely to be strong in the pediatric and adolescent population, and hence, the potential to be effective and cost-effective (ie, through scale-up) may be high [8]. The cost-saving potential of DHIs is associated in particular with behavioral interventions for chronic conditions in this population, such as the management of obesity and anxiety through web-based CBT [10]. However, the overall cost-effectiveness of DHIs for this population is unclear as evidence seems to differ according to the clinical setting and intervention type [8,11]. For instance, web portals and telephone support programs for obesity and SMS text messaging interventions for mental health and maternal, neonatal, and child care settings are more likely to be cost-effective than telemonitoring and videoconferencing systems for posttraumatic stress disorder and cardiovascular conditions [11].
Most published economic evaluations of DHIs follow standard guidelines for the evaluation of health technologies, such as those for pharmaceutical products and interventions [2]. This includes taking a health system or payer perspective, considering only health-related benefits, and conducting cost-utility analyses (CUAs) [2]. However, economic evaluations of DHIs may raise distinct methodological issues compared with pharmaceuticals and medical devices, including the measurement of non–health care benefits and costs, user involvement, and the choice of comparator [2]. The design of economic evaluations of DHIs tailored to pediatric populations may face additional challenges as DHIs originally designed for adult populations may not offer the type of interactions, self-monitoring features, and user involvement that are appropriate to address the needs of children and adolescents [3,12]. In general, the overall methodological quality of published economic evaluations in pediatric settings is unknown, including whether economic evaluations for this population face distinct methodological issues such as the measurement of costs and effects compared with DHIs for adults.
Objectives
To address these research gaps, we systematically reviewed economic evaluations of DHIs targeted at pediatric and adolescent populations. In addition, we examined and categorized the methodological issues reported in the reviewed economic evaluations with the purpose of informing methodological areas where future research might need to be prioritized.
Methods
Data Sources and Search Strategy
We used free-text terms to search for articles indexed in PubMed (MEDLINE database) from November 2011 to November 2021. This period was selected because of the emerging nature of DHIs, particularly mobile health and eHealth, which have experienced a growth in uptake in recent years. Search term combinations paired “cost-effect*,” “cost benefit,” “cost utility,” “economic evaluation,” “health economic analysis,” “value for money,” “decision model*,” and “cost consequence” with each of “child*,” “paediatric” and “adolescen*,” “infant*,” “neonat*,” “newborn*,” “baby,” and “babies” and each of the following terms: “telemedicine,” “remote* deliver*,” “telehealth,” “digital health,” “mobile health,” “m-health,” “ehealth,” “internet,” and “online” (Textbox 1). The selection of search terms was made based on a review of previously published literature on economic evaluations of DHIs, as well as in the pediatric and adolescent populations [9,10,13,14]. We also manually searched the bibliographies of eligible articles to identify other articles of interest. We conducted a systematic search of articles using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist and reporting recommendations (Textbox 1 and Multimedia Appendix 1) [15].
Search strategies.
PubMed or MEDLINE
Cost effective AND child AND telemedicine
Cost effective AND child AND remote monitoring
Cost effective AND child AND remote
Cost effective AND child AND telehealth
Cost effective AND child AND digital health
Cost effective AND child AND digital
Cost effective AND child AND mobile health
Cost effective AND child AND mhealth
Cost effective AND child AND ehealth
Cost effective AND child AND internet
Cost effective AND child AND online
Subsequent iterations substituted “cost effective” for other economic evaluation terms (“cost benefit,” “cost utility,” “economic,” “value,” and “cost consequence”) and substituted “child” for “paediatric” and “adolescent” to yield a total of 198 search terms.
Inclusion and Exclusion Criteria and Study Selection
In total, 2 reviewers (TS and TSA) independently screened the titles and abstracts of selected articles according to the prespecified inclusion and exclusion criteria: age range of the population of children and adolescents between 1 and 18 years, type of economic evaluation, intervention (ie, telephone, audiovisual consultation, SMS text messaging, mobile phone app, or web-based portal), and outcomes (effects, costs, and cost-effectiveness results). After screening titles and abstracts, we excluded the following types of studies: studies not covering pediatric and adolescent populations, studies that were not economic evaluations, reviews or systematic reviews, clinical study protocols, feasibility studies, surveys, qualitative studies, case reports, opinion articles, and clinical guidelines (Figure 1 and Table 1). In line with the economic evaluation definition commonly used in the literature [16], cost minimization, cost-consequence, and simple cost comparison studies were excluded from this review (Figure 1 and Table 1). We included articles for data extraction if consensus among the reviewers was reached after reviewing the full texts. A third reviewer was used to help resolve any disagreements. The types of economic evaluations that we included in this review were cost-effectiveness analyses (costs per clinical effect), cost-benefit analyses (costs and effects expressed in monetary terms as net benefit), and CUAs (cost per quality-adjusted life year [QALY] or disability-adjusted life year [DALY]) [16,17]. Although cost-per-QALY analyses are a very common type of economic evaluation for adult interventions, they are often not feasible for children and adolescents, primarily because of the methodological challenges and lack of data on valuing health states and deriving QALYs specific to these populations [18,19].
Figure 1.
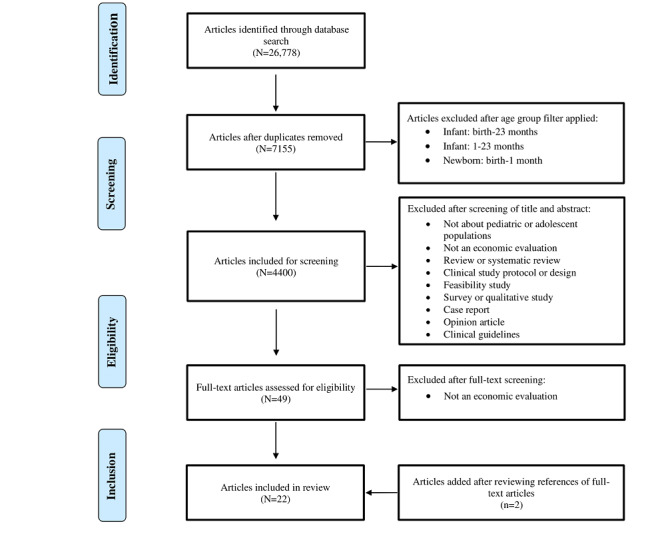
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) study flow diagram. The PubMed search resulted in 26,778 articles from November 2011 to November 2021. Following the removal of duplicates, screening of titles and abstracts and full-text screening based on predefined inclusion and exclusion criteria, a total of 22 articles were selected for inclusion.
Table 1.
Inclusion and exclusion criteria.
| Category | Inclusion criteria | Exclusion criteria |
| Language |
|
|
| Year of publication in PubMed (MEDLINE database) |
|
|
| Age |
|
|
| Article type |
|
|
| Type of economic evaluation |
|
|
Data Extraction
We generated a data abstraction procedure (Textbox 2) based on recommendations from the Panel on Cost-Effectiveness in Health and Medicine [20]. A narrative descriptive analysis was used to synthesize the quantitative data. We did not attempt to meta-analyze the results because of the high levels of heterogeneity across the studies. The extracted data of interest included (1) characteristics of the reviewed studies (eg, population, country, disease area, sample size, and DHI type); (2) main results of the studies (costs, outcomes, and incremental cost-effectiveness ratio [ICER]); (3) methodological issues regarding the health economic evaluations; and (4) key cost-effectiveness drivers, which were defined as input parameters that had the most impact on cost-effectiveness results.
Standard operating procedure for data abstraction.
Framework and background information
Reference (article number, first author, year, title, and journal)
Diseases under assessment
Study design (eg, randomized trial or retrospective analysis)
Perspective from which costs were evaluated (eg, society, patient, health care sector, or a combination of these)
Interventions
Comparators
Tool used (eg, decision tree or analysis, mathematical model, computer simulation model, or expert consultation)
Data and methods
Cost-benefit: what was monetized (eg, lost productivity) and how it was monetized
Cost-effectiveness: outcomes of interest
Cost-utility: factors included in utility quantification and how they were quantified in terms of utility
Description of sensitivity analyses conducted
Estimates and estimate development
Inputs
-
Population
Demographic, behavioral, and clinical characteristics
Subpopulations, if applicable
Size
Location of study (country and setting)
-
Costs
Currency
Year
Inflation adjustment method
Discount rates
Results
Costs and effectiveness results in aggregate
Costs and effectiveness results disaggregated by groups, if relevant
Incremental cost-effectiveness ratio
Variables to which determination of cost-effectiveness was sensitive
Discussion
Distributive effects (ie, who pays for the intervention)
Recommendations to specific audiences, if any
Limitations
References
First author, year, and title of relevant articles gathered from references
To facilitate the summary of the main results of the economic evaluations and comparisons between studies, we converted costs and cost-effectiveness estimates from Great British pound, Euro, and Australian dollar to 2021 US dollar using the following conversion rates: £1=US $1.364, €1=US $1.183, and Aus $1=US $0.751 [21-23].
Methodological Issues
Most economic evaluations of DHIs follow methodology guidelines used for standard health technologies because of a lack of DHI-specific guidelines and published research [2]. However, DHIs are complex interventions with their own features that pose different types of methodological challenges to economic evaluations compared with pharmaceuticals and medical devices [2]. We reported key methodological challenges and issues concerning the conduct of the reviewed economic evaluations. Drawing on previously published literature [2], we grouped all identified issues into 4 distinct categories related to DHIs, data (clinical or and cost), economic evaluation, and study design.
Results
Article Selection
The database search yielded a total of 26,778 articles as of November 2021 (Figure 1). Following the removal of duplicates and studies that did not include populations aged 1 to 18 years, 16.43% (4400/26,778) of the articles remained. We next screened the titles and abstracts and excluded 98.89% (4351/4400) as they were not about pediatric or adolescent populations; were not an economic evaluation; or were a systematic review, clinical study protocol and design, feasibility study, survey and qualitative study, case report, opinion article, or clinical guidelines. We reviewed the full texts of the remaining 49 articles and excluded 29 (59%) as they were not economic evaluations (ie, exclusion of studies that conducted only a simple cost comparison, cost minimization, or cost-consequence analysis). We added 2 articles after screening the references of the reviewed full-text articles. Therefore, we selected 22 articles for inclusion in this review [24-45].
Overview of Key Characteristics
DHIs administered through an web portal were the most frequent among the included studies (8/22, 36%), followed by SMS text messaging (6/22, 27%) and mobile phone apps (3/22, 14%). Other types of DHIs included telephone consultations (2/22, 9%), audiovisual consultations (2/22, 9%), and web-based symptom monitoring (1/22, 5%). Most studies were conducted in Europe (8/22, 36%) and Africa (6/22, 27%). The most frequently evaluated health care specialties were mental health (7/22, 32%) and maternal, newborn, and child health (MNCH; 7/22, 32%). Other disease areas included asthma, malaria, gastrointestinal disorders, sleeping disorders, child immunization, and conditions requiring emergency care.
Of the reviewed articles, 64% (14/22) included economic evaluations that were based on individual patient data (IPD) [23,24,28,30,32,34-39,41-43], with the remaining focusing on decision analytical modeling (7/22, 32%). Only 5% (1/22) of the economic evaluations used a combination of IPD and decision modeling [31]. Of the 14 IPD-based studies, 13 (93%) were randomized controlled trials, and only 1 (7%) was a non-RCT. The average follow-up duration of the IPD-based and model-based economic evaluations was 11 (range 0.5-36) months and 7 (range 3-10) years, respectively. All studies entailed a cost-effectiveness analysis (6/22, 27%), a CUA (9/22, 41%), or a combination of both (7/22, 32%). In Table 2, we provide a detailed overview of the key characteristics of the studies included in this review.
Table 2.
Overview of the key characteristics of the economic evaluation studies included in this review.
| Article author, year, and journal | DHIa type | Target population | Health care setting | Country | Sample size | Economic evaluation (model or trial based) | Follow-up period (trials) or time horizon (models) |
| Yang et al [24], 2015, Medical Decision Making | Audiovisual consultation | Children presenting to highest triage emergency category | Rural emergency care/pediatric critical care | United States | 135 | Model based; CEAb | 5 years |
| Chatterton et al [25], 2019, Australian and New Zealand Journal of Psychiatry | Telephone consultation | Children aged 7-17 years with a diagnosed anxiety disorder | Specialist referral centers | Australia and New Zealand | 281 | Trial based; CEA and CUAc | 1 year |
| Olthuis et al [26], 2018, Journal of Abnormal Child Psychology | Audiovisual and telephone consultation | Primary caregivers of children aged 6 to 12 years with disruptive behavior disorders | Community children’s mental health clinics | Canada | 172 | Trial based; CEA | 22 months |
| LeFevre et al [27], 2018, JMIR mHealth and uHealth | SMS text messaging | Pregnant women and infants | Maternal and neonatal care | South Africa | 356 | Model based; CUA | 5 years |
| Jo et al [28], 2021, BMJ Open | SMS text messaging | Pregnant women | Maternal and neonatal care | Bangladesh | 1 million | Model based; CUA | 10 years |
| Willcox et al [29], 2019, JMIR | SMS text messaging | Pregnant women, postpartum women, and their children aged <5 years | MNCHd facilities | Ghana | 1000 | Model based; CUA | 10 years |
| Kawakatsu et al [30], 2020, Vaccine | SMS text messaging | Pregnant women as well as children and their parents | Primary care center | Nigeria | 9368 | Trial based; CEA | 3 months |
| Jo et al [31], 2019, PLOS ONE | SMS text messaging | Pregnant women | MNCH facilities | Bangladesh | 610 | Model based; CUA | 4 years |
| Zurovac et al [32], 2012, PLOS ONE | SMS text messaging | Health workers in charge of patients aged <5 years | Pediatric outpatient clinic | Kenya | 119 | Trial based; CEA | 6 months |
| Modi et al [33], 2020, JMIR mHealth and uHealth | Mobile phone app | Pregnant women and infants | MNCH facilities | India | 5754 | Model and trial based; CUA | 3 years |
| Bowser et al [34], 2018, Annals of Global Health | Mobile phone app | Pregnant women and neonates | MNCH facilities | Nigeria | 339,475 | Trial based; CUA | 1 year |
| Prinja et al [35], 2018, Cost Eff and Resource Alloc | Mobile phone app | Pregnant women and neonates | MNCH facilities | India | 300,000 | Model based; CUA | 10 years |
| Jolstedt et al [36], 2018, Lancet Child Adolesc Health | Web portal | Children aged 8-12 years with a principal anxiety disorder diagnosis | Pediatric mental health centers | Sweden | 131 | Trial based; CEA and CUA | 3 months |
| De Bruin et al [37], 2016, Sleep | Web portal | Adolescents with insomnia | Mental health specialist centers | Netherlands | 62 | Trial based; CUA | 1 year |
| Lalouni et al [38], 2019, Clinical Gastroenterol & Hepatology | Web portal | Children aged 8-12 years with functional abdominal pain disorder | Mental health specialist centers | Sweden | 90 | Trial based; CEA and CUA | 1.5 years |
| Lenhard et al [39], 2017, BMJ Open | Web portal | Adolescents aged 12-17 years with OCDe | Mental health specialist centers | Sweden | 67 | Trial based; CEA and CUA | 3 months |
| Nordh et al [40], 2021, JAMA Psychiatry | Web portal | Children and adolescents aged 10 to 17 years with diagnosis of SADf and their parents | Mental health specialist centers | Sweden | 103 | Trial based; CEA and CUA | 3 months |
| Aspvall et al [41], 2021, JAMA Network Open | Web portal | Children and adolescents aged 8 to 17 years with OCD | Mental health specialist centers | Sweden | 152 | Trial based; CEA and CUA | 10-month trial+6-month follow-up |
| Lee et al [42], 2017, Epidemiology and Psychiatric Sciences | Web portal | Schoolchildren and adolescents aged 11-17 years (subcohort: students with subthreshold depression) | Primary and secondary schools | Australia | 23 per class | Model based; CUA | 10 years |
| Sampaio et al [43], 2019, BMJ Open | Web portal | Adolescents aged 13-17 years diagnosed with IBSg | Primary, secondary, and tertiary care clinics | Sweden | 101 | Trial based; CEA and CUA | 10 weeks |
| Wasil et al [44], 2021, J of Consulting & Clin Psychology | Web portal | Kenyan high school students (regardless of baseline depression symptoms) | High school | Kenya | 101 | Trial based; CEA | 2 weeks |
| van den Wijngaart et al [45], 2017, Euro. Respiratory Journal | Web-based symptom monitoring | Teenagers (aged 12-16 years) and young children (aged 6-12 years) with asthma and their caregivers | Asthma clinic | Netherlands | 210 | Trial based; CEA | 16 months |
aDHI: digital health intervention.
bCEA: cost-effectiveness analysis.
cCUA: cost-utility analysis.
dMNCH: maternal, newborn, and child health.
eOCD: obsessive-compulsive disorder.
fSAD: social anxiety disorder.
gIBS: irritable bowel syndrome.
Clinical Effectiveness Estimates
We report key effectiveness outcomes of DHI interventions and comparators in the reviewed studies in Table 3. The most frequently reported health outcomes included the number of deaths averted or lives saved, QALYs, and DALYs. Although most studies (17/22, 77%) suggested that the DHIs were effective, 23% (5/22) of the studies reported not statistically significant differences in health outcomes between the DHI and control groups [25,37,40,41,43]. The most common comparators in the reviewed studies were paper-based, in-person consultations in outpatient settings and the absence of an intervention. Relevant clinical outcomes included care coverage, disease remission rates, immunization rates, treatment response, anxiety and depressive symptoms, and other disease-specific quality-of-life (QoL) metrics. The effectiveness of the DHIs did not seem to differ according to the children’s age cohort (eg, children vs adolescents). Only 5% (1/22) of the studies, which evaluated a web-based asthma monitoring program, reported statistically significant improvements in health outcomes for young children but not for teenagers [45].
Table 3.
Clinical and economic outcomes of the economic evaluation studies included in this review.
| Study | Intervention | Comparator | Effects | Costs (original year and currency) | Costs (converted to 2021 US $) | ICERa; DHIb CEc (yes or no); values inside parentheses are converted to 2021 US Dollars to facilitate comparison across studies | Key cost-effectiveness drivers |
| Yang et al [24] | Telemedicine consultation with remote physician or nurse | Telephone consultation | 31% reduction in patient transfers with DHI | Annual cost savings with DHI: US $4662 per patient (2013) | Annual cost savings with DHI: US $5423 per patient | Cost savings: US $46,620 (US $54,227) per EDd per 10 pediatric patients; CE: yes (dominant from payer perspective) | Reduction in ambulance patient transfer rates |
| Chatterton et al [25] | Stepped care: telephone-delivered 3-stage CBTe | Empirically validated face-to-face CBT program with a therapist | No statistically significant QALYf differences between the 2 study arms | Mean cost savings through stepped care: Aus $1334 in total societal cost, Aus $198 in intervention delivery, and Aus $563 in health sector costs (2015) | Mean cost savings through stepped care: US $1249 in total societal cost, US $185 in intervention delivery, and US $527 in health sector cost | Reported visually as a CE plane; CE: yes (dominant from social perspective) | Reduction in indirect (caregiver) costs and increase in insurance reimbursement availability (Medicare) |
| Olthuis et al [26] | Written material, skill-based videos, and telephone coaching sessions for caregivers | Usual care—mental health services offered by referring agency or other providers | 0.56 improvement with DHI in child behavior checklist scores | Cost savings with DHI: CAD $1059 (2016) | Cost savings with DHI: US $861 | Average bootstrapped ICER: −US $2128 (US $1730) of DHI compared with usual care; CE: yes (dominant) | Reduction in costs of educational and health care services |
| LeFevre et al [27] | SMS text messaging service for pregnant women | No intervention | 95% vs 90% immunization rate in DHI vs control group | US $1.2 million 5-year DHI cost (2015) | US $1.37 million 5-year DHI cost | US $1985 (US $2269) per DALYg in first year; US $200 (US $229) per DALY in fifth year; CE: yes | Increase in number of lives saved and reduction in programmatic costs |
| Jo et al [28] | Comprehensive and basic pregnancy surveillance intervention | SOCh; paper based | 3076 averted deaths in 10 years with DHI | US $43 million (US $115 million) incremental (societal) DHI cost (2018) | US $46.4 million incremental DHI cost and US $124 million incremental societal DHI cost | US $327 (US $353) and US $462 (US $499) per DALY averted; CE: yes | Increase in number of lives saved, population coverage, and implementation duration and reduction in program costs |
| Willcox et al [29] | Interactive voice messages on pregnancy and infant care; appointment reminders for clinical visits | SOC | 59,906 lives saved and cumulative 1,550,028 DALYs averted in 10 years with DHI | DHI cost: US $66,166 per district per year (2014) | DHI cost: US $75,734 per district per year | US $20.94 (US $23.97) per DALY averted and US $586.72 (US $671.56) per death averted; CE: yes | Reduction in still deaths and maternal deaths, personnel time, and program start-up costs (training and equipment) |
| Kawakatsu et al [30] | SMS text message reminder 2 days before in-person appointments | No intervention | 4.8%-6% increase in return rate with DHI | DHI development: US $26,466 (65%); mobile phones: US $6314 (2019) | DHI development: US $28,051 (65%); mobile phones: US $6629 | US $7.90 (US $8.40) per return case; CE: N/Ai | Reduction in number of appointments and increase in geographic coverage of SMS text message reminders |
| Jo et al [31] | Comprehensive pregnancy surveillance intervention | Basic pregnancy surveillance | 354 averted newborn deaths per 1 million with DHI | Total 2-year incremental cost of DHI: US $319,000 (2016) | Total 2-year incremental cost of DHI: US $360,154 | US $31 (US $35) per DALY averted and US $901 (US $1017) per death averted; CE: yes | Reduction in program costs (mainly supervision and training); increase in population coverage |
| Zurovac et al [32] | SMS text message reminders sent to health workers on pediatric malaria case management | No intervention | 25% of additional children correctly managed; additional number of febrile children correctly managed—under study conditions: 38,435, under implementation by the Ministry of Health: 38,435, and under national implementation: 2,955,250 | Total costs—under study conditions: US $19,342, under implementation by the Ministry of Health: US $13,920, and under national implementation: US $97,350 (2010) | Total costs—under study conditions: US $24,036, under implementation by the Ministry of Health: US $17,298, and under national implementation: US $120,973 | Cost per additional child correctly treated—under study conditions: US $0.50 (US $0.62), under implementation by the Ministry of Health: US $0.36 (US $0.45), and under national implementation: US $0.03 (US $0.04); CE: yes | Results robust to changes in input parameters in sensitivity analyses |
| Modi et al [33] | Mobile phone app reminders; health promotion and decision support with web interface | SOC | 11 averted infant deaths per 1000 live births with DHI | Annual incremental cost of DHI: US $163,841 (2016) | Annual incremental cost of DHI: US $184,978 | US $84 (US $95) per life years saved and US $5709 (US $6446) per death averted; CE: yes | Increase in district scale-up and program effectiveness |
| Bowser et al [34] | Mobile devices, phones, and tablets for case management and decision support | No intervention | Higher care coverage and 4661 lives saved with DHI, including women, neonates, and stillbirths | Incremental cost savings with DHI: US $610 (2014) | Incremental cost savings with DHI: US $699 | US $13,155 (US $15,057) per life saved and US $568 (US $650) per DALY averted; CE: no (in base case) | Number of unassisted deliveries |
| Prinja et al [35] | Routine care+mobile phone app used by community health workers in MNCHj care | SOC | Reduction of 0.2% and 5.3% in maternal and neonatal deaths, respectively, over 10 years with DHI | Incremental cost of DHI: US $982 million (2015) | Incremental cost of DHI: US $1.1 billion (of which 90% implementation) | Health system perspective: US $205 (US $234) per DALY averted and US $5865 (US $6705) per death averted; societal perspective: DHI is cost saving; CE: yes | Increase in uptake of preventive services and reduction in number of maternal and neonatal illnesses |
| Jolstedt et al [36] | iCBTk | Internet-delivered child-directed play | 48% vs 15% remission rate in DHI vs control | Average societal cost saving with DHI: €493 (2016) | Average societal cost saving with DHI: US $606 | ICER not calculated because of minimal differences in QALYs (0.02 years); CE: yes for CEAl and no for CUAm | Reduction in intervention costs |
| De Bruin et al [37] | iCBTIn | fCBTIo | No significant differences in sleep efficiency and quality of life | No significant cost differences | No significant cost differences | DHI intervention dominates; CE: yes | Reduction in intervention costs and ongoing intervention costs (after trial period) and reduction in willingness-to-pay threshold |
| Lalouni et al [38] | iCBT | Usual treatment (in health and school system) | Significant and substantial improvement in gastrointestinal symptoms, quality of life, and avoidance behaviors in DHI group | Average societal cost savings per patient with DHI: US $974 (2016) | No significant cost differences | DHI intervention dominant; US $1050 (US $1186) cost savings per patient treated with DHI; CE: yes | Results robust to changes in input parameters in sensitivity analyses |
| Lenhard et al [39] | iCBT | Untreated condition (patients on waitlist) | 27% and 0% treatment response in iCBT and control group, respectively | Average societal cost savings per patient with DHI: US $145 (2016) | Average societal cost savings per patient with DHI: US $164 | Societal perspective: iCBT dominant; health care perspective: ICER of US $78 (US $86) per responder; CE: yes | Reduction in health care resource use |
| Nordh et al [40] | iCBT | iSUPPORTp—active comparator | Nonsignificant QALY differences | Average societal cost savings per patient with DHI: €1076 (2018) | Average societal cost savings per patient with DHI: US $1393 | ICER: €17,901 (US $23,167) per QALY (iCBT dominant over the active comparator); CE: yes; however, from HCPq perspective, iCBT more costly but more effective | Reduction in education costs and increase in school productivity |
| Aspvall et al [41] | Guided iCBT implemented within a stepped-care model (iCBT+in person) | In-person CBT | 68% treatment response in both groups; mean QALY difference=−0.029 | Average cost savings per patient with DHI: US $2052 (2020) | Average cost savings per patient with DHI: US $2148 | Mean cost savings of US $2104 (US $2203) per participant (39% relative savings) from health care sector perspective and US $1748 (US $1830) per participant from societal perspective; CE: yes | Not reported |
| Lee et al [42] | (1) Internet-delivered depression prevention—uDHIr and iDHIs and (2) face-to-face depression prevention—uF2Ft and iF2Fu | No intervention | uF2F: 3367 DALYs averted; iF2F: 4083 DALYs averted | Incremental net cost of DHI: Aus $37,041 (2013) | Incremental net cost of DHI: US $44,719 | uF2F: ICER of Aus $7350 (US $8874) per DALY averted; iF2F: ICER of Aus $19,550 (US $23,602) per DALY averted; uDHI and iDHI were highly cost-effective when assuming 50%-100% relative effect size compared with F2F; CE: yes | Increase in intervention effect size and long-term health impacts and reduction in intervention costs and indirect costs (time and travel) |
| Sampaio et al [43] | Exposure-based iCBT | Waitlist control | iCBT group had small QALY gains (0.0031) and average improvement of 5.647 points on PedsQLv compared with control | Average incremental cost of DHI per participant: US $170 (2016) | Average incremental cost of DHI per participant: US $192 | CUA: ICER of US $54,916 (US $62,001) per QALY gained with DHI; CEA: US $85.29 (US $96.29) per PedsQL point improvement with DHI; CE: undetermined | Reduction in intervention costs and resource use |
| Wasil et al [44] | Online single-session depression intervention | Online study skills active control | Greater reduction in depressive symptoms in DHI group (PHQ-8w score standardized mean difference=0.5) | Incremental cost of DHI per student: US $3.6 (2020) | Incremental cost of DHI per student: US $3.77 | US $25.35-$34.62 (US $26.54-$36.25) per case; CE: N/A | Reduction in cost components |
| van den Wijngaart et al [45] | VACx—outpatient visits reduced by 50% and monthly web-based asthma control test for monitoring | Usual care—routine 4-monthly outpatient visits including an ACTy | Asthma control higher with VAC than usual care for young children (mean difference=1.17); nonsignificant difference for teenagers | Mean cost saving per patient: €352 for young children and €852 for teenagers (2014) | Mean cost saving per patient: US $556 for young children and US $1345 for teenagers | DHI dominant in all subcohorts for asthma control outcome and in caregiver subcohort for quality-of-life outcome; CE: yes | Number of outpatient clinic visits and reduction in travel expenses |
aICER: incremental cost-effectiveness ratio.
bDHI: digital health intervention.
cCE: cost-effective.
dED: emergency department.
eCBT: cognitive behavioral therapy.
fQALY: quality-adjusted life year.
gDALY: disability-adjusted life year.
hSOC: standard of care.
iN/A: not applicable.
jMNCH: maternal, neonatal, and child health.
kiCBT: internet-delivered CBT.
lCEA: cost-effectiveness analysis.
mCUA: cost-utility analysis.
niCBTI: iCBT for insomnia.
ofCBTI: face-to-face CBT for insomnia.
piSUPPORT: internet-based supportive therapy.
qHCP: health care professional.
ruDHI: universal DHI.
siDHI: indicated DHI.
tuF2F: universal face-to-face depression prevention.
uiF2F: indicated face-to-face depression prevention.
vPedsQL: Pediatric Quality of Life Inventory.
wPHQ-8: 8-item Patient Health Questionnaire.
xVAC: virtual asthma clinic.
yACT: asthma control test.
Cost Estimates
We summarized the cost estimates associated with DHI implementation and the associated health care costs for children and adolescents. Further details regarding the exact cost components included in each of the reviewed studies can be found in Table 4. Many (9/22, 41%) economic evaluation studies took both health system and societal perspectives, whereas the remaining studies were distributed evenly between the health care system and societal perspectives.
Table 4.
Estimates of direct and indirect costs associated with the digital health interventions (DHIs) included in this review.
| Study | DHI type | Currency and year | Study perspective | Cost components | Non–health care costs included? |
| Yang et al [24] | Audiovisual consultation | US dollar, 2013 | Health care payer |
|
No |
| Chatterton et al [25] | Telephone consultation | Australian dollar, 2015-2016 | Health care system and societal |
|
Yes |
| Olthuis et al [26] | Audiovisual and telephone consultation | Canadian dollar, 2016 | Health care system |
|
No |
| LeFevre et al [27] | SMS text messaging | US dollar, 2015 | Societal |
|
No |
| Jo et al [28] | SMS text messaging | US dollar, 2018 | Societal |
|
No |
| Willcox et al [29] | SMS text messaging | US dollar, 2014 | Health care payer or program |
|
No |
| Kawakatsu et al [30] | SMS text messaging | US dollar, 2019 | Health care payer or government |
|
No |
| Jo et al [31] | SMS text messaging | US dollar, 2016 | Program |
|
No |
| Zurovac et al [32] | SMS text messaging | US dollar, 2010 | Program implementer |
|
No |
| Modi et al [33] | Mobile phone app | US dollar, 2016-2017 | Health care payer (government) |
|
No |
| Bowser et al [34] | Mobile phone app | US dollar, 2014 | Societal |
|
Yes |
| Prinja et al [35] | Mobile phone app | Indian rupee, 2015 | Health care system and societal |
|
Yes |
| Jolstedt et al [36] | Online portal | Euro, 2016 (converted from Swedish krona) | Societal |
|
Yes |
| De Bruin et al [37] | Online portal | Euro, 2014 | Societal |
|
Yes |
| Lalouni et al [38] | Online portal | US dollar, 2016 | Societal |
|
Yes |
| Lenhard et al [39] | Online portal | Swedish krona, 2014, and US dollar, 2016 | Societal and health care payer |
|
Yes |
| Nordh et al [40] | Online portal | Swedish krona and Euro, 2018-2019 | Societal, health care payer, and health care professional |
|
Yes |
| Aspvall et al [41] | Online portal | US dollar, 2020 | Societal and health care sector |
|
Yes |
| Lee et al [42] | Online portal | Australian dollar, 2013 | Health care payer (public sector) and societal |
|
Yes |
| Sampaio et al [43] | Online portal | Swedish krona, 2016, converted to US dollar, 2016 | Societal and health care system |
|
Yes |
| Wasil et al [44] | Online portal | US dollar, 2020 | School, researcher, and societal |
|
Yes |
| van den Wijngaart et al [45] | Online symptom monitoring | Euro, 2014 | Societal and health care system |
|
Yes |
aGP: general practitioner.
bAPI: application programming interface.
cM&E: monitoring and evaluation.
dCBTI: cognitive behavioral therapy for insomnia.
eiCBT: internet-delivered cognitive behavioral therapy.
fVAC: virtual asthma clinic.
Most studies (18/22, 82%) reported detailed direct cost components broken up into 3 phases of integrating the intervention into the health care system: DHI development, DHI start-up, and DHI implementation. Approximately 55% (12/22) of the studies reported indirect costs, which typically included productivity losses because of missed hours from school for children and from paid and unpaid work for parents and caregivers, parental out-of-pocket expenditures on travel, informal care, and educational expenses associated with tutoring and mentoring of the target population.
DHIs were associated with cost savings compared with the standard of care in 45% (10/22) of the studies [24-26,34,36,38-41,45], ranging from a mean of US $164 to US $5423 per patient (Table 3) [24,39]. Other studies reported the cumulative annual incremental cost of DHIs compared with the standard of care, which ranged from US $184,978 to US $1.1 billion depending on the scale-up of the intervention program [33,35] (Table 3). The relative proportion of each of the DHI-related cost components (reported only in 9/22, 41% of the studies) was as follows: 12% average start-up cost (range 5%-24%), 15% average development cost (range 6%-23%), and 73% average implementation cost (range 63%-89%) [24,27-31,33-35].
Cost-Effectiveness Outcomes
We reported key findings of the economic evaluations in Table 3 grouped by DHI type. In 82% (18/22) of the reviewed studies, DHIs were cost-effective (15/18, 83%) or cost saving (3/18, 17%) compared with the standard of care, with an ICER ranging from US $24 to US $23,602 per DALY averted [29,40,42,43]. In 32% (7/22) of the studies, DHIs were a dominant strategy.
Cost-Effectiveness Drivers
The most frequent drivers of cost-effectiveness across the studies were population coverage, cost components, and intervention effect size (Table 3). In particular, the cost components associated with the implementation of DHI programs and supervision/training of health care professionals had the most impact on cost-effectiveness results [26-29,36,37,40,42-44]. For instance, the cost-effectiveness of internet-delivered universal prevention of major depression was highly sensitive to variations in implementation cost (ie, small changes to the average implementation cost per person led to large changes in the resulting ICER, with the intervention becoming not cost-effective when such cost was >US $90 per person [42]).
The mean differences in health outcomes between DHIs and comparators were most sensitive to factors such as population coverage [28,30,35], assumptions about the number of deaths averted or lives saved [27-29], and number of clinic visits [30,45]. For example, the annual coverage increase from 5% to 10% for antenatal and postnatal care was associated with twice as many lives saved over 10 years of implementation of an SMS text messaging program for pregnant women attending MNCH facilities [28].
Overview of Methodological Issues
DHI-Related Methodological Issues
Challenges directly related to data collection or modeling of the evaluated DHI interventions were reported in 36% (8/22) of the articles and included the following types of issues (Table 5):
Table 5.
Summary of the limitations and methodological issues of the reviewed studies.
| DHIa type, reference, and type of methodological issue | Description | ||
| Audiovisual consultation [24] | |||
|
|
Study design | Low generalizability; selection bias—telephone and telemedicine consultations not randomly assigned; children in DHI group were younger than those in the control group | |
|
|
DHI related | Low telemedicine use and likely overestimated operation cost because of small cohort | |
|
|
Clinical data | No patient follow-up data to monitor potential postdischarge health problems | |
| Telephone consultation [25] | |||
|
|
Study design | Low generalizability (a single specialist referral center with high socioeconomic status); measuring differences in clinical outcomes but not in cost outcomes; double counting of parental time costs | |
|
|
Clinical data | Some information collected in self-reported questionnaires was subject to recall bias | |
| Audiovisual and telephone consultation [26] | |||
|
|
Clinical data | Parent self-reported measures leading to incidental misreporting because of memory errors (long trial period); no data collected on diagnostic remission; missing demographic data for a large percentage of the sample | |
|
|
Cost data | Costs associated with accessing mental health services not included | |
|
|
DHI related | Inconsistency between treatment arms as DHI was delivered one-to-one and usual care was delivered in group format | |
|
|
Study design | No blinding to random allocation; DHI self-selection bias; generalizability limited (narrow age range) | |
| SMS text messaging [27] | |||
|
|
Clinical and cost data | Lack of primary data (patient recruitment challenges); incomplete data records for approximately 50% of participating women upon exit interviews | |
|
|
DHI related | Most of the fixed costs of DHI did not vary with changing program scale | |
| SMS text messaging [28] | |||
|
|
DHI -related | Limited empirical data and evidence on large-scale mHealthb programs for pregnancy; thus, numerous assumptions about population and service coverage inputs | |
|
|
Economic evaluation | Model does not incorporate complexities between preventive and curative care | |
| SMS text messaging [29] | |||
|
|
Cost data | Cost data collected from a single district and did not include costs incurred by pregnant or postpartum women to seek care or to the health system to collect data | |
|
|
Study design | Methodological weaknesses in study design and data collection methods (sampling and survey tool) | |
| SMS text messaging [30] | |||
|
|
Study design | Number of participants divided into 2 groups was not equal or adequately balanced | |
|
|
DHI related | Study not able to verify whether SMS text message reminders were received and further read by clients in the DHI group | |
| SMS text messaging [31] | |||
|
|
Study design | Quasi-experimental design—not enough statistical power and adjustment for confounding factors | |
|
|
Cost data | Cost adjustment for standardized estimations to 1 million population may not incorporate changes with scaling up; household and service provision costs associated with DHI not included | |
|
|
DHI related | Intervention was a reminder, not provision of care, implying that the health outcome could have been influenced by other determinants of access and quality of care in the system | |
| SMS text messaging [32] | |||
|
|
Study design | Short follow-up; new “test and treat” malaria case management policy not tested under trial conditions; patients aged ≥5 years not included | |
| Mobile phone app [33] | |||
|
|
Cost data | Did not assess health care input cost or time spent by health workers in training and supportive supervision by medical officers and health care professionals; total cost of implementing DHI assumed to be the same in per-protocol and intention-to-treat analyses, which may be an overestimation in the per-protocol analysis | |
| Mobile phone app [34] | |||
|
|
Cost and clinical data | Not able to track provision of services for all 10 antenatal care interventions | |
|
|
Clinical data | No follow-up data to monitor compliance with hypertension management; no appropriate data to track subsequent second tetanus toxoid vaccination | |
|
|
Economic evaluation | Assumed full compliance of women given iron folate and malaria prophylaxis | |
| Mobile phone app [35] | |||
|
|
DHI related | Not clear whether the effect of several simultaneous interventions was additive, multiplicative, or otherwise | |
|
|
Cost data | Intervention scale-up costs estimated in ideal conditions without bottlenecks in implementation | |
|
|
Study design | Intervention was not randomly assigned, leading to possible confounding | |
| Web portal [36] | |||
|
|
Clinical data | Available measures of quality of life may lack validity for children and adolescents with anxiety disorders | |
|
|
Study design | Active control condition rated as being less credible at week 3; results may not be generalizable to the entire patient population (most patients were self-referred and from educated families); participants with missing data were more severely ill at baseline and in the comparator vs DHI group; short follow-up | |
| Web portal [37] | |||
|
|
Cost data | Uncertainties on ongoing vs sunk costs; ongoing costs of DHI may have been overestimated because of small sample size | |
|
|
Study design | No conclusions on noninferiority can be drawn (this was an intention-to-treat rather than per-protocol analysis) | |
| Web portal [38] | |||
|
|
Study design | Crossover from usual treatment to iCBTc after 10-week follow-up; high educational level of parents may reduce external validity; inclusion criterion of basic reading and writing skills excluded newly arrived or marginalized immigrants; not possible to blind patients and therapists to treatment assignment | |
| Web portal [39] | |||
|
|
Study design | Moderate sample size; measurements at 2 time points (before and after the intervention); short follow-up | |
| Web portal [40] | |||
|
|
Study design | Most participants were self-referred, with potential confounding effects of higher motivation to work compared with typical patients with SADd | |
|
|
Economic evaluation | Comparator less credible than iCBT | |
| Web portal [41] | |||
|
|
Cost data | Tax-funded universal health system in Sweden may affect interpretation of the results; other health care resources and societal costs were assessed retrospectively with a parent-reported measure | |
|
|
Study design | Stepped care may result in delayed treatment response and, thus, is not the preferred choice for policy makers | |
| Web portal [42] | |||
|
|
Study design | Study narrowly focused on health benefits linked to prevention of incidence of depression only | |
|
|
Economic evaluation | Model assumed that preventive interventions for depression led to a reduction in depression incidence based on the outcomes of meta-analyzed RCTe studies with short time frames; excluded evidence from RCT studies assessing depression symptom changes | |
|
|
Cost and clinical data | Data limitations (old intervention pathways, effectiveness data with high risk of bias, and lack of cost data) | |
| Web portal [43] | |||
|
|
Economic evaluation | Short time horizon because of lack of evidence on longer-term effects of DHI; no active comparator | |
|
|
Clinical data | No multi-attribute utility instrument for dimensions affected by IBSf in adolescents | |
| Web portal [44] | |||
|
|
Cost data | Cost data estimated retroactively; prospective monitoring of costs could yield more precise estimates | |
|
|
Study design | Short follow-up data collection period (2 weeks) | |
| Web-based symptom monitoring [45] | |||
|
|
Study design | Frequency of outpatient visits may differ in clinical practice compared with RCT conditions | |
|
|
DHI related | DHI partly combined with usual care in the intervention arm | |
aDHI: digital health intervention.
bmHealth: mobile health.
ciCBT: internet-delivered cognitive behavioral therapy.
dSAD: social anxiety disorder.
eRCT: randomized controlled trial.
fIBS: irritable bowel syndrome.
Lack of a well-defined comparator was observed in 75% (6/8) of the studies [26,35,36,38,40,45]. This included potential biases arising from differences in the delivery format of the DHI and comparator interventions [26] as well as the mixing of DHI and comparator interventions in certain child groups [35,45].
Lack of user involvement was reported to weaken the effectiveness of DHI interventions. This was mostly because of difficulties in maintaining the expected level of user involvement during the study period that would be desirable for the specific DHI [24,25,30].
Difficulty in measuring the impact of DHIs on costs and outcomes. This was because of either the complexity of DHI programs that was not captured by the economic evaluation [28,31] or the lack of a clear understanding of the pathways to impact, where outcomes may have been influenced by non-DHI components (eg, complementary services) [31].
Issues With Measuring Costs and Outcomes
Limitations of measuring outcomes included biases in self-reported patient information, lack of primary data on resource use, and lack of appropriate QoL measures adjusted to younger age groups (ie, use of generic rather than pediatric EQ-5D and 36-item Short Form Health Survey instruments). The most frequent challenges with cost data were incomplete resource use data, costing intervention scale-up because of difficulties in estimating the likely population size, and uncertainties regarding distinguishing and measuring operational costs versus sunk costs.
Economic Evaluation Methodological Assumptions
We identified methodological issues related to decision modeling in 23% (5/22) of the economic evaluation studies [28,34,40,42,43]. This included failure to appropriately model preventive and curative care in complex treatment pathways, short model time horizons because of lack of evidence on longer-term effects of DHIs, and issues with the validity of the input parameters (Table 5). Another prevalent issue with modeling of DHIs in child settings was the difficulty of summarizing results into a single metric, such as cost per QALY, because of the lack of generic health outcomes in this population [25,32,41] (Table 2).
Study Design Issues
The reviewed studies also faced methodological issues related to the study design (17/22, 77%). These issues primarily related to studies that used individual patient-level data (14/17, 82%) rather than decision analytic models. At the clinical trial initiation/enrollment stage, these issues included selection bias and the absence of blinding to random allocation. For instance, 12% (2/17) of the studies faced potential confounding because of age differences between the intervention and control groups [24,35]. In addition, participants in some of the studies were permitted to self-refer, resulting in self-selection bias [24,26,40]. Other limitations related to the study design included low generalizability/external validity, small sample size because of the difficulty of recruiting children and adolescents for DHI programs, short study follow-up period, and incomplete data because of loss to follow-up (Table 5). Low generalizability was also a common issue in the included studies [24-26,36]. For instance, a narrow age range of the trial population posed a barrier to generalizing the findings of the economic evaluations to a wider health care setting or patient population [26].
Discussion
Principal Findings
This systematic review identified 22 economic evaluations of DHI interventions for children and adolescents. Most studies (14/22, 64%) evaluated interventions delivered through online portals or SMS text messaging, most frequently within the health care specialties of mental health and MNCH. In 82% (18/22) of the reviewed studies, the DHI was cost-effective or cost saving compared with the standard of care. The studies reported various levels of granularity of cost components used in the economic evaluations; however, most studies (18/22, 82%) included direct medical costs and DHI costs broken up into 3 major components: DHI development, DHI start-up, and DHI implementation. More than half (12/22, 55%) of the reviewed studies also included indirect costs, such as productivity losses because of missed hours from school for children and from paid and unpaid work for parents and caregivers. The methodological challenges commonly identified in the reviewed studies suggest that issues with study design were the most prevalent (17/22, 77%), followed by issues with cost data (11/22, 50%) and challenges related to decision modeling (5/22, 23%). The most frequent drivers of cost-effectiveness included population coverage, implementation and user support costs, and intervention effect size.
Comparison With the Existing Literature and Method Guidelines
This study provides the first systematic review of economic evaluations of DHIs targeting pediatric and adolescent populations. In line with the published literature on adult populations, we found that many of the economic evaluations of DHIs for children and adolescents focused on the management of long-term conditions and mental health, primarily in the form of CBT, cardiac monitoring, and weight loss and diabetes management [5,11,46,47]. In these areas, our findings are very much in line with those for adult populations. For example, our review found SMS text message reminders used for weight loss management to be cost-effective, similar to findings for adult populations [11]. However, we identified other areas where DHIs for children and adolescents are often cost-effective or cost saving compared with usual care in contrast to related literature in adult settings [11]. For example, videoconferencing sessions for children and adolescents were found to be cost-effective, whereas similar videoconferencing interventions in adult populations are rarely cost-effective, as well as having no significant impact on health-related QoL [11].
Most of the studies (19/22, 86%) included in this review are unlikely to comprehensively meet the evidence standards framework published by the National Institute for Health and Care Excellence (NICE) and the World Health Organization [3,48]. For example, NICE guidelines suggest the need for generalizability of economic evaluation assumptions beyond the local context, but most of the reviewed studies (15/22, 68%) focused on a narrow patient population with specific characteristics that are not directly generalizable. In addition, many of the reviewed studies (12/22, 55%) did not have a sufficiently long time horizon to capture the relevant health outcomes and costs and did not provide a clear justification for the chosen sensitivity analysis as per NICE recommendations [48].
Strengths
This review adds to the current body of literature by identifying some key methodological issues in the reviewed economic evaluations that are relevant to the pediatric setting. These include mixing of DHI and comparator interventions across treatment arms, inconsistencies in accounting for different cost components of DHIs (eg, maintenance, sunk, and scale-up costs), and difficulties maintaining user involvement.
The methodological issues specific to the nature of DHIs and the difficulties of comparing economic evaluations of DHIs because of heterogeneity align well with those previously identified in the published literature [1,2,11,49,50]. These include lack of established measures for clinical effectiveness of DHIs, difficulty in distinguishing DHIs from “standard of care,” and inability to measure and model broader costs and effects of DHIs beyond the direct impact on the health system (ie, production losses, travel costs, absenteeism, and presenteeism for parents and caregivers). In the context of the published literature, many of the study design issues were similar to those faced in clinical studies of health care interventions more broadly and included selection bias, small sample size, low generalizability, short or no follow-up period, and no blinding to random allocation.
Limitations
This systematic review has some limitations. The database search was limited to studies published between 2011 and 2021 and indexed in PubMed. Considering that DHIs have received increasing attention in the last decade, this time restriction may be reasonable. This review was based on the MEDLINE database and has not been comprehensively combined with other databases. However, when we performed a high-level search in Embase, it did not retrieve any additional relevant studies, suggesting that the PubMed search strategy likely covered the vast majority of the relevant economic evaluation studies.
This review did not assess the reporting quality of the reviewed studies in line with reporting guidelines such as CHEERS (Consolidated Health Economic Evaluation Reporting Standards) [51] for 2 reasons: first, published economic evaluations are typically required to follow the CHEERS checklist, and second, our quality assessment focused on a wider range of methodological issues regarding economic evaluations, which included aspects more relevant to the conduct of economic evaluations of DHIs as well as generic quality aspects such as study design.
Suggestions for Further Research
This review provides a critique of methodological challenges that may arise in economic evaluations of DHIs tailored to pediatric and adolescent populations. Numerous DHIs are produced and adopted at pace, and economic evaluations considering the specific features of DHIs are needed. Economic evaluations with significant limitations may lead to suboptimal decisions and poor use of limited health care resources. In this section, we highlight some methodological areas that, in our view, would constitute interesting avenues for further research.
First, a common issue highlighted across the reviewed studies was the use of health outcome measures that are clinically relevant but less likely to meet the requirements for cost-effectiveness assessments, such as generic QoL measures. The development and validation of generic QoL measures for children and adolescents is beginning to emerge [52], but further research is warranted. For example, it is important to understand whether the measures are consistent across different age groups. Second, a broader perspective capturing indirect and non–health care costs and benefits is generally a preferred perspective in economic evaluations, but our review shows that it has not yet been used consistently for the evaluation of DHIs for children and adolescents. This could be addressed through more carefully designed data collection of indirect costs that account for health- and non–health-related burden to both children and their caregivers [11]. Third, although there have been some efforts to measure user participation (eg, user time) in the evaluation of DHIs [2,49,53], further research is needed to develop a more holistic value framework for formally valuing and incorporating user involvement in the cost and outcome analysis. In addition, following international guidelines and recommendations may improve the quality and consistency of economic evaluations of DHIs.
Conclusions
Our study found that most (18/22, 82%) DHIs for children and adolescents are cost-effective or cost saving compared with the nondigital standard of care from either the health system/payer or societal viewpoint. However, there was a substantial degree of variability in cost-effectiveness results depending on the type of DHI, health care setting, and inclusion of certain input parameters in the economic evaluations. The cost-effectiveness of these DHIs appeared to be sensitive to the inclusion of certain cost components (particularly DHI implementation and supervision/training of health care professionals), intervention effect size, and its potential to be scaled up. This study highlights common methodological challenges directly related to the conduct of economic evaluations of DHIs. These included failure to measure user involvement, generic QoL outcomes, and indirect effects of DHIs, highlighting areas where further methodological research is required.
Acknowledgments
This work was supported by the National Institute for Health and Care Research (). The content is solely the responsibility of the authors, and the study’s findings and conclusions do not necessarily represent the official position of the National Institute for Health and Care Research.
Abbreviations
- CBT
cognitive behavioral therapy
- CHEERS
Consolidated Health Economic Evaluation Reporting Standards
- CUA
cost-utility analysis
- DALY
disability-adjusted life year
- DHI
digital health intervention
- ICER
incremental cost-effectiveness ratio
- IPD
individual patient data
- MNCH
maternal, newborn, and child health
- NICE
National Institute for Health and Care Excellence
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
quality-adjusted life year
- QoL
quality of life
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist [15].
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during this study.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Murray E, Hekler EB, Andersson G, Collins LM, Doherty A, Hollis C, Rivera DE, West R, Wyatt JC. Evaluating digital health interventions: key questions and approaches. Am J Prev Med. 2016 Nov;51(5):843–51. doi: 10.1016/j.amepre.2016.06.008. https://europepmc.org/abstract/MED/27745684 .S0749-3797(16)30229-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes M, Murray E, Raftery J. Economic evaluation of digital health interventions: methodological issues and recommendations for practice. Pharmacoeconomics. 2022 Apr 08;40(4):367–78. doi: 10.1007/s40273-022-01130-0. https://europepmc.org/abstract/MED/35132606 .10.1007/s40273-022-01130-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO guideline: recommendations on digital interventions for health system strengthening. World Health Organization. 2019. [2022-05-08]. https://apps.who.int/iris/bitstream/handle/10665/311941/9789241550505-eng.pdf?ua=1 . [PubMed]
- 4.Soobiah C, Cooper M, Kishimoto V, Bhatia RS, Scott T, Maloney S, Larsen D, Wijeysundera HC, Zelmer J, Gray CS, Desveaux L. Identifying optimal frameworks to implement or evaluate digital health interventions: a scoping review protocol. BMJ Open. 2020 Aug 13;10(8):e037643. doi: 10.1136/bmjopen-2020-037643. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=32792444 .bmjopen-2020-037643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang X, Ming W-K, You JH. The cost-effectiveness of digital health interventions on the management of cardiovascular diseases: systematic review. J Med Internet Res. 2019 Jun 17;21(6):e13166. doi: 10.2196/13166. https://www.jmir.org/2019/6/e13166/ v21i6e13166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanyal C, Stolee P, Juzwishin D, Husereau D. Economic evaluations of eHealth technologies: a systematic review. PLoS One. 2018 Jun 13;13(6):e0198112. doi: 10.1371/journal.pone.0198112. https://dx.plos.org/10.1371/journal.pone.0198112 .PONE-D-17-32371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darling KE, Sato AF. Systematic review and meta-analysis examining the effectiveness of mobile health technologies in using self-monitoring for pediatric weight management. Child Obes. 2017 Oct;13(5):347–55. doi: 10.1089/chi.2017.0038. [DOI] [PubMed] [Google Scholar]
- 8.Hollis C, Falconer CJ, Martin JL, Whittington C, Stockton S, Glazebrook C, Davies EB. Annual research review: digital health interventions for children and young people with mental health problems - a systematic and meta-review. J Child Psychol Psychiatry. 2017 Apr 10;58(4):474–503. doi: 10.1111/jcpp.12663. [DOI] [PubMed] [Google Scholar]
- 9.Rooksby M, Elouafkaoui P, Humphris G, Clarkson J, Freeman R. Internet-assisted delivery of cognitive behavioural therapy (CBT) for childhood anxiety: systematic review and meta-analysis. J Anxiety Disord. 2015 Jan;29:83–92. doi: 10.1016/j.janxdis.2014.11.006.S0887-6185(14)00172-8 [DOI] [PubMed] [Google Scholar]
- 10.Brigden A, Anderson E, Linney C, Morris R, Parslow R, Serafimova T, Smith L, Briggs E, Loades M, Crawley E. Digital behavior change interventions for younger children with chronic health conditions: systematic review. J Med Internet Res. 2020 Jul 31;22(7):e16924. doi: 10.2196/16924. https://www.jmir.org/2020/7/e16924/ v22i7e16924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentili A, Failla G, Melnyk A, Puleo V, Tanna GL, Ricciardi W, Cascini F. The cost-effectiveness of digital health interventions: a systematic review of the literature. Front Public Health. 2022 Aug 11;10:787135. doi: 10.3389/fpubh.2022.787135. https://europepmc.org/abstract/MED/36033812 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchesson MJ, Rollo ME, Krukowski R, Ells L, Harvey J, Morgan PJ, Callister R, Plotnikoff R, Collins CE. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015 May;16(5):376–92. doi: 10.1111/obr.12268. [DOI] [PubMed] [Google Scholar]
- 13.de la Torre-Díez I, López-Coronado M, Vaca C, Aguado JS, de Castro C. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemed J E Health. 2015 Feb;21(2):81–5. doi: 10.1089/tmj.2014.0053. https://europepmc.org/abstract/MED/25474190 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mistry H. Systematic review of studies of the cost-effectiveness of telemedicine and telecare. Changes in the economic evidence over twenty years. J Telemed Telecare. 2012 Jan;18(1):1–6. doi: 10.1258/jtt.2011.110505.jtt.2011.110505 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. https://dx.plos.org/10.1371/journal.pmed.1000097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergmo TS. How to measure costs and benefits of eHealth interventions: an overview of methods and frameworks. J Med Internet Res. 2015 Nov 09;17(11):e254. doi: 10.2196/jmir.4521. https://www.jmir.org/2015/11/e254/ v17i11e254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummond M, Stoddart, GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford, UK: Oxford University Press; 1987. [Google Scholar]
- 18.van IJzendoorn MH, Bakermans-Kranenburg MJ. Problematic cost-utility analysis of interventions for behavior problems in children and adolescents. New Dir Child Adolesc Dev. 2020 Jul 10;2020(172):89–102. doi: 10.1002/cad.20360. https://europepmc.org/abstract/MED/32909695 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Do child QALYs = adult QALYs? Five reasons why they might not. London Office of Health Economics. 2020. Feb 4, [2023-01-12]. http://www.ohe.org/news/do-child-qalysadult-qalys-five-reasons-why-they-might-not .
- 20.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, Salomon JA, Sculpher MJ, Trikalinos TA, Russell LB, Siegel JE, Ganiats TG. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016 Sep 13;316(10):1093–103. doi: 10.1001/jama.2016.12195.2552214 [DOI] [PubMed] [Google Scholar]
- 21.HMRC exchange rates for 2021: monthly. United Kingdom Government. 2020. Dec 21, [2022-12-22]. https://www.gov.uk/government/publications/hmrc-exchange-rates-for-2021-monthlywww.gov.uk)
- 22.US dollar (USD) European Central Bank. [2022-12-22]. https://www.ecb.europa.eu/stats/policy_and_exchange_rates/euro_reference_exchange_rates/html/eurofxref-graph-usd.en.html .
- 23.Australian Taxation Office Foreign currency exchange rates for the calendar year ending December 2021. Foreign currency equivalent to $1 Aust. Australian Taxation Office. 2022. [2022-12-22]. https://www.ato.gov.au/Tax-professionals/TP/Calendar-year-ending-31-December-2021/
- 24.Yang NH, Dharmar M, Yoo B-K, Leigh JP, Kuppermann N, Romano PS, Nesbitt TS, Marcin JP. Economic evaluation of pediatric telemedicine consultations to rural emergency departments. Med Decis Making. 2015 May 07;35(6):773–83. doi: 10.1177/0272989x15584916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatterton ML, Rapee RM, Catchpool M, Lyneham HJ, Wuthrich V, Hudson JL, Kangas M, Mihalopoulos C. Economic evaluation of stepped care for the management of childhood anxiety disorders: results from a randomised trial. Aust N Z J Psychiatry. 2019 Jul 18;53(7):673–82. doi: 10.1177/0004867418823272. [DOI] [PubMed] [Google Scholar]
- 26.Olthuis JV, McGrath PJ, Cunningham CE, Boyle MH, Lingley-Pottie P, Reid GJ, Bagnell A, Lipman EL, Turner K, Corkum P, Stewart SH, Berrigan P, Sdao-Jarvie K. Distance-delivered parent training for childhood disruptive behavior (Strongest Families™): a randomized controlled trial and economic analysis. J Abnorm Child Psychol. 2018 Nov 8;46(8):1613–29. doi: 10.1007/s10802-018-0413-y.10.1007/s10802-018-0413-y [DOI] [PubMed] [Google Scholar]
- 27.LeFevre A, Cabrera-Escobar MA, Mohan D, Eriksen J, Rogers D, Neo Parsons A, Barre I, Jo Y, Labrique A, Coleman J. Forecasting the value for money of mobile maternal health information messages on improving utilization of maternal and child health services in Gauteng, South Africa: cost-effectiveness analysis. JMIR Mhealth Uhealth. 2018 Jul 27;6(7):e153. doi: 10.2196/mhealth.8185. https://mhealth.jmir.org/2018/7/e153/ v6i7e153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jo Y, LeFevre A, Ali H, Mehra S, Alland K, Shaikh S, Haque R, Pak ES, Chowdhury M, Labrique AB. mCARE, a digital health intervention package on pregnancy surveillance and care-seeking reminders from 2018 to 2027 in Bangladesh: a model-based cost-effectiveness analysis. BMJ Open. 2021 Apr 01;11(4):e042553. doi: 10.1136/bmjopen-2020-042553. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=33795294 .bmjopen-2020-042553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willcox M, Moorthy A, Mohan D, Romano K, Hutchful D, Mehl G, Labrique A, LeFevre A. Mobile technology for community health in ghana: is maternal messaging and provider use of technology cost-effective in improving maternal and child health outcomes at scale? J Med Internet Res. 2019 Feb 13;21(2):e11268. doi: 10.2196/11268. https://www.jmir.org/2019/2/e11268/ v21i2e11268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawakatsu Y, Oyeniyi Adesina A, Kadoi N, Aiga H. Cost-effectiveness of SMS appointment reminders in increasing vaccination uptake in Lagos, Nigeria: a multi-centered randomized controlled trial. Vaccine. 2020 Sep 29;38(42):6600–8. doi: 10.1016/j.vaccine.2020.07.075.S0264-410X(20)31019-7 [DOI] [PubMed] [Google Scholar]
- 31.Jo Y, LeFevre AE, Healy K, Singh N, Alland K, Mehra S, Ali H, Shaikh S, Haque R, Christian P, Labrique AB. Costs and cost-effectiveness analyses of mCARE strategies for promoting care seeking of maternal and newborn health services in rural Bangladesh. PLoS One. 2019;14(10):e0223004. doi: 10.1371/journal.pone.0223004. https://dx.plos.org/10.1371/journal.pone.0223004 .PONE-D-19-14266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zurovac D, Larson BA, Sudoi RK, Snow RW. Costs and cost-effectiveness of a mobile phone text-message reminder programmes to improve health workers' adherence to malaria guidelines in Kenya. PLoS One. 2012 Dec 18;7(12):e52045. doi: 10.1371/journal.pone.0052045. https://dx.plos.org/10.1371/journal.pone.0052045 .PONE-D-12-30752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modi D, Saha S, Vaghela P, Dave K, Anand A, Desai S, Shah P. Costing and cost-effectiveness of a mobile health intervention (ImTeCHO) in improving infant mortality in tribal areas of Gujarat, India: cluster randomized controlled trial. JMIR Mhealth Uhealth. 2020 Oct 14;8(10):e17066. doi: 10.2196/17066. https://mhealth.jmir.org/2020/10/e17066/ v8i10e17066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowser DM, Shepard DS, Nandakumar A, Okunogbe A, Morrill T, Halasa-Rappell Y, Jordan M, Mushi F, Boyce C, Erhunmwunse OA. Cost effectiveness of mobile health for Antenatal care and facility births in Nigeria. Ann Glob Health. 2018 Nov 05;84(4):592–602. doi: 10.9204/aogh.2364. https://europepmc.org/abstract/MED/30779506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prinja S, Bahuguna P, Gupta A, Nimesh R, Gupta M, Thakur JS. Cost effectiveness of mHealth intervention by community health workers for reducing maternal and newborn mortality in rural Uttar Pradesh, India. Cost Eff Resour Alloc. 2018 Jun 25;16(1):25. doi: 10.1186/s12962-018-0110-2. https://resource-allocation.biomedcentral.com/articles/10.1186/s12962-018-0110-2 .110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jolstedt M, Wahlund T, Lenhard F, Ljótsson B, Mataix-Cols D, Nord M, Öst L-G, Högström J, Serlachius E, Vigerland S. Efficacy and cost-effectiveness of therapist-guided internet cognitive behavioural therapy for paediatric anxiety disorders: a single-centre, single-blind, randomised controlled trial. Lancet Child Adolesc Health. 2018 Nov;2(11):792–801. doi: 10.1016/S2352-4642(18)30275-X.S2352-4642(18)30275-X [DOI] [PubMed] [Google Scholar]
- 37.De Bruin EJ, van Steensel FJ, Meijer AM. Cost-effectiveness of group and internet cognitive behavioral therapy for insomnia in adolescents: results from a randomized controlled trial. Sleep. 2016 Aug 01;39(8):1571–81. doi: 10.5665/sleep.6024. https://europepmc.org/abstract/MED/27306272 .sp-00634-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lalouni M, Ljótsson B, Bonnert M, Ssegonja R, Benninga M, Bjureberg J, Högström J, Sahlin H, Simrén M, Feldman I, Hedman-Lagerlöf E, Serlachius E, Olén O. Clinical and cost effectiveness of online cognitive behavioral therapy in children with functional abdominal pain disorders. Clin Gastroenterol Hepatol. 2019 Oct;17(11):2236–44.e11. doi: 10.1016/j.cgh.2018.11.043. https://linkinghub.elsevier.com/retrieve/pii/S1542-3565(18)31320-X .S1542-3565(18)31320-X [DOI] [PubMed] [Google Scholar]
- 39.Lenhard F, Ssegonja R, Andersson E, Feldman I, Rück C, Mataix-Cols D, Serlachius E. Cost-effectiveness of therapist-guided internet-delivered cognitive behaviour therapy for paediatric obsessive-compulsive disorder: results from a randomised controlled trial. BMJ Open. 2017 May 17;7(5):e015246. doi: 10.1136/bmjopen-2016-015246. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=28515196 .bmjopen-2016-015246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordh M, Wahlund T, Jolstedt M, Sahlin H, Bjureberg J, Ahlen J, Lalouni M, Salomonsson S, Vigerland S, Lavner M, Öst L-G, Lenhard F, Hesser H, Mataix-Cols D, Högström J, Serlachius E. Therapist-guided internet-delivered cognitive behavioral therapy vs internet-delivered supportive therapy for children and adolescents with social anxiety disorder: a randomized clinical trial. JAMA Psychiatry. 2021 Jul 01;78(7):705–13. doi: 10.1001/jamapsychiatry.2021.0469. https://europepmc.org/abstract/MED/33978699 .2779637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aspvall K, Sampaio F, Lenhard F, Melin K, Norlin L, Serlachius E, Mataix-Cols D, Andersson E. Cost-effectiveness of internet-delivered vs in-person cognitive behavioral therapy for children and adolescents with obsessive-compulsive disorder. JAMA Netw Open. 2021 Jul 01;4(7):e2118516. doi: 10.1001/jamanetworkopen.2021.18516. https://europepmc.org/abstract/MED/34328501 .2782562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YY, Barendregt JJ, Stockings EA, Ferrari AJ, Whiteford HA, Patton GA, Mihalopoulos C. The population cost-effectiveness of delivering universal and indicated school-based interventions to prevent the onset of major depression among youth in Australia. Epidemiol Psychiatr Sci. 2017 Oct 11;26(5):545–64. doi: 10.1017/S2045796016000469. https://europepmc.org/abstract/MED/27509769 .S2045796016000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sampaio F, Bonnert M, Olén O, Hedman E, Lalouni M, Lenhard F, Ljótsson B, Ssegonja R, Serlachius E, Feldman I. Cost-effectiveness of internet-delivered cognitive-behavioural therapy for adolescents with irritable bowel syndrome. BMJ Open. 2019 Jan 24;9(1):e023881. doi: 10.1136/bmjopen-2018-023881. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=30679293 .bmjopen-2018-023881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wasil A, Kacmarek C, Osborn T, Palermo EH, DeRubeis RJ, Weisz JR, Yates BT. Economic evaluation of an online single-session intervention for depression in Kenyan adolescents. J Consult Clin Psychol. 2021 Aug;89(8):657–67. doi: 10.1037/ccp0000669.2021-80268-001 [DOI] [PubMed] [Google Scholar]
- 45.van den Wijngaart LS, Kievit W, Roukema J, Boehmer AL, Brouwer ML, Hugen CA, Niers LE, Sprij AJ, Rikkers-Mutsaerts ER, Rottier BL, Verhaak CM, Pijnenburg MW, Merkus PJ. Online asthma management for children is cost-effective. Eur Respir J. 2017 Oct 05;50(4):1701413. doi: 10.1183/13993003.01413-2017. http://erj.ersjournals.com/cgi/pmidlookup?view=long&pmid=28982768 .50/4/1701413 [DOI] [PubMed] [Google Scholar]
- 46.Hedman E, Ljótsson B, Lindefors N. Cognitive behavior therapy via the internet: a systematic review of applications, clinical efficacy and cost-effectiveness. Expert Rev Pharmacoecon Outcomes Res. 2012 Dec;12(6):745–64. doi: 10.1586/erp.12.67. [DOI] [PubMed] [Google Scholar]
- 47.Arnberg FK, Linton SJ, Hultcrantz M, Heintz E, Jonsson U. Internet-delivered psychological treatments for mood and anxiety disorders: a systematic review of their efficacy, safety, and cost-effectiveness. PLoS One. 2014;9(5):e98118. doi: 10.1371/journal.pone.0098118. https://dx.plos.org/10.1371/journal.pone.0098118 .PONE-D-14-08409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evidence standards framework for digital health technologies. National Institute for Health and Care Excellence. 2018. Dec 10, [2023-01-07]. https://www.nice.org.uk/corporate/ecd7 .
- 49.Babigumira JB, Dolan S, Shade S, Puttkammer N, Bale J, Tolentino H, Santas XM. Applied economic evaluation of digital health interventions. Centers for Disease Control and Prevention, International Training & Education Center for Health. 2021. Jan, [2022-12-06]. https://www.go2itech.org/wp-content/uploads/2021/02/I-TECH_HIS_Economic_Evaluation.pdf .
- 50.Puleo V, Gentili A, Failla G, Melnyk A, Di Tanna G, Ricciardi W, Cascini F. Digital health technologies: a systematic review of their cost-effectiveness. Eur J Public Health. 2021 Oct;31(Supplement_3):ckab164.273. doi: 10.1093/eurpub/ckab164.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, Caulley L, Chaiyakunapruk N, Greenberg D, Loder E, Mauskopf J, Mullins CD, Petrou S, Pwu R-F, Staniszewska S, CHEERS 2022 ISPOR Good Research Practices Task Force Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022 Jan;25(1):3–9. doi: 10.1016/j.jval.2021.11.1351. https://linkinghub.elsevier.com/retrieve/pii/S1098-3015(21)03146-6 .S1098-3015(21)03146-6 [DOI] [PubMed] [Google Scholar]
- 52.Rowen D, Rivero-Arias O, Devlin N, Ratcliffe J. Review of valuation methods of preference-based measures of health for economic evaluation in child and adolescent populations: where are we now and where are we going? Pharmacoeconomics. 2020 Apr 06;38(4):325–40. doi: 10.1007/s40273-019-00873-7.10.1007/s40273-019-00873-7 [DOI] [PubMed] [Google Scholar]
- 53.Pagliari C. Design and evaluation in eHealth: challenges and implications for an interdisciplinary field. J Med Internet Res. 2007 May 27;9(2):e15. doi: 10.2196/jmir.9.2.e15. https://www.jmir.org/2007/2/e15/ v9i2e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist [15].
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during this study.


